Abstract
Evidence in the literature supports the hypothesis that the T1R2+3 heterodimer binds to compounds that humans describe as sweet. Here, we assessed the necessity of the T1R2 and T1R3 subunits in the maintenance of normal taste sensitivity to carbohydrate stimuli. We trained and tested water-restricted T1R2 knockout (KO), T1R3 KO and their wild-type (WT) same-sex littermate controls in a two-response operant procedure to sample a fluid and differentially respond on the basis of whether the stimulus was water or a tastant. Correct responses were reinforced with water and incorrect responses were punished with a time-out. Testing was conducted with a modified descending method of limits procedure across daily 25-min sessions. Both KO groups displayed severely impaired performance and markedly decreased sensitivity when required to discriminate water from sucrose, glucose, or maltose. In contrast, when Polycose was tested, KO mice had normal EC50 values for their psychometric functions, with some slight, but significant, impairment in performance. Sensitivity to NaCl did not differ between these mice and their WT controls. Our findings support the view that the T1R2+3 heterodimer is the principal receptor that mediates taste detection of natural sweeteners, but not of all carbohydrate stimuli. The combined presence of T1R2 and T1R3 appears unnecessary for the maintenance of relatively normal sensitivity to Polycose, at least in this task. Some detectability of sugars at high concentrations might be mediated by the putative polysaccharide taste receptor, the remaining T1R subunit forming either a homodimer or heteromer with another protein(s), or nontaste orosensory cues.
Keywords: Tas1r2, Tas1r3, sweet taste, polysaccharide taste, gustatory system
the evidence supporting the necessity of the T1R2 and T1R3 proteins in the mediation of sweet taste is compelling. Intracellular calcium imaging responses in HEK-293-derived cells transfected with rat, mouse, or human T1R2 and T1R3, suggest that the subunits combine to form a heterodimer broadly tuned to bind with a range of compounds that humans describe as sweet (33, 39, 69). Consistent with the view that the T1R2 and T1R3 proteins function in heterodimeric form, there is a high degree of coexpression of T1R3 with T1R2 (or T1R1) in taste receptor cells (30, 34–35, 39). With the caveat of potential developmental compensation, knockout (KO) mice, in which specific T1R genes have been genetically ablated, have provided an effective strategy to test the functional necessity of these receptor proteins. Electrophysiological recordings from the chorda tympani nerve (CT), which innervates the anterior tongue, and the glossopharyngeal nerve (GL), which innervates the posterior tongue, show that T1R2 KO and T1R3 KO mice are unresponsive to artificial sweeteners (8, 71), and although the GL of T1R3 KO mice responds to acesulfame potassium (AceK), this is most likely attributable to the contribution of the potassium cation. The CT and GL of T1R2 and T1R3 KO mice also show markedly attenuated responses to natural sugars compared with WT mice but display weak residual responses, particularly at the higher concentrations (8, 46, 71–72).
In most cases, the electrophysiological measures correspond with behavioral findings. In 24-h two-bottle intake tests in which a taste solution and water are presented and relative intake is measured, T1R3 KO mice do not show a preference for artificial sweeteners over water, whereas WT mice do (8). In brief-access taste tests, T1R2 KO and T1R3 KO mice are completely unresponsive to artificial sweeteners in contrast to the concentration-dependent responses of WT mice (63, 65, 71). Like the electrophysiological findings, both T1R2 and T1R3 KO mice show severely blunted unconditioned licking to sucrose, glucose and maltose but display some residual responsiveness at the higher concentrations (71).
Regarding the possibility that some degree of responsiveness to sugars may be maintained in the absence of T1R2 or T1R3, there are other examples in the literature in which T1R2 KO and T1R3 KO mice display partial or even normal behavioral responses to sugars. In 24-h two-bottle intake tests T1R3 KO mice show some degree of preference at higher concentrations of sucrose and display relatively normal preference for glucose (8). However, the implications of outcomes from long-term intake tests regarding gustatory function must be viewed with caution because these tests are known to be influenced by postingestive factors (e.g., 37, 53, 67). Furthermore, with repeated exposure to caloric compounds, animals can associate motivationally neutral orosensory cues with positive postingestive feedback, resulting in a flavor preference (e.g., 1, 15, 51, 53). Indeed, compared with naive KO mice, those with previous test experience with the sugar show a preference at lower concentrations of sucrose (71–72), suggesting the involvement of learning.
The brief-access taste test circumvents some of these interpretive limitations. This procedure involves the measurement of unconditioned licking responses across individual presentations of more than one stimulus in brief (several seconds) trials across a single session, thus minimizing the ability of the animal to associate postingestive consequences with a given concentration during a trial (e.g., 6, 9, 21, 57, 59, 70). Nevertheless, even responses in a brief-access taste test, under certain conditions, can be influenced by postingestive cues after repeated exposure (63, 71). For example, we previously reported that although T1R2 and T1R3 KO mice showed flat concentration-response functions to some sugars when assessed on the first session of a brief-access test, in subsequent sessions of testing they displayed concentration-dependent licking. This is in contrast to what was observed for nonnutritive sodium saccharin for which responding by the KO mice remained low regardless of test experience (63, 65).
Although learned associations between a remaining orosensory cue and the positive postingestive consequences of sugar ingestion can potentially explain the eventual emergence of preferences for natural sweeteners by T1R2 and T1R3 KO mice, the fact that T1R3 KO mice display absolutely normal detection thresholds as assessed psychophysically in a shock-avoidance task is much more difficult to reconcile with the existing literature (10). In light of the persuasive evidence supporting the role of T1R2 and T1R3 in mediating sucrose taste, one might predict that T1R3 KO mice would have at least elevated detection thresholds for sucrose. It is surprising that the deletion of the gene would not result in large effects on sensitivity, considering detection thresholds to sucrose and glucose have been shown to be dependent on T1R3 allele status (17).
There is also growing evidence in the literature supporting the hypothesis that the T1R2+3 heterodimer does not play a large role in signaling all behaviorally salient carbohydrate taste stimuli. This evidence comes from findings indicating that, with some minor differences from WT mice, T1R2 and T1R3 KO mice showed relatively normal motivated behavior in both short-term two-bottle tests and brief-access tests with Polycose, a mixture of glucose polymers, that appeared early in testing and so could not easily be explained by postingestive-based learning (63, 65, 72). These very same animals, nonetheless, displayed severely blunted responses to sugar stimuli. Moreover, mice missing both T1R2 and T1R3 have been shown to generate completely flat concentration-response functions to sugar stimuli, but nonetheless display concentration-dependent responsiveness to Polycose in a brief-access test (65). Finally, CT responses to Polycose are very similar between T1R3 KO and WT mice (72). Indeed, there are more than two decades of research suggesting that rodents respond to Polycose in a manner that is distinguishable from other sweeteners (14, 18, 20, 25–26, 38, 42–45, 47, 49, 58, 66). Collectively, these findings suggest that maltooligosaccharides may activate a taste receptor different from those to which sugars bind (50).
Here, in an attempt to further psychophysically assess the necessity of T1R2 and T1R3 in the maintenance of normal taste sensitivity to carbohydrate stimuli, we used an operant-conditioning procedure in which taste serves as a signal for other reinforcing events. The procedure involves the delivery of small volumes of a given taste stimulus and the measurement of immediate responses, thus increasing confidence that the behavior is under orosensory control. This task does not rely on the hedonic characteristics of the stimulus to drive responding, and thus represents a pure measure of taste signal sensitivity independent of affect. Similar paradigms have been shown to be exquisitely sensitive in revealing the effects of a variety of manipulations of the gustatory system (4–5, 16–17, 32, 62, 64). Because there is no evidence that T1R2 or T1R3 deletion affects responsiveness to NaCl, this salt was chosen as the stimulus for training the animals in the task and also as an internal control. Sucrose was chosen as a test stimulus because it is the prototypical natural sweetener and also because of the vexing evidence in the literature demonstrating that T1R3 KO mice have normal sucrose detection thresholds (10). Of the polysaccharides, Polycose has been the most studied in taste experiments and thus was also chosen as a test stimulus to address the question of whether maltooligosaccharides may bind to receptors other than the T1R2+3 heterodimer. Polycose contains glucose polymers of varying chain lengths, including a small amount of glucose and maltose (29). It was previously shown that although T1R2 KO and T1R3 KO mice displayed relatively flat concentration-response functions to glucose and maltose on the first session of a brief-access test, by the third session, the mice displayed concentration-dependent licking responses to maltose but not glucose (65); accordingly, these compounds were also chosen as test stimuli as a comparison for Polycose.
MATERIALS AND METHODS
Subjects.
Male and female breeding pairs of mice that were homozygous null for the Tas1r2 or Tas1r3 gene (initially derived from 129X1/SvJ and backcrossed with C57BL/6 mice for at least three generations) were provided by Dr. Charles Zuker (University of California, San Diego; now at Columbia University). Wild-type C57BL/6J (B6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Wild-type mice and homozygous null mice were bred to generate mice that were heterozygous for Tas1r2 or Tas1r3. Mice heterozygous (−/+) for Tas1r2 or Tas1r3 were paired to generate mice heterozygous (−/+), homozygous null (−/−), and wild-type (+/+). From these mice, only homozygous null (T1R2 KO, n = 10; T1R3 KO, n = 10) and wild-type (T1R2 WT, n = 10; T1R3 WT, n = 10) same-sex littermate controls (T1R2: 5 female, 5 male pairs; T1R3: 3 female, 7 male pairs) were used as subjects in the behavioral tests.
The mice were housed in polycarbonate shoebox cages in a room with automatically controlled temperature, humidity and a 12:12-h light-dark cycle. Except where noted, mice were provided ad libitum chow (Purina Laboratory Chow 5001, St. Louis, MO) and deionized reverse-osmosis water. Mice were single-housed and at least 7 wk of age at the start of behavioral training. During training and testing, mice were put on a restricted water-access schedule for which water was available only during the daily test sessions. Water bottles were removed on Sunday, no more than 23 h before testing. Water bottles were returned to the home cages of the mice after the last session on Friday. Food was freely available in the home cage at all times. Mice that fell below 85% of their free-drinking body weight during the water-restriction schedule received 1 ml supplemental water 1 h after the end of the testing session. All procedures were approved by the Florida State University Animal Care and Use Committee.
Taste stimuli.
All solutions were prepared daily with deionized reverse-osmosis water and presented at room temperature. Test stimuli consisted of NaCl (0.00075, 0.003, 0.006, 0.01, 0.02, 0.04, 0.07, 0.15, 0.3, and 0.6 M; Mallinckrodt Chemicals, Phillipsburg NJ), sucrose (0.001, 0.008, 0.015, 0.03, 0.06, 0.13, 0.25, 0.5, and 1.0 M; BDH Chemicals, West Chester, PA), Polycose (1.0, 2.0, 4.0, 8.0, 10.0, 12.0, 16.0, 19.0, 25.0, and 32.0%; Ross Laboratories, Columbus, OH), maltose (0.001, 0.0075, 0.015, 0.03, 0.06, 0.125, 0.25, 0.5, and 1.0 M; Sigma-Aldrich, St. Louis, MO; Alfa Aesar, Heysham, Lancashire, UK; EMD Chemicals, Gibbstown, NJ), and glucose (0.015, 0.03, 0.06, 0.125, 0.25, 0.5, 1.0, and 2.0 M; BDH Chemicals). On the basis of data from various behavioral studies, the concentrations used to begin training and testing were 0.6 M for NaCl (11, 12, 16), 1.0 M for sucrose and maltose (8, 11, 12, 71), 2.0 M for glucose (65), and 32% for Polycose (63, 72). The concentrations selected were purposely very high so as to favor acquisition of the task and accommodate mice that could have difficulty detecting the compounds depending on the stimulus and genotype.
Apparatus.
Mice were trained and tested in a new specially designed computer-controlled testing apparatus referred to as a gustometer. The testing cage is enclosed in a sound-attenuating chamber in which a broadband masking noise serves to minimize extraneous auditory cues. The gustometer is designed to deliver the stimulus via a centrally positioned borosilicate glass ball (0.95 cm diameter) that rotates around a horizontal axis behind a slot (1.97 cm high and 0.45 cm wide) in the side of the test chamber wall. An adjustable shelf runs across the testing chamber wall, 2 cm above the floor to support the animal's front paws when it is licking. The stimulus solution is delivered to the glass sample ball via polytetrafluoroethylene (PTFE) (Teflon) tubes (ID 0.13 cm, OD 0.19 cm) connected to syringes. The initial lick causes ∼10 μl of fluid to be delivered to coat the ball. The specially machined plungers of the syringes are driven by individual stepping motors. After the stimulus delivery (sample phase), the sample ball is retracted into a ball washer and is rinsed with water, which is evacuated into a collection reservoir and dried with pressurized air. The animals were also trained to lick the two reinforcement balls (1-cm diameter, polyoxymethylene) positioned behind slots located on either side of the central slot. Water is delivered to these reinforcement balls via PTFE tubing delivery lines to infusion pumps. Each reinforcement ball has a vertically positioned hole drilled all the way through it. The tubing is connected to a stainless-steel tube that is threaded from the top of the ball and stops short of the bottom, so that the mouse has access to the fluid by licking the hole at the bottom of the ball. The apparatus uses force transducers to record licks that preclude the need to pass current through the animal. The new design also permits fine control of lick volume and stimulus delivery timing by the use of infusion pumps allowing the delivery of smaller volumes per lick than had been used in our prior gustometer design (e.g., 12, 16, 60, 64). In this study, the volume was decreased to 1 μl/lick (except where noted), which more closely resembles unconditioned licking from sipper tubes in B6 mice (13).
Trial structure.
Water-restricted mice were trained and tested in daily 25-min sessions. The mouse was required to lick the sample ball two times within 250 ms to initiate a trial. This ensured the mouse was engaged in active licking. The trial began with the “sample phase,” during which the fluid stimulus was presented via the centrally positioned sample ball for 2 s or up to 5 licks (whichever came first). The sample ball was then retracted away from the reach of the mouse and cleaned. During the “decision phase,” the mouse had 10 s (limited hold) to lick one of the two reinforcement balls. As soon as contact was made with one of the two reinforcement balls, the “reinforcement phase” began. If a correct response (see below) was made, the mouse received up to 15 licks or 4-s access to the water reinforcer, whichever came first. If an incorrect response or no response was made during the allocated time, the mouse received a 30-s time-out, during which no fluid was presented. The intertrial interval lasted ∼6 s.
Training.
“Spout training” consisted of training the mice to lick the different fluid delivery balls in the gustometer by presenting the mice with access to only one ball for 25 min each day. Water was delivered on all 6 days of this training phase and was available ad libitum throughout the session.
“Side training” involved training the mice to associate one reinforcement ball with 0.6 M NaCl and the other with water. In each session, only one stimulus was presented, and only the reinforcement ball associated with that stimulus was available. The stimulus presented and the reinforcement spout available were alternated each session.
“Alternation” involved presenting 0.6 M NaCl or water repeatedly until the correct response was made a predetermined number of times. The other stimulus was then presented until an equivalent number of correct responses was made. Alternation training continued over six sessions with the criterion number of correct responses decreasing every second day. During the first two sessions, the stimulus alternated after four correct responses, during the third and fourth sessions after two correct responses, and during the fifth and sixth sessions after one correct response. The reinforcement period remained the same. A time-out was introduced, and the limited hold was decreased. Because of some apparatus problems, some adjustments were made, and animals were trained in “Alternation” and “Discrimination” phases twice, as described in Table 1.
Table 1.
Training and testing schedule
| Sessions | Phase | Timeout, s | Limited Hold, sa | Stimuli | Schedule |
|---|---|---|---|---|---|
| 6 | Spout training | None | None | water | Constant |
| 6 | Side training | None | 180 | 0.6 M NaCl or water | Constant |
| 6 | Alternationb | 10, 20, 30c | 15 | 0.6 M NaCl or water | Alternated after x correct responses |
| 31 | Discrimination training | 30 | 15 | 0.6 M NaCl or water | Semirandomd |
| 25 | Alternatione | 10, 20, 30 | 15 | 0.6 M NaCl or water | Alternated after x correct responses |
| 10 | Discrimination training | 30 | 15 | 0.6 M NaCl or water | Semirandom |
| 28 | Detection testing | 30 | 10 | NaCl or water | Semirandom |
| 40-42f | Discrimination training | 30 | 10 | 1.0 M sucrose or water | Semirandom |
| 23 | Detection testing | 30 | 10 | sucrose or water | Semirandom |
| 8 | Stimulus control | 30 | 10 | 0.6 M NaCl or water | Semirandom |
| 23 | Discrimination training | 30 | 10 | 32% Polycose or water | Semirandom |
| 25 | Detection testing | 30 | 10 | Polycose or water | Semirandom |
| 10 | Detection testing IIg | 30 | 10 | Polycose or water | Semirandom |
| 10 | Stimulus control | 30 | 10 | 0.6 M NaCl or water | Semirandom |
| 34h | Discrimination training | 30 | 10 | 1.0 M maltose or water | Semirandom |
| 23 | Detection testing | 30 | 10 | maltose or water | Semirandom |
| 10 | Stimulus control | 30 | 10 | 0.6 M NaCl or water | Semirandom |
| 10 | Discrimination training | 30 | 10 | 2.0 M glucose or water | Semirandom |
| 20 | Detection testing | 30 | 10 | glucose or water | Semirandom |
| 9 | Stimulus control | 30 | 10 | 0.6 M NaCl or water | Semirandom |
| 10 | Discrimination training | 30 | 10 | 1.0 M sucrose or water | Semirandom |
| 20 | Detection testing | 30 | 10 | sucrose or water | Semirandom |
Limited hold is defined as the amount of time the animal had to respond on a reinforcement ball after the sample phase.
A stimulus was presented repeatedly until a predetermined number of correct responses was made. It was not necessary that the correct responses be successive.
On the first 2 days of alternation training, the timeout was 10 s, which was increased to 20 s for days 3 and 4 and increased again to 30 s for days 5 and 6.
Three reservoirs of water and three reservoirs of a taste solution were presented in randomized blocks.
Until this phase, the sample solution delivered to the sample ball following the animal's trial initiation was insufficient to coat the ball. Priming volume was increased. Also, from alternation day 7, volume per lick was increased from ∼1 μl to 1.6 μl and then returned to ∼1 μl from discrimination day 3 onward.
Because of apparatus problems, some mice developed side biases, and thus, they were reintroduced to “side-training” for 1 or 2 sessions.
Lick volume was increased from ∼1 to ∼1.6 μl, sample licks from 5 to 7 licks, reinforcement licks from 15 to 20 licks during this phase.
Maltose powder was out of stock, so animals were given ad libitum food and water for 2 wk and not trained between sessions 10 and 11 of maltose discrimination training.
Discrimination training involved the use of three reservoirs filled with 0.6 M NaCl and three reservoirs filled with water, from which the compounds were dispensed in randomized blocks. The probability of receiving 0.6 M NaCl as the sample solution was 0.5. The limited hold was systematically decreased. Animals that performed significantly above chance (determined by the normal approximation of the binomial distribution, one-tailed test) across the last two discrimination training sessions for a given stimulus progressed to concentration-response testing. This criterion was chosen as sufficiently high to attempt to establish a psychophysical function without disrupting stimulus control. Mice that did not reach this criterion were reintroduced to NaCl vs. water control sessions in an attempt to maintain stimulus control.
Detection threshold testing.
During each NaCl session, one concentration of NaCl was tested against water. Testing was conducted with a modified descending method of limits procedure across sessions, as previously used (5). The NaCl concentration was systematically decreased with a single concentration presented on Tuesday and Thursday sessions. Stimulus control sessions were interspersed on Mondays, Wednesdays, and Fridays, during which the concentration presented to each mouse was based on its individual performance in prior sessions. The lowest concentration at which an individual mouse performed consistently at 80% was presented so that stimulus control could be maintained. If the animal performed lower than 80% but had reached the criteria for concentration series testing, the highest concentration was presented for the stimulus control sessions. The testing phase for each compound was complete when performance of every mouse dropped to ∼50% correct. In this fashion, the procedure was custom-tailored to the specific sensitivity of a given subject.
When testing for NaCl was complete, NaCl was replaced with another stimulus. EC50 values (operationally defined as the detection threshold) for an array of stimuli were obtained (Table 1). Stimulus phases were interspersed with reintroduction of NaCl tests to serve as a baseline reference and to maintain stimulus control.
Control tests.
After testing was completed, a water control test session was conducted in which all reservoirs were filled with water with half arbitrarily assigned to the “water” reinforcement ball and the other half to the “tastant” reinforcement ball. This test was performed to exclude the possibility that extraneous cues contributed to responses during threshold testing.
After the water control test, water bottles were returned to the animals for 2 days for rehydration. A 48-h two-bottle intake test was then conducted in the home cages of the mice. One bottle was filled with 10 mM Na-saccharin and the other with water. Intake was measured and the position of the bottles was swapped after 24 h. The two-bottle preference test was conducted to serve as a functional confirmation of the genotypes.
Data analysis.
For a particular compound, trials with a response were collapsed across sessions for each concentration, and a percentage-correct measure was calculated. Performance was compared across the genotypes using a two-way ANOVA with repeated measures (genotype × concentration) for all animals that performed significantly above chance (determined by the normal approximation of the binomial distribution, one-tail test) on the last two discrimination training sessions for a given stimulus. Analysis of data from these animals is referred to as the concentration-response analysis.
Animals that performed at 80% or better to the highest concentration of a given stimulus were included in two additional analyses collectively referred to as threshold testing. A separate two-way ANOVA was conducted for this subset of mice. In addition, nonlinear regression was used to fit the following logistic equation to the data representing performance as a function of concentration:
where a is the maximum asymptote of performance, b is the slope, c is the log10 stimulus concentration corresponding to one-half of the maximum asymptote of performance (i.e., EC50) and x is the stimulus concentration in log10 units. Curves were fit to the performance scores for each mouse. The detection threshold was operationally defined as the EC50 because this value can effectively represent lateral shifts in the psychometric function. The parameters of the logistic functions were then compared across genotypes using t-tests.
For the water control test session, the normal approximation of the binomial distribution (one-tailed test) was used to determine any positive deviation of performance from chance.
For the two-bottle preference test, a preference ratio score was calculated using the following equation:
where a preference ratio score of 0.5 indicates equal intake of the Na-saccharin solution and water, and a score of 1.0 indicates exclusive intake of the Na-saccharin solution. A value of P ≤ 0.05 was considered significant in all statistical tests.
Five animals (1 from T1R2 KO, 1 from T1R2 WT, and 3 from T1R3 KO) out of the 40 mice did not learn the discrimination task. These animals and their littermate controls were removed from the entire experiment. Throughout the duration of the experiment, seven mice died (2 T1R2 KO, 1 T1R2 WT, 1 T1R3 KO, and 3 T1R3 WT). When these animals died, the littermate controls were also removed from the experiment from the beginning of that particular testing phase.
Genotyping.
PCR analysis was conducted to confirm the genotypes. Tail samples were obtained, DNA was extracted, and PCR was performed in a final reaction mixture volume of 12.5 μl, including 1 μl of the isolated DNA. The primers used for T1R2 were 5′-TTG GAG GAG GGG GCA GTG GGA GTG-3′, 5′-ATA ATC CTC TCC TGC CAC CCT AAC-3′, and 5′-CTG CCC CAA AGG CCT ACC CGC TTC-3′. PCR conditions were as follows: a preheating step for 30 s at 95°C followed by 42 cycles of 30 s at 95°C, 15 min at 60°C, 1 min at 72°C, and an autoextension step of 10 min at 72°C. For T1R3, the primers used were 5′-CCC CAC ACA CCC ATC TAT TGT TAG-3′ and 5′-GAC TTG AAT GCT TCT GCC CCC TAG-3′. PCR conditions were as follows: a preheating step for 30 s at 95°C followed by 40 cycles of 30 s at 95°C, 15 min at 50°C, and 2.5 min at 72°C and an autoextension step of 10 min at 72°C. The PCR products were separated using electrophoresis on a 1% agarose gel.
RESULTS
NaCl.
All animals that reached the criterion for the concentration-response analysis (i.e., performed significantly above chance on the last two discrimination training sessions for NaCl) also reached the criterion for threshold analysis (i.e., performed at 80% or better at the highest NaCl concentration tested). A two-way ANOVA (genotype × concentration) comparing performance across NaCl concentrations of KO groups with their WT controls did not reveal a main effect of genotype, but did reveal a main effect of concentration with no significant interaction indicated (Tables 2 and 3). The KO and WT groups did not significantly differ in their sensitivity to NaCl (Fig. 1). Two-sample t-tests revealed that the curve parameter representing EC50 (c-parameter, operationally defined as threshold) for NaCl did not significantly differ between the T1R2 KO and T1R2 WT mice, nor between the T1R3 KO and T1R3 WT mice. The T1R2 KO and T1R3 KO mice did not significantly differ from their respective WT groups in regard to asymptotic performance (a-parameter), or slope (b-parameter) of the NaCl detectability function (Tables 4 and 5). The individual animal curves reasonably fit the performance data as reflected by the mean R2 value of 0.90 ± 0.01 across animals.
Table 2.
Concentration-response analysis with ANOVA values comparing all animals that learned the discrimination task
| Stimulus and Group | Genotype | Concentration | Genotype × Concentration |
|---|---|---|---|
| NaCl | |||
| T1R2 | F (1,16) = 1.214, P = 0.287 | F (9,144) = 82.842, P < 0.001 | F (9,144) = 1.026, P = 0.423 |
| T1R3 | F (1,12) = 0.162, P = 0.695 | F (9,108) = 48.000, P < 0.001 | F (9,108) = 0.417, P = 0.924 |
| Polycose 1 | |||
| T1R2 | F (1,14) = 2.500, P = 0.136 | F (9,126) = 51.554, P < 0.001 | F (9,126) = 2.613, P = 0.008 |
| T1R3 | F (1,12) = 5.499, P = 0.037 | F (9,108) = 39.944, P < 0.001 | F (9,108) = 3.469, P = 0.001 |
| Polycose 2 | |||
| T1R2 | F (1,14) = 0.095, P = 0.762 | F (6,84) = 84.084, P < 0.001 | F (6,84) = 1.235, P = 0.297 |
| T1R3 | F(1,12) = 0.535, P = 0.478 | F (6,72) = 2.283, P < 0.001 | F (6,72) = 2.283, P = 0.045 |
| Maltose | |||
| T1R2 | F (1,12) = 7.346, P = 0.019 | F (8,96) = 71.126, P < 0.001 | F (8,96) = 10.931, P < 0.001 |
| T1R3 | F (1,8) = 9.510, P = 0.015 | F (8,64) = 48.468, P < 0.001 | F (8,64) = 11.650, P < 0.001 |
| Glucose | |||
| T1R2 | F (1,11) = 29.190, P < 0.001 | F (7,77) = 55.068, P < 0.001 | F (7,77) = 7.611, P < 0.001 |
| T1R3 | |||
| Sucrose 2 | |||
| T1R2 | F (1,10) = 92.035, P < 0.001 | F (7,70) = 24.291, P < 0.001 | F (7,70) = 7.371, P < 0.001 |
| T1R3 |
Table 3.
Threshold analysis with ANOVA values comparing all animals that performed at least 80% to the highest concentration for a given stimulus
| Stimulus and Group | Genotype | Concentration | Genotype × Concentration |
|---|---|---|---|
| NaCl | |||
| T1R2 | F (1,16) = 1.214, P = 0.287 | F (9,144) = 82.842, P < 0.001 | F (9,144) = 1.026, P = 0.423 |
| T1R3 | F (1,12) = 0.162, P = 0.695 | F (9,108) = 48.000, P < 0.001 | F (9,108) = 0.417, P = 0.924 |
| Polycose 1 | |||
| T1R2 | F (1,10) =1.202, P = 0.299 | F (9,90) = 47.845, P < 0.001 | F (9,90) = 2.783, P = 0.006 |
| T1R3 | F (1,8) =1.649, P = 0.235 | F (9,72) = 38.107, P < 0.001 | F (9,72) = 2.869, P = 0.006 |
| Polycose 2 | |||
| T1R2 | F (1,13) = 0.433, P = 0.522 | F (6,78) = 77.867, P < 0.001 | F (6,78) = 1.423, P = 0.217 |
| T1R3 | F (1,12) = 0.535, P = 0.478 | F (6,72) = 2.283, P < 0.001 | F (6,72) = 2.283, P = 0.045 |
| Maltose | |||
| T1R2 | |||
| T1R3 | |||
| Glucose | |||
| T1R2 | F (1,7) = 22.819, P = 0.002 | F (7,49) = 45.586, P < 0.001 | F (7,49) = 3.213, P = 0.007 |
| T1R3 | |||
| Sucrose 2 | |||
| T1R2 | |||
| T1R3 |
Fig. 1.
A: mean (± SE) performance as a function of NaCl concentration for the T1R2 wild type (WT; top, ●) and T1R2 knockout (KO; top, ○), T1R3 WT (bottom, ●) and T1R3 KO (bottom, ○) groups. B: threshold distribution for individual female (○) and male (△) mice and the means (solid lines) and SE (dashed lines) for the T1R2 KO, T1R2 WT, T1R3 KO, and T1R3 WT genotype groups.
Table 4.
Curve parameter values for T1R2 animals included in threshold analysis
| Compound and Parameter | WT Mean | KO Mean | Genotype t-Test |
|---|---|---|---|
| NaCl | |||
| Asymptotic Performance (a) | 88.48 ± 1.40 | 92.20 ± 1.83 | t (16) = 1.617, P = 0.125 |
| Slope (b) | −4.86 ± 1.98 | −1.79 ± 0.41 | t (16) = 1.518, P = 0.148 |
| Log10 Threshold (c) | −2.03 ± 0.10 | −1.69 ± 0.17 | t (16) = 1.725, P = 0.104 |
| Sucrose | |||
| Asymptotic Performance (a) | 90.35 ± 1.84 | ||
| Slope (b) | −2.03 ± 0.50 | ||
| Log10 Threshold (c) | −1.21 ± 0.11 | ||
| Polycose 1 | |||
| Asymptotic Performance (a) | 92.42 ± 0.85 | 88.28 ± 3.29 | t (10) = −1.426, P = 0.184 |
| Slope (b) | −3.63 ± 0.46 | −29.44 ± 14.33 | t (10) = −2.172, P = 0.055 |
| Log10 Threshold (c) | 0.97 ± 0.04 | 1.03 ± 0.06 | t (10) = 0.917, P = 0.381 |
| Polycose 2 | |||
| Asymptotic Performance (a) | 92.49 ± 2.27 | 91.54 ± 2.88 | t (13) = −0.253, P = 0.804 |
| Slope (b) | −2.38 ± 0.23 | −3.72 ± 1.55 | t (13) = −0.800, P = 0.438 |
| Log10 Threshold (c) | 0.87 ± 0.09 | 0.95 ± 0.06 | t (13) = 0.765, P = 0.458 |
| Maltose | |||
| Asymptotic Performance (a) | 90.40 ± 1.88 | ||
| Slope (b) | −3.52 ± 0.20 | ||
| Log10 Threshold (c) | −0.83 ± 0.04 | ||
| Glucose | |||
| Asymptotic Performance (a) | 94.50 ± 1.41 | 93.88 ± 3.96 | t (7) = 0.188, P = 0.856 |
| Slope (b) | −2.70 ± 0.55 | −1.35 ± 0.31 | t (7) = 1.68, P = 0.150 |
| Log10 Threshold (c) | −0.72 ± 0.03 | −0.20 ± 0.10 | t (7) = 6.47, P < 0.001 |
| Sucrose 2 Asymptotic | |||
| Performance (a) | 91.51 ± 1.72 | ||
| Slope (b) | −2.05 ± 0.28 | ||
| Log10 Threshold (c) | −1.49 ± 0.07 |
Table 5.
Curve parameter values for T1R3 animals included in threshold analysis
| Compound and Parameter | WT Mean | KO Mean | Genotype t-Test |
|---|---|---|---|
| NaCl | |||
| Asymptotic Performance (a) | 91.23 ± 3.14 | 91.64 ± 2.57 | t (12) = 0.102, P = 0.920 |
| Slope (b) | −1.55 ± 0.33 | −2.23 ± 0.55 | t (12) = −1.071, P = 0.305 |
| Log10 Threshold (c) | −1.65 ± 0.21 | −1.83 ± 0.14 | t (12) = −0.705, P = 0.494 |
| Sucrose | |||
| Asymptotic Performance (a) | 87.30 ± 3.40 | ||
| Slope (b) | −13.91 ± 9.90 | ||
| Log10 Threshold (c) | −1.48 ± 0.21 | ||
| Polycose 1 | |||
| Asymptotic Performance (a) | 95.28 ± 2.20 | 87.29 ± 4.63 | t (8) = −1.745, P = 0.119 |
| Slope (b) | −5.92 ± 2.61 | −2.43 ± 0.32 | t (8) = 1.065, P = 0.318 |
| Log10 Threshold (c) | 0.99 ± 0.03 | 0.88 ± 0.09 | t (8) = −1.404, P = 0.198 |
| Polycose 2 | |||
| Asymptotic Performance (a) | 92.78 ± 2.01 | 95.63 ± 2.42 | t (12) = 0.904, P = 0.384 |
| Slope (b) | −4.29 ± 1.14 | −1.67 ± 0.47 | t (12) = 2.124, P = 0.055 |
| Log10 Threshold (c) | 0.78 ± 0.07 | 0.92 ± 0.14 | t (12) = 0.869, P = 0.402 |
| Maltose | |||
| Asymptotic Performance (a) | 92.82 ± 1.98 | 83.62 | |
| Slope (b) | −2.30 ± 0.31 | −5.25 | |
| Log10 Threshold (c) | −0.99 ± 0.05 | −0.73 | |
| Glucose | |||
| Asymptotic Performance (a) | 94.82 ± 1.88 | ||
| Slope (b) | −33.93 ± 28.65 | ||
| Log10 Threshold (c) | −0.67 ± 0.16 | ||
| Sucrose 2 | |||
| Asymptotic Performance (a) | 93.08 ± 2.54 | ||
| Slope (b) | −2.98 ± 0.41 | ||
| Log10 Threshold (c) | −1.48 ± 0.08 |
Sucrose 1.
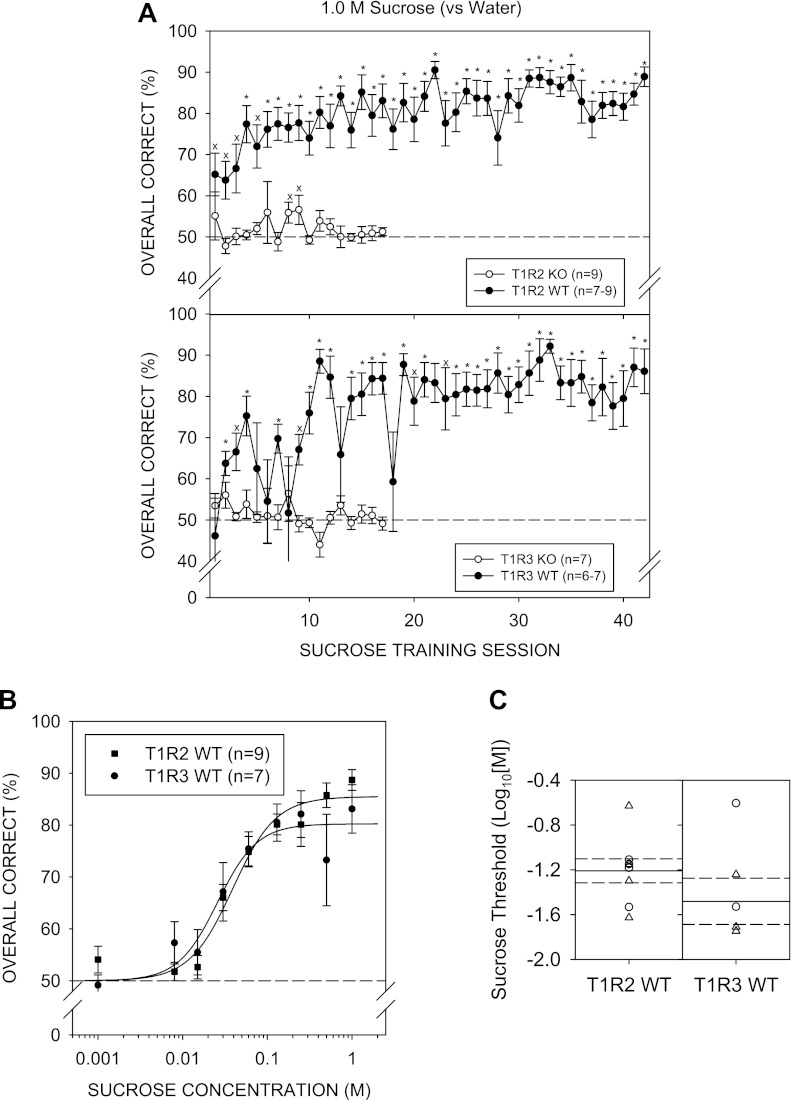
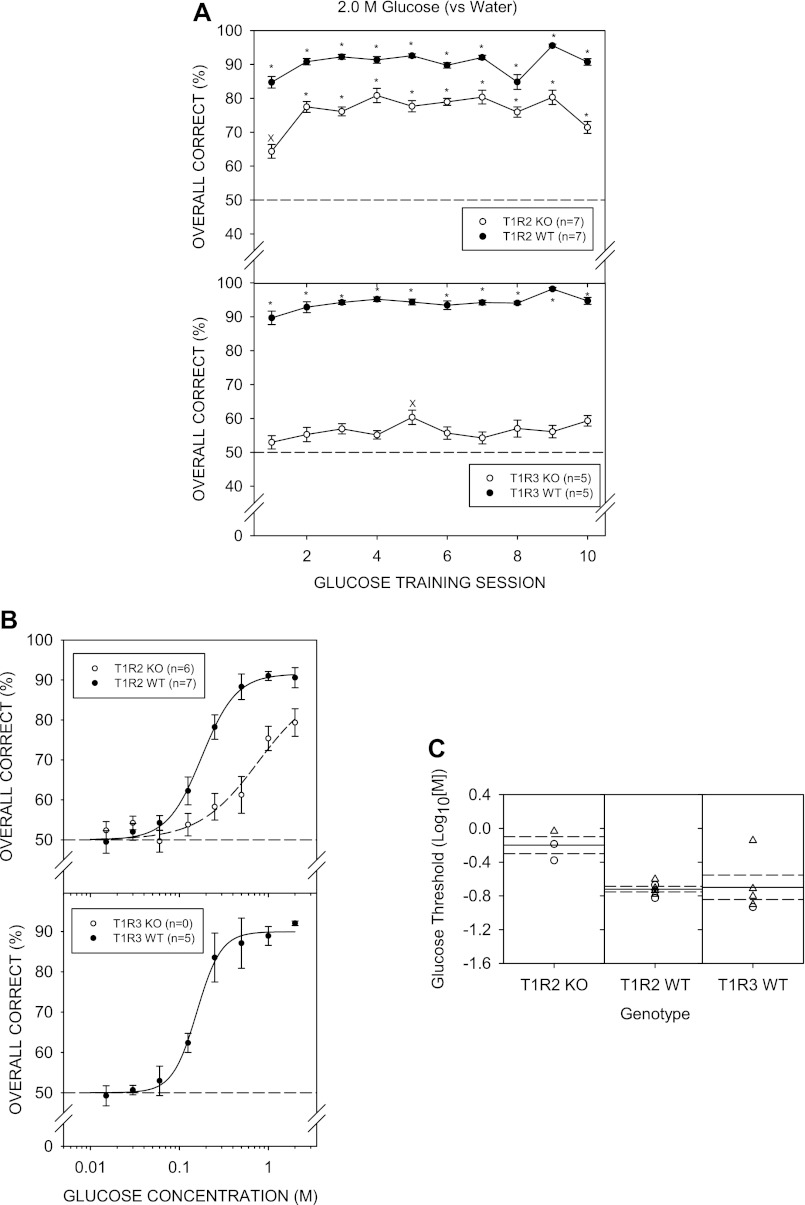
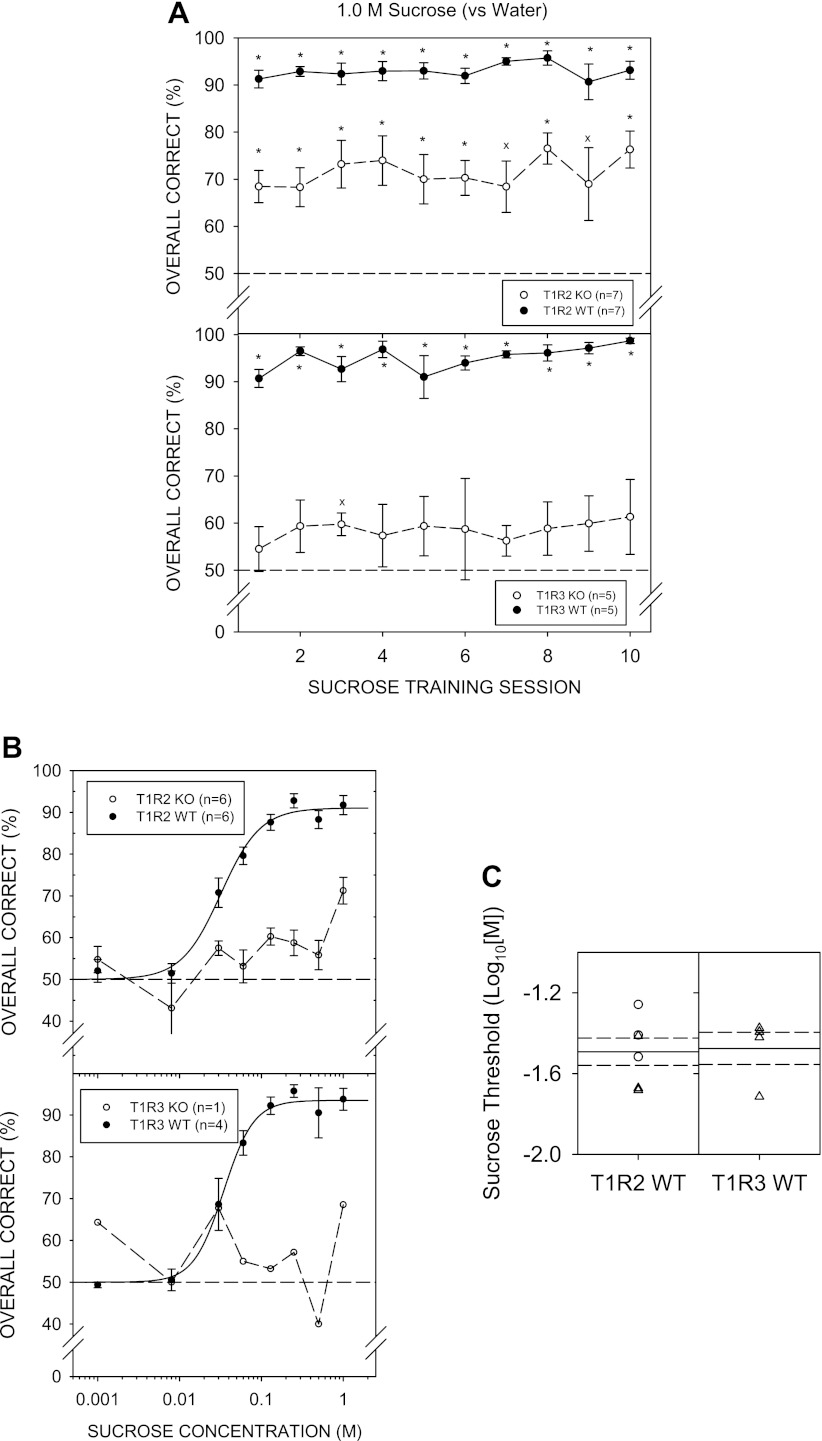
By day 4 of 1.0 M sucrose vs. water discrimination training, both T1R2 WT and T1R3 WT mice showed overall performance significantly higher than 50% [T1R2 WT: t (8) = 6.049, P = 0.003; T1R3 WT t (6) = 5.243, P = 0.020, with Bonferroni adjustment]. On Monday sessions (e.g., sessions 13 and 18), performance generally dropped, but this was more noticeable for the T1R3 WT group. Nevertheless, as training sessions progressed, performance for both WT groups gradually improved (Fig. 2A). In contrast, T1R2 KO and T1R3 KO mice did not perform above chance, indicating these animals did not learn the 1.0 M sucrose vs. water discrimination task, even over 17 sessions. On training session days 8 and 9, T1R2 KO mice showed performance statistically higher than 50% (chance), but this did not survive Bonferroni adjustment. Furthermore, performance for the sessions before and following these two sessions, were at chance. The T1R3 KO group did not perform significantly above chance on any of the sucrose training sessions. Therefore, we stopped testing the KO mice with sucrose at that point and advanced them to NaCl control sessions so as not to lose stimulus control of behavior.
Fig. 2.
A: mean (± SE) performance on the 1.0 M sucrose vs. water discrimination task across training sessions for the T1R2 WT (top, ●) and T1R2 KO (top, ○), T1R3 WT (bottom, ●) and T1R3 KO (bottom, ○) groups. An asterisk (*) denotes performance that was significantly greater than 50%, while “x” denotes statistical significance that did not survive Bonferroni adjustment. B: mean ± SE performance as a function of sucrose concentration for the T1R2 WT (■) and T1R3 WT (●) groups. C: threshold distribution for individual female (○) and male (△) animals and the means (solid lines) and SE (dashed lines) for the T1R2 WT and T1R3 WT genotype groups.
A two-way ANOVA (group × concentration) comparing performance across sucrose concentrations of T1R2 WT and T1R3 WT groups did not reveal a main effect of group [F (1, 14) = 0.199, P = 0.662] but did reveal a main effect of concentration [F (8,112) = 31.711, P < 0.001], with no significant interaction [F (8,112) = 1.338, P = 0.232] (Fig. 2B). Two WT (1 T1R2 WT and 1 T1R3 WT) mice did not reach the 80% performance criterion for 1.0 M sucrose across testing sessions to be included in the threshold analysis. A two-way ANOVA (group × concentration) comparing performance of the WT groups, excluding the animals that did not reach criterion, produced similar outcomes: Group: [F (1,13) = 0.060, P = 0.810], concentration: [F (8,104) = 32.839, P < 0.001], and interaction: [F (8,104) = 1.025, P = 0.422]. The WT groups did not significantly differ in their sensitivity to sucrose (Fig. 2C). Two-sample t-tests did not reveal significant differences for any of the parameters of the logistic functions (Table 6). The mean R2 value of 0.78 ± 0.05 indicates moderate curve fits to the data across animals and was not higher largely because some of the mice had very steep psychometric functions dropping from over 75% to chance, especially in the T1R3 WT group, as indicated by its large negative slope value (i.e., b), making some curves difficult to fit.
Table 6.
t-test values comparing curve parameter values for between WT and KO groups
| Compound and Parameter | WT t-test | KO t-test |
|---|---|---|
| NaCl | ||
| Asymptotic Performance (a) | t (14) = −0.865, P = 0.401 | t (14) = 0.184, P = 0.856 |
| Slope (b) | t (14) = −1.454, P = 0.168 | t (14) = 0.656, P = 0.522 |
| Log10 Threshold (c) | t (14) = −1.796, P = 0.094 | t (14) = 0.588, P = 0.566 |
| Sucrose | ||
| Asymptotic Performance (a) | t (12) = 0.845, P = 0.415 | |
| Slope (b) | t (12) = 1.432, P = 0.178 | |
| Log10 Threshold (c) | t (12) = 1.254, P = 0.234 | |
| Polycose 1 | ||
| Asymptotic Performance (a) | t (11) = −1.287, P = 0.224 | t (7) = 0.179, P = 0.863 |
| Slope (b) | t (11) = 0.935, P = 0.370 | t (7) = −1.661, P = 0.141 |
| Log10 Threshold (c) | t (11) = −0.503, P = 0.625 | t (7) = 1.424, P = 0.197 |
| Polycose 2 | ||
| Asymptotic Performance (a) | t (12) = −0.098, P = 0.924 | t (13) = −1.067, P = 0.305 |
| Slope (b) | t (12) = 1.644, P = 0.126 | t (13) = −1.191, P = 0.255 |
| Log10 Threshold (c) | t (12) = 0.764, P = 0.459 | t (13) = 0.184, P = 0.857 |
| Maltose | ||
| Asymptotic Performance (a) | t (12) = −0.394, P = 0.701 | |
| Slope (b) | t (12) = −3.493, P = 0.004 | |
| Log10 Threshold (c) | t (12) = 2.106, P = 0.057 | |
| Glucose | ||
| Asymptotic Performance (a) | t (9) = 0.091, P = 0.930 | |
| Slope (b) | t (9) = 1.082, P = 0.308 | |
| Log10 Threshold (c) | t (9) = −0.157, P = 0.879 | |
| Sucrose 2 | ||
| Asymptotic Performance (a) | t (8) = −0.535, P = 0.607 | |
| Slope (b) | t (8) = −1.926, P = 0.090 | |
| Log10 Threshold (c) | t (8) = −0.157, P = 0.879 |
Polycose.
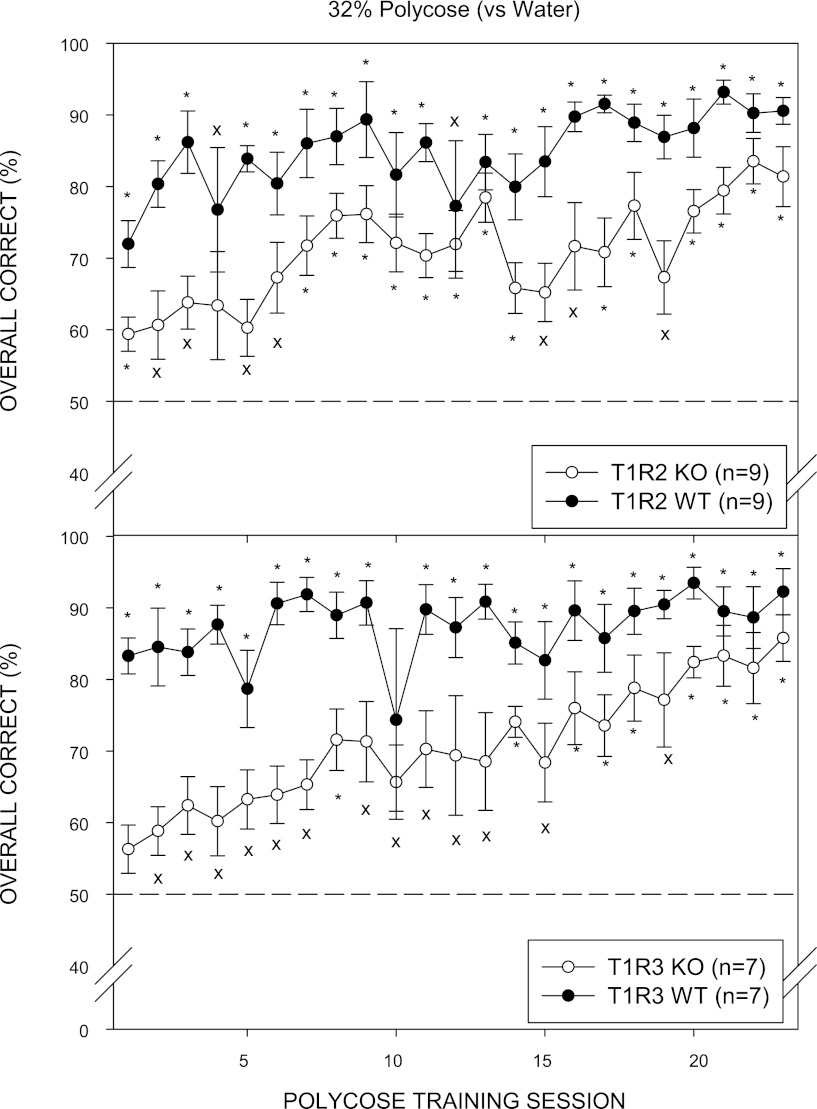
On day 1 of Polycose discrimination training, both T1R2 WT and T1R3 WT groups performed significantly above chance (50%) and continued to gradually improve across the 32% Polycose vs. water training sessions. Performance of T1R2 KO and T1R3 KO mice began lower than their WT control groups. As training progressed, both KO groups improved and reached an overall average performance above 80%, and each individual animal performed significantly above chance on the last 2 days of Polycose discrimination training (Fig. 3). The improvement in performance across training sessions to Polycose in KO mice resembled the acquisition curve of WT mice across sucrose training sessions. The slower acquisition of the Polycose vs. water discrimination task by the KO mice may be attributed to differences in training history in which the KO mice were subjected to an extremely difficult and seemingly impossible discrimination between sucrose and water in contrast to their WT counterparts.
Fig. 3.
Mean (± SE) performance on the 32% Polycose vs. water discrimination task across training sessions for the T1R2 WT (top, ●), T1R2 KO (top, ○), T1R3 WT (bottom, ●), and T1R3 KO (bottom, ○) groups. An asterisk (*) denotes performance that was significantly greater than 50%, while “x” denotes statistical significance that did not survive Bonferroni adjustment.
Two-way ANOVAs (genotype × concentration) comparing performance of KO groups and their WT control groups included in the concentration-response analysis across Polycose concentrations did not reveal a main effect of genotype for T1R2 but did show a main effect of concentration and a significant interaction (Table 2). For T1R3, there was a main effect of genotype, main effect of concentration, and a significant interaction. Several animals did not reach the ≥80% performance criterion and were not included in threshold analysis. When the data from animals that did not reach this performance criterion were excluded, the genotype × concentration interaction remained significant, but the main effect of genotype in the T1R3 group disappeared (Polycose 1; Table 3).
Parameter values for T1R2 KO and T1R3 KO groups did not significantly differ from those of their WT controls. Although the KO mice performed well in the task, they differed from the WT mice, as indicated by a significant genotype × concentration interaction when percent-correct values were analyzed both in the concentration-response analysis and the threshold analysis (Table 2). Thus, although asymptote parameter values did not significantly differ, there was evidence that the KO groups showed slightly lower performance, particularly at the higher concentrations, compared with their WT controls. The mean R2 values indicate tighter fits of the curves to the performance data for the WT mice (0.89 ± 0.03) compared with those of the KO mice (0.78 ± 0.07).
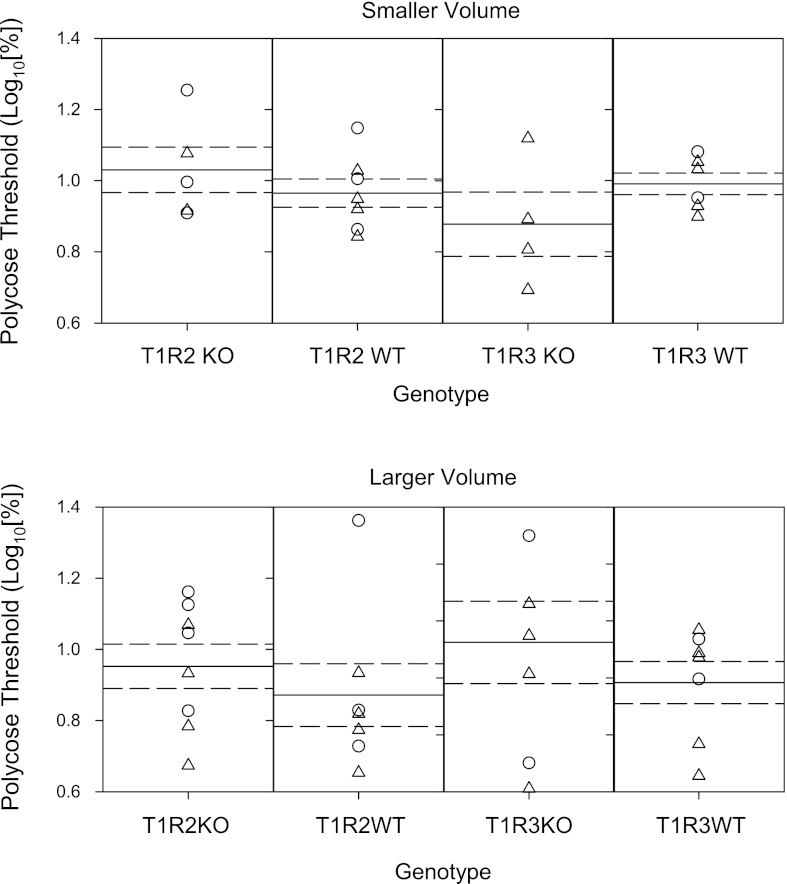
It is surprising that the concentration that corresponds to the EC50 obtained in the detection procedure is not below the dynamic range of concentrations at which there is a change in responsiveness to Polycose in a brief-access test (63, 65). One difference between the two procedures is that in the task here, volume is restricted to 5 licks, whereas in the brief-access test mice can potentially take 10–20 times more licks of the stimulus. Although polysaccharide receptors have not been identified, it is possible that receptors that bind to Polycose are not densely populated in the anterior tongue. To address this issue, we retested the mice with a subset of the concentration in addition to 0.2% and attempted to expand the oral area of stimulation by increasing the volume per lick to ∼1.6 μl/lick and increasing the number of sample licks from 5 to 7. This essentially doubled the sample volume. At the higher volume, comparisons of performance to Polycose no longer revealed main effects of genotype. However, a significant genotype × concentration interaction was still evident for the T1R3 KO and WT groups (Polycose 2; Tables 2 and 3). It appears that these effects are due to slightly poorer performance of the KO groups, especially at the higher concentrations (Fig. 4), but two-sample t-tests did not reveal significant differences in any of the curve parameter values (Table 5, Fig. 5). Compared with mean R2 values at the lower sample volume, the mean R2 values obtained when animals were tested at the higher volume are higher but still indicate a more accurate fit for the performance for the WT mice (0.93 ± 0.01) compared with the KO mice (0.82 ± 0.04).
Fig. 4.
Mean (± SE) performance when the sample size was 5 licks at ∼1 μl/lick (left) and when the sample size was increased to 7 licks at ∼1.6 μl/lick (right) as a function of Polycose concentration for the T1R2 KO (top, ○), T1R2 WT (top, ●), T1R3 KO (bottom: ○), and T1R3 WT (bottom, ●) groups.
Fig. 5.
Threshold distribution for individual female (○) and male (△) animals that performed at least at 80% correct to 32% Polycose and the means (solid lines) and SE (dashed lines) for the T1R2 WT and T1R3 WT genotype groups for Polycose presented either at 5 licks at ∼1 μl/lick (top) or at 7 licks at ∼1.5 μl/lick (bottom).
Maltose.
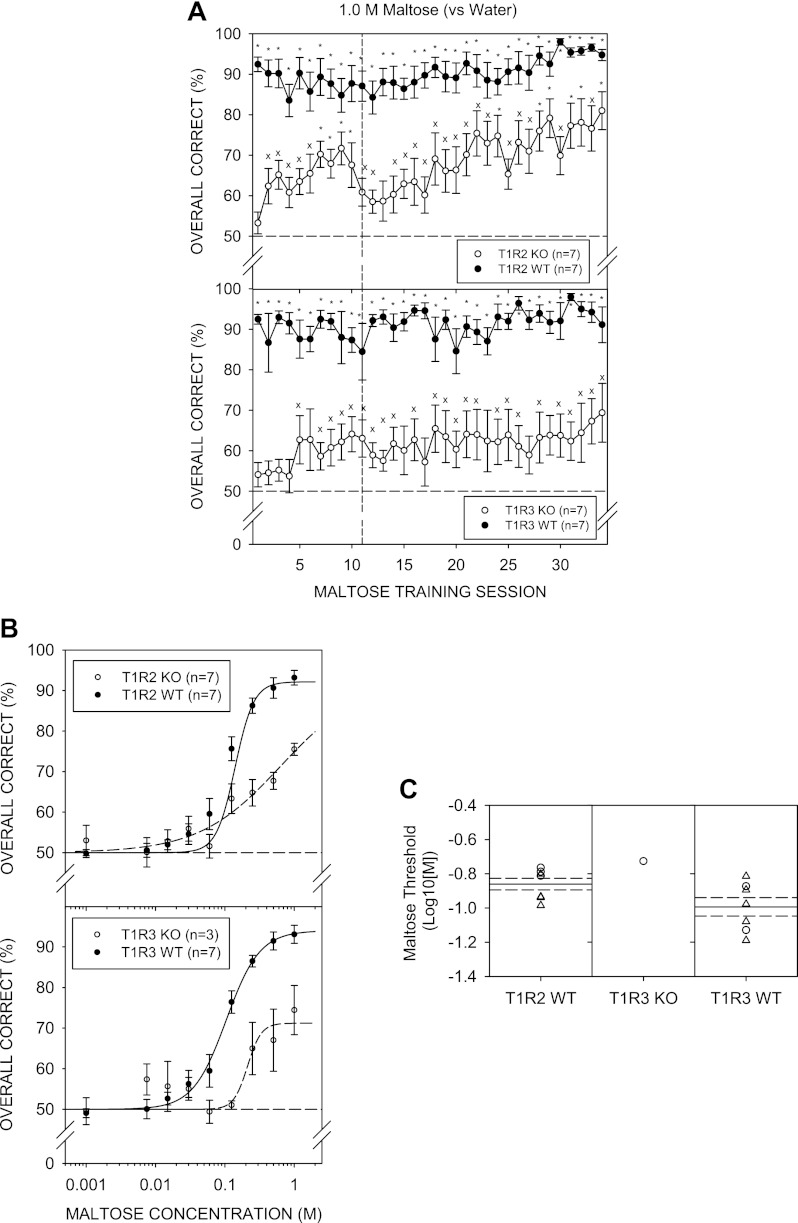
Both WT groups performed significantly above chance during maltose discrimination training with mean performance around 90%. Four T1R3 KO mice did not perform significantly above chance on the last 2 days of maltose discrimination training and were returned to NaCl control sessions. Mean performance for the KO groups was initially poor but steadily improved across training sessions. Nevertheless, performance never reached that observed in their respective WT control groups (Fig. 6A).
Fig. 6.
A: mean (± SE) performance on the 1.0 M maltose vs. water discrimination task across training sessions for the T1R2 WT (top, ●) and T1R2 KO (top, ○), T1R3 WT (bottom, ●) and T1R3 KO (bottom, ○) groups. An asterisk (*) denotes performance that was significantly greater than 50%, while an “x” denotes statistical significance that did not survive Bonferroni adjustment. Vertical dashed line represents a break in training for 2 wk during which animals were given ad libitum food and water. B: mean (± SE) performance as a function of maltose concentration for the T1R2 WT (top, ●) and T1R3 WT (bottom, ●) groups. No T1R2 KO mice and only 1 T1R3 KO mouse performed at least 80% correct to the highest maltose concentration. C: threshold distribution for individual female (○) and male (△) mice and the means (solid lines) and SE (dashed lines) for the T1R2 WT and T1R3 WT genotype groups.
Particularly to the higher concentrations of maltose, the performance of T1R2 KO and T1R3 KO mice was significantly lower compared with their WT controls, as revealed by a main effect of genotype and a significant genotype × concentration interaction in the respective ANOVAs (Fig. 6B; Table 2). Furthermore, none of the T1R2 KO mice and only 1 T1R3 KO mouse performed at least 80% to the highest concentration of maltose tested, and thus, no KO mice were included for threshold analysis (Fig. 6C). Asymptotic performance (a-parameter) and sensitivity (c-parameter) did not appear to be significantly different across the two WT groups, but t-tests did reveal a steeper slope (b-parameter) for the T1R2 WT compared with the T1R3 WT mice (Table 6). The curves tightly fit the individual performance data, as reflected by the mean R2 value of 0.96 ± 0.02 across WT animals.
Glucose.
As observed for the other stimuli, both WT groups performed significantly above chance during glucose discrimination training. Performance of T1R2 KO mice was significantly above chance across the glucose discrimination training sessions, although poorer than that of their WT controls. In contrast, T1R3 KO mice performed above chance only on day 5, and this did not survive Bonferroni adjustment (Fig. 7A). During glucose discrimination training, there was evidence that one T1R3 KO mouse was not under stimulus control; thus, data from that animal and its littermate control were eliminated from the experiment from that phase onward. Six mice (1 T1R2 KO and all T1R3 KO) did not reach the criterion of performance above chance on the last 2 days of discrimination training and thus were not tested with the glucose concentration series. Only three T1R2 KO mice performed above 80% at the highest glucose concentration tested. Performance across glucose concentrations of the T1R2 KO mice was significantly poorer than that of their WT controls, as revealed by the main effect of genotype and a significant interaction (Fig. 7B; Tables 2 and 3). Furthermore, EC50 values for the psychometric functions of T1R2 KO mice were significantly higher than those obtained from T1R2 WT mice (Fig. 7C; Table 4). The mean R2 value of 0.92 ± 0.02 across animals indicates the curves tightly fit the performance data.
Fig. 7.
A: mean (± SE) performance on the 2.0 M glucose vs. water discrimination task across training sessions for the T1R2 WT (top, ●), T1R2 KO (top, ○), T1R3 WT (bottom, ●), and T1R3 KO (bottom, ○) groups. An asterisk (*) denotes performance that was significantly greater than 50%, while an “x” denotes statistical significance that did not survive Bonferroni adjustment. B: mean (± SE) performance as a function of glucose concentration for the T1R2 KO (top, ○), T1R2 WT (top, ●), and T1R3 WT (bottom, ●) groups. None in the T1R3 KO group performed significantly above chance in the 2.0 M glucose vs. water discrimination task. C: threshold distribution for individual female (○) and male (△) mice and the means (solid lines) and SE (dashed lines) for the T1R2 KO, T1R2 WT, and T1R3 WT genotype groups.
Sucrose 2.
When animals were initially trained in the 1.0 M sucrose vs. water discrimination task (i.e., Sucrose 1), T1R2 KO and T1R3 KO mice did not perform significantly above chance. When animals were retested with 1.0 M sucrose (Sucrose 2), both WT groups, as well as a subset of the KO mice, performed significantly above chance (Fig. 8A). Across the last two sucrose discrimination training days, three T1R3 KO mice did not perform significantly above chance and were thus returned to NaCl control sessions, but across all the training days, one-sample t-tests showed the T1R2 KO group performed significantly above chance. During testing, one T1R3 WT mouse died, and thus, data from its T1R3 KO littermate control were removed from the experiment at the beginning of that phase onward. This left only 1 T1R3 KO mouse for sucrose testing. When the remaining mice were tested in a concentration series, all KO mice showed clearly impaired performance compared with their WT control groups. Two-way ANOVAs revealed main effects of genotype and concentration and a significant genotype × concentration interaction for the respective ANOVAs (Fig. 8B; Table 2). None of the KO mice displayed performance of at least 80% to the highest concentration of sucrose tested and thus no KO mice were included for threshold analysis. The curves for the WT groups fit the performance data well, as indicated by the mean R2 value of 0.95 ± 0.01.
Fig. 8.
A: mean (± SE) performance during the second phase of 1.0 M sucrose vs. water discrimination training sessions for the T1R2 WT (top, ●), T1R2 KO (top, ○), T1R3 WT (bottom, ●), and T1R3 KO (bottom, ○) groups. An asterisk (*) denotes performance that was significantly greater than 50%, while an “x” denotes statistical significance that did not survive Bonferroni adjustment. B: mean (± SE) performance as a function of sucrose concentration during the second phase of sucrose testing for the T1R2 KO (top, ○), T1R2 WT (top, ●), T1R3 KO (bottom, ○) and T1R3 WT (bottom, ●) groups. C: threshold distribution for individual male (△) and female (○) animals and the means (solid lines) and SE (dashed lines) for the T1R2 WT and T1R3 WT genotype groups for the second phase of sucrose testing.
Analysis by sex.
Further breakdown by sex resulted in low sample sizes precluding any meaningful data analysis, but qualitatively, there were no striking effects of sex, as evident by the threshold distributions across the testing stimuli (Figs. 1B, 2C, 5, 6C, 7C, 8C).
Water control test.
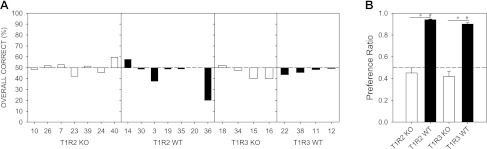
During the water control test, no mouse responded significantly above chance (50%; all P values >0.05), with performance averaging 46.78 ± 1.71%. Thus, there was no evidence to suggest that the animals could perform the task in the absence of chemical cues (Fig. 9A).
Fig. 9.
A: overall correct response during the water control test for each individual animal. Chance is 50%. B: mean (± SE) preference ratio to 10 mM Na-saccharin (vs. water) in a 48-h two-bottle preference test for each genotype group. An asterisk (*) denotes preference ratio scores between KO and WT groups were significantly different, while # denotes preference ratio scores that were significantly greater than 0.5.
Two-bottle preference test.
Both T1R2 KO [t (6) = −1.007, P = 0.824] and T1R3 KO [t (3) = −1.656, P = 0.902] groups showed preference ratio scores that were not significantly different from 0.5 during the two-bottle preference test, indicating no significant difference between intake of Na-saccharin and water. In contrast, the T1R2 WT [t (6) = 82.527, P < 0.001] and T1R3 WT [t (3) = 31.905, P < 0.001] showed substantial preference for 10 mM Na-saccharin, as evidenced by preference ratio scores significantly higher than 0.5 (Fig. 9B). Preference ratio scores for both the T1R2 [t (12)=−10.148, P < 0.001] and T1R3 KO [t (6)=−9.520, P < 0.001] groups were significantly different from those of their respective WT control groups. The results of this test functionally confirm the genotypes.
DISCUSSION
In the current study, T1R2 and T1R3 KO mice showed severely impaired taste detection performance to sucrose, glucose, and maltose but were remarkably competent in the task when Polycose was the stimulus, albeit, with some diminished accuracy. As expected, both KO groups learned the task and displayed normal detection thresholds when NaCl was the stimulus compared with their WT control groups. On the basis of electrophysiological measures from the CT nerve and behavioral data from a brief-access test, it has been shown that mice missing various T1R receptor proteins respond normally to NaCl, compared with WT mice (71). Accordingly, the Tas1r gene family is not thought to be related to the mediation of NaCl taste and served as an ideal control stimulus for this experiment. Indeed, T1R2 KO and T1R3 KO mice did not significantly differ from their respective WT control groups, in any of the parameters of the NaCl detectability function, which rules out learning and general performance factors as the basis of the deficits observed in the KO mice when the sugars were tested. Functional confirmation of the genotype status was provided by the fact that the KO animals displayed indifference to sodium saccharin in contrast to pronounced preference demonstrated by the WT mice in a 24-h two-bottle intake test (vs. water). Finally, the results of the water control test indicated that the animals could not competently perform the task in the absence of chemical cues. The findings of this study demonstrate that T1R2 and T1R3 are individually necessary for normal orosensory sensitivity to sucrose, maltose, and glucose to be maintained, whereas the combined presence of these protein subunits is not necessary for the relatively normal detection of Polycose in mice. These outcomes support the view that the T1R2+3 heterodimer is the principal receptor that mediates “sweet” taste (3, 23, 30, 33–36, 39, 41, 48, 69). At the same time, these results also buttress the evidence in the literature pointing to a novel taste receptor(s) that mediates polysaccharide taste (50, 52). To our knowledge, this is the first time a psychometric function for Polycose detectability has been measured.
If the T1R2+3 heterodimer mediates sucrose taste sensibility, then it would be expected that T1R3 KO mice should be unable to detect sucrose, at least at low concentrations, but contrary to this prediction, it has been previously reported that T1R3 KO mice possess normal sucrose detection thresholds compared with WT mice (10). The basis of the disparity in the outcomes of the Delay et al. (10) study and those of the current study remains to be understood. The different outcomes have different ramifications regarding the necessity of the T1R2 and T1R3 subunits for sucrose detection. One possible explanation is that although operant procedures were used both here and in the Delay et al. (10) study, the discrepancy between the respective results may be due to detailed differences in the methods employed. For example, here a two-response operant procedure was used in which the animal was reinforced with water for correct responses and punished with time-outs, whereas Delay et al. (10) used a shock avoidance task, in which correct responses were reinforced with water and incorrect responses were punished with a mild shock to the tongue. Another possibility may be the difference in the way the gene was deleted. Mouse (and human) T1R3 belongs to a G protein-coupled receptor (GPCR) family that contains a large extracellular domain. It is thought that the large N-terminal domain is involved in ligand binding and dimerization (see Refs. 34, 40, 41). The mice used in the Delay et al. (10) study had the entire T1R3 coding region deleted (8), whereas the animals used in the current study were missing the extracellular ligand binding domain, but the remainder of the gene was retained (71). Although both gene deletion procedures should render the Tas1r3 gene nonfunctional, it has been suggested (10) that if a truncated form of T1R3 containing the transmembrane domain is expressed, it may be capable of forming a dimer with T1R2 and inhibit the response to sucrose, thus acting as a dominant negative. Nevertheless, in other studies, in which T1R3 KO mice were tested that had the entire T1R3 coding region deleted, as in the Delay et al. study (10), both electrophysiological and behavioral responses to sucrose in KO mice were severely impaired (8, 72–73). Furthermore, detection thresholds to sucrose have been previously shown to be dependent on T1R3 allele status (17); thus, it is surprising that the deletion of the gene would not yield large effects on threshold. Even if a dominant-negative effect is rendering our T1R3 KO mice more impaired than when the entire coding sequence is deleted, it would still suggest that the taste receptor cells expressing T1R2 are critical in the maintenance of normal sensitivity to sucrose, and given that expression of T1R2 is relatively segregated from other taste receptor proteins (2, 24, 39), this finding is conceptually relevant from a neural coding perspective.
Overall, the degree of impairment was greater in the T1R3 KO mice compared with that in the T1R2 KO mice. Modest performance was observed in the KO mice at the higher maltose and glucose concentrations, more so in the T1R2 KO group. Furthermore, during the second phase of sucrose testing, a subset of the KO mice discriminated 1.0 M sucrose from water at levels significantly above chance, suggesting that at the higher concentrations, there is a detectable signal. Whether this detectable cue is a taste or other signal such as viscosity or odor, remains unclear. Detectability of maltose and glucose at the higher concentrations could potentially be attributed to the postulated polysaccharide taste receptor (52) or to the remaining T1R subunit forming a functional receptor. In the two-bottle intake tests or even in the brief-access test, animals have more access to the stimulus. In this procedure, orthonasal olfaction was minimized by requiring the animal to be engaged in licking before the stimulus was presented, coupled with the delivery of very small stimulus volumes. These features likely reduce the potency of these other cues. It has been suggested in the literature that there are different ligand binding domains for the T1Rs (27, 28, 31, 68, 69), which may explain these differences. Although conformational changes in both the T1R2 and T1R3 N-terminal domains were observed in response to sucrose and glucose, suggesting the binding of these ligands involves both units (41), the relative contribution of each may differ. Another possibility is that T1R3 might be more effective at forming functional homodimers compared with T1R2 (71).
Compared with the WT controls, both KO groups showed some impairment in performance to Polycose, but this was not as severe as in the performance to sucrose, glucose, or maltose. Although the Polycose EC50 values did not significantly differ between KO mice and their WT control groups, the KO groups differed from their WT controls, as evident from the significant genotype × concentration interaction when performance values were compared. Asymptotic parameter values did not significantly differ, but it appears the KO groups showed slightly lower performance compared with their WT controls, particularly at the higher concentrations. It is plausible that the small amounts of lower-molecular-weight glucose polymers make Polycose a slightly less salient stimulus to the KO groups. Alternatively, the T1R2 or T1R3 subunit might heteromerize with another protein to form a functional glucose polymer receptor. These are not mutually exclusive possibilities.
The EC50 values for Polycose obtained in the present study using a two-response operant procedure is comparable to the concentration at which there is an inflection in the affective response curve to Polycose in a brief-access test (63, 65). It is surprising that the EC50 value was not lower. The EC50 values for NaCl obtained in a two-response operant procedure are lower than the inflection point on a NaCl response curve obtained in a brief-access test for both mice (11, 16, 64) and rats (19, 22). Similarly, the detection threshold for sucrose obtained in a two-response operant procedure in mice in the current study and previously (17) is lower than the point of inflection in the sucrose concentration-response curve assessed in a brief-access test (11, 63). The EC50 values obtained for NaCl and sucrose in the current study are lower than those previously reported, confirming the sensitivity of the procedure (16, 17, 64). Thus, it appears that it is something about the features of Polycose that is contributing to the right-shifted psychometric function obtained in the detection task compared with that derived from licking in the brief-access test. Although polysaccharide receptors have not been identified, there is evidence that lends support to the possibility that receptors more sensitive to Polycose may be located in the posterior tongue (25, 66). Also, the number of receptors that bind to Polycose may be fewer compared with those that respond to other taste compounds. In regard to these possibilities, the stimulus volume in this study (up to 5 μl per trial) may have been less than optimal for detection of the compound, especially if, indeed, the receptors are located in the crypts of the circumvallate and foliate papillae. In contrast, in the brief-access test, at higher concentrations, the animals took ∼35 μl per trial, and the stimulus may have more effectively reached the taste receptors. Indeed, perceived taste intensity in humans has been previously shown to be a function not only of concentration, but also of area of stimulation (56). Furthermore, it has been shown that larger stimulus volumes correlate with lower NaCl detection in rats (e.g., 55). When volume per lick was increased in the current study, asymptotic performance of both KO groups did improve but had little effect on detection threshold values.
Previously, we reported that T1R2 KO, T1R3 KO, T1R2, and T1R3 KO mice display concentration-dependent responses to Polycose, as assessed in a brief-access taste test (63, 65), which measures unconditioned licking responses that are driven by the hedonic component of the stimulus. Here, T1R2 KO and T1R3 KO mice have been shown to be able to detect this polysaccharide mixture in a psychophysical task. The qualitative percept of Polycose in these KO mice has yet to be assessed. Polycose may possess, in part, a “sweet” taste quality component that is abolished with the genetic ablation, but the KO groups may still display relatively normal performance to Polycose in the detection threshold procedure based on the remaining qualitative characteristics of the stimulus. This could potentially be tested in a conditioned taste aversion generalization procedure, which has been used to make inferences about how similar various test stimuli are to the conditioned stimulus. In this procedure, a novel tastant is paired with administration of a toxin (often LiCl) to induce visceral illness and on subsequent presentations, the animal will avoid the conditioned stimulus. The generalization of the avoidance to other chemical compounds is taken as an index of qualitative similarity (e.g., 18, 26, 44, 45, 49). If KO animals were conditioned to avoid Polycose, it is unlikely that the mice would generalize the aversion to sucrose, in light of the evidence that the KO mice show severely impaired detection of this sugar, but it would still be meaningful to determine whether the animals significantly avoided licking stimulus representatives of other prototypical taste qualities. Such an analysis would also be meaningful in WT mice, especially given the fact that rats do not treat Polycose and sucrose as similar (44, 49). In addition, the use of an operant conditioning-based taste generalization procedure could be revealing in this regard as well (22).
Perspectives and Significance
The present report supports the view that the T1R2+3 heterodimer is the principal taste receptor that mediates the detection of natural sweeteners, but not of all carbohydrate stimuli. The combined presence of T1R2 and T1R3 does not appear necessary for the maintenance of relatively normal sensitivity to Polycose, lending evidence to the hypothesis that a novel receptor(s) that binds to longer-chain polysaccharides exists in the oral cavity of at least rodents (52). The functional role of the putative glucose polymer receptor has been proposed to allow animals to detect complex carbohydrates (52). In rats, bilateral transection of the glossopharyngeal nerve, which innervates taste receptor fields in the posterior tongue, results in a decrease in Polycose, but not sucrose intake (66), thus suggesting the yet-to-be-identified glucose polymer receptor may be located in the posterior tongue. In terms of taste transduction mechanisms, α-gustducin and Trpm5 appear to be necessary for the maintenance of responsiveness to Polycose (54). Although longer-chain polysaccharides, such as Polycose, have been reported to be bland to humans (see Ref. 50), there are findings in the literature that suggest there may be oral receptors in humans that respond to “nonsweet” carbohydrates (7). Thus, in humans, rather than taste perception, activation of the putative glucose polymer receptor may play a role in other physiological functions (see Ref. 61).
GRANTS
This study was supported by National Institutes of Health R01-DC004574 (to A. C. Spector).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.T. and A.C.S. conception and design of research; Y.T. performed experiments; Y.T. analyzed data; Y.T. and A.C.S. interpreted results of experiments; Y.T. prepared figures; Y.T. drafted manuscript; Y.T. and A.C.S. edited and revised manuscript; Y.T. and A.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to thank Thomas Shakar and Ginger Blonde for their technical help in this experiment. We would also like to thank Dr. Charles Zuker for generously supplying the T1R2 and T1R3 knock-out breeding pairs. Parts of this paper were presented in a dissertation in partial fulfillment of a Doctor of Philosophy degree from Florida State University and were also presented at the 19th Annual Meeting of the Society for the Study of Ingestive Behavior, Clearwater FL, USA July 2011. Current address of Yada Treesukosol: Department of Psychiatry and Behavioral Sciences, School of Medicine, Johns Hopkins University, Baltimore, MD 21205.
REFERENCES
- 1. Ackroff K, Manza L, Sclafani A. The rat's preference for sucrose, Polycose and their mixtures. Appetite 21: 69– 80, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS. A novel family of mammalian taste receptors. Cell 100: 693– 702, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26: 925– 933, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blonde G, Jiang E, Garcea M, Spector AC. Learning-based recovery from perceptual impairment in salt discrimination after permanently altered peripheral gustatory input. Am J Physiol Regul Integr Comp Physiol 299: R1027– R1036, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blonde GD, Garcea M, Spector AC. The relative effects of transection of the gustatory branches of the seventh and ninth cranial nerves on NaCl taste detection in rats. Behav Neurosci 120: 580– 589, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Boughter JDJ, St. John SJ, Noel DT, Ndubuizu O, Smith DV. A brief-access test for bitter taste in mice. Chem Senses 27: 133– 142, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Chambers ES, Bridge MW, Jones DA. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. J Physiol 587: 1779– 1794, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850– 853, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Davis JD. The effectiveness of some sugars in stimulating licking behavior in the rat. Physiol Behav 11: 39– 45, 1973 [DOI] [PubMed] [Google Scholar]
- 10. Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses 31: 351– 357, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Dotson CD, Roper SD, Spector AC. PLCβ2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Senses 30: 593– 600, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dotson CD, Spector AC. Behavioral discrimination between sucrose and other natural sweeteners in mice: Implications for the neural coding of T1R ligands. J Neurosci 27: 11242– 11253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dotson CD, Spector AC. Drinking spout orifice size affects licking behavior in inbred mice. Physiol Behav 85: 655– 661, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Dugas du Villard X, Her C, Mac Leod P. Qualitative discrimination of sweet stimuli: behavioural study on rats. Chem Senses 6: 143– 148, 1981 [Google Scholar]
- 15. Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric Polycose infusions: A detailed analysis using an electronic esophagus preparation. Physiol Behav 47: 63– 77, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Eylam S, Spector AC. The effect of amiloride on operantly conditioned performance in an NaCl taste detection task and NaCl preference in C57BL/6J mice. Behav Neurosci 116: 149– 159, 2002 [PubMed] [Google Scholar]
- 17. Eylam S, Spector AC. Stimulus processing of glycine is dissociable from that of sucrose and glucose based on behaviorally measured taste signal detection in sac ‘taster’ and ‘non-taster’ mice. Chem Senses 29: 639– 649, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Formaker BK, Kearns CE, Frank ME. The taste of polycose in hamsters. Chem Senses 23: 675– 682, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Geran LC, Spector AC. Sodium taste detectability in rats is independent of anion size: the psychophysical characteristics of the transcellular sodium taste transduction pathway. Behav Neurosci 114: 1229– 1238, 2000 [PubMed] [Google Scholar]
- 20. Giza BK, Scott TR, Sclafani A, Antonucci RF. Polysaccharides as taste stimuli: their effect in the nucleus tractus solitarius of the rat. Brain Res 555: 1– 9, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with abberant taste and oromotor function. Chem Senses 27: 461– 474, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Grobe CL, Spector AC. Constructing quality profiles for taste compounds in rats: A novel paradigm. Physiol Behav 95: 413– 424, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJP, Zuker CS. Putative mammalian taste receptors: A class of taste-specific GPCRs with distinct topographic selectivity. Cell 96: 541– 551, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJP, Zuker CS. The cells and logic for mammalian sour taste detection. Nature 442: 934– 938, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129. B6-Tas1r3 congenic mice. Physiol. Genomics 32: 82– 94, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jakinovich W., Jr Taste aversion to sugars by the gerbil. Physiol Behav 28: 1065– 1071, 1982 [DOI] [PubMed] [Google Scholar]
- 27. Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LMJ, Osman R, Margolskee RF, Max M. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem 280: 15238– 15246, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Jiang P, Ji Q, Liu Z, Snyder LA, Benard LMJ, Margolskee RF, Max M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J Biol Chem 279: 45068– 45075, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Kennedy JF, Knill CJ, Taylor DW. Malodextrins. In: Handbook of Starch Hydrolysis Products and their Derivatives, edited by Kearsley MW, Dziedzic SZ. London, UK: Blackie Academic & Professional, 1995, p. 65– 82 [Google Scholar]
- 30. Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun 283: 236– 242, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Koizumi A, Nakajima Ki Asakura T, Morita Y, Ito K, Shmzu-Ibuka A, Misaka T, Abe K. Taste-modifying sweet protein, neoculin, is received at human T1R3 amino terminal domain. Biochem Biophys Res Commun 358: 585– 589, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kopka SL, Spector AC. Functional recovery of taste sensitivity to sodium chloride depends on regeneration of the chorda tympani nerve after transection in the rat. Behav Neurosci 115: 1073– 1085, 2001 [PubMed] [Google Scholar]
- 33. Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99: 4692– 4696, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28: 58– 63, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4: 492– 497, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Montmayeur JP, Matsunami H. Receptors for bitter and sweet taste. Curr Opin Neurobiol 12: 366– 371, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Mook DG. Oral and postingestional determinants of the intake of various solutions. J Comp Physiol Psychol 56: 645– 659, 1963 [Google Scholar]
- 38. Nakamura K, Norgren R. Taste responses of neurons in the nucleus of the solitary tract of awake rats: An extended stimulus array. J Neurophysiol 70: 879– 891, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJP, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381– 390, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Nie Y, Hobbs JR, Vigues S, Olson WJ, Conn GL, Munger SD. Expression and purification of functional ligand-binding domains of T1R3 taste receptors. Chem Senses 31: 505– 513, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol 15: 1948– 1952, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Ninomiya Y, Funakoshi M. Qualitative discrimination of gustatory stimuli in three different strains of mice. Brain Res 322: 83– 92, 1984 [DOI] [PubMed] [Google Scholar]
- 43. Nishijo H, Norgren R. Parabrachial neural coding of taste stimuli in awake rats. J Neurophysiol 78: 2254– 2268, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: A two-carbohydrate taste model. Neurosci Biobehav Rev 11: 187– 196, 1987 [DOI] [PubMed] [Google Scholar]
- 45. Nowlis GH, Frank ME, Pfaffman C. Specificity of acquired aversions to taste qualities in hamsters and rats. J Comp Physiol Psychol 94: 932– 942, 1980 [DOI] [PubMed] [Google Scholar]
- 46. Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways reveal in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol 296: R960– R971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramirez I. Thresholds for starch and Polycose are lower than for sucrose in rats. Physiol Behav 50: 699– 703, 1991 [DOI] [PubMed] [Google Scholar]
- 48. Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 77: 896– 903, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Sako N, Shimura T, Komure M, Mochizuki R, Matsuo R, Yamamoto T. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiol Behav 56: 741– 745, 1994 [DOI] [PubMed] [Google Scholar]
- 50. Sclafani A. Carbohydrate taste, appetite, and obesity: An overview. Neurosci Biobehav Rev 11: 131– 153, 1987 [PubMed] [Google Scholar]
- 51. Sclafani A. Oral and postoral determinants of food reward. Physiol Behav 81: 773– 779, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Sclafani A. The sixth taste? Appetite 43: 1– 3, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 289: R712– R720, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of α-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol 293: R1504– R1513, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Slotnick BM, Wittich AR, Henkin RI. Effect of stimulus volume on taste detection threshold for NaCl. Chem Senses 13: 345– 353, 1988 [Google Scholar]
- 56. Smith DV. Taste intensity as a function of area and concentration: Differentiation between compounds. J Exp Psychol 87: 163– 171, 1971 [DOI] [PubMed] [Google Scholar]
- 57. Smith JC. The history of the “Davis Rig”. Appetite 36: 93– 98, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Somenarain L, Jakinovich W., Jr Antagonism of the gerbil's sweetener and Polycose gustatory responses by copper chloride. Brain Res 522: 83– 89, 1990 [DOI] [PubMed] [Google Scholar]
- 59. Spector AC. The functional organization of the peripheral gustatory system: Lessons from behavior. In: Progress in Psychobiology and Physiological Psychology, edited by Fluharty SJ, Grill HJ. Bingley, UK: Emerald Group Publishing, 2003, p. 101–161 [Google Scholar]
- 60. Spector AC, Andrews-Labenski J, Letterio FC. A new gustometer for psychophysical taste testing in the rat. Physiol Behav 47: 795– 803, 1990 [DOI] [PubMed] [Google Scholar]
- 61. Spector AC, Glendinning JI. Linking peripheral taste processes to behavior. Curr Opin Neurobiol 19: 370– 377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. St. John SJ, Spector AC. Combined glossopharyngeal and chorda tympani nerve transection elevates quinine detection thresholds in rats (Rattus norvegicus). Behav Neurosci 110: 1456– 1468, 1996 [DOI] [PubMed] [Google Scholar]
- 63. Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol 296: R855– R865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol 292: R799– R809, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Treesukosol Y, Smith KR, Spector AC. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neurosci 31: 13527– 13534, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vigorito M, Sclafani A, Jacquin MF. Effects of gustatory deafferentation on Polycose and sucrose appetite in the rat. Neurosci Biobehav Rev 11: 201– 209, 1987 [DOI] [PubMed] [Google Scholar]
- 67. Weingarten H, Watson S. Sham feeding as a procedure for assessing the influence of diet palatability on food intake. Physiol Behav 28: 401– 407, 1982 [DOI] [PubMed] [Google Scholar]
- 68. Winnig M, Bufe B, Kratochwil NA, Slack JP, Meyerhof W. The binding site for neohesperidin dihydrochalcone at the human sweet taste receptor. BMC Struct Biol 7: 66, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu H, Straszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA 101: 14258– 14263, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Young PT, Trafton CL. Activity countour maps as related to preference in four gustatory stimulus areas of the rat. J Comp Physiol Psychol 58: 68– 75, 1964 [DOI] [PubMed] [Google Scholar]
- 71. Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255– 266, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol 296: R866– R876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses 34: 685– 394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]