Abstract
A single exposure to mechanical unloading can result in significant bone loss, but the consequences of multiple exposures are largely unknown. Within a 18-wk period, adult C57BL/6 male mice were exposed to 2 wk of hindlimb unloading (HLU) followed by 4 wk of reambulation (RA) once (1x-HLU), twice (2x-HLU), or three times (3x-HLU), or served as ambulatory age-matched controls. In vivo μCT longitudinally tracked changes in trabecular and cortical compartments of the femur. Normally ambulating control mice experienced significant age-related loss in trabecular bone volume fraction throughout the course of the experiment. This loss was compounded by HLU with 2x- and 3x-HLU mice experiencing a 27% and 24% greater reduction in trabecular bone and a 60% and 63% inhibition of age-related trabecular thickening. The recovery of cortical bone was also incomplete during each 4-wk RA period and, at completion of the experiment, cortical area in 3x-HLU mice was 5% smaller than in control and 1x-HLU. When eliminating age as a confounding variable, comparison between individual HLU/RA cycles showed that the magnitude of the response diminished during subsequent exposures. The extent of trabecular thinning in mice unloaded for the first time was 1.6-fold greater than the second time and nearly twofold greater than the third time. Similarly, the increase in trabecular thickness during the first RA cycle was twofold greater than during the second and third RA cycle. Together, our data demonstrate that even though multiple exposures to mechanical unloading are more detrimental than a single unloading period, bone's mechanosensitivity is reduced with consecutive unloading/reambulation cycles.
Keywords: skeleton, disuse, hindlimb unloading, reambulation
bone structure and composition evolve in response to mechanical cues from the environment. Mechanical unloading, or disuse, as experienced during spaceflight, prolonged bedrest, or spinal injury results in marked bone atrophy, jeopardizing the mechanical integrity and long-term health of bones (5, 16, 32). The unloading-induced tissue waste is typically compartment-specific with more pronounced losses in the trabecular than in the cortical compartment (27). The limited knowledge gleaned from human studies has been supplemented with results from ground-based animal and in vitro studies, demonstrating decreased osteoblast (9) and increased osteoclast activity (46), changes that are modulated by many factors, including genetic make-up (23), age (12, 37), sex (43, 44), and the duration of unloading (1, 40).
The deleterious effects of unloading persist despite the adoption of physical (36, 45, 47), and/or pharmacological (31) countermeasures, and both preclinical and clinical studies show that full recovery from unloading-induced losses may not occur within months or even years after returning to normal load-bearing activity (30). For instance, trabecular volumetric bone mineral density in the proximal femur declined by almost 20% after 4–6 mo in space but recovered only 6% 1 yr after return to planetary gravitational fields (28). Further, at 6 mo of mobilizing after 17 wk of bedrest, healthy males still had 3–4% lower bone mineral density in the lumbar spine and the femoral neck (30a). Similarly, in mouse models of mechanical unloading, trabecular bone volume in the proximal tibia can remain 40% lower than age-matched controls after a 3-wk unloading/3-wk reambulation cycle with 30% smaller bone formation rates (36). Thus, unloading-induced deficits may persist, potentially leading to musculoskeletal complications, such as accelerated osteoporosis later in life.
Although astronauts have performed multiple space missions in the past and some patients must endure multiple periods of bedrest, most studies have applied only a single unloading period with or without a recovery phase of normal weight-bearing (3, 6, 10, 28, 36). Consequently, the long- and short-term consequences of repetitive exposures to unloading and reloading remain largely unknown. Whether the loss of bone suffered during a previous unloading cycle will potentiate the response to subsequent unloading exposures or whether an adaptive mechanism will diminish the sensitivity of the musculoskeleton with each additional cycle has yet to be determined.
Here, using the well-developed rodent hindlimb unloading (HLU) model (35, 43), we investigated changes in the femur and soleus of C57BL/6J mice during multiple unloading/reambulation periods and specifically asked: What is the overall impact on bone morphology, formation, resorption, and soleus mass when the mouse hindlimb is unloaded either once, twice, or three times over an 18-wk period? and Do prior unloading/reambulation cycles affect changes in trabecular and cortical bone morphology during subsequent cycles?
METHODS
Experimental design.
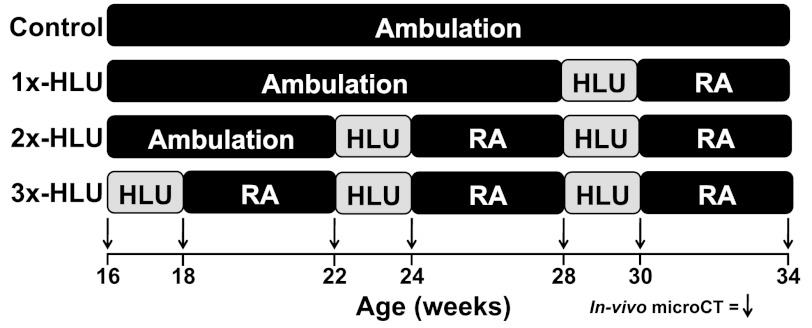
All procedures were approved by the Stony Brook University Animal Care and Use Committee. Forty-two, 16-wk-old, young adult male C57BL/6J (B6) mice (Jackson Laboratory, Bar Harbor, ME) were weighed and randomly assigned to either age-matched controls (control; n = 9) or subject to a single (1x-HLU), double (2x-HLU), or triple (3x-HLU) unloading/reambulation cycle (n = 11/group). Weight-bearing was removed through hindlimb unloading, a ground-based model that simulates many of the physiological effects of microgravity, including hypokinesia and a cephalic fluid shift (35).
During each cycle, mice were subjected to HLU continuously for 2 wk, a duration sufficient to cause musculoskeletal atrophy in several inbred mouse strains, including male B6 mice (1, 41). Each 2-wk HLU period was followed by 4 wk of reambulation (RA). Even though bone recovery is most pronounced in the early weeks following the return to weight-bearing, the recovery of tissue during reambulation is much slower than the loss during disuse (39). The 4-wk recovery period was chosen as a time frame during which significant, but not complete, recovery should occur, while still maintaining a study duration that minimizes age-related changes in bone morphology. 3x-HLU mice were subjected to HLU at 16 wk, 22 wk, and 28 wk of age. Because bone's remodeling activity is age-dependent, differences in the response to HLU between consecutive HLU cycles may not be exclusively attributed to the existence of a prior HLU/RA cycle but also to the disparate ages at which these cycles are applied. To control for potentially concomitant effects of advanced age and prior HLU/RA exposure to unloading, the start of the first HLU/RA cycle was staggered; 2x-HLU mice were subject to HLU at 22 wk and 28 wk while 1x-HLU animals were unloaded for 2 wk at 28 wk (Fig. 1). Thus, this study design permitted an assessment of how age modulates changes in tissue morphology during the first HLU/RA cycle and by comparing the response of the three experimental groups to HLU at 28 wk enabled an age-independent analysis of the effects of multiple exposures. In addition, staggering the start of the first suspension ensured that all experimental groups had the same reambulation duration prior to death.
Fig. 1.
Hindlimb unloading (HLU) and re(ambulation) schedule for control, 1x-, 2x-, and 3x-HLU animals.
Mice were euthanized at 34 wk of age by cardiac puncture and subsequent decapitation. The right soleus and femur were dissected and weighed. At 3, 4, 11, and 12 days before death, mice were injected with calcein (15 mg/kg ip) fluorochrome labels. Animals were individually housed and given free access to a standard rodent chow and water during ambulation, HLU, and RA.
μCT.
Longitudinal changes in the mid-diaphyseal and distal metaphyseal region of the femur were tracked by in vivo μCT at 20.5-μm resolution (VivaCT 75, Scanco Medical, SUI). These regions were scanned 7 times in each mouse: at baseline (16 wk), 18 wk, 22 wk, 24 wk, 28 wk, 30 wk, and at death (34 wk). During each scan, mice were sedated via inhalational anesthesia with isoflurane, as described previously (24). Imaging was performed at an energy, intensity, and integration time of 45 kVp, 177 μA, and 200 ms, respectively, delivering a CT dose index of 550 mGy per scan as estimated by the manufacturer with a 35-mm PMA cylinder that mimicked soft tissue of the mouse. Previous studies showed that single and multiple doses at much higher radiation levels do not exacerbate unloading induced bone loss in mice (22, 26). Furthermore, radiation doses comparable or higher than in this study did not adversely affect trabecular and cortical bone morphology in adult mice and rats (8, 29), even though four weekly scans at 846 mGy deteriorated trabecular bone volume in the tibia of rapidly growing mice (25). Thus, radiation effects were unlikely in this study but cannot be discounted.
Seventy-four transverse slices, covering a 1.5-mm-long region of interest (ROI) that started ∼0.75 mm proximal of the growth plate, were used to assess three-dimensional bone morphology in the distal metaphysis of the right femur. A customized algorithm separated trabecular from cortical bone compartments (34). Trabecular morphology was described via bone volume fraction (BV/TV), trabecular number (Tb.N), thickness (Tb.Th), separation (Tb.Sp), and connectivity (Conn.D). In the mid-diaphysis, a 0.6-mm-thick section located at 50% of the femur length was used to evaluate cortical area (Ct.Ar), thickness (Ct.Th), marrow area (Ma.Ar), total area (Tt.Ar), polar moment of inertia (Ct.Ip), and tissue mineral density (Ct.TMD) (7). Intracortical porosities within the resolution of the images were estimated as the percentage of bone tissue present within Ct.Ar. A bending strength index (BSI), defined as the product of Ct.TMD and the second moment of area for bending in the anterior-posterior direction, was determined (15, 42).
Histomorphometry and histology.
Right femurs were excised, removed of soft tissue, dehydrated, and embedded undecalcified in polymethylmethacrylate. In a metaphyseal ROI comparable to that examined with μCT, indices of bone formation, including mineralizing surface to bone surface (MS/BS), mineral apposition rate (MAR), and bone formation rate (BFR/BS) were assessed across three nonconsecutive unstained frontal sections (5 μm thick) from each sample. In a subset of the same frontal sections (n = 6/group), endocortical and periosteal MS/BS, MAR, and BFR/BS were quantified over a 600-μm length along the anterior and posterior edge of the diaphyseal cortex. For those sections that did not contain double labels, MAR was set to the lowest MAR detected in all sections (0.03 μm/day), as previously suggested (21). To quantify osteoclast surface in the same ROIs, two additional sections (6–8 μm thick) from the same blocks were decalcified and stained with tartrate-resistant alkaline phosphatase. All sections were analyzed at ×20 magnification with Osteomeasure software (Osteometrics, Decatur, GA).
Statistics.
Longitudinal, pairwise differences within groups were compared via repeated-measures ANOVA with Student-Newman-Keuls (SNK) post hoc tests. One-way ANOVA with SNK post hoc tests assessed differences among groups in baseline characteristics and in endpoint histomorphometric and muscle data. The same test was used for between-group comparisons in the changes in bone mass morphology between baseline and death and between different time points throughout the 18-wk experiment. Linear regressions tested for associations between the starting value of any given morphologic variable and the response of this variable during HLU or RA. All data were presented as means ± SD. P values <0.05 were considered significant.
RESULTS
Animals.
At baseline, mice assigned to experimental and normal ambulation control groups had similar body mass (average across groups: 27.5 ± 0.5 g) and bone morphology. All mice remained healthy and active throughout the protocol. At death, differences in body mass between control and experimental animals remained insignificant (Table 1). However, unloaded mice experienced a significant weight loss of 5–10% (P < 0.001) during the first week of each HLU. Some of the weight loss was recovered during the latter part of the HLU cycle, while the rest was regained to levels comparable to that of age-matched controls in the subsequent RA period. Further, no between-group differences in soleus mass were noted at death; the wet weight of the soleus muscle at 34 wk was 8.6 ± 2.8 g in control animals, and 9.3 ± 1.3 g, 9.4 ± 1.9 g, and 9.8 ± 0.9 g in 1x-, 2x-, and 3x-HLU mice, respectively.
Table 1.
Trabecular morphology of the femoral metaphysis and cortical morphology of the femoral mid-diaphysis at baseline and the relative change in morphology between baseline (16 wk) and time of death (34 wk) in control mice and those subject to hindlimb unloading once (1x-HLU), twice (2x-HLU), or three times (3x-HLU)
| Average Baseline | Change Between Baseline and Death, % |
||||
|---|---|---|---|---|---|
| Value | Control | 1x-HLU | 2x-HLU | 3x-HLU | |
| Metaphysis | |||||
| Body Mass, g | 28 ± 0.53 | 15 ± 4.5* | 14 ± 5.1* | 9.8 ± 5.5* | 11 ± 4.8* |
| BV/TV, % | 8.6 ± 0.35 | −36 ± 6.4* | −41 ± 15* | −47 ± 9.4*,a | −46 ± 9.0*,a |
| Tb.N, 1/mm | 4.9 ± 0.08 | −20 ± 5.3* | −20 ± 5.3* | −24 ± 4.0* | −20 ± 4.6* |
| Tb.Th, μm | 41 ± 0.73 | 10 ± 6.1* | 8.8 ± 5.1* | 4.1 ± 3.6*a | 3.8 ± 4.6*a |
| Tb.Sp, mm | 0.20 ± 0.003 | 26 ± 8.8* | 24 ± 9.0* | 31 ± 6.5* | 25 ± 7.5* |
| Conn.D, 1/mm3 | 35 ± 4.2 | 77 ± 17* | 77 ± 21* | 76 ± 18* | 78 ± 17* |
| Diaphysis | |||||
| Ct.Ar, mm2 | 0.82 ± 0.01 | 2.1 ± 5.1 | 4.0 ± 5.5* | 0.49 ± 5.4 | −2.8 ± 5.1a,b |
| Ct.Th, μm | 180 ± 2.8 | −3.4 ± 6.3 | 0.95 ± 4.8 | −3.2 ± 6.4 | −4.3 ± 6.3 |
| Ma.Ar, mm2 | 1.1 ± 0.02 | 4.5 ± 5.0* | 3.1 ± 3.9* | 3.7 ± 4.3* | 1.6 ± 3.5 |
| Tt.Ar, mm2 | 1.9 ± 0.03 | 3.3 ± 3.1* | 3.7 ± 3.6* | 2.1 ± 2.4* | −0.23 ± 1.6a,b |
| Ct.Ip, mm4 | 0.42 ± 0.01 | 5.0 ± 8.1* | 7.1 ± 8.8* | −3.0 ± 6.0b | −1.1 ± 5.5b |
| Ct.TMD, mgHa/ccm | 1100 ± 12 | 1.2 ± 1.9* | 4.2 ± 1.8* | 0.18 ± 2.6b | 1.0 ± 2.7b |
| ICP, % | 98 ± 0.74 | 0.44 ± 1.1 | −0.21 ± 0.98 | −0.11 ± 0.55 | −0.04 ± 0.63 |
| BSI, mm4 mgHA/ccm | 160 ± 21 | 13 ± 12* | 17 ± 7* | 2.8 ± 6.7a,b | 1.1 ± 13a,b |
Data are shown as means ± SD. Letters denote significant differences (P < 0.05) between groups.
Significantly different from baseline, P < 0.05. aAny group significantly different from control. b2x- or 3x-HLU significantly different from 1x-HLU. c3x-HLU different from 2x-HLU. BV/TV, trabecular bone volume fraction; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation; Conn.D, connectivity density; Ct.Ar, cortical bone area; Ct.Th, cortical bone thickness; Ma.Ar, marrow area; Tt.Ar, total area; Ct.TMD, cortical tissue mineral density; Ct.Ip, cortical polar moment of inertia; ICP, intracortical porosity; BSI, cortical bending strength index.
Total 18-wk changes in bone with either 1x, 2x, or 3x unloading/reambulation.
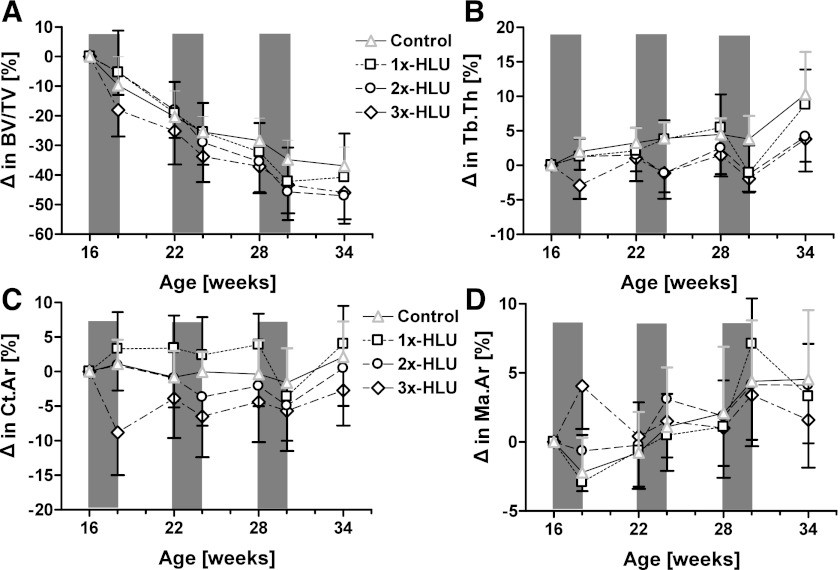
Changes in bone morphology over the 18-wk experimental protocol were compared among the four groups to establish the overall impact of the number of HLU/RA cycles. Control mice experienced significant age-related changes in trabecular bone morphology during the 18-wk course of the experiment (Figs. 2 and 3). For instance, trabecular thickness increased on average by 10% (P < 0.001), but the number of trabeculae decreased by 20% (P < 0.001), giving rise to a 36% (P < 0.001) loss of trabecular bone volume fraction (Table 1, Fig. 2). Morphological changes produced by unloading/reloading cycles were, in part, distinct from those produced by aging. Qualitatively, each unloading cycle inhibited age-induced trabecular thickening, had no effect on trabecular number, and accentuated the loss of bone volume fraction (Figs. 2 and 3). At death and compared with age-matched controls, the increase in Tb.Th from baseline was 60% (P < 0.05) and 63% (P < 0.05) smaller in 2x-HLU and 3x-HLU mice, while the decrease in BV/TV was 27% (P < 0.05) and 24% (P < 0.05) greater. Changes in Tb.N, Tb.Sp, and Conn.D were not significantly different across groups (Table 1). One cycle of HLU/RA within the experimental period did not significantly alter trabecular morphology relative to controls.
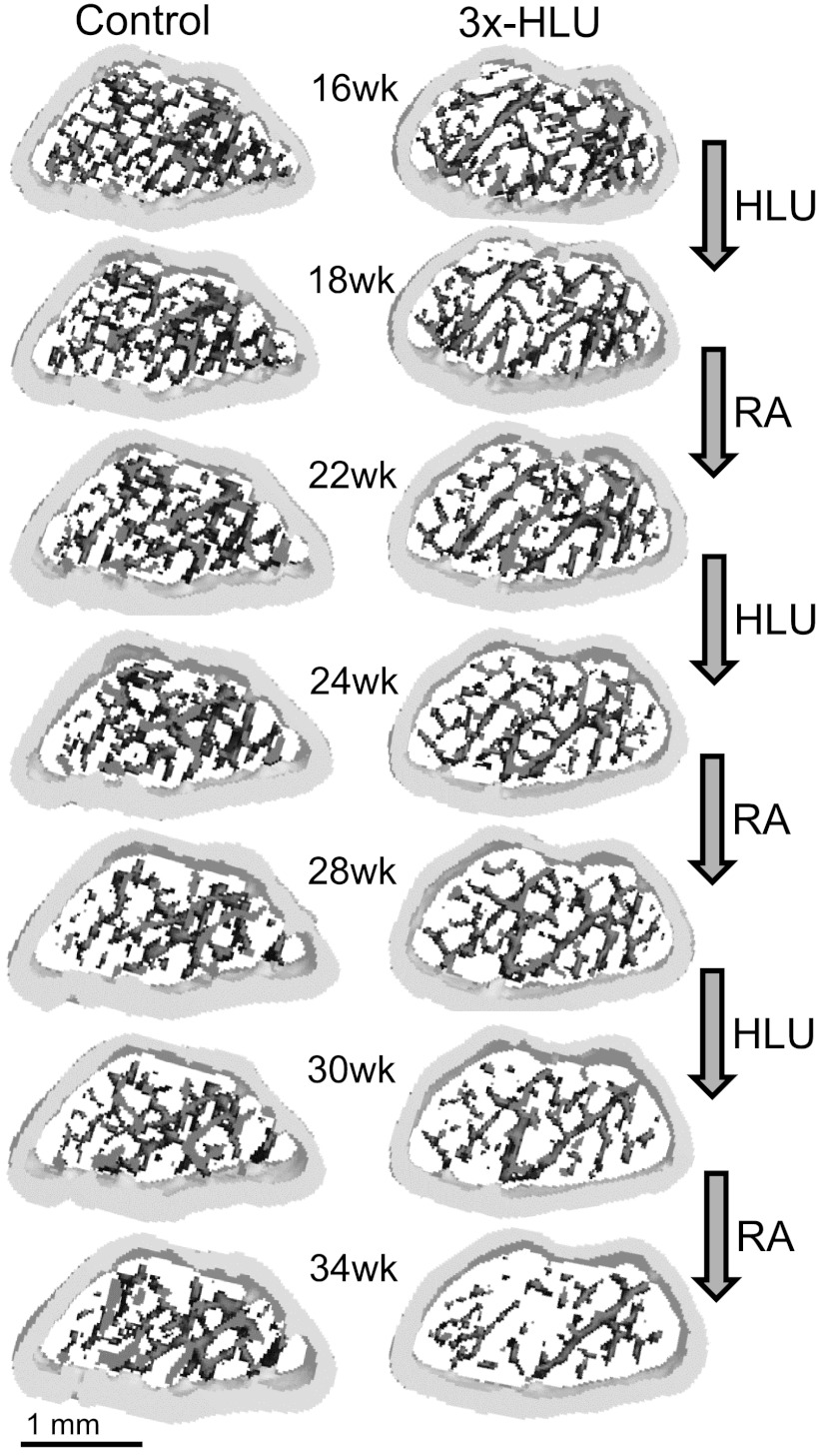
Fig. 2.
Volumetrically rendered three-dimensional morphology of the metaphyseal femur between 16 wk and 34 wk of age of a normally ambulating control mouse and a mouse that was subjected to three consecutive cycles of 2 wk HLU followed by 4 wk of RA. The consequences of superimposing HLU and RA onto the age-related decrease of trabecular number and increase in trabecular thickness are visible. For better visualization of individual trabeculae, only the central 25 (out of 74) slices of the region of interest are shown.
Fig. 3.
Longitudinal relative changes relative to baseline values (means + SD) in metaphyseal trabecular bone volume fraction (A) and trabecular thickness (B), as well as diaphyseal cortical area (C) and endocortical area (D). Time periods during which at least one of the groups was exposed to HLU are shaded.
The number of unloading cycles that the mice experienced also influenced indices of trabecular bone formation measured at the end of the last reambulation period. A trend in successively decreasing MAR and BFR/BS values was noted among 1x-, 2x-, and 3x-HLU animals (Table 2). The number of unloading periods affected, in particular, the rate at which osteoblasts were producing tissue (MAR), rather than the relative surface area covered by active osteoblasts (MS/BS); after three HLU cycles, both MAR (P < 0.01) and BFR/BS (P < 0.05) were 83% lower than controls. In contrast to the reductions in bone's formative cell activity, trabecular osteoclast surface (Oc.S/BS) was similar among all groups.
Table 2.
Indices of trabecular and cortical bone formation and resorption measured in the distal femoral metaphysis and middiaphysis at time of death (34 wk)
| Values at Time of Death |
||||
|---|---|---|---|---|
| Control | 1x-HLU | 2x-HLU | 3x-HLU | |
| Metaphysis | ||||
| Trabecular | ||||
| MS/BS, % | 2.9 ± 1.0 | 2.7 ± 0.84 | 2.1 ± 1.0 | 2.1 ± 0.67 |
| MAR, μm/day | 0.31 ± 0.17 | 0.23 ± 0.21 | 0.16 ± 0.14 | 0.06 ± 0.07a |
| BFR/BS × 10−3, | 11 ± 9.1 | 6.4 ± 6.8 | 3.9 ± 3.8 | 1.8 ± 2.3a |
| μm3•μm−2•day−1 | ||||
| Oc.S/BS, % | 13 ± 4.5 | 15 ± 4.1 | 18 ± 2.8 | 16 ± 5.6 |
| # Without Double Label | 0 | 3 | 3 | 5 |
| Diaphysis | ||||
| Endocortical | ||||
| MS/BS, % | 9.1 ± 5.0 | 16 ± 10 | 9.4 ± 9.3 | 14 ± 3.5 |
| MAR, μm/day | 0.22 ± 0.13 | 0.15 ± 0.16 | 0.17 ± 0.18 | 0.30 ± 0.24 |
| BFR/BS × 10−3, | 22 ± 20 | 26 ± 27 | 28 ± 48 | 44 ± 30 |
| μm3•μm−2•day−1 | ||||
| Oc.S/BS, % | 15 ± 7.9 | 13 ± 5.9 | 16 ± 10.9 | 12 ± 8.2 |
| # Without Double Label | 1 | 1 | 2 | 1 |
| Periosteal | ||||
| MS/BS, % | 3.7 ± 6.1 | 13 ± 10 | 7.3 ± 5.2 | 11 ± 12 |
| MAR, μm/day | 0.08 ± 0.11 | 0.12 ± 0.08 | 0.14 ± 0.17 | 0.19 ± 0.19 |
| BFR/BS × 10−3, | 8.5 ± 18 | 20 ± 21 | 17 ± 29 | 26 ± 25 |
| μm3•μm−2•day−1 | ||||
| Oc.S/BS, % | 0.00 ± 0.00 | 0.31 ± 0.58 | 0.52 ± 1.0 | 0.32 ± 0.72 |
| # Without Double Label | 4 | 2 | 2 | 2 |
Data are shown as means ± SD. Groups were compared using ANOVA followed by SNK. Letters denote significant differences (P < 0.05) between groups. aAny group different from control. MS/BS, % mineralizing surface; MAR, mineralization apposition rate; BFR/BS, bone formation rate; Oc.S/BS, osteoclast surface.
In cortical bone, ambulatory controls experienced significant age-associated changes in mid-diaphyseal shape over the 18-wk experimental protocol. A concomitant expansion of the marrow cavity and periosteum by 5% (P < 0.001) and 3% (P < 0.001) largely maintained cortical bone mass but produced a 5% (P < 0.05) and 13% (P < 0.01) increase in polar moment of inertia and bending strength index, respectively (Table 1, Fig. 2). Qualitatively, each HLU cycle accelerated endosteal expansion, while reambulation caused a contraction of bone marrow space (Figs. 2 and 3). Over the 18-wk experimental period, the three HLU/RA cycles in 3x-HLU mice largely prevented the age-induced increase in periosteal expansion and BSI seen in control and 1x-HLU (P < 0.05, Table 1). The 3% loss in Ct.Ar in 3x-HLU was significantly different from changes in control and 1x-HLU (P < 0.05, Table 1). Reductions in Ct.Ip in 2x-HLU (P < 0.01) and 3x-HLU (P < 0.05) mice were significantly different from the 7% increase (P < 0.01) in 1x-HLU mice (Table 1). These differences were not reflected in indices of bone formation measured on endocortical and periosteal surfaces at the end of the experiment. No differences in MS/BS, MAR, and BFR/BS were observed in the femoral diaphysis just prior to death (Table 2).
Effect of initial age on first unloading/reambulation response.
Since bone's morphology and remodeling activity change with age, the effect of age on the magnitude of the response to HLU/RA was evaluated independently by comparing morphological changes occurring during the first unloading/reloading cycle at 16 wk, 22 wk, and 28 wk in 3x-, 2x-, and 1x-HLU mice, respectively. During the first unloading cycle at 16–18 wk, 22–24 wk, and 28–30 wk, bone volume fraction decreased by 18%, 13% and 15%, respectively (P < 0.001, Table 3), with no significant differences among groups.
Table 3.
Relative changes in metaphyseal trabecular bone morphology and diaphyseal cortical bone morphology during the first period of hindlimb unloading at 16–18 wk (3x-HLU), 22–24 wk (2x-HLU), or 28–30 wk (1x-HLU), during the first reambulation period at 18–20 wk (3x-HLU), 24–26 wk (2x-HLU), or 30–32 wk (1x-HLU), and during the entire first HLU/RA cycle
| Change Between Time Points, % |
|||
|---|---|---|---|
| 3x-HLU | 2x-HLU | 1x-HLU | |
| HLU Period | 16–18 wk | 22–24 wk | 28–30 wk |
| RA Period | 18–22 wk | 24–28 wk | 30–34 wk |
| HLU/RA Period | 16–22 wk | 22–28 wk | 28–34 wk |
| Metaphysis | |||
| BV/TV | |||
| HLU | −18 ± 9.0* | −13 ± 7.8* | −15 ± 8.3* |
| RA | −8.8 ± 7.5* | −8.7 ± 14* | 2.0 ± 9.1 |
| HLU/RA | −25 ± 11* | −21 ± 13* | −13 ± 15* |
| Tb.N | |||
| HLU | −6.4 ± 3.1*b,c | −3.8 ± 2.7* | −2.2 ± 1.8 |
| RA | −4.2 ± 2.1* | −4.1 ± 4.0* | −3.2 ± 3.3* |
| HLU/RA | −10.1 ± 4.0*b | −7.7 ± 5.0* | −5.3 ± 2.4* |
| Tb.Th | |||
| HLU | −2.9 ± 2.0b | −2.5 ± 2.5*b | −6.2 ± 2.7* |
| RA | 4.0 ± 2.8*b | 3.7 ± 2.8*b | 10 ± 5.7* |
| HLU/RA | 1.0 ± 3.2 | 1.0 ± 2.9 | 3.3 ± 5.3* |
| Tb.Sp | |||
| HLU | 7.0 ± 3.3*b,c | 4.1 ± 2.8* | 2.2 ± 2.0 |
| RA | 4.6 ± 2.0* | 4.8 ± 4.6* | 2.8 ± 4.2 |
| HLU/RA | 12 ± 4.5*b | 9.2 ± 5.9*b | 5.0 ± 3.1* |
| Conn.D | |||
| HLU | −40 ± 33* | −24 ± 30* | −54 ± 18* |
| RA | −6.8 ± 59 | −2.5 ± 96 | 25 ± 46 |
| HLU/RA | −47 ± 35* | −32 ± 50 | −42 ± 38 |
| Diaphysis | |||
| Ct.Ar | |||
| HLU | −7.8 ± 5.4*c | −2.7 ± 3.7b | −7.2 ± 3.9* |
| RA | 4.5 ± 4.8*b | 1.7 ± 3.2b | 9.2 ± 4.9* |
| HLU/RA | −3.6 ± 6.0*b | −1.5 ± 3.3 | 1.1 ± 2.8 |
| Ct.Th | |||
| HLU | −9.1 ± 6.0*c | −1.4 ± 5.3b | −7.5 ± 4.3* |
| RA | 5.3 ± 6.0c | −0.22 ± 2.8b | 9.0 ± 5.1* |
| HLU/RA | −4.5 ± 6.6b | −1.7 ± 3.4 | 0.64 ± 3.2 |
| Ma.Ar | |||
| HLU | 4.0 ± 3.1* | 3.3 ± 2.0*b | 6.0 ± 1.9* |
| RA | −3.5 ± 2.4*c | −1.1 ± 1.9b | −3.4 ± 2.5* |
| HLU/RA | 0.43 ± 3.8 | 2.1 ± 0.9* | 2.3 ± 2.1* |
| Tt.Ar | |||
| HLU | −1.1 ± 2.4 | 0.79 ± 1.0 | 0.25 ± 1.3 |
| RA | −0.32 ± 1.3 | −0.03 ± 1.25 | 1.5 ± 1.7 |
| HLU/RA | −1.4 ± 2.4b,c | 0.76 ± 1.6 | 1.8 ± 1.9 |
| Ct.Ip | |||
| HLU | −7.0 ± 6.4*c | −1.4 ± 4.0 | −5.7 ± 4.9* |
| RA | 3.3 ± 4.4c | 1.5 ± 3.4b | 9.7 ± 6.3* |
| HLU/RA | −4.1 ± 6.9b | 0.1 ± 4.7 | 3.2 ± 5.1 |
| Ct.TMD | |||
| HLU | −1.0 ± 2.0 | −0.40 ± 1.4 | −2.0 ± 1.5* |
| RA | 0.94 ± 1.2 | 0.93 ± 1.2 | 1.4 ± 1.9 |
| HLU/RA | −0.08 ± 1.9 | 0.36 ± 1.2 | −0.64 ± 0.8 |
| ICP | |||
| HLU | −0.04 ± 0.4 | −0.03 ± 0.23 | 0.01 ± 0.32 |
| RA | 0.01 ± 0.3 | 0.04 ± 0.23 | −0.00 ± 0.62 |
| HLU/RA | −0.03 ± 0.57 | 0.01 ± 0.20 | 0.00 ± 0.68 |
| BSI | |||
| HLU | −8.1 ± 8.4 | −0.42 ± 11 | −4.3 ± 11.5 |
| RA | 8.9 ± 10 | 2.7 ± 8.9 | 7.3 ± 9.5 |
| HLU/RA | −0.48 ± 7.1 | 1.7 ± 7.9 | 1.9 ± 9.0 |
Data are shown as means ± SD. The relative change during HLU was computed as the % change from the beginning to the end of the 2-wk HLU period; the relative change during RA is the % change from the beginning to the end of the 4-wk RA period; the relative change in HLU/RA is the % change from the beginning of the HLU period to the end of the RA period. Letters denote significant differences (P < 0.05) between groups. b2x- or 3x-HLU different from 1x-HLU; c3x-HLU different from 2x-HLU.
Significant within group change (P < 0.05) during the time period for which the relative % change is measured (HLU, RA, or HLU/RA) .
In spite of these similar volumetric changes, microarchitectural changes accompanying the first unloading cycle were distinct for the different groups. At 16–18 wk of age, bone loss in 3x-HLU mice occurred though a preferential deterioration (P < 0.01) in trabecular number (−6%) over thickness (−3%) while at 22–24 wk, Tb.N (−4%, P < 0.01) and Tb.Th (−3%, P < 0.05) experienced a similar deterioration in 2x-HLU mice (Table 3). In contrast, at 28–30 wk, the significant decrease in trabecular thickness (−6%) drove the bone atrophy in 1x-HLU (P < 0.001, Table 3). Trabecular separation increased by 7% (P < 0.001) during the first HLU period at 16–18 wk, a change that was significantly greater than during the first HLU at 22–24 wk (4%, P < 0.01) and at 28–30 wk (2%, P > 0.05). The first reambulation cycle at 18–22 wk and 24–28 wk continued to decrease BV/TV by 9% (P < 0.01) and 8% (P < 0.01), contrasting with the RA cycle at 30–34 wk during which bone volume was maintained (Table 3). There was also a greater (P < 0.001) recovery in Tb.Th during the first RA at 30–34 wk (10%, P < 0.001) compared with the recoveries during the first RA at 18–22 wk (4%, P < 0.001) and 24–28 wk (4%, P < 0.01). For the first RA period, neither changes in Tb.N nor Tb.Sp were significantly different across the three time points. When considering an entire 6-wk unloading/reambulation cycle, changes in Tb.N and Tb.Sp were significantly greater at 16–22 wk than at 28–34 wk (Table 3).
Changes in cortical morphology were also dependent on age during the first HLU/RA cycle. Mice that were exposed to unloading at 22–24 wk experienced significantly less (P < 0.01) cortical thinning (1%, P > 0.05) than those from 16–18 wk (9%, P < 0.001) and 28–30 wk (8%, P < 0.01), concomitant with a smaller reduction in Ct.Ar. As a consequence, the change in the polar moment at 22–26 wk was, in contrast to the other two groups, not significant (Table 3). Ma.Ar increased significantly during the first HLU at all three time points, but this increase was smaller (P < 0.05) at 22–24 wk than at 28–30 wk. The smaller cortical loss (P < 0.05) during the first HLU at 22–24 wk was followed by smaller gains in cortical tissue than in the other two groups. Similar to Ct.Th, Ct.Ar increased by 5% (P < 0.001) during the first RA cycle at 18–22 wk and by 9% (P < 0.001) at 30–34 wk, contrasting with a lack of change in Ct.Ar at 24 wk. Similarly, Ma.Ar contracted by 4% (P < 0.001) at 18–22 wk and 3% (P < 0.001) at 30–34 wk but not at 22 wk. The polar moment of area increased by 10% (P < 0.05) at 30–34 wk, a greater response than at 18 wk (3%, P > 0.05) and 24 wk (2%, P > 0.05). For any of the three HLU and RA periods, no significant changes in Tt.Ar, ICP, and BSI were observed. For the aggregate of the entire 6-wk unloading/reambulation cycle, changes in Ct.Ar, Ct.Th, Tt.Ar, and Ct.Ip were significantly greater at 16–22 wk than at 28–34 wk (Table 3).
Although the relative response of several trabecular and cortical variables to the initial HLU cycle varied with age, morphological changes in normally ambulating age-matched controls also varied with age. To adjust for these differences, the average change in a given variable over a given time period of HLU or RA was normalized by subtracting the average change in that variable for ambulatory control animals over the same time period. After accounting for these age-related changes, the magnitude of the response to the first HLU and first RA cycle was independent of age for all trabecular parameters. On the other hand, the significantly smaller changes in cortical area, cortical thickness, and marrow area in mice subject to HLU at 22 wk and RA at 24 wk remained. In addition, changes in Ct.TMD became significantly different among the three experimental groups.
Linear regressions were used to test whether morphological differences at the beginning of any given experimental period may have influenced the response during HLU and RA. When including the starting values prior to commencing any of the six HLU periods administered across the three groups, a moderate negative correlation was found only for Tb.Th (R2 = 0.58, P < 0.0001). In other words, mice with thicker trabeculae experienced a higher relative loss. The same analysis produced a significant, but weak, negative correlation (R2 = 0.09, P < 0.05) between starting values and loss in Ct.Ar across all HLU periods, and a weak positive correlation (R2 = 0.09, P < 0.05) between starting values and the gain in Ct.Ar across all RA periods. When the correlations were restricted to only the last HLU (at 28–30 wk) and RA (at 30–34 wk) period, the starting value/response correlations for Tb.Th (R2 = 0.60, P < 0.0001) and Ct.Ar. (R2 = 0.15, P < 0.05) during HLU were preserved, but no significant correlations were found for RA.
Magnitude of the response during multiple unloading/reambulation cycles.
Because age modulated, at least in part, bone's response to HLU/RA, the impact of the number of previous HLU/RA cycles on the magnitude of the response in subsequent cycles was evaluated independent of age by comparing the relative changes in bone morphology for the HLU cycle at 28–30 wk and the RA cycle at 30–34 wk of age. For most trabecular and cortical indices, the magnitude of the response tended to diminish with the number of prior cycles.
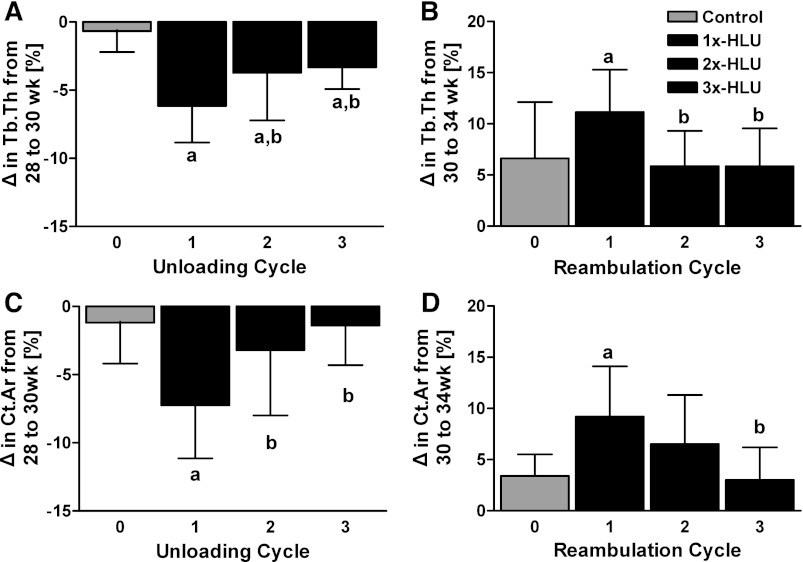
During the unloading cycle at 28 wk of age, the extent of trabecular thinning in mice unloaded for the first time was 1.6-fold greater than the second time (2x-HLU, P < 0.05) and nearly twofold greater than the third time (3x-HLU, P < 0.05) (Fig. 4). Similar to the decreased mechanosensitivity observed in mice during multiple unloading periods, the degree of tissue recovery during reambulation diminished with the number of RA cycles at 30 wk of age; the increase in trabecular thickness in both 2x- and 3x-HLU was only half (P < 0.05) of 1x-HLU (Fig. 4).
Fig. 4.
Relative changes (means ± SD) measured during the first, second, and third unloading (A, C) and reambulation (B, D) cycle for trabecular thickness (A, B) and diaphyseal cortical area (C, D). The magnitude of the response, measured at the same age across groups, diminished with the number of prior exposures to unloading or reambulation. aAny group different from control. bDifferent from #1 unloading (reambulation) cycle.
Similar to trabecular thinning, the reduction in cortical bone volume and thickness in the mid-diaphysis at 28 wk of age was most pronounced for mice undergoing the first cycle, suffering losses that were more than twice (P < 0.05) 2x-HLU and about five times greater (P < 0.01) than 3x-HLU losses (Fig. 4). Marrow expansion during the first HLU (6%, P < 0.001) was also accelerated (P < 0.001) relative to mice in their second (2%, P < 0.05) or third (2%, P < 0.01) cycle of unloading. During the following reambulation cycle, mice in their first RA increased their cortical area at a rate that was three times greater than of age-matched controls (P < 0.01), while 2x- and 3x-HLU mice added tissue that was similar to normally ambulating controls (Fig. 4). The large increase in Ct.Ar during the first RA was associated with a contraction in Ma.Ar that was significantly greater than in control and mice during their second RA.
DISCUSSION
Although clinical and preclinical studies have examined the sensitivity of both bone quantity and architecture to a single exposure of unloading and reambulation, here, we longitudinally quantified bone's response to repetitive unloading and reloading cycles. In vivo μCT scans taken at regular intervals of the femur in B6 mice demonstrated that even after the second exposure to unloading, indices of tissue morphology remained responsive to both catabolic pressure induced by the loss of weight-bearing, as well as the anabolic mechanical signals associated with reambulation. Even though bone was responsive to the second and third unloading/reloading cycle, the magnitude of the response was diminished. For the entire experimental period, detrimental morphological consequences of a single exposure to mechanical unloading were compounded by multiple exposures, emphasizing the additional risk accrued to long bones.
Even though the age of the mice corresponded to young adulthood, during which skeletal mass in mice typically plateaus (4), ambulatory control animals experienced significant changes in metaphyseal trabecular volume and thickness, but not mid-diaphyseal cortical area, over the 18-wk protocol. These changes are consistent with the age-related skeletal evolution of male B6 mice documented in previous studies, which also observed a marked loss in BV/TV, an increase in Tb.Th, and an concurrent expansion of the total and marrow area between 16 wk and 34 wk of age (14, 20). Considering that these age-related changes were not constant over the experimental time frame and that baseline morphology and cellular activity can influence bone's response to unloading (43), it is not surprising that bone's response to the first cycle of HLU/RA cycle was somewhat dependent on the age of the mice. Although this age-dependency did not introduce bias in our multiple HLU/RA cycle analysis, as we only relied on data that were independent of age, results from this study need to be interpreted within the context of large age-related changes in B6 mice, particularly those in the trabecular bone.
Morphological changes induced by unloading and reambulation were sensitive to the number of prior exposures. For several indices modulated by the removal of weight-bearing, the magnitude of the response to HLU/RA between 28 wk and 34 wk of age was diminished during the second and third cycle compared with the first cycle. Many of the microarchitectural and cellular changes that accompany mechanical unloading are also observed during aging, albeit over a much longer timescale. The mechanosensitivity of the musculoskeleton to both anabolic (38) and catabolic stimuli (37) may markedly decrease with advanced age. For instance, 2 wk of HLU reduced the osteoprogenitor population in bone marrow by 71% in 6-wk-old mice and only by 17% in 6-mo-old mice (2). The same length of HLU also decreased BV/TV by more than 20% in 6-mo-old rats but left what little tissue mass was remaining in 32-mo-old animals almost unchanged. Thus, it is conceivable that multiple exposures to unloading transformed bone into a state similar to accelerated aging, thereby decreasing its mechanosensitivity during subsequent HLU/RA cycles. In addition, studies tracking the loss of bone in response to continuous catabolic signals, such as paraplegia (13, 17) or prolonged hindlimb unloading (33), indicate that bone atrophy does not persist indefinitely; it is generally acute in the early period following immobilization, but eventually plateaus or reaches a steady state, after which the bone tissue becomes refractory to the continued presence of catabolic signals. Thus, it is also possible that previously unloaded B6 mice may be approaching such an adaptive plateau, thereby exhibiting a diminished response to further exposures to HLU.
Similar to prior investigations, BV/TV and Tb.Th were the only trabecular indices to be significantly reduced compared with age-matched controls in male B6 mice subject to a single 2-wk exposure to unloading (1). Perhaps as a consequence of the unloading-related suppression of the osteoprogenitor population in this model, BV/TV remained depressed, even after return to normal weight-bearing (36). Generally, trabecular thickness is the only microarchitectural index to recover during relatively short reambulation periods (2, 6, 36). Removal of weight-bearing causes underloading of trabecular struts, initiating bone resorption at the trabecular surface and reducing overall strut thickness, if not entirely eliminating them. The subsequent thickening of the remaining struts during RA may be the result of overload upon reintroduction to weight-bearing, in addition to small age-related increases. As very thin and disconnected struts may remain underloaded and continue to resorb during RA, the recovery in Tb.Th may be accompanied by a loss of Tb.N and an increase in Tb.Sp. These architectural changes emphasize that trabecular bone architecture following unloading and reambulation cycles can be significantly different from baseline and age-matched controls, even when bone quantity is comparable.
In contrast to hindlimb unloading in other mouse strains, in which changes in the diaphysis are minimal even after several weeks of HLU (44), the diaphyseal cortex of B6 mice is sensitive to disuse (23), and as shown here, continues to respond to changes in weight-bearing over multiple cycles of HLU and RA. The reduction in and partial recovery of Ct.Ar is similar to the tibial changes observed in male B6 animals following temporary hindlimb muscle paralysis with botulin (19). However, the botulin-induced changes in tibial Ct.Ar were driven almost exclusively by the lability of the endocortical surface, while changes in the femur coupled marrow expansion and contraction with a suppression of periosteal growth. Although the marrow area of the botulin-injected limbs remained significantly greater than in controls, even weeks after the return of normal muscle activity, the difference did not translate to reduced bone strength and stiffness in the paralyzed limbs. On the other hand, since periosteal area has a greater influence on the load-bearing capacity of cortical bone than marrow area, even the modest blunting of periosteal expansion seen in 3x-HLU was sufficient to reduce the bending strength index relative to ambulatory controls and 1x-HLU.
Multiple cycles of HLU significantly reduced dynamic indices of trabecular bone formation in 3x-HLU animals compared with controls at death, while cortical bone formation activity was similar among groups. In both compartments, osteoclast surface also remained comparable among all groups, although it is possible that unloading may have increased osteoclastic activity without an accompanying increase in osteoclast number (33). While the timing of our fluorochrome markers did not allow for conclusions regarding cellular activity during the unloading cycle, histomorphometric data from previous studies indicate that disuse-induced bone atrophy is primarily driven by a decrease in bone formation and mineralization in this model (11, 18), and only to a lesser, though conflicting extent, by an elevation in bone resportion (48, 49). The decrease in trabecular MAR and BFR with three unloading cycles seen here suggests a similar recovery pattern as that observed for trabecular thickness and cortical bone volume—indices of bone formation, which are significantly depressed during HLU cycles, remain attenuated even after periods of remobilization (6, 36), and after multiple incomplete recovery cycles, become significantly reduced relative to ambulatory controls.
Perspectives and Significance
The present work represents a critical step toward establishing the skeletal risks associated with multiple unloading cycles. The results show that when applied over a given period in B6 mice, multiple exposures to mechanical unloading were more detrimental to trabecular and cortical bone than a single unloading period. However, because the magnitude of the response to unloading decreased with the number of exposures for many outcome parameters, most of the erosion in bone morphology and architecture was sustained during the initial unloading cycle. Consequently, bone's quantity and quality in mice unloaded twice and three times were comparable at death. While trabecular bone volume continued to deteriorate during periods of normal ambulation, indices of cortical bone quantity exhibited limited recovery, and longer recovery periods may allow these parameters to normalize to values comparable to ambulatory controls, even for mice exposed to multiple HLU cycles. Taken together, the sensitivity of bone to weight-bearing across multiple exposures to disuse and its failure to fully recover during reambulation suggest an increased likelihood of short-term catastrophic failure, particularly upon return to normal weight-bearing, and a predisposition to long-term musculoskeletal complications. Future studies investigating how flight schedules, including the number of flights or the length and distribution of recovery periods between flights, can be optimized will be critical for maintaining skeletal health during multiple missions.
GRANTS
This research was kindly funded by the National Aeronautics and Space Administration and a Kirchman T32 postdoctoral fellowship from the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.G. and S.J. conception and design of research; S.G., S.V., and G.U. performed experiments; S.G., S.V., and S.J. analyzed data; S.G. and S.J. interpreted results of experiments; S.G. and S.J. prepared figures; S.G. drafted manuscript; S.G. and S.J. edited and revised manuscript; S.G. and S.J. approved final version of manuscript.
ACKNOWLEDGMENTS
Technical support from Alyssa Tuthill, Andrea Trinward, Jonathan Gillis, and Svetlana Lublinsky was greatly appreciated.
REFERENCES
- 1. Amblard D, Lafage-Proust MH, Laib A, Thomas T, Ruegsegger P, Alexandre C, Vico L. Tail suspension induces bone loss in skeletally mature mice in the C57BL/6J strain but not in the C3H/HeJ strain. J Bone Miner Res 18: 561–569, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Basso N, Bellows CG, Heersche JN. Effect of simulated weightlessness on osteoprogenitor cell number and proliferation in young and adult rats. Bone 36: 173–183, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Basso N, Jia Y, Bellows CG, Heersche JN. The effect of reloading on bone volume, osteoblast number, and osteoprogenitor characteristics: studies in hind limb unloaded rats. Bone 37: 370–378, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone 18: 397–403, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 29: 197–206, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Boudignon BM, Bikle DD, Kurimoto P, Elalieh H, Nishida S, Wang Y, Burghardt A, Majumdar S, Orwoll BE, Rosen C, Halloran BP. Insulin-like growth factor I stimulates recovery of bone lost after a period of skeletal unloading. J Appl Physiol 103: 125–131, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25: 1468–1486, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Brouwers JE, Van RB, Huiskes R. No effects of in vivo micro-CT radiation on structural parameters and bone marrow cells in proximal tibia of wistar rats detected after eight weekly scans. J Orthop Res 25: 1325–1332, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Carmeliet G, Nys G, Bouillon R. Microgravity reduces the differentiation of human osteoblastic MG-63 cells. J Bone Miner Res 12: 786–794, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Carpenter RD, LeBlanc AD, Evans H, Sibonga JD, Lang TF. Long-term changes in the density and structure of the human hip and spine after long-duration spaceflight. Acta Astronaut 67: 71–81, 2010 [Google Scholar]
- 11. Dehority W, Halloran BP, Bikle DD, Curren T, Kostenuik PJ, Wronski TJ, Shen Y, Rabkin B, Bouraoui A, Morey-Holton E. Bone and hormonal changes induced by skeletal unloading in the mature male rat. Am J Physiol Endocrinol Metab 276: E62–E69, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Deschenes MR, Britt AA, Chandler WC. A comparison of the effects of unloading in young adult and aged skeletal muscle. Med Sci Sports Exercise 33: 1477–1483, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34: 869–880, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone 33: 387–398, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Ferretti JL, Capozza RF, Zanchetta JR. Mechanical validation of a tomographic (pQCT) index for noninvasive estimation of rat femur bending strength. Bone 18: 97–102, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord 38: 26–32, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Frotzler A, Berger M, Knecht H, Eser P. Bone steady-state is established at reduced bone strength after spinal cord injury: A longitudinal study using peripheral quantitative computed tomography (pQCT). Bone 43: 549–555, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Globus RK, Bikle DD, Morey-Holton E. The temporal response of bone to unloading. Endocrinology 118: 733–742, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Grimston SK, Goldberg DB, Watkins M, Brodt MD, Silva MJ, Civitellit R. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J Bone Miner Res 26: 2151–2160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res 17: 1044–1050, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Hauge E, Mosekilde LE, Melsen F. Missing observations in bone histomorphometry on osteoporosis: Implications and suggestions for an approach. Bone 25: 389–395, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Judex S, Chung H, Torab A, Xie L, Rubin CT, Donahue LR. Micro-CT-induced radiation does not exacerbate disuse-related bone loss. In Proceedings of the Annual Meeting of the Orthopaedic Research Society, Washington, DC, 2005 [Google Scholar]
- 23. Judex S, Garman R, Squire M, Busa B, Donahue LR, Rubin C. Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res 19: 607–613, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Judex S, Luu YK, Ozcivici E, Adler B, Lublinsky S, Rubin CT. Quantification of adiposity in small rodents using micro-CT. Methods 50: 14–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klinck RJ, Campbell GM, Boyd SK. Radiation effects on bone architecture in mice and rats resulting from in vivo micro-computed tomography scanning. Med Eng Phys 30: 888–895, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Kondo H, Yumoto K, Alwood JS, Mojarrab R, Wang A, Almeida EA, Searby ND, Limoli CL, Globus RK. Oxidative stress and gamma radiation-induced cancellous bone loss with musculoskeletal disuse. J Appl Physiol 108: 152–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 19: 1006–1012, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Lang TF, LeBlanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res 21: 1224–1230, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Laperre K, Depypere M, van Gastel N, Torrekens S, Moermans K, Bogaerts R, Maes F, Carmeliet G. Development of micro-CT protocols for in vivo follow-up of mouse bone architecture without major radiation side effects. Bone 49: 613–622, 2011 [DOI] [PubMed] [Google Scholar]
- 30. LeBlanc A, Schneider V. Can the adult skeleton recover lost bone? Exp Gerontol 26: 189–201, 1991 [DOI] [PubMed] [Google Scholar]
- 30a. LeBlanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res 5: 843–850, 1990 [DOI] [PubMed] [Google Scholar]
- 31. LeBlanc AD, Driscol TB, Shackelford LC, Evans HJ, Rianon NJ, Smith SM, Feeback DL, Lai D. Alendronate as an effective countermeasure to disuse induced bone loss. J Musculoskelet Neuronal Interact 2: 235–243, 2002 [PubMed] [Google Scholar]
- 32. LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact 7: 33–47, 2007 [PubMed] [Google Scholar]
- 33. Li XJ, Jee WSS, Chow SY, Woodbury DM. Adaptation of cancellous bone to aging and immobilization in the rat—A single photon-absorptiometry and histomorphometry study. Anat Record 227: 12–24, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Lublinsky S, Ozcivici E, Judex S. An automated algorithm to detect the trabecular-cortical bone interface in micro-computed tomographic images. Calcif Tissue Int 81: 285–293, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol 92: 1367–1377, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Ozcivici E, Luu YK, Rubin CT, Judex S. Low-level vibrations retain bone marrow's osteogenic potential and augment recovery of trabecular bone during reambulation. PLos One 5: e11178, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perrien DS, Akel NS, Dupont-Versteegden EE, Skinner RA, Siegel ER, Suva LJ, Gaddy D. Aging alters the skeletal response to disuse in the rat. Am J Physiol Regul Integr Comp Physiol 292: R988–R996, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int 50: 306–313, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Sibonga JD, Evans HJ, Sung HG, Spector ER, Lang TF, Oganov VS, Bakulin AV, Shackelford LC, LeBlanc AD. Recovery of spaceflight-induced bone loss: Bone mineral density after long-duration missions as fitted with an exponential function. Bone 41: 973–978, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Sievanen H. Immobilization and bone structure in humans. Arch Biochem Biophys 503: 146–152, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Simske SJ, Luttges MW, Allen KA, Gayles EC. The role of sex and genotype on antiorthostatic suspension effects on the mouse peripheral skeleton. Aviat Space Environ Med 65: 123–133, 1994 [PubMed] [Google Scholar]
- 42. Siu WS, Qin L, Leung KS. pQCT bone strength index may serve as a better predictor than bone mineral density for long bone breaking strength. J Bone Miner Metab 21: 316–322, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Squire M, Brazin A, Keng Y, Judex S. Baseline bone morphometry and cellular activity modulate the degree of bone loss in the appendicular skeleton during disuse. Bone 42: 341–349, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Squire M, Donahue LR, Rubin C, Judex S. Genetic variations that regulate bone morphology in the male mouse skeleton do not define its susceptibility to mechanical unloading. Bone 35: 1353–1360, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Swift JM, Nilsson MI, Hogan HA, Sumner LR, Bloomfield SA. Simulated resistance training during hindlimb unloading abolishes disuse bone loss and maintains muscle strength. J Bone Miner Res 25: 564–574, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Tamma R, Colaianni G, Camerino C, Di Benedetto A, Greco G, Strippoli M, Vergari R, Grano A, Mancini L, Mori G, Colucci S, Grano M, Zallone A. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J 23: 2549–2554, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehaillia M, Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355: 1607–1611, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Vico L, Novikov VE, Very JM, Alexandre C. Bone histomorphometric comparison of rat tibial metaphysis after 7-day tail suspension vs 7-day spaceflight. Aviat Space Environ Med 62: 26–31, 1991 [PubMed] [Google Scholar]
- 49. Zerwekh JE, Ruml LA, Gottschalk F, Pak CYC. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res 13: 1594–1601, 1998 [DOI] [PubMed] [Google Scholar]