Abstract
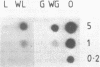
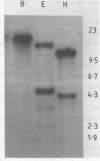
Messenger RNAs homologous to a cDNA clone (pTOM 13) derived from a ripe-tomato-specific cDNA library are expressed during tomato fruit ripening and after the wounding of leaf and green fruit material. Both responses involve the synthesis of the hormone ethylene. Accumulation of the pTOM 13--homologous RNA during ripening is rapid and sustained, and reaches its maximum level in orange fruit. Following mechanical wounding of tomato leaves a pTOM 13--homologous RNA shows rapid induction within 30 minutes, which occurs before maximal ethylene evolution (2-3 h). This RNA also accumulates following the wounding of green tomato fruit. Northern blot analysis of poly(A)+ RNA indicates that the length of the mRNA is about 1400 nucleotides. Nucleotide sequence analysis showed the cDNA insert to contain the complete coding region of the pTOM 13 protein (33.5 kD) and an unusual 5' structure of ten dT-nucleotides. Hybridisation of the pTOM 13 cDNA insert to Southern blots of tomato DNA indicates the presence of only a small number of homologous sequences in the tomato genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acaster M. A., Kende H. Properties and Partial Purification of 1-Aminocyclopropane-1-carboxylate Synthase. Plant Physiol. 1983 May;72(1):139–145. doi: 10.1104/pp.72.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen R. E., Laties G. G. Ethylene regulation of gene expression in carrots. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4060–4063. doi: 10.1073/pnas.79.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Tamaki S., Dunsmuir P., Favreau M., Katayama C., Dooner H., Bedbrook J. mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucleic Acids Res. 1986 Mar 11;14(5):2229–2240. doi: 10.1093/nar/14.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. I. The cDNA-deduced primary structure of pre-inhibitor I and its post-translational processing. J Biol Chem. 1985 Jun 10;260(11):6555–6560. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitrit Y., Riov J., Blumenfeld A. Regulation of Ethylene Biosynthesis in Avocado Fruit during Ripening. Plant Physiol. 1986 May;81(1):130–135. doi: 10.1104/pp.81.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]