Abstract
Intermittent hypoxia (IH) and sleep fragmentation (SF) are major manifestations of sleep apnea, a frequent condition in aging humans. Sleep perturbations are frequent in Alzheimer's disease (AD) and may underlie the progression of disease. We hypothesized that acute short-term IH, SF, and their combination (IH+SF) may reveal unique susceptibility in sleep integrity in a murine model of AD. The effects of acute IH, SF, and IH+SF on sleep architecture, delta power, sleep latency, and core body temperature were assessed in adult male human ApoE4-targeted replacement mice (hApoE4) and wild-type (WT) controls. Slow wave sleep (SWS) was significantly reduced, and rapid eye movement (REM) sleep was almost abolished during acute exposure to IH alone and IH+SF for 6 h in hApoE4, with milder effects in WT controls. Decreased delta power during SWS did not show postexposure rebound in hApoE4 unlike WT controls. IH and IH+SF induced hypothermia, which was more prominent in hApoE4 than WT controls. Mice subjected to SF also showed sleep deficits but without hypothermia. hApoE4 mice, unlike WT controls, exhibited increased sleep propensity, especially following IH and IH+SF, suggesting limited ability for sleep recovery in hApoE4 mice. These findings substantiate the potential impact of IH and SF in modulating sleep architecture and sleep homeostasis including maintenance of body temperature. Furthermore, the increased susceptibility and limited recovery ability of hApoE4 mice to sleep apnea suggests that early recognition and treatment of the latter in AD patients may restrict the progression and clinical manifestations of this frequent neurodegenerative disorder.
Keywords: sleep apnea, neurodegenerative disease, sleep fragmentation
the episodic changes in upper airway resistance during sleep in patients with sleep apnea induce numerous physiological disturbances, including fluctuations in oxygenation (intermittent hypoxia, IH), sleep fragmentation (SF), episodic oscillations in carbon dioxide tension, increased intrathoracic pressure swings, altered acid-base status, and disruptions in autonomic function and behavioral state (10).
In addition to sleep apnea, fragmented or disrupted sleep is also a common occurrence in many other clinical conditions, including neurodegenerative diseases, such as Alzheimer's disease (AD), and has been shown to contribute to the progression of neurodegenerative processes via mechanisms that remain poorly understood (13). Apolipoprotein E (ApoE) is a cholesterol transport protein that exists as three alleles: ϵ2 (E2), ϵ3 (E3), and ϵ4 (E4). An excess of ApoE4 allele was reported in patients with memory disorders and in late-onset familial and sporadic AD (∼65–75%) (7, 47, 50). The presence of ApoE4 has been estimated as contributing to 20% of all dementias (22, 40, 52). ApoE4 has been directly implicated in AD via increased amyloid deposition and impaired neuronal repair (1). The ϵ4 allele is associated with reduced levels of APOE, conferring increased susceptibility and reduced response to neuronal injury (56). Recent reports on the increased prevalence of the ApoE4 in sleep-disordered breathing (SDB) (15, 26, 35), particularly in the presence of cognitive deficits (8, 17, 27, 30, 38), leads to the assumption that ϵ4 carriers might be more vulnerable to any sleep perturbation. It is important to note that these studies do not differentiate between two working hypotheses, namely 1) AD elicits sleep disruption or sleep apnea, or 2) sleep disruption/sleep apnea may either cause or otherwise facilitate AD progression.
To further examine these issues, we hypothesized that both of these working hypotheses coexist, and therefore that the presence of components of a sleep disorder such as sleep apnea (i.e., IH, SF, or both) would lead to a more pronounced disruption of sleep integrity in a murine model of AD, and that the homeostatic sleep recovery processes to such perturbations would be reduced in the AD mice.Since hApoE4 mice start to develop aberrant pathophysiological responses at around 4 mo of age (9, 67), and since C57BL/6 mice have been previously used as controls (9), we chose to study these strains at the age 6 mo.
MATERIALS AND METHODS
Animals
Adult male human ApoE4-targeted replacement mice [B6.129P2-Apoetm3(APOE*4)MaeN8] (hApoE4) and C57BL/6NTac (wild-type, WT) mice, purchased from Taconic (Germantown, NY), were housed in a 12-h light/dark cycle (light on 7:00 AM to 7:00 PM) at a constant temperature (26 ± 1°C) and were allowed access to food and water ad libitum. The mean body weights were comparable between the groups: group 1, 25.60 ± 0.86 g; group 2, 26.0 ± 0.66 g; and group 3, 25.68 ± 0.56 g. ApoE4 expression reduces activity of ApoE and disrupts cholesterol deposition and transport in brain. These mice express human apoliprotein E4 isoform under the control of the murine ApoE regulatory sequences (29). The experimental protocols were approved by the Institutional Animal Use and Care Committee and are in close agreement with the National Institutes of Health Guide in the Care and Use of Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Surgical Procedure and Implantation of Telemetric Transmitter and Electrodes
All surgical procedures were performed under sterile conditions and using isoflurane for general anesthesia: induction, 3% isoflurane, and 1 l/min of supplemental O2 and maintenance, 2% isoflurane, and 0.5 l/min of supplemental O2. First, the animals were positioned in sternal recumbency, and a dorsal neck incision of 2–3 cm was made through the skin along the dorsal midline, after which, a 1.5- to 2-cm incision was performed through the skin and abdominal wall along the ventral midline. A telemetric transmitter weighing 3.5 g [F20-EET (DSI)], which allows for simultaneous monitoring of two biopotential channels [electroencephalogram (EEG) and electromyogram (EMG)], temperature, and locomotor activity, was inserted; biopotential leads were exteriorized; and the abdominal wall was closed using 4-0 nonabsorbable suture with a simple interrupted pattern. The two pairs of biopotential leads were then advanced subcutaneously from the ventral abdomen incision to the dorsal neck incision using a trocar. Animals were then fixed in a stereotaxic apparatus for implantation of EEG electrodes, with the first pair of biopotential leads being fixed to the skull above the frontal area (1 mm anterior to bregma and 2 mm lateral to midsagittal suture for one of the leads, and 1 mm anterior to lambda and 2.5 mm lateral to midsagittal suture for the other lead). The other pair of biopotential leads was placed within the same bundle of dorsal neck muscles for the recording of nuchal EMG.
General Experimental Set Up
Animals were housed in two identical commercially designed chambers (30 × 20 × 20 in3) (Oxycycler model A44XO, Biospherix, Redfield, NY) that accommodate two mouse cages equipped with a custom-designed sleep fragmenter device (44). The sleep fragmenter cages were placed on top of DSI telemetry receivers (RPC-1), which were in turn connected to an acquisition computer through a data exchange matrix. All exposures were conducted under a 12-h light/dark (light 7:00 AM to 7:00 PM) cycle. In all experimental protocols, following at least 1 wk of acclimatization in the cages, the magnetic switch of the transmitter was activated, and polygraphic recordings were begun at 7.00 AM. This set up enabled us to subject the animals to either IH alone, SF alone, or IH and SF combined. All experimental exposures were carried out between 1 PM and 7 PM. Physiological data were continuously acquired for 24 h using Dataquest ART acquisition software (version 3.1, DSI), at a sampling rate of 500 Hz. Data were first scored automatically using SleepSign software (Kissei Comtec) (11, 34, 44), and records were then visually confirmed or corrected as needed by an investigator who was blinded to the experimental condition.
Behavior was classified into three different states: wake, slow wave sleep (SWS), and rapid eye movement (REM) sleep. EEG during wake had low-amplitude, high-frequency (desynchronized) waves. During wake, EMG records showed gross body movement artifacts and, behaviorally, animals had grooming, scratching, and orienting activity. The SWS stage was characterized by low-frequency, high-amplitude (synchronized) EEG with a considerable reduction in EMG amplitude. The mice assumed a curled recumbent posture during this period. REM sleep was characterized by desynchronized EEG and a drastic reduction in EMG (muscle atonia). Sleep-related, low-frequency (delta) activity was also derived from the records using bandpass filtering of 1–4.0 Hz. Delta power was computed offline by using SleepSign software by Fast Fourier Transform (FFT), which was based on 512 points corresponding to 10-s epochs, at a sampling rate of 250 Hz with Hanning as the window filter of FFT. Those SWS epochs that showed movement artifacts were excluded when computing delta power, since EEG signals are especially sensitive to movement, with the resulting artifact specifically enhancing signals in the delta band. The mean wake episodes were computed throughout 24 h in 2-h bins.
Intermittent Hypoxia
Gas was circulated around each of the chambers at 60 l/min (i.e., one complete change per 10 s). The O2 concentration was continuously measured by an O2 analyzer and was changed from 1:00 PM until 7:00 PM by a computerized system controlling the gas valve outlets, such that the moment-to-moment desired oxygen concentration of the chamber was programmed and adjusted automatically. Deviations from the desired concentration were met by addition of N2 or room air (21% O2) through solenoid valves. For the remaining 12 h of dark period (7:00 PM to 7:00 AM), O2 concentrations were kept at 21%. The IH profile (18, 65) consisted of 90 s of 5.7% O2 alternating with 90 s room air for 12 h during the light period for 1 day. Ambient CO2 in the chamber was periodically monitored and maintained at 0.03% by circulating the gas through soda lime. The gas was also circulated through a molecular sieve (Type 3A, Fisons) so as to remove ammonia. Humidity was measured and maintained at 40–50% by circulating the gas through a freezer and silica gel. Ambient temperature was kept at 26 ± 1°C. The cycling oxygenation profiles aimed to reproduce repetitive oxyhemoglobin desaturations to mimic moderate to severe apneic episodes during sleep and consisted of 5.7% nadir FiO2 alternating with 21% FiO2 every 90 s. Indeed, in a separate group of our mice, oxyhemoglobin saturation (SpO2) was measured in restrained waking conditions while exposing the animals to the IH protocol. Mean nadir SpO2 values revolved around 72–78% for all mice.
Sleep Fragmentation
SF was performed by switching on the sweeper to a timer mode in the cage. In this mode, the sweeper requires around 9 s to sweep the floor of the cage one way. When it reaches the end of the cage, a relay engages the timer to pause for 2 min before enabling the sweeper to move in the opposite direction (34, 44). Between the two intervals, the animal remained undisturbed. During sweeper motion, awake animals have to step over the sweeper,and then can continue with their unrestrained behaviors. If the mouse is asleep during the sweeper crossings, the brief tactile stimulation elicited by the sweeper will elicit a brief arousal as required to step over the sweeper. This methodological approach prevents the need for human contact and intervention, minimizes physical activity during the entire sleep disruption procedure, and closely mimics patterns of sleep fragmentation seen in sleep disorders such as sleep apnea. Since on average, 30 episodes of arousal per hour occur in patients with severe apnea (i.e., every 2 min), our aim was to mimic closely the severe disease condition, and thus, chose 2-min intervals for the SF paradigm.
Murine Multiple Sleep Latency Test
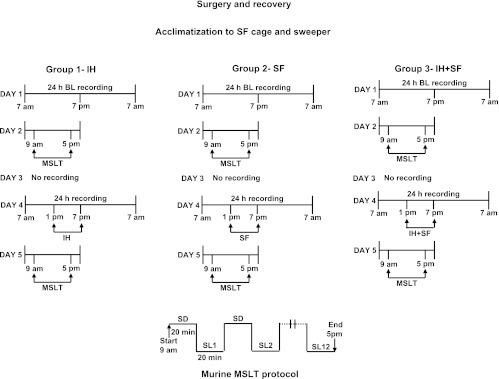
After the experimental paradigm was completed, animals were subjected to a modified multiple sleep latency test (MSLT) the next day, as previously described by Veasey and collaborators (60). Briefly, one cycle of MSLT consisted of keeping mice awake for 20 min followed by 20 min of allowable sleep time. A total of 12 such cycles was carried out starting from 9:00 AM (Fig. 1).
Fig. 1.
Experimental protocol diagram. Schematic diagram illustrating sleep, behavior, and multiple sleep latency test (MSLT) experiments with exposures to either intermittent hypoxia (IH), sleep fragmentation (SF), or IH+SF in both wild-type (WT) and human apolipoprotein E (ApoE)4-targeted replacement (hApoE4) mice. 12:12-h light:dark cycle represents light period from 7 AM to 7 PM and dark period from 7 PM to 7 AM.
Experimental Design
The various phases of the experimental paradigm are illustrated in Fig. 1.
Group 1: IH.
After a 7-day acclimatization period, on experimental day 1, baseline recordings for 24 h (7 AM to 7 AM next day) were carried out for both WT and ApoE4 (n = 6 each). The next day (day 2), following baseline recording, MSLT was performed from 9 AM to 5 PM as described above. There were no recordings performed on day 3, and on day 4, recordings were carried out for 24 h during which the mice were subjected to IH from 1 PM to 7 PM, followed by a second MSLT from 9 AM to 5 PM on day 5.
Group 2: SF.
After a 7-day acclimatization period, on day 1, 24-h baseline recordings (7 AM to 7 AM next day) were carried out for both WT and ApoE4 (n = 6 each). The next day (day 2), following baseline recordings, MSLT was performed from 9 AM to 5 PM as described above. On day 4, mice were subjected to SF from 1 PM to 7 PM, followed by MSLT from 9 AM to 5 PM on day 5.
Group 3: IH+SF.
An identical process was followed and performed in both WT and ApoE4 (n = 6 each), except that the mice were subjected to concomitant IH and SF from 1 PM to 7 PM on day 4, followed by MSLT from 9 AM to 5 PM on day 5.
Body temperature.
Body temperature was recorded every 10 s throughout all experiments. To increase the precision of recording, the lower limit of temperature records was set at 34°C and the upper limit at 41°C. The transmitter underwent three point calibration at 35°C, 37°C, and 39°C before beginning of each recording.
Data Analysis
In all the experimental conditions, the sleep-wake data were divided into 10-s epochs and scored and then summarized into 2-h bins. To elucidate the nature of identified interactions for the baseline and intervention, the data were analyzed by one-way ANOVA. First, overall statistical significance was determined for the 24-h period between the treatment groups (BL and IH, BL and SF, and BL and IH+SF). In addition, intergroup comparisons (WT and hApoE4) were also carried out. The statistical significance for 2-h bins for 24 h was assessed, followed by post hoc Holm-Sidak analyses, as needed. Similar statistical approaches were used to compare delta power during SWS, wake episodes, and core body temperature. For all comparisons, a P value <0.05 was considered to achieve statistical significance.
RESULTS
After surgical procedures, hApoE4 mice recovered similarly to WT mice with no differences recorded in eating, drinking, motor activity, and sleep postures. None of the mice showed atypical features in the EEG and EMG tracings.
Group 1 (IH)
Behavioral state.
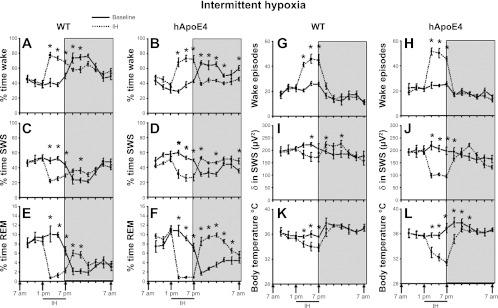
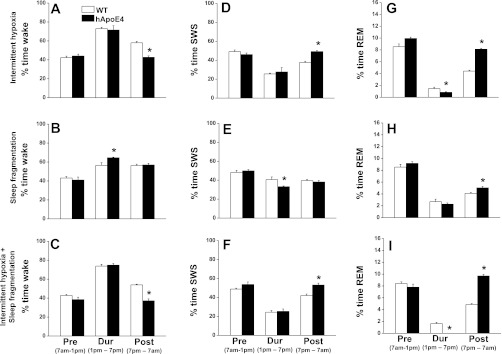
Overall analysis of the wake state for a period of 24 h revealed significant changes between baseline and IH in both WT [F(5,23) = 13.48, P < 0.001] and hApoE4 [F(5,23) = 15.31, P < 0.001]. Similar changes were seen in SWS in both WT [F(5,23) = 10.46, P < 0.001] and hApoE4 [F(5,23) = 9.98, P < 0.001] and in REM sleep WT [F(5,23) = 17.18, P < 0.001] and hApoE4 [F(5,23) = 40.17, P < 0.001], indicating that IH had influenced state in both WT and hApoE4 mice. The baseline values for wake (48.49 ± 3.70%), SWS (45.3 ± 3.31%), and REM sleep (6.20 ± 0.70%) in hApoE4 mice showed no significant differences from WT mice (50.60 ± 4.20%, 43.8 ± 3.90%, and 5.83 ± 0.70%, respectively) (Fig. 2, A–F). All mice subjected to acute IH spent more time in the wake state during the IH period (Fig. 2, A and B). There was a significant reduction in wake following IH during the dark period, which was more prominent in hApoE4 mice (42.67 ± 1.56% vs. 58.01 ± 1.47%) (Fig. 5A) [F(1,10) = 51.26, P < 0.001]. SWS during acute IH showed that the mice spent significantly less time in SWS (Fig. 2, C and D). After cessation of IH, there was a significant rebound in SWS, which was more prominent in hApoE4 mice (49.22 ± 1.00% vs. 37.57 ± 1.45%) (Fig. 5D) [F(1,10) = 44.10, P < 0.001]. hApoE4 mice showed marked reductions in REM sleep compared with WT mice (0.83 ± 0.10% vs. 1.44 ± 0.25%) (Fig. 2, E and F, and Fig. 5G) [F(1,10) = 5.22, P < 0.04]. During the dark period, there was a significant rebound in REM sleep, which was more prominent in hApoE4 mice (8.11 ± 0.30% vs. 4.42 ± 0.15%) (Fig. 5G) [F(1,10) = 267.15, P < 0.001].
Fig. 2.
Sleep wakefulness, electroencephalogram (EEG) delta power, and body temperature (IH). All graphs are plotted per 2 h for a 24-h period: percent time waking during baseline (black line) and IH (dotted line) in WT mice (A) and hApoE4 mice (B); percent time in nonrapid eye movement (NREM) sleep during baseline (black line) and IH (dotted line) in WT mice (C) and hApoE4 mice (D); percent time in REM sleep during baseline (black line) and IH (dotted line) in WT mice (E) and hApoE4 mice (F); number of wake episodes during baseline (black line) and IH (dotted line) in WT mice (G) and hApoE4 mice (H); NREM sleep delta power during baseline (black line) and IH (dotted line) in WT mice (I) and hApoE4 mice (J); body temperature during baseline (black line) and IH (dotted line) in WT mice (K) and hApoE4 mice (L). Black line indicates IH period (1 PM to 7 PM). Shaded area represents dark period, 7 PM to 7 AM. SWS, slow wave sleep. *P < 0.05. Error bar represent mean SE. See text for more details.
Fig. 5.
Changes in sleep following IH, SF, and IH+SF. All graphs are plotted as preintervention (6 h; 7 AM to1 PM), during intervention (6 h; 1 PM to 7 PM), and postintervention (12 h; 7 PM to 7 AM): percent time in wake in WT mice (open bar) and hApoE4 mice (filled bar) on IH (A), SF (B) and IH+SF (C); percent time in NREM sleep in WT mice (open bar) and hApoE4 mice (filled bar) on IH (D), SF (E), and IH+SF (F); percent time in REM sleep in WT mice (open bar) and hApoE4 mice (filled bar) on IH (G), SF (H), and IH+SF (I). *P < 0.05. Error bar represent mean SE.
EEG delta power during SWS.
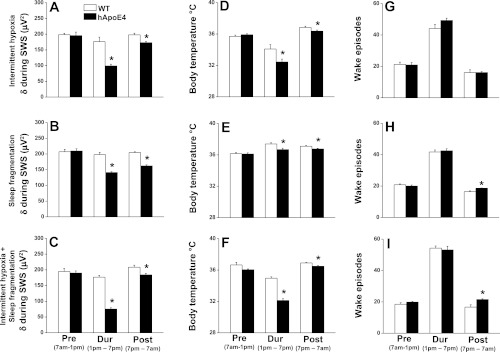
Overall analysis for a period of 24 h revealed significant changes between baseline and IH in both WT [F(5,23) = 1.91, P < 0.02] and hApoE4 [F(5,23) = 4.25, P < 0.001], indicating that IH imposed significant effects on global EEG delta power. The baseline delta power values for hApoE4 mice were comparable with WT (Fig. 2, I and J). Although all mice showed significant reduction in delta power during IH, hApoE4 mice were more affected (Fig. 6A) [F(1,10) = 25.24, P < 0.001]. The WT mice showed a significant rebound delta increase immediately following cessation of IH (P < 0.001) during the dark period, which was absent in hApoE4 mice (Fig. 6A) [F(1,10) = 14.03, P < 0.004].
Fig. 6.
Changes in delta power, body temperature, and wake episodes following IH, SF, and IH+SF. All graphs are plotted as preintervention (6 h; 7 AM to1 PM), during intervention (6 h; 1 PM to 7 PM), and postintervention (12 h; 7 PM to 7 AM): delta power during NREM sleep in WT mice (open bar) and hApoE4 mice (filled bar) on IH (A), SF (B), and IH+SF (C); changes in body temperature in WT mice (open bar) and hApoE4 mice (filled bar) on IH (D), SF (E), and IH+SF (F); number of wake episodes in WT mice (open bar) and hApoE4 mice (filled bar) on IH (G), SF (H) and IH+SF (I). *P < 0.05. Error bar represent mean SE.
Wake episodes.
The number of wake episodes at baseline in WT was 21.28 ± 1.39 and in hApoE4 was 20.83 ± 1.62. There was a significant increase in wake episodes from 1 PM to 7 PM during the IH procedures, indicating that IH elicited perturbations in sleep integrity in both WT and hApoE4 mice (Fig. 2, G and H). During exposure to IH, WT mice showed significant increases in episodes of wake (44.10 ± 2.58; P < 0.001) and hApoE4 mice had 49.28 ± 1.44; P < 0.001 (Fig. 6G). There were no differences in the number of wake episodes following cessation of IH exposures.
Body temperature.
Overall analysis for a period of 24 h revealed significant changes between baseline and IH in both WT [F(5, 23) = 18.55, P < 0.001] and hApoE4 [F(5,23) = 32.99, P < 0.001], indicating IH had significant effects on core body temperature. The baseline values for hApoE4 mice were comparable with WT (Fig. 2, K and L). During exposure to IH, all mice showed significant reductions in body temperature (WT: 34.10 ± 0.56°C) with hApoE4 mice showing more prominent core temperature declines (32.40 ± 0.37°C compared with WT; Fig. 6D; [F(1,10) = 6.06, P < 0.03]). The body temperature returned to baseline levels following cessation of IH during the dark period in WT mice (Fig. 2K) unlike hApoE4 mice, which took longer to recover (Fig. 2L) [F(1,10) = 5.03, P < 0.04].
Group 2 (SF)
Behavioral state.
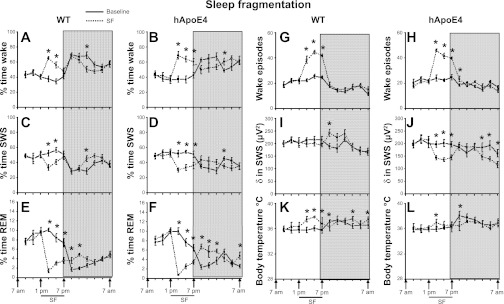
As in group 1, overall analysis of the wake, SWS, and REM sleep for a period of 24 h revealed significant changes between baseline and SF in both WT and hApoE4. The baseline values for wake (49.7 ± 4.27%), SWS (44.32 ± 3.73%), and REM sleep (5.85 ± 0.63%) in hApoE4 mice showed no significant differences from those recorded in WT mice (51.37 ± 3.71%, 42.62 ± 3.77%, and 5.86 ± 0.31% time in wake, SWS, and REM sleep, respectively; Fig. 3, A–F). All mice subjected to SF spent more time in the wake state during the initial period of SF (Fig. 3, A and B) and showed no significant changes in wake following SF in either WT or hApoE4 mice (56.32 ± 1.40% vs. 56.89 ± 1.81%) (Fig. 5B). SWS during SF showed that the mice spent significantly less time in SWS (Fig. 3, C and D), an effect that was more prominent in hApoE4 mice {40.68 ± 2.79% vs. 33.2 ± 1.75%; [F(1, 10) = 6.72, P < 0.027]}. After cessation of SF, SWS did not show significant rebound (WT, 39.52 ± 1.28% vs. hApoE4, 38.07 ± 1.63%; Fig. 5E). During SF, both mice spent significantly less time in REM sleep (Fig. 3, E and F). During the dark period, there was a significant rebound in REM sleep, which was more prominent in hApoE4 mice (5.03 ± 0.23%; Fig. 5G) [F(1,10) = 85.53, P < 0.007].
Fig. 3.
Sleep wakefulness, EEG delta power, and body temperature (SF). All graphs are plotted per 2 h for a 24-h period: percent time waking during baseline (black line) and SF (dotted line) in WT mice (A) and hApoE4 mice (B); percent time in NREM sleep during baseline (black line) and SF (dotted line) in WT mice (C) and hApoE4 mice (D); percent time in REM sleep during baseline (black line) and SF (dotted line) in WT mice (E) and hApoE4 mice (F); number of wake episodes during baseline (black line) and SF (dotted line) in WT mice (G) and hApoe4 mice (H); NREM sleep delta power during baseline (black line) and SF (dotted line) in WT mice (I) and hApoE4 mice (J); body temperature during baseline (black line) and SF (dotted line) in WT mice (K) and hApoE4 mice (L). Black line indicates SF period (1 PM to 7 PM). Shaded area represents dark period, 7 PM to 7 AM. *P < 0.05. Error bar represent mean SE. See text for more details.
EEG delta power during SWS.
A 24-h period revealed significant changes between baseline and SF in both WT [F(5, 23) = 1.72, P < 0.03] and hApoE4 [F(5,23) = 5.44, P < 0.001]. Although the baseline values for hApoE4 mice were comparable with WT (Fig. 3, I and J), during SF both mice showed significant reduction in delta power. However, hApoE4 mice showed a more prominent reduction in delta power compared with WT (Fig. 6B) [F(1,10) = 53.31, P < 0.001]. The WT mice showed significant rebound delta increases immediately following cessation of SF (P < 0.001) during the dark period, and such responses were absent in hApoE4 mice (Fig. 6B).
Wake episodes.
The number of wake episodes at baseline was comparable in both mice. SF increased the wake episodes both in WT (41.72 ± 1.31; P < 0.001) and hApoE4 mice (42.5 ± 1.28; P < 0.001; Fig. 6H), indicating SF procedure did elicit periodic arousal in both WT and hApoE4 mice (Fig. 3, G and H). After cessation of SF, during delta power, the hApoE4 mice continued to exhibit significantly higher episodes of wake (18.58 ± 0.23 vs. 16.44 ± 0.51 WT, P < 0.004).
Body temperature.
A 24-h period revealed significant changes between baseline and SF in both WT [F(5, 23) = 11.12, P < 0.001] and hApoE4 [F(5,23) = 8.08, P < 0.001]. The baseline values for hApoE4 mice were comparable to WT mice (Fig. 3, K and L). Significant increase in body temperature seen during exposure to SF was prominent in WT (37.34 ± 0.16°C) than hApoE4 mice (36.67 ± 0.18°C; Fig. 6E) [F(1,10) = 8.85, P < 0.01]. hApoE4 mice required longer time to recover from SF-induced hyperthermia (Fig. 2L) [F(1,10) = 5.03, P < 0.04] compared with the WT mice (Fig. 3K).
Group 3 (IH+SF)
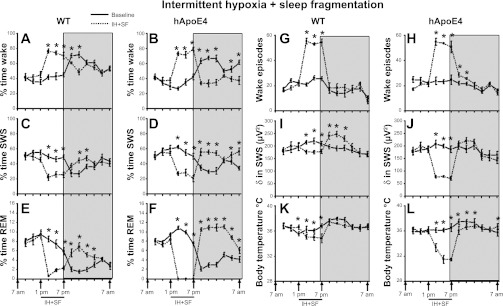
Behavioral state.
Wake, SWS, and REM sleep showed significant changes for a 24-h period, indicating that IH+SF influenced state in both WT and hApoE4 mice. The baseline values for wake (47.82 ± 3.47%), SWS (46.0 ± 3.29%), and REM sleep (6.25 ± 0.43%) in hApoE4 mice were comparable to WT mice (Fig. 4, A–F). During exposure to IH+SF, mice spent more time in the wake state (Fig. 4, A and B). The post-IH+SF reduction in wake was more prominent in hApoE4 mice (37.14 ± 2.05%) compared with WT (53.98 ± 0.76%; Fig. 5C) [F(1,10) = 59.11, P < 0.001]. During acute IH+SF the mice spent significantly less time in SWS (Fig. 4, C and D). The rebound in SWS, following exposure to IH+SF, was more prominent in hApoE4 mice (53.11 ± 2.00% vs. 42.03 ± 1.55% WT; Fig. 5F) [F(1,10) = 19.10, P < 0.001]. IH+SF totally abolished REM sleep in hApoE4 mice [F(1,10) = 61.91, P < 0.001] (Fig. 4, E and F, and Fig. 5I). The post-IH+SF REM sleep showed a significant rebound that was more prominent in hApoE4 mice (9.68 ± 0.26% vs. 4.86 ± 0.16% WT; Fig. 5I) [F(1,10) = 242.77, P < 0.001].
Fig. 4.
Sleep wakefulness, EEG delta power, and body temperature (IH+SF). All graphs are plotted per 2 h for a 24-h period: percent time waking during baseline (black line) and IH+SF (dotted line) in WT mice (A) and hApoE4 mice (B); percent time in NREM sleep during baseline (black line) and IH+SF (dotted line) in WT mice (C) and hApoE4 mice (D); percent time in REM sleep during baseline (black line) and IH+SF (dotted line) in WT mice (E) and hApoE4 mice (F); number of wake episodes during baseline (black line) and IH+SF (dotted line) in WT mice (G) and hApoe4 mice (H); NREM sleep delta power during baseline (black line) and IH+SF (dotted line) in WT mice (I) and hApoE4 mice (J); body temperature during baseline (black line) and IH+SF (dotted line) in WT mice (K) and hApoE4 mice (L). Black line indicates IH+SF period (1 PM to 7 PM). Shaded area represents dark period, 7 PM to 7 AM. *P < 0.05. Error bar represent mean SE. See text for more details.
EEG delta power during SWS.
Significant effect on global 24 h EEG delta power revealed significant changes between baseline and IH+SF in both WT [F(5, 23) = 2.22, P < 0.003] and hApoE4 [F(5,23) = 28.7, P < 0.001]. The baseline values for hApoE4 mice were comparable with WT (Fig. 4, I and J). During exposure to IH+SF, hApoE4 mice showed a marked reduction in delta power compared with WT (Fig. 6C) [F(1,10) = 208.6, P < 0.001]. The rebound increase in delta power following IH+SF observed in WT mice (P < 0.001) was absent in hApoE4 mice (Fig. 6C) [F(1,10) = 9.16, P < 0.013].
Wake episodes.
The increase in wake episodes during the IH+SF procedures were similar to group 1 and 2 (WT, 54.22 ± 1.44 and hApoE4, 53.11 ± 2.33; Fig. 4, G and H, and Fig. 6G). However, following cessation of IH+SF, ApoE4 mice had more wake episodes compared with WT mice (21.46 ± 0.5 vs.16.68 ± 1.47; P < 0.012).
Body temperature.
Although the baseline values were comparable (Fig. 4, K and L), IH+SF exerted significant effects on core body temperature in both WT [F(5, 23) = 20.07, P < 0.001] and hApoE4 [F(5,23) = 61.40, P < 0.001]. hApoE4 mice showed a significant reduction in body temperature (32.11 ± 0.23°C) compared with WT (34.96 ± 0.17°C; Fig. 6D) [F(1,10) = 97.01, P < 0.001]. Moreover, hApoE4 mice took a longer time to recover from IH+SF-induced hypothermia (Fig. 4L) [F(1,10) = 15.43, P < 0.003] compared with WT mice (Fig. 4K).
Comparison between experimental groups.
Overall (24 h) comparisons between groups showed significant differences in all three behavioral states wake [F(8,45) = 36.13, P < 0.001], SWS [F(8,45) = 23.09, P < 0.001], and REM sleep [F(8,45) = 226.54, P < 0.001]. In all groups, the preintervention period was comparable. However, wake state showed a significant decrease [F(2,15) = 31.37, P < 0.001] in both IH (q = 10.85, P < 0.001) and IH+SF (q = 7.822, P < 0.001) compared with SF during the postintervention period. SWS showed a significant increase [F(2,15) = 23.90, P < 0.001] in both IH (q = 6.98, P < 0.001) and IH+SF (q = 9.42, P < 0.001) compared with SF during the postintervention period. However, REM showed a significant decrease both during intervention [F(2,15) = 67.13, P < 0.001], IH vs. SF (q = 10.24, P < 0.001), IH vs. IH+SF (q = 5.95, P < 0.002), and SF vs. IH+SF (q = 16.19, P < 0.001) and a significant rebound during postintervention period [F(2,15) = 67.13, P < 0.001], IH vs. SF (q = 13.84, P < 0.001), IH vs. IH+SF (q = 7.06, P < 0.001), and SF vs. IH+SF (q = 2.091, P < 0.001). Delta power during SWS showed a significant decrease during intervention across groups [F(2,15) = 61.92, P < 0.001]. Body temperature also showed a significant decrease during intervention [F(2,15) = 86.93, P < 0.001] in both IH and IH+SF compared with SF.
MSLT.
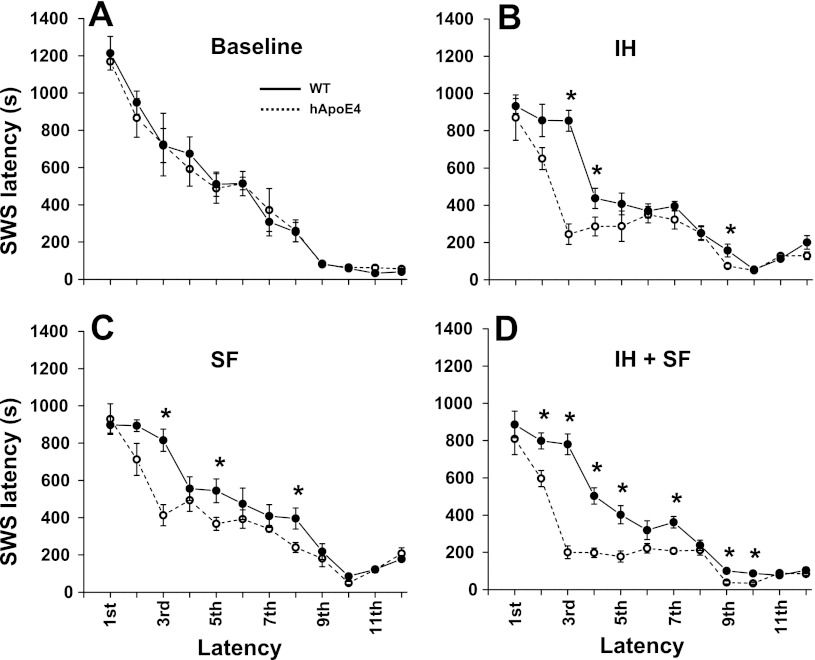
MSLT showed comparable SWS duration during each 20-min bin of recovery periods in both hApoE4 and WT mice during baseline conditions (Fig. 7). However, after every intervention (IH, SF, and IH+SF), hApoE4 mice spent markedly higher time in SWS during the recovery time compared with WT mice for the first eight cycles. MSLT performed after exposures to IH [F(5,23) = 30.3, P < 0.001], SF [F(5,23) = 28.76, P < 0.001], and IH+SF [F(5,23) = 53.3, P < 0.001] (Fig. 7) revealed significant reductions in SWS latency in hApoE4 mice, which were more pronounced after exposures to IH+SF. Starting at the ninth cycle, WT mice showed similar sleep latencies compared with the hApoE4 mice, indicating increased sleep pressure.
Fig. 7.
Data shown are mean NREM sleep latency values (seconds) obtained using murine multiple sleep latency test. A: baseline NREM sleep latency in WT (black line) and hApoE4 mice (dotted line). B: NREM sleep latency in WT (black line) and hApoE4 mice (dotted line) during IH. C: NREM sleep latency in WT (black line) and hApoE4 mice (dotted line) during SF. D: NREM sleep latency in WT (black line) and hApoE4 mice (dotted line) during IH+SF. Note latency to NREM sleep is significantly reduced in hAPoE4 mice compared with WT. *P < 0.05. Error bar represent mean SE.
DISCUSSION
In the present study, we report on persistent increases in wake and decreases in SWS and REM sleep during a 6-h exposure to IH and IH +SF, as well as reductions in delta power and hypothermia. Such deficits were also seen in mice after SF procedures, albeit to a lesser extent, and without delta power suppression and hypothermia. Murine MSLT on the following day after such interventions showed a reduction of the average latency to sleep onset (a direct measure of sleepiness) in hApoE4 mice. The magnitude of the effects seen following any of the interventions was significantly higher in hApoE4 mice compared with WT controls. Based on such findings, hApoE4 mice exhibited more pronounced hypersomnolence, as evidenced by rebound SWS and REM sleep and reduced sleep latencies, independently from the type of exposure.
The exposures to IH, SF, and IH+SF were designed to reproduce some of the characteristics of the sleep profile of patient suffering form sleep apnea, in whom sleep is fragmented, thereby resulting in chronic deficits in the amount of stages 3–4 of NREM sleep and deficits in REM sleep (19, 39, 46, 55). The exposures to IH, SF, and IH+SF effectively fragmented sleep and displayed potentiated effects during IH+SF. Such synergistic effect was particularly prominent in REM sleep, which was completely abolished in IH+SF mice. It is worth noting that acute IH alone did elicit fragmented sleep, thereby increasing wake episodes, and IH+SF potentiated this effect. The acute short-term nature of the present study is a clear limitation. Despite the novelty and exploratory nature of such experiments, it is likely that both earlier and later time points, as well as assessment of long-term chronic exposures to IH, SF, and IH+SF conditions, may elicit differentially expressed effects in both their magnitude and characteristics.
hApoE4 mice exposed to IH, SF, and IH+SF exhibited abnormalities in their ability to regulate sleep that are akin to the sleep regulatory deficits observed in AD patients. However, the exact nature of ApoE4-mediated neurochemical and physiological underpinnings for disruption of sleep in AD remains unknown. ApoE is associated with the intracellular neurofibrillary tangles, which are composed of cytoskeletal elements, especially phosphorylated τ and extracellular amyloid plaques (36, 64). Many AD-like features have been reproduced in murine models expressing human ApoE4, including increased plaques (24, 25), reduced presynaptic terminals with ApoE4 (4–6), increased phosphorylated τ with neuronal expression of ApoE4 (3, 21, 59), and impaired spatial learning and memory (28, 42, 43). The present study provides evidence on the unique susceptibility of sleep integrity and homeostasis to disruption in hApoE4 mice. However, comparing mice with transgenic human ApoE4 with mice in which a human ApoE3 would be expressed would definitely provide a more suitable control, even if such comparisons have been already reported (9).
Sleep disturbances that have been reported in AD patients include significant loss of SWS and REM sleep, as well as increases in the latency to the first REM sleep episode and an increase in the amount of wakefulness and EEG slowing during wake and during REM sleep (2, 32, 41). Such perturbations in sleep structure lead to the breakdown of sleep/wake circadian rhythms and marked attenuations in delta sleep (31, 41, 48, 61, 62). The current experiments were conducted in younger mice, and as such, the magnitude of sleep alterations was not observed during baseline conditions but became apparent during the recovery period from any of the three experimental sleep-disrupting paradigms used herein. Of note, sustained IH may independently have resulted in oxidative stress injury, especially to the hippocampal formation and prefrontal cortex (18, 33), basal forebrain (60), noradrenergic locus ceruleus and dopaminergic ventral periaqueductal gray wake neurons (66). Thus, in the context of the oxidative stress elicited by either IH (33) or SF (34), the increased susceptibility both during and following IH, SF, or IH-SF in hAPOE4 mice was not surprising (28).
In AD patients, a phase delay in body temperature (later time of peak) and lower amplitude of body temperature rhythm have been described (48, 63), and there is also evidence that the suprachiasmatic nucleus and its output pathways are disrupted in AD (16, 54, 57). We are unaware of any published study on the effects of IH and SF on thermoregulation, especially in hApoE4 mice. In rats, medial preoptic α-2 adrenoceptors are implicated in sleep and thermoregulation (45). Previous studies have shown that application of sustained hypoxic stimuli decrease body temperature and reduce metabolism in rats (12, 37). Sleep efficiency is maximal under thermoneutral conditions (51) and hence the quantity of sleep, especially REM sleep, is decreased when ambient temperature is raised or lowered outside the thermoneutral range (58), a phenomenon that is corroborated by our findings in WT mice. It has been previously shown that sustained hypoxia elicits a reduction in the behavioral thermoregulatory set point in rats (14), and, at subthermoneutral ambient temperatures, such changes are accompanied by reductions in both body temperature and metabolic heat production (12). In a 2001 study (20), application of hypoxic stimuli during sleep led to body temperature decreases, with continuous hypoxia promoting lower body temperatures than when intermittent hypoxic stimuli were applied and timed to coincide with the sleep episodes. However, these studies that were carried out in normal rats cannot be extrapolated to murine models of neurodegenerative disorders such as hApoE4 mice. Our findings further reinforce the concept of unique vulnerabilities being present in multiple neuronal populations in hApoE4 mice and their restricted ability to recover from any disruption, including sleep and thermoregulation. Hence, further studies are needed to determine the contributions of IH to decreases in body temperature, and conversely the hyperthermic consequences of SF, and their relationship to the alterations in sleep architecture reported here in both WT and hApoE4 mice.
Taken together, the present study shows that even a short-term exposure to some of the constitutive elements of sleep apnea, namely IH, SF, and IH+SF, is sufficient to elicit sleep deficits, especially in terms of delta power and excessive sleepiness, and that the presence of hApoE4 further exacerbates such effects. These findings further suggest that the neurobehavioral impairments and the residual excessive sleepiness in AD patients carrying ϵ4 allele with OSA may stem from IH- and SF-induced alterations in sleep homeostatic responses.
Perspectives and Significance
The ϵ4 allele of the ApoE gene is a known risk factor for AD that is identified in ∼20–25% of the population and in 40–50% of late-onset AD cases (49). Although ApoE is directly implicated in AD pathology and etiology, the specific function of ApoE in the brain and the mechanism(s) by which this genotype facilitates progression of AD need further investigation. Although some studies reported no differences between patients with AD and controls (53), other cohort surveys reported higher prevalence of OSA in AD and in ApoE ϵ4 allele-positive individuals compared with the rest of the population (23). These cross-sectional studies should be construed as testing one of two plausible (but indistinguishable) working hypotheses, namely, 1) AD causes sleep apnea, perhaps by disruption of motor outflow from medullary centers controlling breathing at either the diaphragmatic (central events) or the upper airway motor level (obstructive or mixed events); or 2) sleep apnea could have caused or otherwise facilitated AD progression via effects on higher cortical function. Our present findings support that hApoE4 mice experience higher degrees of hypersomnolence, reduced sleep latencies, and poorer thermoregulatory responses during short-term exposures to SF and IH, suggesting that interactions between OSA and AD may lead to enhanced central nervous system susceptibility and disease progression. The deficits in sleep regulatory mechanisms in the context of murine models of OSA lay out an intricate web of neurochemical and neurophysiological pathways that hamper homeostatic recovery processes in hApoE4 mice.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-086662.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.K. and V.R. performed experiments; N.K., V.R., and D.G. analyzed data; N.K. and V.R. prepared figures; N.K. and V.R. drafted manuscript; N.K., V.R., and D.G. approved final version of manuscript; D.G. conception and design of research; D.G. interpreted results of experiments; D.G. edited and revised manuscript.
ACKNOWLEDGMENTS
Part of this work was conducted while the authors were in the Department of Pediatrics, Kosair Children's Hospital Research Institute, University of Louisville School of Medicine, Louisville, KY 40202.
REFERENCES
- 1. Baum L, Chen L, Ng HK, Pang CP. Apolipoprotein E isoforms in Alzheimer's disease pathology and etiology. Microsc Res Tech 50: 278–281, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bliwise DL, Tinklenberg J, Yesavage JA, Davies H, Pursley AM, Petta DE, Widrow L, Guilleminault C, Zarcone VP, Dement WC. REM latency in Alzheimer's disease. Biol Psychiatry 25: 320–328, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Dee Fish J, Wyss-Coray T, Buttini M, Mucke L, Mahley RW, Huang Y. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci 24: 2527–2534, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buttini M, Akeefe H, Lin C, Mahley RW, Pitas RE, Wyss-Coray T, Mucke L. Dominant negative effects of apolipoprotein E4 revealed in transgenic models of neurodegenerative disease. Neuroscience 97: 207–210, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mucke L, Mahley RW. Expression of human apolipoprotein E3 or E4 in the brains of Apoe-/- mice: isoform-specific effects on neurodegeneration. J Neurosci 19: 4867–4880, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci 22: 10539–10548, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261: 921–923, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Cosentino FI, Bosco P, Drago V, Prestianni G, Lanuzza B, Iero I, Tripodi M, Spada RS, Toscano G, Caraci F, Ferri R. The APOE epsilon4 allele increases the risk of impaired spatial working memory in obstructive sleep apnea. Sleep Med 9: 831–839, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, Olson SJ, Montine TJ, Amarnath V, Roberts LJ., 2nd Treatment with a γ-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice. J Alzheimers Dis 27:49–59, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espana RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep 30: 1417–1425, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol Regul Integr Comp Physiol 262: R1040–R1046, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Gaig C, Iranzo A. Sleep-disordered breathing in neurodegenerative diseases. Curr Neurol Neurosci Rep 2: 205–217, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Gordon CJ, Fogelson L. Comparative effects of hypoxia on behavioral thermoregulation in rats, hamsters, and mice. Am J Physiol Regul Integr Comp Physiol 260: R120–R125, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Gottlieb DJ, DeStefano AL, Foley DJ, Mignot E, Redline S, Givelber RJ, Young T. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology 63: 664–668, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Goudsmit E, Hofman MA, Fliers E, Swaab DF. The supraoptic and paraventricular nuclei of the human hypothalamus in relation to sex, age and Alzheimer's disease. Neurobiol Aging 11: 529–536, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Gozal D, Capdevila OS, Kheirandish-Gozal L, Crabtree VM. APOE epsilon 4 allele, cognitive dysfunction, and obstructive sleep apnea in children. Neurology 69: 243–249, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med 27: 465–484, 1976 [DOI] [PubMed] [Google Scholar]
- 20. Hamrahi H, Stephenson R, Mahamed S, Liao KS, Horner RL. Selected Contribution: Regulation of sleep-wake states in response to intermittent hypoxic stimuli applied only in sleep. J Appl Physiol 90: 2490–2501, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E, Hopkins PC, Scearce-Levie K, Weisgraber KH, Mucke L, Mahley RW, Huang Y. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer's disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA 100: 10966–10971, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He J, Gui JH, Zhang YH, Yu WZ, Chou DH, Xu JT. Relationship between apolipoprotein E gene and the risk for onset of Alzheimer disease in aged adults in Urumqi. Chin J Clin Rehabil 9: 207–209, 2005 [Google Scholar]
- 23. Hoch CC, Reynolds CF, 3rd, Kupfer DJ, Houck PR, Berman SR, Stack JA. Sleep-disordered breathing in normal and pathologic aging. J Clin Psychiatry 47: 499–503, 1986 [PubMed] [Google Scholar]
- 24. Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 97: 2892–2897, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizarry MC, Hyman BT. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer's disease model. Ann Neurol 47: 739–747, 2000 [PubMed] [Google Scholar]
- 26. Kadotani H, Kadotani T, Young T, Peppard PE, Finn L, Colrain IM, Murphy GM, Jr, Mignot E. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA 285: 2888–2890, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Kalra M, Pal P, Kaushal R, Amin RS, Dolan LM, Fitz K, Kumar S, Sheng X, Guha S, Mallik J, Deka R, Chakraborty R. Association of ApoE genetic variants with obstructive sleep apnea in children. Sleep Med 9: 260–265, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E-deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep 28: 1412–1417, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest 103: 1579–1586, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kripke DF, Shadan FF, Dawson A, Cronin JW, Jamil SM, Grizas AP, Koziol JA, Kline LE. Genotyping sleep disorders patients. Psychiatry Investig 7: 36–42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loewenstein RJ, Weingartner H, Gillin JC, Kaye W, Ebert M, Mendelson WB. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol Aging 3: 371–377, 1982 [DOI] [PubMed] [Google Scholar]
- 32. Montplaisir J, Petit D, Lorrain D, Gauthier S, Nielsen T. Sleep in Alzheimer's disease: further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. Sleep 18: 145–148, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLos One 6: e19847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, Gozal D. Sleep fragmentation induces cognitive deficits via NADPH oxidase-dependent pathways in mouse. Am J Respir Crit Care Med 184: 1305–1312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nalivaevaa NN, Fisk L, Kochkina EG, Plesneva SA, Zhuravin IA, Babusikova E, Dobrota D, Turner AJ. Effect of hypoxia/ischemia and hypoxic preconditioning/reperfusion on expression of some amyloid-degrading enzymes. Ann NY Acad Sci 1035: 21–33, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res 541: 163–166, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Pappenheimer JR. Sleep and respiration of rats during hypoxia. J Physiol 266: 191–207, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pellegrino R, Mazzotti DR, Guindalini C, Santos-Silva R, Bittencourt LR, Tufik S. Apolipoprotein E polymorphisms and sleep quality in obstructive sleep apnea syndrome. Clin Chim Acta 412: 2223–2227, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Penzel T, Kantelhardt JW, Lo CC, Voigt K, Vogelmeier C. Dynamics of heart rate and sleep stages in normals and patients with sleep apnea. Neuropsychopharmacology 28, Suppl 1: S48–S53, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Ponomareva NV, Korovaitseva GI, Rogaev EI. EEG alterations in non-demented individuals related to apolipoprotein E genotype and to risk of Alzheimer disease. Neurobiol Aging 29: 819–827, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, Zemcuznikov N, Gerber CJ. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc 30: 86–93, 1982 [DOI] [PubMed] [Google Scholar]
- 42. Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci USA 95: 10914–10919, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature 404: 352–354, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Ramesh Kaushal N, Gozal D. Sleep fragmentation in mice differentially modifies EEG delta power during slow wave sleep in socially isolated and paired mice. Sleep Sci 2: 64–75, 2009 [Google Scholar]
- 45. Ramesh V, Kumar VM. The role of alpha-2 receptors in the medial preoptic area in the regulation of sleep-wakefulness and body temperature. Neuroscience 85: 807–817, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Roehrs T, Conway W, Wittig R, Zorick F, Sicklesteel J, Roth T. Sleep-wake complaints in patients with sleep-related respiratory disturbances. Am Rev Respir Dis 132: 520–523, 1985 [DOI] [PubMed] [Google Scholar]
- 47. Roses AD. On the discovery of the genetic association of Apolipoprotein E genotypes and common late-onset Alzheimer disease. J Alzheimers Dis 9: 361–366, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Satlin A, Volicer L, Stopa EG, Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer's disease. Neurobiol Aging 16: 765–771, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Saunders AM, Schmader K, Breitner JC, Benson MD, Brown WT, Goldfarb L, Goldgaber D, Manwaring MG, Szymanski MH, McCown N, Dole KC, Schmechel DE, Strittmatter WJ, Pericak-Vance MA, Roses AD. Apolipoprotein E epsilon 4 allele distributions in late-onset Alzheimer's disease and in other amyloid-forming diseases. Lancet 342: 710–711, 1993. [DOI] [PubMed] [Google Scholar]
- 50. Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43: 1467–1472, 1993. [DOI] [PubMed] [Google Scholar]
- 51. Schmidek WR, Hoshino K, Schmidek M, Timo-Iaria C. Influence of environmental temperature on the sleep-wakefulness cycle in the rat. Physiol Behav 8: 363–371, 1972 [DOI] [PubMed] [Google Scholar]
- 52. Sleegers K, Roks G, Theuns J, Aulchenko YS, Rademakers R, Cruts M, van Gool WA, Van Broeckhoven C, Heutink P, Oostra BA, van Swieten JC, van Duijn CM. Familial clustering and genetic risk for dementia in a genetically isolated Dutch population. Brain 127: 1641–1649, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Smallwood RG, Vitiello MV, Giblin EC, Prinz PN. Sleep apnea: relationship to age, sex, and Alzheimer's dementia. Sleep 6: 16–22, 1983 [DOI] [PubMed] [Google Scholar]
- 54. Standaert DG, Lee VM, Greenberg BD, Lowery DE, Trojanowski JQ. Molecular features of hypothalamic plaques in Alzheimer's disease. Am J Pathol 139: 681–691, 1991 [PMC free article] [PubMed] [Google Scholar]
- 55. Stepanski EJ. The effect of sleep fragmentation on daytime function. Sleep 25: 268–276, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer's disease. Annu Rev Neurosci 19: 53–77, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Swaab DF, Fisser B, Kamphorst W, Troost D. The human suprachiasmatic nucleus: neuropeptide changes in senium and Alzheimer's disease. Basic Appl Histochem 32: 43–54, 1988 [PubMed] [Google Scholar]
- 58. Szymusiak R, Satinoff E, Schallert T, Whishaw IQ. Brief skin temperature changes towards thermoneutrality trigger REM sleep in rats. Physiol Behav 25: 305–311, 1980 [DOI] [PubMed] [Google Scholar]
- 59. Tesseur I, Van Dorpe J, Spittaels K, Van den Haute C, Moechars D, Van Leuven F. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am J Pathol 156: 951–964, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27: 194–201, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Vitiello MV, Prinz PN. Alzheimer's disease. Sleep and sleep/wake patterns. Clin Geriatr Med 5: 289–299, 1989 [PubMed] [Google Scholar]
- 62. Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer's disease. J Gerontol 45: M131–M138, 1990 [DOI] [PubMed] [Google Scholar]
- 63. Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer's disease. Am J Psychiatry 158: 704–711, 2001 [DOI] [PubMed] [Google Scholar]
- 64. Wisniewski T, Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett 135: 235–238, 1992 [DOI] [PubMed] [Google Scholar]
- 65. Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 126: 313–323, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Zhu Y, Fenik P, Zhan G, Mazza E, Kelz M, Aston-Jones G, Veasey SC. Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci 27: 10060–10071, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu Y, Nwabuisi-Heath E, Dumanis SB, Tai LM, Yu C, Rebeck GW, LaDu MJ. APOEgenotype alters glial activation and loss of synaptic markers in mice. Glia 60: 559–569, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]