Abstract
Background acupuncture (AP) has been shown to have a therapeutic potential for gastrointestinal motility disorders. The aims of this study were to investigate the effects and possible mechanisms of acupuncture on postprandial upper and lower abdominal symptoms induced by rectal distension (RD). Twenty healthy volunteers were involved in a two-session study (AP and sham-AP, AP and no-AP, or sham-AP and no-AP). In 12 of the volunteers, RD was performed for 60 min in the postprandial state, and AP at ST36 or sham-AP was performed during the second 30-min period of RD. Gastric slow waves and heart rate variability (HRV) were recorded using the electrogastrogram and electrocardiogram, respectively. Upper and lower abdominal symptoms were scored during RD with AP and sham-AP. In five of the subjects, an additional experiment with two sessions (with AP and no-AP) was performed. In the remaining eight volunteers, the same experiment was performed with sham-AP and no-AP was performed. The results were, first, RD at an average volume of 171 ml induced upper and lower abdominal symptoms (P < 0.01). AP, but not sham-AP or no-AP, reduced both upper and lower abdominal symptoms (P < 0.05). Second, RD decreased the percentage of normal gastric slow waves (P < 0.05). AP improved gastric slow waves compared with sham-AP or no-AP (P < 0.05). Third, in the larger, but not smaller, sample size experiment, the vagal activity during the RD plus AP period was significantly higher than that during the RD alone period in the same session and the corresponding period with sham-AP or no-AP in other sessions (P < 0.05). Neither sham-AP nor no-AP showed any effects on vagal activity (P > 0.05). Finally, in the experiment with eight volunteers, neither sham-AP nor no-AP showed any effects on RD-induced impairment in gastric slow waves, abdominal symptoms, or vagal activity (P > 0.05). The conclusions are RD induces upper or lower abdominal symptoms and impairs gastric slow waves in healthy volunteers. AP at ST36 is able to improve upper and lower abdominal symptoms and impaired gastric slow waves induced by RD, possibly mediated via the vagal pathway.
Keywords: functional gastrointestinal diseases, slow waves, gastrointestinal motility
chronic constipation and constipation predominant irritable bowel syndrome (IBS) are common diseases, affecting more than 10% of population in United States (16, 20, 32, 50). These patients usually complain of a series of symptoms such as excretion difficulty and lower abdominal discomfort/pain. More than 30% of the IBS patients are reported to present upper abdominal symptoms such as bloating, upper abdominal discomfort, or pain and even nausea and vomiting (25, 26). Delayed gastrointestinal transit and impaired upper gastrointestinal myoelectrical and motility activities were frequently recorded in patients with IBS and constipation (5, 23). It is believed that the main cause for upper gastrointestinal dysmotility is rectal distension (RD) attributed to fecal stasis in the colon-rectum region, resulting in reflexive inhibition of proximal gastrointestinal motility (6, 17, 33, 57). RD-induced gastric motility disorders include impaired gastric accommodation, slow wave dysrhythmia, attenuated antral contractions, and delayed gastric emptying (4, 14, 34).
Diet, laxative, prokinetics, and biofeedback training are main methods for the treatment of constipation dominant IBS (C-IBS) and chronic constipation. The symptoms that overlap with upper abdominal symptoms are resistant to the treatment because of the refractory constipation, and most of therapeutics have no or little effects on upper gastrointestinal motility; some of therapies are limited due to their side effects (42, 44).
Acupuncture (AP), an ancient Chinese traditional method, or electroacupuncture (EA) has been shown to be effective in the treatment of various functional diseases and the normalization of abnormal physiological conditions in humans (18, 36). AP or EA at ST36 (Zusanli) has been widely reported to improve gastrointestinal symptoms such as nausea, vomiting, and visceral pain in various clinical settings (19, 52). AP or EA has also been shown to accelerate gastric emptying (41, 55) and colon transit (30), restoring vagotomy-induced impaired gastric accommodation (40), and improving rectal perception (54) in humans or animal models. Recently, a canine study in our lab showed that EA at ST36 was able to restore RD-induced impairment in antral motility and vagal activity and suggested that EA at ST36 might be used to treat upper gastrointestinal motility disorders induced by RD (14). However, it is unknown whether the findings in the dogs can be replicated in humans.
Therefore, the aims of this study were to investigate the effects of RD on upper and lower abdominal symptoms and gastric slow waves, and further more, to study the effects and mechanisms of AP at ST36 on RD-induced upper and lower abdominal symptoms and impairment in gastric slow waves in healthy volunteers.
MATERIALS AND METHODS
Subjects.
Twenty healthy volunteers (11 male and 9 female, mean age 22 yr, range 21–23 yr) were recruited from the medical staff at the Tongji Medical College for this study. The volunteers were mostly first-year students just graduated from high schools and had little or no knowledge of AP. None of the subjects had any symptoms or any history of gastrointestinal surgeries or took any medications during the 2 wk before the study. Physical examination was performed on all subjects to rule out any systemic diseases. For the female subjects, to avoid the possible influence of the menses on gastrointestinal functions, the study was performed during the follicular phase of the menses. Written consent was obtained from every participant, and the study protocol was approved by the ethical review board of the institution of Tongji Medical College.
Experimental protocol.
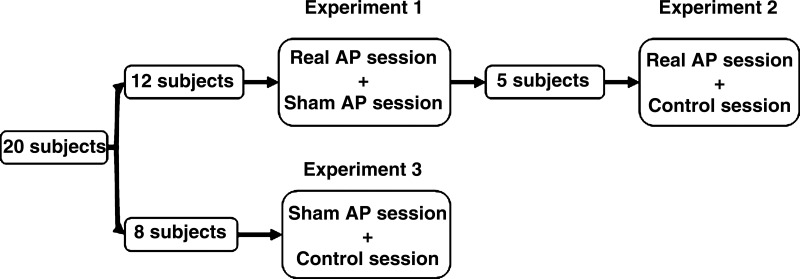
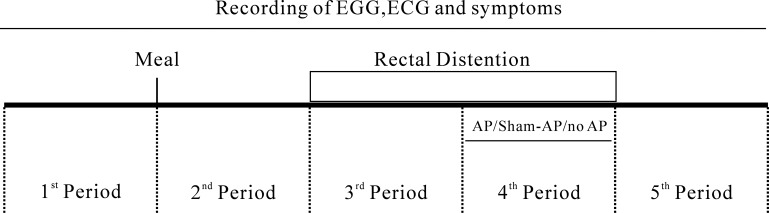
The study was composed of three experiments. The flow chart of the subjects is shown in Fig. 1. In experiment 1, two sessions (sham-AP and AP) were performed in all 12 subjects. The order of the two sessions was randomized, and the interval between the sessions was 3 days. To avoid the possible effect of overeating on gastrointestinal functions, larger than an individual's regular meals were forbidden during 3 days before the study and between the two experimental sessions. The subjects were fasted for at least 8 h before the initiation of each session. In a preliminary test about 3 days before the initiation of the formal study, rectal distension was performed in each subject to determine the maximum tolerable volume that was used in the formal experiments. The study was performed in a quiet room and each subject was advised to visit the bathroom 30 min before the initiation of each session. Each session was composed of five consecutive 30-min periods as shown in Fig. 2: after a 30-min baseline recording in the first period, a 600-ml test meal (200 Nutrition powder dissolved in 600 ml distilled water, 600 cal, Nutricia Pharm, Holland) was consumed within 10 min. After a 30-min postprandial period (the second period), RD was continuously applied during the third and fourth periods. AP or sham-AP was performed during the fourth period. The electrogastrogram (EGG) and electrocardiogram (ECG) were recorded during the entire five 30-min periods.
Fig. 1.
Flowchart of subjects.
Fig. 2.
Experimental protocol. In experiment 1, there were two sessions with the same protocol except that in one session real acupuncture (AP) was performed, whereas in the other session, sham-AP was administrated. Experiment 2 was composed by AP and control sessions. In the control session, no AP or sham-AP was performed during the fourth period, called no-AP.
Experiment 2 was designed to include a session in which no-AP (no needle insertion at all) was performed, compared with AP, and also to investigate the reproducibility of the ameliorating effect of AP. It was performed 6 mo after experiment 1 in 5 of the 12 subjects. The experiment was composed of two sessions (AP and control). The protocol of the control session was exactly the same as experiment 1 except no needle was inserted into the skin during the fourth period. This period, called no-AP was used as the control for AP or sham-AP. The protocol of AP session in experiment 2 was the same as the AP session in experiment 1; this session was repeated to study the reproducibility of the effects of AP.
Experiment 3 was designed to rule out the possible bias of the De Qi sensation on the evaluation of acupuncture treatment. In this experiment, sham-AP and no-AP were performed in two separate sessions on eight healthy volunteers who did not participate in experiments 1 or 2. The protocol of the experiment was the same as experiment 1 except sham-AP or no-AP was performed during the fourth period.
Rectal distension (RD).
A manometric catheter with a balloon attached to its distal end was inserted into the rectum and positioned so that the caudal pole of the balloon lay 6 cm from the anal verge. The balloon was inflated with air using a 60-ml syringe. To make sure that the subject could tolerant prolong rectal distension (60 min), the distension volume was chosen to be 20 ml lower than the maximum tolerance volume, which was measured in the preliminary test.
Acupuncture.
Manual AP was performed for 30 min during the fourth 30-min period or the second 30-min period of RD in both experiments. A professional acupuncturist performed the procedure. The steel acupuncture needle was of a diameter of 0.25 mm and a length of 40 mm (lot number: 060719, Suzhou Acupuncture Supplies, Suzhou, China). Bilateral acupoints, ST36 (Stomach 36), was used in this study since ST36 was one of the most frequently used AP points for the treatment of gastric diseases and has been used in our previous studies in both humans and dogs (14, 55). The location of ST36 was at the proximal one-fifth of the craniolateral surface of the leg distal to the head of the tibia in a depression between the muscles of the cranial tibia and the long digital extensor (55). The AP was applied as follows: the AP needles were inserted into the points with an appropriate depth of 2 cm and manual manipulations (slight thrusting, slight withdrawal, and twirling) were performed for 1-min every 15 min. The subject may have a feeling of De Qi with AP, including “suan” (aching or soreness), “ma” (numbness or tingling), “zhang” (fullness/distention or pressure), and “zhong” (heaviness). Meanwhile, the acupuncturist may feel increased resistance of the needle (needle grasping) as tense, tight, and full like “a fish biting onto the bait”(28). The feeling of De Qi was not asked in this project. The needles remained in the points during the entire 30-min AP period. Sham-AP was performed using the same method with the needles inserted at nonacupoint, located 2 cm lateral to ST36. To ensure blindness, a special feeling related to AP called “De Qi” was not asked as the subject would not have a sensation of “De Qi” when AP was applied to sham points.
Recording and analysis of gastric slow waves.
The EGG was recorded via three abdominal surface electrodes using a portable EGG device (Digitrapper EGG; Synetics Medical, Irving, TX). After a thorough preparation of the abdominal skin, three ECG electrodes were placed on the abdomen: one at the midpoint between the xiphoid and the navel, one at 4–5 cm to the left and 4–5 cm above this point, and a reference electrode in the lower quadrant close to the left costal margin. The EGG signal was recorded with low and high cutoff frequencies of 1 and 18 cycles per minute (cpm), respectively, and sampled at a frequency of 1 Hz (13).
At the completion of the each session, the EGG signal was uploaded to the PC, and the previously validated spectral analysis methods were used to derive the following EGG parameters (13): 1) the percentage of normal slow waves; and 2) dominant frequency and dominant power of the EGG that represent the frequency and amplitude of the gastric slow waves. Gastric slow waves in the range of 2–4 cpm were designated as normal. Slow waves of 0–2 cpm were termed bradygastria and 4–9 cpm were termed tachygastria.
Recording and analysis of HRV.
The autonomic function was assessed based on spectral analysis of the HRV signal derived from the ECG recording. The ECG was recorded using a special one-channel amplifier with a cut-off frequency of 100 Hz (model 2283 Fti Universal Fetrode Amplifier, UFI, Morro Bay, CA) through three surface electrodes placed at the following locations: the right arm electrode on the manubrium of sternum, the left arm electrode at the surface marking of the V5 position (just above the fifth interspace in the anterior axillary line), and the ground electrode at right chest. The data were digitized online at 1,000 Hz using a PC and a data acquisition package (Alice 3, Healthdyne Technologies, Marietta, GA). The HRV signal was derived from the ECG recording using a special program developed and validated in our laboratory by identifying R peaks, calculating R-R intervals, interpolating the R-R intervals so that the time interval between consecutive samples was equal, and finally downsampling the interpolated data to a frequency of 1 Hz (37).
Power spectral analysis was applied to the HRV signal, and the power in each frequency subband was calculated using a previously validated method (33). It has been well established that the power in the low frequency band (0.04–0.15 Hz, LF) represents mainly sympathetic activity and the power in the high frequency band (0.15–0.50 Hz, HF) stands purely for parasympathetic or vagal activity. LF was defined as the area under the curve in the frequency range of 0.04–0.15 Hz, and HF was defined as the area under the curve in the frequency range of 0.15–0.50 Hz. The HF-to-(LF+HF) ratio was considered as vagal activity.
Symptoms score.
Upper abdominal symptoms (fullness, satiety, upper abdominal pain, bloating, nausea, vomiting) and lower abdominal symptoms (lower abdominal fullness, lower abdominal pain, urgency) were scored by the subjects immediately before the initiation of AP or sham-AP (the third period) and at the end of AP or sham-AP or no-AP. These symptoms were assessed based on their severity: 0, none; 1, mild; 2, moderate; and 3, severe (3). To make the evaluation more objective, the subjects were blinded on the nature of intervention (AP, sham-AP, or no-AP).
Statistics.
All values were expressed as means ± SD. The analysis of variance (ANOVA) was applied to study the difference in any of the measurements among different time periods. Paired Student's t-test was used to investigate the difference in any of the measurements/parameters between AP and sham-AP or no-AP or between RD before the intervention (third period) and RD with AP, sham-AP, or no-AP (fourth period). P < 0.05 was considered significant.
RESULTS
Rectal distention volume.
The mean maximum tolerable volume for RD among the subjects was 190 ± 34 ml, and therefore the means RD pressure was 170 ± 34 ml. All subjects developed upper and lower abdominal symptoms but were able to tolerate RD for 60 min with this volume. There were no dropouts between two sessions or adverse effects.
Effects of RD and AP on upper and lower abdominal symptoms.
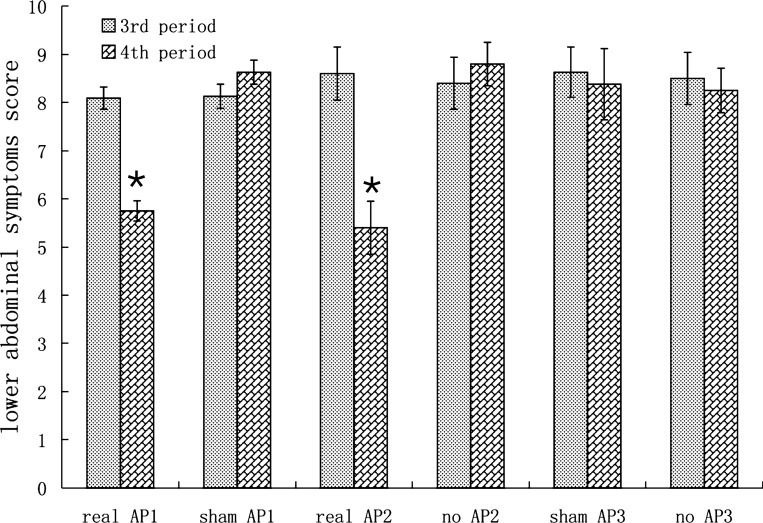
In experiment 1, AP but not sham-AP, significantly improved both upper and lower abdominal symptoms induced by rectal distension (P < 0.05, ANOVA). As shown in Fig. 3, in the sham-AP session, the lower abdominal symptom scores was 8.13 ± 0.25 during RD (third period) and 8.63 ± 0.25 during RD plus sham-AP (fourth period) (P > 0.05). Lower abdominal fullness, lower abdominal pain, and urgency were the major symptoms. No subjects presented the symptom of dejection. In the AP session, however, the lower abdominal score was reduced from 8.09 ± 0.23 during RD without intervention (third period) to 5.75 ± 0.21 during RD plus AP (fourth period) (P < 0.01).
Fig. 3.
Effects of rectal distension (RD) and AP on lower abdominal symptoms. Real AP1: real AP session in experiment 1. Sham AP1: sham AP session in experiment 1. Real AP2: real AP session in experiment 2. No AP2: control session in experiment 2. Sham AP3: sham acupuncture in experiment 3. No AP3: control session in experiment 3. *P < 0.01 vs. third period in the same session.
The ameliorating effect of AP on RD-induced lower abdominal symptoms was found reproducible. In experiment 2, the lower abdominal symptom score was 8.60 ± 0.55 during RD without intervention (third period) and 5.40 ± 0.55 during RD plus AP (fourth period) in the AP session (P < 0.01). In the control session, the symptom score was 8.40 ± 0.54 during RD without intervention (third period) and remained unchanged during the no-AP period (fourth period) (8.80 ± 0.45, P > 0.05; see Fig. 3).
In experiment 3 (subjects treated only with sham-AP and no-AP), the lower abdominal symptoms score were 8.63 ± 0.52 without intervention (third period) and 8.38 ± 0.74 during RD plus sham-AP (fourth period) in the sham AP session (P > 0.05). In the no-AP session, the symptom score was 8.50 ± 0.54 during RD without intervention (third period) and remained unchanged during the no-AP period (fourth period) (8.25 ± 0.46, P > 0.05; see Fig. 3).
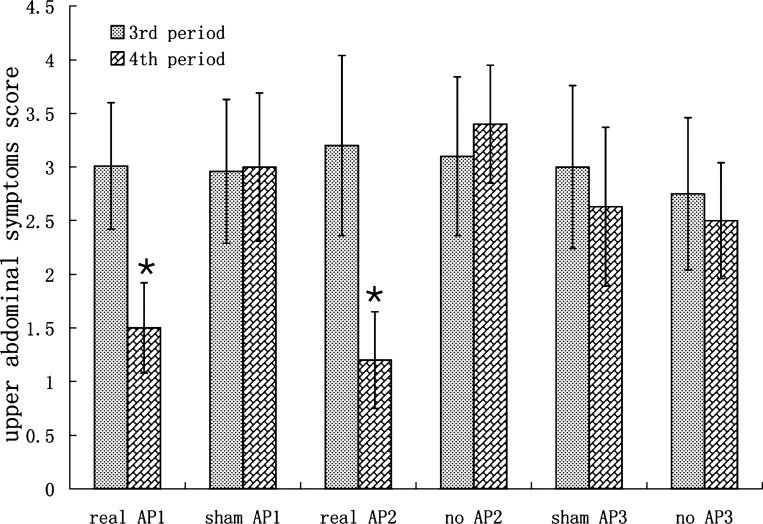
AP also improved the upper abdominal symptoms induced by RD and the effect was also reproducible. As shown in Fig. 4 (experiment 1), in the sham-AP session, the upper abdominal symptom score was 2.96 ± 0.67 during RD alone and remained unchanged (3.00 ± 0.69) during RD plus sham-AP (P > 0.05). Fullness, satiety, and bloating were main symptoms. AP, however, reduced the RD-induced upper abdominal symptom score from 3.01 ± 0.59 during the third period without intervention to 1.5 ± 0.42 during the fourth period with AP (P < 0.01). In experiment 2, AP still significantly decreased the upper abdominal symptom score from 3.20 ± 0.84 during RD only (the third period) to 1.2 ± 0.45 during RD+AP (the fourth period) (P < 0.01) in the AP session. In control session, the upper abdominal symptom score was 3.10 ± 0.74 during the third period (baseline) and 3.40 ± 0.55 during the fourth period (no-AP) (P > 0.05) (see Fig. 4). In experiment 3, neither sham-AP nor no-AP showed any effects on the upper abdominal symptoms score (P > 0.05) (see Fig. 4).
Fig. 4.
Effects of RD and AP on upper abdominal symptoms (see Fig. 3 for group descriptions). *P < 0.01 vs. third period in the same session.
Effects of RD and AP on gastric slow waves.
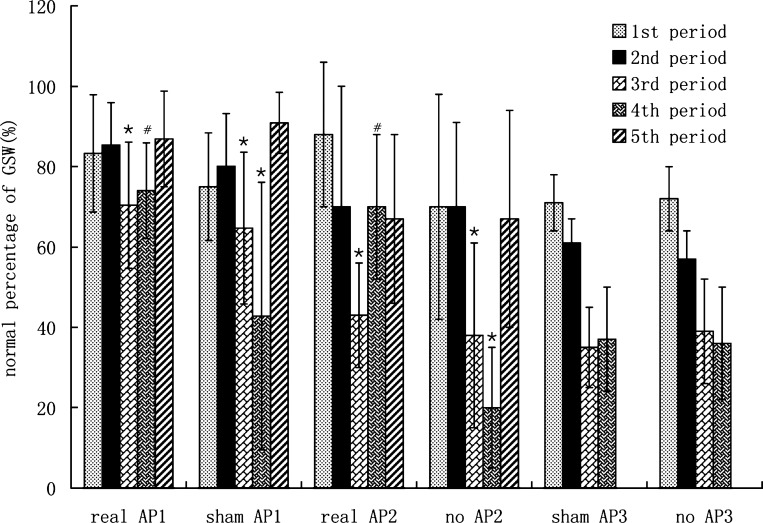
RD impaired gastric slow wave rhythmicity and AP, but not sham-AP, improved gastric slow waves. In experiment 1, in the AP session, the percentage of normal slow waves was 83.3% ± 14.6% in the fasting state (first period), remained unchanged at 85.4% ± 10.5% after the test meal (second period), and significantly decreased to 70.4% ± 15.7% during the first 30 min of RD (P < 0.05). In the sham-AP sessions, the percentages of normal slow waves during these three periods were similar to the AP session (see Fig. 5). AP, but not sham-AP, prevented further impairment in gastric slow waves during the second 30-min period of RD. In the sham-AP session, the percentage of normal slow waves during the second 30-min RD with sham-AP was further decreased to 42.8% ± 33.3% (P < 0.05 vs. RD without sham-AP); in the AP session, this value during the second 30-min RD with AP remained unchanged at 74.0% ± 11.9% (P > 0.05 vs. the third period) and was significantly higher than that in the corresponding time period in the sham-AP session (P < 0.01). The difference in the percentage of normal slow waves between the fourth 30-min period and third 30-min period in the AP session was also significantly higher than that in sham-AP session (3.6% ± 15.6% vs. −21.9% ± 40.7%, P < 0.01), demonstrating the preventive effect of AP on further impairment of gastric slow waves with prolonged RD. The dominant frequency and power among different time periods were not significantly different (P > 0.05, ANOVA).
Fig. 5.
Effects of RD and AP on gastric slow waves (GSW) (see Fig. 3 for group descriptions). *P < 0.05 vs. previous period. #P < 0.05 vs. sham AP or control in the same experiment.
The ameliorating effect of AP on gastric slow waves was also reproducible. In experiment 2, in the AP session, the percentage of normal slow waves was 88% ± 18% in the fasting period, 70% ± 30% after the test meal, and significantly decreased to 43% ± 13% during the first 30 min of RD (P < 0.05). In the control session, the percentages of normal slow waves in the fasting period and fed periods without RD were 70% ± 28% and 70% ± 21%, respectively, significantly decreased to 38% ± 23% during the first 30 min of RD (P < 0.05). In the control session, the normal percentage of gastric slow waves during the second 30-min RD with no-AP (fourth period) was further decreased to 20% ± 15% (P < 0.05 vs. third period). In the AP session, the percentage of normal slow waves maintained the same level as that without RD (70% ± 18%) during the RD+AP period, which was significantly higher than that without AP (third period) and corresponding period (RD+no-AP) in the control session (P < 0.05) (Fig. 5).
In experiment 3 (subjects treated only with sham-AP and no-AP), in the sham-AP session, the percentage of normal slow waves was 71 ± 7% in the fasting period, 61 ± 6% after the test meal, significantly decreased to 35 ± 10% during the first 30 min of RD, and 37 ± 13% during the second 30-min RD with sham-AP (P > 0.05). In the no-AP session, the percentages of normal slow waves in the fasting period and fed periods without RD were 72 ± 8% and 57 ± 7%, respectively, significantly decreased to 39 ± 13% during the first 30 min of RD, and remain unchanged (36 ± 14%) during the second 30 min with no-AP (P > 0.05) (Fig. 5).
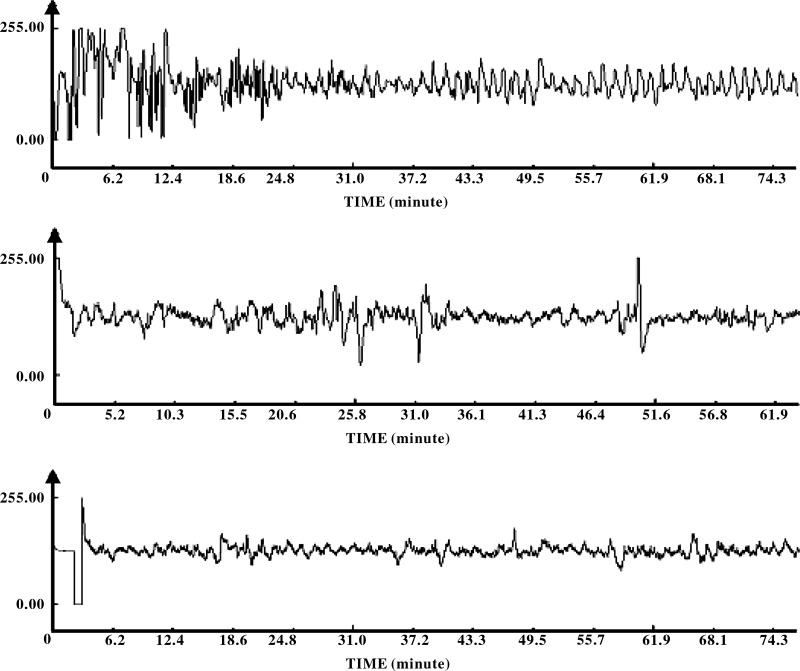
Figure 6 presents the original tracings of gastric slow waves during the RD and RD+intervention (AP, sham-AP, or no-AP) periods (the third and fourth periods) in three different sessions. It can been seen that gastric slow waves were interrupted by RD (first 30-min in each tracing) and remained interrupted during RD+sham-AP (middle) or RD+no-AP (bottom) but improved during RD+AP (top).
Fig. 6.
GSW recordings in one subject. Top: recordings from the AP session in experiment 1; the GSW were interrupted during the first 30 min (the third period: RD only) but were regular during the second 30 min (the fourth period: RD+AP). Middle: recordings from the sham-AP session in experiment 1; irregular slow waves were noted during both the first 30-min period (the third period: RD only) and the second 30-min period (the fourth period: RD+sham-AP). Bottom: recordings from the control session in experiment 2; irregular slow waves were noted during both the first 30-min period (the third period: RD only) and the second 30-min period (the fourth period: RD+no-AP or RD only).
Effects of RD and AP on autonomic functions.
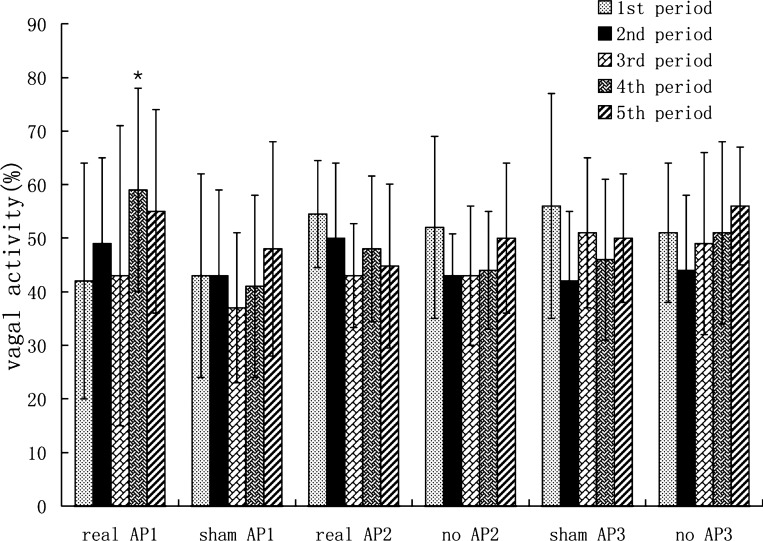
The vagal activity (HF) assessed by the spectral analysis of the HRV showed no difference among the first three periods (fasting period, fed period, and fed + RD period) in either session (ANOVA P > 0.05). Although RD showed a trend in decreasing HF, the decrease was not statistically significant (P = 0.29). AP significantly increased the value of HF from 0.43 ± 0.28 during RD to 0.59 ± 0.19 during RD plus AP (P < 0.01). The HF during RD plus AP in real AP session was also significantly higher than that during RD plus sham-AP in the sham-AP session (P < 0.01). Whereas sham-AP showed no such an effect on the value of HF (0.41 ± 0.17 during RD vs. 0.37 ± 0.14 during RD plus sham-AP, P > 0.05) (Fig. 7). RD did not significantly alter the sympathetic activity. AP reduced the sympathetic activity during RD (0.52 ± 0.27 during RD vs. 0.41 ± 0.19 during RD+AP, P < 0.05). Sham-AP showed no such an effect on the sympathetic activity (0.59 ± 0.17 during RD vs. 0.62 ± 0.14 during RD + sham-AP, P > 0.05).
Fig. 7.
Vagal activity (high frequency, HF) during different time periods (see Fig. 3 for group descriptions). *P < 0.01 vs. third period in the same session.
In experiment 2, however, AP resulted in a trend of increase in vagal activity, the increase was, however, not statistically significant (P = 0.29), possibly attributed to the small sample size (N = 5) (Fig. 7).
In experiment 3, neither sham-AP nor no-AP showed any effects on vagal activity (P > 0.05 vs. RD period without intervention) (Fig. 7).
DISCUSSION
In the present study we demonstrated that RD with an average volume of 170 ml induced upper/lower abdominal symptoms and impaired gastric slow waves. Compared with the sham-AP or no-AP, AP at ST36 showed an ameliorating effect on the upper and lower abdominal symptoms induced by RD, prevented the impairment of gastric slow waves due to prolonged RD, and increased vagal activity assessed from the spectral analysis of HRV. The ameliorating effects of AP on RD-induced upper and lower abdominal symptoms and RD-induced impairment in gastric slow waves were found reproducible.
Combined upper and lower gastrointestinal symptoms are commonly presented in C-IBS and functional gastrointestinal disorders in clinical practice. It has been reported that 55–87% C-IBS patients complain of dyspepsia symptoms (2, 47). Some studies reported that 30–50% patients with functional gastrointestinal disorders had both dyspepsia and IBS symptoms (25, 26, 39). Bouin et al. (7) reported that 91% patients with overlapping symptoms presented rectal intolerance to distension. Therefore, the fecal or gas distension in the colon-rectum can directly cause reflexive inhibition of proximal gastrointestinal motility, which is believed to be the major cause of symptoms of dyspepsia. Clinically, overlapping symptoms between IBS and functional dyspepsia (FD) are difficult to treat due to the lack of effective drugs or side effects. Development of new methods to resolve both upper and lower gastrointestinal symptoms is of great clinical importance.
Since IBS patients are heterogeneous, healthy volunteers were used in this study as the first step in developing an AP therapy for treating the abovementioned overlap symptoms. To mimic the clinical setting, rectal balloon distension was used in the present study. RD is a commonly used model on inducing both the upper and lower gastrointestinal motility disorders. Previous studies have demonstrated that RD is capable of inducing dyspepsia symptoms, inhibiting gastric accommodation, and delaying gastric emptying in humans and animals (17, 34, 58). A recent canine study in our lab showed that RD was able to impair gastric slow waves and anal contractions in healthy dogs (14). RD with a low distention volume had no effects on gastric slow waves and autonomic functions, whereas RD with a high distention volume impaired gastric slow waves and increased sympathetic activity. Accordingly, in the present study, before the formal study, a preliminary test was performed and the maximum tolerable volume of RD was assessed in each subject. In the formal study, the RD volume was set at 20 ml below the maximum tolerable volume. Therefore, all the subjects tolerated the distension and there were no dropouts or adverse effects. It should also be noted that this was an acute study and therefore there were no dropouts. In chronic studies, however, dropout is common, especially in patients who are treated with sham-AP.
A number of previous studies have reported the ameliorating effects of AP on gastrointestinal motility and symptoms. Xu et al. (55) reported that AP at ST36 and PC6 significantly improved gastric emptying in FD patients with delayed gastric emptying and relieved dyspeptic symptoms in FD patients with normal gastric emptying. Xing et al. (54) showed that AP at ST36 and PC6 improved the compliance perception in IBS patients. Generally, ST36 is believed to improve gastrointestinal motility, and PC6 can prevent emesis or vomiting (51). Our recent canine study showed that EA at ST36 significantly improved antral hypomotility induced by RD (14). These studies all suggested that AP might be effective in treating both upper and lower gastrointestinal symptoms. However, most of previous studies were performed in animals.
AP at ST36 in this study was found capable of reducing both upper and lower gastrointestinal symptoms induced by RD. With the distention volume of 20 ml lower than the maximum rectal tolerable volume, all subjects presented obvious lower and upper gastrointestinal symptoms. The major upper symptoms were satiety and fullness, whereas the main lower symptoms were lower abdominal fullness, lower abdominal pain, and urgency. The RD-induced upper abdominal symptoms are believed to be attributed to the inhibitory rectogastric reflex (6, 7). The ameliorating effect of AP on RD-induced symptoms of both upper and lower gut suggests a therapeutic potential of AP for patients with excessive colorectal retention, such as C-IBS, and chronic constipation attributed to pelvic floor dysfunctions. Although this study was performed in healthy volunteers, further clinical studies in relevant patients are warranted.
Consistent with previous findings, RD in the present study impaired gastric slow waves. One interesting and novel finding of this study was the observation of a further decrease in the percentage of normal slow waves during the second 30-min RD. As it can be appreciated in Fig. 4A, the percentage of normal slow waves during the second 30-min RD was reduced from 85% immediately before RD to 42%, a 50% reduction, which has rarely been reported in the literature. This finding suggests that the impairment of gastric slow waves is also dependent on the duration of RD. In healthy volunteers, it is hard to study mechanisms involved in the detrimental effect of RD on gastric slow waves. However, based on the present and previous findings, the impairment of gastric slow waves was believed to be attributed to the intrinsic autonomic functions. In a previous canine study (14), the RD-induced slow wave dysrhythmias were blocked by guanethidine. The same sympathetic mechanism is anticipated in this human study. The immediately recovery of gastric slow waves after the termination of RD (compare periods 4 and 5 in Fig. 4A) also suggests the involvement of a neural pathway. If certain hormones were involved, one would expect a slow recovery of gastric slow waves after RD. Previously, RD was found to impair gastric slow waves in dogs (1, 14) and healthy volunteers (43). However, none of these previous studies have reported a severe detrimental effect of prolonged RD on gastric slow waves. Abnormal gastric slow waves have been linked to impaired gastric motility, such as delayed gastric emptying (11, 12).
It was also interesting to note that AP was able to prevent the inhibitory effect of prolonged RD on gastric slow waves. While the percentage of normal slow waves was reduced to 42% during the second 30-min RD with sham-AP, it remained unchanged at 74% when AP at ST36 was performed. Previously, AP or EA was reported to improve gastric slow waves in subjects with motion sickness (27), healthy volunteers (9, 15, 36), and patients with diabetes (10). The ameliorating effect of EA on gastric slow waves reported in various clinical studies has been consistent and reproducible, indicating the robust role of AP or EA for the treatment of gastric slow wave dysrhythmia. In the animal model, EA was reported to improve or normalize gastric dysrhythmia by increasing the vagal activity, suggesting the involvement of the vagal pathway (14, 41). The spectral analysis of the HRV signal reveals vagal efferent activity. Accordingly, the vagal efferent pathway was believed to be involved in the present study. However, it remains uncertain whether vagal afferent pathway was also involved in the ameliorating effect of AP on RD-induced impairment in gastric slow waves.
In experiment 2, the AP showed a trend of increase in vagal activity compared with the control (Fig. 7, fourth period); however, the increase did not reach statistical significance. We believe that this was mainly attributed to the reduced sample size. However, a significant improvement was noted with AP in the EGG measurement (Fig. 5) with the same reduced sample size. This might be explained as follows: the EGG measurement might be more robust than the HRV measurement and rarely affected by the environment or mood of the subject. Whereas, the vagal activity of the subject might be more subjective to factors other than AP, such as the environment and mood of the subject, etc.; for example, Sloan et al. (46) reported that mental stress effectively changed vagal activity throughout a day, and Egizio et al. (21) found that the psychological stress-induced changes in vagal activity was associated with social functioning. Increased variations in vagal activity attributed to factors other than AP, and reduced sample size might have led to the insignificant change shown in Fig. 7. The gastric slow wave, however, is less affected by factors other than AP, and therefore a significant change was still noted even with the reduced sample size.
The concurrent increase in vagal activity observed during AP in experiment 1 suggests the vagal mechanism involved in the improvement of gastric slow waves. Similar vagal mechanisms have been previously reported in both animal and human studies (29, 45, 56). In both regular and diabetic rats, electroacupuncture at ST36 was reported to improve gastric motility and increase vagal activity measured using the same method–spectral analysis of HRV (29, 56). In patients with scleroderma, transcutaneous electrical stimulation at ST36 was found to improve gastric dysrhythmia and increase vagal activity (45). In the current study, vagal activity was increased with AP. It is interesting to note that the current and previous studies showed concurrent increase in vagal activity and gastric motility, and the increase in vagal activity was consistent no matter if AP was performed using actual needle insertion at ST36 with or without electrical stimulation or via surface electrodes placed at ST36 with electrical stimulation. In a recent rodent study, the effect of AP at ST36 on vagal activity was reported to be blocked by central glial metabolic inhibitor, suggesting the involvement of vagal nuclei (53).
In the present study, the main upper and lower abdominal symptoms were fullness, satiety, lower abdominal pain, and urgency. Lorena et al. (38) reported a decreased vagal activity in a group of FD patients whose dominant dyspeptic symptoms were fullness and satiety, suggesting the association between the symptoms of upper fullness and satiety and the vagal activity. Abnormal gastric slow waves are commonly presented in patients with gastric dysmotility, such as antral hypomotility and delayed gastric emptying, which are associated with the symptoms of upper abdominal fullness and satiety (38). The symptoms of fullness and satiety may be attributed to impaired gastric motility mediated via the vagal pathway. Accordingly, the improvement in fullness and satiety with AP is believed to be mediated via the somatosensory vagal pathway (49). Similarly, Burr et al. (8) found a decreased vagal tone in women with IBS, suggesting that the lower abdominal pain, fullness, and urgency might also be associated with the vagal activity. On the other hand, the analgesic effect of AP has been reported to be mediated by an endogenous opioid pathway (19, 48). A previous canine study reported that the painful sensation induced by rectal distension was attenuated by EA at ST-36, and the effect was abolished by pretreatment with naloxone, but not naloxone methiodide, suggesting a central opioid pathway (31). The relationship between the vagal pathway and opioid pathway is still unclear. In a previous canine study (14), RD similar to that applied in this study was found to inhibit vagal activity and increase sympathovagal balance assessed using the same spectral analysis of HRV as in this study. EA at ST36 prevented the inhibition in vagal activity and activation in sympathetic activity induced by RD, and this preventive effect was not blocked by naloxone, suggesting two independent pathways involved in the ameliorating effect of EA. Although EA or AP may act on the opioid and vagal pathways independently, dependency between these two pathways has been frequently reported in the literature. In a rodent study, activation of subdiaphragmatic vagal afferents was reported to play a role in opioid-dependent antinociceptive pathways activated by noxious RD. On the other hand, central opioids were reported to control (inhibit) sympathetic outflow (22, 35).
Since AP may induce a special sensation of De Qi, whereas sham-AP may not induce such a sensation, a better way to control the study is to perform AP and sham-AP in two different groups of subjects. To achieve this, AP was performed in 12 subjects in experiment 1 and sham-AP was performed in 8 different subjects in experiment 3. In the subjects treated with AP, significant improvement was noted during the intervention period in RD-induced symptoms, impairment in gastric slow waves, and autonomic functions. On the contrary, the subjects treated with sham-AP showed no changes during the intervention in RD-induced symptoms, impairment in gastric slow waves, or autonomic functions. These data suggested that the ameliorating effects of AP were not attributed to placebo.
Whereas AP has been shown to have therapeutic effects on abdominal symptoms in animal and human studies, a number of clinical studies also reported similar effects with sham-AP. The effectiveness of sham-AP could be from two facts: 1) a placebo effect and 2) a true effect due to sham AP. In the present study, however, sham-AP was not found to be effective and the discrepancy might be attributed to fact 1. In previous clinical studies, the subjects were patients suffering from functional gastrointestinal disorders. We believe that patients in general are expecting symptom improvement from a study and this may produce a placebo effect. Second, true disorders the patients have may provide a room of improvement with sham-AP, whereas, in the present study, all subjects were healthy and they might not expect any symptom relief with AP. Also, in the present study, symptoms were induced by RD in healthy volunteers, which were different from the symptoms in previous clinical studies. For example, there was no obvious upper abdominal pain in the present study, whereas it is commonly presented in patients. Third, most of previous clinical studies were chronic, whereas the current study was acute. We believe that the placebo and sham-effects are enhanced when a treatment is given chronically and repetitively.
Perspectives and Significance
Overlap of upper and lower gastrointestinal symptoms is a common clinical problem in C-IBS and functional gastrointestinal disorders with a lack of optimal therapies. The present human results, together with our previous animal studies, demonstrated that AP at ST36 was capable of reducing both upper and lower gastrointestinal symptoms, improving impaired gastric slow waves, accelerating gastric emptying and small bowel transit, and decreasing visceral hyperalgesia. These findings suggest that AP may have a therapeutic potential for overlapping symptoms of the upper and lower gut. This was an acute study and the clinical meaning of the data is subjected to further verification with chronic studies in C-IBS or functional gastrointestinal disorder patients. We anticipate that the acceleration in gastrointestinal emptying and improvement in gastric slow waves would lead to a reduction in dysmotility-like symptoms, and that reduced visceral perception would ameliorate symptoms of hyperalgesia. Chronic study with AP at ST36 should be performed to access the therapeutic potential of AP for overlap symptoms in C-IBS and functional gastrointestinal disorders.
In conclusion, AP at ST36 is able to improve RD-induced upper and lower gastrointestinal symptoms and impairment in gastric slow waves by possibly enhancing vagal activity. AP may have a therapeutic potential for functional gastrointestinal disorders.
GRANTS
This work was partially supported by a grant from the National Institutes of Health (AT004489).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L. performed experiments; J.L. drafted manuscript; H.H. interpreted results of experiments; H.H. prepared figures; X.X. analyzed data; J.D.C. conception and design of research; J.D.C. edited and revised manuscript; J.D.C. approved final version of manuscript.
REFERENCES
- 1. Abo M, Kono T, Wang Z, Chen JDZ. Impairment of gastric and jejunal myoelectrical activity during rectal distension in dogs. Dig Dis Sci 45: 1731–1736, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Agreus L, Svardsudd K, Nyren O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology 109: 671–680, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Arts J, Holvoet L, Caenepeel P, Bisschops R, Sifrim D, Verbeke K, Janssens J, Tack J. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther 26: 1251–1258, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Azpiroz F, Malagelada JR. Perception and reflex relaxation of the stomach in response to gut distention. Gastroenterology 98: 1193–1198, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Bassotti G, Stanghellini V, Chiarioni G, Germani U, De Giorgio R, Vantini I, Morelli A, Corinaldesi R. Upper gastrointestinal motor activity in patients with slow-transit constipation. Further evidence for an enteric neuropathy. Dig Dis Sci 41: 1999–2005, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Bojo L, Cassuto J. Gastric reflex relaxation by colonic distension. J Auton Nerv Syst 38: 57–64, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Bouin M, Lupien F, Riberdy M, Boivin M, Plourde V, Poitras P. Intolerance to visceral distension in functional dyspepsia or irritable bowel syndrome: an organ specific defect or a pan intestinal dysregulation? Neurogastroenterol Motil 16: 311–314, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Burr RL, Heitkemper M, Jarrett M, Cain KC. Comparison of autonomic nervous system indices based on abdominal pain reports in women with irritable bowel syndrome. Biol Res Nurs 2: 97–106, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chang CS, Chou JW, Ko CW, Wu CY, Chen GH. Cutaneous electrical stimulation of acupuncture points may enhance gastric myoelectrical regularity. Digestion 66: 106–111, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Chang CS, Ko CW, Wu CY, Chen GH. Effect of electrical stimulation on acupuncture points in diabetic patients with gastric dysrhythmia: a pilot study. Digestion 64: 184–190, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Chen JDZ, Lin ZY, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci 41: 1538–1545, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Chen JDZ, Pan J, McCallum RW. Clinical significance of gastric myoelectrical dysrhythmias. Dig Dis 13: 275–290, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Chen J, McCallum RW. Electrogastrography: principles and applications. New York: Raven, 45–73, 1994 [Google Scholar]
- 14. Chen J, Song GQ, Yin J, Koothan T, Chen JD. Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol 295: G614–G620, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Chou JW, Chang YH, Chang CS, Chen GH. The effect of different frequency electrical acu-stimulation on gastric myoelectrical activity in healthy subjects. Hepatogastroenterology 50: 582–586, 2003 [PubMed] [Google Scholar]
- 16. Cook IJ, Talley NJ, Benninga MA, Rao SS, Scott SM. Chronic constipation: overview and challenges. Neurogastroenterol Motil 21, Suppl 2: 1–8, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Coremans G, Geypens B, Vos R, Tack J, Margaritis V, Ghoos Y, Janssens J. Influence of continuous isobaric rectal distension on gastric emptying and small bowel transit in young healthy women. Neurogastroenterol Motil 16: 107–111, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Dickman R, Schiff E, Holland A, Wright C, Sarela SR, Han B, Fass R. Clinical trial: acupuncture vs. doubling the proton pump inhibitor dose in refractory heartburn. Aliment Pharmacol Ther 26: 1333–1344, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Diehl DL. Acupuncture for gastrointestinal and hepatobiliary disorders. J Altern Complement Med 5: 27–45, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari EUS. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 38: 1569–1580, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Eqizio VB, Jennings JR, Christie IC, Sheu LK, Matthews KA, Gianaros PJ. Cardiac vagal activity during psychological stress varies with social functioning in older women. Psychophysiology 45: 1046–1054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gabella G. Meeting report: the autonomic nervous system in health and disease. J Auton Nerv Syst 19: 175–178, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Glia A, Lindberg G. Antroduodenal manometry findings in patients with slow transit constipation. Scand J Gastroenterol 33: 55–62, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Gschossmann JM, Mayer EA, Miller JC, Raybould HE. Subdiaphragmatic vagal afferent innervation in activation of an opioidergic antinociceptive system in response to colorectal distension in rats. Neurogastroenterol Motil 14: 403–408, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Halder SL, Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case-control study. Aliment Pharmacol Ther 1519: 233–242, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Holtmann G, Goebell H, Talley NJ. Functional dyspepsia and irritable bowel syndrome: is there a common pathophysiological basis? Am J Gastroenterol 92: 954–959, 1997 [PubMed] [Google Scholar]
- 27. Hu S, Stern RM, Koch KL. Electrical acustimulation relieves vection-induced motion sickness. Gastroenterology 102: 1854–1858, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Hui KK, Sporko TN, Vangel MG, Li M, Fang J, Lao L. Perception of Deqi by Chinese and American acupuncturists: a pilot survey. Chin Med 6: 2, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Imai K, Ariga H, Chen C, Mantyh C, Pappas TN, Takahashi T. Effects of electroacupuncture on gastric motility and heart rate variability in conscious rats. Auton Neurosci 29: 91–98, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. Electroacupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J Physiol Gastrointest Liver Physiol 290: G285–G292, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Iwa M, Strickland C, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture reduces rectal distension-induced blood pressure changes in conscious dogs. Dig Dis Sci 50: 1264–1270, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 25: 599–608, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Kellow JE, Gill RC, Wingate DL. Modulation of human upper gastrointestinal motility by rectal distension. Gut 28: 864–868, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lei Y, Zhu H, Xing J, Chen JDZ. Rectal distension modulates canine gastric tone and accommodation. Dig Dis Sci 50: 2134–2140, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Li P, Sun FY, Zhang AZ. The effect of acupuncture on blood pressure: the interrelation of sympathetic activity and endogenous opioid peptides. Acupunct Electrother Res 8: 45–56, 1983 [DOI] [PubMed] [Google Scholar]
- 36. Lin X, Liang J, Ren J, Mu F, Zhang M, Chen JD. Electrical stimulation of acupuncture points enhances gastric myoelectrical activity in humans. Am J Gastroenterol 92: 1527–1530, 1997 [PubMed] [Google Scholar]
- 37. Liu S, Peng S, Hou X, Ke M, Chen JDZ. Transcutaneous electroacupuncture improves dyspeptic symptoms and increases high frequency heart rate variability in patients with functional dyspepsia. Neurogastroenterol Motil 20: 1204–1211, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Lorena SL, Figueiredo MJ, Almeida JR, Mesquita MA. Autonomic function in patients with functional dyspepsia assessed by 24-hour heart rate variability. Dig Dis Sci 47: 27–31, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Minocha A, Wigington WC, Johnson WD. Racial differences in epidemiology of irritable bowel syndrome alone, uninvestigated dyspepsia alone, and “Overlap Syndrome” among African Americans compared to caucasians: a population-based study. Dig Dis Sci 51: 218–226, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Ouyang H, Xing J, Chen JDZ. Electroacupuncture restores impaired gastric accommodation in vagotomized dogs. Dig Dis Sci 49: 1418–1424, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Ouyang H, Yin J, Wang Z, Pasricha PJ, Chen JDZ. Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. Am J Physiol Gastrointest Liver Physiol 282: G390–G396, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Preston DM, Lennard-Jones JE. Severe chronic constipation of young women: idiopathic slow transit constipation Gut 27: 4–8, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qian L, Orr WC, Chen JDZ. Inhibitory reflexive effect of rectal distension on postprandial gastric myoelectrical activity. Dig Dis Sci 47: 2473–2479, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Reynolds JC, Ouyang A, Lee CA, Baker L, Sunshine AG, Cohen S. Chronic severe constipation. Prospective motility studies in 25 consecutive patients. Gastroenterology 92: 414–420, 1987 [PubMed] [Google Scholar]
- 45. Sallam H, McNearney TA, Doshi D, Chen JD. Transcutaneous electrical nerve stimulation (TENS) improves upper GI symptoms and balances the sympathovagal activity in scleroderma patients. Dig Dis Sci 52: 1329–1337, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Sloan RP, Shapiro PA, Bagiella E, Boni SM, Paik M, Bigger JT, Jr, Steinman RC, Gorman JM. Effect of mental stress throughout the day on cardiac autonomic control. Biol Psychol 37: 89–99, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Stanghellini V, Tosetti C, Barbara G, De Giorgio R, Cogliandro L, Cogliandro R, Corinaldesi R. Dyspeptic symptoms and gastric emptying in the irritable bowel syndrome. Am J Gastroenterol 97: 2738–2743, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Stux G, Pomeranz B. Basics of Acupuncture (4th ed). New York: Springer, 1995 [Google Scholar]
- 49. Takahashi T. Mechanism of acupuncture on neuromodulation in the gut: A review. Neuromodulation 14: 8–12, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology 101: 927–934, 1991 [DOI] [PubMed] [Google Scholar]
- 51. Tatewaki M, Strickland C, Fukuda H, Tsuchida D, Hoshino E, Pappas TN, Takahashi T. Effects of acupuncture on vasopressin-induced emesis in conscious dogs. Am J Physiol Regul Integr Comp Physiol 288: R401–R408, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Tian XY, Bian ZX, Hu XG, Zhang XJ, Liu L, Zhang H. Electro-acupuncture attenuates stress-induced defecation in rats with chronic visceral hypersensitivity via serotonergic pathway. Brain Res 1088: 101–108, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Wang SZ, Liu XD, Huang YX, Ma QJ, Wang JJ. Disruption of glial function regulates the effects of electro-acupuncture at Tsusanli on gastric activity in rats. Am J Chin Med 37: 647–656, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Xing J, Laeive B, Mekhail N, Soffer E. Transcutaneous electrical acustimulation can reduce visceral perception in patients with irritable bowel syndrome: a pilot study. Altern Ther Health Med 10: 38–42, 2003 [PubMed] [Google Scholar]
- 55. Xu S, Hou X, Zha H, Gao Z, Zhang Y, Chen JDZ. Electroacupuncture accelerates solid gastric emptying and improves dyspeptic symptoms in patients with functional dyspepsia. Dig Dis Sci 51: 2154–2159, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Yin J, Chen J, Chen JD. Ameliorating effects and mechanisms of electroacupuncture on gastric dysrhythmia, delayed emptying, and impaired accommodation in diabetic rats. Am J Physiol Gastrointest Liver Physiol 298: G563–G670, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Youle MS, Read NW. Effect of painless rectal distension on gastrointestinal transit of solid meal. Dig Dis Sci 29: 902–906, 1984 [DOI] [PubMed] [Google Scholar]
- 58. Zighelboim J, Talley NJ, Phillips SF. Response of gastric fundus to rectal distension in healthy persons. Dig Dis Sci 39: 1441–1445, 1994 [DOI] [PubMed] [Google Scholar]