Abstract
Previous work from our group (Ravasio A, Hobi N, Bertocchi C, Jesacher A, Dietl P, Haller T. Am J Physiol Cell Physiol 300: C1456–C1465, 2011.) showed that contact of alveolar epithelial type II cells with an air-liquid interface (IAL) leads to a paradoxical situation. It is a potential threat that can cause cell injury, but also a Ca2+-dependent stimulus for surfactant secretion. Both events can be explained by the impact of interfacial tensile forces on cellular structures. Here, the strength of this mechanical stimulus became also apparent in microarray studies by a rapid and significant change on the transcriptional level. Cells challenged with an IAL in two different ways showed activation/inactivation of cellular pathways involved in stress response and defense, and a detailed Pubmatrix search identified genes associated with several lung diseases and injuries. Altogether, they suggest a close relationship of interfacial stress sensation with current models in alveolar micromechanics. Further similarities between IAL and cell stretch were found with respect to the underlying signaling events. The source of Ca2+ was extracellular, and the transmembrane Ca2+ entry pathway suggests the involvement of a mechanosensitive channel. We conclude that alveolar type II cells, due to their location and morphology, are specific sensors of the IAL, but largely protected from interfacial stress by surfactant release.
Keywords: cell deformation, cell injury, mechanotransduction, microarray, stretch
in the alveoli, mechanical forces are a constant modulator of tissue geometry and function (4). These forces arise from multiple sources, such as cyclic stretching and compression during respiration, changes in transcapillary pressure, or surface tension at the air-liquid interface (IAL) (22). They are probably nonuniform in space and time (26, 51, 67) and impose either physiological responses of the cells, or pathological ones, depending on the type of tissue or the magnitude or abnormality of forces that are at work (reviewed in Refs. 18, 66, 69, 72). In particular, tissue stretch (= tensile strain) has already been shown to be an important determinant of surfactant secretion in alveolar type II (AT II) cells (2, 15, 36, 47, 71, 73). Studies on single cells revealed that stretch induces an elevation of the intracellular Ca2+ concentration ([Ca2+]i) (23, 73), perhaps via mechanosensitive ion channels like the well-characterized transient receptor potential (TRP) vanilloids (reviewed in Refs. 16, 77). Recently, the panoply of mechanosensitive mechanisms was expanded by the finding that the IAL, the “normal” microenvironment of the AT II cells (6), may be a strong mechanical incentive as well. Two recent independent studies (55, 56) described a stimulating, but also harmful, effect of an IAL on the AT II cell function. Interestingly, interfacial contact is followed by an increase in [Ca2+]i and release of ATP, both of which stimulate secretion (reviewed in Refs. 1, 44, 59). It has even been speculated that this response could act within a feedback loop to continuously monitor and to adjust the physical properties of the interface or the hypophase below.
In addition to surfactant secretion, mechanical forces have been discussed to profoundly alter cell metabolism and tissue differentiation (18). Particularly, the AT II cells seem to be very adaptable to changes in their local environment and to the state of alveolar micromechanics. After tissue damage, for example, they proliferate (in vivo) and differentiate into AT I cells to restore the structural integrity (reviewed in Refs. 18, 19, 43). Notably, more than 600 genes have been indentified so far to be differentially expressed in either of these two cell types subject to various stretching or hyperinflation protocols (30). These studies have been performed in animal models, isolated lungs, or AT II-like cell types. However, until now, no such experiments have been performed with primary cultures of AT II cells.
In this report, transcriptomic analysis was performed to test the concept of an IAL as a mechanical stimulus, stress factor, and/or regulator of the AT II cells. For this purpose, cultured AT II cells were exposed to two different interfacial conditions. A second aim was to further elucidate possible mechanisms in the sensation of an IAL by using agonists and inhibitors for various Ca2+-dependent channels and pathways. The results support the concept that the IAL is a strong stimulus for AT II cells, inducing cellular responses that seem to be closely associated with mechanotransduction, stress response, and alveolar repair. Furthermore, we show that surfactant protects the AT II cells from the detrimental contact with the interface and prevents cell damage.
METHODS
Cell isolation and culture conditions.
This study was reviewed and approved by the Austrian Government, and cell preparations were conducted in conformity with the Austrian rules for animal care and testing (a license from the Austrian Government has been granted to T. Haller). The AT II cells were isolated from male Sprague-Dawley rats according to standard protocols described elsewhere (17, 34). The cells were plated in sterile multiwell tissue culture plates (all Greiner) and left for 48 h in DMEM (Sigma-Aldrich) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 24 mM NaHCO3, and 10% FCS (Biochrom) in a humidified 5% CO2 atmosphere of 37°C. Culture media were changed after 24 and 36 h to reduce the number of macrophages (45). When verified by microscopy, their fraction always amounted to ≤1%. NIH3T3 fibroblasts (3T3) were grown in culture dishes in MEM (Sigma-Aldrich) supplemented with 10% FCS, 4 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Subcultures were routinely established on every second to third day of cultivation by 0.5% trypsin/EDTA treatment. The immortalized human dermal microvascular endothelial cell-line (HMEC-1) was maintained in MCDB-131 medium (Gibco BRL) supplemented with 10% FCS, 2 mM glutamax, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.2 μg/ml hydrocortisone, and 10 ng/ml EGF. Confluent cells were subcultivated by trypsinization (see above) every week. Cell medium supplements (hydrocortisone, EGF) were obtained from Gibco BRL.
Conventional interface experiments.
We defined conventional interface experiments as those in which cells were exposed to an IAL by aspiration of the cell medium. This was performed by tilting the respective cell culture plates and by careful removal of the fluid, from a corner, with a pipette. Then the wells were exposed for the indicated times to ambient air at room temperature and a relative humidity (RH) of 50%, adjusted by an automated humidifier (Monsun 300, Suntec) within the entire laboratory space (adjustment of the RH was crucial to obtain a defined rate of water evaporation). Conventional interface experiments comprise the Ca2+, lactate dehydrogenase (LDH), and ATP measurements and the LSM microscopy. Microarrays were performed with both the conventional and the inverted interface approaches. For the conventional interface microarray studies, cells were exposed to air for 7 min (see above) and then incubated for 1 and 4 h, respectively, in cell medium. Afterwards, cells were lysed by adding 350 μl RLT buffer (Qiagen). For every group (control, 1 h, 4 h), lysed cells from 16 wells were pooled to obtain sufficient amounts for RNA extraction (∼5 × 105 cells). The experiment was run in triplicate (= independent preparations). Cells were stored at −80°C.
Inverted interface experiments.
Our inverted interface model was recently used to analyze calcium signals and surfactant adsorption at the IAL. For the details, we thus refer to these publications (8, 33, 57). In the present study, we used a specially designed, slightly different kind of chamber (200-μm holes made in stainless steel, 45 holes/plate; see Fig. 4, A and B) to run multiple experiments in parallel to obtain enough total RNA. Cells from primary culture were detached from the dishes by mild trypsinization (0.25%; 5 min), followed by a stopping reaction with DMEM + 10% FCS and a gentle centrifugation (800 g, 3 min). Cells were resuspended in 5% CO2 enriched medium without FCS, and 30 μl of cell suspension, containing ∼100 cells, were pipetted into each hole of the inverted interface plate, as shown in Fig. 4A. After sedimentation of the cells at the IAL (10 min; see Fig. 4, C and D), medium above the cells was exchanged (to exclude slow sedimenting and/or buoyant material), and the plate was incubated with an atmosphere of 5% CO2 and a RH <10% at 37°C for 4 h. Afterwards, cells were collected by applying a compression (see Fig. 4E), which pushed all cells at the IAL into a collecting chamber containing small amounts of RLT lysis buffer (Qiagen). Samples were stored at −80°C.
Fig. 4.
Inverted interface experiments. A: a chamber with a circular 200-μm aperture at its bottom keeps an IAL in a planar position above a long-distance objective of an inverted microscope. Cells, when applied from top of the fluid-filled chamber (volume 30 μl), settle down to the IAL by sedimentation (arrow). B: entire plate containing 45 chambers. C: bright-field image of the aperture (= IAL), demonstrating cells in interface contact. D: time dependence of cell arrival at the IAL (n = 3; mean ± SD). E: cell collection. After 4-h IAL contact, medium above the cells was removed up to the indicated level (dotted line), and cells were collected, by applying a pressure (P) onto the silicon membrane, in the lysis buffer below.
Confocal microscopy.
Time-resolved z-sectioning was performed with an SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) equipped with a fast resonant scanner. We used a HCX PL APO lambda blue 63 × 1.2 water immersion objective. Imaging was performed using the 488-nm laser line for the FM 1–43 staining. Fluorescence emission was detected from 496 to 600 nm; image sequence was acquired every 5 s for 10 min and further processed using the LAS AF acquisition software version 2.1.0. (Leica Microsystems).
RNA isolation and microarray analysis.
Sample processing (RNA extraction and quantification) and array hybridization were performed at an Affymetrix service provider and core facility (KFB Center of Excellence for Fluorescent Bioanalytics, Regensburg, Germany). Total RNA was extracted using RNAeasy Mini Kit (Qiagen), according to manufacturer's instructions. Concentration and quality of samples were determined spectrophotometrically using Nanodrop (Peqlab) and gel electrophoresis on a Bioanalyzer 2100 (Agilent). RNA sample processing and generation of biotinylated single-stranded DNA fragments were performed by the Affymetrix GeneChip Whole Transcript Sense Target Labeling Assay protocol (Affymetrix). Samples were hybridized to an Affymetrix Rat Gene 1.0 ST microarray, washed on a Fluidics Station 450, and scanned using the GeneChip Scanner 3000–7G (Affymetrix). For the inverted interface experiments (small yield of RNA), a specially designed processing protocol was used. Here, the preparation of the extracted RNA was performed with following kits from NuGEN: Pico SL WTA (synthesis of single-stranded SPIA DNA); Exon Module (synthesis of the double-stranded DNA); and Encore Biotin Module (labeling of the DNA). All of the following steps were performed with the standard Affymetrix protocol with some modification suggested by NuGEN.
The microarray enables expression profiling for ≥27,000 transcripts and up to 40 probes per gene. Basic data analysis (microarray quality report, background adjustment, quantile normalization, probe set signal summarization) was done using Affymetrix Expression Console software. Fold change calculations and statistical tests (Student's t-test) were performed in Excel. First the log2 signal of the treated sample was subtracted by the log2 signal of the control to obtain a log signal ratio. Ratios of the three replicates were averaged, and the fold change calculated. Significance was considered at P < 0.05 and fold changes of ±1.5 (conventional interface) or ±2.0 (inverted interface). Heat map was designed with Java TreeView and Excel. Analysis of Gene Ontology (GO terms) was performed using DAVID 6.7. GO terms are defined categories representing gene product properties, which covers the domain's cellular component, molecular function, and biological process. DAVID discovers enriched functionally related genes by comparing the background population with the uploaded gene list (37). DAVID poses predesigned Affymetrix background (RaGene-1–0-st-v1). Threshold was set at 0.05 Ease Score. Text-based data mining, using search terms shown in Fig. 3 and 6, was made with Pubmatrix (PubMed, National Institutes of Health). Microarray data are available at MIAMExpress of the EMBL-EBI Database, ArrayExpress accession: E-MEXP-3363 (conventional interface) and E-MEXP-3372 (inverted interface).
Fig. 3.
Text-based data mining of conventional interface experiments. Significant regulated genes are shown that are reported in the literature and associated in Pubmatrix with the indicated search terms. Separate analyses were performed for up- and downregulated genes. Results are reported as the percentage of genes, available in the Pubmatrix database and associated with the given search terms, of all up- (11 = 100%) and all downregulated genes (27 = 100%). C-IAL, conventional IAL; ALI, acute lung injury; VALI, ventilator-associated lung injury; VILI, ventilator-induced lung injury.
Fig. 6.
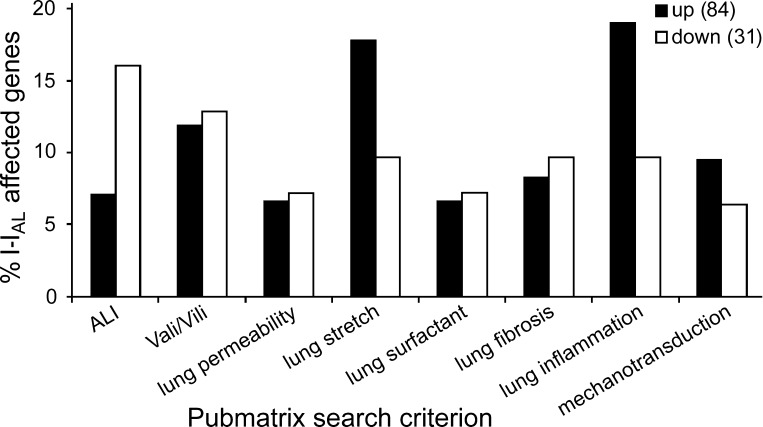
Text-based data mining of inverted interface results. Significant regulated genes are shown that are reported in the literature and associated in Pubmatrix with the indicated search terms. Separate analyses were performed for up- and downregulated genes. Results are reported as the percentage of genes, available in the Pubmatrix database and associated with the given search terms, of all up- (84 = 100%) and downregulated genes (31 = 100%). I-IAL, inverted IAL.
Ca2+ measurements.
Cells were seeded, in high density (106 × cm−2), into flat, transparent 96-wells. After 48 h, cells were washed and supplemented with 5 μM fura 2-AM in DMEM for 20 min at 37°C. Drugs were applied, as further described in the legend to Fig. 8, within the same time. Subsequently, wells were washed twice with standard experimental solution and placed into a Tecan M200pro microplate reader (Tecan). After 5 min, measurements were started using excitation wavelength = 335/380 nm and emission wavelength = 510 nm. Following the recording of baseline cytosolic [Ca2+]i values under submerged conditions, the plate was shortly moved out of the instrument, the buffer gently removed (= time 0), and the measurements continued for 10 min. Results are presented as background-corrected fura 2 ratios (335/380).
Fig. 8.
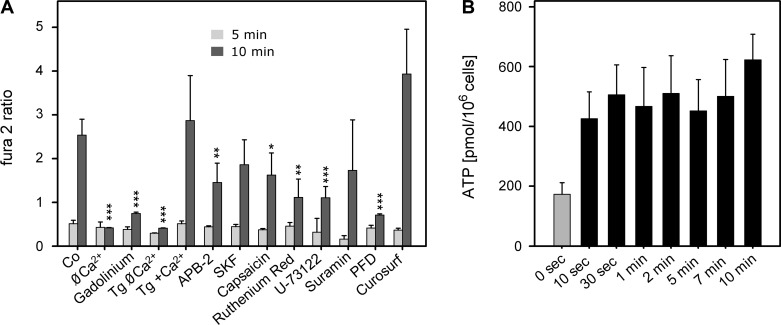
Mechanisms of IAL-induced cell signaling. A: Ca2+ signaling. Cells were preincubated under the indicated conditions. Co (= standard experimental solution), Ø Ca2+ (Ca2+-free + 1 mM EGTA, 5 min), gadolinium (50 μM, 20 min), TG Ø Ca2+ and TG + Ca2+ (100 nM thapsigargin, 5 min), APB-2 (2-aminoethyl diphenylborinate, 100 μM, 20 min), SKF (SKF-96365, 50 μM, 20 min), capsaicin (5 μM, 20 min), ruthenium red (5 μM, 20 min), U-73122 (10 μM, 5 min), suramin (100 μM, 20 min), PFD (perfluorodecalin, 100%, added at time 0), and Curosurf (0.1 mg/ml, 5 min). From continuous Ca2+ recordings (= fura 2 ratios; see also Fig. 1D, thick line), only the values at 5 and 10 min after fluid aspiration (= surface contact) were noted. Levels of significance: *P < 0.05; **P < 0.01; ***P < 0.001 (n = 4, means ± SD). B: ATP release at various times after surface contact. 0 sec, Without surface contact (= control). Solid bars denote significance (P < 0.05; n = 18, means ± SE).
ATP release experiments.
Cells were grown as described for the Ca2+ measurements. After 48 h, culture medium was replaced by standard experimental solution, and cells were exposed to air (see conventional interface experiments). Thereafter, 20 μl PBS were added. Control cells were treated in the same way, without air exposure. In addition to the 20-μl PBS, 80 μl luciferin-luciferase solution, prepared according to manufacturer's instructions (Invitrogen), were pipetted into the wells to obtain a total supernatant volume of 100 μl and subsequently transferred together with the standards into a new white 96-well (Greiner), and luciferase luminescence was recorded with the multiplate reader (Tecan M200pro). Luminescence measurements were taken every minute for 10 min with an integration time of 1,000 ms/well. The ATP content in each sample was calculated as the mean signal obtained within the 10 min of measurement calibrated to a standard curve.
LDH measurements.
Cells (AT II, 3T3, HMEC-1) were seeded in 24-well plates and grown to confluence. They were gently washed two times with experimental solution and exposed to air (see conventional interface experiments), followed by re-addition of 600 μl experimental solution. Cells were incubated with Ca2+ channel inhibitors, as further described in the legend to Fig. 9. After 2-h incubation (37°C, 5% CO2), supernatants were collected and stored at 4°C. Samples were analyzed for LDH release according to kit instructions (Roche). Fifty microliters of each sample were transferred, in duplicates, into a 96-well plate and mixed with 100 μl assay reagent. The reaction mix was incubated for 15 min at room temperature under light protection. Absorbance was measured at wavelength = 492 nm with a microplate reader (GENios Plus, Tecan).
Fig. 9.
Resistance of cells with respect to IAL challenge. A: measurements of cytotoxicity. Cytotoxicity is expressed as LDH release in percentage of LDH release using 1% Triton. AT II, AT II cells in standard experimental solution (n = 4 duplicates); AT II-Ca2+, AT II cells in Ca2+-free (+1 mM EGTA, 5 min preincubation; n = 6 triplicates) solution; AT II + gadolinium, AT II cells preincubated with gadolinium (50 μM, 30 min; n = 4); AT II + APB-2, AT II cells preincubated with APB-2 (100 μM, 30 min; n = 4); 3T3, 3T3 fibroblasts (n = 5 duplicates); 3T3 + Curosurf (0.1 mg/ml, 5 min preincubation; n = 4 duplicates); HMEC-1: human dermal microvascular endothelial cells (n = 6 duplicates). Significant (P < 0.05) levels compared with controls (without air exposure = 0 sec) are marked as solid bars (means ± SD). B: evaporative water loss in wells containing a free surface (buffer), or surfaces covered with surfactant from AT II cells (cell lysate), a monolayer of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), or multilayers of native surfactant (NS). Values are not significant (n = 4 triplicates ± SD).
Solutions and reagents.
The standard experimental solution contained the following (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 5 glucose, and 10 HEPES (pH 7.4 at 25°C). Fura 2-AM and FM 1–43 were from Invitrogen; gadolinium(III) chloride hydrate, thapsigargin, APB-2 (2-aminoethyl diphenylborinate), capsaicin, SKF-96365 hydrochloride, suramin sodium salt, EGTA, IgG, Triton X-100, streptomycin, penicillin, and trypsin from Sigma-Aldrich; the LDH detection kit from Roche; elastase (for the AT II cell preparation) from Elastin Products; Curosurf from Nycomed; 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) from Avanti Polar Lipids; and perfluorodecalin from F2 Chemicals. Purified surfactant was kindly provided by J. Pérez-Gil (Madrid, Spain).
RESULTS
Conventional interface experiments.
See Fig. 1. Confocal imaging was performed to visualize cell-IAL contacts, as they are likely to occur in the conventional interface experiments. Z-sectioning revealed that, after aspiration, a small but variable liquid layer is always present above the cells. During further thinning of this layer (due to evaporative water loss), the IAL remained continuous for at least 10 min. Furthermore, the IAL became increasingly bended over the cell apex when the thickness of the bulk liquid layer fell below that of the cell height. Concurrently with the bending of the IAL, the cells became increasingly compressed (Fig. 1B), and, with ongoing time, up to extents that might be the cause of cell damage (∼20 min; Fig. 1C). Since bending of the IAL started at about the same time as cell flattening, this suggests that the surface tension of the IAL is in a comparable range as the reported elastic modulus of AT II cells (3). Furthermore, since cell flattening occurred considerably earlier than cell damage, we conclude that, at least at the beginning, it is not a harmful event (56). Importantly, these data directly show that the IAL exerts a mechanical deforming stress on the cells, which is in accordance with what has been postulated and shown by different methods in recent studies before (55, 56).
Fig. 1.
Conventional interface experiments. A: confocal z-sectioning of a single alveolar type II (AT II) cell. FM 1–43 preincubation (in high concentrations) led to intense staining of lamellar bodies (bright patches), together with a faint staining of cytosol, membranes, the upper surface of the glass coverslip, and the contour of the air-liquid interface (IAL) (arrows). 0 min, Immediately after aspiration of fluid; medium was still present above the cells, although locally to very variable extents. 0.5 min, Reducing the thickness (height) of the water space (by evaporation) enforced a first cell-IAL contact. Further water loss (8 min) led to a bending of the IAL (arrow) and a massive compression of the cell. Arrowhead marks a possibly exocytosed lamellar body (surfactant) that disappeared in the following images, probably by adsorption at the IAL. Images were enhanced by brightness and contrast adjustments. Blurred outlines in the air space were due to optical reflections. B: after surface contact (distance to the IAL = 0 μm), the cell became increasingly compressed (increase in the length-to-height aspect ratio). Data in A and B are exemplary, but highly consistent observations. C: lactate dehydrogenase (LDH) release was not significant up to 10 min surface contact, but started to increase at 20 and 40 min (see also Fig. 9; n = 4 duplicates; mean ± SD). D: surface contact led to a steep increase in intracellular calcium concentration ([Ca2+]i) (= fura 2 ratio; black thick line), ∼4 min after aspiration of the fluid (at time 0). An increase in [Ca2+]i, however, was not observed by increasing buffer osmolarity without surface contact (dashed lines; n = 3 duplicates). Measurements in C and D were performed in a microplate reader.
To rule out changes of osmolarity in the cell culture medium, we used a dye dilution technique. We found that osmolarity typically increased by only ∼8 mosM/min (at 50% RH) in the fluid remaining above the cells. Additionally, direct hyperosmotic treatments, using different osmotically adjusted buffers up to 1,000 mosM, did not lead to Ca2+-signaling events when analyzed in cells under submerged conditions (Fig. 1D). Thus the characteristic Ca2+ increase shown in Fig. 1D, which is probably the decisive sensory event in cell-interface contacts (56) and starts as early as ∼4 min after fluid aspiration (= IAL contact), is unlikely due to osmotic effects. It is also unlikely that this Ca2+ increase is due to plasma membrane damage, as LDH release was detected only after 20 min (see Figs. 1C and 9).
These results, taken together with previous findings (56), demonstrate that conventional interface experiments (aspiration of cell medium followed by evaporation to enforce a close interface contact) can be used as a simple model to study cell-interface responses. However, these experiments can only be performed under defined conditions, e.g., within a short interval of exposure due to unspecific conditions at longer times (dry out, cell damage, and/or severe cell deformation), or undefined conditions before (no interface contact and/or no Ca2+ signal at shorter periods).
Gene expression in the conventional interface experiments.
See Fig. 2. For the reasons outlined above, 7-min air exposure was chosen to perform the analysis of transcripts. At this time, cytosolic [Ca2+]i is already high (Fig. 1D), and cell damaged can be excluded (Figs. 1C and 9). To capture early and late transcriptional events, RNA extraction was performed 1 and 4 h after re-addition of serum-free medium to the air-exposed cells. From ∼27,000 genes analyzed, the 1-h group showed 6 up- and 5 downregulated transcripts, several with unknown biological functions. In the 4-h group, we identified 41 transcripts with significant fold changes (11 up- and 30 downregulated), 3 of them have not been described by their function. All responding genes (52 genes) out of both groups (1 h, 4 h) were submitted to DAVID. Most of them (49 genes) were available in the database and analyzed with the annotation chart tool. The heat map in Fig. 2 demonstrates the expression pattern of 44 responding genes, in the 3 replicates, and highlights the most important GO terms. Eight genes with unknown biological function were not shown in the heat map. GO terms were selected by the number of involved genes and their biological relevance. Further Pubmatrix analysis with 38 genes was performed by a pairwise comparison of gene symbols with clinical and biological relevant terms, similar to the recent study by Yerrapureddy et al. (76). The results (Fig. 3) tentatively identified a genomic response associated with all search terms, with most genes linked to lung inflammation (36% up), lung surfactant (36% up), and ventilator-associated lung injury (VALI)/ventilator-induced lung injury (VILI) (36% up). Four genes were associated with all search terms (except mechanotransduction): Ptgs2 (up), Cxcl1 (up), Nos2 (down), and Alox5 (down), all of which are involved in defense response, response to stress, and response to stimulus pathways. Notably, three downregulated genes are members of the solute carrier family, one of them, SLC34a2, is highly expressed in AT II cells, where it regulates phosphate transport associated with surfactant phospholipid synthesis (35, 65).
Fig. 2.
Heat map of the conventional interface experiment demonstrates the expression pattern of individual replicates (= columns). Upregulated genes are indicated in red; downregulated in green. Shown are genes with fold changes ±1.5 and P < 0.05. Gene transcripts with unknown functions were excluded. GO (gene ontology) terms (bottom left corner) were selected by the number of participating genes. Vertical bars indicate involved genes. Upregulated genes associated with GO terms are grouped within the light red box; downregulated genes in the light green box. *Gene also regulated in Ref. 76.
Inverted interface experiments.
See Fig. 4. The inverted interface served as alternative approach to study cell-interface responses. Compared with the method above, several factors are different. 1) Cells contact the IAL by mere sedimentation through a constant volume of medium, minimizing potential mechanical, rheological, osmotic, or even dry out effects. 2) Before the experiments, cells are detached from the substratum and applied in a very dilute suspension, so they were largely lacking direct intercellular contacts and probably not subject to any paracrine interactions. 3) By microscopy, we could prove that all cells came into interface contact (Fig. 4, C and D). 4) Microscopy also served as an additional control of cell purity. 5) Interface contact was 4 h. We have chosen this time for three reasons. 1) Time point of interface contact is not sudden, but accomplished for all cells after <10 min (Fig. 4D). 2) The cellular response is weaker than in conventional interface experiments or may be completely absent (56). 3) We have shown that cells tolerate contact with the inverted interface for much longer times, at least for 24 h (56). Thus this approach is not a mere replication of the conventional interface experiments, and additionally allowed the study of the consequences of a more prolonged interface challenge, which is not possible with the former approach.
Gene expression in the inverted interface experiments.
See Fig. 5. Here, we used a specially designed technique (NuGEN) for low-concentrated RNA samples (500 pg to 100 ng). Cells with 4-h IAL contact showed 153 up- and 52 downregulated transcripts. All responding genes (205) were analyzed with DAVID in the same way as described before. The 141 genes, recognized by DAVID, are shown in Fig. 5 (except those of unknown functions). Again, GO terms were selected by the number of involved genes. Further Pubmatrix analysis of 115 genes (Fig. 6) revealed that most genes are associated with following search terms. Lung inflammation (19% up), lung stretch (18% up), and acute lung injury (ALI; 16% down). Six genes were associated with almost all search terms: Igfb3 (down), Tlr3 (down), Ngf (up), Serpine1 (up), Ctgf (up), and Cyr61 (up). Both downregulated genes (Idfb3 and Tlr3) were previously indentified to be involved in pathogenesis of ALI and acute respiratory distress syndrome (13, 61). Moreover, Tlr3 has also been shown to be altered in surfactant protein C-deficient mice (28). Recently, it has been reported that Ctgf and Cyr61 are early responding genes of VILI (70). Additionally, Ctgf and Serpine1 have been indentified to be involved in the newly discovered cell deformation signaling cascade (60). The highest upregulated gene (fold change of 6.3) with known biological function was Ankrd1 (cardiac ankyrin repeat domain 1). This gene has been proposed to play a critical role in the titin-based, stretch-sensing complex in muscle cells and wound healing in skin cells (42, 62) and was recently identified to be highly upregulated in 5-day cultured AT II cells compared with freshly isolated ones (5). The highest downregulated gene (fold change of −3.0) with known biological function was Sema4C, involved in cell-cell communication in nonneural tissue (79).
Fig. 5.
Heat map of the inverted interface experiment demonstrates the expression pattern of individual replicates (= columns). Upregulated genes are indicated in red; downregulated in green (arrow: continuation of the gene list). Shown are genes with fold changes ±2.0 and P < 0.05. Gene transcripts with unknown functions were excluded. GO terms were selected by the number of participating genes. Vertical bars indicate involved genes. Upregulated genes associated with GO terms are grouped within the light red box; downregulated genes in the light green box. *Genes also regulated in Ref. 76.
Summary of conventional vs. inverted interface experiments.
See Fig. 7. As outlined before, both interface approaches are similar, although cells experience IAL contact in probably quite different ways. This might be reflected by the number of regulated genes, which was higher (205 vs. 52) with the inverted interface treatment, the ratio of up- vs. downregulated ones (also higher with the inverted interface approach), and also by the fact that no genes in common were comparably regulated in both protocols. However, analyzing all genes from both experiments with DAVID, similar pathways involved in cell wounding and cell defense were identified, both of which, in turn, stimulate repair mechanisms, cell proliferation, and programmed cell death. The hierarchical compilation in Fig. 7 highlights the most enriched GO terms of the conventional interface (C-IAL, 16 terms), the inverted interface (I-IAL, 13 terms), and those that were enriched in both treatments (9 terms). Notably, interfacially treated cells (conventional and inverted) showed a different expression pattern in five members of the solute carrier family (Slc10a1, Slc16a14, Slc2a3, Slc34a2, Slc38a2) and in three dual-specificity phosphatase coding genes (Dusp1, Dusp8, Dusp16), suggesting an involvement in inorganic phosphate homeostasis and MAPK activation/inactivation. In summary, both results provide strong evidence that interfacial stress leads to transcriptional changes. This is likely to occur, via stretch/cell deformation signaling, followed by activation of cellular responses, such as cell proliferation, wound healing, and cellular repair.
Fig. 7.
The hierarchical compilation indicates the most enriched GO terms of the conventional interface (C-IAL, 16 terms), the inverted interface (I-IAL, 13 terms), and those that were enriched in both treatments (9 terms). Indentation denotes subordinate categories, X indicates enriched GO terms.
Signaling mechanisms.
Previously, it has been shown that cells respond in conventional and in inverted interface experiments with intracellular Ca2+ signals (55, 56, 63). Here we extended those studies using agonists and inhibitors for various channels and pathways (Fig. 8). The source of the Ca2+ increase was demonstrated to be extracellular. Ca2+ removal before air exposure totally abolished the rise in the fura 2 ratio, similar to the effect of gadolinium [a nonspecific stretch-activated channel inhibitor (7, 11, 14)] in Ca2+-containing buffer. Furthermore, thapsigargin, which depletes intracellular Ca2+ stores (64), had no inhibitory effect. Instead, it consistently increased Ca2+, probably due to a parallel activation of store-operated Ca2+ entry. This pathway was indeed inhibitable by APB-2, an inhibitor of store-operated Ca2+ entry at high concentrations (10, 53), but not by SKF, which ought to do the same (20, 40). However, APB-2 also modulates various TRP channels (54), and, indeed, inhibition was also found with the TRP vanilloid 1 agonist capsaicin, recently identified to block stretch-activated Ca2+ signals in AT II cells (Ref. 68 and G. Fois, personal communication) and ruthenium red acting in the same way (54). Finally, U-73122, a known PLC inhibitor, also blocked the rise in [Ca2+]i, suggesting a contribution of a purinergic receptor (1). However, suramin, a nonselective P2 purinergic antagonist (12, 25), had no effect. This was also the case for Curosurf, which was used to decrease surface tension of the IAL. Perfluorodecalin, which served as a model for a liquid-liquid interface, did not lead to a detectable [Ca2+]i increase.
Since the source of Ca2+ was extracellular, and suramin had no inhibitory effect, a paracrine action of released ATP (via activating P2Y2 receptors) appears unlikely. Nevertheless, we measured ATP in the conventional interface experiments because of its suggested functions in mechanotransduction (49). A small ATP release was indeed detectable, starting as early as 10 s after IAL exposure, but without time-dependent change afterwards (Fig. 8B). Furthermore, the rapid release of ATP compared with the slow onset in Ca2+ increase (≥4 min) additionally argue against a prominent role of ATP in interfacial sensing.
Tolerance toward IAL contact.
See Fig. 9. Gene analysis revealed activation of pathways related to defense response, wounding, and repair. Furthermore, our laboratory demonstrated previously that IAL contact may be a harmful event upon which cells respond with increased surfactant secretion (56). Thus we investigated whether changes on the intracellular level are reflected in a physiological setting. First, we compared viability of AT II cells with two other cell models that do not experience an IAL in vivo: endothelial cells (HEMC-1) and 3T3 fibroblasts. Whereas LDH release in AT II cells was not detectable before 20-min air exposure, as in our laboratory's previous study (56), it was significant at 5 and 1 min in HEMC-1 and 3T3 fibroblasts, respectively. This suggests that AT II cells might be constitutively more robust than those of endothelial or mesenchymal origin. On the other hand, their higher tolerance could be the result of a constitutive release of surfactant. Removal of bath Ca2+, known to completely inhibit agonist and stretch-induced exocytosis in AT II cells (23, 24), indeed rendered their susceptibility comparable to that of the fibroblasts, whereas direct addition of purified surfactant to 3T3 cells clearly prolonged the time to cytotoxic damage. A further evidence for the role of Ca2+-induced surfactant secretion was observed by preincubation of AT II cells with gadolinium. APB-2, however, had no effect.
Nevertheless, the supposed protective effect of surfactant is likely to be complex, maybe involving reduction of evaporative water loss above the cells and thus delaying air contact. However, this was not measurable (Fig. 9B). Evaporation rates were not different between pure water, or surfaces covered either by surfactant released from AT II cells or obtained from lung lavages, or a surface with a monomolecular film of spread DPPC. Taking these findings together, we speculate that surfactant does not delay the time to air contact, but probably alleviates the stress effects exerted by air contact. Thus increased surfactant secretion, as a result of interface contact, might be one of the protective roles of AT II cells in alveolar defense and repair.
DISCUSSION
Our laboratory reported previously that the IAL represents a distinct element in alveolar micromechanics, particularly by conveying physical forces to the AT II cells, which respond to them by Ca2+ signals and surfactant secretion (56). We hypothesized that the underlying mechanism is a mechanical sensation of that interface, transmitted via the same pathways and mechanisms that are generally discussed for tissue stretch and strain. Here, we extended these investigations by studying the cell response on the transcriptional level, and by seeking possible mechanisms for signal transduction in the course of interface contact.
Interface contact was accomplished in two ways. The first we called conventional interface experiments, because, in principle, cells were brought into contact with the IAL by removing the bulk fluid, a simple procedure used in other studies (e.g., Ref. 55). However, this approach alone is not sufficient to enforce an instantaneous and “real” cell-IAL contact, as it just reduces the amount of fluid above the cells, and thus their distance to the IAL. This we demonstrated by confocal z-sectioning, where we found that cells are still submerged by medium immediately after aspiration of the fluid, and that the contour of the IAL was essentially flat over the cell apex. These image-based experiments confirm our previous observation of slower evaporation of water in wells containing (wet) cells compared with wells containing pure buffer (56). Both results point to the fact that cells always retain a covering of water, and interface contact can only be provoked by an additional brief period of evaporation to further reduce the thickness of the water shell. As discussed before (see results), the interface then became increasingly bended over the cells, and the cells became increasingly compressed onto the substrate; in fact, a similar procedure was used to press T cells onto a substratum (50). This demonstrates that the surface tension of the IAL (in our experiments) is in a range to overwhelm the elastic modulus of AT II cells (3), and that neither cellular surface tension nor any internal scaffolding is able to sustain the loads induced by a curved IAL (50). Thus these data directly show that the IAL is able to exert a mechanical deforming stress on the cells, which is in accordance to what has been postulated and shown by different methods in recent studies before (55, 56). Additionally, these data show that cell response is due neither to simple fluid removal nor to dry out phenomena, but due to a critical compression of the cells preceding an actual loss of cell water.
For the second approach, we used the so-called inverted interface (8, 33, 52, 56). This method circumvents any additional forces except sedimentation, but still guarantees intimate interface contact that can even be analyzed by microscopy (56). Potential side effects or even destructive effects (dramatic increase in osmolarity, dry out, rheological effects) could be ruled out completely. Importantly, AT II cells seem to tolerate this environment without noticeable changes in cell function or morphology for much longer periods of time than used here (56).
Although the cells in this system are not adherent to any sort of substrate (except the IAL itself), and thus should not experience any counterforce to surface tension at all (except gravitation minus buoyancy), they respond to a contact with the IAL, as shown previously (56). This finding can be explained in various ways. 1) There may be a shear stress acting on micrometer- or nanoscaled cell-surface structures, which is caused by the minuscule pressure the cell is exerting toward the IAL. 2) There may also be a tensile strain acting along the cell membrane whenever surface structures break the IAL [the same mechanism we proposed for the adsorption and spreading of lamellar bodies when contacting the IAL (33)]. 3) Finally, the IAL may be subject to localized, dynamic flows, especially on adsorption of surface active compounds, probably creating normal (pressure) and shear forces on the surface attached cells.
Mechanical stress on cells are complex, especially near phase boundaries, in dynamic rheological situations, and in the presence of complex surface topographies, as has been shown in experimental and theoretical models for pulmonary airway reopenings (Refs. 9, 39 and reviewed in Ref. 27). From these models, assuming Newtonian fluids and stiff cell substrates, pressure gradients over the length of the cells dominate over shear stress and shear stress gradients as the decisive factors to generate cell-deformation-induced cell injury. In fact, local pressure imbalances are most obvious in the conventional interface model, where positive and negative curvatures of the IAL are forming from the cell apex to the cell periphery (Fig. 1A). Apart from this, pressure gradients, as well as shear stress and shear stress gradients, may arise not only from meniscus forces, but also in consequence of barely noticeable draining flows, which are described in fluid mechanics of thin films (39). Since, in both systems (conventional and inverted interface model), cells are covered by thin films, we have to take into consideration that flows exist, irrespective of the specific model used, probably imposing similar or even identical mechanical stress profiles onto the cells. We acknowledge, however, that an analytic approach to dissect the relative contribution of each of these factors in both models still has to be evaluated.
Under both experimental conditions we observed an altered transcriptomic pattern, with no apparent overlap, supporting our laboratory's previous findings that the IAL contact is a trigger of cellular events (56). This result is also in accordance with the reports of Ramsingh et al. (55) on IAL-dependent cell deformation and subsequent Ca2+ transients in A549 cells when air bubbles were passed over the cell monolayers, or by tilting the culture dishes to drain the fluid. Indirectly, our result is also in accordance with those many studies, which used air-liquid cultures of cells, and found significant effects on cell functions, which, however, have not been interpreted on the basis of a clear mechanistic or a physical model.
Specifically, our microarray analyses demonstrated enhanced or attenuated expression of a variety of genes, including those which are linked to ALI, VALI/VILI, fibrosis, inflammation, and lung stretch, according to an extended Pubmatrix search (Figs. 3 and 6). Due to the lack of other studies on the same or a similar topic, we conducted this analysis according to that published by Yerrapureddy et al. (76). These authors used isolated AT II cells that have been differentiated to some extent into an AT I-like pheno- and genotype and subject the cells, grown on a Silastic membrane, to cyclic stretch for up to 6 h. Cells thus experienced stress (force per unit area) and stretch (change in length), followed by compression in between the cycles. In contrast, we used phenotypically and functionally characterized AT II cells (32), which were forced, by aspiration/evaporation of the fluid or by sedimentation, into a close but probably static contact with an IAL. Interestingly, despite this relatively vast difference in the experimental protocols, it seems that interfacial stress sensation is followed by similar transcriptional events as induced with cyclic stretch protocols (76). In particular, Serpine1, Fosl1, and Ppp1r10 were significantly regulated in both studies: Ppp1r10, described to depress cellular apoptosis and to have a protective effect (76), Fosl1, involved in alveolar epithelial cell injury and repair (78); and Serpine1 (synonym: plasminogen activator inhibitor-1), known to be highly responsive to cell shape altering stimuli and to be upregulated in intact lungs at large tidal volume ventilation (74). Serpine1 is coding for a clinically important profibrotic protein, as is the case for Ctgf, which was also upregulated in our study. Both genes were assigned by Samarakoon (60) as the two model responding genes linking cell deformation to signaling events, probably through Rho activation. Rho-associated kinase is a promising candidate for transmitting extracellular mechanical stimuli (stretch, interfacial forces) into the alveolar type cell gene expression due to its pivotal role in regulating the actin cytoskeleton and in mechanotransduction-mediated differentiation (21, 60). Since plasminogen activator inhibitor-1 and Ctgf contribute to the pathogenesis of several interstitial lung diseases like lung fibrosis, detailed studies focusing on the nature of the stimuli and its related downstream mechanism could be essential for the invention of novel therapeutic targets (75).
Mechanical stress can lead to a reprogramming of the cell's functional state and impact a variety of biological responses, including growth, migration, and differentiation, likely to follow in IAL-treated cells at a long term. On the other hand, mechanical stress can also have an immediate effect, depending on the cell type and the nature of the forces that are acting on them. In AT II cells, for example, cell stretch is a potent stimulus for surfactant secretion and proceeds via Ca2+-dependent pathways (23, 73). In most studies, the intracellular Ca2+ elevation is mediated by a transmembrane Ca2+ influx, which, in turn, activates a secondary Ca2+-induced intracellular Ca2+ release mechanism. Mechanosensitive ion channels, like members of the TRP channel family, are particularly promising candidates in promoting this signaling cascade. Such a transmembrane Ca2+ influx also occurred in the IAL-treated cells and was found to be inhibitable by gadolinium, APB-2, capsaicin, and ruthenium red. Therefore, a high consistency with stretch-induced mechanisms could be found, both of which provide ample evidence for a Ca2+-permeable mechanosensitive ion channel whose molecular identity and mode of activation, however, are still obscure.
Nonetheless, Ca2+ channel activation could be secondary. ATP is released by AT II cells in response to stretch and interacts with purinergic P2Y2 and P2X4 receptors to increase [Ca2+]i (46, 49). Within the alveolar lining fluid, ATP might reach millimolar concentrations (58), but the source of it is essentially unknown. Beside a supposed release from scattered purinergic nerve terminals, and release due to cell membrane stress failures, a channel-gated release from AT II cells, as well as a mechanosensitive ATP-exocytosis, has been proposed (16, 55). Here, a release of ATP, in concentrations similar to those reported by Patel et al. (49) for cyclic stretch, was detected after a brief IAL exposure (∼10 s). Inhibition of Ca2+ increase by U-73122, a PLC inhibitor, also suggests a contribution of a purinergic receptor. However, it is also reported that U-73122 inhibits calcium influx (31), an effect that is more likely to apply for our studies. Furthermore, the lack of Ca2+ entry in Ca2+-free solutions and the lack of inhibition by suramin argue against a role of ATP. In line with this, the rapid release of ATP compared with the slow onset in Ca2+ increase (≥4 min) does not support a purinergic signaling, which was proposed by Ramsingh et al. (55) as the decisive mechanism in A549 cells subject to passage of air bubbles or perfusion with air.
As previously discussed in more detail (56), and also in the work of Ramsingh et al. (55), the prominent morphology of AT II cells (cuboideal with microvilli), their placement within the alveoli (projecting out of the smooth plane of AT I cells and the alveolar corners, probably bulging the interface into the air-filled lumen), and their main function (replenishment of the surfactant pool, fluid resorption) would provide all prerequisites to design them as the preferred sensors of the status of the interface and the forces acting there, so, for example, to synergistically amplify the lung inflation-induced signals from the AT I cells (2, 38). Such alterations in the status of the interface can be easily envisaged, considering, for example, that the lung parenchyma cannot be the exclusive structure that is subject to periodic stretch. The hypophase has to follow these geometric changes in some way, and most likely to the same extent as the surface of the tissue itself. This hypothesis, however, remains elusive as long as vital lung microscopy, employing sophisticated techniques, will allow it to be proven or disproven (41, 66). In contrast to this hypothesis, experiments provided evidence that interfacial stress is a determinant of cell wounding in pathophysiological settings, e.g., in surfactant-compromised lungs or as a consequence of alveolar edema (9, 48).
Prolonged air exposure (>10 min) leads to cell damage as previously shown. The results in this study also suggest that AT II cells are constitutively more robust than those of endothelial or mesenchymal origin with regard to stresses associated with an IAL. Their higher resistance could be explained by the release of specific extracellular matrix components (e.g., surfactant lipids), rapidly transferring to the IAL. This is supported by the observation that addition of purified surfactant to 3T3 fibroblasts clearly prolonged the time to cytotoxic damage, and that inhibition of surfactant secretion (Ø Ca2+ and gadolinium) shortened this time in AT II cells. Taken together, we conclude that surfactant does not delay the time to IAL contact, but makes a cell more resistant to the stress resulting from IAL contact, most likely by a reduction of surface tension that also reduces the magnitude of compressional or shear forces exerted by the IAL. However, the Ca2+ signal in AT II cells exposed to an IAL in the presence of Curosurf was unaffected. Thus a “physiologically conditioned” IAL, containing a surface film, is a stimulus for the cells, but its harmful nature appears to be largely alleviated. This conclusion is in correspondence with other studies relating the severity of a damaging interfacial stress with the presence/absence of surfactant in various models of pulmonary airway reopenings related to atelectrauma (9, 29). Interestingly, even small amounts of surfactant (0.1 mg/ml) significantly improved the viability of epithelial cells exposed to an advancing IAL, as it is probably also the case for the degree of interfacial compression of the surface films or the amount of surface-associated material (29). Consequently, one could speculate that pulmonary surfactant, in addition to its well-known functions, also serves as a protective surface coat, and that the ability of cells to produce such a coat might have been an important prerequisite in the evolution of air-exposed epithelia to further allow the development of entire gas-exchanging organs, a hypothesis also similarly raised elsewhere (55).
GRANTS
The research was funded by the Austrian Science Fund (FWF): P70472.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.H., A.R., and T.H. conception and design of research; N.H. and A.R. performed experiments; N.H. analyzed data; N.H. and T.H. interpreted results of experiments; N.H. and T.H. prepared figures; N.H. and T.H. drafted manuscript; N.H., A.R., and T.H. approved final version of manuscript.
ACKNOWLEDGMENTS
Technical assistance by G. Siber and I. Öttl is gratefully acknowledged. We thank P. Dietl for reading the manuscript and valuable suggestions, and J. Fürst and S. Signorelli for providing the 3T3 and the HMEC-1 cell cultures.
Present address of A. Ravasio: Mechanobiology Institute, National University of Singapore, Singapore.
REFERENCES
- 1. Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 293: L259– L271, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ashino Y, Ying XY, Dobbs LG, Bhattacharya J. [Ca2+]i oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am J Physiol Lung Cell Mol Physiol 279: L5– L13, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Azeloglu EU, Bhattacharya J, Costa KD. Atomic force microscope elastography reveals phenotypic differences in alveolar cell stiffness. J Appl Physiol 105: 652– 661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bachofen H, Schurch S. Alveolar surface forces and lung architecture. Comp Biochem Physiol A Mol Integr Physiol 129: 183– 193, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Ballard PL, Lee JW, Fang XH, Chapin C, Allen L, Segal MR, Fischer H, Illek B, Gonzales LW, Kolla V, Matthay MA. Regulated gene expression in cultured type II cells of adult human lung. Am J Physiol Lung Cell Mol Physiol 299: L36– L50, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bastacky J, Lee CYC, Goerke J, Koushafar H, Yager D, Kenaga L, Speed TP, Chen Y, Clements JA. Alveolar lining layer is thin and continuous–low-temperature scanning electron-microscopy of rat lung. J Appl Physiol 79: 1615– 1628, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol 82: 25– 42, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Bertocchi C, Ravasio A, Bernet S, Putz G, Dietl P, Haller T. Optical measurement of surface tension in a miniaturized air-liquid interface and its application in lung physiology. Biophys J 89: 1353– 1361, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bilek AM, Dee KC, Gaver DP. Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 94: 770– 783, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Broad LM, Braun FJ, Lievremont JP, Bird GSJ, Kurosaki T, Putney JW. Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem 276: 15945– 15952, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol Cell Physiol 275: C619– C621, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Charlton SJ, Brown CA, Weisman GA, Turner JT, Erb L, Boarder MR. PPADS and suramin as antagonists at cloned P-2Y- and P-2U-purinoceptors. Br J Pharmacol 118: 704– 710, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chun CD, Liles WC, Frevert CW, Glenny RW, Altemeier WA. Mechanical ventilation modulates Toll-like receptor-3-induced lung inflammation via a MyD88-dependent, TLR4-independent pathway: a controlled animal study. BMC Pulm Med 10: 57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clemo HF, Baumgarten CM. Stretch-activated channel blockers Gd3+ and 9-Ac alter cardiac cell-volume and the response to osmotic stretch (Abstract). FASEB J 6: A442, 1992 [Google Scholar]
- 15. Dietl P, Frick M, Mair N, Bertocchi C, Haller T. Pulmonary consequences of a deep breath revisited. Biol Neonate 85: 299– 304, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Dietl P, Liss B, Felder E, Miklavc P, Wirtz H. Lamellar body exocytosis by cell stretch or purinergic stimulation: possible physiological roles, messengers and mechanisms. Cell Physiol Biochem 25: 1– 12, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type-II cells in high-yield and purity. Am Rev Respir Dis 134: 141– 145, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Edwards YS. Stretch stimulation: its effects on alveolar type II cell function in the lung. Comp Biochem Physiol A Mol Integr Physiol 129: 245– 260, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res 2: 33– 52, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flemming R, Xu SZ, Beech DJ. Pharmacological profile of store-operated channels in cerebral arteriolar smooth muscle cells. Br J Pharmacol 139: 955– 965, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foster CD, Varghese LS, Gonzales LW, Margulies SS, Guttentag SH. The Rho pathway mediates transition to an alveolar type I cell phenotype during static stretch of alveolar type II cells. Pediatr Res 67: 585– 590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fredberg JJ, Kamm RD. Stress transmission in the lung: pathways from organ to molecule. Annu Rev Physiol 68: 507– 541, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Frick M, Bertocchi C, Jennings P, Haller T, Mair N, Singer W, Pfaller W, Ritsch-Marte M, Dietl P. Ca2+ entry is essential for cell strain-induced lamellar body fusion in isolated rat type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 286: L210– L220, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Frick M, Eschertzhuber S, Haller T, Mair N, Dietl P. Secretion in alveolar type II cells at the interface of constitutive and regulated exocytosis. Am J Respir Cell Mol Biol 25: 306– 315, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Garcia-Verdugo I, Ravasio A, de Paco EG, Synguelakis M, Ivanova N, Kanellopoulos J, Haller T. Long-term exposure to LPS enhances the rate of stimulated exocytosis and surfactant secretion in alveolar type II cells and upregulates P2Y(2) receptor expression. Am J Physiol Lung Cell Mol Physiol 295: L708– L717, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Gatto LA, Fluck RR. Alveolar mechanics in the acutely injured lung: role of alveolar instability in the pathogenesis of ventilator-induced lung injury. Respir Care 49: 1045– 1055, 2004 [PubMed] [Google Scholar]
- 27. Ghadiali SN, Gaver DP. Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol 163: 232– 243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glasser SW, Witt TL, Senft AP, Baatz JE, Folger D, Maxfield MD, Akinbi HT, Newton DA, Prows DR, Korfhagen TR. Surfactant protein C-deficient mice are susceptible to respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 297: L64– L72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glindmeyer HW, Smith BJ, Gaver DP. In situ enhancement of pulmonary surfactant function using temporary flow reversal. J Appl Physiol 112: 149– 158, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 288: L179– L189, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Grierson JP, Meldolesi J. Calcium homeostasis in mouse fibroblast cells–affected by U-73122, a putative phospholipase C-beta blocker, via multiple mechanisms. Br J Pharmacol 115: 11– 14, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haller T, Dietl P, Pfaller K, Frick M, Mair N, Paulmichl M, Hess MW, Furst J, Maly K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J Cell Biol 155: 279– 289, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haller T, Dietl P, Stockner H, Frick M, Mair N, Tinhofer I, Ritsch A, Enhorning G, Putz G. Tracing surfactant transformation from cellular release to insertion into an air-liquid interface. Am J Physiol Lung Cell Mol Physiol 286: L1009– L1015, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Haller T, Ortmayr J, Friedrich F, Volkl H, Dietl P. Dynamics of surfactant release in alveolar type II cells. Proc Natl Acad Sci U S A 95: 1579– 1584, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hashimoto M, Wang DY, Kamo T, Zhu Y, Tsujiuchi T, Konishi Y, Tanaka M, Sugimura H. Isolation and localization of type IIb Na/Pi cotransporter in the developing rat lung. Am J Pathol 157: 21– 27, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hildebran JN, Goerke J, Clements JA. Surfactant release in excised rat lung is stimulated by air inflation. J Appl Physiol 51: 905– 910, 1981 [DOI] [PubMed] [Google Scholar]
- 37. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44– 57, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Ichimura H, Parthasarathi K, Lindert J, Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol 291: L596– L601, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Jacob AM, Gaver DP. An investigation of the influence of cell topography on epithelial mechanical stresses during pulmonary airway reopening. Phys Fluids 17: 031502, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang N, Zhang ZM, Liu L, Zhang C, Zhang YL, Zhang ZC. Effects of Ca2+ channel blockers on store-operated Ca2+ channel currents of Kupffer cells after hepatic ischemia/reperfusion injury in rats. World J Gastroenterol 12: 4694– 4698, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuebler WM, Parthasarathi K, Lindert J, Bhattacharya J. Real-time lung microscopy. J Appl Physiol 102: 1255– 1264, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Lehti TM, Silvennoinen M, Kivela R, Kainulainen H, Komulainen J. Effects of streptozotocin-induced diabetes and physical training on gene expression of titin-based stretch-sensing complexes in mouse striated muscle. Am J Physiol Endocrinol Metab 292: E533– E542, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Mason RJ. Biology of alveolar type II cells. Respirology 11: S12– S15, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Mason RJ, Voelker DR. Regulatory mechanisms of surfactant secretion. Biochim Biophys Acta 1408: 226– 240, 1998 [DOI] [PubMed] [Google Scholar]
- 45. McRitchie DI, Isowa N, Edelson JD, Xavier AM, Cai L, Man HY, Wang YT, Keshavjee SH, Slutsky AS, Liu MY. Production of tumour necrosis factor alpha by primary cultured rat alveolar epithelial cells. Cytokine 12: 644– 654, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Miklavc P, Mair N, Wittekindt OH, Haller T, Dietl P, Felder E, Timmler M, Frick M. Fusion-activated Ca(2+) entry via vesicular P2X(4) receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc Natl Acad Sci U S A 108: 14503– 14508, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nicholas TE, Power JHT, Barr HA. The pulmonary consequences of a deep breath. Respir Physiol 49: 315– 324, 1982 [DOI] [PubMed] [Google Scholar]
- 48. Oeckler RA, Lee WY, Park MG, Kofler O, Rasmussen DL, Lee HB, Belete H, Walters BJ, Stroetz RW, Hubmayr RD. Determinants of plasma membrane wounding by deforming stress. Am J Physiol Lung Cell Mol Physiol 299: L826– L833, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patel AS, Reigada D, Mitchell CH, Bates SR, Margulies SS, Koval M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am J Physiol Lung Cell Mol Physiol 289: L489– L496, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Patrick SM, Kim S, Braunstein NS, Maldarelli CM, Thomas JL, Leonard EF. Controlled cell deformation produces defined areas of contact between cells and ligand-coated surfaces. Ann Biomed Eng 29: 1– 8, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Perlman CE, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol 103: 1037– 1044, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Possmayer F, Hall SB, Haller T, Petersen NO, Zuo YY, de la Serna JB, Postle AD, Veldhuizen RAW, Orgeig S. Recent advances in alveolar biology: some new looks at the alveolar interface. Respir Physiol Neurobiol 173: S55– S64, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol 536: 3– 19, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 68: 619– 647, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Ramsingh R, Grygorczyk A, Solecki A, Cherkaoui LS, Berthiaume Y, Grygorczyk R. Cell deformation at the air-liquid interface induces Ca2+-dependent ATP release from lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L587– L595, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Ravasio A, Hobi N, Bertocchi C, Jesacher A, Dietl P, Haller T. Interfacial sensing by alveolar type II cells: a new concept in lung physiology? Am J Physiol Cell Physiol 300: C1456– C1465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ravasio A, Olmeda B, Bertocchi C, Haller T, Perez-Gil J. Lamellar bodies form solid three-dimensional films at the respiratory air-liquid interface. J Biol Chem 285: 28174– 28182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rice WR, Burhans M, Wispe JR. Effect of oxygen exposure on Atp content of rat bronchoalveolar lavage. Pediatr Res 25: 396– 398, 1989 [DOI] [PubMed] [Google Scholar]
- 59. Rooney SA. Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol 129: 233– 243, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Samarakoon R, Goppelt-Struebe M, Higgins PJ. Linking cell structure to gene regulation: signaling events and expression controls on the model genes PAI-1 and CTGF. Cell Signal 22: 1413– 1419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schnapp LM, Donohoe S, Chen JZ, Sunde DA, Kelly PM, Ruzinski J, Martin T, Goodlett DR. Mining the acute respiratory distress syndrome proteome: Identification of the insulin-like growth factor (IGF)/IGF-binding protein-3 pathway in acute lung injury. Am J Pathol 169: 86– 95, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shi Y, Reitmaier B, Regenbogen J, Slowey RM, Opalenik SR, Wolf E, Goppelt A, Davidson JM. CARP, a cardiac ankyrin repeat protein, is up-regulated during wound healing and induces angiogenesis in experimental granulation tissue. Am J Pathol 166: 303– 312, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sobolewski P, Kandel J, Klinger AL, Eckmann DM. Air bubble contact with endothelial cells in vitro induces calcium influx and IP3-dependent release of calcium stores. Am J Physiol Cell Physiol 301: C679– C686, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific-inhibition of the endoplasmic-reticulum Ca2+-Atpase. Proc Natl Acad Sci U S A 87: 2466– 2470, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Traebert M, Hattenhauer O, Murer H, Kaissling B, Biber J. Expression of type II Na-Pi cotransporter in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 277: L868– L873, 1999 [DOI] [PubMed] [Google Scholar]
- 66. Tschumperlin DJ, Boudreault F, Liu F. Recent advances and new opportunities in lung mechanobiology. J Biomech 43: 99– 107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol 86: 2026– 2033, 1999 [DOI] [PubMed] [Google Scholar]
- 68. Usmani SM, Fois G, Albrecht S, von Aulock S, Dietl P, Wittekindt OH. 2-APB and capsazepine-induced Ca(2+) influx stimulates clathrin-dependent endocytosis in alveolar epithelial cells. Cell Physiol Biochem 25: 91– 102, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Vlahakis NE, Hubmayr RD. Response of alveolar cells to mechanical stress. Curr Opin Crit Care 9: 2– 8, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Wallace MJ, Probyn ME, Zahra VA, Crossley K, Cole TJ, Davis PG, Morley CJ, Hooper SB. Early biomarkers and potential mediators of ventilation-induced lung injury in very preterm lambs. Respir Res 10: 19, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang PM, Fujita E, Bhattacharya J. Vascular regulation of type II cell exocytosis. Am J Physiol Lung Cell Mol Physiol 282: L912– L916, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol 119: 1– 17, 2000 [DOI] [PubMed] [Google Scholar]
- 73. Wirtz HRW, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial-cells. Science 250: 1266– 1269, 1990 [DOI] [PubMed] [Google Scholar]
- 74. Wurfel MM. Microarray-based analysis of ventilator-induced lung injury. Proc Am Thorac Soc 4: 77– 84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang JL, Tan Y, Zhao FL, Ma ZS, Wang YH, Zheng SR, Epstein PN, Yu J, Yin X, Zheng Y, Li XK, Miao LN, Cai L. Angiotensin II plays a critical role in diabetic pulmonary fibrosis most likely via activation of NADPH oxidase-mediated nitrosative damage. Am J Physiol Endocrinol Metab 301: E132– E144, 2011 [DOI] [PubMed] [Google Scholar]
- 76. Yerrapureddy A, Tobias J, Margulies SS. Cyclic stretch magnitude and duration affect rat alveolar epithelial gene expression. Cell Physiol Biochem 25: 113– 122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem Biophys 56: 1– 18, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Zhang Q, Kleeberger SR, Reddy SP. DEP-induced fra-1 expression correlates with a distinct activation of AP-1-dependent gene transcription in the lung. Am J Physiol Lung Cell Mol Physiol 286: L427– L436, 2004 [DOI] [PubMed] [Google Scholar]
- 79. Zielonka M, Xia JJ, Friedel RH, Offermanns S, Worzfeld T. A systematic expression analysis implicates Plexin-B2 and its ligand Sema4C in the regulation of the vascular and endocrine system. Exp Cell Res 316: 2477– 2486, 2010 [DOI] [PubMed] [Google Scholar]