Abstract
Preeclampsia is defined as new-onset hypertension with proteinuria after 20 wk gestation and is hypothesized to be due to shallow trophoblast invasion in the spiral arteries thus resulting in progressive placental ischemia as the fetus grows. Many animal models have been developed that mimic changes in maternal circulation or immune function associated with preeclampsia. The model of reduced uterine perfusion pressure in pregnant rats closely mimics the hypertension, immune system abnormalities, systemic and renal vasoconstriction, and oxidative stress in the mother, and intrauterine growth restriction found in the offspring. The model has been successfully used in many species; however, rat and primate are the most consistent in comparison of characteristics with human preeclampsia. The model suffers, however, from lack of the ability to study the mechanisms responsible for abnormal placentation that ultimately leads to placental ischemia. Despite this limitation, the model is excellent for studying the consequences of reduced uterine blood flow as it mimics many of the salient features of preeclampsia during the last weeks of gestation in humans. This review discusses these features.
Keywords: pregnancy, soluble fms-like tyrosine kinase-1, angiotensin type 1 receptor autoantibodies, endothelin, angiotensin II, immune system, tumor necrosis factor-α
this review article is part of a collection on Cardiovascular Regulation in Pregnancy. Other articles appearing in this collection, as well as a full archive of all Review collections, can be found online at http://ajpheart.physiology.org/.
Introduction
Preeclampsia is a multisystem disorder that typically occurs during the second half of pregnancy and complicates ∼3–7% for nulliparous and 1–3% for multiparous pregnancies (96). It is a major cause of maternal morbidity and mortality, prenatal death, and intrauterine growth restriction (IUGR) and contributes to ∼15% of all preterm pregnancies (73, 96). Preeclampsia is characterized by new-onset (pregnancy-specific) maternal hypertension, proteinuria, widespread maternal endothelial dysfunction, an imbalance of angiogenic factors and typically occurs after 20 wk gestation (72). Although the mechanism(s) responsible for preeclampsia are unclear, hypertension associated with preeclampsia remits after delivery, implicating the placenta as the culprit in the disease progression. In support of this notion, Magann and colleagues (67) reported that curettage immediately following delivery was more efficacious in the resolution of the hypertension in severely hypertensive women with preeclampsia than antihypertensive treatment with nifedipine.
Preeclampsia is Associated with Abnormal Uteroplacental Blood Flow
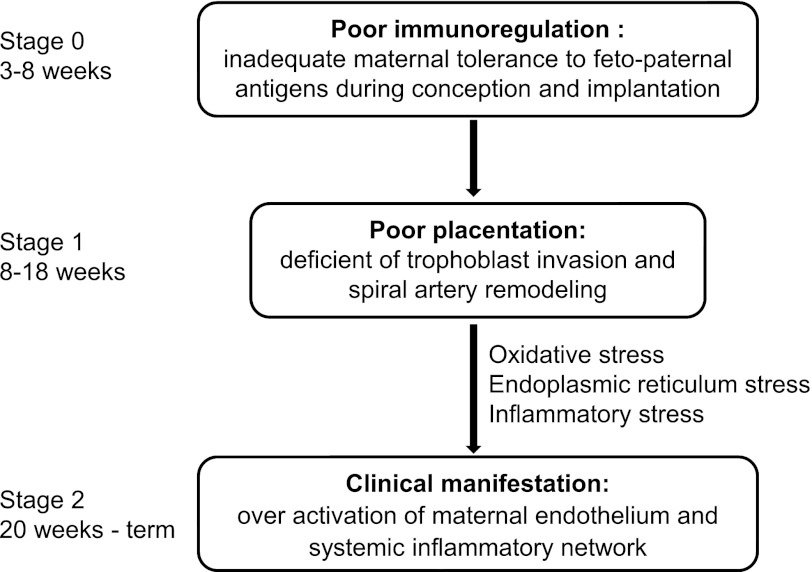
It has been hypothesized that preeclampsia arises from reductions in uteroplacental perfusion, leading to fetoplacental ischemia (82, 83). The mechanisms leading to reduced placental perfusion in preeclampsia are not clear, but studies in humans implicate impaired first-trimester cytotrophoblast invasion of spiral arterioles as an important factor. During weeks 8–18 of normal pregnancy, the villous cytotrophoblasts originating from the fetus invade the uterine wall through the entire depth of the endometrium and the inner third of the myometrium (42, 82) (see Fig. 1). As a result, the spiral arteries are extensively remodeled, replacing their smooth muscle and endothelium by the invading cytotrophoblasts. These arteries become widely dilated, with low resistance, and are less responsive, or even refractory, to vasoconstrictor substances (45). This normal invasive vascular remodeling occurring early in placentation is abnormal in pregnant women that develop preeclampsia (45). Thus preeclampsia is thought to be caused by a reduction in uterine blood flow due to abnormal trophoblast invasion of the spiral arteries, a concept first suggested by Young in 1914 (107). In support of this hypothesis, preeclamptic human placental bed biopsies indicate shallow trophoblast invasion, compared with normal pregnancy. As a result, the corresponding arterial segments remain undilated, leading to insufficient blood flow to the uteroplacental unit, and the maternal circulation remains responsive to vasoconstrictors, such as angiotensin II (ANG II) (18) and endothelin (ET-1) (51). The ischemic placenta releases cytokines, reactive oxygen species, and anti-angiogenic factors, such as soluble fms-like tyrosine kinase-1 (sFlt-1) (35), soluble endoglin (69, 70), and ANG II type 1 receptor autoantibodies (AT1-AA) (100), into the circulation, making this an aggravated interaction between maternal and fetal circulation. It should be mentioned that studies in double-knockout Rag2−/−II2rg−/− mice, in which there is no spiral artery remodeling during pregnancy, placental ischemia is present, but this does not result in IUGR or hypertension (25). These data suggest that other mechanisms are involved in the development of preeclampsia in addition to poor spiral artery remodeling at least in the mouse.
Fig. 1.
The three-stage model of preeclampsia. Stages 1 and 2 of preeclampsia are well accepted, whereas stage 0 is hypothetical. Based on this model, preeclampsia is proposed to arise from abnormal maternal immune response to fetopaternal antigens in the early stage of pregnancy (stage 0), leading to poorly perfused placenta in stage 1, which further causes multiple stresses and the final clinical manifestations in stage 2 of preeclamplsia (82, 83).
Animal Models for the Study of Preeclampsia
An ideal animal model to study preeclampsia should duplicate the pathogenesis of preeclampsia in women, including failure of early immune mechanisms, impaired first-trimester trophoblast invasion and placentation, reduced uteroplacental perfusion, and fetoplacental ischemia, thus resulting in systemic inflammation, proteinuria, and endothelial dysfunction in the mother and restricted growth in the fetus. However, preeclampsia is thought to occur spontaneously only in women, and only very few cases have been reported in great apes, including chimpanzees (49, 91) and gorillas (15, 95). The use of nonhuman primates and other animal species as models for preeclampsia are discussed at length in a review by Podjarny et al. (80) and McCarthy et al. (72).
There is evidence that hypertensive inbred BPH-5 mice, derived from brother-sister mating of borderline hypertensive BPH/2 mice, exhibit further increases in blood pressure (BP) in late gestation (from ∼130 to 160 mmHg, compared with ∼105 mmHg in control C57BL-6) that resolves within 2 days of delivery and is accompanied by increases in proteinuria and IUGR of the pups (26, 28). The mechanisms responsible for the increased BP during pregnancy in this model have not been elucidated, but adenoviral vector-mediated vascular endothelial growth factor (VEGF) 121 (46) and tempol (103) prevented the increase in BP (103), suggesting that placental ischemia and oxidative stress may contribute to the further elevated BP.
Models to Evaluate a Single Pathway in the Progression of Preeclampsia
There are several genetically manipulated mouse models of preeclampsia that affect a single gene, such as sFlt-1, the renin-angiotensin system, VEGF, endothelin, endothelial nitric oxide synthase, etc. (47). These models have varying levels of similarity with human preeclampsia, although, interestingly, few develop new-onset hypertension close to term. Readers are referred to a recent review by Ishida and colleagues (47) for more information on mouse models of preeclampsia. Below we discuss studies performed in models other than mice to learn more about the role of specific pathways hypothesized to contribute to the pathogenesis of preeclampsia.
Nitric Oxide Inhibition
Nitric oxide (NO) is an important mediator in controlling vascular tone, endothelial function, and oxidative stress. Increased NO production plays a critical role in the maternal BP and glomerular hemodynamic adaptation to pregnancy. NO production is reduced in preeclampsia (22, 27). Chronic NO synthase (NOS) inhibition in pregnant rats causes a dose-dependent increase in BP, renal vasoconstriction, proteinuria, maternal morbidity and mortality, and IUGR of pups (19, 74, 106). However, plasma from women with preeclampsia increases, rather than decreases, endothelial cell NO (16), and the effect of pregnancy on BP in endothelial NOS knockout mice is controversial, with one study reporting typical pregnancy-induced decreases in BP (89) while another reported a further increase of BP during pregnancy (43).
Anti-Angiogenic Factors
Women with preeclampsia have elevated circulating sFlt-1, a soluble VEGF receptor binding to and inactivating VEGF and placental growth factor (PIGF), critical players in angiogenesis and placentation. Placental and amniotic sFlt-1 levels are elevated while plasma levels of free VEGF and PIGF are decreased (70). Adenoviral vector-mediated sFlt-1 in pregnant rats resulted in increased BP, proteinuria, glomerular endotheliosis, and decreases in plasma free VEGF and PIGF (70). Similar studies performed in mice resulted in similar vascular effects with significantly lower pup and placental weight (66). In later studies performed by Murphy et al. (17, 76), sFlt-1 infusion in rats increased vascular/placental oxidative stress, decreased vasodilatory responses to agonists, reduced placenta and fetal weight, decreased maternal circulating VEGF and NO, and increased renal preproendothelin (prepro-ET-1). Importantly, the effects of sFlt-1 also occurred in a dose-dependent manner in nonpregnant controls, indicating these results are not specific for pregnancy, suggesting that increases in sFlt-1 under any circumstances compromise the cardiovascular system.
Models Based on Inflammatory Mediators
Women with preeclampsia exhibit increased systemic inflammation, heightened oxidative stress, and circulating autoantibodies (AT1-AA) that bind and activate ANG II type 1 receptor (AT1R) (52, 86, 87, 100). Pregnant mice injected with human AT1-AA developed progressive hypertension, proteinuria, and glomerular endotheliosis, all of which were blocked by AT1R antagonism (108). More specific studies indicated that infusion of rat AT1-AA from day 12 to day 19 of gestation into pregnant rats resulted in hypertension, elevated plasma sFlt-1 and renal and placental ET-1, and oxidative stress (54, 79). However, infusion of rat AT1-AA has no vascular or tissue effects in nonpregnant rats. These findings are interesting, since they suggest that the milieu of pregnancy is necessary for AT1-AA to cause adverse cardiovascular effects.
Preeclamptic women also have elevated inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and CD4+ T cells (52). Pregnant rats receiving TNF-α infusion from days 14 to 19 of gestation displayed hypertension and increases in prepro-ET-1 in placenta, aorta, and kidneys (60). Pretreatment with the ET-1 receptor A (ETA) antagonist abolished the BP response to TNF-α. Infusion of IL-6 in pregnant rats resulted in the development of hypertension and renal vasoconstriction without affecting ET-1 levels (20). Interestingly, later studies demonstrated that infusion of TNF-α or IL-6 stimulated AT1-AAs, and the resulting hypertension was completely abolished by AT1R blockade (56, 100). Importantly, CD4+ T helper cells, shown to be elevated in preeclamptic women and in reduced uterine perfusion pressure (RUPP) rats, cause hypertension when transferred from RUPP into normal pregnant rats (99). It is important to emphasize that none of these factors, elevated TNF-α, IL-6, CD4+ T cells, or AT1-AA, causes hypertension in nonpregnant rats, indicating the importance of inflammatory mediators in the progression of hypertension in response to placental ischemia and preeclampsia.
The RUPP Model
Because preeclampsia is thought to be caused by a reduction in uterine blood flow due to abnormal trophoblast invasion of the spiral arteries, the development of a model that exploits this reduction in uterine perfusion pressure and flow is a natural alternative for study. RUPP models have been performed in dogs (104, 105), rabbits (3, 65), sheep (23, 61), guinea pigs (39), primates (4, 20) (see Table 1), and rats (1, 11, 29). It should be noted that differences in placentation between animal models (e.g., sheep) and humans may alter the cardiovascular responses to the RUPP maneuvers. Furthermore, in sheep, increased BP only occurs with RUPP when dams are given a high-salt diet (61). The most well-characterized and utilized is the RUPP pregnant rat model (see Table 2). To our knowledge, there have been no studies using the RUPP model in mice.
Table 1.
Models of reduced uterine perfusion pressure in animals other than rat
| Species | Method | Characteristics | Ref. No. |
|---|---|---|---|
| Dog | Occlusion of abdominal aorta below the renal arteries | Inc BP, UPrV, glomerular lesions, fluid retention diffuse hemorrhagic infarction of the placenta | 3, 5, 77, 104, 105 |
| Rabbit | Clamp or ligation of aorta proximal to the ovarian and distal to the renal arteries | Inc BP, UPrV, PVR, IUGR; endothelial swelling, inc glomerular fibrinogen RVR, placental lesions | 2, 3, 65 |
| Nonhuman primate | Red, abdominal aortic blood flow below kidneys | Inc BP, UPrV, RVR, serum uric acid, dec PRA, endtheliosis of glomeruli, inc sFlt-1 | 4, 20, 68, 91 |
| Guinea pig | Banding uterine arteries, ovarian arteries | Inc BP, UPrV, creatinine | 39 |
| Sheep | Occluder around internal iliac artery or abdominal aorta distal to renal arteries | Inc BP only with high-salt diet, IUGR | 23, 61, 94 |
Inc, increased; dec, decreased; BP, blood pressure; UPrV, proteinuria; PRV, peripheral vascular resistance; RVR, renal vascular resistance; PRA, plasma renal activity; sFLT-1, soluble fms-like tyrosine kinase-1; IUGR, intrauterine growth restriction.
Table 2.
Summary of studies done in rat model of RUPP
| Year | Methods Employed | Results of the Study | Ref. No. |
|---|---|---|---|
| 2001 | Characterization of the model | Inc. BP, dec GFR, RPF, nNOS | 11 |
| 2001 | Blockade of endothelin ETA receptor | RUPP causes inc. prepro-ET mRNA, dec BP with Abt627 | 9 |
| 2001 | Blockade with CEI | RUPP causes inc PRA | 8 |
| Enalapril has no effect on BP | |||
| 2002 | Blockade of thromboxane receptor | RUPP causes inc TxB2 | |
| SQ-29548, no effect on BP | |||
| 2003 | Characterization of IUGR with RUPP | Inc BP at 4 wk, sex difference in BP at 12 wk, small for gestational age catch-up growth by 12 wk | 7 |
| 2004 | Blockade of eicosanoid, 20-HETE | 1-ABT dec BP, 20-HETE in renal cortex | 63 |
| 2004 | l-Arginine infusion | Dec BP > in NP | 12 |
| 2005 | Measurement of TNF-α | RUPP inc serum TNF-α | 53 |
| 2005 | Uterine arcuate artery function | Inc response to vasoconstrictor, dec response to vasodilators | 13 |
| 2005 | Measurement leptin, leptin receptors | RUPP inc plasma leptin, inc placental leptin receptor | 14 |
| 2005 | Treatment with N-acetylcysteine | N-AC dec BP, no effect on pups | 21 |
| 2006 | Measurement of interleukin-6 | RUPP causes threefold inc IL-6 | 30 |
| 2007 | Sympathetic nerve activity (SNA) | RUPP inc renal SNA, shifts baroreflex sensitivity | 44 |
| 2007 | Measurement ANG-(1-7), ACE2 | RUPP dec intrarenal ANG-(1-7), no change renal ACE2 activity | 50 |
| 2007 | Measurement sFlt-1 | RUPP inc sFlt-1 | 35 |
| 2008 | Measurement of oxidative stress infusion of tempol | RUPP inc urinary F2-IsoP, MDA, dec renal SOD activity, tempol dec BP | 88 |
| 2008 | AT1 receptor autoantibodies (AT1-AA) | RUPP causes inc AT1-AA, and TNF-α losartan dec BP | 58 |
| 2008 | blockade of TNF-α | Etanercept dec BP | 55 |
| 2008 | Soluble endoglin | RUPP inc soluble endoglin | 37 |
| 2009 | 17α-Progesterone infusion | Dec BP, TNF-α, IL-6, ET-1 | 97 |
| 2009 | Treatment with RUPP plasma | Dec mesenteric vasorelaxation | 102 |
| 2010 | Infusion of VEGF121 | Dec BP, inc GFR, RPF | 38 |
| 2010 | TNF-α, AT1-AA treatment of placental explants | Inc sFlt-1, s-endoglin | 79 |
| 2011 | Role of B lymphocytes | Nituximab dec BP, AT1-AA, ET-1 | 57 |
| 2011 | Infusion of CD4+ cells from RUPP into NP | CD4+ cells in NP inc sFlt-1, TNF-α | 99 |
| 2011 | Infusion of TNF-α soluble receptor | Etanercept dec cardiomegaly, fibrosis dec BNP, inc eNOS, oxytocin receptor | 41 |
| 2011 | Middle cerebral artery myogenic response | RUPP dec myogenic response, causes brain edema | 85 |
| 2011 | Upregulate heme oxygenase 1 (HO-1) with cobalt protoporphyrin (CoPP) | CoPP dec placental SOD, prepro-ET-1 dec placental sFlt-1-to-VEGF ratio | 32, 34 |
| 2011 | PPAR-γ agonist ± HO-1 antagonist | Rosiglitazone dec BP, prevented by Tin protophorphyrin | 71 |
| 2011 | Mesenteric myogenic response | RUPP inc mesenteric myogenic response | 81 |
| 2012 | CoPP inc HO-1 | CO and biliverdin dec placental explant sFlt-1 and reactive oxygen species | 34 |
| 2012 | Poly-ADP-ribose polymerase inhibition | PJ34 before RUPP surgery, dec BP dec mesenteric nitrotyrosine | 101 |
RUPP, reduced uterine perfusion pressure; GFR, glomerular filtration rate; RPF, renal plasma flow; nNOS, neuronal nitric oxide synthase; prepro-ET, preproendothelin; ET-1, endothelin-1; ETA, ET-1 receptor A; TxB2, thromboxane B2; NP, normal pregnant; ACE2, angiotensin converting enzyme 2; eNOS, endothelial nitric oxide synthase; SOD, superoxide dismutase; VEGF, vascular endothelial growth factor; CO, carbon monoxide.
Method to Induce the RUPP Model in Pregnant Rats
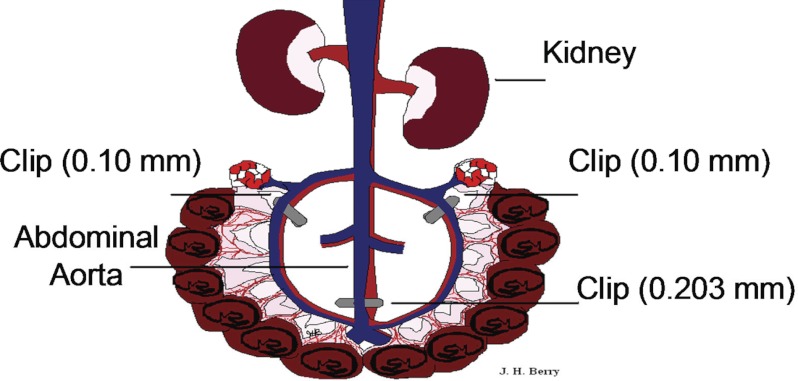
The RUPP rat model of preeclampsia was adapted by Crews and colleagues (24), and the clipping procedure is tightly controlled, in terms of gestation time and silver clip position and size. Pregnant rats weighing ∼200–250 g undergo clipping at day 14 of gestation, in which a silver clip (0.203 mm ID) is placed around the aorta above the iliac bifurcation (see Fig. 2). Because compensatory blood flow to the placenta occurs via an adaptive increase in ovarian blood flow, both right and left uterine arcades are also clipped (silver clip, 0.100 mm ID) (11). These procedures reduce uterine blood flow in the gravid rat by ∼40% (24).
Fig. 2.
Induction of reduced uterine perfusion pressure (RUPP) model in pregnant rats. In the rat RUPP model, laparotomy is performed through an abdominal incision on day 14 of gestation. A silver clip with a 0.203-mm internal diameter is placed around the aorta right above the iliac bifurcation, and silver clips with 0.1 mm internal diameter were placed around the left and right uterine arcade at the ovarian artery before the first segmental artery. Uterine perfusion pressure in the gravid rat is reduced by ∼40%. Blood pressure is measured via a carotid arterial catheter.
Characteristics of the RUPP Model
The RUPP rat mimics numerous physiological features of preeclampsia in women (see Table 3), including hypertension (∼20–30 mmHg increase in mean arterial pressure), proteinuria (∼5-fold increase in urinary protein excretion), impaired renal function, as indicated by reduction in glomerular filtration rate (GFR) (<40%) and renal plasma flow (<23%), an increase in vascular reactivity, a reduction in cardiac index (90), and increases in leptin (14) and blood lactate (36). Fetal IUGR also occurs in RUPP rats, with decreased litter size and pup weight (10, 40).
Table 3.
Comparison of characteristics in women with preeclampsia and the model of RUPP in the rat
| Characteristic | Women with Preeclampsia | RUPP in Rats | Ref. No. |
|---|---|---|---|
| New-onset hypertension | Yes | Yes | 11 |
| Abnormal placentation | Yes | No | 42, 45, 82, 83 |
| Proteinuria | Yes | Yes | 11 |
| Renal plasma flow | Dec | Dec | 90 |
| GFR | Dec | Dec | 11 |
| Inflammation (TNF-α, IL-6, CD4+ T cells) | Inc | Inc | 30, 59, 99 |
| Oxidative stress | Inc | Inc | 88 |
| Endothelin-1 | Inc | Inc | 33 |
| sFlt-1 | Inc | Inc | 35 |
| VEGF, PIGF | Dec | Dec | 35 |
| Soluble endoglin | Inc | Inc | 37 |
| Placental HIF-1α | Inc | Inc | 37 |
| AT1-AA | Inc | Inc | 58 |
| Glomerular endotheliosis | Yes | No | 62 |
| Podocyte shedding | Yes | 6, 31 | |
| HELLP syndrome | In severe cases | No | 48 |
| Fetal IUGR | Yes | Yes | 7, 52 |
TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; PIGF, placental growth factor; HIF-1α, hypoxia-inducible factor-1α; AT1-AA, agonistic autoantibodies against angiotensin type 1 receptor; HELLP syndrome, hemolysis, elevated liver enzymes, low platelets.
RUPP rats have reductions in NO (24) and increases in vasoconstrictor substances or response (84). Vascular smooth muscle cells isolated from renal interlobular arteries displayed increased contractility to ANG II via enhanced calcium entry (75). Inhibition of ANG II synthesis with a converting-enzyme inhibitor had no effect on the BP in RUPP rats (8), but, in contrast, treatment of RUPP rats with losartan, the AT1R antagonist, significantly reduced their BP (58). These findings lead to the discovery that, just as in women with preeclampsia, RUPP rats have increased AT1-AA that causes activation of AT1R and is a contributor to hypertension in the model (100). RUPP rats also exhibit increased tissue ET-1, and ETA receptor antagonists attenuate the BP increases (93). Furthermore, as in women with preeclampsia, sera from RUPP rats causes endothelial cell activation that is attenuated by AT1R blockade, suggesting a role for the AT1-AA in the circulation of RUPP rats to activate the AT1R on the vascular endothelium (84).
As discussed previously, women with preeclampsia have increases in the levels of inflammatory cytokines (52). In RUPP rats, serum levels of TNF-α and IL-6 are increased, and infusion of TNF-α or IL-6 increases BP in normal pregnant rats, suggesting that inflammation could contribute to the hypertension of RUPP rats (30, 59). Supporting this notion, administration of a TNF-α soluble receptor, etanercept, attenuated the hypertension and reduced ET-1 transcript expression and endothelial cell activation from RUPP rats (55). Sunderland and colleagues (92) recently reported similar findings with infusion of TNF-α in pregnant baboons that also resulted in increases in sFLT-1 and soluble engdoglin.
Angiostatic factors, such as sFlt-1 and soluble endoglin, may be important in development and progression of preeclampsia in women (69, 98). RUPP rats exhibit increased plasma and placental sFlt-1 and decreased plasma VEGF and PIGF (35). A recent study demonstrated that chronic infusion of recombinant VEGF-121 during late gestation restored GFR and endothelial function and reduced BP in RUPP rats (38). Serum and placental soluble endoglin are increased along with placental hypoxia-inducible factor-1α expression, whereas placental heme oxygenase-1 (HO-1) is decreased in the RUPP rats (37). An HO-1 inducer attenuates the increases in BP, placental superoxide, placental sFlt-1-to-VEGF ratios, and prepro-ET-1 mRNA in RUPP rats (33). Oxidative stress, as measured by 8-isoprostane and malondialdehyde, is also increased, and renal superoxide dismutase activity is decreased in RUPP rats. Chronic treatment with tempol attenuates the hypertension associated with RUPP (88). George and colleagues (32, 34) have recently shown that the reduction in sFlt-1 with heme oxygenase induction is mediated via production of antioxidants, biliverdin and carbon monoxide.
Advantages of the RUPP Model
The RUPP model of preeclampsia resembles numerous features exhibited by women with preeclampsia (Table 3) and serves as a good tool for investigating potential mechanisms underlying hypertension induced by placental ischemia in pregnancy. Importantly, RUPP models also successfully develop fetal IUGR, which is not always seen in other animal models of preeclampsia, and is a very useful model for studying fetal programming of cardiovascular disease. The RUPP model has also been exploited in nonhuman primates by Makris and colleagues (68), who find similar changes such as hypertension and elevated sFlt-1. The RUPP model provides the opportunity to study potential therapeutic approaches for management of preeclampsia that may not be appropriate for study in women due to the consideration of the health of the fetus.
Limitations of the RUPP Model
Despite the utility of the RUPP model of preeclampsia, the model does have several limitations. First, an increase in BP with RUPP does not always occur in all species or even within the same species (5). The RUPP model developed by Granger (40), LaMarca (54–57), Alexander (7), and colleagues in rats requires the clipping of both the lower abdominal aorta and the uterine arteries on day 14 of gestation in ∼250 g female rats (78). Thus the conditions to produce the model must be rigorous to have a successful outcome of hypertension that recedes after delivery of the pups. The use of rats as a RUPP model is cost effective, but somewhat limited, since the clipping is performed at day 14 of gestation, which only lasts 21–22 days. Thus the window for study is narrow in the rat. The use of nonhuman primates for the RUPP model, as characterized by Makris and colleagues (68), provides a longer gestation time, placentation, and trophoblast invasion closer to humans than rats but is significantly more costly to maintain the animals.
A second important limitation of the RUPP model is that it only mimics the pathogenesis responsible for the hypertension in preeclampsia downstream of midgestational placental ischemia. Thus the RUPP model is not useful to investigate the early immune mechanisms, trophoblast invasion, and vascular remodeling abnormalities. Furthermore, aortic clipping may have a hemodynamic impact on sympathetic nervous system and cardiac output, since Sholook and colleagues (90) reported that the RUPP procedure results in reductions in blood flow to the heart, stomach, intestine, and skeletal muscle, without differences in blood flow to the brain, liver, kidney, or spleen compared with the normal pregnant rats.
A third limitation is that the RUPP model in the rat does not mimic the glomerular endotheliosis that is one of the hallmarks of preeclampsia (62). This may be important because several investigators find that podocyte shedding occurs in women with preeclampsia and may be an early marker for preeclpamsia (6, 31).
Finally, the RUPP model is not successful in mimicking severe preeclampsia, which is characterized by the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. RUPP rats do not have changes in hemoglobin, platelets, or liver function (48).
Summary
In conclusion, pregnancy in women involves unique fetal villous trophoblast invasion and placental vascular remodeling, namely placentation. This process is only seen in women and great apes. Thus to our knowledge, preeclampsia occurs spontaneously only in women and great apes due to abnormal placentation and the resulting placental ischemia. Thus no animal model of preeclampsia can mimic the entire pathogenesis of the disease as seen in women. A variety of animal models, including genetically manipulated mouse models, have been developed based on a single mediator of preeclampsia that only reflects limited aspects of the mechanisms underlying the disease; however, these are useful to identify mechanisms that could potentially contribute to the pathogenesis of preeclampsia. The RUPP model has been performed in various species (see Table 1) and involves induction of placental ischemia by reducing uterine blood flow by some maneuver, such as placement of clips on the uterine arteries and abdominal aorta, that results in a preeclamptic state. The RUPP model in the rat has been the most extensively studied (see Table 2) and exhibits many of the manifestations of preeclampsia (see Table 3). Unfortunately, the greatest limitation of the RUPP model is its lack of suitability for the study of early placentation and the subsequent pathogenesis responsible for placental ischemia, such as early occurring abnormal immune mechanisms or trophoblast invasion and vascular remodeling. However, the RUPP model is excellent for the study of many of the consequences of placental ischemia, including hypertension, vascular dysfunction, and immune dysfunction. The ability to develop the RUPP model in both rats and nonhuman primates makes it advantageous for many future studies. Whether the RUPP model could be developed in mice is not clear.
GRANTS
This work was supported by National Institutes of Health (NIH) RO1s HL-66072, HL-69194, and PO1 HL-51971 (to J. F. Reckelhoff), American Heart Association Frant SDG 0835472N, and NIH Grant HD-067541. (to B. B. LaMarca).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.L. and B.L. prepared figures; J.L. drafted manuscript; B.L. and J.F.R. edited and revised manuscript; J.F.R. approved final version of manuscript.
REFERENCES
- 1.Abitbol MM. Simplified technique to produce toxemia in the rat: considerations on cause of toxemia. Clin Exp Hypertens B 1: 93–103, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Abitbol MM, Driscoll SG, Ober WB. Placental lesions in experimental toxemia in the rabbit. Am J Obstet Gynecol 125: 942–948, 1976 [DOI] [PubMed] [Google Scholar]
- 3.Abitbol MM, Gallo GR, Pirani CL, Ober WB. Production of experimental toxemia in the pregnant rabbit. Am J Obstet Gynecol 124: 460–470, 1976 [DOI] [PubMed] [Google Scholar]
- 4.Abitbol MM, Ober MB, Gallo GR, Driscoll SG, Pirani CL. Experimental toxemia of pregnancy in the monkey, with a preliminary report on renin and aldosterone. Am J Pathol 86: 573–590, 1977 [PMC free article] [PubMed] [Google Scholar]
- 5.Abitbol MM, Pirani CL, Ober WB, Driscoll SG, Cohen MW. Production of experimental toxemia in the pregnant dog. Obstet Gynecol 48: 537–548, 1976 [PubMed] [Google Scholar]
- 6.Aita K, Etoh M, Hamada H, Yokoyama C, Takahashi A, Suzuki T, Hara M, Nagata M. Acute and transient podocyte loss and proteinuria in preeclampsia. Nephron Clin Pract 112: c65–c70, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension 38: 742–745, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 37: 485–489, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 15: 170–175, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. l-Arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension 43: 832–836, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Anderson CM, Lopez F, Zhang HY, Pavlish K, Benoit JN. Reduced uteroplacental perfusion alters uterine arcuate artery function in the pregnant Sprague-Dawley rat. Biol Reprod 72: 762–766, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Anderson CM, Lopez F, Zhang HY, Pavlish K, Benoit JN. Characterization of changes in leptin and leptin receptors in a rat model of preeclampsia. Am J Obstet Gynecol 193: 267–272, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Baird JN., Jr Eclampsia in a lowland gorilla. Am J Obstet Gynecol 141: 345–346, 1981 [DOI] [PubMed] [Google Scholar]
- 16.Baker PN, Davidge ST, Roberts JM. Plasma from women with preeclampsia increases endothelial cell nitric oxide production. Hypertension 26: 244–248, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens 22: 564–568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown CE, Gant NF, Cox K, Spitz B, Rosenfeld CR, Magness RR. Low-dose aspirin. II Relationship of angiotensin II pressor responses, circulating eicosanoids, and pregnancy outcome. Am J Obstet Gynecol 163: 1853–1861, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Cadnapaphornchai MA, Ohara M, Morris KG, Jr, Knotek M, Rogachev B, Ladtkow T, Carter EP, Schrier RW. Chronic NOS inhibition reverses systemic vasodilation and glomerular hyperfiltration in pregnancy. Am J Physiol Renal Physiol 280: F592–F598, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Cavanagh D, Rao PS, Knuppel RA, Desai U, Balis JU. Pregnancy-induced hypertension: development of a model in the pregnant primate (Papio anubis). Am J Obstet Gynecol 151: 987–999, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Chang EY, Barbosa E, Paintlia MK, Singh A, Singh I. The use of N-acetylcysteine for the prevention of hypertension in the reduced uterine perfusion pressure model for preeclampsia in Sprague-Dawley rats. Am J Obstet Gynecol 193: 952–956, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Choi JW, Im MW, Pai SH. Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann Clin Lab Sci 32: 257–263, 2002 [PubMed] [Google Scholar]
- 23.Clark KE, Durnwald M, Austin JE. A model for studying chronic reduction in uterine blood flow in pregnant sheep. Am J Physiol Heart Circ Physiol 242: H297–H301, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 35: 367–372, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Croy BA, Burke SD, Barrette VF, Zhang J, Hatta K, Smith GN, Bianco J, Yamada AT, Adams MA. Identification of the primary outcomes that result from deficient spiral arterial modification in pregnant mice. Pregnancy Hypertens 1: 87–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, Sethi S, Weiss RM, Bates JN. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension 39: 337–342, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Deng A, Engels K, Baylis C. Impact of nitric oxide deficiency on blood pressure and glomerular hemodynamic adaptations to pregnancy in the rat. Kidney Int 50: 1132–1138, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Dokras A, Hoffmann DS, Eastvold JS, Kienzle MF, Gruman LM, Kirby PA, Weiss RM, Davisson RL. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol Reprod 75: 899–907, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Eder DJ, Macdonald MT. A role for brain angioension II in experimental pregnancy-induced hypertesnion in laboratory rats. Clin Exp Hypertens Preg B6: 431–451, 1988 [Google Scholar]
- 30.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension 48: 711–716, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Garovic VD, Wagner SJ, Turner ST, Rosenthal DW, Watson WJ, Brost BC, Rose CH, Gavrilova L, Craigo P, Bailey KR, Achenbach J, Schiffer M, Grande JP. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol 196: e1–e7, 2007 [DOI] [PubMed] [Google Scholar]
- 32.George EM, Arany M, Cockrell K, Storm MV, Stec DE, Granger JP. Induction of heme oxygenase-1 attenuates sFlt-1-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol 301: R1495–R1500, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George EM, Cockrell K, Arany M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57: 941–948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George EM, Colson D, Dixon J, Palei AC, Granger JP. Heme oxygenase-1 attenuates hypoxia-induced sFlt-1 and oxidative stress in placental villi through its metabolic products CO and bilirubin. Int J Hypertens In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Gilbert JS, Bauer AJ, Gingery A, Banek CT, Chasson S. Circulating and utero-placental adaptations to chronic ischemia in the rat. Placenta 33: 100–105, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 53: 399–403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55: 380–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golden JG, Hughes HC, Lang CM. Experimental toxemia in the pregnant guinea pig (Cavia porcellus). Lab Anim Sci 30: 174–179, 1980 [PubMed] [Google Scholar]
- 40.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 9: 147–160, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Gutkowska J, Granger JP, Lamarca BB, Danalache BA, Wang D, Jankowski M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: effect of tumor necrosis factor blockade. J Hypertens 29: 1203–1212, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Harris LK. IFPA Gabor Than Award lecture: Transformation of the spiral arteries in human pregnancy: key events in the remodelling timeline. Placenta 32, Suppl 2: S154–S158, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Hefler LA, Tempfer CB, Moreno RM, O'Brien WE, Gregg AR. Endothelial-derived nitric oxide and angiotensinogen: blood pressure and metabolism during mouse pregnancy. Am J Physiol Regul Integr Comp Physiol 280: R174–R182, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Hines T, Beauchamp D, Rice C. Baroreflex control of sympathetic nerve activity in hypertensive pregnant rats with reduced uterine perfusion. Hypertens Pregnancy 26: 303–314, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol 2: 543–549, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann DS, Weydert CJ, Lazartigues E, Kutschke WJ, Kienzle MF, Leach JE, Sharma JA, Sharma RV, Davisson RL. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension 51: 1058–1065, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Ishida J, Matsuoka T, Saito-Fujita T, Inaba S, Kunita S, Sugiyama F, Yagami K, Fukamizu A. Pregnancy-associated homeostasis and dysregulation: lessons from genetically modified animal models. J Biochem 150: 5–14, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Isler CM, Bennett WA, Rinewalt AN, Cockrell KL, Martin JN, Jr, Morrison JC, Granger JP. Evaluation of a rat model of preeclampsia for HELLP syndrome characteristics. J Soc Gynecol Investig 10: 151–153, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Johne J. Pregnancy toxaemia in a multiparous chimpanzee at Dallas Zoo. Int Zoo Yearb 11: 239–241, 1971 [Google Scholar]
- 50.Joyner J, Neves LA, Granger JP, Alexander BT, Merrill DC, Chappell MC, Ferrario CM, Davis WP, Brosnihan KB. Temporal-spatial expression of ANG-(1–7) and angiotensin-converting enzyme 2 in the kidney of normal and hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol 293: R169–R177, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol 283: R29–R45, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol 170: 1752–1759, 1994 [PubMed] [Google Scholar]
- 53.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 54.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 54: 905–909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension 52: 1161–1167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LaMarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. Inter J Interferon Cytokine Med Res 3: 65–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN, Jr, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension 57: 865–871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 52: 1168–1172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 60.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46: 82–86, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Leffler CW, Hessler JR, Green RS, Fletcher AM. Effects of sodium chloride on pregnant sheep with reduced uteroplacental perfusion pressure. Hypertension 8: 62–65, 1986 [DOI] [PubMed] [Google Scholar]
- 62.Lindheimer MD, Conrad KP, Karumanchi SA. Renal Physiology and Disease in Pregnancy. In: Seldin and Giebisch's The Kidney; Physiology and Pathophysiology (4th ed.), edited by Alpern RJ, Hebert SC. San Diego, CA: Academic, 2008, p. 2339–2398 [Google Scholar]
- 63.Llinás MT, Alexander BT, Capparelli MF, Carroll MA, Granger JP. Cytochrome P-450 inhibition attenuates hypertension induced by reductions in uterine perfusion pressure in pregnant rats. Hypertension 43: 623–628, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Llinás MT, Alexander BT, Seedek M, Abram SR, Crell A, Granger JP. Enhanced thromboxane synthesis during chronic reductions in uterine perfusion pressure in pregnant rats. Am J Hypertens 15: 793–797, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Losonczy G, Brown G, Venuto RC. Increased peripheral resistance during reduced uterine perfusion pressure hypertension in pregnant rabbits. Am J Med Sci 303: 233–240, 1992 [DOI] [PubMed] [Google Scholar]
- 66.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol 196: 396 e391–e397, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Magann EF, Bass JD, Chauhan SP, Perry KG, Jr, Morrison JC, Martin JN., Jr Accelerated recovery from severe preeclampsia: uterine curettage versus nifedipine. J Soc Gynecol Investig 1: 210–214, 1994 [DOI] [PubMed] [Google Scholar]
- 68.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 71: 977–984, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Correlation between soluble endoglin, vascular endothelial growth factor receptor-1, and adipocytokines in preeclampsia. J Clin Endocrinol Metab 92: 2672–2679, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK, Kenny LC. Peroxisome proliferator-activated receptor-γ as a potential therapeutic target in the treatment of preeclampsia. Hypertension 58: 280–286, 2011 [DOI] [PubMed] [Google Scholar]
- 72.McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta 32: 413–419, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Meis PJ, Goldenberg RL, Mercer BM, Iams JD, Moawad AH, Miodovnik M, Menard MK, Caritis SN, Thurnau GR, Bottoms SF, Das A, Roberts JM, McNellis D. The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol 178: 562–567, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Molnar M, Suto T, Toth T, Hertelendy F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am J Obstet Gynecol 170: 1458–1466, 1994 [DOI] [PubMed] [Google Scholar]
- 75.Murphy JG, Herrington JN, Granger JP, Khalil RA. Enhanced [Ca2+]i in renal arterial smooth muscle cells of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Heart Circ Physiol 284: H393–H403, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Murphy SR, Lamarca B, Cockrell K, Arany M, Granger JP. l-Arginine supplementation abolishes the blood pressure and endothelin response to chronic increases in plasma sFlt-1 in pregnant rats. Am J Physiol Regul Integr Comp Physiol 302: R259–R263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ogden E, Hildebrand GJ, Page EW. Rise of blood pressure during ischemia of the gravid uterus. Proc Soc Exp Biol Med 43: 49–51, 1940 [Google Scholar]
- 78.Ojeda NB, Grigore D, Alexander BT. Intrauterine growth restriction: fetal programming of hypertension and kidney disease. Adv Chronic Kidney Dis 15: 101–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 23: 911–916, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Podjarny E, Losonczy G, Baylis C. Animal models of preeclampsia. Semin Nephrol 24: 596–606, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramirez RJ, Debrah J, Novak J. Increased myogenic responses of resistance-sized mesenteric arteries after reduced uterine perfusion pressure in pregnant rats. Hypertens Pregnancy 30: 45–57, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Redman CW. Preeclampsia: a multi-stress disorder. Rev Med Interne 32, Suppl 1: S41–S44, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol 63: 534–543, 2010 [DOI] [PubMed] [Google Scholar]
- 84.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension 47: 615–618, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Ryan MJ, Gilbert EL, Glover PH, George EM, Masterson CW, McLemore GR, Jr, LaMarca B, Granger JP, Drummond HA. Placental ischemia impairs middle cerebral artery myogenic responses in the pregnant rat. Hypertension 58: 1126–1131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med 28: 192–209, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Schiessl B. Inflammatory response in preeclampsia. Mol Aspects Med 28: 210–219, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shesely EG, Gilbert C, Granderson G, Carretero CD, Carretero OA, Beierwaltes WH. Nitric oxide synthase gene knockout mice do not become hypertensive during pregnancy. Am J Obstet Gynecol 185: 1198–1203, 2001 [DOI] [PubMed] [Google Scholar]
- 90.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol 293: H2080–H2084, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Stout C, Lemmon WB. Glomerular capillary endothelial swelling in a pregnant chimpanzee. Am J Obstet Gynecol 105: 212–215, 1969 [DOI] [PubMed] [Google Scholar]
- 92.Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A. Tumor necrosis factor α induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 56: 192–199, 2011 [DOI] [PubMed] [Google Scholar]
- 93.Tam Tam KB, George E, Cockrell K, Arany M, Speed J, Martin JN, Jr, Lamarca B, Granger JP. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol 204: 330 e331–e334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thatcher CD, Keith JC., Jr Pregnancy-induced hypertension: development of a model in the pregnant sheep. Am J Obstet Gynecol 155: 201–207, 1986 [DOI] [PubMed] [Google Scholar]
- 95.Thornton JG, Onwude JL. Convulsions in pregnancy in related gorillas. Am J Obstet Gynecol 167: 240–241, 1992 [DOI] [PubMed] [Google Scholar]
- 96.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, management. Vasc Health Risk Manag 7: 467–474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Veillon EW, Jr, Keiser SD, Parrish MR, Bennett W, Cockrell K, Ray LF, Granger JP, Martin JN, Jr, LaMarca B. 17-Hydroxyprogesterone blunts the hypertensive response associated with reductions in uterine perfusion pressure in pregnant rats. Am J Obstet Gynecol 201: e1–e6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 12: 642–649, 2006 [DOI] [PubMed] [Google Scholar]
- 99.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN, Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103: 945–952, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walsh S, English F, Crocker I, Johns E, Kenny L. Contribution of poly(ADP-ribose) polymerase to endothelial dysfunction and hypertension in a rat model of pre-eclampsia. Br J Pharmacol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walsh SK, English FA, Johns EJ, Kenny LC. Plasma-mediated vascular dysfunction in the reduced uterine perfusion pressure model of preeclampsia: a microvascular characterization. Hypertension 54: 345–351, 2009 [DOI] [PubMed] [Google Scholar]
- 103.Woods AK, Hoffmann DS, Weydert CJ, Butler SD, Zhou Y, Sharma RV, Davisson RL. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension 57: 94–102, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woods LL. Importance of prostaglandins in hypertension during reduced uteroplacental perfusion pressure. Am J Physiol Regul Integr Comp Physiol 257: R1558–R1561, 1989 [DOI] [PubMed] [Google Scholar]
- 105.Woods LL, Brooks VL. Role of the renin-angiotensin system in hypertension during reduced uteroplacental perfusion pressure. Am J Physiol Regul Integr Comp Physiol 257: R204–R209, 1989 [DOI] [PubMed] [Google Scholar]
- 106.Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol 169: 1316–1320, 1993 [DOI] [PubMed] [Google Scholar]
- 107.Young J. The aetiology of eclampsia and albuminuria and their relation to accidental haemorrhage: an anatomical and experimental investigation. Proc R Soc Med 7: 307–348, 1914 [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 14: 855–862, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]