Abstract
A decline in mitochondrial biogenesis and mitochondrial protein quality control in skeletal muscle is a common finding in aging, but exercise training has been suggested as a possible cure. In this report, we tested the hypothesis that moderate-intensity exercise training could prevent the age-associated deterioration in mitochondrial biogenesis in the gastrocnemius muscle of Wistar rats. Exercise training, consisting of treadmill running at 60% of the initial V̇o2max, reversed or attenuated significant age-associated (detrimental) declines in mitochondrial mass (succinate dehydrogenase, citrate synthase, cytochrome-c oxidase-4, mtDNA), SIRT1 activity, AMPK, pAMPK, and peroxisome proliferator-activated receptor gamma coactivator 1-α, UCP3, and the Lon protease. Exercise training also decreased the gap between young and old animals in other measured parameters, including nuclear respiratory factor 1, mitochondrial transcription factor A, fission-1, mitofusin-1, and polynucleotide phosphorylase levels. We conclude that exercise training can help minimize detrimental skeletal muscle aging deficits by improving mitochondrial protein quality control and biogenesis.
Keywords: mitochondrial fission/fusion, LON protease, sirtuins, oxidative stress
during high-intensity exercise, the metabolic rate of skeletal muscles can increase up to 100-fold, compared with that of cells at rest, and the mitochondrial network in the skeletal muscle exhibits a high degree of plasticity as a result of exercise, including biogenesis, fission, and fusion (3, 6, 12, 24, 28). Successful mitochondrial biogenesis requires a well-orchestrated signaling process, in which many factors are involved.
Mitochondrial transcription factor A (TFAM) is a major regulator of the mitochondrial genome, while nuclear respiratory factors (Nrf1, Nrf2) regulate the expression of many mitochondrial proteins whose genes are located in the cell nucleus (23, 49); TFAM, Nrf1, and Nrf2 are all encoded by nuclear genes. The coordination and activation of TFAM, Nrf1, and Nrf2, in turn, are controlled by peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) (39). Indeed, the coactivator PGC-1α induces mitochondrial biogenesis, once it has been activated (by phosphorylation) by the mitogen-activated protein kinase (p38 MAPK) or AMP-activated protein kinase (AMPK) (23, 39, 60). In addition, it has been shown that SIRT1 catalyzes PGC-1α deacetylation, which also activates this coactivator (36, 62). It is known that PGC-1α is readily induced by acute and regular exercise (25, 56).
Recent work indicates that the type 1 IFN-β inducible polynucleotide phosphorylase (PNPase), an evolutionarily conserved 3′,5′ exoribonuclease with an expanding repertoire of functions, also plays a crucial role in mitochondrial biogenesis (61). PNPase localized to the mitochondrial intermembrane space (also present in the cytosol), is vital for mitochondrial homeostasis (9) and can induce cellular senescence (52). Free radicals, and other reactive oxygen species, can downregulate PNPase expression (21), but the effects of exercise on PNPase are currently unknown.
The ability for mitochondria to fuse with one another enables them to mix their contents, thereby minimizing the effects of dysfunctional mitochondria. Mitofusins, Mfn1 and Mfn2, are located in the outer membrane, and regulate the initial steps of mitochondrial fusion (7, 8). These steps are critical and have been suggested to protect the function and integrity of mitochondrial DNA (14, 57). Fission of mitochondria segregates them from the mitochondrial network (2). Mitochondrial fission downregulates mitochondrial elongation and may have a protective role in cellular senescence (31). However, the effects of exercise on fusion and fission of aged skeletal muscle is not well known.
Oxygen radicals, and oxidants in general, seem to play a role in modulating mitochondrial biogenesis (13), mitochondrial fragmentation (15), and oxidative damage to mitochondrial proteins (4, 5, 37). To maintain mitochondrial function under normal and stressful conditions, the integrity of proteins is under constant surveillance by mitochondrial chaperones. Lon is the major protease responsible for the degradation of oxidized proteins in mitochondria and is part of the oxidative stress adaptive response repertoire (4, 5, 37, 58). Interestingly, Lon is required to maintain the integrity of mitochondrial DNA and has also been correlated with enhanced mitochondrial biogenesis (34). The molecular chaperone Hsp78 has been shown to reinstate mitochondrial DNA replication following thermal inactivation (17). HSP78 may also be required for the efficient Lon-mediated proteolysis of damaged proteins (48).
Pioneering work by Holloszy (22) showed that exercise can significantly increase mitochondrial enzyme activity in skeletal muscle, and work by Davies and colleagues (11, 13) demonstrated that endurance exercise training increases the mitochondrial mass of skeletal muscles. In aging, there is a marked decrease in muscle endurance, force production, and muscle mass. There is also a marked decline in Lon mRNA and protein levels in skeletal muscles (4, 30) and increased oxidative damage (29). These properties are associated with the attenuated mitochondrial function observed in inactive humans and in laboratory rodents maintained in standard housing without exercise “facilities”.
We have suggested that the exercise-induced adaptation decreases the aging-associated impairment of mitochondrial biogenesis and quality control of mitochondrial proteins.
MATERIALS AND METHODS
Exercise training.
Twelve young (3 mo) and twelve old (26 mo) male Wistar rats were used in the study, randomly assigned into young control (YC), young-exercised (YE), old control (OC) and old-exercised (OE) groups. The investigation was carried out according to the requirements of “The Guiding Principles for Care and Use of Animals, EU”, and was approved by the Semmelweis University Ethics Committee. Exercise-trained rats were first introduced to treadmill running for 3 days. Then for the next 2 wk, they were trained for 30 min daily at a running speed of 10 m/min, on a 5% incline. The running speed and duration of the daily exercise sessions were then gradually increased to 60% of the animals' V̇o2max such that, on the last week of the 6-wk training program, young animals ran at 22 m/min, on a 10% incline, for 60 min; in contrast, old animals ran at 13 m/min, on a 10% incline for 60 min. The intensity of the exercise program was carefully matched in these two groups by V̇o2 max data, as described previously (43, 45). Earlier studies have shown that this intensity of running significantly alters the biochemical profile of gastrocnemius muscle (16, 45, 53, 55). The animals were killed two days after the last exercise training session to avoid the metabolic effects of the final run. Gastrocnemius muscles were carefully excised, homogenized in buffer containing 137 mM NaCl, 20 mM Tris·HCl (pH 8.0), 2% NP 40, 10% glycerol, and protease inhibitors.
Western blots.
Ten to 50 μg of protein were electrophoresed on 8–12% vol/vol polyacrylamide SDS-PAGE gels. Proteins were electrophoretically transferred onto PVDF membranes. The membranes were subsequently blocked and after blocking, were incubated at room temperature with the following antibodies: 1:500, sc-13067 [Santa Cruz PGC-1 (H-300); Santa Cruz Biotechnology, Santa Cruz, CA], 1:1,400, 2532 (Cell Signaling, Beverly, MA) AMPKα, 1:500, 2535 Cell Signaling p-AMPKα (Thr172) (40H9), 1:1,000, sc-33771 Santa Cruz NRF-1 (H-300), 1:500 sc-30963 Santa Cruz mtTFA (E-16) /TFAM/, 1:500, sc-98900 Santa Cruz Fis1 (Fl-152), 1:10,000, sc-50330 Santa Cruz Mfn1 (H-65), 1:1,000, sc-99006 Santa Cruz PNPase (H-124), 1:200, U7757 Sigma-Aldrich UCP3, 1:5,000 ab87253 Abcam CLPB /HSP78/, 1:5,000, sc-98253 Santa Cruz SDH (H-204), 1:1,000, sc-69359 Santa Cruz COX4 (D-20), 1:500, ab53517 Abcam SIRT1, and 1:15,000, T6199 Sigma α-tubulin. The antibody for Lon protease was generated by us, as described previously (38). After incubation with primary antibodies, membranes were washed in TBS-Tween-20 and incubated with horseradish peroxidase-conjugated secondary antibodies. After incubation with secondary antibody, membranes were repeatedly washed. Membranes were incubated with an ECL plus reagent (RPN 2132; Amersham, Piscataway, NJ), and protein bands were visualized on X-ray films. The bands were quantified by ImageJ software, and normalized to tubulin, which served as an internal control.
Estimation of oxidant levels and redox active iron.
Intracellular oxidant and redox-active iron levels (26) were estimated using modifications of the dichlorodihydrofluorescein diacetate (H2DCFDA) staining method (44). The oxidative conversion of stable, nonfluorometric, DCF-DA to highly fluorescent 2′7′-dichlorofluoroescein (DCF) was measured in the presence of esterases, as previously reported (43). This assay approximates levels of reactive species, such as superoxide radical, hydroxyl radical, and hydrogen peroxide. The method has been widely used in the literature but does have the problem of not being particularly specific, and results can be strongly affected by release of labile iron or copper (26). Thus, the DCF fluorescence assay measures the production of (unknown) oxidants, probably including peroxide, and the release of redox-active iron that together cause the fluorescence (26). Briefly, the H2DCFDA (Invitrogen-Molecular Probes, D399) was dissolved at a concentration of 12.5 mM in ethanol and kept at −80°C in the dark. The solution was freshly diluted with potassium phosphate buffer to 125 μM before use. For fluorescence reactions, 96-well black microplates were loaded with potassium phosphate buffer (pH 7.4) to a final concentration of 152 μM/well. Then 8 μl of diluted tissue homogenate and 40 μl 125 μM dye were added to achieve a final dye concentration of 25 μM. The change in fluorescence intensity was monitored every 5 min for 30 min with excitation and emission wavelengths set at 485 nm and 538 nm (Fluoroskan Ascent FL), respectively. The fluorescence intensity unit was normalized with the protein content and expressed in relative unit production per minute.
Assessment of SIRT1 activity.
The Cyclex SIRT1/Sir2 deacetylase fluorometric assay kit (Cyclex, CY-1151) was used to measure SIRT1 deacetylase activity, according to the manufacturer's established protocol, including the separation of nuclear extract. For our assays, 10 μl of cytosolic and nuclear extracts of quadriceps muscle was mixed with a reaction mixture (40 μl) containing 50 mM Tris·HCl pH 8.8, 4 mM MgCl2, 0.5 mM DTT, 0.25 mAU/ml lysyl endopeptidase, 1 μM trichostatin A, 20 μM fluoro-substrate peptide, and 200 μM NAD+ in a microplate. The samples were mixed well and incubated for 10 min at RT, and the fluorescence intensity (excitation: 355 nm, emission: 460 nm) was read for 2 h every 10 min and normalized by the protein content.
Citrate synthase activity assay.
To appraise the effect of aging and physical exercise, citrate synthase activity was measured as described by Sheperd and Garland (54).
Mitochondrial DNA measurement.
To estimate the mtDNA content, we quantified the mtDNA to nuclear DNA (nDNA) ratio (mtDNA/nDNA). Total DNA was extracted (fast DNA kit, 6540–400, BIO 101 Systems Qbiogene) and quantitated spectrophotometrically. The mtDNA content was measured by PCR (Rotor-Gene 6000; Corbett Research, Sydney, Australia) and corrected by the simultaneous measurement of a single copy nuclear BDNF gene. Primers used for the analysis of mtDNA were R-CYTB-F (5′-CCC CAG AGG ATT AAA CTC CAA CGC A-3′), and R-CYTB-R (5′-GGG TGG GGT CAG GGG GT-3′). Primers used for the analysis of nDNA were R-BDNF-genome-exon-IV-F (5′-TTG GGA TGG GAA AGA TGG G-3′) and R-BDNF-genome-exon-IV-R (5′- CAG AGT AGG AGG GAA CAA GTG TGA C-3′). Then the mtDNA content was normalized to nDNA. Data are expressed as the means of three measurements.
Statistical analyses.
Statistical significance was assessed by one-way ANOVA, followed by Tukey's post hoc test. Correlation matrices were applied to evaluate the relationship between different variables. The significance level was set at P < 0.05.
RESULTS
Exercise training modulates many factors in the PGC1-α repertoire.
An acute bout of exhaustive exercise increases production of reactive oxygen species, but this response is ameliorated by exercise training (11, 12, 50). Factors, such as AMP-K and SIRT1, are also affected by exercise training. These factors are upstream of PGC1-α, and can regulate the activity of this master gene. PGC-1α, in turn, can initiate the transcription of NRF1, which can then initiate the transcription of proteins that regulate mitochondrial biogenesis, including nuclear DNA-coded TFAM.
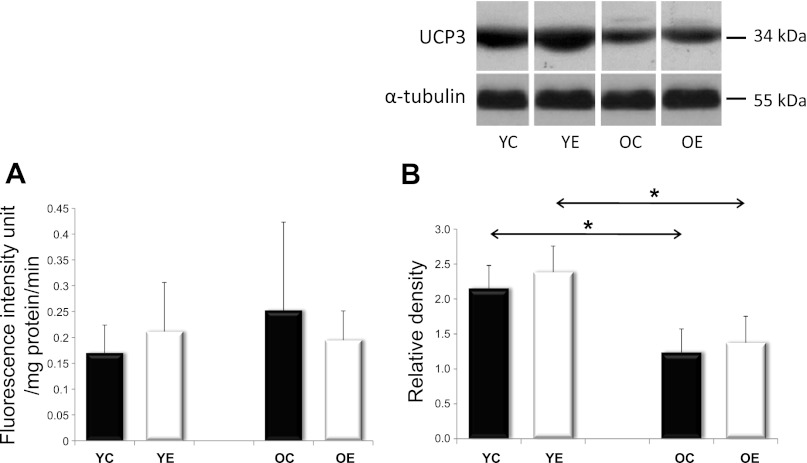
We first estimated intracellular oxidant levels and reactive iron release levels in young and old rats that were either exercised or not exercised-trained. Young rats were tested at 3 mo old, while old rats were tested at 26 mo old. The gastrocnemius muscle was isolated and stained for 2′7′-dichlorofluorescein oxidation. Although a trend toward higher DCF fluorescence was seen in older animals, no statistically significant difference was found between samples (Fig. 1A), possibly suggesting that neither aging nor exercise training had consistently strong effects on intracellular oxidant levels and reactive iron release levels. Alternative explanations for the negative results of Fig. 1A are that the 2-day rest between final exercise session, and euthanasia of the animal was sufficient to reverse most evidence of tissue oxidation or that, as previously reported (50), exercise training actually causes cellular adaptations that include a higher threshold than the training stimulus for stress responses to be activated. Uncoupling protein 3 (UCP3) has been shown to be protective against aging and protein damage through the modulation of reactive oxygen species. We analyzed UCP3 levels in our rats and found that UCP3 decreased with aging and that this decline could not be completely reversed by exercise training (Fig. 1B).
Fig. 1.
Oxidant levels/redox active iron release, and UCP3 levels in rat skeletal muscle. Rat skeletal muscle was stained with H2DCFDA to gain a rough estimate of relative steady-state oxidant levels and redox-active iron release levels [both of which can increase DCF fluorescence, (26)] in young control (YC), young exercised (YE), old control (OC), old exercised (OE), as depicted in A. Statistically significant differences were not observed. B: Western Blot analysis of UCP3 revealed significant decreases with age in both the sedentary and the exercise-trained groups. Values are expressed as means ± SD for six animals per group (*P < 0.05). All samples were run on the same gel; therefore, representative lanes were removed for final presentation.
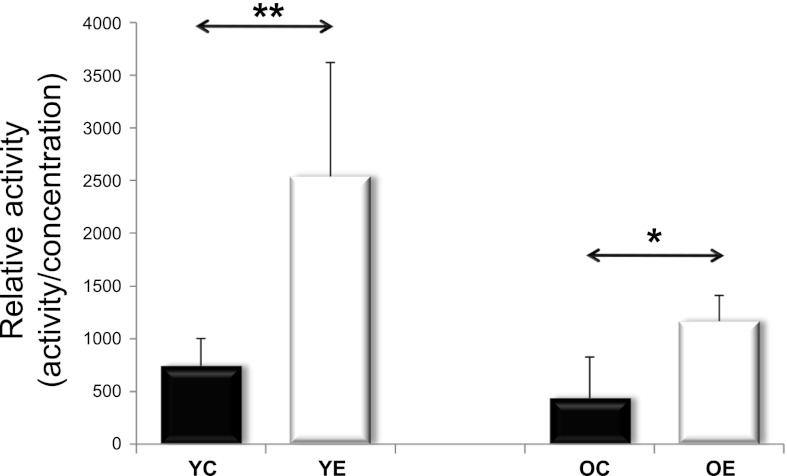
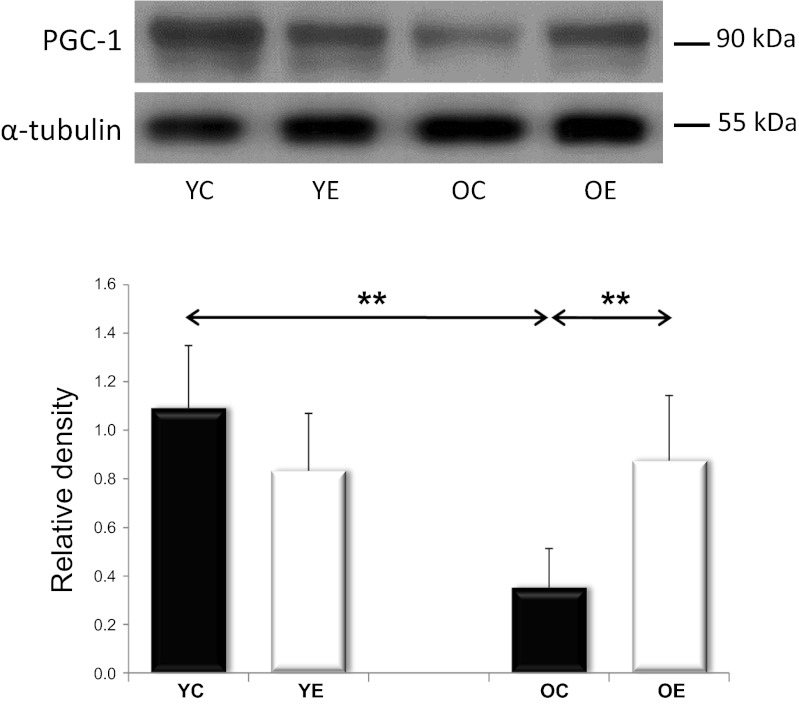
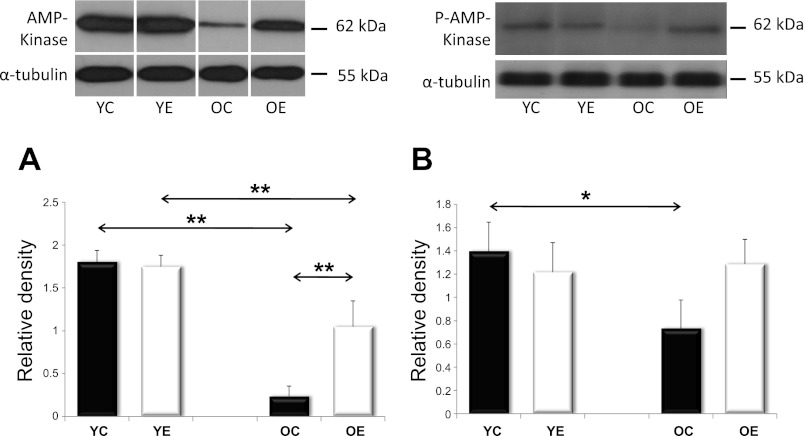
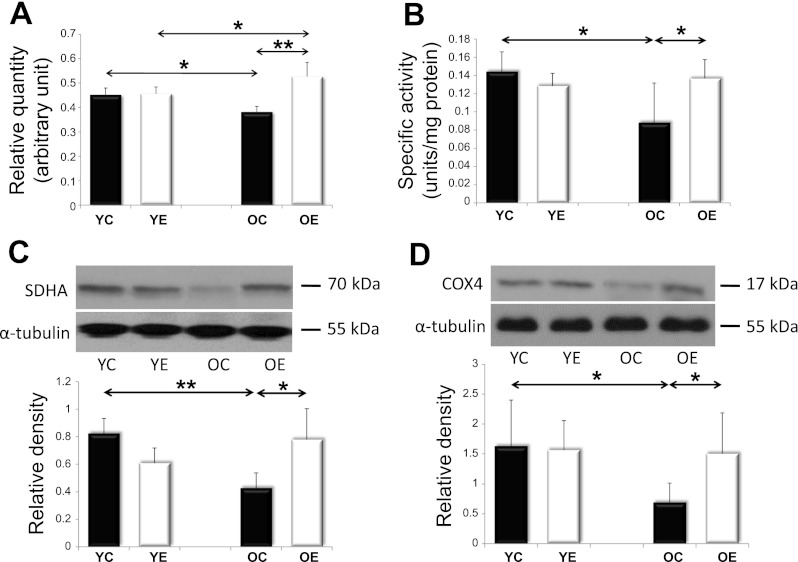
SIRT1 and AMPK are both capable of activating PGC1α, a master regulator of mitochondrial protein expression in the nuclear genome. SIRT1 can activate PGC1α by deacetylation. We analyzed SIRT1 activity in young and old rats, with and without exercise training. We found that SIRT1 activity tended to decrease with aging; however, exercise training increased the activity in both the young and the old age groups (Fig. 2). The PGC-1α content, on the other hand, decreased with aging, and this was reversed by exercise training (Fig. 3). We next studied AMPK levels. Because AMPK phosphorylation results in AMPK activation, we studied both unphosphorylated and phosphorylated AMPK. We found that total and phosphorylated AMPK content decreased with aging; however, regular exercise reversed this decrease (Fig. 4). We also studied the factors downstream of PGC1α, the mitochondrial TFAM, and NRF1. We found that the levels of TFAM and NRF1 were significantly increased by aging in murine skeletal muscles, but that these increases were blocked in rats that were exercise trained (Fig. 5). We have apprised the level of mitochondrial biogenesis by mtDNA content, the activity of citrate synthase, and the contents of succinate dehydrogenase, as well as cytochrome-c oxidase-4, and all of these markers showed age-associated decline (P < 0.05), which was reversed by exercise training (Fig. 6).
Fig. 2.
Relative activity of SIRT1. SIRT1 deacetylase activity was analyzed in YC, YE, OC, and OE rat skeletal muscle. In both age groups, exercise training significantly increased SIRT1 activity. SIRT1 activity was measured fluorometrically (see materials and methods) and divided by sample protein content. Values are means ± SD for six animals per group (*P < 0.05, **P < 0.01).
Fig. 3.
PGC-1a levels. Western blots of YC, YE, OC, and OE skeletal muscles show significant reductions in PGC-1a levels with age, which was prevented by exercise training. Values for PGC-1a reported as “relative densities,” which have corrected for loading level differences, as judged by α-tubulin levels. Values are expressed as means ± SD for six animals per group (** P < 0.01).
Fig. 4.
The levels of AMPK and pAMPK. Skeletal muscle from YC, YE, OC, and OE rats were probed with either AMPK in A or phosphorylated AMPK in B. Values for AMPK and pAMPK are reported as “relative densities,” which have been corrected for loading-level differences, as judged by α-tubulin levels. Both AMPK and pAMPK showed decreases with age, which were partially prevented by exercise training. Values are expressed as means ± SD for six animals per group (*P < 0.05, **P < 0.01). All samples were run on the same gel; therefore, representative lanes were removed for final presentation.
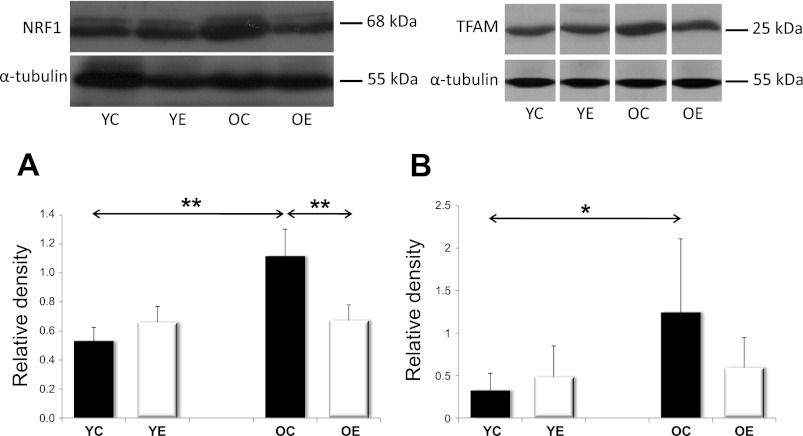
Fig. 5.
The levels of transcription factors, NRF1, and TFAM. YC, YE, OC, and OE rats were probed with the NRF1 antibody depicted in panel A, or the TFAM antibody in B. Values for NRF1 and TFAM are reported as relative densities that have been corrected for loading level differences, as judged by α-tubulin levels. Aging increased both the NRF1 and TFAM levels, while exercise training attenuated these increases. Values are expressed as means ± SD for six animals per group (*P < 0.05, **P < 0.01). All samples were run on the same gel; therefore, representative lanes were removed for final presentation.
Fig. 6.
Markers of mitochondrial biogenesis. mtDNA levels (A), as well as activity of citrate synthase (CS) (B), contents of succinate dehydrogenase (SDH) (C), and cytochrome-c oxidase-4 (COX4) (D) were measured to apprise the level of mitochondrial biogenesis. Values for each marker protein are reported as relative densities that have been corrected for loading-level differences, as judged by α-tubulin levels. The age-associated decreases in all of these markers were reversed by exercise training. Values are expressed as means ± SD for six animals per group (*P < 0.05, **P < 0.01).
Mitochondrial fission and fusion is maintained with exercise training.
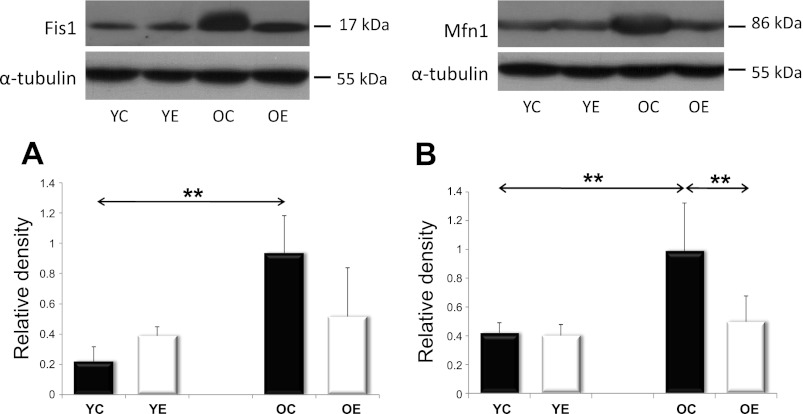
Mitochondrial plasticity depends on the ability of mitochondria to fuse and then separate from one another rapidly, especially during high-intensity exercise. The two most important proteins responsible for mitochondrial fusion and fission are mitofusin-1 (Mfn1) and fission-1 (Fis1). Our results indicate that both Mfn1 and Fis1 protein levels increased with aging, but that this increase was prevented by exercise training (Fig. 7).
Fig. 7.
Analysis of mitochondrial biogenesis factors, Fis1 and Mfn1. Levels of mitochondrial fission and fusion proteins were assessed in YC, YE, OC, and OE rats. Values for Fis1 and Mfn1 are reported as relative densities that have been corrected for loading-level differences, as judged by α-tubulin levels. In both the mitochondrial fission regulator Fis1, as depicted in A, and the fusion controller Mfn1, as depicted in B, there was an age-associated increase that was attenuated with exercise training. Values are means ± SD for six animals per group (**P < 0.01).
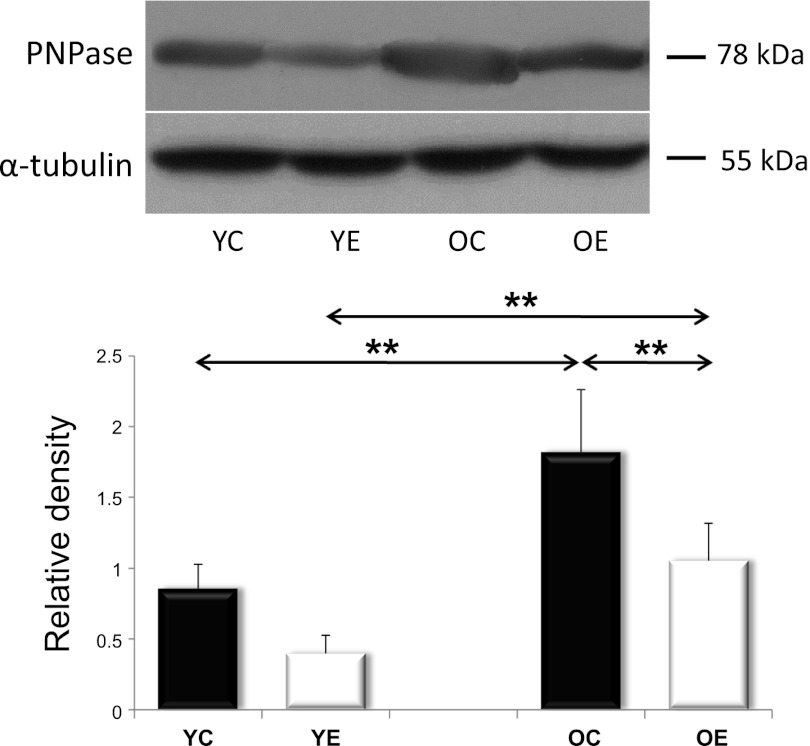
PNPase is also an important regulator of mitochondrial biogenesis. Interestingly, PNPase protein levels showed a similar profile to Mfn1 and Fis1: in other words, PNPase exhibited an age-associated increase of that was attenuated by exercise training (Fig. 8).
Fig. 8.
The levels of PNPase. Western blot analysis of YC, YE, OC, and OE muscles. Values for PNPase are reported as relative densities that have been corrected for loading-level differences, as judged by α-tubulin levels. PNPase levels increased with age; however, exercise blocked this induction. Values are expressed as means ± SD for six animals per group (**P < 0.01).
Mitochondrial quality control proteins are rescued with exercise training.
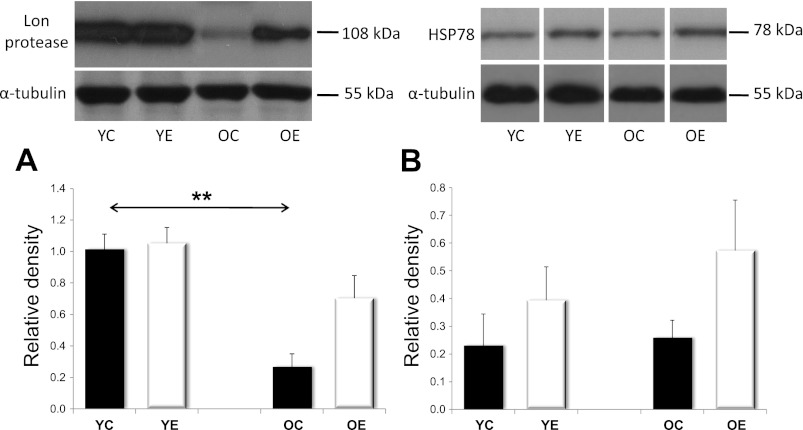
We also wanted to look at mitochondrial protein quality control by assessing both the Lon protease and HSP78, also known as ClpB. We found that Lon levels decreased significantly with age, but that this reduction was, in part, rescued with exercise training (Fig. 9A). HSP78, previously suggested to work in coordination with Lon protease, was also elevated as a result of exercise training (Fig. 9B).
Fig. 9.
The levels of Lon protease and HSP78. Analysis of YC, YE, OC, and OE skeletal muscles revealed that the Lon protease decreased with age. This decrease, however, was blunted in the OE group (A). The levels of the molecular chaperone HSP78 were increased, as expected, although, not significantly with exercise training (B). Values for Lon and HSP78 are reported as relative densities that have been corrected for loading level differences, as judged by α-tubulin levels. Values are expressed as means ± SD for six animals per group (**P < 0.01). All samples were run on the same gel; therefore, representative lanes were removed for final presentation.
DISCUSSION
One of the deficits associated with aging is a loss of mitochondrial function, leading to higher levels of superoxide and hydrogen peroxide production, which cause mitochondrial damage and, eventually, perpetuate the process (27). Interestingly, oxidative stress has been proposed as one of the regulators of mitochondrial biogenesis (11, 13, 27). In this study, we aimed to look at the effects of exercise training on mitochondrial biogenesis and aging. We found that steady-state oxidant levels and reactive iron release levels do not seem to be affected by exercise training or aging significantly. It seems that although single bouts of exhaustive exercise do transiently increase oxidative stress (11, 13), exercise training causes adaptations that minimize the stress (19, 45, 46, 50).
It should also be noted that the animals in the present study were given 2 days to recover from their last exercise training session before being studied. These results are also in accordance with some previous reports (1, 10, 20, 42).
Since exercise training did not significantly increase steady-state oxidant levels and reactive iron release levels, we did not expect to see a concomitant induction in UCP3 with the exercise training used for these rats. In fact, we actually found a decrease in UCP3 that was not reversed by exercise training.
We were interested in studying the PGC1α repertoire, as this coactivator is one of the major controllers of mitochondrial protein expression and biogenesis. AMPK and SIRT are two known regulators of PGC1α, and it has been reported previously that aging decreases the levels of both of these factors (27, 47). Our results here are in accordance with these findings, and we show that aging is associated with decreased levels of PGC-1α in skeletal muscle of rats, as well as its upstream regulators AMPK and SIRT1, which confirms earlier observations (27, 47). We also look at two downstream factors of the PGC1α master regulator that is NRF1 and TFAM (18). Although there was an age-associated downregulation of AMPK, pAMPK, SIRT1, and PGC-1α, it was not accompanied by the same trend in NRF1 and TFAM. On the contrary, the levels of NRF-1 and TFAM 1 actually increased with age, which is in line with previous observations that were also performed in human skeletal muscle. It is suggested that the upregulation of both NRF1 and TFAM results in the upregulation of mtDNA content (33). Indeed, the increase in mitochondrial DNA content (35) might be part of an adaptive response that will help the cell compensate for the age-associated loss of mitochondrial function. Our data suggest that the observed increases in both NRF1 and TFAM are independent of PGC-1a activation and reactive oxygen species, suggesting the involvement of other regulatory mechanisms.
To study mitochondrial biogenesis more directly, we also investigated the two main regulatory proteins of mitochondrial fusion and fission, Mfn1 and Fis1. The fission and fusion of mitochondria are not only protective against the accumulation of mitochondrial damage, but are a major part of the continuous reorganization of the mitochondrial network, essential in providing efficient structure for energy production and signaling (40). As observed with NRF1 and TFAM, Mfn1 and Fis1 also increased with age. This might be a compensatory mechanism toward the increasing levels of dysfunctional mitochondria, as it is known that fission and fusion can dilute out mitochondrial damage. Exercise training, however, seemed to block the age-associated increase in all of these factors. Since exercise training significantly decreased the gap between young and aged muscles in the levels of various mitochondrial markers, NRF1, TFAM, Mfn1 and Fis1, we propose that regular exercise could help delay many of the age-associated changes in muscle mitochondria.
PNPase is an exoribonuclease that is localized to the mitochondrial intermembrane space. PNPase plays a crucial role in mitochondrial RNA import (61); however, the upregulation of PNPase could induce inflammation by interferon-mediated degradation of specific mRNAs and small noncoding RNAs (51). Interestingly, NRF1 overexpression has also been associated with inflammation (59). Aging is associated with increased levels of inflammation (41), and there is limited data suggesting that PNPase could play a role in cellular senescence (32). The correlation between our NRF1 and PNPase data (r = 0.6115, P = 0.009) further support this suggestion. We found that exercise training partially prevented the induction of PNPase that occurred in old animals. The effects of regular exercise on PNPase levels may be even more pronounced, since exercise also decreased PNPase levels in young animals (P = 0.0798). Whether NRF1 and PNPase are actually working together to modulate age-associated inflammation needs further investigation.
The Lon protease is important for the quality control of mitochondrial proteins (4, 5, 37, 58), and it has been proposed that HSP78 may assist Lon in the degradation of damaged proteins (48). The levels of Lon decline with age, which could result in the accumulation of oxidized or dysfunctional proteins in the mitochondria (4, 5, 30), as well as result in the loss of mitochondrial function with age. We present novel data that suggest that regular exercise can prevent the age-related decline in Lon and, therefore, rejuvenate proper quality control of proteins in older cells (58). We also present the first study in which exercise training induced HSP78 levels in both young and old groups. The induction of both Lon and HSP78 indicates that regular exercise has beneficial effects on mitochondrial protein quality control. Although not directly studied in this paper, it is quite clear that Lon plays a major role in maintaining mitochondrial quality control (4, 5, 37). It has also been reported that Lon levels and activity decline with age in skeletal muscles (5, 58) and that the inducibility of Lon and its ability to cope with stress-induced protein damage, declines with senescence (37). Thus, our finding that the normal age-associated decline in Lon may be reversed by exercise training gives hope that exercise therapy may be able to help older individuals correct mitochondrial pathologies. Clearly, however, much more detailed and specific research will be needed to properly address this exciting possibility.
Taken together, our data suggest that regular exercise training stimulates mitochondrial biogenesis through the PGC1α system, a rejuvenation of the mitochondrial network via fission and fusion, and an improved quality control of mitochondrial proteins by the Lon protease. All of these properties, working in conjunction with one another, would improve the overall functionality of mitochondria in aged cells.
GRANTS
The present work was supported by Hungarian grants from Health Science Committee Grant 38388, Science and Technology Foundation JAP13/02, JSPS (L-10566), National Science and Research Foundation (K75702) awarded to Z. Radák. K. J. A. Davies and J. Ngo were supported by Grant RO1-ES003598 and by American Recovery and Reinvestment Act Supplement 3RO1-ES 003598-22S2, both from the National Institutes of Health/National Institute of Environmental Health Sciences to K. J A. Davies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.K., N.H., and Z.R. performed experiments; E.K., N.H., A.W.T., and Z.R. analyzed data; E.K., S.G., J.K.N., K.J.A.D., and Z.R. interpreted results of experiments; E.K. prepared figures; E.K., J.K.N., K.J.A.D., and Z.R. edited and revised manuscript; E.K., N.H., A.W.T., S.G., J.K.N., K.J.A.D., and Z.R. approved final version of manuscript; A.W.T. and Z.R. drafted manuscript; Z.R. conception and design of research.
REFERENCES
- 1. Aldred S, Rohalu M. A moderate intensity exercise program did not increase the oxidative stress in older adults. Arch Gerontol Geriatr 53: 350–353, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Benard G, Karbowski M. Mitochondrial fusion and division: Regulation and role in cell viability. Semin Cell Dev Biol 20: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bo H, Zhang Y, Ji LL. Redefining the role of mitochondria in exercise: a dynamic remodeling. Ann N Y Acad Sci 1201: 121–128, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol 4: 674–680, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bota DA, Van Remmen H, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett 532: 103–106, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med 44: 224–229, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep 11: 678–684, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen H, Chan DC. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18: R169–R176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen HW, Rainey RN, Balatoni CE, Dawson DW, Troke JJ, Wasiak S, Hong JS, McBride HM, Koehler CM, Teitell MA, French SW. Mammalian polynucleotide phosphorylase is an intermembrane space RNase that maintains mitochondrial homeostasis. Mol Cell Biol 26: 8475–8487, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Close GL, Kayani AC, Ashton T, McArdle A, Jackson MJ. Release of superoxide from skeletal muscle of adult and old mice: an experimental test of the reductive hotspot hypothesis. Aging Cell 6: 189–195, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Davies KJ, Hochstein P. Ubisemiquinone radicals in liver: implications for a mitochondrial Q cycle in vivo. Biochem Biophys Res Commun 107: 1292–1299, 1982 [DOI] [PubMed] [Google Scholar]
- 12. Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol Endocrinol Metab 242: E418–E427, 1982 [DOI] [PubMed] [Google Scholar]
- 13. Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107: 1198–1205, 1982 [DOI] [PubMed] [Google Scholar]
- 14. Ding H, Jiang N, Liu H, Liu X, Liu D, Zhao F, Wen L, Liu S, Ji LL, Zhang Y. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta 1800: 250–256, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Fan X, Hussien R, Brooks GA. H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med 49: 1646–1654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD. Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J Appl Physiol 88: 1192–1198, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Germaniuk A, Liberek K, Marszalek J. A bichaperone (Hsp70-Hsp78) system restores mitochondrial DNA synthesis following thermal inactivation of Mip1p polymerase. J Biol Chem 277: 27801–27808, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25: 1354–1366, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44: 126–131, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Gomez-Cabrera MC, Vina J, Ji LL. Interplay of oxidants and antioxidants during exercise: implications for muscle health. Phys Sportsmed 37: 116–123, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Hayakawa H, Sekiguchi M. Human polynucleotide phosphorylase protein in response to oxidative stress. Biochemistry 45: 6749–6755, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967 [PubMed] [Google Scholar]
- 23. Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol 59 Suppl 7: 5–18, 2008 [PubMed] [Google Scholar]
- 24. Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol 209: 2265–2275, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Ikeda S, Kawamoto H, Kasaoka K, Hitomi Y, Kizaki T, Sankai Y, Ohno H, Haga S, Takemasa T. Muscle type-specific response of PGC-1 alpha and oxidative enzymes during voluntary wheel running in mouse skeletal muscle. Acta Physiol (Oxf) 188: 217–223, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Kalyanaraman B, Darley-Usmar V, Davies KJA, Dennery P, Forman HJ, Grisham M, Mann G, Moor Roberts JLJI, Ischiropoulos H. The use of fluorescence probes to measure reactive oxygen and nitrogen species in cell: challenges, potentials and caveats. Free Radic Biol Med 52: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev 131: 21–28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflügers Arch 459: 277–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic Biol Med 25: 1089–1097, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science 285: 1390–1393, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem 282: 22977–22983, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Leszczyniecka M, Kang DC, Sarkar D, Su ZZ, Holmes M, Valerie K, Fisher PB. Identification and cloning of human polynucleotide phosphorylase, hPNPase old-35, in the context of terminal differentiation and cellular senescence. Proc Natl Acad Sci USA 99: 16636–16641, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lezza AM, Pesce V, Cormio A, Fracasso F, Vecchiet J, Felzani G, Cantatore P, Gadaleta MN. Increased expression of mitochondrial transcription factor A and nuclear respiratory factor-1 in skeletal muscle from aged human subjects. FEBS Lett 501: 74–78, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Luciakova K, Sokolikova B, Chloupkova M, Nelson BD. Enhanced mitochondrial biogenesis is associated with increased expression of the mitochondrial ATP-dependent Lon protease. FEBS Lett 444: 186–188, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61: 534–540, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280: 16456–16460, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic Biol Med 46: 1042–1048, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic Biol Med 46: 1042–1048, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olesen J, Kiilerich K, Pilegaard H. PGC-1α-mediated adaptations in skeletal muscle. Pflügers Arch 460: 153–162, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem 149: 241–251, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 298: R1485–R1495, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev 7: 34–42, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Radak Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, Goto S. Age-associated increase in oxidative stress and nuclear factor κB activation are attenuated in rat liver by regular exercise. FASEB J 18: 749–750, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Radak Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, Goto S. Age-associated increase in oxidative stress and nuclear factor κB activation are attenuated in rat liver by regular exercise. FASEB J 18: 749–750, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflügers Arch 445: 273–278, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Radak Z, Sasvari M, Nyakas C, Pucsok J, Nakamoto H, Goto S. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch Biochem Biophys 376: 248–251, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5: 151–156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rottgers K, Zufall N, Guiard B, Voos W. The ClpB homolog Hsp78 is required for the efficient degradation of proteins in the mitochondrial matrix. J Biol Chem 277: 45829–45837, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1α content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem 86: 10605–10617, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Salo DC, Donovan CM, Davies KJ. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med 11: 239–246, 1991 [DOI] [PubMed] [Google Scholar]
- 51. Sarkar D, Fisher PB. Human polynucleotide phosphorylase (hPNPase old-35): an RNA degradation enzyme with pleiotrophic biological effects. Cell Cycle 5: 1080–1084, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Sarkar D, Park ES, Emdad L, Randolph A, Valerie K, Fisher PB. Defining the domains of human polynucleotide phosphorylase (hPNPaseOLD-35) mediating cellular senescence. Mol Cell Biol 25: 7333–7343, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLos One 5: e13307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sheperd D, Garland S. Citrate synthase from rat liver. In: Methods in Enzymology, edited by Lowenstein JM. San Diego, CA: Academic Press, 1969, p. 11–16 [Google Scholar]
- 55. Spirduso WW, Farrar RP. Effects of aerobic training on reactive capacity: an animal model. J Gerontol 36: 654–662, 1981 [DOI] [PubMed] [Google Scholar]
- 56. Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun 296: 350–354, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta 1777: 1092–1097, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ugarte N, Petropoulos I, Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal 13: 539–549, 2010 [DOI] [PubMed] [Google Scholar]
- 59. van Tienen FH, Lindsey PJ, van der Kallen CJ, Smeets HJ. Prolonged Nrf1 overexpression triggers adipocyte inflammation and insulin resistance. J Cell Biochem 111: 1575–1585, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Vina J, Gomez-Cabrera MC, Borras C, Froio T, Sanchis-Gomar F, Martinez-Bello VE, Pallardo FV. Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliv Rev 61: 1369–1374, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Wang G, Chen HW, Oktay Y, Zhang J, Allen EL, Smith GM, Fan KC, Hong JS, French SW, McCaffery JM, Lightowlers RN, Morse HC, 3rd, Koehler CM, Teitell MA. PNPASE regulates RNA import into mitochondria. Cell 142: 456–467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, Kaasik A. PGC-1α and PGC-1β regulate mitochondrial density in neurons. J Biol Chem 284: 21379–21385, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]