Abstract
This study examined the effects of anti-TGF-β antibody (1D11) therapy in Dahl S (S) rats fed a 4% NaCl diet. Baseline renal expression of TGF-β1 and the degree of injury were lower in female than male S rats maintained on a 0.4% NaCl diet. 4% NaCl diet increased mean arterial pressure (MAP), proteinuria, and renal injury to the same extent in both male and female S rats. Chronic treatment with 1D11 had renoprotective effects in both sexes. The ability of 1D11 to oppose the development of proteinuria when given alone or in combination with antihypertensive agents was further studied in 6-wk-old female S rats, since baseline renal injury was less than that seen in male rats. 1D11, diltiazem, and hydrochlorothiazide (HCT) attenuated the development of hypertension, proteinuria, and glomerular injury. 1D11 had no additional effect when given in combination with these antihypertensive agents. We also explored whether 1D11 could reverse renal injury in 9-wk-old male S rats with preexisting renal injury. MAP increased to 197 ± 4 mmHg and proteinuria rose to >300 mg/day after 3 wk on a 4% NaCl diet. Proteinuria was reduced by 30–40% in rats treated with 1D11, HCT, or captopril + 1D11, but the protective effect was lost in rats fed the 4% NaCl diet for 6 wk. Nevertheless, 1D11, HCT, and captopril + 1D11 still reduced renomedullary and cardiac fibrosis. These results indicate that anti-TGF-β antibody therapy reduces renal and cardiac fibrosis and affords additional renoprotection when given in combination with various antihypertensive agents in Dahl S rats.
Keywords: hypertension, renal injury, kidney, glomerulus, chronic kidney disease
hypertension is one of the leading causes of chronic kidney disease (CKD) that affects nearly 20 million Americans (9, 17). Despite effective medications, compliance and drug costs remain a problem, and only a small percentage of patients achieve adequate blood pressure control (9). As a result, the incidence of CKD is still increasing, and the cost to the federal government now exceeds $42 billion/yr. Clearly, there is a need for more effective therapies to slow the progression of hypertension-induced CKD.
Transforming growth factor-β (TGF-β) is a multifunctional cytokine with profibrogenic properties that has been implicated in the pathogenesis of renal, cardiac, and vascular end-organ damage associated with hypertension (5, 24, 29, 36, 37, 44). TGF-β increases the deposition (27) and reduces the degradation of matrix proteins (14) and facilitates a profibrotic state (4). Previous studies have indicated that circulating and/or renal concentrations of TGF-β1 are elevated in man and experimental animal models of glomerulonephritis (39, 42), diabetic nephropathy (32, 37–39), and hypertension-induced glomerular disease (4, 28). Chronic administration of TGF-β2 or upregulation of the expression of TGF-β1 induces renal fibrosis (19, 22) and glomerular disease in experimental animal models, similar to that seen in patients with diabetes and/or hypertension (5, 20, 21, 23, 27). Other studies have suggested that the renal production of TGF-β is stimulated by elevations in dietary sodium intake and in salt-sensitive forms of hypertension (35, 40, 41). This is associated with a rise in proteinuria and renal hypertrophy and fibrosis (2, 6, 31, 33, 43). Chronic inhibition of TGF-β with decorin or knockdown of the expression of TGF-β has been reported to reduce the degree of glomerulosclerosis in models of experimental proliferative glomerulonephritis (3, 4, 18). Downregulation of TGF-β expression with either antisense oligodeoxynucleotides or inhibition of the actions of TGF-β with a neutralizing antibody also have been shown to decrease proteinuria in animal models of renal injury (1, 7, 8, 15, 44).
More recently, chronic administration of an anti-TGF-β antibody was found to reduce mean arterial pressure (MAP), proteinuria, and the degree of renal medullary interstitial fibrosis in 12-wk-old male Dahl S rats fed a high-salt diet (10). However, it did not reduce the degree of glomerulosclerosis, in part, because the male animals in this study already exhibited rather severe glomerular injury, even when they were maintained on a low-salt diet to prevent the development of hypertension. Thus, it remains to be determined whether chronic blockade of TGF-β1 can delay or prevent the development of hypertension-induced renal injury if it is given to younger animals prior to the development of glomerular disease. Moreover, little is known as to whether there are any sex differences in the renal expression of TGF-β or the effectiveness of anti-TGF-β therapy on the development of proteinuria and glomerular disease in Dahl S rats. Thus, the present study compared the renal expression of TGF-β1, TGF-β2, and TGF-β3 and the development of hypertension, proteinuria, and glomerular injury in young (6 wk old) male and female Dahl S rats and then examined the renoprotective effects of chronic administration of a murine monoclonal antibody (1D11) that neutralizes all isoforms of TGF-β (12), when given alone or in combination with other antihypertensive agents.
MATERIALS AND METHODS
General.
Experiments were performed on 234 Dahl S (SS/Jr) rats obtained from a colony maintained at the Medical College of Wisconsin and that were later transferred to University of Mississippi Medical Center. The rats were housed in the animal care facility approved by the American Association for Accreditation of Laboratory Animal Care at both institutions. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin, as well as the University of Mississippi Medical Center. The rats had free access to food and water throughout the study.
Protocol 1. comparison of the expression of the TGF-β isoforms in male and female Dahl S rats.
These experiments were performed on 24 male and 24 female Dahl S rats that were maintained from weaning on a control purified AIN-76 diet (Dyets, Bethlehem, PA) containing 0.4% NaCl instead of standard rat chow containing 0.9–1.0% NaCl by weight. At 6 wk of age, half the rats were switched to a high-salt AIN-76 diet containing 4% NaCl, while the rest of the animals remained on the control diet. After 1 or 3 wk on these diets, the rats were then anesthetized with isoflurane, and the left kidney was collected for measurement of the expression of TGF-β proteins by Western blot, and the right kidney was collected for immunohistochemistry.
Western blot analysis.
The kidney was separated into renal cortex and medulla and then homogenized in a solution containing 20 mM HEPES, 10 mM sodium chloride, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 10 mM EDTA, and a protease inhibitor cocktail. The homogenates were centrifuged at 5,000 g for 5 min and 9,000 g for 15 min, and the supernatant was collected. Western blot analysis for TGF-β isoforms was performed by incubating the blots with TGF-β1 (sc-146, 1:250; Santa Cruz Biotechnology, Santa Cruz, CA), β2 (sc-90, 1:250), and β3 (ab-15537, 1:250; Abcam, Cambridge, MA) primary antibodies overnight followed by a 1:10,000 dilution of a horseradish peroxidase-coupled secondary antibody (sc-2004) for 1 h. The blots were exposed to SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, cat. no. 34076) and imaged using a ChemiDoc photodocumentation system (Bio-Rad, Hercules, CA). Equal loading of protein was confirmed by stripping and reprobing the membranes with a β-actin primary antibody (Sigma, 1:5,000) overnight followed by a 1:20,000 dilution of horseradish peroxidase-coupled secondary antibody (sc-2004) for 1 h.
Immunohistochemistry.
The expression of TGF-β1, TGF-β2, and TGF-β3 proteins was assessed by indirect immunofluoresence staining of frozen kidney sections obtained from both male and female Dahl S rats maintained on the control 0.4% NaCl diet or exposed to a 4% NaCl diet for 1 or 3 wk. The rats were anesthetized with 2% isoflurane, and the kidneys were perfused with 20 ml of 4% paraformaldehyde/4% sucrose solution. The kidneys were extracted, hemisected, and fixed in a 4% paraformaldehyde/4% sucrose solution for 2 h before stored in 30% sucrose solution overnight at 4°C. The kidneys were embedded in Tissue-Tek OCT embedding medium and frozen by liquid nitrogen and stored at −80°C. Frozen sections (8 μM thick) were cut and mounted on poly-l-lysine-coated slides. The sections were fixed in ice-cold acetone for 5 min and air-dried. After a 1-min wash in PBS and two 2-min washes in TBS-Tween 20, the slides were blocked with 1% goat serum + 1% milk for 1 h at room temperature. The slides were incubated overnight at 4°C in a sealed container with isoform specific primary antibody (TGF-β1; β2 or β3) at a 1:100 dilution. The slides were rinsed twice with TBS-Tween 20 for 5 min and incubated for 1 h with secondary antibody (Alexa Fluor 488 goat anti-rabbit, 1:1,000 in 1% goat serum). The slides were rinsed 3 times for 2 min in TBS-Tween 20 and counterstained with 0.001% Evan's Blue dye for 5 min to quench the green autofluorescence of the tissue. Finally, the slides were washed twice for 1 min in distilled water, cover slipped with ProLong Gold antifade mounting medium (Invitrogen no. p36930), and stored at 4°C in the dark until analyzed. Images were captured using a Nikon fluorescence microscope using a 20× objective and a color camera using both FITC and rhodamine filter sets and the images overlaid.
Protocol 2: comparison of the sex-dependent effects of anti-TGF-β therapy on the development of hypertension-induced renal injury in Dahl S rats.
These experiments were performed on 28 male and 35 female Dahl S rats maintained from weaning on a 0.4% NaCl diet. At 6 wk of age, the male rats were switched to the 4% NaCl diet for 3 wk, while the female rats were fed the 4% NaCl diet for 4 wk, until they achieved the same level of proteinuria. A TGF-β neutralizing antibody (1D11) was administered via intraperitoneal injection at a dose of 0.5 mg/kg, every other day to 15 of the male and female rats, while 7 male and 13 female control rats received vehicle. An additional six male and seven female rats remained on the control 0.4% NaCl diet for the duration of the study. The rats were placed in metabolic cages weekly to collect overnight urine samples for the measurement of protein excretion. Prior to the end of the study, arterial pressure was measured via an indwelling femoral catheter in male and female rats after 3 and 4 wk on the 4% NaCl diet, respectively (10). Briefly, 1 wk prior to measurement of arterial pressure, the animals were anesthetized with an intramuscular injection of ketamine (40 mg/kg), xylazine (2.5 mg/kg), and acepromazine (0.6 mg/kg). A catheter was implanted in the femoral artery and tunneled to the back of the neck for direct measurement of arterial pressure. At the end of the experiments, the rats were euthanized with sodium pentobarbital, and the kidneys were collected and weighed to assess the degree of hypertrophy and fixed in a 10% buffered formalin solution for the histological assessment of renal injury. Thin paraffin sections (3 μm) were prepared and stained with Masson's trichrome. The degree of glomerular injury was assessed on ∼30 glomeruli per section. The percentage of the glomerular capillary area filled with matrix material was scored as described previously (30) on a 0–4 scale, with 0 representing no injury, 2 indicating loss of 50% of glomerular capillary area, and 4 representing the complete loss of capillaries. The sections were also examined for the degree of fibrosis in the outer medulla. Images of the outer medulla were obtained, and the percentage of fibrotic area filled in the outer medulla was determined using the Metamorph imaging program (Universal Imaging, Downingtown, PA).
Protocol 3: prevention study: comparison of anti-TGFβ antibody therapy given alone or in combination with various antihypertensive agents on the progression of hypertension-induced renal injury in young female Dahl S rats with minimal renal injury.
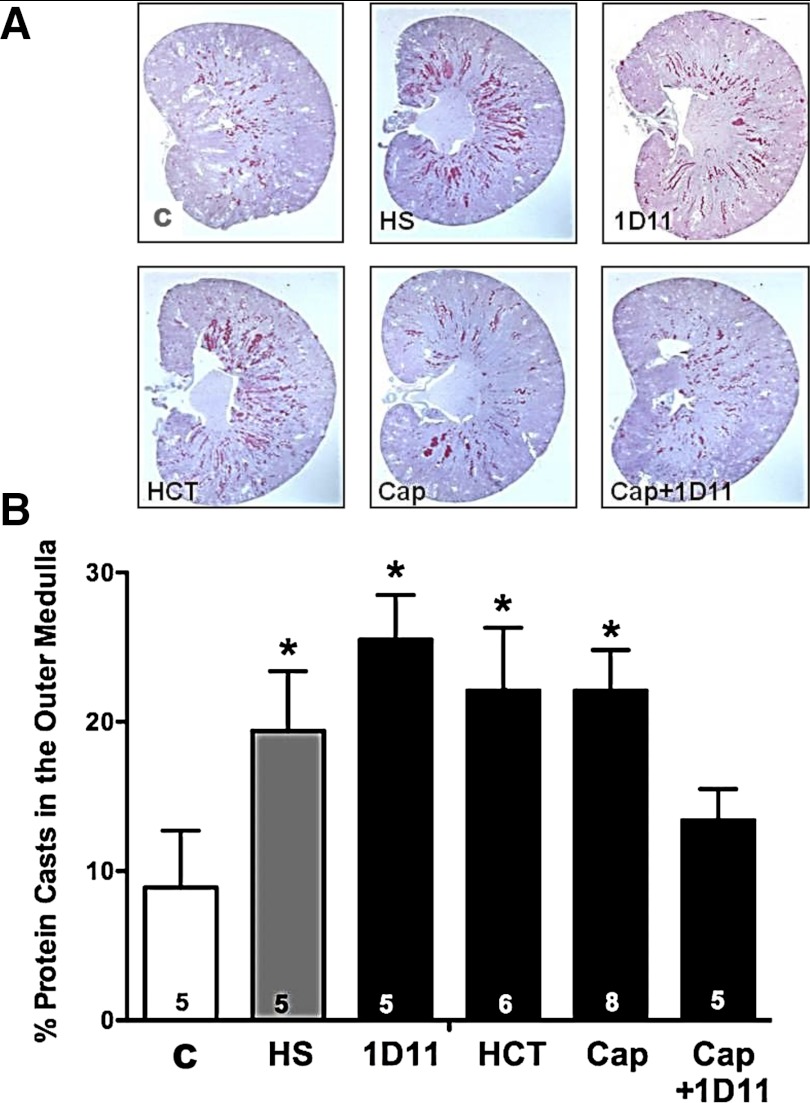
These experiments were performed on 85 six-wk-old female Dahl S rats maintained on a 0.4% NaCl diet, since the results of the experiments in protocol 2 indicated that they have much less baseline renal injury compared with the male rats when maintained on this diet. Following control measurements of proteinuria, the rats were given a 4% NaCl diet for 4 wk. The rats received one of eight treatments: group 1 (n = 12) received vehicle; group 2 (n = 10) was given the angiotensin-converting enzyme inhibitor, captopril (50 mg·kg−1·day−1); group 3 (n = 11) received a calcium channel blocker, diltiazem (100 mg·kg−1·day−1); group 4 (n = 9) was treated with the thiazide diuretic, hydrochlorothiazide (HCT; 100 mg·kg−1·day−1); group 5 (n = 11) received an injection of a TGF-β neutralizing antibody, 1D11 (0.5 mg/kg ip, every other day); groups 6, 7, and 8 (n = 7–13 per group) received combination therapy of 1D11 plus captopril, diltiazem, or HCT in the drinking water. Protein excretion was measured weekly, and MAP was measured directly via an indwelling arterial catheter after 4 wk on a 4% NaCl diet. At the end of the study, the rats were euthanized with sodium pentobarbital (100 mg/kg ip), and the kidneys were harvested, weighed, and fixed in 10% buffered formalin for the histological assessment of renal injury, as described above. The sections were also examined for the percentage of protein casts in the outer medulla.
Protocol 4. reversal study: effects of anti-TGF-β antibody therapy given alone or in combination with antihypertensive agents in male Dahl S rats with preexisting injury.
These studies were conducted using 48 nine-wk-old male Dahl S rats, maintained on a 0.4% NaCl diet from weaning, since the results obtained in protocol 2 and our previous study (10) indicated that they already exhibit an elevated baseline protein excretion and a relatively high degree of preexisting glomerular injury at this age. The rats were anesthetized by an intramuscular injection of ketamine (40 mg/kg im), xylazine (2.5 mg/kg im), and acepromazine (0.6 mg/kg im) and were instrumented with a radiotelemetry transmitter via the femoral artery for chronic monitoring of MAP. Overnight urine samples were collected weekly for assessment of protein excretion. After the determination of baseline MAP and proteinuria, the rats were assigned to one of six treatment groups: group 1 (n = 6) remained on the control diet for the duration of the study; group 2 (n = 12) was placed on a 4% NaCl diet; group 3 (n = 10) was fed a 4% NaCl diet and received intraperitoneal injections of 1D11 (0.5 mg/kg ip) every other day; group 4 (n = 11) was fed a 4% NaCl diet and received HCT (100 mg·kg−1·day−1) in the drinking water; group 5 (n = 12) was fed a 4% NaCl diet and received captopril (50 mg·kg−1·day−1) in the drinking water; and group 6 (n = 9) was fed a 4% NaCl diet and was treated with captopril plus 1D11. The rats were studied for up to 6 wk or until either MAP exceeded 200 mmHg or proteinuria exceeded 400 mg/day, at which time the animals had to be euthanized to recover the telemetry probes. At the end of the study, a final blood sample was collected, and kidneys and hearts were harvested, weighed, and fixed in 10% buffered formalin for histological evaluation. Images of heart and kidney sections were analyzed for the degree of cardiac and vasa recta fibrosis by thresholding the area of blue color (fibrosis) using Metamorph imaging software (Universal Imaging), as previously described (10).
Statistics.
Mean values ± SE are presented. Significance of difference between mean values was determined using a repeated-measures ANOVA with multiple groups or a two-way ANOVA (factored for sex and treatment) followed by the Student-Newman-Keul's post hoc test for protocols 1, 2, and 3. ANOVA followed by the Student-Newman-Keul's post hoc test was used to determine significant differences in degrees of fibrosis and protein casts in protocol 3. P < 0.05 was considered to be significant.
RESULTS
Protocol 1. expression of TGF-β isoforms in the kidney of male and female Dahl S rat fed a 4% NaCl diet.
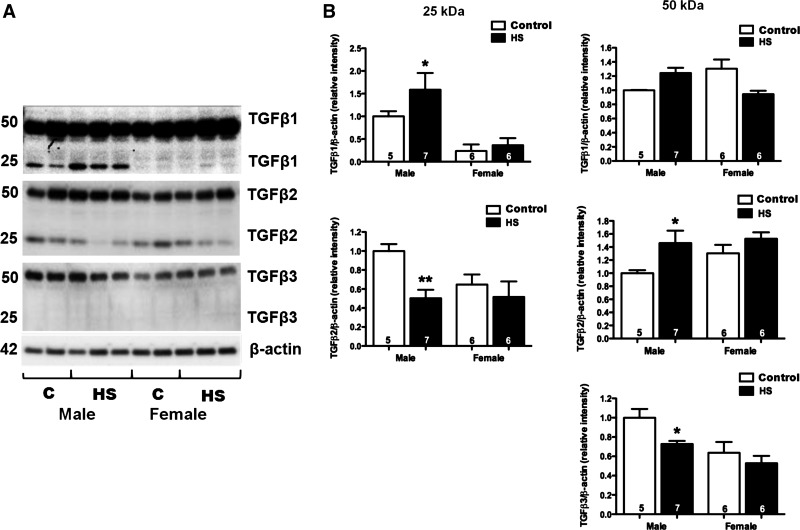
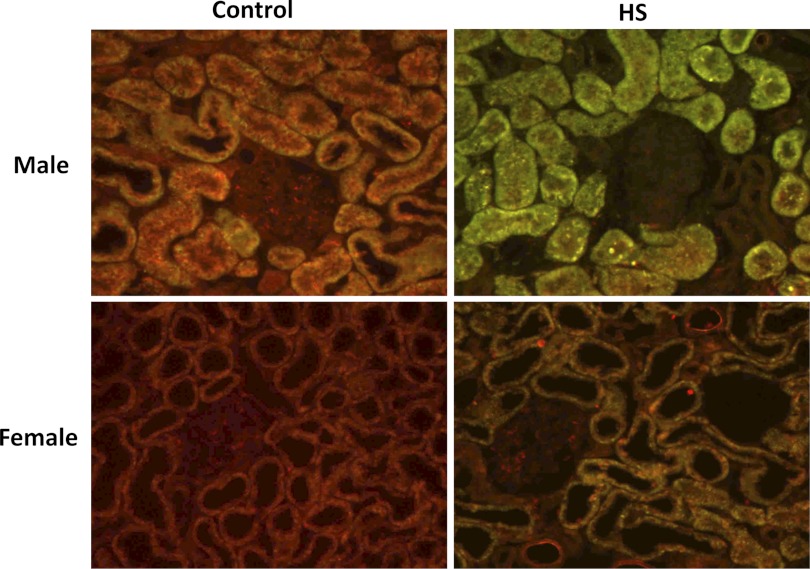
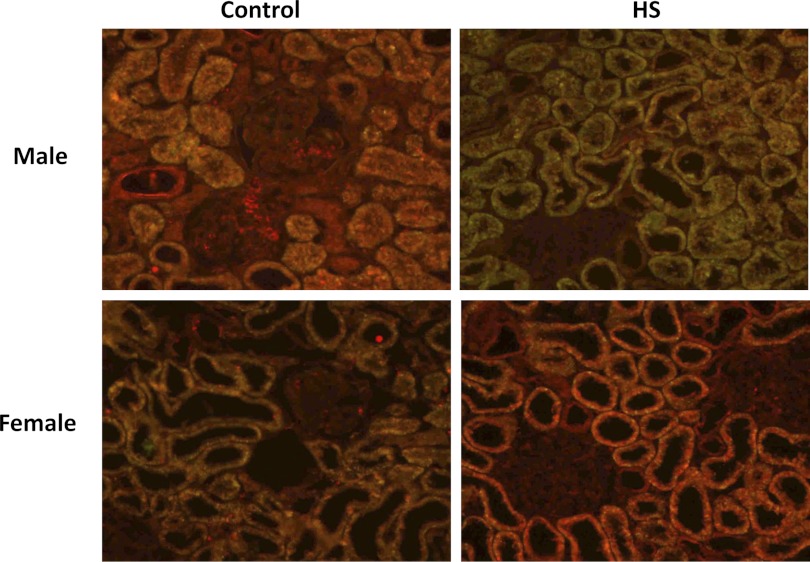
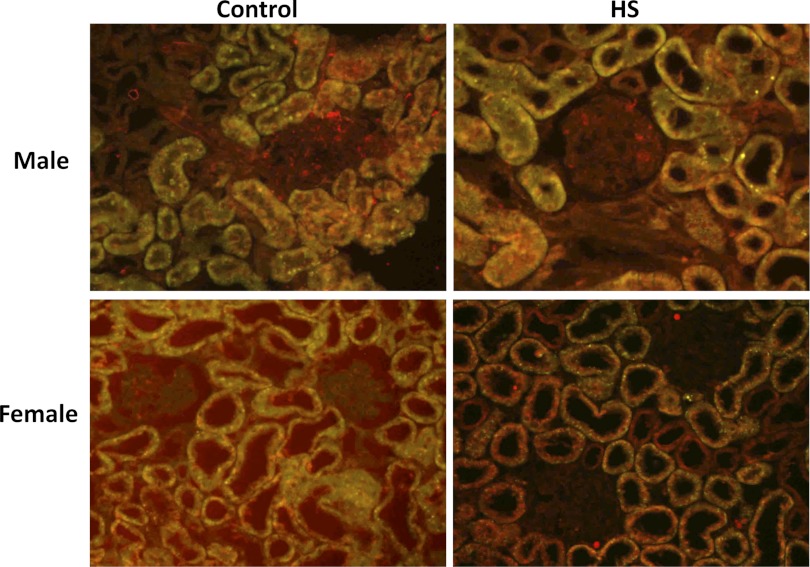
Expression of TGF-β isoforms were assessed by Western blot and immunohistochemical analysis in male and female Dahl S rats fed a 4% NaCl diet for 1 and 3 wk (Fig. 1). After 3 wk on a 4% NaCl diet, activated TGF-β1 (25-kDa band) was significantly increased in male Dahl S rats (P < 0.05). The levels of the latent form of TGF-β2 (50 kDa) also increased in the male rats fed a 4% NaCl diet, but the levels of the active form decreased. We did not detect any expression differences in the active form of TGF-β3. However, the latent form of TGF-β3 was significantly decreased in male rats fed a 4% NaCl diet for 3 wk. The expression of the active forms of both TGF-β1 and TGF-β2 was significantly lower in the kidneys of female vs. male Dahl S rats fed a 0.4% NaCl diet. However, the renal expression of the latent forms of TGF-β1, TGF-β2, and TGF-β3 were not significantly different in the kidneys of the male and female rats. Moreover, after 3 wk on a 4% NaCl diet, females displayed significantly less active TGF-β1 compared with males. Unlike the male animals, the expression of the latent forms of TGF-β1, TGF-β2, or TGF-B3 did not change in the female Dahl S rats fed a 4% NaCl diet for 3 wk. Similar directional changes were seen in the expression of the TGF-β isoforms in male and female rats fed the 4% NaCl diet for 1 wk (data not shown). Using immunohistochemistry, we determined that all 3 isoforms were largely expressed in the proximal tubules of the kidney (Figs. 2–4). We also found that the expression of TGF-β1 protein increased markedly in the proximal tubules in male Dahl S rats fed a 4% NaCl diet for 1 wk and returned toward control in animals fed the 4% NaCl diet for 3 wk (data not shown). In contrast, the expression of TGF-β1 protein was markedly less in female than in male rats fed a 0.4% NaCl diet and the expression of TGF-β1 did not increase as much in female rats challenged with the 4% NaCl diet. No significant differences were noted in staining of male and female kidneys for TGF-β2 and TGF-β3 expression after exposure to a 4% NaCl diet.
Fig. 1.
Western blot analysis of activated (25 kDa) or inactivated (50 kDa) TGF-β1, TGF-β2, and TGF-β3 isoforms in renal cortical homogenates from male and female Dahl S rats fed a control diet containing 0.4% NaCl (C) or a 4% NaCl (HS) diet for 3 and 4 wk, respectively. Numbers in the bars indicate the number of animals per group. *Significant difference (P < 0.05) from the corresponding value in rats fed the control diet within the same sex. **Significant difference (P < 0.01) from the corresponding value in male rats within the same diet.
Fig. 2.
Expression of TGF-β1 in kidneys of male and female Dahl S rats fed a control (0.4% NaCl) or 4% NaCl (HS) diet for 1 wk. Sections were labeled with an isoform-specific primary antibody (sc-146, 1:250 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), an Alexa Fluor 488 secondary antibody, and counterstained with 0.001% Evan's Blue dye to quench the green autofluorescence of the tissue. Green and yellow fluorescence indicates regions of TGF-β expression, whereas red fluorescence indicates background levels of staining.
Fig. 3.
Expression of TGF-β2 in kidneys of male and female Dahl S rats fed a control (0.4% NaCl) or 4% NaCl (HS) for 1 wk. Sections were labeled with an isoform-specific primary antibody (sc-90, 1:250 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), an Alexa Fluor 488 secondary antibody, and counterstained with 0.001% Evan's Blue dye to quench the green autofluorescence of the tissue. Green and yellow fluorescence indicates regions of TGF-β expression, whereas red fluorescence indicates background levels of staining.
Fig. 4.
Expression of TGF-β3 in kidneys of male and female Dahl S rats fed a control (0.4% NaCl) or 4% NaCl (HS) for 1 wk. Sections were labeled with an isoform-specific primary antibody (ab-15537, 1:250 dilution; Abcam, Cambridge, MA), an Alexa Fluor 488 secondary antibody, and counterstained with 0.001% Evan's Blue dye to quench the green autofluorescence of the tissue. Green and yellow fluorescence indicates regions of TGF-β expression, whereas red fluorescence indicates background levels of staining.
Protocol 2. comparison of the effectiveness of anti-TGF-β antibody therapy on the development of hypertension-induced renal injury in male and female Dahl S rats.
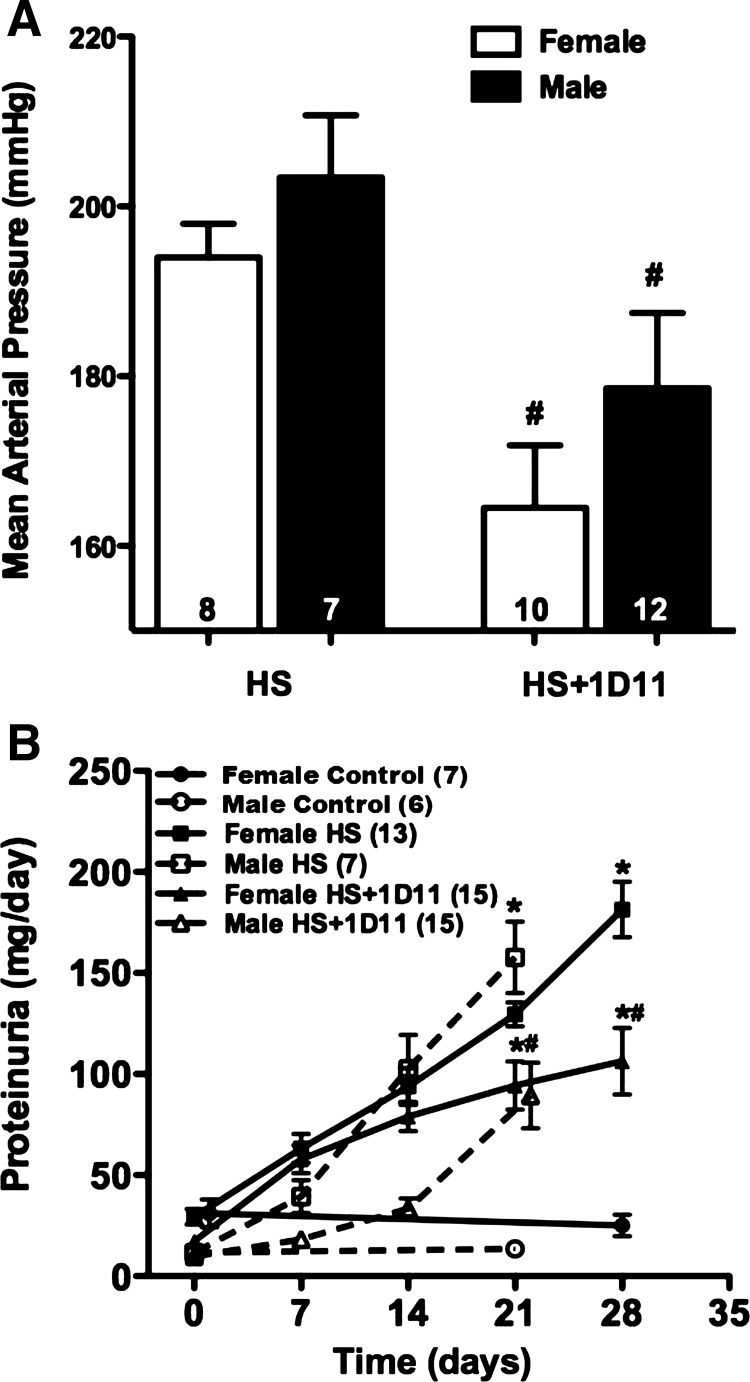
The effects of 1D11 on MAP and urinary protein excretion in male vs. female Dahl S rats are presented in Fig. 5. MAP increased in both male (203 ± 7 mmHg) rats and female (194 ± 4 mmHg) Dahl S rats and was not significantly different between sexes after 3 and 4 wk on a 4% NaCl diet (Fig. 5A). Chronic administration of the TGF-β-neutralizing antibody, 1D11, significantly attenuated the rise in arterial pressure in both male (179 ± 9 mmHg) and female (165 ± 7 mmHg) rats.
Fig. 5.
Effects of anti-TGF-β therapy, 1D11 (0.5 mg/kg ip every other day), on mean arterial pressure (MAP) (A) and proteinuria (B) in female Dahl S rats fed a 4% NaCl (HS) diet for 4 wk and male Dahl S rats fed a 4% NaCl diet for 3 wk. Numbers in bars or in parentheses indicate the number of animals studied per group. Values are presented as means ± SE. *Significant difference (P < 0.05) from the corresponding value in rats fed a control diet within the same sex. #Significant difference (P < 0.05) from the corresponding value in rats fed a 4% NaCl diet within the same sex.
Urinary protein excretion increased to 172 ± 21 mg/day in males and 181 ± 14 mg/day in females after 3 and 4 wk, respectively, on the 4% NaCl diet (Fig. 5B). Treatment with 1D11 reduced proteinuria to a similar degree in both male (93 ± 15 mg/day) and female (91 ± 15 mg/day) Dahl S rats.
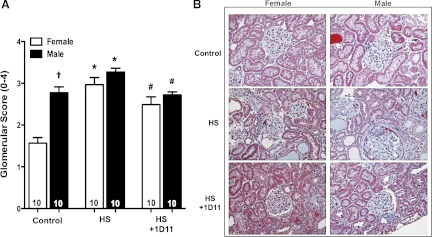
Effect of chronic anti-TGF-β antibody therapy on glomerular injury and fibrosis in female and male Dahl S rats.
The degree of baseline glomerular injury was significantly greater in 9-wk-old male rats compared with female Dahl S rats maintained on a 0.4% NaCl diet (Fig. 6). The glomerular injury score increased significantly in both male and female rats fed a 4% NaCl diet, but the increase was more apparent in females because of the lower baseline injury. Chronic treatment with 1D11 significantly reduced the degree of glomerular injury when given to both the younger male and female rats in this study.
Fig. 6.
Effects of the treatment of anti-TGF-β therapy, 1D11 (0.5 mg/kg ip every other day), on the degree of glomerular injury of male and female Dahl S rats fed a 4% NaCl (HS) diet for 3 and 4 wk, respectively. Numbers in the bars indicate the number of animals per group. Values are presented as means ± SE. *Significant difference (P < 0.05) within the same sex from the corresponding value in rats fed a control diet containing 0.4% NaCl. †Significant difference (P < 0.05) from the corresponding value in female rats within the same diet. #Significant difference (P < 0.05) from the corresponding value in rats fed a 4% NaCl diet within the same sex.
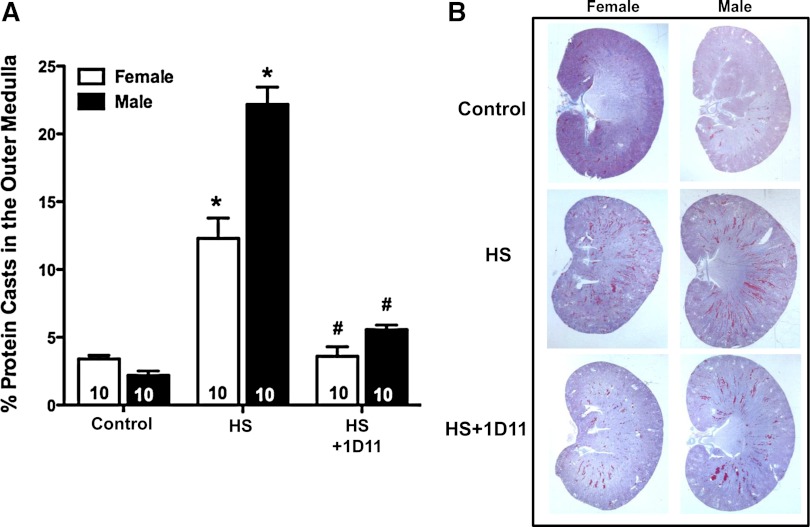
The percentage of tubular necrosis and protein casts in the outer medulla of male and female Dahl S rats is presented in Fig. 7. The percentage of protein casts in the outer medulla was significantly elevated in male and female Dahl S rats fed a 4% NaCl diet. Chronic blockade of TGF-β with 1D11 significantly reduced the percentage of protein casts by 75% in male rats and 70% in the female animals.
Fig. 7.
Effects of the treatment of anti-TGF-β therapy, 1D11 (0.5 mg/kg ip every other day), on the degree of percentage of tubular necrosis and protein casts in the outer medulla of male and female Dahl S rats fed a 4% NaCl (HS) diet for 3 and 4 wk, respectively. Numbers in the bars indicate the number of animals per group. Values are presented as means ± SE. *Significant difference (P < 0.05) within the same sex from the corresponding value in rats fed a control diet containing 0.4% NaCl. #Significant difference (P < 0.05) from the corresponding value in rats fed a 4% NaCl diet within the same sex.
Protocol 3. effect of anti-TGF-β antibody therapy on preventing renal injury in young female Dahl S rats.
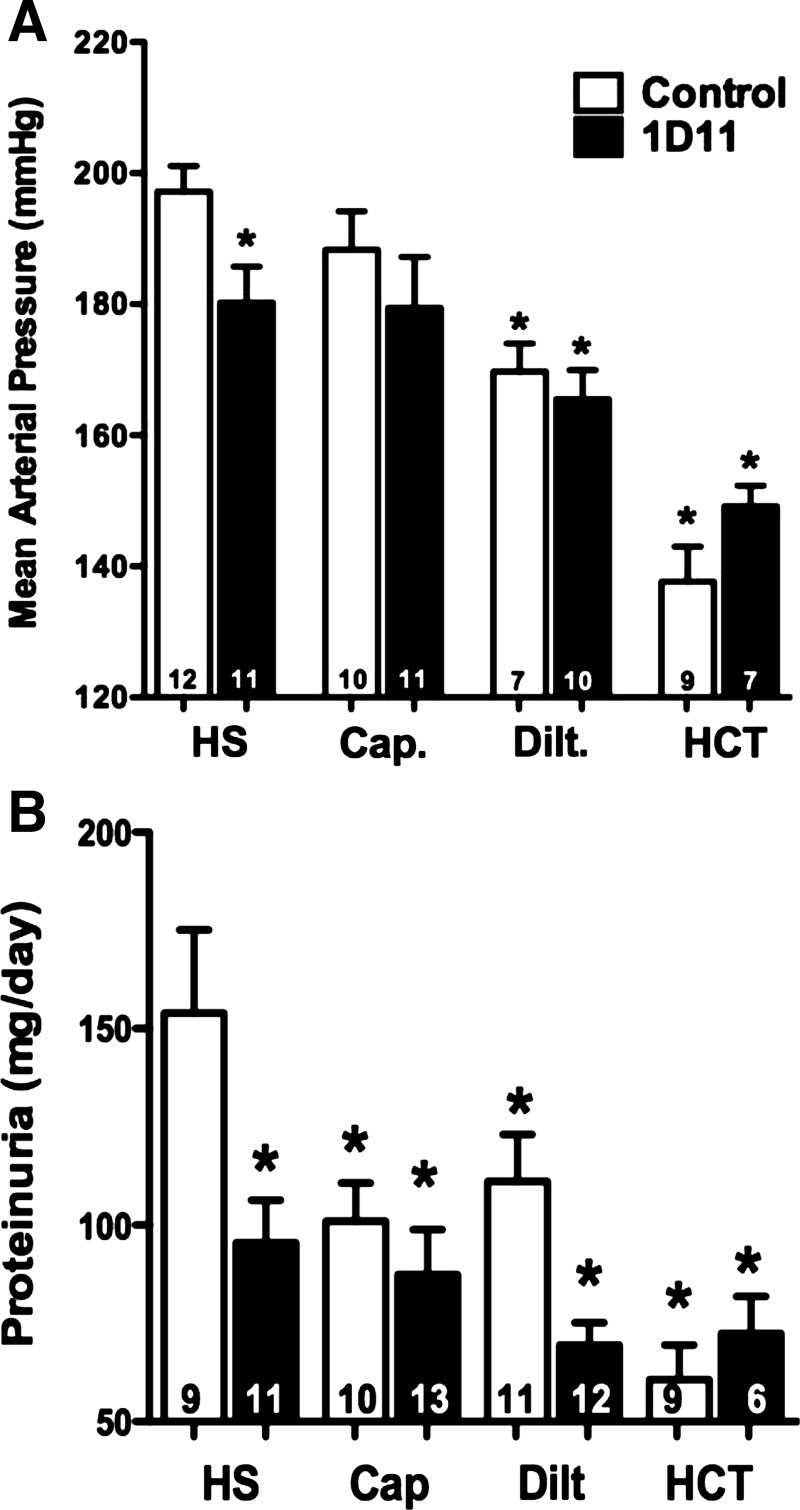
We next studied whether chronic administration of anti-TGF-β antibody when given alone or in combination with other antihypertensive agents would afford additional antihypertensive or renoprotective effects in 6-wk-old female Dahl S rats. Female rather than male rats were studied, since the results in protocol 1 indicated that they exhibit less baseline renal injury at 9 wk of age than male rats when maintained on the 0.4% NaCl diet to prevent the development of hypertension. Increases in dietary sodium intake for 4 wk increased MAP to 197 ± 4 mmHg in the female S rats (Fig. 8A). Administration of the calcium channel blocker diltiazem and the thiazide diuretic HCT significantly reduced MAP to 170 ± 4 and 138 ± 5 mmHg, respectively. Chronic administration of anti-TGF-β antibody, alone, lowered MAP ∼15 mmHg. However, add-on therapy of 1D11 with captopril, diltiazem, and HCT had no additional effect to lower MAP over the effects of each of these agents given alone.
Fig. 8.
Comparison of the effect of coadministration of 1D11 with other antihypertensive drugs on MAP (A) and proteinuria (B) in 6-wk-old female Dahl S rats fed a 4% NaCl (HS) diet for 4 wk. The rats were treated with captopril (Cap; 50 mg·kg−1·day−1), diltiazem (Dilt.; 100 mg·kg−1·day−1), or hydrochlorothiazide (HCT; 100 mg·kg−1·day−1) in the drinking water and received either intraperitoneal injections of 1D11 (0.5 mg/kg) or vehicle every other day for 4 wk. Numbers in the bars indicate the number of animals per group. Values are presented as means ± SE. *Significant difference (P < 0.05) from the corresponding value in control rats fed a 4% NaCl diet for 4 wk.
The effects of 1D11 given alone or in combination with other antihypertensive agents on the development of proteinuria are presented in Fig. 8B. Chronic treatment of female Dahl S rats with captopril reduced proteinuria by 35% compared with control rats fed a 4% NaCl diet. Diltiazem (111 ± 12 mg/day) was equally effective as captopril in reducing proteinuria. Chronic administration of HCT, which largely prevented the development of hypertension, was the most effective agent in opposing the development of proteinuria (61 ± 9 mg/day). Chronic blockade of TGF-β with 1D11, alone, significantly reduced proteinuria, but it did not enhance the beneficial effects of diltiazem, HCT, or captopril.
Effect of anti-TGF-β therapy on glomerulosclerosis and fibrosis in 6-wk-old female Dahl S rats.
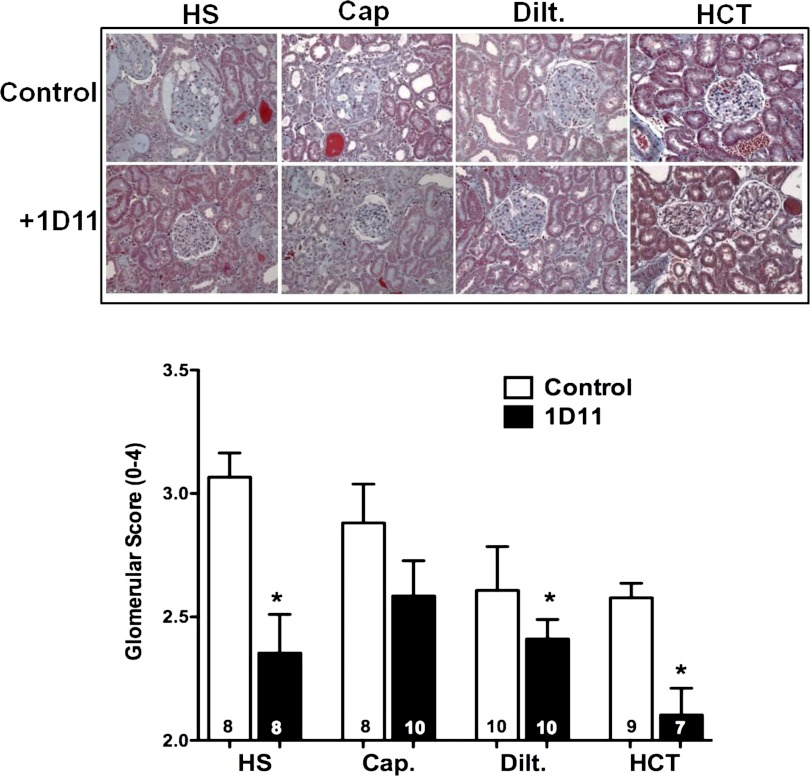
The effects of 1D11 on the development of glomerulosclerosis are presented in Fig. 9. Female Dahl S rats fed a 4% NaCl diet for 4 wk exhibited severe glomerulosclerosis, with expansion of mesangial matrix, and collapse of most of the glomerular capillaries. The glomerular injury score averaged 3.1 ± 0.03, indicating loss of 75% of the filtration area in most of the glomeruli examined. Chronic administration of captopril, diltiazem, or HCT had no significant effect on the glomerular injury score, even though proteinuria was reduced. Chronic blockade of TGF-β with 1D11, alone and when given in combination with diltiazem or HCT, but not captopril, reduced glomerular injury in female Dahl S rats fed a 4% NaCl diet for 4 wk. However, the degree of renal protection was not greater than that seen in the animals given 1D11 alone.
Fig. 9.
Effects of add-on therapy with 1D11 with other antihypertensive drugs on the degree of glomerular injury in 6-wk-old female Dahl S rats fed a 4% NaCl (HS) diet for 4 wk. The rats were treated with captopril (Cap; 50 mg·kg−1·day−1), diltiazem (Dilt.; 100 mg·kg−1·day−1), or hydrochlorothiazide (HCT; 100 mg·kg−1·day−1) in the drinking water and received either intraperitoneal injections of 1D11 (0.5 mg/kg) or vehicle every other day for 4 wk. Numbers in the bars indicate the number of animals per group. Values are presented as means ± SE. *Significant difference (P < 0.05) from the corresponding value in control rats fed a 4% NaCl diet for 4 wk.
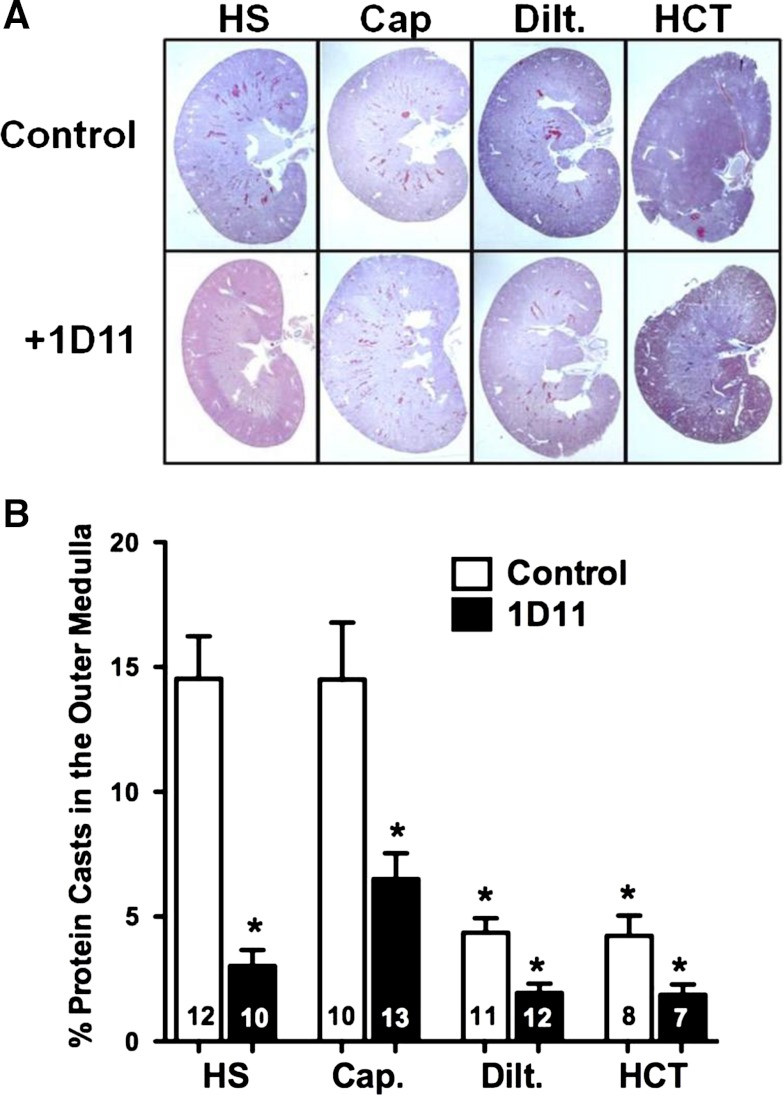
The percentage of protein casts within the outer medulla of the kidneys of female Dahl S rats fed a 4% NaCl diet are presented in Fig. 10. Administration of diltiazem or HCT significantly reduced the percentage of protein casts within the outer medulla of female rats fed a 4% NaCl diet for 4 wk. Similar to glomerular injury, administration of captopril, alone, did not lower the development of protein casts in the outer medulla. Chronic treatment of female Dahl S rats with 1D11 alone and when given in combination with captopril, diltiazem, or HCT decreased the percentage of protein casts found in the outer medulla.
Fig. 10.
Effects of add-on therapy with 1D11 with other antihypertensive drugs on the percentage of protein casts within the outer medulla of 6-wk-old female Dahl S rats fed a 4% NaCl (HS) diet for 4 wk. The rats were treated with captopril (Cap; 50 mg·kg−1·day−1), diltiazem (Dilt.; 100 mg·kg−1·day−1), or hydrochlorothiazide (HCT; 100 mg·kg−1·day−1) in the drinking water and received either intraperitoneal injections of 1D11 (0.5 mg/kg) or vehicle every other day for 4 wk. Numbers in the bars indicate the number of animals per group. Values are presented as means ± SE. *Significant difference (P < 0.05) from the corresponding value in rats fed a 4% NaCl diet.
Protocol 4: effects of add-on anti-TGF-β antibody therapy in male Dahl S rats with preexisting renal injury.
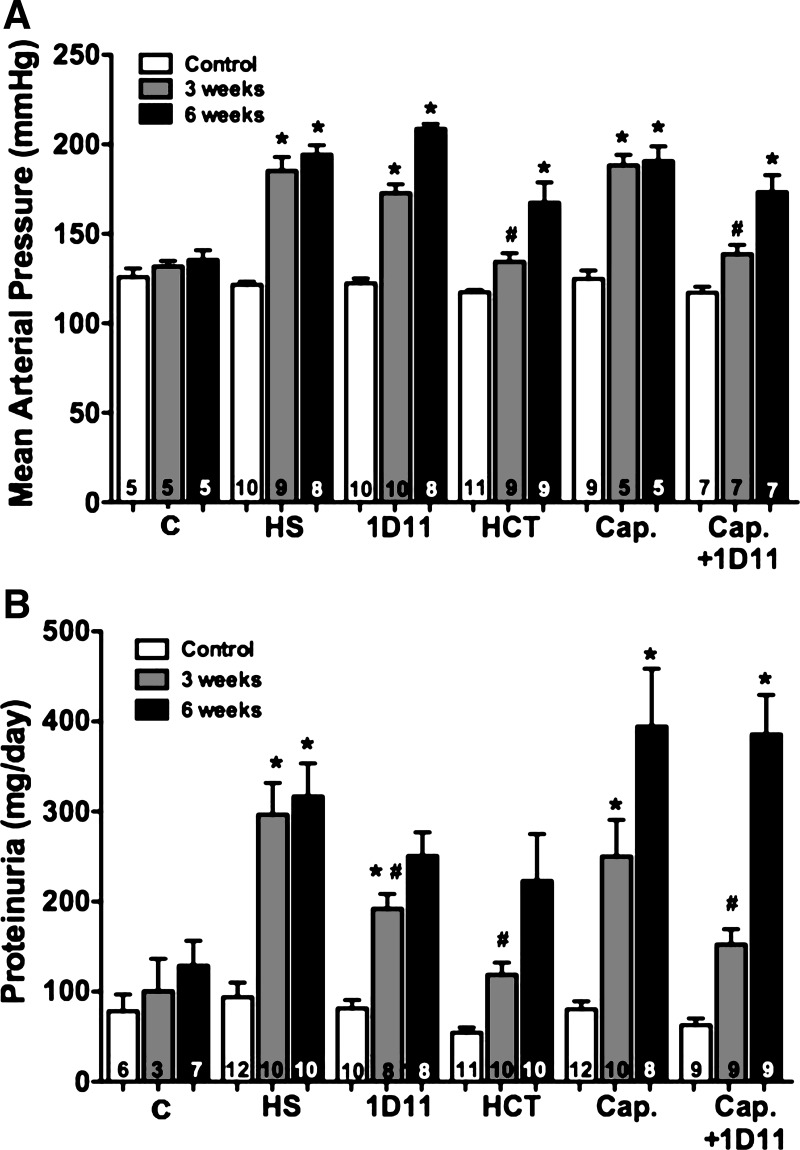
A comparison of the effects of various antihypertensive therapies vs. 1D11 on the progression of hypertension and proteinuria in 9-wk-old male Dahl S rats with preexisting renal injury is presented in Fig. 11. MAP was increased in control Dahl S rats fed a 4% NaCl diet for 3 wk (∼180 mmHg) compared with rats fed the 0.4% NaCl diet (Fig. 11A). After 3 wk on a 4% NaCl diet, chronic treatment with 1D11 or captopril had no significant effect on the development of hypertension. However, administration of HCT (134 ± 5 mmHg) and the combined administration of captopril and 1D11 (139 ± 5 mmHg) significantly reduced blood pressure. Proteinuria increased from 100 to 300 mg/day in Dahl S rats fed 4% NaCl diet for 3 wk (Fig. 11B). Administration of 1D11 (192 ± 16 mg/day), HCT (119 ± 13 mg/day), and captropril + 1D11 (161 ± 18 mg/day) significantly reduced proteinuria in rats fed a 4% NaCl diet for 3 wk.
Fig. 11.
Comparison of the effects of 1D11 vs. other antihypertensive drugs on MAP (A) and proteinuria (B) in 9-wk-old male Dahl S rats with preexisting renal injury fed either a control (C) or 4% NaCl (HS) diet for 6 wk. The rats were treated with captopril (Cap; 50 mg·kg−1·day−1), or hydrochlorothiazide (HCT; 100 mg·kg−1·day−1) in the drinking water, or intraperitoneal injection of 1D11 (0.5 mg/kg) every other day. Numbers in the bars indicate the number of animals per group. Values are presented as means ± SE. *Significant difference (P < 0.05) from the corresponding value in rats fed a control diet. #Significant difference (P < 0.05) from the corresponding value in rats fed a 4% NaCl diet.
We continued to follow the rats for an additional 3 wk to determine whether the renoprotective or antihypertensive actions of these treatments would be sustained. After 6 wk on a 4% NaCl diet, MAP rose to 209 ± 3, 191 ± 3, and 173 ± 9 mmHg in rats treated with captopril, 1D11, and captopril + 1D11, respectively. Similarly, the rats became resistant to the antihypertensive effects of HCT, and MAP rose to 167 ± 11 mmHg. After 6 wk on the 4% NaCl diet, proteinuria was not significantly different in the rats treated with 1D11 (251 ± 26 mg/day), HCT (223 ± 52 mg/day), captopril (394 ± 64 mg/day), or captopril + 1D11 (286 ± 44 mg/day) vs. the control rats.
Effect of chronic anti-TGF-β antibody therapy on glomerulosclerosis and renal medullary fibrosis in male Dahl S rats.
The glomerular injury score averaged 2.7 ± 0.1 in 9-wk-old Dahl S rats fed a 0.4% NaCl diet, indicating these animals have rather severe baseline glomerular damage. The glomerular injury score increased slightly to 3.0 ± 0.1, in male Dahl S rats fed a 4% NaCl diet for 6 wk. Treatment with 1D11 (2.7 ± 0.2), captopril (2.97 ± 0.1), HCT (2.9 ± 0.2), and captopril plus 1D11 (2.81 ± 0.1) combination therapy did not protect against glomerular injury compared with male Dahl S rats fed 4% NaCl diet alone, indicating treatment of the rats with 1D11, captopril, HCT, or captopril plus 1D11 did not protect against hypertension-induced damage in older male animals that had preexisting renal injury.
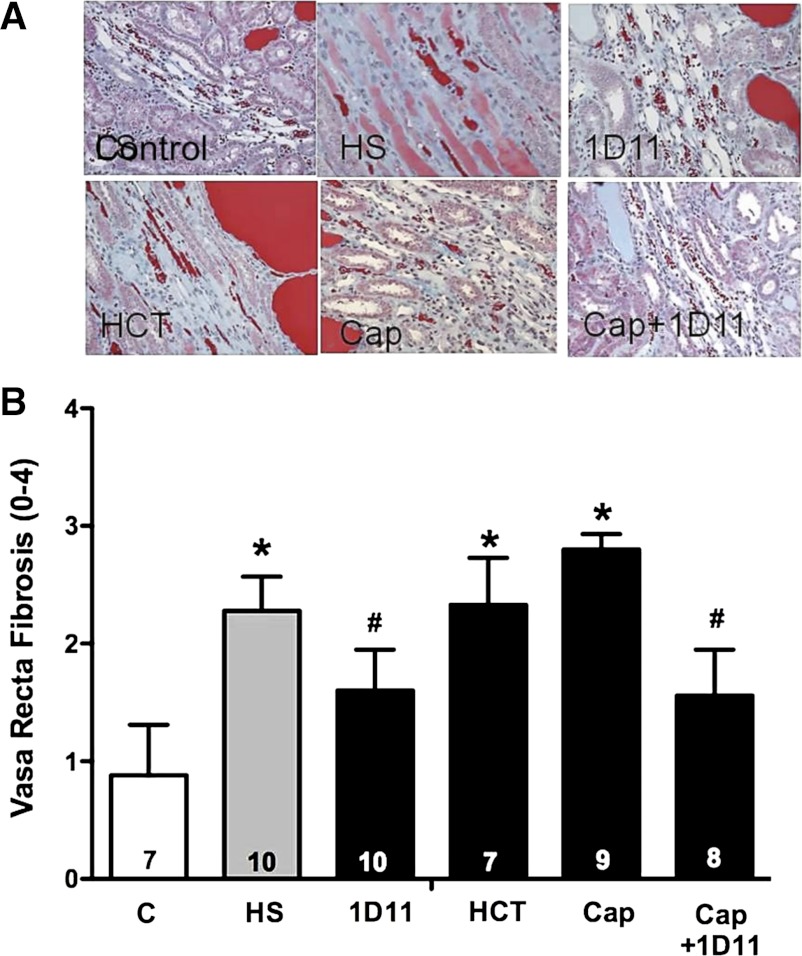
The degree of fibrosis in vasa recta capillaries and the percentage of protein casts and tubular necrosis in the outer medulla of male Dahl S rats fed a 4% NaCl diet for 6 wk are presented in Figs. 12 and 13. After 6 wk on a 4% NaCl diet, the degree of vasa recta fibrosis and area of tubular necrosis and protein casts in the outer medulla was significantly increased compared with that seen in control rats maintained on the 0.4% NaCl diet for the duration of the study. Captopril and HCT did not reduce the degree of vasa recta fibrosis (Fig. 12); however, 1D11 given alone or in combination with captopril significantly reduced the degree of vasa recta fibrosis in male Dahl S rats fed a 4% NaCl diet for 6 wk. Chronic treatment with HCT, captopril, or 1D11 alone did not reduce the area of tubular necrosis and protein casts in the outer medulla, but rats treated with captopril and 1D11 exhibited less tubular necrosis (Fig. 13).
Fig. 12.
Effects of add-on therapy with 1D11 with other antihypertensive drugs on the degree of vasa recta fibrosis in 9-wk-old male Dahl S rats with preexisting renal injury fed a 4% NaCl (HS) diet for 6 wk. The rats were treated with captopril (Cap; 50 mg·kg−1·day−1) or hydrochlorothiazide (HCT; 100 mg/kg/day) in the drinking water, or received either intraperitoneal injections of 1D11 (0.5 mg/kg ip) or vehicle every other day for 6 wk. Numbers in the bars indicate the number of animals per group. Values are presented as means ± SE. *Significant difference (P < 0.05) from the corresponding value in rats fed a control diet. #Significant difference (P < 0.05) from the value in rats fed a 4% NaCl diet.
Fig. 13.
Effects of add-on therapy with 1D11 with other antihypertensive drugs on the percentage of protein casts in the outer medulla in 9-wk-old male Dahl S rats with preexisting renal injury fed a 4% NaCl (HS) diet for 6 wk. The rats were treated with captopril (Cap; 50 mg·kg−1·day−1), or hydrochlorothiazide (HCT; 100 mg·kg−1·day−1) in the drinking water or received either intraperitoneal injections of 1D11 (0.5 mg/kg) or vehicle every other day for 6 wk. Numbers in the bars indicate the number of animals per group. Values are presented as means ± SE. *Significant difference (P < 0.05) from the corresponding value in rats fed a control diet.
Effect of chronic anti-TGF-β antibody therapy on cardiac hypertrophy and fibrosis.
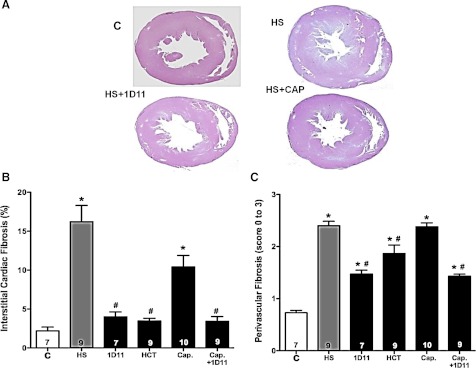
The effects of captopril, HCT, and 1D11 on the degree of cardiac hypertrophy and fibrosis are presented in Fig. 14. Male Dahl S rats fed a 4% NaCl diet for 6 wk exhibited a marked increase in left ventricular (LV) wall thickness from a control value of 16.7 ± 1.2 mm2/100 g to 23.1 ± 1.7 mm2/100 g (Fig. 14A). Chronic treatment of the rats with HCT, 1D11, captopril, or captopril plus 1D11 had no significant effect on the degree of left ventricular hypertrophy in Dahl S rats fed a 4% NaCl diet for 6 wk.
Fig. 14.
Comparison of the effects of 1D11 vs. other antihypertensive drugs on the degree of left ventricular wall thickening (A) and cardiac interstitial (B) and perivascular fibrosis (C) in 9-wk-old Dahl S rats with preexisting renal injury fed either a control (C) or 4% NaCl (HS) diet for 6 wk. Animals were treated with intraperitoneal injections of 1D11 (0.5 mg/kg ip), captopril (Cap; 50 mg·kg−1·day−1), or hydrochlorothiazide (HCT; 100 mg·kg−1·day−1) in the drinking water. Numbers in bars indicate the number of animals per group. Values are presented as mean ± SE. *Significant difference (P < 0.05) from the corresponding value in rats fed a control diet. #Significant difference (P < 0.05) from the corresponding value in rats fed a 4% NaCl diet.
The degree of cardiac fibrosis increased from 2.2 ± 0.5% to 16.2 ± 2.1% of the area of the left ventricle in male Dahl S rats fed a 4% NaCl diet for 6 wk (Fig. 14B). Chronic treatment with 1D11 significantly reduced the degree of cardiac fibrosis. Administration of HCT, which was most effective at lowering MAP, reduced the degree of cardiac fibrosis. Captopril did not reduce the degree of cardiac fibrosis when given alone, but it was equally effective as 1D11 and HCT when given in combination with 1D11.
A comparison of the degree of perivascular fibrosis in the heart is presented in Fig. 14C. Perivascular fibrosis increased from 0.7 ± 0.1 to 2.40 ± 0.1 in male Dahl S rats fed a 4% NaCl diet for 6 wk. Chronic administration of 1D11, HCT, or the combination of captopril + 1D11 significantly reduced the degree of perivascular fibrosis. However, treatment with captopril had no significant effect on the degree of perivascular fibrosis.
DISCUSSION
This study compared sex differences in the renal expression of TGF-β and the development of proteinuria and glomerular injury in male and female Dahl S rats fed a 4% NaCl diet and the effectiveness of anti-TGF-β antibody (1D11) therapy on the development of proteinuria and renal injury in these animals. The results indicate that the baseline renal expression of active (25 kDa) TGF-β1 and TGF-β2 is lower in normotensive female than male Dahl S rats maintained on a 0.4% NaCl diet, while the levels of the latent 50-kDa forms are not significantly different. We found that the expression of active TGF-β1 increases in male Dahl S rats fed a 4% NaCl diet, but it did not increase in female animals, and it remained significantly lower than that seen in the males. The upregulation of the expression of TGF-β1 in the male animals fed a 4% NaCl diet was largely in the proximal tubules. The immunostaining for TGF-β1 in the female animals fed a 4% NaCl diet was also much less than that seen in the males. These results are consistent with previous reports suggesting that a high-salt diet upregulates the expression of TGF-β in the kidney of male Dahl S rats (11). Previous studies have indicated that the expression of TGF-β increases in the first few days of a high-salt diet before the development of a marked elevation in arterial pressure (10), but as we found in the present study, the upregulation of the expression of TGF-β tends to return back toward control following the development of hypertension and renal injury in male rats fed a 4% NaCl diet for several weeks. This suggests that the change in salt load and associated neurohumoral factors, rather than hypertension and renal injury, likely triggers the upregulation of the expression of TGF-β.
To determine a sex-dependent effect of TGF-β blockade on the development of hypertension, young 6-wk-old male and female Dahl S rats fed a 4% NaCl diet for 3 and 4 wk, respectively, were studied. Both male and female Dahl S rats developed severe hypertension and proteinuria when fed a 4% NaCl diet. Females displayed proteinuria levels statistically different from the 1D11-treated rats after 4 wk on a 4% NaCl diet, a response similar to that seen in males after 3 wk on a 4% NaCl diet. Additionally, blood pressure measurements were similar in males fed a 4% NaCl diet for 3 wk and females fed a 4% NaCl diet for 4 wk. The degree of baseline glomerular injury was significantly less in female compared with male rats maintained on a 0.4% NaCl diet throughout the study. However, after 4 wk on a 4% NaCl diet, female Dahl S rats exhibited a similar degree of glomerular damage as that seen in the male rats. Treatment with 1D11 was equally effective in reducing proteinuria, glomerular injury, and renal fibrosis in both male and female Dahl S rats fed a 4% NaCl diet. This finding is somewhat surprising in that unlike male rats fed a 4% NaCl diet, TGF-β levels failed to increase in female Dahl S rats. This may suggest that the basal expression of TGF-β in female Dahl S rats is permissive and sufficient to promote renal injury. Alternatively, we may not have been able to detect important changes in the levels of TGF-β in some critical renal cell populations such as podocytes, fibroblasts, or infiltrating macrophages because the basal levels in the proximal tubules that make up the bulk of the cortical samples were unchanged.
Overall, our findings are the first to show a lack of a sex difference in the development of hypertension and proteinuria in male and female Dahl S rats fed a high-salt diet for 3–4 wk. Our findings differ from the conclusion of a previous study by Hinojosa-Laborde et al. (16), who reported that blood pressure increased to 154 mmHg in male vs. 141 mmHg in female Dahl S rats fed an 8% NaCl diet for 2 wk. The reasons for the difference in these two studies are unknown; but there are differences in the experimental design. First, we studied Dahl SS/Jr rats obtained from a colony provided by John Rapp at the Medical College of Ohio and fed them a 4% NaCl diet for 3 or 4 wk, whereas the rats in the previous study were purchased from Harlan Sprague Dawley and were only challenged with high salt for 2 wk. Our rats were maintained on AIN76-defined diet for 3–4 wk, while the rats in the previous study were fed a grain-based diet. In this regard, Mattson and colleagues (13, 26) reported that grain-based diets and the type of protein in the diet, casein vs. gluten, affect the development of hypertension and renal injury. Indeed, the degree of hypertension was far more severe in our rats, and MAP increased to more than 180 mmHg, whereas it only reached ∼150 mmHg in the male rats in the previous study.
We also evaluated the ability of chronic TGF-β antibody therapy to oppose the development of hypertension and renal injury when given alone or in combination with commonly prescribed antihypertensive therapies. These studies were first performed in young 6-wk-old female Dahl S rats, since they exhibited much less preexisting renal damage than age-matched males. Administration of 1D11 lowered MAP and significantly reduced proteinuria, glomerular injury, and the percentage of tubular necrosis and protein casts in the outer medulla after 4 wk on a 4% NaCl diet. Administration of HCT and diltiazem markedly attenuated the development of hypertension and greatly reduced the development of proteinuria. Captopril had no effect on MAP but significantly reduced proteinuria. The renoprotective effects of diltiazem and HCT are likely due to their antihypertensive properties, whereas the beneficial effects of 1D11 are likely due to its ability to oppose the profibrotic effects of TGF-β. The effect of captopril on proteinuria may result from a reduction in glomerular capillary pressure and the filtered load of protein, since blockade of the renin-angiotensin system would be expected to selectively reduce the resistance of the efferent arteriole. Even though captopril, diltiazem, and HCT reduced protein excretion and the percentage of protein casts within the outer medulla, they did not significantly reduce the degree of glomerulosclerosis. Administration of 1D11 did significantly reduce the degree of glomerular injury in the rats given diltiazem and HCT, but the degree of renal protection was not greater than that seen in the animals receiving 1D11 alone. A recent study by Ma et al. (25) shows inhibition of infiltrating macrophages reduces progressive fibrosis following anti-TGF-β antibody therapy. These effects were noted with an increase in MMP-9 activity and decrease in plasminogen activator inhibitor-1, suggesting that inactivation of TGF-β with the neutralizing anti-body, 1D11, promotes degradation of ECM formation. It should be noted that chronic administration of anti-TGF-β shows no immune dysregulation or alteration in immune parameters in mice. This is in contrast to TGF-β knockout mice, which develop widespread systemic inflammation and autoantibody formation (34). Together, these data suggest that blocking TGF-β with 1D11 may be beneficial in opposing the progression of renal fibrosis and glomerular damage in patients with salt-sensitive forms of hypertension and renal disease associated with elevated renal levels of TGF-β.
The present study also examined whether chronic blockade of TGF-β with the anti-TGF-β antibody, 1D11, could reverse the progression of proteinuria and glomerular injury when given to 9-wk-old male Dahl S rats with preexisting proteinuria and renal injury. Despite having no effect on MAP, treatment with 1D11 lowered protein excretion after 3 wk of a 4% NaCl diet. However, this renoprotective effect waned after the rats were maintained on the 4% NaCl diet for 6 wk. A similar initial reduction in proteinuria was noted in rats treated with HCT and captopril plus 1D11, but again the renoprotective effects of these treatments were lost with more prolonged exposure to the 4% NaCl diet as MAP rose. None of the treatments reduced the degree of glomerulosclerosis in these animals, but treatment with 1D11 and captopril plus 1D11 markedly reduced the degree of vasa recta fibrosis. This suggests that antihypertensive or anti-TGF-β therapy, unlike the effects seen in female rats to delay the development of glomerulosclerosis, cannot reverse the degree of glomerular injury in male rats with preexisting renal injury. Despite the fact that long-term treatment with 1D11 only delayed, but could not prevent, the development of proteinuria and glomerulosclerosis, it was still very effective at reducing the cardiac interstitial and perivascular fibrosis. This protective effect was observed in the absence of a reduction of blood pressure. Captopril was the least effective agent, but add-on therapy with 1D11 enhanced its ability to reduce cardiac fibrosis in the absence of a fall in MAP after 6 wk of a 4% NaCl diet. The marked improvement in cardiac fibrosis in rats treated with the anti-TGF-β antibody would be expected to better preserve diastolic function and may delay the onset of cardiac failure in these animals.
Overall, the results of the present study indicate that neutralizing TGF-β with 1D11 may be beneficial in opposing the development of renal fibrosis and glomerular damage in patients with salt-sensitive forms of hypertension. This occurs through increased degradation and reduced deposition of ECM, reducing progressive fibrosis in both renal and cardiac tissues. These findings suggest that the addition of TGF-β antibody therapy may offer some supplementary protection against the progression of proteinuria and glomerular disease over other antihypertensive therapies in patients with salt-sensitive forms of hypertension.
Significance and Perspectives
The present study indicates that basal renal expression of TGF-β is reduced in female vs. male Dahl S rats and that it increases in male rats fed a HS diet for 1–3 wk, but it does not increase in female rats. Sex differences in the regulation of the renal expression of TGF-β may contribute to increased severity of renal disease in males. Nevertheless, chronic treatment with an anti-TGF-β antibody was equally effective at reducing proteinuria, cardiac and renal fibrosis, and glomerular damage in both male and female Dahl S rats fed a HS diet, suggesting that anti-TGF-β therapy might afford additional renoprotection opposing the progression of renal fibrosis and glomerular damage in patients with salt-sensitive forms of hypertension and renal disease associated with elevated renal levels of TGF-β.
GRANTS
This study was partially supported by National Institutes of Health Grants HL-36279 and HL-29587.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.R.M., A.J.D.-V., K.M.J.D., C.C.A.C., and S.R.L. performed experiments; S.R.M., A.J.D.-V., C.C.A.C., and R.J.R. analyzed data; S.R.M., A.J.D.-V., C.C.A.C., J.M.W., and R.J.R. interpreted results of experiments; S.R.M. and C.C.A.C. prepared figures; S.R.M. drafted manuscript; S.R.M., C.C.A.C., J.M.W., and R.J.R. edited and revised manuscript; S.R.M., J.M.W., and R.J.R. approved final version of manuscript; A.J.D.-V. and R.J.R. were responsible for conception and design of research.
REFERENCES
- 1.Benigni A, Zoja C, Corna D, Zatelli C, Conti S, Campana M, Gagliardini E, Rottoli D, Zanchi C, Abbate M, Ledbetter S, Remuzzi G. Add-on anti-TGF-β antibody to ACE inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol 14: 1816–1824, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bitzer M, Sterzel RB, Bottinger EP. Transforming growth factor-beta in renal disease. Kidney Blood Press Res 21: 1–12, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360: 361–364, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature 346: 371–374, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Chen PY, St, John PL, Kirk KA, Abrahamson DR, Sanders PW. Hypertensive nephrosclerosis in the Dahl/Rapp rat. Initial sites of injury and effect of dietary l-arginine supplementation. Lab Invest 68: 174–184, 1993 [PubMed] [Google Scholar]
- 7.Chen S, Iglesias-de la Cruz MC, Jim B, Hong SW, Isono M, Ziyadeh FN. Reversibility of established diabetic glomerulopathy by anti-TGF-beta antibodies in db/db mice. Biochem Biophys Res Commun 300: 16–22, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 38: 606–611, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. Antihypertensive effects of chronic anti-TGF-beta antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 283: R757–R767, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension 45: 643–648, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol 142: 1536–1541, 1989 [PubMed] [Google Scholar]
- 13.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 57: 269–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douthwaite JA, Johnson TS, Haylor JL, Watson P, El Nahas AM. Effects of transforming growth factor-beta1 on renal extracellular matrix components and their regulating proteins. J Am Soc Nephrol 10: 2109–2119, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Han DC, Hoffman BB, Hong SW, Guo J, Ziyadeh FN. Therapy with antisense TGF-beta1 oligodeoxynucleotides reduces kidney weight and matrix mRNAs in diabetic mice. Am J Physiol Renal Physiol 278: F628–F634, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of Dahl hypertension. Hypertension 35: 484–489, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Jones CA. Hypertension and renal dysfunction: NHANES III. J Am Soc Nephrol 14: S71–S75, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kasuga H, Ito Y, Sakamoto S, Kawachi H, Shimizu F, Yuzawa Y, Matsuo S. Effects of anti-TGF-beta type II receptor antibody on experimental glomerulonephritis. Kidney Int 60: 745–755, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Kelly FJ, Anderson S, Thompson MM, Oyama TT, Kennefick TM, Corless CL, Roman RJ, Kurtzberg L, Pratt BM, Ledbetter SR. Acute and chronic renal effects of recombinant human TGF-β2 in the rat. J Am Soc Nephrol 10: 1264–1273, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Ketteler M, Noble NA, Border WA. Increased expression of transforming growth factor-beta in renal disease. Curr Opin Nephrol Hypertens 3: 446–452, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Kopp JB, Factor VM, Mozes M, Nagy P, Sanderson N, Bottinger EP, Klotman PE, Thorgeirsson SS. Transgenic mice with increased plasma levels of TGF-β1 develop progressive renal disease. Lab Invest 74: 991–1003, 1996 [PubMed] [Google Scholar]
- 22.Ledbetter S, Kurtzberg L, Doyle S, Pratt BM. Renal fibrosis in mice treated with human recombinant transforming growth factor-β2. Kidney Int 58: 2367–2376, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Li B, Khanna A, Sharma V, Singh T, Suthanthiran M, August P. TGF-β1 DNA polymorphisms, protein levels, and blood pressure. Hypertension 33: 271–275, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Luft FC. Transforming growth factor beta-angiotensin II interaction: implications for cardiac and renal disease. J Mol Med 77: 517–518, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Ma LJ, Jha S, Ling H, Pozzi A, Ledbetter S, Fogo AB. Divergent effects of low versus high dose anti-TGF-β antibody in puromycin aminonucleoside nephropathy in rats. Kidney Int 65: 106–115, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 45: 736–741, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Mozes MM, Bottinger EP, Jacot TA, Kopp JB. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-β) isoforms in TGF-β transgenic mice. J Am Soc Nephrol 10: 271–280, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Noble NA, Border WA. Angiotensin II in renal fibrosis: should TGF-beta rather than blood pressure be the therapeutic target? Semin Nephrol 17: 455–466, 1997 [PubMed] [Google Scholar]
- 29.Peters H, Noble NA, Border WA. Transforming growth factor-beta in human glomerular injury. Curr Opin Nephrol Hypertens 6: 389–393, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Raij L, Chiou XC, Owens R, Wrigley B. Therapeutic implications of hypertension-induced glomerular injury. Comparison of enalapril and a combination of hydralazine, reserpine, and hydrochlorothiazide in an experimental model. Am J Med 79: 37–41, 1985 [DOI] [PubMed] [Google Scholar]
- 31.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 4: 753–763, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Reeves WB, Andreoli TE. Transforming growth factor beta contributes to progressive diabetic nephropathy. Proc Natl Acad Sci USA 97: 7667–7669, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roman RJ, Alonso-Galicia M, Wilson TW. Renal P450 metabolites of arachidonic acid and the development of hypertension in Dahl salt-sensitive rats. Am J Hypertens 10: 63S–67S, 1997 [PubMed] [Google Scholar]
- 34.Ruzek MC, Hawes M, Pratt B, McPherson J, Ledbetter S, Richards SM, Garman RD. Minimal effects on immune parameters following chronic anti-TGF-β monoclonal antibody administration to normal mice. Immunopharmacol Immunotoxicol 25: 235–257, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol 297: F237–F243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma K, McGowan TA. TGF-β in diabetic kidney disease: role of novel signaling pathways. Cytokine Growth Factor Rev 11: 115–123, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Sharma K, Ziyadeh FN. Renal hypertrophy is associated with upregulation of TGF-β1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1094–F1101, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA 90: 1814–1818, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto T, Noble NA, Cohen AH, Nast CC, Hishida A, Gold LI, Border WA. Expression of transforming growth factor-β isoforms in human glomerular diseases. Kidney Int 49: 461–469, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-β1. Am J Physiol Renal Physiol 295: F406–F414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-β in rats. Am J Physiol Renal Physiol 274: F635–F641, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Yoshioka K, Takemura T, Murakami K, Okada M, Hino S, Miyamoto H, Maki S. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest 68: 154–163, 1993 [PubMed] [Google Scholar]
- 43.Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 98: 2621–2628, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]