Abstract
Right heart catheterization is often required to monitor intra-cardiac pressures in a number of disease states. Ultrasound contrast agents can produce pressure modulated subharmonic emissions that may be used to estimate right ventricular (RV) pressures. A technique based on subharmonic acoustic emissions from ultrasound contrast agents to track RV pressures noninvasively has been developed and its clinical potential evaluated. The subharmonic signals were obtained from the aorta, RV, and right atrium (RA) of five anesthetized closed-chest mongrel dogs using a SonixRP ultrasound scanner and PA4-2 phased array. Simultaneous pressure measurements were obtained using a 5-French solid state micromanometer tipped catheter. Initially, aortic subharmonic signals and systemic blood pressures were used to obtain a calibration factor in units of millimeters of mercury per decibel. This factor was combined with RA pressures (that can be obtained noninvasively) and the acoustic data from the RV to obtain RV pressure values. The individual calibration factors ranged from −2.0 to −4.0 mmHg/dB. The subharmonic signals tracked transient changes in the RV pressures within an error of 0.6 mmHg. Relative to the catheter pressures, the mean errors in estimating RV peak systolic and minimum diastolic pressures, and RV relaxation [isovolumic negative derivative of change in pressure over time (−dP/dt)] by use of the subharmonic signals, were −2.3 mmHg, −0.8 mmHg, and 2.9 mmHg/s, respectively. Overall, acoustic estimates of RV peak systolic and minimum diastolic pressures and RV relaxation were within 3.4 mmHg, 1.8 mmHg, and 5.9 mmHg/s, respectively, of the measured pressures. This pilot study demonstrates that subharmonic emissions from ultrasound contrast agents have the potential to noninvasively track in vivo RV pressures with errors below 3.5 mmHg.
Keywords: contrast echocardiography, subharmonic microbubble signals, noninvasive pressure estimation, contrast media
the right ventricle (rv) has limited ability to cope with abnormal hemodynamic loading or chronic pressure loading before severe RV dysfunction or RV failure occurs (26). Various cardiac abnormalities affect RV functionality (16). RV dysfunction is a presage for a poorer prognosis in patients with various cardiac abnormalities (36). The RV output is extremely sensitive to changes in the afterload (26), and the histological changes associated with RV pressure overload states are more pronounced compared with the volume overload states (22, 25). Furthermore, moderate to severe episodes of biopsy negative rejections following heart transplants are accompanied by changes in the intra-cardiac pressures (12). Invasive hemodynamic monitoring with right heart catheterization provides absolute pressure measurements to diagnose and/or monitor therapy outcomes (6). However, the potential complications associated with this invasive technique underscore the need for reliable methods to generate noninvasive measurements of intra-cardiac pressures (7, 28, 30).
Microbubble-based ultrasound contrast agents, approved in the United States for left ventricular opacification (2), have the potential to act as ambient pressure sensors, due to their characteristic volume pulsations (32). Previous experiments have revealed the presence of subharmonic signals (at half the insonation frequency) from the insonated bubbles in liquid and that the subharmonic signal generation occurs only when the incident acoustic power levels exceed a certain threshold (9, 27). Once the threshold power is exceeded and the subharmonic signal is generated, this subharmonic signal may be characterized into three stages-occurrence, growth, and saturation (32, 33). This classification of subharmonic emissions into three stages has also been characterized by a Newtonian rheological model that revealed a steep growth of subharmonic signal after the occurrence stage (29). In the occurrence stage the subharmonic signal amplitude is probably too low to be detected above the noise level. In the saturation stage the subharmonic signal is the highest mostly due to strong subharmonic emissions by the collapsing bubbles. The concept of subharmonic aided pressure estimation (SHAPE) revolves around the subharmonic signal emissions in the intermediate stage, i.e., the growth stage. In the growth stage, the subharmonic response can be used to track ambient pressure changes in vitro (17, 32) and in vivo (13, 18). An increase in ambient pressures when the subharmonic emissions are in the growth phase may dampen the nonlinear oscillations causing the subharmonic emissions, and this may result in a decrease in subharmonic signal amplitude as the ambient pressure increases. Alternatively, it has been postulated that the subharmonic emissions may also be dependent on the ratio of the excitation frequency (transmit frequency) to the bubbles′ natural resonance frequency and that the bubbles′ natural resonance frequency is affected by ambient pressure changes (23). The concept to use ambient pressure modulated subharmonic signals for subharmonic imaging is also being discussed (10).
Overall, theoretical and experimental studies have revealed the presence of ambient pressure modulated subharmonic emissions (1, 10, 13–15, 17, 18, 23, 32, 33); these subharmonic emissions are harnessed in the current manuscript to estimate RV pressures. The application of SHAPE in open-chest canines provided an initial proof-of-concept, but the use of single element transducers (without imaging capability) and the invasive approach precluded clinical investigations (13). Hence, this pilot study evaluates the potential of SHAPE to measure RV pressures noninvasively in a closed-chest model using a commercially available ultrasound scanner.
MATERIALS AND METHODS
Animal Preparation
This study was approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University and conducted in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Five mongrel dogs (weight range, 21 to 23 kg) were studied in this project to evaluate the efficacy of noninvasively estimating and tracking pressures in the RV using SHAPE. Initially, an intravenous injection of Propofol (Abbott Laboratories, Chicago, IL; dose of 7 ml/kg) was used as the anesthetic. During the course of the experiments, the animals were intubated and anesthesia was maintained with 0.5% to 2% isoflurane (Iso-thesia; Abbott Laboratories) via an endotracheal tube. A warming blanket was also used to maintain normal body temperature. An 18-gauge catheter was placed in a forelimb vein for infusion of Sonazoid microbubbles (GE Healthcare, Oslo, Norway) at a concentration of 0.015 μl·kg−1·min−1. Sonazoid microbubbles were selected for this study, because the subharmonic emissions from this agent were found to be the most sensitive to ambient pressures when compared with the subharmonic emissions from other contrast agents (17). This concept was verified through simulations and experimental studies conducted by independent laboratories working with Sonazoid and other contrast agents (1, 15). Sonazoid is not yet approved by the US Food and Drug Administration for human use, but this agent has been used for 5 years to detect liver lesions in Japan and other numerous animal and human studies have documented the safety of Sonazoid microbubbles for in vivo use (24). Sonazoid microbubbles have a volume median diameter of about 3 μm, enabling them to traverse the entire vasculature, and they can withstand ambient pressure changes up to 300 mmHg (19, 34). A 5F solid state micromanometer tipped catheter (SPR 350; Millar Instruments, Houston, TX) was used as the reference standard and was introduced at the site for pressure measurements under ultrasound guidance. At the end of the experiments the canines were euthanized by an intravenous injection of Beuthanasia (0.25 mg/kg).
Data Acquisition
A commercial SonixRP ultrasound scanner (Ultrasonix, Richmond, BC, Canada) with a PA4-2 phased array probe (2.5-MHz center frequency) was used to image the canines (closed chest) in pulse inversion grayscale mode. A synchronization signal from the ultrasound scanner was used to trigger an oscilloscope (9350 AM; LeCroy, Chestnut Ridge, NY) for simultaneous acquisition of the pressure catheter data on a computer through LabView (Version 8.0; National Instruments, Austin, TX). The subharmonic response from the Sonazoid microbubbles and their ability to track ambient pressures depend on the insonation frequency and the incident acoustic power. Based on in vitro studies, the ultrasound transmit frequency was maintained at 2.5 MHz (17), whereas the incident acoustic output power from the scanner was varied from −8 to 0 dB (i.e., from 39% to maximum scanner output in increments of 2 dB or from 76 to 897 kPa peak to peak corresponding to a peak mechanical index of 0.38). This range of incident power levels was selected to elicit the subharmonic response from the microbubbles in the growth phase, which is the ambient pressure sensitive phase. Varying the power levels is required, because the attenuation affecting the incident beam will vary on a case-by-case basis and will depend on the site and depth of the measurement location. The unprocessed ultrasound radio frequency (RF) data were acquired in triplicate for 5 s simultaneously with the pressure catheter data for each of the five discreet acoustic power levels [−8 dB (39%), −6 dB (50%), −4 dB (63%), −2 dB (79%), and 0 dB (100%)]. These data were first acquired from the aorta, then from the RV, and then from the right atrium (RA) of all five canines cumulating to 225 data sets. The ultrasound data acquisitions were performed in B/pulsed wave mode (simultaneous display of live B-mode images and pulsed wave Doppler waveform; B-mode pulses interleaved with pulsed wave pulses) to avoid possible signal corruption due to motion artifacts.
Data Processing and Statistical Analysis
Subharmonic signal extraction and pressure analyses were performed offline using Matlab (Version 7.8.0; The Mathworks, Natick, MA). The RF data for each accumulated pulse (Fig. 1, A and B) was transformed to the Fourier domain (Fig. 1, C and D) and the subharmonic signal amplitude (in dB) was extracted as the average signal in a 40% bandwidth around the subharmonic frequency (i.e., 1.25 MHz) as shown in Fig. 1, C and D. This process was repeated for all the pulses for each acquisition. The extracted data were subsequently processed using a median filter to eliminate noise spikes. After noisy pulses were eliminated, the amplitudes of the subharmonic signal in the systolic and diastolic phases were determined using time points obtained from the synchronized pressure catheter data. The resulting range of the subharmonic signals (i.e., the difference between maximum and minimum subharmonic amplitudes) were compared to identify the most sensitive incident acoustic power setting for aortic and RV pressure tracking in each canine based on two criteria: the subharmonic signal was residing in the growth stage of subharmonic emissions and the range of subharmonic signals was not influenced by bubble destruction (which occurs at relatively high incident acoustic power levels) (17, 32). These comparisons were performed with a one-way ANOVA and Bonferroni corrections for multiple comparisons (4) (P values less than 0.05 were considered significant) using PASW Statistics 18 (SPSS, Chicago, IL). After the most sensitive incident acoustic power setting was identified, a calibration factor in millimeters of mercury per decibel was calculated from the aorta using the subharmonic data as well as systolic and diastolic pressure values (i.e., systemic pulse pressure). This calibration factor was calculated for each individual canine. The calibration factor and the RA pressure values were then used with the subharmonic data from the RV to obtain RV pressure contours. Based on the subharmonic pressure contours, the minimum RV diastolic pressure (RVDmin), RV systolic pressure (RVSP), and RV peak −dP/dt values were determined and compared with the manometer pressures. Values are indicated as means ± SD.
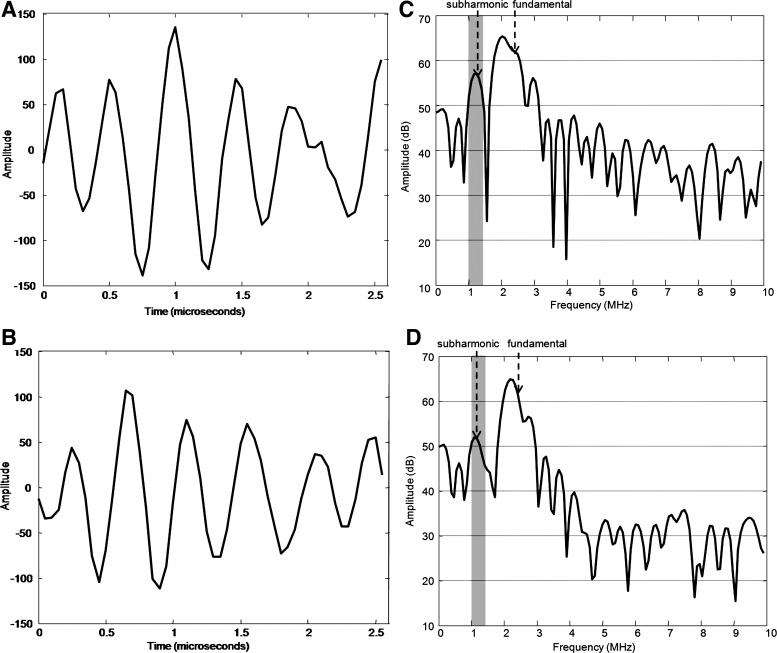
Fig. 1.
Ultrasound radio frequency data acquired from the pulsed wave Doppler gate. The pulsed wave gate data acquired during minimum diastolic (A) and peak systolic right ventricular (RV) pressure (B) phases are shown. The Fourier domain representations of the pulses in A and B are shown in C and D, respectively. The bandwidth from which the subharmonic signal was extracted from each pulse is shaded in gray in C and D. The subharmonic signal amplitude decreases from about 56 dB (C) to about 51 dB (D) as the pressure increases during systole. During the systolic pressure rise, there is no change in fundamental signal amplitude confirming that subharmonic signal alone can be calibrated to indicate absolute pressure values, consistent with an inverse relationship between subharmonic signals and ambient pressure values (1, 13, 15, 17, 18, 32).
RESULTS
Determination of Optimum Incident Power Levels for SHAPE
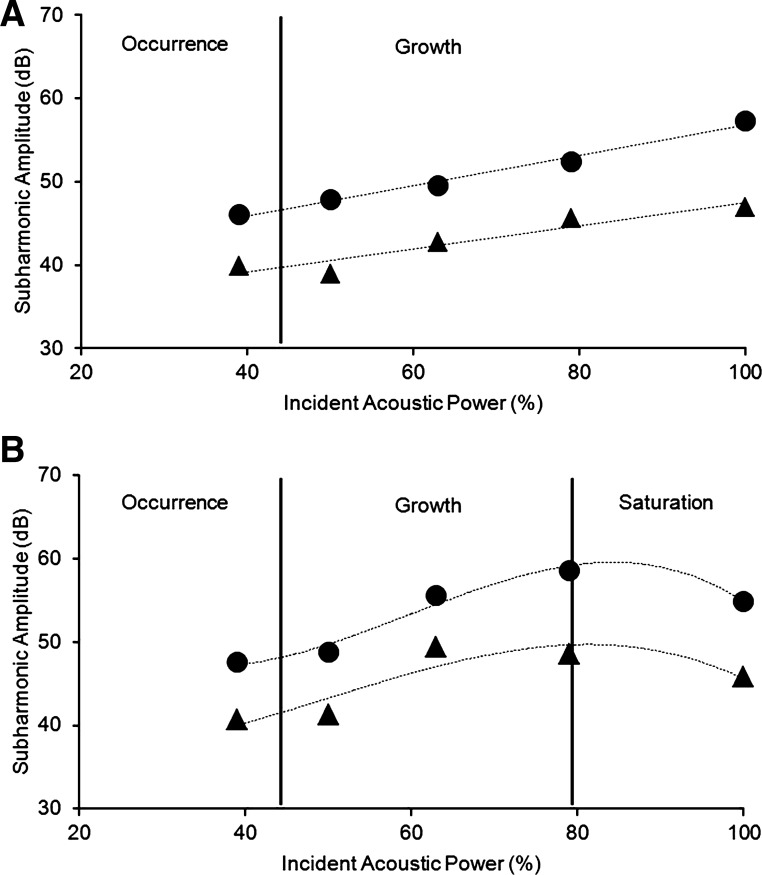
A representative data set of subharmonic amplitudes obtained from the aorta and the RV of a single canine is represented in Fig. 2. Figure 2A depicts the subharmonic data acquired from the aorta, specifically with the subharmonic amplitudes corresponding to the peak systolic and minimum diastolic pressures, and Fig. 2B shows the data acquired from the RV. From the aortic data (Fig. 2A), it can be seen that the subharmonic signals are either in the occurrence stage or the growth phase, because the subharmonic amplitudes did not achieve a steady state value (characteristic of the saturation phase) even with 100% power. Statistically, this was confirmed by results obtained from the ANOVA, which showed significant main effect of the acoustic power level [F (4, 23) = 50.7, and F (4, 28) = 27.8, P < 0.001] with subharmonic amplitudes at both 79% and 100% greater than at other power levels (indicating that saturation was not reached). Thus 100% power level was selected based on the aortic data from this canine. On the other hand, the RV data exhibited subharmonic signals in all three phases: occurrence (up to 50% power level), growth (up to 79% power level), and saturation followed by bubble destruction (marked by a decreased in subharmonic amplitude at 100% power) (Fig. 2B). Statistically, this was confirmed by results obtained from ANOVA, which showed significant main effect of the acoustic power level [F (4, 31) = 129.2 and F (4, 36) = 98.5, p < 0.001]. However, post hoc analyses revealed that subharmonic amplitudes at 100% were less than at 79% power levels (P < 0.001), indicating saturation at 79% power level and possibly bubble destruction at 100% power level. Thus 63% power level was selected for the RV data from this canine. Similarly, data from all other canines were analyzed with ANOVA revealing the optimum power levels to be used for SHAPE from the aorta and the RV, and these are presented in Table 1.
Fig. 2.
Sample subharmonic signal amplitudes from the aorta and RV of 1 canine. The subharmonic amplitudes corresponding to the peak systolic (closed triangles) and minimum diastolic (closed circles) pressures in the aorta (A) and RV (B) are shown. The distinct phases of subharmonic emissions from microbubbles identified using ANOVA and post hoc comparisons are indicated.
Table 1.
Optimum incident power levels obtained in five canines
| Optimum Power Levels |
||||
|---|---|---|---|---|
| For Aortic Data |
For RV Data |
|||
| Canines | % | As indicated on scanner, dB | % | As indicated on scanner, dB |
| 1 | 79 | −2 | 79 | −2 |
| 2 | 63 | −4 | 63 | −4 |
| 3 | 63 | −4 | 63 | −4 |
| 4 | 63 | −4 | 50 | −6 |
| 5 | 100 | 0 | 63 | −4 |
RV, right ventricular.
Calculation of Calibration Factor (in mmHg/dB) in the Aorta
The systemic pulse pressures and the subharmonic amplitudes corresponding to the peak systolic and minimum diastolic pressures are given in Table 2. The SDs were derived from about 5 to 7 pressure contours per 5 s acquisition, and, because there are data from three runs, this corresponds to about 15 to 21 values for each canine. The resulting calibration factors for each canine are also shown in Table 2. Note that this calibration factor varied by as much as a factor of 2, encompassing a range from −2 mmHg/dB to −4 mmHg/dB and underscoring the need to obtain individual calibration factors for each case. The mean peak systolic and minimum diastolic pressures in the aorta were 86.9 ± 20.1 mmHg and 57.5 ± 14.2 mmHg, respectively. The corresponding mean subharmonic amplitudes were 51.5 ± 6.1 dB and 61.6 ± 7.0 dB with higher subharmonic amplitudes associated with lower ambient pressures (minimum diastolic) and lower subharmonic amplitudes associated with higher ambient pressures (peak systolic), as reported in the literature (1, 13, 15, 17, 18, 32). The calibration factor was successfully obtained for a wide range of systemic pulse pressures, i.e., from 21.1 mmHg to 41.8 mmHg.
Table 2.
Calibration factor calculations from the aorta
| Specific Aortic Pressure Phases | Catheter, mmHg | Subharmonic Amplitude, dB | Calibration Factor, mmHg/dB |
|---|---|---|---|
| Canine 1 | |||
| Peak systolic | 74.8 ± 1.2 | 53.0 ± 1.1 | |
| Minimum diastolic | 49.6 ± 0.4 | 65.5 ± 1.3 | |
| Systemic pulse | 25.2 ± 1.0 | −12.5 ± 1.0 | −2.0 |
| Canine 2 | |||
| Peak systolic | 81.0 ± 1.1 | 48.6 ± 0.2 | |
| Minimum diastolic | 59.9 ± 1.0 | 54.1 ± 0.3 | |
| Systemic pulse | 21.1 ± 0.3 | −5.5 ± 0.1 | −3.8 |
| Canine 3 | |||
| Peak systolic | 76.7 ± 1.8 | 61.6 ± 2.4 | |
| Minimum diastolic | 45.3 ± 3.0 | 71.6 ± 1.9 | |
| Systemic pulse | 31.4 ± 3.5 | −10.0 ± 3.1 | −3.1 |
| Canine 4 | |||
| Peak systolic | 79.3 ± 0.6 | 47.6 ± 0.9 | |
| Minimum diastolic | 51.7 ± 0.8 | 59.3 ± 1.3 | |
| Systemic pulse | 27.6 ± 1.0 | −11.7 ± 1.6 | −2.4 |
| Canine 5 | |||
| Peak systolic | 122.8 ± 0.8 | 46.9 ± 1.7 | |
| Minimum diastolic | 81.0 ± 1.0 | 57.3 ± 1.1 | |
| Systemic pulse | 41.8 ± 1.3 | −10.4 ± 2.0 | −4.0 |
Values are means ± 1 SD. Subharmonic signal amplitudes at optimum incident acoustic power setting are indicated. Systemic pulse values are calculated as the difference between the systolic and diastolic values. The calibration factor values are calculated by dividing the systemic pulse pressures by the systemic pulse subharmonic amplitudes.
RV Pressure Estimation and Comparison with the Catheter Pressures
RV pressure profiles.
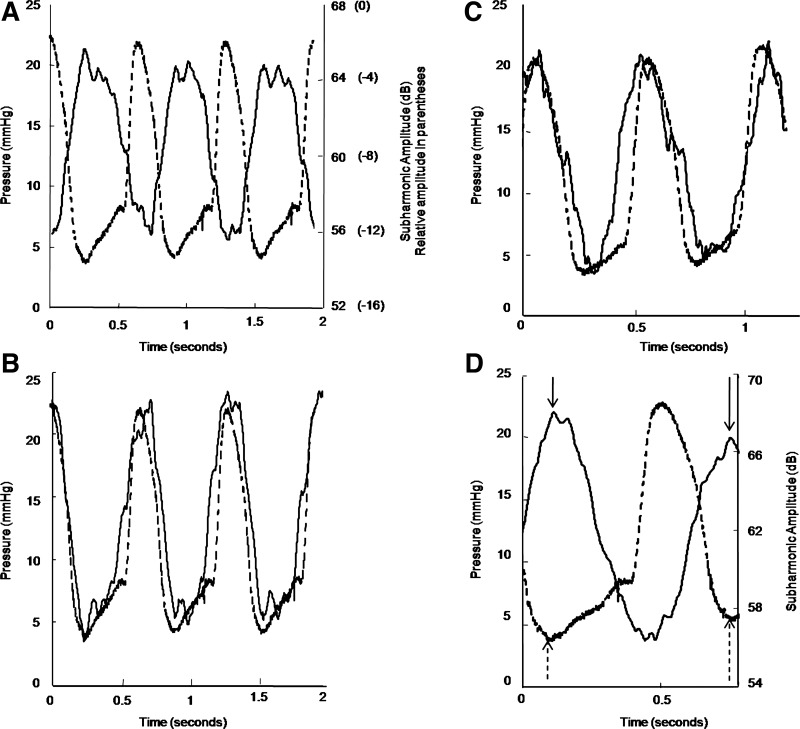
Figure 3 shows the subharmonic signal variations and SHAPE in the RV compared with the measured pressure, along with the SHAPE-generated RV pressure profiles from two canines (Fig. 3, B and C). Figure 3D shows a transient change in the minimum RV pressure from 3.7 to 5.7 mmHg for one of the canines. The peak subharmonic signal amplitude showed a corresponding decrease from 68.0 dB to 66.7 dB, i.e., a drop of 1.3 dB, equivalent to a pressure increase of 2.6 mmHg (calculated with a calibration factor of −2.0 mmHg/dB) compared with 2.0 mmHg recorded by the pressure catheter. This suggests that at optimum incident acoustic output from the scanner the subharmonic amplitudes may be very sensitive to ambient pressure changes exhibiting errors on the order of 0.6 mmHg in vivo.
Fig. 3.
RV pressure waveform using subharmonic aided pressure estimation (SHAPE) and the reference standard (manometer catheter). The inverse relationship between the subharmonic signal variation (solid line) and the pressure (dashed line) is shown (A). Sample RV pressure waveforms obtained using SHAPE (solid line) and pressure catheter (dashed line) from 2 canines are depicted in B and C, respectively. The sensitivity of the subharmonic signal (solid line) to changes in the pressure (dashed line) is demonstrated in D; note the decrease in the subharmonic signal amplitude (solid arrows) as the minimum RV diastolic pressure increases (dashed arrows).
RV pressures.
Table 3 lists the RVSP and RVDmin obtained using the SHAPE pressure contours and using the pressure catheter data. For the five canines the mean RVSP and RVDmin pressures obtained using the pressure catheter were 22.4 ± 4.6 mmHg and 4.7 ± 1.1 mmHg, respectively, and the mean RVSP and RVDmin pressures obtained using the SHAPE pressures were 24.7 ± 4.8 mmHg and 5.5 ± 1.7 mmHg, respectively. Note that the absolute errors in RVSP and RVDmin measurements ranged from 0.0 to 3.4 mmHg and from 0.1 to 1.8 mmHg. Table 3 also lists the RV relaxation rate (peak isovolumic −dP/dt) with a mean value of −132.7 ± 39.8 mmHg/s obtained with the pressure catheter data and a mean value of −135.7 ± 38.7 mmHg/s obtained with the SHAPE data; here the maximum error relative to the catheter pressure data was 5.9 mmHg/s. Finally, the results of paired comparisons for RVSP, RVDmin, and RV relaxation rate obtained with the pressure catheter data and the SHAPE data are presented in Table 4. Although the RVSP measurements showed a significant difference (P = 0.02) between the pressure catheter data and SHAPE data, the absolute difference was less than 4 mmHg (mean: 2.3 ± 1.3 mmHg; Table 4). For RVDmin and RV relaxation rates these differences were not significant (P > 0.05), and the absolute mean differences were 0.8 ± 0.7 mmHg and 2.9 ± 3.1 mmHg/s, respectively.
Table 3.
SHAPE in the RV compared with the manometer catheter
| Catheter | SHAPE | Absolute Error | Percent Error | |
|---|---|---|---|---|
| Canine 1 | ||||
| RVSP (mmHg) | 22.2 ± 1.1 | 24.5 ± 1.1 | −2.3 | 10.4 |
| RVDmin pressure (mmHg) | 4.5 ± 1.0 | 5.4 ± 0.9 | −0.9 | 20.0 |
| RV relaxation (mmHg/s) | −154.1 | −155.2 | 1.2 | 0.8 |
| Canine 2 | ||||
| RVSP (mmHg) | 21.3 ± 0.6 | 21.3 ± 1.0 | 0.0 | 0.0 |
| RVDmin pressure (mmHg) | 4.2 ± 1.0 | 5.0 ± 0.7 | −0.8 | 19.0 |
| RV relaxation (mmHg/s) | −162.5 | −161.0 | −1.5 | 0.9 |
| Canine 3 | ||||
| RVSP (mmHg) | 20.2 ± 0.9 | 23.6 ± 0.6 | −3.4 | 16.8 |
| RVDmin pressure (mmHg) | 5.0 ± 0.1 | 5.3 ± 1.2 | −0.3 | 6.0 |
| RV relaxation (mmHg/s) | −113.1 | −118.6 | 5.5 | 4.9 |
| Canine 4 | ||||
| RVSP (mmHg) | 18.1 ± 0.3 | 21.2 ± 0.6 | −3.1 | 17.1 |
| RVDmin pressure (mmHg) | 3.5 ± 0.2 | 3.6 ± 1.2 | −0.1 | 2.9 |
| RV relaxation (mmHg/s) | −71.5 | −75.4 | 3.9 | 5.5 |
| Canine 5 | ||||
| RVSP (mmHg) | 30.2 ± 3.6 | 32.8 ± 2.8 | −2.6 | 8.6 |
| RVDmin pressure (mmHg) | 6.4 ± 0.9 | 8.2 ± 1.8 | −1.8 | 28.1 |
| RV relaxation (mmHg/s) | −162.2 | −168.1 | 5.9 | 3.6 |
Values are means ± 1 SD. RV relaxation corresponds to the late peak minus derivative of change in pressure over time. Errors are indicated as catheter values-subharmonic aided pressure estimation (SHAPE) values. Percent errors are calculated with respect to the catheter pressure values.
Table 4.
Paired comparisons of SHAPE in the RV with the manometer catheter
| Catheter Pressure-SHAPE Pressures |
95% CI of the Difference |
||||
|---|---|---|---|---|---|
| Measurement | Mean | SD | Bottom | Top | Significance (2-tailed) |
| RVSP (mmHg) | −2.3 | 1.3 | −3.9 | −0.6 | 0.02 |
| RVDmin pressure (mmHg) | −0.8 | 0.7 | −1.6 | 0.0 | 0.06 |
| RV relaxation (mmHg/s) | 2.9 | 3.1 | −0.9 | 6.9 | 0.10 |
CI indicates confidence interval.
DISCUSSION
This pilot study represents the first in vivo application of subharmonic signals from microbubbles for noninvasive quantification of RV pressures. Techniques to use microbubbles for quantifying ambient pressures based on dissolution time of microbubbles (5), shift in resonance frequency (11), detection of single bubble echoes (20), and excitation using dual frequencies (31) have been proposed. However, these techniques have produced errors in the range of 10 to 15 mmHg under ideal in vitro conditions (clearly unacceptable clinically), and thus no in vivo results have been published. Other attempts to monitor pressures by using subharmonic emissions from in-house contrast media and ultrasound scanner also have not reported any in vivo results (14). Conversely, in this study, the SHAPE technique based on subharmonic emissions from a commercially available ultrasound agent and scanner showed good agreement (maximum error of 3.4 mmHg) with the reference standard in vivo, albeit based on a limited data set. Furthermore, the use of the SHAPE technique is not limited in terms of implementation on any specific hardware platform or use of any specific contrast agent, because the efficacy of using the SHAPE technique for pressure estimation has been demonstrated with other combinations of commercially available ultrasound agents and scanners (17, 18). As evident from Tables 1 and 2, the subharmonic signal amplitudes will differ on a case-by-case basis depending on the attenuation of the incident ultrasound beam. Thus the SHAPE technique uses an individual calibration factor (in mmHg/dB) for each canine to estimate RV pressures from the RV subharmonic waveform (in dB) to reduce estimation errors.
For the RV, calculation of ejection fraction has been a primary echocardiographic measurement to evaluate the systolic function (26). This ejection fraction can be measured noninvasively and accurately only by cardiac MRI, which is expensive and not always readily available (26). Two-dimensional echocardiographic size measurements from the apical four-chambered view do not correlate well with three-dimensional volumes, whereas three-dimensional echocardiographic assessments await standardization before clinical acceptance (3, 26). High RVSPs portend decreased survival in cardiac resynchronization therapy patients (35), and RV pressure waveforms may assist in monitoring wave reflection and cardiac index in patients with pulmonary arterial hypertension (21). In this pilot study, the SHAPE technique recorded errors ranging from 0.0 to 3.4 mmHg in measuring RVSPs and provided reproducible RV pressure waveforms for contour-based analysis. Recently, a new technique to monitor the RV filling pressure difference has shown that impairment in RV relaxation is marked by a reduced RV driving force (8). Thus measuring the RV filling pressures may identify RV diastolic dysfunction. In this regard, the SHAPE measurements yielded errors on the order of 0.1 to 1.8 mmHg in estimating RV diastolic pressures. These errors are sufficiently small to explore clinical applications of SHAPE. Finally, unlike the Doppler technique for estimating RV systolic pressures, the SHAPE technique may be used for pressure estimation even in the absence of tricuspid regurgitation, and because the SHAPE technique is based on acoustic reflections from the bubbles, it is independent of the angle of ultrasound interrogation.
Limitations
The major limitation of this study is that data were obtained from only five canines. The RV pressure range (peak systolic-minimum diastolic) in these canines extended from 14.6 to 23.8 mmHg, but the use of the SHAPE technique may still require additional validation across a broader range of RV pressures. The processing to obtain the SHAPE pressures was performed offline, but a new version of the subharmonic extraction and processing algorithm will permit real-time pressure estimation in future studies. The calibration factor from the aorta also was obtained based on pressure catheter values; this probably contributed to the small errors reported in the study. For clinical use, the efficacy of this technique needs to be investigated with systemic pulse pressures obtained from cuff-sphygmomanometer. In addition, no hemodynamic alterations were studied in these canines, but the subharmonic-based pressure contours tracked pressure catheter values indicating that the subharmonic signal is sensitive to changes in pressures that may be a characteristic of the underlying disease state. Finally, in this study we utilized Sonazoid, which is not yet approved for human use by the US Food and Drug Administration. However, another preliminary study showed that subharmonic emissions from Definity (Lantheus Medical Imaging, N. Billerica, MA; clinically approved to opacify the left ventricle for improved delineation of the left ventricular endocardial border) may also be used for in vivo pressure estimation (18); thus the SHAPE technique is not solely limited to the use of Sonazoid.
Conclusions
The favorable results of this pilot study are promising. Subharmonic emissions from ultrasound contrast agents have the potential to track RV pressures noninvasively with errors ranging from 0.0 to 3.4 mmHg. Maximum errors ranging from 0.1 to 1.8 mmHg in estimating diastolic pressures, if translatable to clinical application in the left heart, would be accurate enough to enable noninvasive determination of left ventricular filling pressures. These results warrant further studies of cardiac pressures as well as validation in clinical populations.
GRANTS
This work was supported by American Heart Association Grant 0655441U and National Heart, Lung, and Blood Institute Grant R21 HL-081892. J. R. Eisenbrey was supported by Grant RC1 DK087365. V. G. Halldorsdottir was supported by U.S. Army Medical Research and Material Command Grant W81XWH-08-1-0503.
DISCLOSURES
K. Dickie and C. Leung are employees of Ultrasonix Medical Corporation.
AUTHOR CONTRIBUTIONS
Author contributions: J.K.D., J.R.E., J.S.R., and F.F. conception and design of research; J.K.D., V.G.H., J.R.E., J.-B.L., M.E.M., K.D., S.W., C.L., and F.F. performed experiments; J.K.D., V.G.H., and J.R.E. analyzed data; J.K.D., V.G.H., J.R.E., J.S.R., J.-B.L., and F.F. interpreted results of experiments; J.K.D. prepared figures; J.K.D. drafted manuscript; J.K.D., V.G.H., J.R.E., J.S.R., J.-B.L., M.E.M., K.D., S.W., C.L., and F.F. edited and revised manuscript; J.K.D., V.G.H., J.R.E., J.S.R., J.-B.L., M.E.M., K.D., S.W., C.L., and F.F. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of S. Wang: Erdos Central Hospital, Erdos, Inner Mongolia, China.
REFERENCES
- 1. Andersen K, Jensen J. Ambient pressure sensitivity of microbubbles investigated through a parameter study. J Acoust Soc Am 126: 3350–3358, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Asch FM, Weissman NJ. Overview of the 2008 Food and Drug Administration advisory committee on safety consideration in the development of ultrasound contrast agents. Circulation 119: 1956–1961, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Avi-Mor V, Sugeng L, Lindner JR. Imaging the forgotten chamber: is the devil in the boundary? J Am Soc Echocardiogr 23: 141–143, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. B M J 310: 170, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouakaz A, Frinking P, de Jong N, Bom N. Noninvasive measurement of the hydrostatic pressure in a fluid filled cavity based on the disappearance time of micrometer-sized free gas bubbles. Ultrasound Med Biol 25: 1407–1415, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Chatterjee K. The Swan-Ganz catheters: past, present and future: a viewpoint. Circulation 119: 147–152, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Connors A, Speroff T, Dawson NV, Thomas C, Harrell FE, Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ, Jr, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA. The effectiveness of right heart catheterization in the initial care of critically ill patients. J Am Med Assoc 276: 889–897, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Cortina C, Bermejo J, Yotti R, Desco MM, Rodríguez-Pérez D, Antoranz JC, Rojo-Álvarez-Rojo JL, Garcia D, García-Fernández A, Avilé Fernández F. Noninvasive assessment of the right ventricular filling pressure gradient. Circulation 116: 1015–1023, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Eller A, Flynn H. Generation of subharmonics of order one-half by bubbles in sound field. J Acoust Soc Am 46: 722–727, 1969 [Google Scholar]
- 10. Faez T, Renaud G, Defontaine M, Calle S, de Jong N. Dynamic manipulation of the subharmonic scattering of phospholipid-coated microbubbles. Phys Med Biol 56: 6459–6473, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Fairbank W, Scully M. A new noninvasive technique for cardiac pressure measurement: resonant scattering of ultrasound from microbubbles. IEEE Trans Biomed Eng 24: 107–110, 1977 [DOI] [PubMed] [Google Scholar]
- 12. Fishbein MC, Kobashigawa J. Biopsy-negative cardiac transplant rejection: etiology, diagnosis, and therapy. Curr Opin Cardiol 19: 166–169, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Forsberg F, Liu JB, Shi WT, Furuse J, Shimizu M, Goldberg BB. In vivo pressure estimation using subharmonic contrast microbubble signals: proof of concept. IEEE Trans Ultrason Ferroelec Freq Contr 52: 581–583, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Frinking PJA, Gaud E, Brochot J, Arditi M. Subharmonic scattering of phospholipid-shell microbubbles at low acoustic pressure amplitudes. IEEE Trans Ultrason Ferroelec Freq Contr 57: 1762–1771, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Ganor Y, Adam D, Kimmel E. Time and pressure dependence of acoustic signals radiated from microbubbles. Ultrasound Med Biol 31: 1367–1374, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 117: 1717–1731, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Halldorsdottir VG, Dave JK, Leodore LM, Eisenbrey JR, Park S, Hall AL, Thomenius K, Forsberg F. Subharmonic contrast microbubble signals for noninvasive pressure estimation under static and dynamic flow conditions. Ultrason Imaging 33: 153–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halldorsdottir VG, Eisenbrey JE, Dave JK, Forsberg F, Machado P, Cavanaugh B, Merton D, Liu JB. Subharmonic-aided pressure estimation for monitoring interstitial fluid pressure in swine melanomas: initial in vitro and in vivo results. Presented at 97th Scientific Assembly and Annual Meeting of the Radiological Society of North America November 2011 (Abstract) [Google Scholar]
- 19. Hogg JC. Neutrophil kinetics and lung injury. Phys Rev 67: 1249–1295, 1987 [DOI] [PubMed] [Google Scholar]
- 20. Hök B. A new approach to non invasive manometry: interaction between ultrasound and bubbles. Med Biol Eng Comput 19: 35–39, 1981 [DOI] [PubMed] [Google Scholar]
- 21. Karamanoglu M, McGoon M, Frantz RP, Benza RL, Bourge RC, Barst RJ, Kjellström Bennett TD. Right ventricular pressure waveform and wave reflection analysis in patients with pulmonary arterial hypertension. Chest 132: 37–43, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Kasimir MT, Seebacher G, Jaksch P, Winkler G, Schmid K, Marta GM, Simon P, Klepetko W. Reverse cardiac remodeling in patients with pulmonary hypertension after isolated lung transplantation. Eur J Cardiothorac Surg 26: 776–781, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Katiyar A, Sarkar K, Forsberg F. Modeling subharmonic response from contrast microbubbles as a function of ambient static pressure. J Acoust Soc Am 129: 2325–2335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Landmark KE, Joansen PW, Johnson JA, Johansen B, Uran S, Skotland T. Pharmacokinetics of perfluorobutane following intravenous bolus injection and continuous infusion of Sonazoid in healthy volunteers and in patients with reduced pulmonary diffusing capacity. Ultrasound Med Biol 34: 494–501, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Marino TA, Kent RL, Uboh CE, Fernandez E, Thomson EW, Cooper G. Structural analysis of pressure versus volume overload hypertrophy of cat right ventricle. Am J Physiol Heart Circ Physiol 249: H371–H379, 1985 [DOI] [PubMed] [Google Scholar]
- 26. Mertens LL, Friedberg MK. Imaging the right ventricle—current state of the art. Nat Rev Cardiol 7: 551–563, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Neppiras E. Subharmonic and other low frequency emission from bubbles in sound field. J Acoust Soc Am 46: 587–601, 1969 [Google Scholar]
- 28. Rose H, Venn R. Recently published papers: dying Swans and other stories. Crit Care 10: 152, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarkar K, Shi WT, Chatterjee D, Forsberg F. Characterization of ultrasound contrast microbubbles using in vitro experiments and viscous and viscoelastic interface models for encapsulation. J Acoust Soc Am 118: 539–550, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Shah MR, Hasselblad V, Stevenson LW, Binanay C, O′Connor CM, Sopko G, Califf RM. Impact of the pulmonary artery catheter in critically ill patients. J Am Med Assoc 294: 1664–1670, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Shankar P, Chapelon J, Newhouse V. Fluid pressure measurement using bubbles insonified by two frequencies. Ultrasonics 24: 333–336, 1986 [Google Scholar]
- 32. Shi WT, Forsberg F, Raichlen J, Needleman L, Goldberg BB. Pressure dependence of subharmonic signals from contrast microbubbles. Ultrasound Med Biol 25: 275–283, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Shi WT, Hoff L, Forsberg F. Subharmonic performance of contrast microbubbles: an experimental and numerical investigation. Proc of IEEE Ultrasonics Symposium 2: 1957–1960, 2002 [Google Scholar]
- 34. Sontum P. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med Biol 34: 824–833, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Tedrow UB, Kramer DB, Stevenson LW, Stevenson WG, Baughman KL, Epstein LM, Lewis EF. Relation of right ventricular peak systolic pressure to major adverse events in patients undergoing cardiac resynchronization therapy. Am J Cardiol 97: 1737–1740, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Voelkel NF, Quaife RA, Leinward LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a national heart, lung and blood institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006 [DOI] [PubMed] [Google Scholar]