Abstract
The choroid plexuses (CPs) form the blood-cerebrospinal fluid (CSF) barrier (BCSFB) and play an important role in maintaining brain normal function and the brain response to injury. Many neurological disorders are associated with oxidative stress that can impact CP function. This study examined the effects of isothiocyanates, an abundant component in cruciferous vegetables, on H2O2-induced BCSFB disruption and CP cell death in vitro. It further examined the potential role of a transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2), in isothiocyanate-induced protection. Sulforaphane (SF) significantly reduced H2O2-induced BCSFB disruption as assessed by transepithelial electrical resistance (29 ± 7% reduction vs. 92 ± 2% decrease in controls) and [3H]mannitol permeability. Allyl-isothiocyanate (AITC) had a similar protective effect. H2O2-induced epithelial cell death was also reduced by these isothiocyanates. In primary CP cells, SF and AITC reduced cell death by 42 ± 3% and 53 ± 10%, respectively. Similar protection was found in a CP cell line Z310. Protection was only found with pretreatment for 12–48 h and not with acute exposure (1 h). The protective effects of SF and AITC were associated with Nrf2 nuclear translocation and upregulated expression of antioxidative systems regulated by Nrf2, including heme oxygenase-1, NAD(P)H quinine oxidoreductase, and cysteine/glutamate exchange transporter. Thus isothiocyanates, as diet or medicine, may be a method for protecting BCSFB in neurological disorders.
Keywords: choroid plexus, blood-cerebrospinal fluid barrier, sulforaphone, allyl-isothiocyante
oxidative stress results from an imbalance between oxidants and antioxidants and can result in oxidative damage to cellular components such as proteins, nucleic acids, and cell membranes. Oxidative stress is involved in many diseases such as stroke, Parkinson's disease, Alzheimer's disease, and multiple sclerosis. Blood-brain barrier (BBB) damage following such stress has been extensively studied. However, less attention has focused on damage at the blood-cerebrospinal fluid (CSF) barrier (BCSFB) located at the choroid plexus (CP) epithelium. The CPs lie within the brain ventricles and act as a unique interface between the peripheral blood and the CSF. Besides secreting CSF, the CPs are also involved in other important aspects of neural function including maintaining the extracellular milieu of the brain by actively modulating chemical exchange between the CSF and brain parenchyma, surveying the chemical and immunological status of the brain, detoxifying the brain, secreting cytokines, and participating in brain repair processes following trauma (5, 15, 32). Because of this diversity of functions, even modest changes in the CPs may have a marked impact on the brain. For example, changes in CP function have been implicated in Alzheimer's disease (24).
Isothiocyanates (ITCs), such as sulforaphane (SF), allyl-isothiocyanate (AITC), benzyl-isothiocyanate (BITC), and phenethyl-isothiocyanate (PEITC), are a group of naturally occurring compounds found in abundance in cruciferous vegetables such as broccoli, cabbage, watercress, Brussel sprouts, and radishes (7). There has been great interest in the potential therapeutic effects of these compounds. ITCs inhibit tumor development in many experimental models and are being investigated as possible chemopreventive agents for specific human cancers. ITCs exert their cancer chemopreventive action by modulating phase I and phase II drug metabolism (6, 21). Recent studies have shown that SF also has neuroprotective effects in the brain, brain microvessels, neurons, and astrocytes by activating a transcription factor called nuclear factor erythroid 2-related factor 2 (Nrf2) (8, 25, 39, 41), and another report has indicated that AITC activates Nrf2 in fibroblasts (13). Under conditions of oxidative stress, Nrf2 is released from an inhibitory protein called Kelch-like ECH-associated protein 1 (KEAP1) and translocates to the nucleus where it regulates transcription of a wide range of antioxidant factors including heme oxygenase-1 (HO-1), H-ferritin, NAD(P)H quinine oxidoreductase (NQO1), and cystine/glutamate exchange transporter (xCT) (34).
The purpose of the current study was, therefore, to examine whether two of the ITCs (SF and AITC) can protect CP epithelial cells from oxidative stress and whether this might be linked to Nrf2 activation and an subsequent upregulation in downstream mediators. Experiments were performed on primary cultures of rat CP epithelial cells and Z310 cells, an immortalized rat CP epithelial cell line. The former form a relatively tight barrier [e.g., transepithelial electrical resistance of 150–200 Ω.cm2, similar to that found for frog choroid plexus that can be mounted in an Ussing chamber, (26)] and enable an examination of the effects of ITCs on barrier function. The latter have advantages in terms of cell growth and number.
MATERIALS AND METHODS
Cell cultures.
All animal studies were performed under the review of the University of Michigan Committee on the Use and Care of Animals. Primary CP epithelial cells cultures were derived from the lateral ventricles of 1- to 2-day-old Sprague-Dawley rats using the method of Strazielle and Ghersi-Egea (17, 33). The CP epithelial cells were collected and seeded on laminin-coated Transwell porous filters (Costar, Cambridge, MA). The culture medium was changed every 48 h after seeding and consisted of Dulbecco's minimum essential medium (DMEM)/F-12 (1:1) supplemented with 10% (vol/vol) fetal bovine serum, 2 mM glutamine, 25 mg/ml of gentamicin, 5 mg/ml of insulin, 5 mg/ml of transferrin, 5 ng/ml of sodium selenite, 10 ng/ml of epidermal growth factor, 2 mg/ml of hydrocortisone, and 5 ng/ml of basic fibroblast growth factor. Cells were grown in a sterile incubator at 37°C, 95% relative humidity, and 5% CO2. Cultures were inspected visually for epithelial growth (i.e., cobblestone appearance) on a regular basis and confluence occurred 5–7 days postseeding.
Z310 cells, an immortalized rat CP epithelial cell line (from Dr Wei Zheng, Purdue University), were cultured as previously described (44). Briefly, cells were grown in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 40 μg/ml of gentamycin in a humidified incubator with 95% air-5% CO2 at 37°C. The cells were passaged (1:12–16) twice a week.
Measurements of transepithelial resistance and permeability of cell monolayers.
After 7 days culture on Transwell filters (24 well plates), confluence of primary CP epithelial cell monolayers and barrier properties were documented by measuring transepithelial resistance (TEER) with a Millipore apparatus. Cells were then pretreated with 10 μM SF or AITC for 24 h before 3 h exposure to 0.25 mM H2O2. TEER was measured again before and after H2O2 exposure. For the transepithelial permeability measurement, 0.1 μCi [3H]mannitol in 500 μl of medium was added to the basolateral chamber and 100 μl (out of 200 μl) was sampled from the apical chamber 60 min later. The samples were counted a liquid scintillation counter (LS 600, Beckman Coulter, Fulleron, CA). The permeability (P; cm/min) of the monolayer to [3H]mannitol was calculated as:
where mannitolapical is the total amount of [3H]mannitol (dpm) that has migrated across the epithelium to the apical chamber, mann.concbasal is the concentration of [3H]mannitol in the basal donor chamber (dpm/ml), A is the surface area of the transwell filter (cm2), and T is the time of the experiment (min).
Lactate dehydrogenase assay.
Cell injury was assessed by measuring extracellular lactate dehydrogenase (LDH) release. Z310 and primary CP cells were grown to confluence on polycarbonate 48-well plates. In the first set of experiments, cells were then pretreated with vehicle, 10 μM SF, or 10 μM AITC for 24 h in culture medium before exposure to 0.5 mM (Z310 cells) or 0.25 mM H2O2 (primary CP cells) in DMEM without FBS for 4 h. LDH release into the medium was assayed using CytoTox96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI), measuring absorbance of 492 nm using a microplate reader (ELX, Bio-Tek, Winooski, VT). The amount of LDH released from each test sample was expressed as a percentage of vehicle-treated cells exposed to H2O2. In a second set of experiments, Z310 cells were treated with 10 μM AITC for 1 to 48 h before exposure to 0.5 mM H2O2 to examine the time course of protection.
l-Cystine transport studies.
For l-[14C]cystine (291.3 mCi/mmol) apical uptake measurements, experiments were performed in triplicate on each individual preparation. Cells were plated on 12-well plates and grown to confluence. Our preliminary experiments showed that l-[14C]cystine uptake was linear over 30 min, and all subsequent experiments employed 5 min as the uptake time. Cells were pretreated with vehicle, 10 μM SF, or AITC for 24 h before uptake. At the start of the uptake experiment, cells were transferred to artificial CSF containing (in mM) 127 NaCl, 20 NaHCO3, 2.4 KCl, 0.5 KH2PO4, 1.1 CaCl2, 0.85 MgCl2, 0.5 Na2SO4, 5.0 glucose, and 0.001 l-cystine (pH 7.4), bubbled with 5% CO2 and 95% O2. After 30 s at 37°C, the buffer was removed and fresh uptake buffer containing l-[14C]cystine and [3H]mannitol (0.2 and 0.1 μCi/ml, respectively; [3H]mannitol 10–20 Ci/mmol) was added to initiate uptake, with the latter added to correct for extracellular isotope. Transport was measured at 37°C. At the end of the experiment, the medium was aspirated, and the cells were rapidly washed three times with ice-cold uptake buffer. The cells were solubilized in methylbenzethonium hydroxide and counted in Beckman LS 600 counter. The protein content of the solubilized cell monolayers was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA). The volume of distribution (VD) for [14C]cystine (corrected for extracellular contamination) was determined as:
where cystinetiss and manntiss are the disintegrations per minute per milligram protein for [14C]cystine and [3H]mannitol, R is the ratio of [14C]cystine to [3H]mannitol radioactivity in the media, and cystinemedia is the disintegrations per minute per microliter of media. Dividing VD (μl/mg) by the duration of uptake equals the influx rate constant (Kin).
Western blot analysis.
Western blot analysis was performed as previously described (29). Cells were harvested after 1 or 24 h treatment with vehicle, 10 μM SF, or 10 μM AITC. The cells harvested at 1 h were used to examine the nuclear translocation of Nrf2. Nuclear fractions were ioslated using a cell fractionation kit (Calbiochem, La Jolla, CA). The cells harvested after 24 h were used for whole cell lysate Western blots to examine HO-1 levels. The primary antibodies were polyclonal rabbit anti-rat HO-1 IgG (1:2,000 dilution; Assay Design, Ann Arbor, MI) and rabbit anti-rat Nrf2 IgG (1:2,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Immumoreactive proteins were visualized using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (GE Healthcare Biosciences, Pittsburgh, PA) and were exposed to Kodak X-OMAT film .The relative densities of bands were analyzed with NIH ImageJ. HO-1 and Nrf2 levels were expressed as percentage of control.
Quantitative real-time PCR.
All of materials were from Applied Biosystems unless otherwise stated. Total RNA was extracted from cultured Z310 cells by TRIzol reagent according to the manufacturer's instructions (Invitrogen). cDNA was synthesized from 1 μg of total RNA in a 20-μl reaction mixture using a cDNA Reverse Transcription Kit with RNase Inhibitor. The mixture was incubated at 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min. The primers used in RT-PCR had the following assay identification numbers: Rn00820640_g1 for rat heavy chain ferritin, Rn00566528_m1 for rat NQO1, and Rn01775763_g1 for rat GAPDH. PCR was carried out in a 30-μl reaction mixture containing 1 μl cDNA, 1.5 μl gene-specific primer and probe, 15 μl TaqMan Universal PCR Master Mix, and 12.5 μl RNase/DNase-free water. A mixture of the cDNA samples was serially diluted to obtain a standard curve for each primer. The sample mixture was incubated at 50°C for 2 min, 95°C for 10 min, and cycled 40 times from 95°C for 15 s to 60°C for 1 min in an Eppendorf Thermal Cycler. The quantified gene expression of ferritin and NQO-1 was normalized to GAPDH.
Statistical analysis.
The results are expressed as means ± SE from at least three separate experiments, and the data were subjected to one-way ANOVA analysis with Dunnett post hoc test for comparisons to a single control or a Tukeys post hoc test for multiple comparisons between groups. For nonparametirc data, a Kruksal-Wallis test with a Dunn's post hoc test was used. A P value <0.05 was considered statistically significant (*P < 0.05, **P < 0.01, and ***P < 0.001). All analysis was performed with Prism 5.0 (Graph Pad, San Diego, CA).
RESULTS
Protection from barrier disruption by ITCs.
TEER, (expressed as Ω.cm2) is a marker of the integrity of the barrier created by primary CP epithelial cells in vitro with barrier damage causing a TEER decrease. As shown in Fig. 1A, 3 h exposure to 0.25 mM H2O2 caused a 92 ± 2% decrease in TEER in vehicle-treated cells. In comparison, in groups pretreated with 10 μM SF or AITC, TEER values were only reduced by 29 ± 7% or 68 ± 1%, respectively (P < 0.001 and P < 0.01 vs. vehicle). The barrier disruption caused by H2O2 is also reflected by an increase in the transepithelial permeability to mannitol. In vehicle-treated cells, H2O2 caused a marked increase in permeability (13-fold). That increase was reduced by 10 μM SF and AITC pretreatment by 69 ± 9% and 56 ± 5%, respectively (P < 0.001; Fig. 1B).
Fig. 1.
Primary choroid plexuses (CP) monolayers were treated with vehicle or 10 μM sulforaphane (SF) or allyl-isothiocyanate (AITC) for 24 h. They were then exposed to media without (control) or with 0.25 mM H2O2 for 3 h, and the (A) transepithelial electrical resistance (TEER) and (B) transepithelial mannitol permeability were measured. Values are means ± SE; n = 3. ** and ***Significant differences from controls at the P < 0.01 and P < 0.001 levels, respectively, NS, not significant. +++Significant difference from control + H2O2 at the P < 0.001 level.
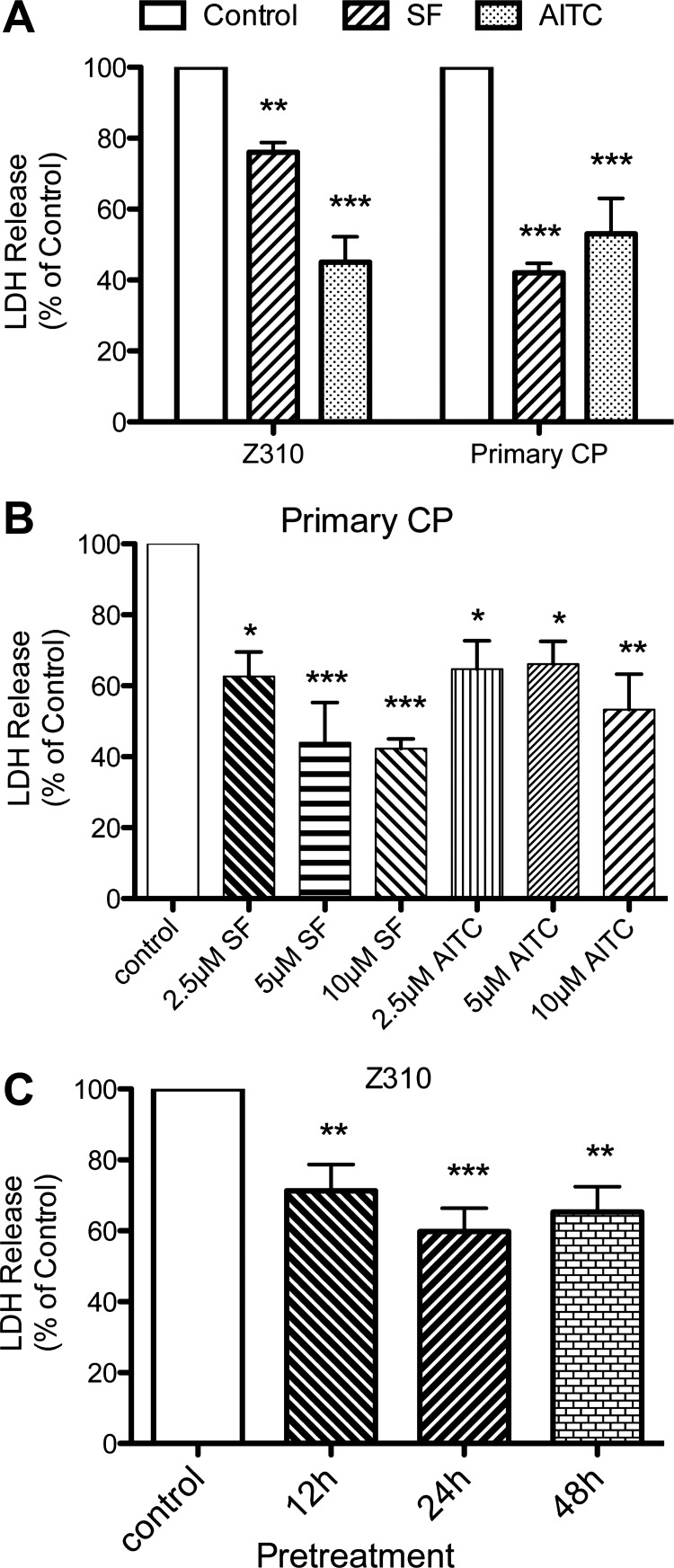
Reduction in H2O2-induced cell death by ITCs.
LDH release serves as an indicator of cell membrane damage and death. As Fig. 2A shows, 24 h pretreatment with 10 μM SF and AITC significantly reduced the LDH release induced by H2O2. Compared with the vehicle-treated cells exposed to H2O2, SF reduced LDH release by 24 ± 3% in Z310 cells and 58 ± 3% in primary CP cells. AITC also reduced LDH release by 40 ± 7% and 47 ± 10% in these two cell types, respectively (Fig. 2A). The dose dependency of the protection was examined in primary CP cells. There was a dose-dependent reduction in H2O2-induced LDH release for both SF and AITC (Fig. 2B).
Fig. 2.
A: lactate dehydrogenase (LDH) release from Z310 and primary CP cells induced by 4 h exposure to 0.25 mM (primary CP cells) or 0.5 mM H2O2 (Z310 cells) with/without 24 h pretreatment of 10 μM SF or AITC. B: in primary CP cells, effects of 24 h pretreatment with different concentrations of SF or AITC on the LDH release induced by 0.25 mM H2O2. Values are expressed as a % of LDH release in controls and vehicle-treated cells exposed to H2O2. C: LDH release from Z310 cells pretreated with 10 μM AITC for different durations (up to 48 h) before exposure to 0.5 mM H2O2. Values are means ± SE, n = 4–6 independent experiments. *, **, and ***Significant differences from controls at the P < 0.05, P < 0.01, and P < 0.001 levels, respectively.
To examine the time course of protection, Z2310 cells were pretreated with 10 μM AITC for 1 to 48 h before H2O2 exposure (Fig. 2C). One hour pretreatment with AITC enhanced rather than reduced LDH release (186 ± 42% of vehicle-treated cells, P < 0.01). In contrast, 12, 24, and 48 h pretreatment with AITC all reduced cell damage (Fig. 2C).
Upregulation of Nrf2 and downstream antioxidant systems.
In response to oxidative stress, cells upregulate a number of cytoprotective genes to increase their antioxidant capacity (36). The transcription factor Nrf2 plays a major role in this response. Under normal conditions, Nrf2 is bound to a specific inhibitory protein KEAP1 and is targeted for proteasomal degradation. However, under conditions of oxidative stress, Nrf2 is released from KEAP1 and enters the nucleus where it upregulates genes that have an antioxidant response element. Therefore, we investigated the possibility that Nrf2 accumulates in the nucleus and upregulation of potential downstream antioxidants (HO-1, NQO-1, ferritin, xCT) after treatment of SF and AITC by Western blot, real-time PCR, and cystine uptake.
Western blotting showed that nuclear levels of Nrf2 in Z310 cells were 11- and 10-fold higher than controls after 1 h treatment with 10 μM SF or AITC, respectively (Fig. 3, A and B). In both Z310 and primary CP cells, 24 h pretreatment with 10 μM SF upregulated cellular HO-1, an enzyme that catalyzes the degradation of heme, by 2.0 ± 0.5- and 2.8 ± 0.2-fold, respectively (Fig. 3, C and D). Similarly, 24 h treatment with 10 μM AITC upregulated HO-1 by 4.3 ± 1.1- and 2.2 ± 0.3-fold in Z310 and primary CP cells, respectively (Fig. 3, C and D). NQO1 is a flavoprotein that catalyses the two-electron reduction of quinones and prevents the production of radical species. Ferritin is a ubiquitous intracellular protein that stores iron and releases it in a controlled fashion. We also examined mRNA express of these two downstream antioxidants of Nrf2 in Z310 cells with real-time PCR. Twenty-four hours exposure of Z310 cells to 10 μM SF and AITC increased NQO1 mRNA levels by five- and threefold (P < 0.001 and 0.05), respectively. However, these two compounds did not induce changes of ferritin mRNA levels.
Fig. 3.
A and B: representative Western blot of nuclear factor erythroid 2-related factor 2 (Nrf2) in Z310 cells after 1 h treatment with vehicle, 10 μM SF, or 10 μM AITC and quantification (fold changes) of the Western blots. Values are means ± SE, n = 3. *Significant difference from vehicle-treated control at the P < 0.05 level. C and D: representative Western blot of whole cell lysate heme oxygenase-1 (HO-1) in Z310 and primary CP cells after 24 h treatment with vehicle, 10 μM SF, or 10 μM AITC and quantification (fold changes) of the Western blots. Values are means ± SE, n = 3–6. * and **Significant differences from vehicle-treated control at the P < 0.05 and P < 0.01 level, respectively.
Glutathione is the main intracellular antioxidant and its synthesis depends on l-cystine transport. In many cells, the cystine/glutamate exchanger (xCT), a transporter regulated by Nrf2 (18), is responsible for transporting l-cystine into cells and is rate limiting for glutathione synthesis (16, 20, 23). Twenty four hours exposure to 20 μM SF enhanced [14C]cystine uptake in both primary CP and Z310 cells to 234 ± 25% and 200 ± 8% of control, respectively (Fig. 4). However, AITC did not have a similar effect in Z310 cells.
Fig. 4.
l-[14C]cystine uptake in Z310 (A) and primary CP (B) cells after 24 h treatment with vehicle, 20 μM SF, or 10 μM AITC. Values are means ± SE, n = 3. **Significant difference from vehicle-treated controls at the P < 0.01 level.
Oxidative stress also enhanced [14C]cystine transport in Z310 cells. H2O2 (0.05 mM) increased uptake by 200% (P < 0.001).
DISCUSSION
The present study demonstrates that SF and AITC can protect the BCSFB in vitro from damage caused by H2O2 as assessed by both TEER and mannitol permeability. In addition, these two ITCs reduced H2O2-induced cell death in primary CP epithelial cells and a CP cell line Z310. This is the first time that protective effects of SF and AITC on the CP/BCSFB have been reported. The protective effects of the ITCs are delayed and were associated with nuclear translocation of Nrf2 and upregulation of a number of antioxidant systems regulated by that transcription factor.
The study used both primary cultures of rat CP epithelial cells and an immortalized rat CP cell line Z310 cells. The latter, developed by Wei Zheng and colleagues (2, 19, 37), have been widely used as an in vitro model of blood-CSF barrier for pharmacological and toxicological studies, although they only produce monolayers with low TEERs. We used primary CP cells for the initial BCSFB studies because of their ability to form a relatively tight barrier [∼150–200 Ω.cm2, (33)]. Such cultures are derived from neonatal rats (17, 33), and they have been used extensively in a variety of studies of CP function (30, 35, 38). While each culture has deficiencies (e.g., being a cell line or being derived from young animals), it is worth noting that the effects of ITCs on cell death, cystine uptake, and HO-1 expression were very similar in the primary cultures and the cell line.
This study focused on two ITCs, SF and AITC. SF is a component of broccoli and AITC is found in mustard seeds (7). Our studies primarily used these ITCs at 10 μM. This concentration is similar to that used in other studies examining neuroprotection (39–41). It also appeared to give near-maximal protection (Fig. 2B). It should be noted, however, that there was evidence of significant CP protection at 2.5 μM for both SF and AITC. Measurements of ITCs in human plasma are limited, but a SF concentration of 1 μM has been reported after ingestion of a cup of broccoli soup (1). It should also be noted that human diet may contain multiple ITCs, and the net effect of combinations of ITCs may be additive. There is also interest in giving pure ITCs to protect against different forms of brain injury (41, 42).
The CP epithelium forms the BCSFB and modulates chemical/fluid exchange between blood and CSF acting as a first line of defense for the brain (9, 10). The CPs also secrete a number of neurotrophic factors that modulate brain function and brain repair after injury (5, 32). Therefore, the integrity of BCSFB and functional CPs play important roles under normal and pathological conditions. Anatomical and physiological changes in the CPs may contribute to and underlie pathological processes. For example, a previous study (12) has showed that brain ischemia induced marked disruption of BCSFB that may enhance the movement of toxic compounds from blood to CSF and areas close to the ventricular system and participate in delayed neuronal death. In Alzheimer's disease, the CP epithelial cells atrophy with resultant decreases in CSF production, enzymatic activity, and polypeptide transport. The consequences of these changes can be widespread, leading to dysfunctional methylation, increased oxidative stress, and augmented accumulation of toxins (27, 28). Distinct alterations in choroidal polypeptide synthesis also occur during brain development and in central nervous system disorders, including Alzheimer's disease, traumatic brain injury, and ischemia (11, 14, 27). Recent studies have shown that CP cells isolated and maintained in vitro and CP cell transplants into brain and CSF exert neuroprotective effects (3, 4). Therefore, CP protection is a potential approach to protecting the brain. It has been reported that SF can protect BBB and reduce brain edema and infarct size after cerebral ischemia (25, 39, 41). However, the protective effects of SF and other ITCs on BCSFB had not been studied previously. In this study, we found that SF and AITC both had marked protective effects against oxidative damage to the CP and the BCSFB. This may provides novel approaches in neurological disorders prevention and treatment.
In this study, protection from oxidative damage was only found when cells were pretreated for 12–48 h. No protection was found when cells were pretreated with AITC for 1 h, suggesting that protection requires protein production. The transcription factor Nrf2 regulates the expression of antioxidative and other cytoprotective genes, and and it plays an important role in cellular defense against a variety of insults (22). Studies have shown that SF has neuroprotective effects in several brain cells by activating Nrf2 (8, 25, 39, 41), and AITC has been found to activate Nrf2 in fibroblasts (13). In this study, we examined the changes of nuclear Nrf2 and four downstream antioxidative proteins HO-1, NQO1, xCT, and ferritin, by Western blot, real-time PCR, and cell cystine uptake (to examine xCT function). We found in this study that both SF and AITC can significantly upregulate Nrf2 , HO-1, and NQO1 (but not ferritin) in Z310 and CP cells. Interestingly, xCT activity increase is only observed after SF treatment, which may indicate a different and selective downstream regulation by these two compounds. All of these changes suggest that the protective effects of SF and AITC in CP are via activation of the Nrf2 pathway. In the current studies, SF and AITC reduced the BCSFB disruption induced by H2O2 and have shown that they reduce the LDH release indicative of cell damage. However, it is possible that these agents also impact epithelial tight junction function. At the blood-brain barrier, alterations in tight junction function may occur through changes in tight junction protein expression, interactions, and cellular location (31). The role of such changes at the CP is being examined.
Oxidative stress is involved in neurological disorders such as stroke, Parkinson's disease, Alzheimer's disease, and multiple sclerosis. Among the many factors causing brain damage in these disorders, oxidative stress plays an important role. Currently, there is no effective treatment for these disorders and a lot of studies have been focusing on developing novel antioxidant compounds as one of the therapeutic strategies. ITCs, a group of naturally occurring compounds in abundance in cruciferous vegetables and widely consumed as human diet, could be potential candidates, as diet or medicine, in preventing and therapy for these disorders since they may protect BCSFB and the brain by activating the intracellular antioxidant system via Nrf2.
Because the ITCs resulted in upregulation of endogenous defense mechanisms, the current study focused on treating cells before the oxidative stress to enable those mechanisms to be upregulated at the time of the stress. As ITCs are natural dietary components, such “pretreatment” may occur. However, SF has also been shown to be protective when given after ischemic and hemorrhagic stroke onset (39, 43). This may reflect protection against delayed injury in those conditions or it could be that some cell types differ in the time course of protection.
In summary, ITCs have a marked protective effect on the CP and the BCSFB. The effects of dietary ITCs on CP function may affect BCSFB function and influence acute (e.g., stroke) and chronic (e.g., Alzheimer's disease) associated with oxidative stress.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.X. and R.F.K. conception and design of research; J.X., G.N.A., and N.Z. performed experiments; J.X. and R.F.K. analyzed data; J.X. and R.F.K. interpreted results of experiments; J.X. and R.F.K. prepared figures; J.X. and R.F.K. drafted manuscript; J.X. and R.F.K. edited and revised manuscript; J.X. and R.F.K. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grant NS-034709 (to R. F. Keep) and by a grant from the Motor City Golf Classic (to J. Xiang).
REFERENCES
- 1. Al Janobi AA, Mithen RF, Gasper AV, Shaw PN, Middleton RJ, Ortori CA, Barrett DA. Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ionisation mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 844: 223–234, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Behl M, Zhang Y, Monnot AD, Jiang W, Zheng W, Behl M, Zhang Y, Monnot AD, Jiang W, Zheng W. Increased beta-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1. Toxicol Appl Pharmacol 240: 245–254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. CNS grafts of rat choroid plexus protect against cerebral ischemia in adult rats. Neuroreport 15: 1543–1547, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke 35: 2206–2210, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Chodobski A, Szmydynger-Chodobska J. Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech 52: 65–82, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Chung FL, Juchatz A, Vitarius J, Hecht SS. Effects of dietary compounds on alpha-hydroxylation of N-nitrosopyrrolidine and N′-nitrosonornicotine in rat target tissues. Cancer Res 44: 2924–2928, 1984 [PubMed] [Google Scholar]
- 7. Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab 3: 233–255, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Danilov CA, Chandrasekaran K, Racz J, Soane L, Zielke C, Fiskum G, Danilov CA, Chandrasekaran K, Racz J, Soane L, Zielke C, Fiskum G. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. GLIA 57: 645–656, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG. The choroid plexus in the rise, fall and repair of the brain. Bioessays 27: 262–274, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Emerich DF, Vasconcellos AV, Elliott RB, Skinner SJ, Borlongan CV. The choroid plexus: function, pathology and therapeutic potential of its transplantation. Exp Opin Biol Therapy 4: 1191–1201, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Engelhardt B, Wolburg-Buchholz K, Wolburg H. Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech 52: 112–129, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Ennis SR, Keep RF. The effects of cerebral ischemia on the rat choroid plexus. J Cereb Blood Flow Metab 26: 675–683, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Ernst IM, Wagner AE, Schuemann C, Storm N, Hoppner W, Doring F, Stocker A, Rimbach G. Allyl-; butyl- and phenylethyl-isothiocyanate activate Nrf2 in cultured fibroblasts. Pharmacol Res 63: 233–240, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Ferrand-Drake M. Cell death in the choroid plexus following transient forebrain global ischemia in the rat. Microsc Res Tech 52: 130–136, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Ghersi-Egea JF, Strazielle N. Brain drug delivery, drug metabolism, and multidrug resistance at the choroid plexus. Microsc Res Tech 52: 83–88, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Hosoya K, Tomi M, Ohtsuki S, Takanaga H, Saeki S, Kanai Y, Endou H, Naito M, Tsuruo T, Terasaki T. Enhancement of l-cystine transport activity and its relation to xCT gene induction at the blood-brain barrier by diethyl maleate treatment. J Pharmacol Exp Ther 302: 225–231, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Hu Y, Ocheltree SM, Xiang J, Keep RF, Smith DE. Glycyl-l-glutamine disposition in rat choroid plexus epithelial cells in primary culture: role of PEPT2. Pharm Res 22: 1281–1286, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M, Uno K, Takarada T, Hinoi E, Yoneda Y. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. Glutamate is a determinant of cellular proliferation through modulation of nuclear factor E2 p45-related factor-2 expression in osteoblastic MC3T3–E1 cells. J Biol Chem 275: 16023–16029, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Kalia K, Jiang W, Zheng W, Kalia K, Jiang W, Zheng W. Manganese accumulates primarily in nuclei of cultured brain cells. Neurotoxicology 29: 466–470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miura K, Ishii T, Sugita Y, Bannai S. Cystine uptake and glutathione level in endothelial cells exposed to oxidative stress. Am J Physiol Cell Physiol 262: C50–C58, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Morse MA, Eklind KI, Hecht SS, Jordan KG, Choi CI, Desai DH, Amin SG, Chung FL. Structure-activity relationships for inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone lung tumorigenesis by arylalkyl isothiocyanates in A/J mice. Cancer Res 51: 1846–1850, 1991 [PubMed] [Google Scholar]
- 22. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284: 13291–13295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okuno S, Sato H, Kuriyama-Matsumura K, Tamba M, Wang H, Sohda S, Hamada H, Yoshikawa H, Kondo T, Bannai S. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br J Cancer 88: 951–956, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ott BR, Cohen RA, Gongvatana A, Okonkwo OC, Johanson CE, Stopa EG, Donahue JE, Silverberg GD. and Alzheimer's Disease Neuroimaging I. Brain ventricular volume and cerebrospinal fluid biomarkers of Alzheimer's disease. J Alzheimers Dis 20: 647–657, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ping Z, Liu W, Kang Z, Cai J, Wang Q, Cheng N, Wang S, Zhang JH, Sun X. Sulforaphane protects brains against hypoxic-ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res 9: 2010 [DOI] [PubMed] [Google Scholar]
- 26. Saito Y, Wright EM. Bicarbonate transport across the frog choroid plexus and its control by cyclic nucleotides. J Physiol 336: 635–648, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer's disease. Front Biosci 8: 1, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Serot JM, Bene MC, Foliguet B, Faure GC. Morphological alterations of the choroid plexus in late-onset Alzheimer's disease. Acta Neuropathol (Berl) 99: 105–108, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Shen H, Smith DE, Keep RF, Xiang J, Brosius FC., 3rd Targeted disruption of the PEPT2 gene markedly reduces dipeptide uptake in choroid plexus. J Biol Chem 278: 4786–4791, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Shu C, Shen H, Teuscher NS, Lorenzi PJ, Keep RF, Smith DE. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: studies in rat choroid plexus epithelial cells in primary culture. J Pharmacol Exp Ther 301: 820–829, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem 284: 19053–19066, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stopa EG, Berzin TM, Kim S, Song P, Kuo-LeBlanc V, Rodriguez-Wolf M, Baird A, Johanson CE. Human choroid plexus growth factors: What are the implications for CSF dynamics in Alzheimer's disease? Exp Neurol 167: 40–47, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Strazielle N, Ghersi-Egea JF. Demonstration of a coupled metabolism-efflux process at the choroid plexus as a mechanism of brain protection toward xenobiotics. J Neurosci 19: 6275–6289, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74: 1526–1539, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Teuscher NS, Shen H, Shu C, Xiang J, Keep RF, Smith DE, Lorenzi PJ. Carnosine uptake in rat choroid plexus primary cell cultures and choroid plexus whole tissue from PEPT2 null mice. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: studies in rat choroid plexus epithelial cells in primary culture. J Neurochem 89: 375–382, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62: 5196–5203, 2002 [PubMed] [Google Scholar]
- 37. Wang X, Li GJ, Zheng W, Wang X, Li GJ, Zheng W. Upregulation of DMT1 expression in choroidal epithelia of the blood-CSF barrier following manganese exposure in vitro. Brain Res 1097: 1–10, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiang J, Ennis SR, Abdelkarim GE, Fujisawa M, Kawai N, Keep RF. Glutamine transport at the blood-brain and blood-cerebrospinal fluid barriers. Neurochem Int 43: 279–288, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett 393: 108–112, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Zhao J, Moore AN, Clifton GL, Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res 82: 499–506, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci 27: 10240–10248, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao X, Sun G, Zhang J, Strong R, Dash P, Kan Y, Grotta J, Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 38: 3280–3286, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 38: 3280–3286, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Zheng W, Zhao Q. Establishment and characterization of an immortalized Z310 choroidal epithelial cell line from murine choroid plexus. Brain Res 958: 371–380, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]