Abstract
A balance between alveolar liquid absorption and secretion is critical for maintaining optimal alveolar subphase liquid height and facilitating gas exchange in the alveolar space. However, the role of cystic fibrosis transmembrane regulator protein (CFTR) in this homeostatic process has remained elusive. Using a newly developed porcine model of cystic fibrosis, in which CFTR is absent, we investigated ion transport properties and alveolar liquid transport in isolated type II alveolar epithelial cells (T2AECs) cultured at the air-liquid interface. CFTR was distributed exclusively to the apical surface of cultured T2AECs. Alveolar epithelia from CFTR−/− pigs failed to increase liquid absorption in response to agents that increase cAMP, whereas cAMP-stimulated liquid absorption in CFTR+/− epithelia was similar to that in CFTR+/+ epithelia. Expression of recombinant CFTR restored stimulated liquid absorption in CFTR−/− T2AECs but had no effect on CFTR+/+ epithelia. In ex vivo studies of nonperfused lungs, stimulated liquid absorption was defective in CFTR−/− alveolar epithelia but similar between CFTR+/+ and CFTR+/− epithelia. When epithelia were studied at the air-liquid interface, elevating cAMP levels increased subphase liquid height in CFTR+/+ but not in CFTR−/− T2AECs. Our findings demonstrate that CFTR is required for maximal liquid absorption under cAMP stimulation, but it is not the rate-limiting factor. Furthermore, our data define a role for CFTR in liquid secretion by T2AECs. These insights may help to develop new treatment strategies for pulmonary edema and respiratory distress syndrome, diseases in which lung liquid transport is disrupted.
Keywords: pig, ion transport, subphase liquid
maintaining a balance between absorption and secretion of alveolar lining liquid is critical in both physiological and pathological processes, including during the transition from fetus to newborn, in homeostasis, and during pulmonary edema. For example, survival of newborns requires the rapid removal of liquid from prenatal lungs to allow newborns to breathe air (28). Under normal in vivo conditions, alveoli maintain an ∼1-μm-deep, tightly regulated apical liquid called the “subphase liquid” (SPL) (27). The SPL has a similar electrolyte composition to serum and contains phospholipids and proteins crucial in maintaining surface tension and the host defense (27). Dysregulation of liquid transport contributes to the development of pulmonary edema. However, the mechanisms underlying regulation of SPL transport in the alveolar epithelium remain poorly defined.
At the molecular level, absorption of fetal lung liquid at birth and resolution of alveolar edema is driven by Na+ and Cl− absorption across the alveolar epithelial barrier (2, 28, 29). Active Na+ transport, energized by the Na+-K+-ATPase expressed on the basolateral membrane, is responsible for generating the osmotic driving force for lung liquid absorption (7, 9, 30). By contrast, the epithelial Na+ channel (ENaC) and the Cl− channel cystic fibrosis transmembrane regulator (CFTR), which are restricted to the alveolar luminal membrane, allow passive Na+ and Cl− transport across the apical membrane. Thus maintenance of homeostasis requires stringent regulation of ion permeability and liquid transport.
Pharmacological interventions intended to increase alveolar liquid absorption in patients with pulmonary edema, including dopamine, β-adrenergic receptor agonists, and steroids, usually affect multiple channels and pumps including ENaC, CFTR, and Na+-K+-ATPase (24, 25). Thus the physiological contribution of CFTR alone to alveolar liquid clearance in pulmonary edema remains incompletely understood. Clinical associations between mutations in ENaC or CFTR and pulmonary phenotypes suggest that disrupting apical Na+ or Cl− transport can alter the volume or composition of airway and alveolar liquid. A subclinical accumulation of liquid in the alveoli is associated with loss-of-function mutations in ENaC (11, 12). In contrast, loss of CFTR function causes cystic fibrosis (CF) and results in chronic airway infection (32, 38), but surprisingly there is no evidence of clinical disease in the alveoli (40). These clinical observations, however, do not exclude a role for CFTR in maintaining liquid homeostasis in the alveolar epithelia.
We hypothesized that CFTR plays a regulatory role in alveolar liquid absorption and secretion in pig lungs. To test this hypothesis, pig type II alveolar epithelial cells (T2AECs) from wild-type (CFTR+/+), heterozygous (CFTR+/−), and null (CFTR−/−) pigs were isolated and cultured by use of an air-liquid interface (ALI) model. Our data in cultured T2AECs as well as ex vivo studies of newborn lungs demonstrate that CFTR is required for maximal cAMP-stimulated liquid absorption, but not for liquid absorption under basal condition. Moreover, lack of CFTR specifically impairs cAMP-stimulated liquid secretion when SPL height was measured in live alveolar epithelia cultured at the ALI, implicating transport of Cl− by CFTR in the mechanisms of both liquid absorption and secretion in the alveolar epithelium.
MATERIALS AND METHODS
Isolation of porcine lung alveolar epithelial cells.
All animal studies were reviewed and approved by the University of Iowa Animal Care and Use Committee. Generation of CFTR−/− pigs has been previously reported (32). Animals were produced by mating CFTR+/− male and female pigs. Newborn littermates including CFTR+/+, CFTR+/−, and CFTR−/− piglets were obtained from Exemplar Genetics (Exemplar Genetics, Sioux Center, IA). Primary porcine T2AECs were isolated according to an adapted procedure originally developed for human T2AECs (10, 37). T2AECs were isolated from lungs of piglets within 12 h after birth, before epithelia manifest changes secondary to CF-related disease. The pulmonary artery was perfused with PBS solution and the distal air spaces were lavaged 10 times with Ca2+- and Mg2+-free PBS solution (0.5 mM EGTA and 0.5 mM EDTA). Lung parenchymal tissue was carefully combed off the bronchioles. Resulting tissue pieces were washed and filtered over a layer of gauze. Tissue cubes of ∼5 mm in length were subjected to a fine manual cutting process to enlarge the surface for enzymatic digestion. A trypsin-elastase combination (0.5 mg/ml elastase in 0.5% trypsin solution) was used to enzymatically digest the distal lung tissue at 37°C for 60 min with shaking. Differential adherence on plastic surfaces served to remove macrophages (incubation at 37°C for 90 min). Blood cells and cell debris were dissociated by use of a discontinuous Percoll density gradient (P = 1.089 g/ml and P = 1.040 g/ml) and centrifuged at 600 g for 20 min. Cells collected from the interface were washed and plated at a density of 106 cells/cm2. Cell viability was assessed by the Trypan blue exclusion method. The purity of isolated porcine T2AECS cells was examined by Lysotrack green staining. Next, cells were seeded onto collagen-coated, semipermeable membranes and grown at the ALI as previously described (44). Specifically, cells were plated at a density of 106 cells/cm2 and grown under liquid covered conditions for 2 days in 10% FBS/DMEM supplemented with gentamicin, ampicillin (50 mg/ml each), and penicillin G (200 U/ml). From day 3 after initial seeding, cells were cultured at the ALI at 37°C in a 5% CO2 atmosphere and the culture medium was replaced at least every 2 days.
Cell marker for porcine T2AEC phenotype.
To confirm the purity of isolated porcine T2AECs, we examined morphological characteristics with Lysotrack green stain, a fluorescent dye that selectively stains lamellar bodies of T2AECs (4, 9). Five days after being seeded on Transwell membranes (Millipore) at the ALI, cells were preincubated for 30 min at 37°C in DMEM with Lysotrack green DND-26 (150 nmol/l; Molecular Probes) and images were obtained with a Nikon Inverted Microscope (TE 2000-E) and a Simple PCI Advance Image Capture system.
Adenoviral infection.
Recombinant adenovirus carrying either the enhanced green fluorescent protein (eGFP) or pig CFTR transgene, under the control of a cytomegalovirus promoter (31), was generated by the University of Iowa Gene Transfer Vector Core. Epithelia were infected apically with virus at a multiplicity of infection of 100 in 50 μl of EMEM for 3 h at 37°C. Virus was aspirated, and the apical surfaces were washed twice with media. Epithelia were returned to the incubator overnight. Infection efficiency was analyzed by visualizing GFP-positive cells on an Olympus FluoView FV1000 confocal microscope. At least three epithelia per condition, from each of the three different genotypes, were studied.
Immunocytochemistry.
Alveolar epithelia were fixed with 4% paraformaldehyde for 15 min, washed extensively with PBS, and permeabilized with 0.2% Triton X-100 (Pierce, Rockford, IL). Nonspecific binding was blocked by 1 h incubation in SuperBlock blocking buffer (Pierce) and the apical surfaces were incubated with primary antibody overnight at 4°C. Primary antibodies used were as follows: 1) mouse anti-CFTR (1:50; R&D); and 2) rabbit anti-zonula occludens-1 (anti-ZO-1; 1:100; Invitrogen-Zymed Laboratories). The following day, epithelia were washed with SuperBlock plus 2% BSA and incubated with secondary antibody (goat anti-rabbit Alexa Fluor 488, goat anti-mouse Alexa Fluor 568, 1:200 in SuperBlock plus 2% BSA; Invitrogen) for 1 h at room temperature protected from light. Following extensive washes with SuperBlock plus 2% BSA, epithelia were counterstained with DAPI, and inserts were then mounted onto glass slides and coverslipped with Vectashield mounting medium (Vector Laboratories).
Alveolar epithelia electrophysiology.
Ussing chamber studies were performed on T2AECs (6–7 days after seeding) from non-CF and CF pigs. Cells were mounted in Ussing chambers and studied as previously described (5, 35, 36). Apical and basolateral chambers contained the same bathing solution with symmetrical Cl− concentrations. CFTR-mediated Cl− current and amiloride-sensitive Na+ current were measured using a previously described protocol (5). First, we inhibited Na+ current with amiloride (100 μM), an ENaC blocker, which hyperpolarizes the apical membrane and increases the driving force for Cl− secretion through CFTR. Second, we added DIDS (100 μM) to block the calcium-activated Cl− channels to specifically examine the CFTR-mediated Cl− current. Next, we increased CFTR activity by elevating cellular levels of cAMP with forskolin (10 μM) and IBMX (100 μM). Then we added GlyH-101(100 μM), a CFTR inhibitor, to block the CFTR-mediated Cl− current (5). Finally, we reduced the transepithelial Cl− current by inhibiting the Na+-K+-Cl− cotransporter with basolateral bumetanide (100 μM). The following parameters were measured: decrease in current after apical addition of amiloride, cAMP-stimulated current after apical addition of forskolin and IBMX to epithelia already in the presence of apical amiloride and DIDS, and decrease in current after addition of GlyH-101 to the previous solutions. Transepithelial electrical conductance was measured with a chopstick ohmmeter (World Precision Instruments, Sarasota, FL).
Liquid absorption rate measurement.
Liquid absorption rate in T2AECs (6–7 days after seeding) was measured by methods similar to those previously described (20, 35). Specifically, the basolateral solution was replaced with fresh cell culture medium (500 μl) and saline (100 μl) was applied to the apical surface. Osmolality of the submucosal solution was adjusted to equal that of the mucosal solution. After incubation for 16 h, the apical solution was collected and the volume was measured. In some experiments forskolin (10 μM each) and IBMX (100 μM), amiloride (100 μM), and GlyH-101 (100 μM) were added to the apical solution.
Measurement of liquid clearance in ex vivo newborn pig lung.
Animals were euthanized (Euthasol, Virbac, Fort Worth, Texas) at 8–15 h of age for study. As previously described, with some modification (33, 34), a bronchus of a piglet lung was cannulated with a catheter and instilled with 8 ml of BSA solution containing 0.1 mg/ml Evans blue dye (EBD) (Sigma, St. Louis, MO). The lung was inflated with 100% oxygen through the catheter at 7 cmH2O airway pressure, placed in a plastic bag, and then submerged in a water bath at 37°C. Alveolar liquid was aspirated at 10 min and 70 min after instillation. EBD concentration of the aspirate samples was assayed by measuring the absorbance at 620 nm in a Hitachi model U2000 Spectrophotometer (Hitachi Instruments, San Jose, CA) and liquid absorption rate was calculated as previously described (6). In some experiments, 0.1 mM isoproterenol was added to the solution.
Wet-to-dry lung weight ratio.
The lung wet-to-dry (W/D) weight ratio was measured as an index of lung water accumulation in wild-type and CF piglets within 12 h of birth. To measure the total amount of lung water, the animals were euthanized and weight of a lung lobe was measured immediately after its excision (wet weight). The lung tissue was then dried in an oven at 60°C for 5 days and reweighed as dry weight. The W/D weight ratio was calculated by dividing the wet by the dry weight as described previously (22).
Measurement of SPL height.
Under ALI conditions, alveolar epithelia cultures were incubated with CellTrace Calcein Green AM (5 μm; Invitrogen) to stain the cytoplasm. SPL was stained with rhodamine B-dextran (70-kDa) by instilling 0.1 μl liquid dye (10 μg/μl) on the apical surface of the culture. After incubation at 37°C for 2 h, perfluorocarbon (1 ml) was added to the apical surface to prevent evaporation. The cells were immediately imaged with x–z view using an inverted confocal microscope and sampling five random sites and avoiding edge effects, as previously described (4). To study the effect of CFTR on SPL secretion, forskolin and IBMX were added to the basolateral culture medium for 48 h. At least five random images were taken from each sample, and the SPL was analyzed by use of Image J [National Institutes of Health (NIH), Bethesda, MD). SPL height was determined by drawing a perpendicular line from the apical membrane of the epithelial cell surface to the outer edge of the SPL. On each image, SPL height measurements were performed at 10 random locations and the average of those measurements was used as the SPL for that image.
Statistics.
Data were analyzed through calculation of group means and SE for each group. The analysis was performed by an unpaired t-test or ANOVA, and P < 0.05 was defined as statistically significant.
RESULTS
Pig alveolar epithelia grown at the ALI develop tight junctions and transepithelial resistance.
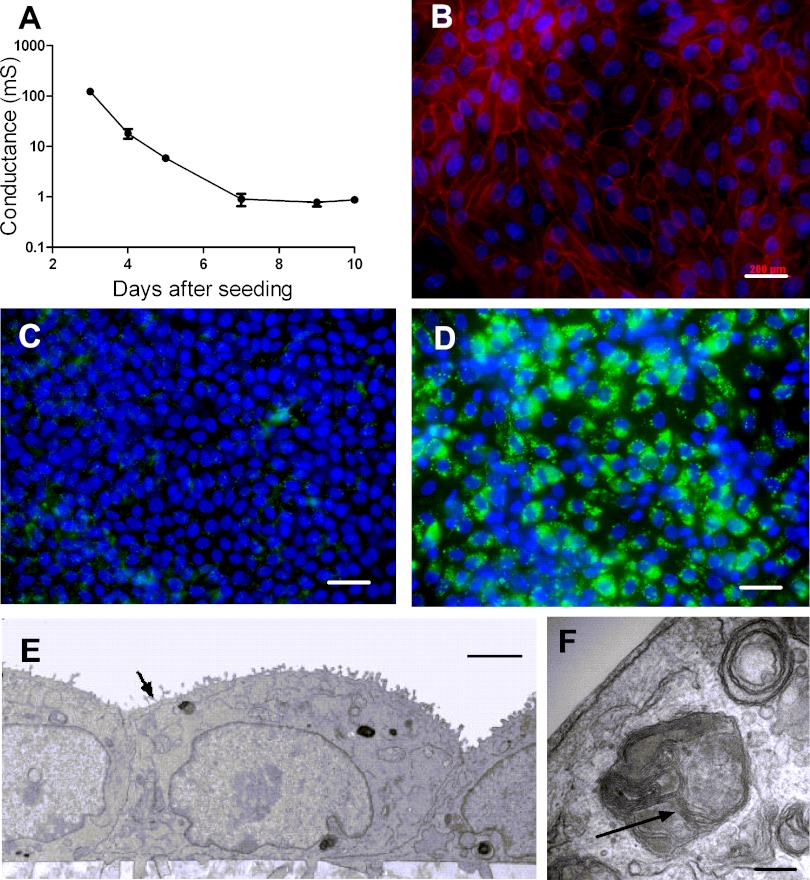
Alveolar epithelial cells from rat, mouse, and human grown in vitro at the ALI on semipermeable filters have been shown to exhibit a well-differentiated alveolar epithelial phenotype, characterized by tight junction formation, lamellar body preservation, and surfactant protein C production (9). We examined whether porcine T2AECs grown under the same conditions develop an alveolar epithelial phenotype. Briefly, T2AECs were isolated from CF (CFTR−/−) and non-CF (CFTR+/+ and CFTR+/−) newborn piglets and cultured on collagen I-coated semipermeable filters. After 72 h, the apical liquid was removed and epithelia were maintained at the ALI. Transepithelial conductance was measured every 48 h for 14 days. The conductance decreased with time (Fig. 1A), and by day 7 the conductance was 0.899 ± 0.249 mS; these results were similar among genotypes (data not shown). We also analyzed expression of the tight junction protein zonula occludens-1 (ZO-1). Figure 1B shows a chicken wire pattern, characteristic of tight junctions, in 4-day-old epithelia. Next, we investigated whether the cells expressed lamellar bodies, which are a hallmark of T2AECs, by staining with Lysotrack green DND-26 4 days after seeding (9). In contrast to pig airway epithelia (Fig. 1C), alveolar epithelial cells stained positive for lamellar bodies (Fig. 1D). To further confirm the identity of the T2AECs, we fixed and processed 4- to 7-day-old epithelia for examination by transmission electron microscopy. At day 4, cultured cells were cuboidal, had microvilli on the apical surface, and contained intracellular lamellar bodies (Fig. 1, E and F). These findings demonstrate that pig alveolar epithelial cells grown at the ALI recapitulate important characteristics of alveolar epithelia in vivo. These observations are also consistent with phenotypes of T2AECs isolated from other species, including human, rat, and mouse (9).
Fig. 1.
Characterization of pig type II alveolar epithelial cells (T2AECs). A: time course of transmembrane electrical conductance (mS, millisiemens) of T2AECs growing on collagen I-coated Transwell membranes (n = 4 at each time point). B: immunostaining of tight junction marker zonula occludens-1 (ZO-1; red) in pig T2AECs 4 days after seeding. Nuclei were counterstained with DAPI (blue). Scale bar = 200 μm. C and D: Lysotrack green DND-26 (150 nmol/l, 30 min) was used to selectively stain lamellar bodies in primary cultures of airway (C) and alveolar (D) epithelial cells. Cells were grown on collagen I-coated Transwell membranes and imaged 4 days after seeding. Lamellar bodies were found in T2AECs (D) but not in tracheal alveolar epithelial cells (C). Nuclei were stained with Hoechst dye (blue). Scale bar = 200 μm. E and F: ultrastructure of pig T2AECs. T2AECs cultured at the air-liquid interface (ALI) for 4 days were fixed and processed for transmission electron microscopy. E: the majority of cells are cuboidal in shape with microvilli on the apical surface (denoted by arrow), consistent with a T2AEC identity. F: at higher magnification, lamellar bodies with a multilayered onionlike ultrastructure were observed (arrow). Scale bars = 2 μm for E and 0.2 μm for F.
CFTR is localized on the apical surface of polarized pig alveolar epithelial cells.
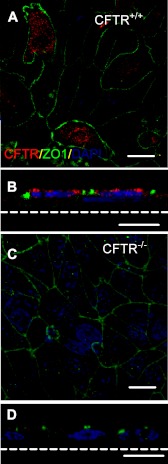
In both airway and alveolar epithelia, CFTR provides both Cl− and HCO3− permeability at the apical surface. Although apical localization of CFTR has been reported in airway epithelial cells, its distribution in alveolar epithelia has been difficult to establish because these cells are very thin and difficult to culture at the ALI. Furthermore, immunostaining has been difficult because of low CFTR expression and poor sensitivity and specificity of available antibodies (15). We stained CF and non-CF polarized alveolar epithelia cultured at the ALI with an anti R-domain monoclonal antibody. CFTR was localized to the apical membrane of 4- to 7-day-old CFTR+/+ alveolar epithelia cultures and absent in the CFTR−/− epithelia (Fig. 2), similar to CFTR distribution in pig airway epithelia (31).
Fig. 2.
CFTR is expressed on the apical surface of differentiated wild-type (WT) pig alveolar epithelia. Immunostaining of CFTR in WT (A and B) or CFTR null (C and D) pig T2AECs cultured at the ALI for 7 days. A and C are enface confocal images; B and D are single vertical sections. CFTR, red; ZO-1, green. Scale bar = 20 μm for (A and C) and 10 μm for (B and D).
CFTR is required for cAMP-stimulated Cl− transport and liquid absorption in pig alveolar epithelia.
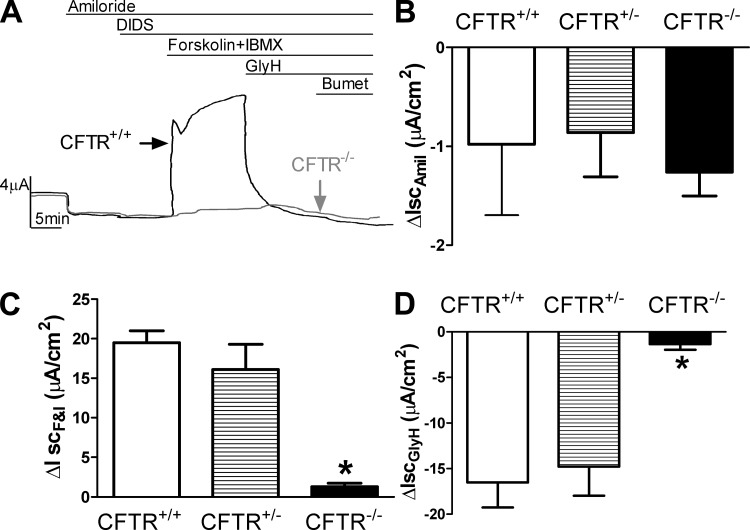
Others have shown that CFTR promotes liquid absorption in alveolar epithelial cells (8, 9, 24), although interpretation of these studies depended on use of CFTR functional blockers/inhibitors. The porcine CF model allowed us to examine the contribution of CFTR to Cl− permeability and liquid absorption. We first isolated alveolar epithelia cells from CFTR+/+ and CFTR−/− piglets and studied their bioelectric properties in Ussing chambers. Figure 3A shows representative short-circuit current (Isc) traces from CFTR+/+ and CFTR−/− T2AECs. Under conditions with symmetrical Cl− concentrations at both apical and basolateral membranes, the amiloride-sensitive current, which reflects ENaC function, was similar in CFTR+/+ and CFTR−/− T2AECs (Fig. 3B). In contrast, CFTR−/− T2AECs had a severely blunted response to agents that increase cAMP levels (forskolin+IBMX, Fig. 3C) and a near absence of GlyH-101 (CFTR inhibitor)-inhibitable current, which reflects CFTR function, compared with CFTR+/+ cells (Fig. 3D). These data are consistent with previous observations using functional CFTR inhibitors in non-CF human and murine alveolar cells (8, 9). Addition of DIDS did not change Isc in either CF or non-CF epithelia, suggesting the absence of calcium-activated Cl− channels in alveolar epithelia (Fig. 3A). However, it remains possible that increases in intracellular calcium might stimulate these channels.
Fig. 3.
Cl− transport is defective in CFTR−/− T2AECs but is restored with expression of one allele of CFTR. A: examples of short-circuit current (Isc) traces from WT (black) and CFTR−/− (gray) T2AECs in response to agents indicated above traces. B: changes in amiloride-sensitive current in CFTR+/+, CFTR+/−, and CFTR−/− T2AECs. C: responses to cAMP agonists (forskolin + IBMX) by CFTR+/+, CFTR+/−, and CFTR−/− T2AECs. D: changes in GlyH 101-inhibitable current in CFTR+/+, CFTR+/−, and CFTR−/− T2AECs. IscAmil, decrease in current after apical addition of amiloride; IscF&I, cAMP-stimulated current after apical addition of forskolin and IBMX to epithelia already in the presence of apical amiloride and DIDS; IscGlyH, decrease in current after addition of GlyH-101 to the previous solutions. Data in B–D are shown as means ± SE; n = 4–6; *P < 0.05 compared with CFTR+/+ and CFTR+/−.
If apical Cl− transport by CFTR is rate limiting for Cl transport, we hypothesized that CFTR+/− epithelia would have an intermediate phenotype. We therefore compared CFTR+/+ and CFTR+/− alveolar epithelia under short-circuit conditions and found no difference. CFTR+/+ and CFTR+/− T2AECs had similar amiloride-sensitive (Fig. 3B), cAMP-induced (Fig. 3C), and GlyH-101-inhibitable Isc (Fig. 3D).
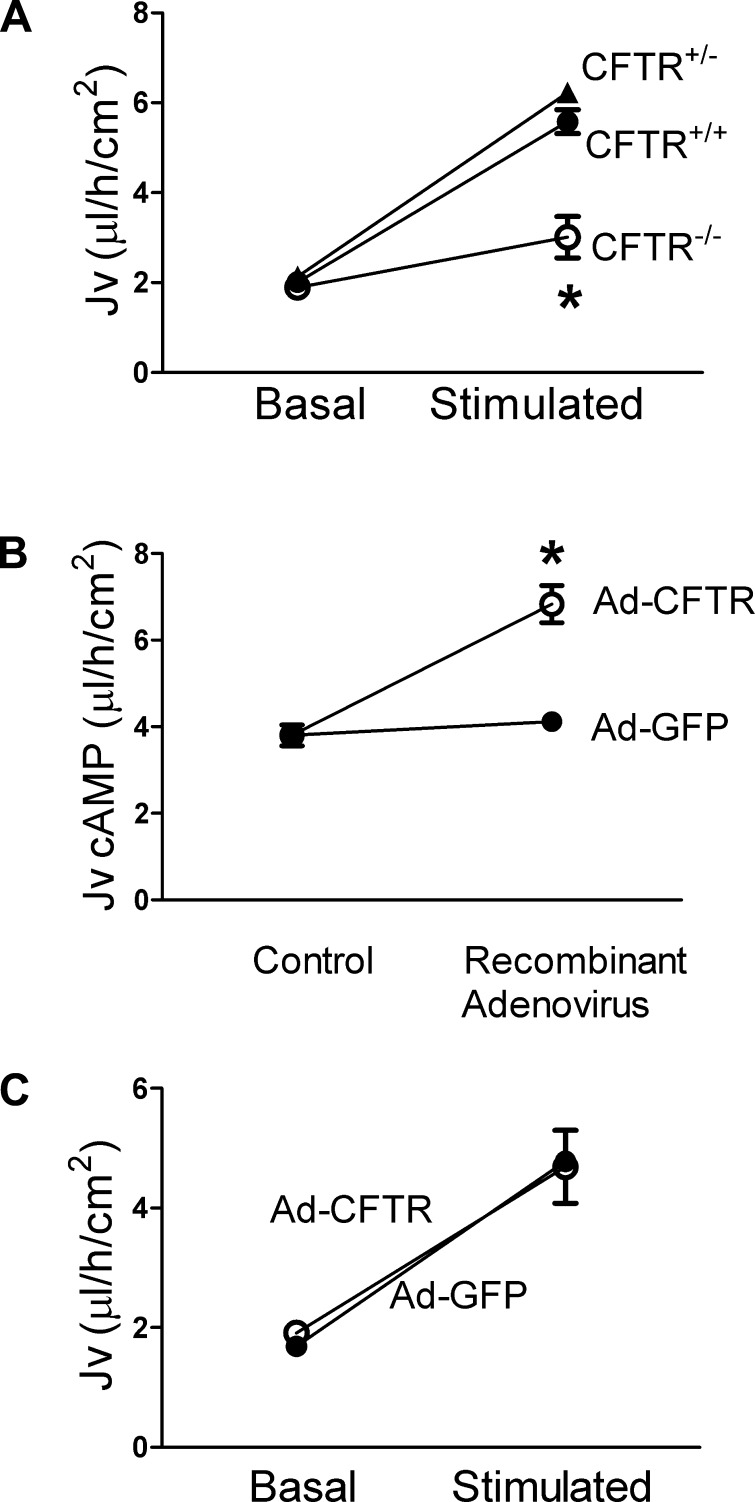
To investigate the effect of CFTR on liquid transport under open-circuit conditions, we applied an isotonic solution to the apical surface of cultured alveolar epithelia and assessed liquid absorption over 16 h. Under basal conditions, we observed no difference in the liquid absorption rate between CFTR+/+ and CFTR−/− T2AECs (Fig. 4A). Elevating cAMP levels increased the liquid absorption rate in CFTR+/+ T2AECs by 280%, whereas only a 50% increase in liquid absorption was detected in cAMP-stimulated CFTR−/− T2AECs. Moreover, the basal and cAMP-stimulated liquid absorption in CFTR+/− alveolar epithelial cells was similar to that of CFTR+/+ cells (Fig. 4A). These results indicate that CFTR is not required for liquid absorption under basal conditions, suggesting the existence of an alternative mechanism to achieve electroneutrality in CFTR−/− alveolar epithelia. In contrast, under cAMP-stimulated conditions, CFTR was required for maximal liquid absorption in pig alveolar epithelia. Moreover, these findings suggest that expression of one normal allele of CFTR is sufficient for maximal liquid absorption, and CFTR is not rate limiting for transepithelial Cl− transport.
Fig. 4.
CFTR is required but is not rate limiting for cAMP-stimulated liquid transport in pig T2AECs. A: basal and cAMP-stimulated (forskolin+IBMX) liquid transport (Jv) was examined in CFTR+/+ (●), CFTR+/− (▲), and CFTR−/− (○) T2AECs cultured at the ALI for 6–7 days. *P < 0.05 compared with cAMP-stimulated CFTR+/+ and CFTR+/−. B: cAMP-stimulated liquid transport was examined in CFTR−/− T2AECs infected with either control adenovirus (Ad-GFP, ●) or adenovirus encoding pig CFTR (Ad-CFTR, ○). *P < 0.05 compared with Ad-GFP. C: basal and cAMP-stimulated liquid transport was assessed in CFTR+/+ T2AECs after infection with either Ad-GFP or Ad-CFTR. Data are shown as means ± SE; n = 4–6.
Adenoviral-mediated expression of CFTR restores cAMP-stimulated liquid absorption in CFTR−/− T2AECs.
To further investigate whether the absence of CFTR is responsible for the decreased cAMP-stimulated liquid absorption in CFTR−/− alveolar epithelial cells, we restored CFTR expression using an adenoviral vector encoding pig CFTR (Ad-CFTR, Ref. 31). We first established the Ad-CFTR dose that restored the cAMP-induced Cl− current in CFTR−/− T2AECs to levels comparable to that of CFTR+/+ T2AECs (data not shown). Next, we found that adenoviral expression of CFTR in CFTR−/− T2AECs increased cAMP-stimulated liquid absorption compared with expression of Ad-GFP control (Fig. 4B). These data further indicate that the absence of CFTR causes defective cAMP-stimulated liquid absorption in alveolar epithelia.
Adenoviral-mediated expression of CFTR in CFTR+/+ T2AECs does not further increase liquid absorption.
We also investigated how expressing CFTR in CFTR+/+ alveolar epithelia affected liquid transport. Basal liquid absorption was not affected by Ad-mediated expression of CFTR compared with Ad-GFP (Fig. 4C). Moreover, Ad-mediated expression of CFTR, which was sufficient to correct liquid absorption in CFTR−/−, did not increase cAMP-stimulated liquid absorption in CFTR+/+ T2AECs (Fig. 4C). These data suggest that further increases in CFTR expression have no additive effect on alveolar liquid absorption.
CFTR−/− newborn pig lungs have decreased cAMP-stimulated alveolar liquid clearance.
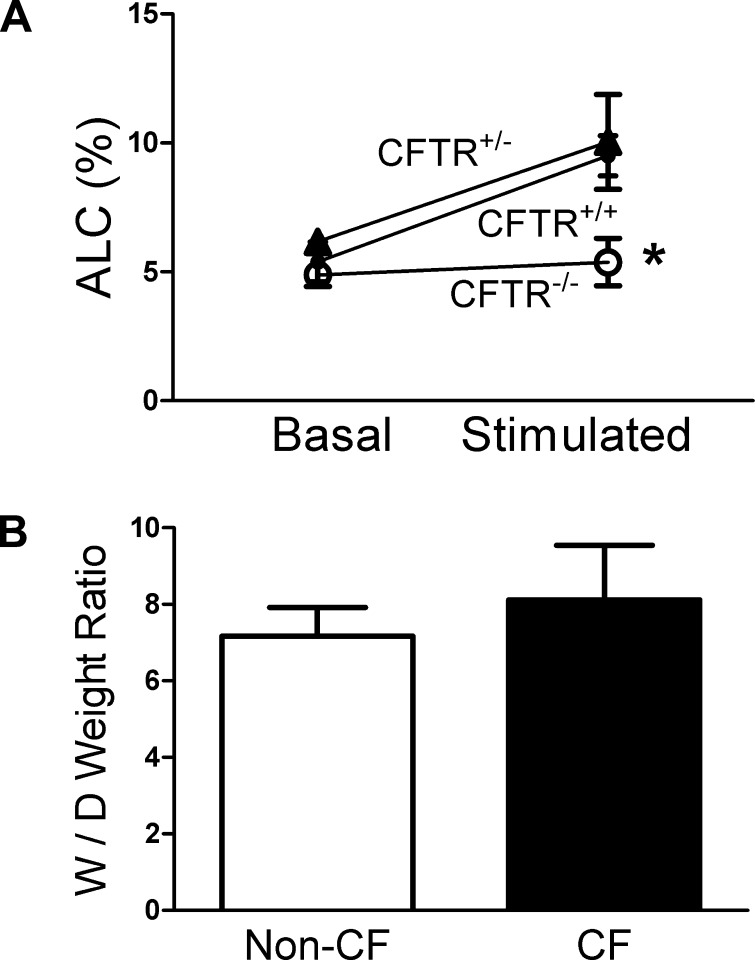
We next studied the basal and cAMP-stimulated liquid clearance in ex vivo nonperfused lung preparations from CFTR+/+, CFTR+/−, and CFTR−/− pigs. The basal alveolar liquid clearance for newborn pig lungs was ∼6% in the first hour for all genotypes (Fig. 5A). Stimulation with isoproterenol increased alveolar liquid clearance by ∼50% in CFTR+/+ and CFTR+/− pigs, which is comparable to other studied species (33). In contrast, CFTR−/− lungs showed no response to isoproterenol. Combined with our in vitro observations, these data suggest a model in which lack of CFTR in alveolar cells causes a defect in Cl− transport, which impairs cAMP-stimulated liquid absorption. Moreover, CFTR expression in CFTR+/− lungs is sufficient for maximal liquid absorption. Interestingly, when we measured the W/D ratios of lung weight from CF and non-CF piglets within 12 h of birth, we did not detect difference (Fig. 5B).
Fig. 5.
Threshold levels of CFTR are necessary for maximal cAMP-stimulated alveolar liquid clearance (ALC) ex vivo. A: ALC was examined in CFTR+/+ (●), CFTR+/− (▲), and CFTR−/− (○) newborn pig lungs under basal and cAMP-stimulated (0.1 mM isoproterenol) conditions. Data are shown as means ± SE; n = 4–6; *P < 0.05 compared with CFTR+/+ and CFTR+/− under stimulated conditions. B: lung wet-to-dry (W/D) weight ratio was used as an index of lung liquid accumulation in CF (CFTR−/−) and non-CF (including CFTR+/+ and CFTR+/−) newborn piglets. W/D weight ratio was calculated by dividing the wet by the dry weight as described previously (22). Data are shown as means ± SE; n = 5.
CFTR regulates SPL secretion at the ALI.
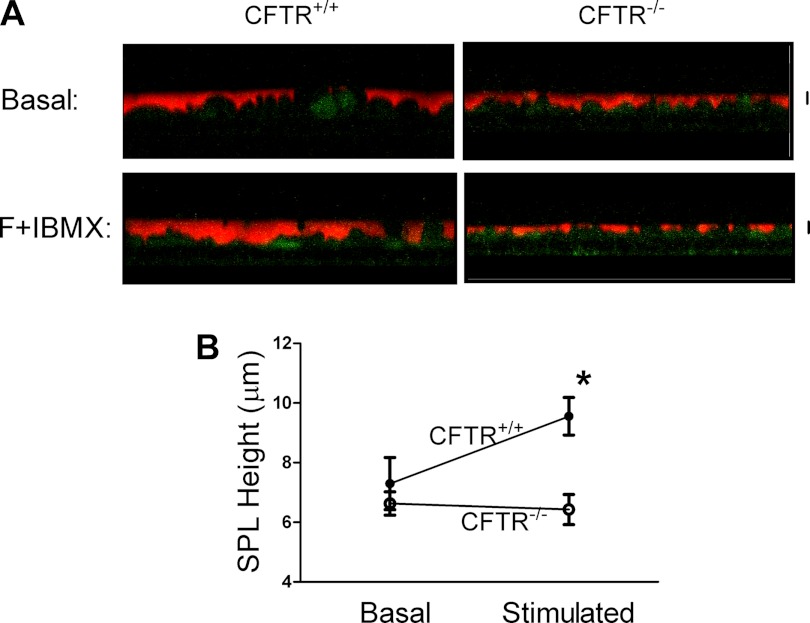
As shown above, alveolar epithelia can absorb liquid, and CFTR is required for maximal liquid absorption. However, these studies were performed on submerged epithelia such as in Ussing chambers and with liquid transport assays that may not reflect the in vivo condition. With respect to liquid secretion, Bove et al. (4) showed that human alveolar epithelia, as studied at the ALI, secrete liquid upon stimulation with agents that increase cAMP levels (i.e., forskolin). In an in vivo model Lindert et al. (19) found that CFTR plays a role in liquid secretion in an air-filled lung. Using our genetic model, we therefore investigated the role of CFTR in SPL secretion in alveolar epithelia cultured at the ALI by visualizing the SPL height with a fluorescent dye, rhodamine B-labeled dextran. Figure 6A shows representative confocal microscopy images of the SPL in either CFTR+/+ or CFTR−/− T2AECs under basal and cAMP-stimulated conditions. Under basal conditions, there was no difference in SPL height between CFTR+/+ and CFTR−/− T2AECs (Fig. 6B). In contrast, stimulation with agents that increase cAMP (forskolin + IBMX) significantly increased the SPL height from 6 μm to ∼10 μm in CFTR+/+ T2AECs. By contrast, addition of forskolin and IBMX treatment had no effect on SPL height in CFTR−/− cells (Fig. 6B). These results indicate an important role for CFTR in liquid secretion in pig alveolar epithelia studied at the ALI.
Fig. 6.
CFTR regulates liquid secretion by pig T2AECs. A: basal or cAMP-stimulated (forskolin, F+IBMX) pig CFTR+/+ and CFTR−/− T2AEC cells were labeled with CellTrack Green; the subphase liquid (SPL) was labeled with a rhodamine-labeled 70-kDa dextran (red). Images are single vertical sections; scale bar = 5 μm. B: SPL height was measured and quantitated in CFTR+/+ and CFTR−/− T2AECs under basal and cAMP-stimulated conditions. Data are shown as means ± SE; n = 3–4; *P < 0.05 compared with CFTR+/+ under cAMP stimulation.
DISCUSSION
Regulation of liquid transport in the alveolar space plays a critical role in gas exchange in the perinatal period. Here, we use a novel animal model of CF that closely mimics human clinical phenotypes (32) to study the regulation of liquid transport by CFTR in alveolar epithelia. Epithelia from CFTR+/+, CFTR+/−, and CFTR−/− pigs have similar amiloride-sensitive current under Isc conditions and similar basal liquid absorption and secretion. However, under conditions of cAMP stimulation, epithelia from CFTR−/− pigs fail to increase liquid absorption compared with both CFTR+/+ and CFTR+/− alveolar epithelia. Moreover, when epithelia were studied at the ALI, SPL height was increased in CFTR+/+ but not CFTR−/− epithelia after cAMP stimulation. Taken together, our data indicate that, by controlling Cl− permeability in alveolar epithelia, CFTR is a key regulator of liquid absorption and secretion.
Maintenance of the ALI in the alveolar space relies on an epithelium that expresses Na+-K+-ATPase at the basolateral side (41), ENaC (21) and CFTR (23) on the apical side, and specific aquaporins (42). Compared with the other channels and pumps, the contribution of CFTR to alveolar liquid absorption is not completely understood. Studies in cultured human and rat alveolar epithelia, in vivo mouse models, and ex vivo isolated human lung preparations have shown that liquid absorption was decreased with pharmacological inhibition of CFTR (8, 9), whereas recombinant CFTR expression increased liquid absorption (24). Interestingly, the phenotype was only observed when conditions led to maximal liquid absorption. For example, Fang and colleagues (9) showed that a CFTR inhibitor, CFTRinh-172, blocked cAMP-stimulated Isc in T2AECs but had no effect on basal liquid transport. When the epithelia were stimulated with reagents increasing cAMP level, CFTRinh-172 prevented the increase in liquid absorption. Similarly, in studies of ex vivo mouse and human lungs, the Cl− channel inhibitor glibenclamide abolished cAMP-stimulated alveolar liquid clearance but also had no effect under basal conditions (8). In contrast to these studies that employed pharmacological approaches to examine the role of CFTR in liquid transport, we used a genetic strategy and also found that CFTR was required for maximal stimulated liquid absorption, whereas basal liquid absorption was independent of CFTR activity.
A majority of the studies of the role of CFTR in liquid transport used either inhibitors of CFTR or a CFTR mouse model, which confounds interpretation of CFTR function. For example, despite abrogation of CFTR function, mice do not develop CF lung disease (5), perhaps because of compensatory activity by other Cl− channels. By contrast, deletion of CFTR in pigs mimics the electrolyte transport properties of human airway epithelia and the pulmonary bacterial infection phenotype of humans with CF (32, 38). In the porcine model, we found that CFTR+/+, CFTR+/−, and CFTR−/− type II alveolar epithelial cells could be cultured at the ALI. Use of this model system allowed specific investigation of the role of CFTR in liquid transport without the use of pharmacological inhibitors. One possible limitation is that pig T2AECs, as grown in these studies, may not reproduce the relative proportion of type I and type II cells seen in vivo. Although most studies of ion channels and liquid absorption have been conducted in type II cells, type I cells have been shown to also play a role in ion and liquid absorption (13). However, the results of our ex vivo studies in an isolated nonperfused piglet lung model validate our in vitro conclusions. Providing further support for our model system, all genotypes studied displayed normal liquid absorption in the transition from fetus to newborn as demonstrated by similar W/D ratios of lung weight (see Fig. 5B), consistent with observations in humans with CFTR mutations (43).
Most studies of alveolar fluid absorption in mice, rats, humans, and now pigs suggest that lack of CFTR results in decreased liquid absorption. These studies seem to contrast with the hypothesis that ENaC “activity” is increased in CF airways. There has been ample support for this hypothesis. First, amiloride luminal perfusion results in a greater change in nasal transepithelial voltage (Vt) in patients with CF (1, 3, 14). Second, studies pioneered by Stutts et al. (39) on MDCK cells and fibroblasts showed that CFTR negatively regulates epithelial Na+ channels. Finally, recent work by Lazrak et al. (16) demonstrated that the open-state probability of ENaC in CFTR−/− mouse alveolar epithelia was higher than in controls. This study was conducted on lung slices and supports the results obtained by others in an ex vivo airway model (39). Although these studies seem to be at odds with our data and may reflect variability inherent in the different models, it is more likely that they highlight the complexity of studying electrical transport properties of epithelia. For example, the data presented herein appear to be a paradoxical result compared with our recently reported data in the airways of a CF pig model (5), even though both studies were performed in the same model. In this previous study, we observed a greater change in voltage and Isc with amiloride in the CF pig nasal epithelia (5). However, Na+ absorption was not increased and liquid absorption was decreased. This is consistent with the fact that adding amiloride hyperpolarizes the apical membrane voltage, thereby increasing the driving force for Cl− secretion and subsequently resulting in smaller changes in Vt and Isc. In the CF nasal epithelia, lack of CFTR prevents Cl− secretion, resulting in measuring only the change in Vt and Isc that was due to blocking ENaC absorption; thus Vt and Isc appear higher. By contrast, when the trachea epithelia were studied, there was no difference in the change in Vt or Isc with amiloride between genotypes, perhaps because CFTR was closed in the wild-type epithelia under basal conditions (5). The amiloride-sensitive Isc was not increased in the CF alveolar epithelia in this study; however, these currents are small compared with airways and we did not stimulate CFTR before adding amiloride.
Although we did not measure the ENaC open-state probability in the pig alveoli, an increased ENaC open-state probability may not result in increased Na+ absorption in CF epithelia. Higher ENaC open-state probability may favor apical membrane Na+ absorption following a chemical gradient while simultaneously increasing Na+ secretion based on an electrochemical gradient created by the higher apical membrane Vt, which is caused by the lack of CFTR. In the future it may be important to investigate ENaC open-state probability in pig alveoli. Measuring electrical properties of epithelia is complex and requires an understanding of the measurements being done, conditions, reagents, concentrations, and models. Moreover, epithelial ion transport is a dynamic and integrated process involving the epithelial apical membrane, basolateral membrane, and a paracellular pathway such that calculations are best suited to mathematical modeling to achieve a complete understanding of epithelial ion transport.
Drugs that augment cAMP levels have been shown to affect Na+-K+-ATPase, ENaC, and CFTR (26), which has important clinical implications for increasing liquid absorption in patients with pulmonary edema. Herein, using CFTR−/− alveolar epithelial cells, we demonstrate that CFTR is required for maximal absorption. On the basis of earlier observations that adenoviral-mediated overexpression of CFTR in vivo further increases liquid absorption (24), we initially hypothesized that CFTR+/− pigs would have an intermediate liquid absorption rate. Unexpectedly, absorption in CFTR+/− T2AECs was identical to CFTR+/+ cells, suggesting that expression of one normal allele of CFTR is sufficient for maximal absorption. Supporting this notion, overexpression of CFTR in CFTR+/+ T2AECs did not provide additional benefit in terms of liquid absorption. Moreover, these observations indicate that apical CFTR functional activity is not the rate-limiting step in cAMP-stimulated liquid reabsorption in alveolar epithelia. The rate-limiting step is most likely at the basolateral membrane; possible rate-limiting factors that control Cl− permeability include KCl cotransporters KCC1, KCC3, and KCC4, which are involved in cAMP-stimulated Cl− efflux across the basolateral membrane (17, 18).
There are two interesting observations from our studies. First, despite a decrease in cAMP-stimulated maximal liquid absorption in newborn CF lungs, we observed no difference in the W/D weight ratios of lungs from CF and non-CF piglets 12 h after birth. This suggests that, at the perinatal period, liquid absorption may be independent of CFTR. Second, in response to cAMP elevation, alveolar epithelia can either absorb or secrete liquid. The direction of net liquid transport is dependent on how much liquid is on top of the epithelia. Bove et al. (4) showed that the net liquid transport is dependent on SPL ATP concentration, which is thought to regulate apical ENaC activity. Alternatively, under certain conditions, different cell populations in distal lung epithelia may be separately responsible for secretion and absorption. Finally, we speculate that alveolar epithelia sense the pressure on the apical membrane via mechanosensitive receptors or by changing the partial pressure of oxygen, which might initiate secondary signaling pathways that then regulate either KCC and NKCC activity at basolateral membrane or apical ENaC activity.
In summary, our study identifies CFTR as a key regulator of liquid absorption and secretion in alveolar epithelia. The mechanism by which the alveolar epithelia sets the balance between absorption and secretion, depending on how much liquid is on top of the epithelia, remains undefined, although our data clearly demonstrate that CFTR has a role in both of these processes. Understanding the precise role of CFTR in each of these processes will have important clinical implications in health and disease.
AUTHOR CONTRIBUTIONS
XL, APC, DAS, PBM, MJW, JZ conceived of study and designed experiments; XL, APC, PHK, SEE, TOM, NDG, PJT, AAP, MVR, NR, DAS performed experiments; XL, APC, MJW, JZ analyzed data, XL, PBM, MJW, JZ wrote and reviewed the manuscript.
GRANTS
This work was supported by the NIH (HL51670, HL091842, and DK54759) and the Cystic Fibrosis Foundation. X. Li was supported by a NIH T32 training grant. M. J. Welsh is an Investigator of the Howard Hughes Medical Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. L. Ostedgaard, P. Hughes, and P. Tan for excellent technical assistance. We thank Dr. Carsten Ehrhardt for sharing the protocol for pig alveolar epithelial cell culture. We thank Dr. Kristina W. Thiel for assistance in manuscript preparation. GlyH-101 was a gift from the Cystic Fibrosis Foundation Therapeutics and R. Bridges. We thank the University of Iowa In Vitro Models and Cell Culture Core and the Gene Transfer Vector Core for assistance, supported, in part, by National Heart, Lung, and Blood Institute Grants HL091842 and HL51670.
REFERENCES
- 1. Alton EW, Currie D, Logan-Sinclair R, Warner JO, Hodson ME, Geddes DM. Nasal potential difference: a clinical diagnostic test for cystic fibrosis. Eur Respir J 3: 922–926, 1990 [PubMed] [Google Scholar]
- 2. Berthiaume Y, Matthay MA. Alveolar edema fluid clearance and acute lung injury. Respir Physiol Neurobiol 159: 350–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boucher RC, Cheng EH, Paradiso AM, Stutts MJ, Knowles MR, Earp HS. Chloride secretory response of cystic fibrosis human airway epithelia. Preservation of calcium but not protein kinase C- and A-dependent mechanisms. J Clin Invest 84: 1424–1431, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, O'Neal WK, Boucher RC. Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem 285: 34939–34949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143: 911–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Comellas AP, Pesce LM, Azzam Z, Saldias FJ, Sznajder JI. Scorpion venom decreases lung liquid clearance in rats. Am J Respir Crit Care Med 167: 1064–1067, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Factor P. Role and regulation of lung Na,K-ATPase. Cell Mol Biol 47: 347–361, 2001 [PubMed] [Google Scholar]
- 8. Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 119: 199–207, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, Lehr CM. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res 311: 31–45, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Hummler E, Horisberger JD. Genetic disorders of membrane transport. V. The epithelial sodium channel and its implication in human diseases. Am J Physiol Gastrointest Liver Physiol 276: G567–G571, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Hummler E, Planes C. Importance of ENaC-mediated sodium transport in alveolar fluid clearance using genetically-engineered mice. Cell Physiol Biochem 25: 63–70, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Johnson M, Allen L, Dobbs L. Characteristics of Cl− uptake in rat alveolar type I cells. Am J Physiol Lung Cell Mol Physiol 297: L816–L827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther 6: 445–455, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell 16: 2154–2167, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: (4) L557–L567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SY, Maniak PJ, Ingbar DH, O'Grady SM. Adult alveolar epithelial cells express multiple subtypes of voltage-gated K+ channels that are located in apical membrane. Am J Physiol Cell Physiol 284: C1614–C1624, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Lee SY, Maniak PJ, Rhodes R, Ingbar DH, O'Grady SM. Basolateral Cl− transport is stimulated by terbutaline in adult rat alveolar epithelial cells. J Membr Biol 191: 133–139, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Lindert J, Perlman CE, Parthasarathi K, Bhattacharya J. Chloride-dependent secretion of alveolar wall liquid determined by optical-sectioning microscopy. Am J Respir Cell Mol Biol 36: 688–696, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mangoo-Karim R, Uchic ME, Grant M, Shumate WA, Calvet JP, Park CH, Grantham JJ. Renal epithelial fluid secretion and cyst growth: the role of cyclic AMP. FASEB J 3: 2629–2632, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 61: 627–661, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Matsuyama H, Amaya F, Hashimoto S, Ueno H, Beppu S, Mizuta M, Shime N, Ishizaka A, Hashimoto S. Acute lung inflammation and ventilator-induced lung injury caused by ATP via the P2Y receptors: an experimental study. Respir Res 9: 79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2: 206–213, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Mutlu GM, Adir Y, Jameel M, Akhmedov AT, Welch L, Dumasius V, Meng FJ, Zabner J, Koenig C, Lewis ER, Balagani R, Traver G, Sznajder JI, Factor P. Interdependency of beta-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res 96: 999–1005, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, Matalon S, Hollenberg S, Factor P. Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling. Circ Res 94: 1091–1100, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Mutlu GM, Factor P. Alveolar epithelial beta2-adrenergic receptors. Am J Respir Cell Mol Biol 38: 127–134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nielson DW, Goerke J, Clements JA. Alveolar subphase pH in the lungs of anesthetized rabbits. Proc Natl Acad Sci USA 78: 7119–7123, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Brodovich H. Epithelial ion transport in the fetal and perinatal lung. Am J Physiol Cell Physiol 261: C555–C564, 1991 [DOI] [PubMed] [Google Scholar]
- 29. O'Brodovich H. Pulmonary edema in infants and children. Curr Opin Pediatr 17: 381–384, 2005 [DOI] [PubMed] [Google Scholar]
- 30. O'Brodovich HM. Immature epithelial Na+ channel expression is one of the pathogenetic mechanisms leading to human neonatal respiratory distress syndrome. Proc Assoc Am Physicians 108: 345–355, 1996 [PubMed] [Google Scholar]
- 31. Ostedgaard LS, Rogers CS, Dong Q, Randak CO, Vermeer DW, Rokhlina T, Karp PH, Welsh MJ. Processing and function of CFTR-DeltaF508 are species-dependent. Proc Natl Acad Sci USA 104: 15370–15375, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321: 1837–1841, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sakuma T, Okaniwa G, Nakada T, Nishimura T, Fujimura S, Matthay MA. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med 150: 305–310, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Sakuma T, Suzuki S, Usuda K, Handa M, Okaniwa G, Nakada T, Fujimura S, Matthay MA. Preservation of alveolar epithelial fluid transport mechanisms in rewarmed human lung after severe hypothermia. J Appl Physiol 80: 1681–1686, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Smith JJ, Karp PH, Welsh MJ. Defective fluid transport by cystic fibrosis airway epithelia. J Clin Invest 93: 1307–1311, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85: 229–236, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Steimer A, Laue M, Franke H, Haltner-Ukomado E, Lehr CM. Porcine alveolar epithelial cells in primary culture: morphological, bioelectrical and immunocytochemical characterization. Pharm Res 23: 2078–2093, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, 4th, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Jr, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2: 29ra31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science 269: 847–850, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Ulrich M, Worlitzsch D, Viglio S, Siegmann N, Iadarola P, Shute JK, Geiser M, Pier GB, Friedel G, Barr ML, Schuster A, Meyer KC, Ratjen F, Bjarnsholt T, Gulbins E, Doring G. Alveolar inflammation in cystic fibrosis. J Cyst Fibros 9: 217–227, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vadasz I, Raviv S, Sznajder JI. Alveolar epithelium and Na,K-ATPase in acute lung injury. Intensive Care Med 33: 1243–1251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verkman AS, Matthay MA, Song Y. Aquaporin water channels and lung physiology. Am J Physiol Lung Cell Mol Physiol 278: L867–L879, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Wilson SM, Olver RE, Walters DV. Developmental regulation of lumenal lung fluid and electrolyte transport. Respir Physiol Neurobiol 159: 247–255, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Zabner J, Wadsworth SC, Smith AE, Welsh MJ. Adenovirus-mediated generation of cAMP-stimulated Cl− transport in cystic fibrosis airway epithelia in vitro: effect of promoter and administration method. Gene Ther 3: 458–465, 1996 [PubMed] [Google Scholar]