Abstract
Cardiac mammalian target of rapamycin (mTOR) is necessary and sufficient to prevent cardiac dysfunction in pathological hypertrophy. However, the role of cardiac mTOR in heart failure after ischemic injury remains undefined. To address this question, we used transgenic (Tg) mice with cardiac-specific overexpression of mTOR (mTOR-Tg mice) to study ischemia-reperfusion (I/R) injury in two animal models: 1) in vivo I/R injury with transient coronary artery ligation and 2) ex vivo I/R injury in Langendorff-perfused hearts with transient global ischemia. At 28 days after I/R, mortality was lower in mTOR-Tg mice than littermate control mice [wild-type (WT) mice]. Echocardiography and MRI demonstrated that global cardiac function in mTOR-Tg mice was preserved, whereas WT mice exhibited significant cardiac dysfunction. Masson's trichrome staining showed that 28 days after I/R, the area of interstitial fibrosis was smaller in mTOR-Tg mice compared with WT mice, suggesting that adverse left ventricular remodeling is inhibited in mTOR-Tg mice. In the ex vivo I/R model, mTOR-Tg hearts demonstrated improved functional recovery compared with WT hearts. Perfusion with Evans blue after ex vivo I/R yielded less staining in mTOR-Tg hearts than WT hearts, indicating that mTOR overexpression inhibited necrosis during I/R injury. Expression of proinflammatory cytokines, including IL-6 and TNF-α, in mTOR-Tg hearts was lower than in WT hearts. Consistent with this, IL-6 in the effluent post-I/R injury was lower in mTOR-Tg hearts than in WT hearts. These findings suggest that cardiac mTOR overexpression in the heart is sufficient to provide substantial cardioprotection against I/R injury and suppress the inflammatory response.
Keywords: myocardial infarction, left ventricular remodeling, transgenic mice, heart failure
acute myocardial infarction (MI) caused by coronary artery occlusion is a common cause of cardiac dysfunction and heart failure (HF). Early restoration of myocardial perfusion is a primary goal of initial treatment for patients with acute MI (30). Advances in pharmacological therapy and percutaneous coronary intervention can restore coronary flow in a majority of patients within a short time after the onset of symptoms (33). However, restoring blood flow itself may extend cardiac injury, referred to as reperfusion injury (41). Adverse left ventricular (LV) remodeling after acute MI, characterized by LV dilatation and fibrosis, is a critical determinant of the subsequent development of HF (32). LV remodeling is also an important pathophysiological feature in ischemia-reperfusion (I/R) injury (38). Therefore, identifying pathways that effectively protect against I/R injury and/or adverse remodeling could lead to novel therapeutic approaches to mitigating LV remodeling and HF after acute MI.
Insulin and the downstream molecules phosphoinositide-3 kinase (PI3K) and Akt confer cardioprotection in many settings (14, 17, 24). The mammalian target of rapamycin (mTOR) is an important mediator of the insulin-PI3K-Akt axis in multiple organs, including the heart (6). mTOR forms two functional complexes: rapamycin-sensitive mTOR complex 1 (mTORC1) and rapamycin-insensitive mTOR complex 2 (mTORC2) (45). mTORC1 activates p70S6 kinase followed by phosphorylation of ribosomal protein S6 and inhibits the binding of 4E-binding protein 1 to eukaryotic translation initiation factor 4E, resulting in the promotion of translation (9). mTORC2 activates Akt, a key regulator of cardiomyocyte survival (21), by phosphorylation at Ser473 (13). Previous studies using pharmacological mTOR inhibitors, including rapamycin, in both in vivo and ex vivo models of MI have found discrepant effects. Some reports (14, 17, 42) have demonstrated beneficial effects of mTOR activation in I/R models; conversely, another (16) saw cardioprotective effects of mTOR inhibition. In some settings, treatment with rapamycin activates PI3K/Akt signaling by suppressing negative feedback inhibition of insulin receptor substrate (IRS)-1 (10). Thus, rapamycin treatment alone cannot definitively determine the biological roles of cardiac mTOR. In addition to phosphorylation of mTOR, the relative amount of the two mTOR complexes plays a critical role in determining mTOR's biological effects (45), suggesting that the level of mTOR expression modulates the activity of the mTOR signaling pathway. We (35) have observed that the level of cardiac mTOR expression is dynamically regulated in the heart and that overexpression of mTOR activates both mTORC1 and mTORC2. In addition, we (35) have shown that overexpression of mTOR prevents cardiac dysfunction in a mouse model of pathological hypertrophy. In the same study, we demonstrated that cardiac mTOR overexpression also suppresses intracellular fibrosis during the development of LV remodeling in cardiac hypertrophy. A previous study (43) using cardiac-specific mTOR knockout mice also indicated that cardiac mTOR plays an important role in cardioprotection in pathological hypertrophy. However, the role of cardiac mTOR in cardiac function and LV remodeling after I/R injury remains undefined.
To explore this potential role of cardiac mTOR, we examined the effect of cardiac mTOR in both in vivo and ex vivo I/R models using cardiac-specific mTOR transgenic (mTOR-Tg) mice. We demonstrated that mTOR overexpression reduces mortality after I/R in vivo and prevents adverse LV remodeling, resulting in preserved cardiac function. In ex vivo Langendorff-perfused hearts, we showed that cardiac mTOR suppresses cardiomyocyte necrosis, inhibits the inflammatory response, and promotes functional recovery. Our results indicate that cardiac mTOR is sufficient to protect the heart against I/R injury.
MATERIALS AND METHODS
Animal models.
Animal experiments in this study were approved by the Institution Animal Care and Use Committees of both Beth Israel Deaconess Medical Center (Boston, MA) and the University of Hawaii (Honolulu, HI). The investigation conformed with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996). Tg mice expressing hemagglutinin-tagged WT rat mTOR under the direction of the murine α-myosin heavy chain promoter have been previously described in detail (35). Line 4 male (mTOR-Tg) mice, in which mTOR expression was about threefold higher than in littermate control (WT) mice (35), were used for these experiments. mTOR-Tg mice were backcrossed to C57BL/6 mice for more than eight generations. Age-matched male WT mice were used as controls unless otherwise indicated.
In vivo I/R model.
Male mice aged 12–14 wk were subjected to I/R as previously described (22). Briefly, animals were anesthetized by ketamine and sevoflulene, intubated, and ventilated. A left thoracotomy was performed, and the left anterior descending coronary artery (LAD) was ligated with 7-0 silk sutures. Five minutes after ischemia, 50 μl of fluorescent microspheres (10-μm FluoSpheres, Molecular Probes) were injected into the LV cavity for the determination of the area at risk. After 30-min ischemia, the LAD ligature was released, and reperfusion was visually confirmed. Operated mice were killed 28 days after I/R for the measurement of the area at risk, fibrotic area, and signaling pathways (see below). Sham-operated mice served as controls.
Histology.
Midventricular short-axis heart sections from WT and mTOR-Tg hearts were prepared and stained with Masson's trichrome as previously described (35). To objectively quantify the amount of tissue fibrosis in the initial infarct zone and remote zone, we measured images selected for analysis of the anterior LV wall, containing the infarct zone, and the posterior LV wall, containing the remote zone of LV wall at each section. Percent fibrosis was determined using ImageJ software to quantify blue (fibrotic) versus nonblue (nonfibrotic) pixels.
Echocardiography.
Echocardiography was performed on nonanesthetized mice using a 13L high-frequency linear transducer (10 MHz, VingMed 5, GE Medical Services) as previously described (35). We measured LV diastolic dimension, LV systolic dimension, and fractional shortening (FS; in %) for analysis of cardiac function before and 2 and 28 days after the I/R operation.
MRI.
Cardiac MRI was performed 28 days after I/R in a 4.7-T experimental system, as previously described (20). A bird-cage coil with an inner diameter of 35 mm (Bruker BioSpin, Billerica, MA) was used for transmit receive. Gated gradient echo sequences were used to acquire sequential true short-axis slices 1 mm in thickness and contiguous to each other, typically requiring seven to eight total slices for the coverage of the entire LV cavity from the apex to the LV outflow tract. For each sequence, 10 cine frames encompassing 1 cardiac cycle were obtained at each slice level.
Langendorff-perfused hearts.
WT and mTOR-Tg mice (12–14 wk old) were subjected to an ex vivo Langendorff apparatus as previously performed (28). After the retrograde perfusion was established at a constant pressure (80 mmHg), hearts were perfused with modified Krebs-Henseleit buffer (11 mM glucose, 118 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, and 0.5 mM EDTA) equilibrated with 95% O2-5% CO2 at 37°C to yield a pH of 7.4. A water-filled balloon catheter was introduced into the LV for recording of LV pressure (PowerLab, ADInstruments, Denver, CO). We measured a volume of the coronary sinus effluent in the perfusate to determine the coronary flow rate. For the ex vivo I/R model, hearts were perfused for 15 min, and flow was then eliminated for the indicated periods of time followed by reperfusion for 40 min.
Western blot analysis.
Hearts were harvested, snap frozen, and crushed in liquid nitrogen. The tissue was then homogenized in cold lysis buffer, as previously performed (35). Protein concentration was measured by the Bradford method (Bio-Rad, Hercules, CA). SDS-PAGE was performed under reducing conditions on 4–20% gradient gels. Proteins were transferred to a nitrocellulose membrane. Blots were incubated with primary antibodies for 18–20 h at 4°C. Blots were then incubated with horseradish peroxidase-conjugated secondary antibody, and the signal was detected using enhanced chemiluminescence (Cell Signaling, Danvers, MA). Primary antibodies to hemagglutinin (12CA5, Roche, Indianapolis, IN), GAPDH (Cell Signaling, Danvers, MA), S6 (Cell Signaling), phospho-S6 (Ser235/236, Cell Signaling), Akt (Cell Signaling), phospho-Akt (Ser473, Cell Signaling), glycogen synthase kinase (GSK)-3β (Cell Signaling), phospho-GSK-3β (Ser9, Cell Signaling), mTOR (Cell Signaling), and LC3 (Novus Biologicals, Littleton, CO) were used for immunoblot analysis.
Quantitative real-time PCR.
Accumulation of the PCR product was monitored in real time, and the threshold cycle (Ct) was determined with the 7900HT Fast Real-Time System (Applied Biosystems, Foster city, CA). The relative change in gene expression was determined using the ΔΔCt method with normalization to β-actin. Quantitative RT-PCRs were performed with the following sets of primers: forward 5′-AGAAGGAGTGGCTAAGGACCAA-3′ and reverse 5′-GCATAACGCACTAGGTTTGCC-3′ for IL-6, forward 5′-CCTTCCAGGATGAGGACATGAG-3′ and reverse 5′-CGTCACACACCAGCAGGTTATC-3′ for IL-1β, forward 5′-AGCAAACCACCAAGTGGAGGA-3′ and reverse 5′-GCTGGCACCACTAGTTGGTTGT-3′ for TNF-α, forward 5′-ATCCCAATGAGTAGGCTGGAGAGC-3′ and reverse 5′-CAGAAGTGCTTGAGGTGGTTGTG-3′ for monocyte chemotactic protein (MCP)-1, forward 5′-ACCTGCTCAACATCATGAAGG-3′ and reverse 5′-AGATGGAGCTATGCAGGTGG-3′ for macrophage inflammatory protein (MIP)-1α, forward 5′-GCCAAGCCTTATCGGAAATG-3′ and reverse 5′-GGGAATTCAAATGCTCCTTGAT-3′ for IL-10, and forward 5′-TGTTACCAACTGGGACGACA-3′ and reverse 5′-GGGGTGTTGAAGGTCTCAAA-3′ for β-actin.
Biological analysis in ex vivo perfused hearts.
The effluent collected at baseline and after 40 min of reperfusion was concentrated with an Amicon Ultra-0.5 centrifugal filter (3K device, Millipore, Billerica, MA). Samples concentrated from the effluent were subjected to an ELISA for IL-6 concentration (R&D Systems, Minneapolis, MN) or enzyme activity kits for creatine kinase (CK; BioAssay Systems, Hayward, CA) and lactate dehydrogenase (LDH; Cayman Chemical, Ann Arbor, MI).
Evans blue staining.
Evans blue staining was performed immediately after ex vivo I/R, as previously described (3). Briefly, at the end of 40 min of reperfusion, hearts were perfused (at 1 ml/min) with 3 ml of ice-cold 0.1% Evans blue (Sigma-Aldrich, St. Louis, MO) diluted in PBS followed by a perfusion with 10 ml of ice-cold PBS. To extract dye from tissue, whole hearts were weighed and minced followed by an immersion in 1 ml formamide at 56°C for 24 h (36). After centrifugation of the samples at 10,000 rpm for 2 min, supernatants were collected, and the color intensity was measured by reading absorbance at 620 nm with a standard solution prepared by a serial dilution of 20 μg/ml Evans blue dissolved in formamide. The content of Evans blue in the tissue was normalized by tissue weight (in μg/mg).
DNA laddering assay.
DNA was isolated by digestion of heart samples with proteinase K as previously described (28). Fragmented DNA was separated by 2% agarose gel electrophoresis with 3 μg of genomic DNA samples followed by staining the gel with ethidium bromide to detect the DNA under UV exposure.
Statistical analysis.
Data are presented as means ± SE. Group differences were analyzed by a two-tailed Student's t-test. For multiple comparisons, one-way or two-way ANOVA with the Bonferroni post hoc test was used. Survival distributions were estimated by the Kaplan-Meier method and compared by a log-rank test. For all analyses, P values of <0.05 were considered significant.
RESULTS
Overexpression of cardiac mTOR reduces mortality in the acute phase and preserves cardiac function in the chronic phase after transient ischemia in vivo.
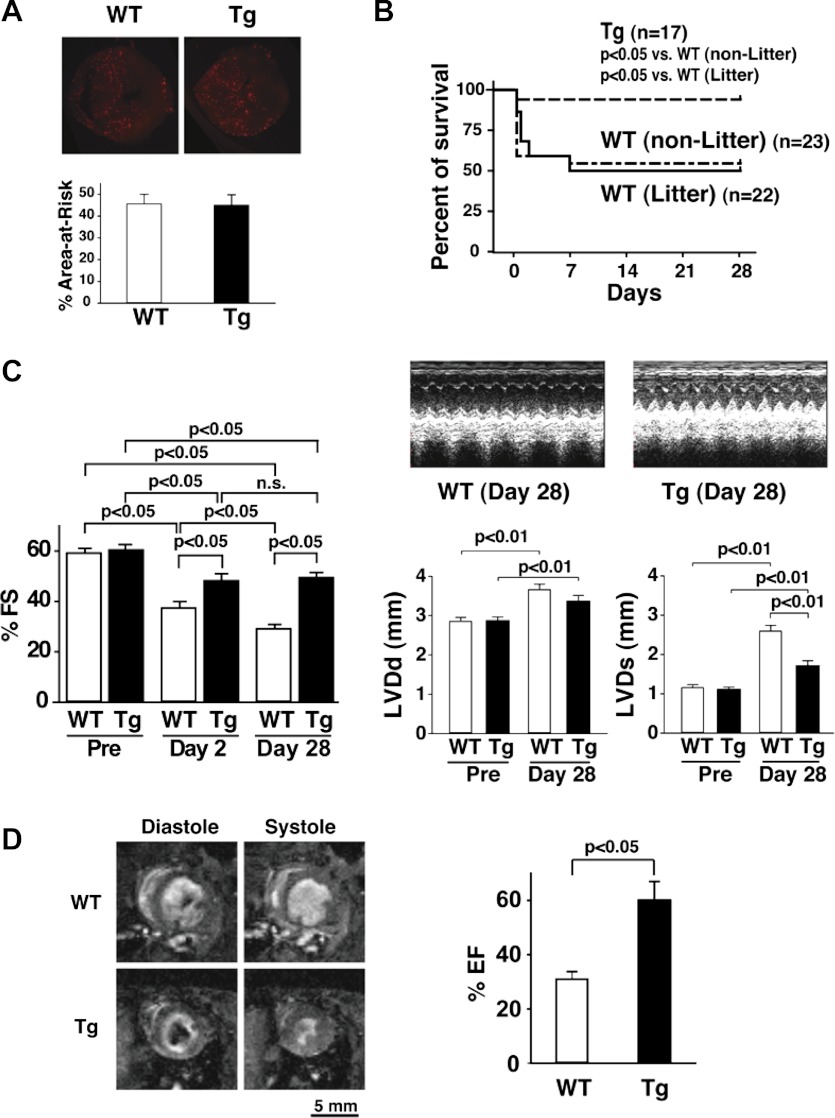
mTOR-Tg mice were subjected to I/R injury generated by LAD ligation as previously performed (20, 22). Echocardiography demonstrated no difference in cardiac function at baseline between WT and mTOR-Tg mice, as previously reported (35) (Fig. 1C, left). Injection of microspheres during LAD ligation indicated no difference in the area at risk between hearts from WT and mTOR-Tg mice (Fig. 1A). Intriguingly, Kaplan-Meier survival curves demonstrated lower mortality in mTOR-Tg mice compared with both littermate and nonlittermate WT mice at 28 days after I/R (P < 0.05; Fig. 1B). The survival benefit of cardiac mTOR overexpression was already evident at 2 days after I/R (Fig. 1B). Echocardiography demonstrated that systolic function in both WT and mTOR-Tg groups was reduced 2 days after I/R compared with baseline (Fig. 1C, left). However, at this time point, FS in the mTOR-Tg group was higher than in the WT group, suggesting again that mTOR overexpression protected hearts during the acute phase after I/R. Of note, whereas FS in WT mice deteriorated progressively from 2 to 28 days after I/R (2 vs. 28 days: 37.5 ± 2.4% vs. 29.4 ± 1.5%, P < 0.05), there was no further change in FS in mTOR-Tg mice after the acute phase (Fig. 1C, left). At 28 days after I/R, systolic function in mTOR-Tg mice was relatively well preserved, whereas in WT mice it was substantially decreased (P < 0.05; Fig. 1C, left and right). MRI confirmed that cardiac dysfunction was preserved in mTOR-Tg mice at 28 days after I/R (ejection fraction: 31.0 ± 2.7% vs. 60.1 ± 6.9% for WT vs. mTOR-Tg mice, P < 0.05; Fig. 1D). These findings indicate that mTOR overexpression was cardioprotective in both acute and chronic phases post-I/R.
Fig. 1.
Mammalian target of rapamycin (mTOR) overexpression protects the heart and preserves cardiac function after in vivo ischemia-reperfusion (I/R). A: the ischemic area (area at risk). Top, representative images of fluorescent microspheres from wild-type (WT) and mTOR-transgenic (Tg) mice. Hearts were harvested at 28 days after I/R. Bottom, percentage of the area at risk of hearts from WT and mTOR-Tg mice. The area at risk was assessed as previously described (22). n = 5 mice/group. B: Kaplan-Meier survival curves from nonlittermate (non-Litter) WT, littermate (Litter) WT, and mTOR-Tg mice after I/R with temporal coronary ligation. n = 17 mTOR-Tg mice, 23 nonlittermate WT mice, and 22 littermate WT mice. C: echocardiographic analyses. Right, representative M-mode images of operated WT and mTOR-Tg mice at 28 days after I/R as well as left ventricular (LV) diastolic dimension (LVDd) and LV systolic dimension (LVDs) at pre-I/R and 28 days after I/R. Left, mean scores for percent fractional shortening (%FS) at pre-I/R and 2 and 28 days after I/R. n = 11 WT mice and 16 mTOR-Tg mice. n.s., Not significant. D: MRI of the heart at 28 days after I/R. Left, representative pair of images from littermate pairs of male mTOR-Tg and WT mice. Right, percent ejection fraction (%EF) calculated from MRI images. n = 3 WT mice and 5 mTOR-Tg mice.
Cardiac mTOR inhibits cardiac fibrosis in adverse LV remodeling.
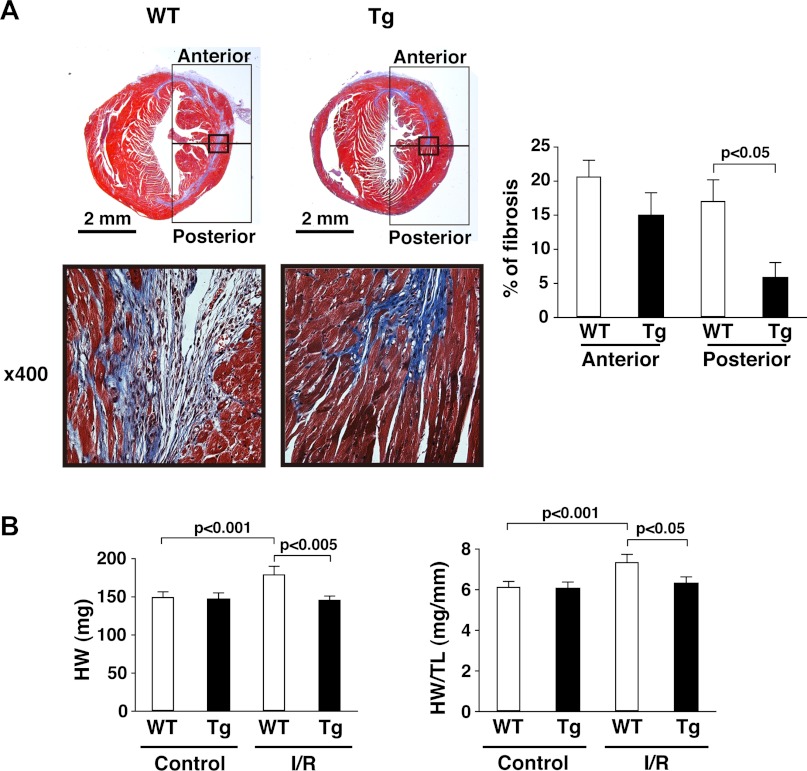
Interstitial fibrosis is an important pathogenic feature of adverse LV remodeling after MI (37). We (20) previously reported that fibrotic scar formation in I/R injury by LAD ligation is located exclusively along the midcardium rather than listing the endocardium and epicardium. Consistent with that, we observed interstitial fibrosis along the midcardium in WT mice (Fig. 2A, bottom left). As previously shown (20), damaged myocytes were located along circumferential myofibers and extended from the initial infarct (anterior in Fig. 2A) to the remote zone (posterior in Fig. 2A); little or no damage occurred to myocytes situated along myofibers with longitudinal orientation. Intriguingly, the fibrotic scar in mTOR-Tg mice was mostly limited to the initial infarct zone (Fig. 2A, left): in fact, there was no significant difference in the size of the fibrotic area in this region between WT and mTOR-Tg mice (WT vs. mTOR-Tg mice: 20.7 ± 2.4% vs. 15.0 ± 3.3%, anterior in Fig. 2A, right). In contrast, the fibrotic area in the remote zone of mTOR-Tg mice was substantially smaller than that in WT mice (WT vs. mTOR-Tg mice: 17.1 ± 3.1% vs. 5.9 ± 2.2%, P < 0.05, posterior in Fig. 2A, right). Cardiac hypertrophy, as measured by heart weight, was also suppressed in mTOR-Tg mice (Fig. 2B). In addition, echocardiography and MRI demonstrated that LV cavity dilatation was limited in mTOR-Tg mice 28 days after I/R (Fig. 1C, right, and D, left). Thus, mTOR overexpression strongly inhibited adverse LV remodeling after transient ischemia in vivo.
Fig. 2.
mTOR overexpression suppresses adverse LV remodeling. A: interstitial myocardial fibrosis. Left, representative photos of Masson's trichrome staining in cardiac sections from WT and mTOR-Tg mice at 28 days after I/R. Bottom, magnified images of the regions indicated by the squares in each heart section of the top images. Right, quantitative analysis of interstitial fibrosis examined by Masson's trichrome staining. n = 5 mice/group. B: heart weight (HW; left) and ratios of HW to tibia length (TL; right). Hearts from each group were harvested after echocardiography was performed. n = 11 control WT mice, 10 control mTOR-Tg mice, 11 I/R WT mice, and 16 I/R mTOR-Tg mice.
mTOR signaling after I/R in vivo.
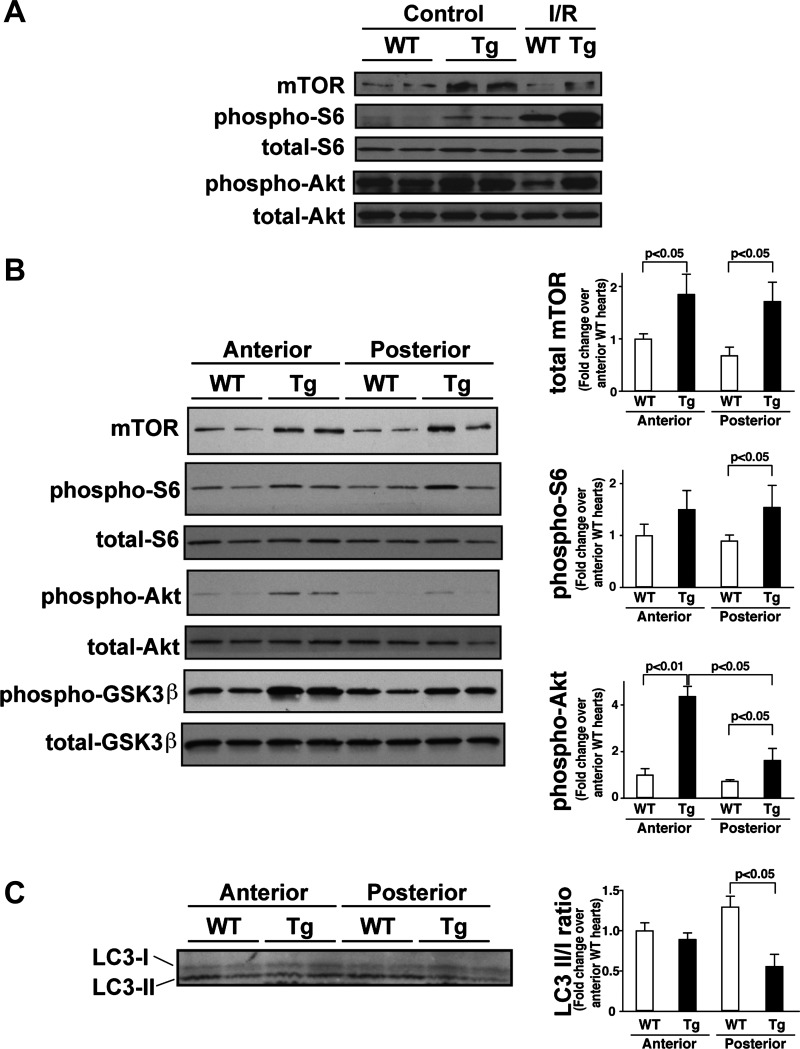
As observed in a previous report (35), mTOR-Tg mice exhibited an increase in both mTORC1 and mTORC2 activation at baseline, as evidenced by S6 and Akt phosphorylation, respectively (Fig. 3A). In both WT and mTOR-Tg mice after I/R, S6 phosphorylation was increased compared with baseline, whereas no change in Akt phosphorylation was observed, suggesting that mTORC1 is the dominant complex stimulated after I/R injury in both WT and mTOR-Tg mice (Fig. 3A). In addition, the data indicate that, although already elevated at baseline, cardiac mTOR signaling in mTOR-Tg mice can be further activated by I/R injury.
Fig. 3.
mTOR-related signaling pathways and autophagy in mTOR-Tg mice after in vivo I/R. A: representative immunoblots of mTOR signaling molecules in the whole heart. Hearts were harvested at 28 days after I/R. Control hearts were harvested from sham-operated mice. Immunoblot analysis was performed with the indicated antibodies. Blots are representative of three independent samples in each group. B: immunoblots of mTOR signaling molecules in cardiac tissues from either the anterior or posterior LV wall. Left, representative immunoblots of mTOR signaling molecules in the anterior and posterior LV wall. The heart was harvested at 28 days after I/R, and the LV wall was split into anterior and posterior sections to produce lysates for Western blot analysis. Right, densitometric quantification of immunoblots. GSK, glycogen synthase kinase. C: analysis of autophagy. Left, representative immunoblots of LC3-I and LC3-II in the anterior and posterior LV wall. Right, ratio of LC3-II to LC3-I (LC3-II/I). Data were normalized to the mean protein level in the anterior LV of WT hearts. n = 5 WT mice and 3 mTOR-Tg mice.
As stated above, we observed less fibrosis at sites remote from the initial infarct zone in mTOR-Tg mice compared with WT mice at 28 days after I/R. To assess the mTOR signaling pathway in the remote zone, we compared mTOR activation in protein lysates from the anterior and posterior myocardium by Western blot analysis. Although the level of Akt phosphorylation in the posterior myocardium of mTOR-Tg mice was lower than that in the anterior myocardium, both mTORC1 and mTORC2 signaling pathways were higher in mTOR-Tg mice than in WT mice, even in the posterior myocardium (Fig. 3B). Phosphorylation of the Akt substrate GSK-3β was higher in mTOR-Tg mice than in WT mice, suggesting that activation of Akt is also higher in mTOR-Tg mice than in WT mice. These results suggest that activated mTOR signaling plays an important role in the prevention of adverse LV remodeling in I/R injury.
mTOR overexpression suppresses autophagy in the remote zone after I/R in vivo.
A previous study (25) of cardiac I/R demonstrated that autophagy is detrimental during reperfusion but protective during ischemia. Since mTOR is a key mediator of autophagy (44), we examined the effect of cardiac mTOR overexpression on autophagy after cardiac I/R by assessing the ratio of LC3-II to LC3-I, an indicator of autophagy (15). In the infarct zone, there was no difference in the LC3-II-to-LC3-I ratio between WT and mTOR-Tg mice (Fig. 3C). However, in the remote zone, the ratio of LC3-II to LC3-I was lower in mTOR-Tg mice than in WT mice. These results suggest that autophagy was suppressed in the remote zone of mTOR-Tg mice after I/R.
mTOR overexpression prevents cardiac dysfunction in ex vivo I/R injury.
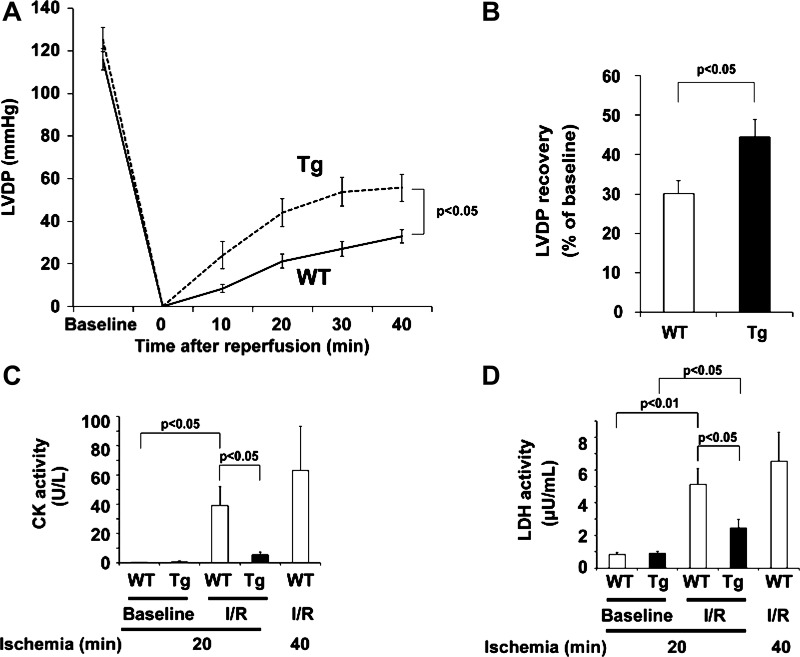
Two to three days after I/R in vivo, we observed reduced mortality and preserved cardiac function in mTOR-Tg mice (Fig. 1, B and C). To further examine the cardioprotective effects of mTOR in the acute phase after I/R, hearts from WT and mTOR-Tg mice were subjected to a Langendorff apparatus, as previously described (28). There was no significant difference in baseline function between WT and mTOR-Tg hearts (Table 1). After 15 min of control perfusion, hearts were exposed to 20 min of global ischemia followed by 40 min of reperfusion. At all time points after global ischemia, we observed greater recovery of LV developed pressure in hearts from mTOR-Tg mice than in hearts from WT mice (maximum LV developed pressure recovery: 30.2 ± 3.1% vs. 44.4 ± 4.5% for WT vs. mTOR-Tg hearts, P < 0.05; Fig. 4, A and B). To assess the magnitude of myocardial injury after global ischemia, we measured the activity of CK and LDH in the effluent during reperfusion. The effluent of WT hearts exposed to longer ischemia (40 min) was used as a positive control and exhibited significant increases in the activity of CK and LDH after I/R compared with baseline controls (Fig. 4, C and D). After I/R, both CK and LDH activities were significantly lower in effluent from mTOR-Tg hearts than in effluent from WT hearts (P < 0.05 each; Fig. 4, C and D), indicating less myocardial damage in mTOR-Tg hearts. These findings indicate that cardiac mTOR protected hearts against transient ischemia in ex vivo perfused hearts.
Table 1.
Baseline cardiac parameters in ex vivo perfused hearts from WT and mTOR-Tg mice
| WT Mice | mTOR-Tg Mice | P Value | |
|---|---|---|---|
| Body weight, g | 25.1 ± 0.6 | 24.0 ± 0.6 | 0.226 |
| Heart weight/tibia length, mg/mm | 5.8 ± 0.3 | 5.4 ± 0.1 | 0.158 |
| LV systolic pressure, mmHg | 121 ± 5 | 131 ± 6 | 0.208 |
| LV end-diastolic pressure, mmHg | 5.3 ± 0.3 | 5.8 ± 0.6 | 0.375 |
| LV developed pressure, mmHg | 116 ± 5 | 125 ± 6 | 0.224 |
| LV dP/dtmax, mmHg/s | 4,794 ± 197 | 4,835 ± 251 | 0.895 |
| LV dP/dtmin, mmHg/s | −2,624 ± 95 | −2,532 ± 109 | 0.533 |
| Coronary flow, ml/min | 2.7 ± 0.2 | 2.4 ± 0.2 | 0.212 |
Values are means ± SE; n = 28 wild-type (WT) mice and 19 mammalian target of rapamycin (mTOR) transgenic (mTOR-Tg) mice. LV, left ventricular; LV dP/dtmax and LV dP/dtmin, maximum and minimum first derivatives of LV pressure.
Fig. 4.
mTOR overexpression preserves cardiac function and prevents cardiac injury during ex vivo I/R. A: LV developed pressure (LVDP) profiles during I/R in WT and mTOR-Tg mice. n = 28 WT mice and 19 mTOR-Tg mice. B: maximum LVDP recovery (percentage of baseline) measured at 40 min of reperfusion. n = 28 WT mice and 19 mTOR-Tg mice. C and D: activity of creatine kinase (CK) and lactate dehydrogenase (LDH) in the effluent collected during the reperfusion period. To determine enzyme activities immediately after ex vivo I/R injury, effluents from hearts exposed to either 20 or 40 min of global ischemia were collected at control perfusion (baseline) and after 40-min reperfusion (I/R). n = 7 WT mice and 8 mTOR-Tg mice in hearts subjected to 20-min ischemia, and n = 3 WT mice in hearts subjected to 40-min ischemia.
Cardiac mTOR suppresses necrosis in ex vivo I/R injury.
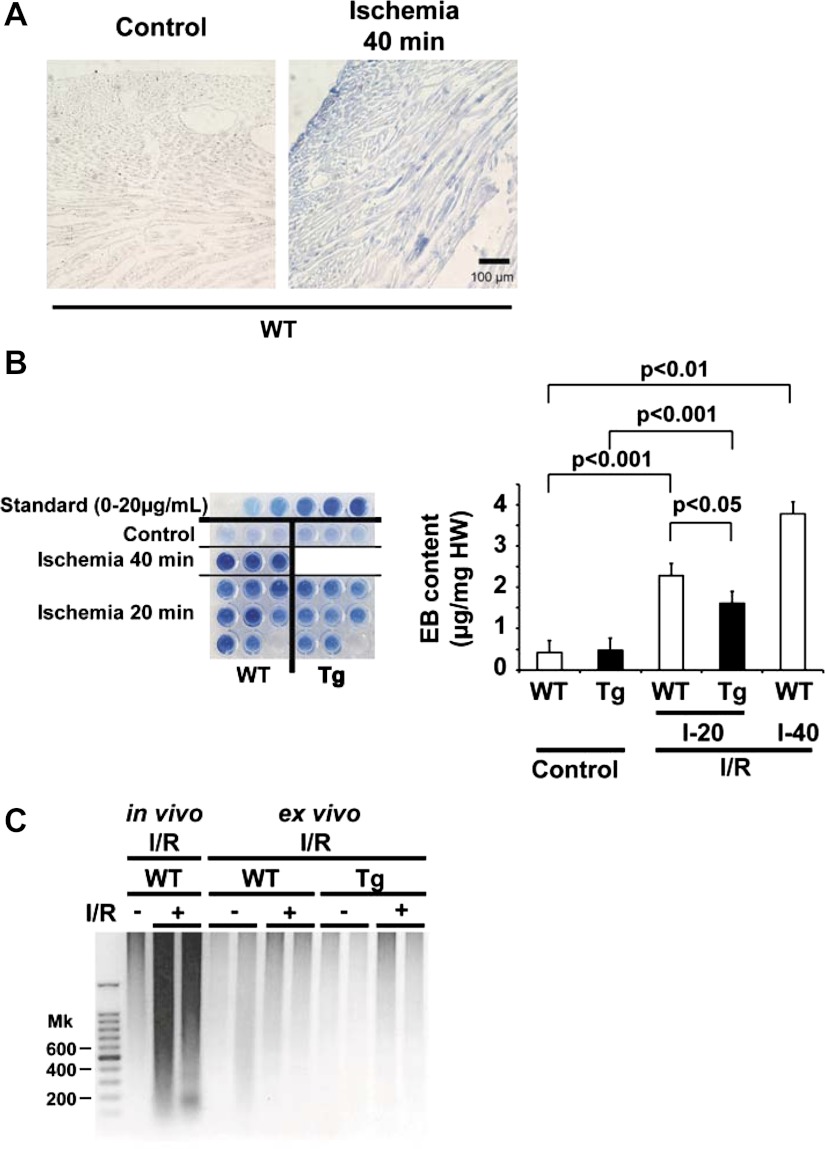
Necrosis is a key pathogenesis of I/R injury as well as apoptosis (39). A previous report (27) demonstrated that Evans blue staining of cardiomyocytes mainly indicates necrosis rather than apoptosis. We perfused hearts with Evans blue after reperfusion and examined the level of incorporation into cardiomyocytes. WT hearts subjected to 40 min of ischemia/40 min of reperfusion had substantial blue staining compared with WT hearts subjected to control perfusion (Fig. 5A). Importantly, the majority of Evans blue staining was located in cardiomyocytes rather than in the luminal side of the vasculature or the extracellular matrix, suggesting that the level of staining with Evans blue indicates an intensity of necrosis predominantly in cardiomyocytes. Quantitative assay of Evans blue incorporation revealed significantly less staining in mTOR-Tg hearts than in WT hearts in ex vivo I/R injury (20 min of ischemia/40 min of reperfusion; 2.28 ± 0.27 vs. 1.61 ± 0.15 μg/mg heart wt for WT vs. mTOR-Tg hearts, P < 0.05; Fig. 5B), indicating that mTOR overexpression suppressed I/R-induced necrosis. Apoptosis contributes to in vivo I/R injury (40) but may not play as important a role in ex vivo I/R injury (28). While genomic DNA from the myocardium at the border zone of in vivo I/R injury exhibited strong DNA laddering, we observed no significant DNA laddering in samples from hearts after ex vivo I/R injury (Fig. 5C). Taken together, these results indicate that necrosis was the predominant form of cardiomyocyte death after ex vivo I/R and that it was significantly suppressed by mTOR overexpression.
Fig. 5.
mTOR overexpression prevents necrosis in ex vivo I/R injury. A: representative tissue sections from hearts perfused with Evans blue (EB). Hearts subjected to 15-min control perfusion or 40-min ischemia/40-min reperfusion (Ischemia 40 min) in WT mice were harvested after perfusion with EB. B, left: EB dye extracted from cardiac tissue. Standard samples (0–20 μg/ml) are shown at the top. WT hearts from 15-min control perfusion (n = 3), mTOR-Tg hearts from 15-min control perfusion (n = 3), WT hearts from 40-min ischemia/40-min reperfusion (I-40; n = 3), and WT (n = 8) and mTOR-Tg (n = 8) hearts from 20-min ischemia/40-min reperfusion (I-20) were examined for this assay. Right, quantification of EB incorporation in the myocardium. The color intensity of the samples on the left was measured as described in methods. C: DNA laddering. Genomic DNA was prepared from hearts subjected to either in vivo or ex vivo I/R injury. Twenty-eight days post-I/R, cardiac tissue from hearts subjected to in vivo I/R injury was harvested at the border zone of the LV, where significant apoptosis has been observed after I/R injury (40). Cardiac tissue from hearts subjected to ex vivo I/R injury was harvested from the LV wall after 20-min ischemia/40-min reperfusion. The DNA laddering shown is representative of results from 3 independent samples/group.
mTOR signaling in ex vivo I/R injury.
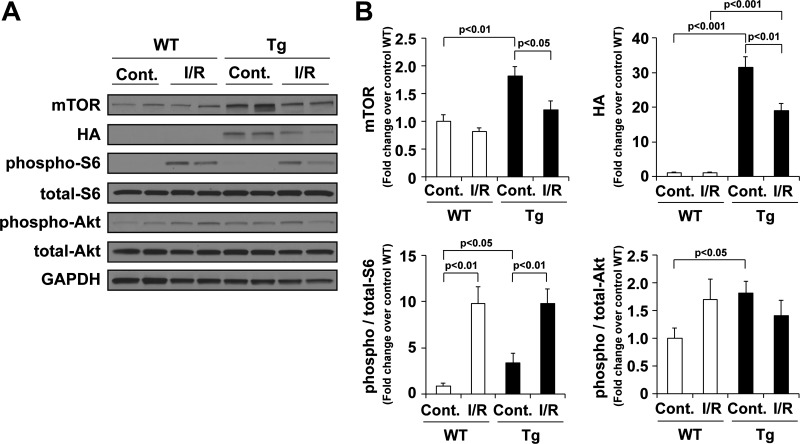
In ex vivo perfused hearts from mTOR-Tg mice at baseline, Western blot analysis demonstrated an increase in both mTORC1 activity, as indicated by S6 phosphorylation, and mTORC2 activity, as indicated by Akt phosphorylation (Fig. 6, A and B). Interestingly, after ex vivo I/R, mTOR expression was decreased in hearts from mTOR-Tg mice but not in hearts from WT mice (Fig. 6). This is in contrast to our earlier findings in mTOR-Tg mice subjected to a model of pressure overload-induced pathological hypertrophy, in which we found an increase in mTOR transgene expression in mTOR-Tg mice (35). We also observed a decrease in mTOR expression in the chronic phase of the in vivo I/R model (Fig. 3A). However, whereas activation of mTORC1 and mTORC2 signaling pathways in mTOR-Tg mice was higher than in WT mice 28 days after in vivo I/R (Fig. 3A), we found no difference in the activation of mTOR signaling between WT and mTOR-Tg mice after reperfusion in the ex vivo I/R model (Fig. 6). These findings imply that baseline activation of mTOR signaling is sufficient for the cardioprotective effect of mTOR overexpression observed in the ex vivo I/R model.
Fig. 6.
mTOR signaling pathways in mTOR-Tg mice after ex vivo I/R. A: representative immunoblots of mTOR signaling molecules in hearts subjected to ex vivo Langendorff perfusion. Hearts were harvested after 20-min ischemia/40-min reperfusion ex vivo. Control hearts were harvested at 15-min control perfusion. Immunoblot analysis was performed with the indicated antibodies. Blots are representative of 4 independent samples/group. B: quantitative analysis of mTOR, hemagglutinin (HA), phospho-S6, and phospho-Akt. Values were normalized to levels in control WT hearts in each experiment. n = 8 mice/group.
Cardiac mTOR attenuates the inflammatory response after ex vivo I/R.
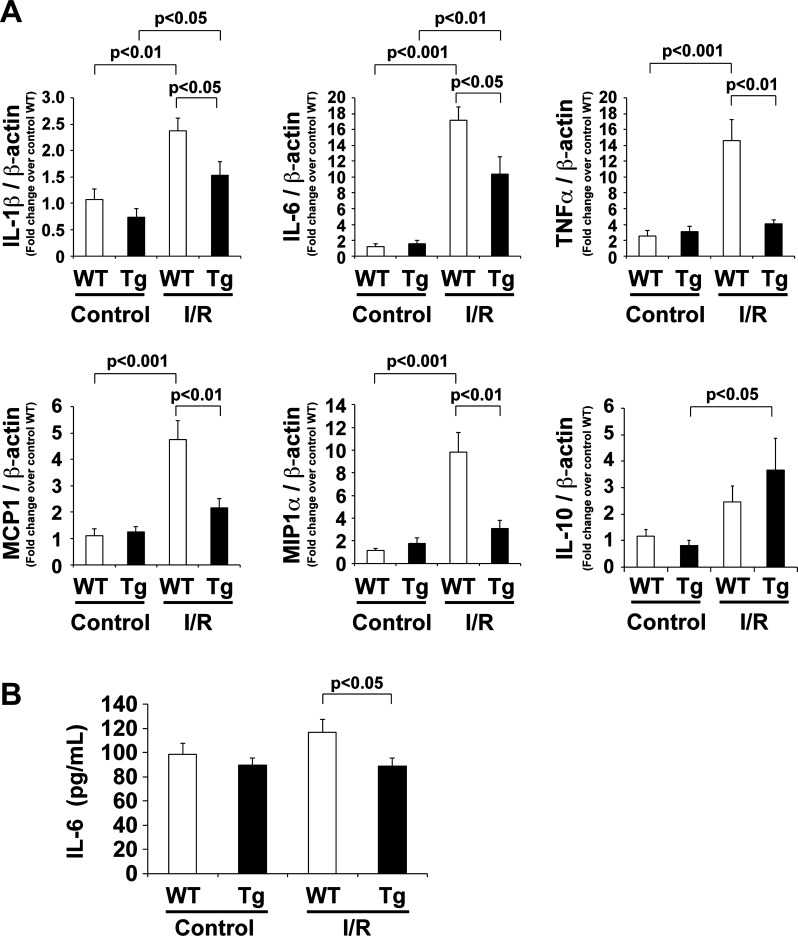
We observed less cardiac fibrosis in mTOR-Tg mice compared with WT mice after in vivo I/R (Fig. 2). The inflammatory response is a key pathophysiological reaction and determines the extent of cardiac injury, including fibrosis (7). To examine the inflammatory response in ex vivo I/R injury, we measured mRNA levels of proinflammatory cytokines (IL-6, IL-1β, and TNF-α), an anti-inflammatory cytokine (IL-10), and chemokines (MCP-1 and MIP-1α) in hearts from WT and mTOR-Tg mice subjected to ex vivo I/R injury (Fig. 7A). Among the cytokines in WT mice, the increase in IL-6 expression after I/R was most marked (17-fold increase compared with control, P < 0.001). IL-6 mRNA levels were also elevated in mTOR-Tg hearts after I/R; however, the increase was significantly smaller than in WT mice (P < 0.05; Fig. 7A). Similarly, induction of mRNA levels of other proinflammatory cytokines (IL-1β and TNF-α) and chemokines (MCP-1 and MIP-1α) was also decreased in mTOR-Tg hearts compared with WT hearts (Fig. 7A). To confirm the effect of mTOR overexpression on cytokine generation, we measured secreted cytokines in the effluent. ELISAs demonstrated that the level of secreted IL-6 protein was suppressed in mTOR-Tg hearts to levels comparable with those seen at baseline (Fig. 7B). In contrast, the mRNA level of the anti-inflammatory cytokine IL-10 was increased in mTOR-Tg hearts compared with WT hearts (Fig. 7A). These data indicate that mTOR overexpression inhibits the inflammatory response in ex vivo I/R injury.
Fig. 7.
Inflammatory response in mTOR-Tg mice after ex vivo I/R. A: expression of IL-1β, IL-6, TNF-α, monocyte chemotactic protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, and IL-10 mRNA levels in the heart. mRNA was measured by quantitative RT-PCR. n = 6–9 mice/group. B: IL-6 protein secreted from ex vivo perfused hearts. Effluents were collected at 15-min control perfusion and after 20-min ischemia/40-min reperfusion (I/R) in each group. n = 27 WT mice and 19 mTOR-Tg mice.
DISCUSSION
The results of this study demonstrate that cardiac mTOR is sufficient to protect the heart and preserves cardiac function in both in vivo and ex vivo models of I/R injury. Our experiments using in vivo I/R models show that mTOR overexpression increases survival during the acute phase of I/R injury and prevents adverse LV remodeling in the chronic phase, resulting in the preservation of cardiac function. To explore this effect further, we investigated how overexpression of mTOR in the heart affects cardiac function after I/R injury in ex vivo Langendorff-perfused hearts. Our results indicate that necrosis is the primary mechanism of cardiomyocyte death in I/R injury and that overexpression of cardiac mTOR inhibits necrosis and suppresses the inflammatory response during reperfusion.
Insulin and IGF-I, which potentially activates PI3K, Akt, and mTOR, inhibit MI in vivo (5, 31). Both treatment with insulin in the ex vivo I/R injury model (14) and myocardial preconditioning in in vivo acute I/R injury model (17) activate the Akt/mTOR signaling pathway and suppress MI. Rapamycin treatment abolished the cardioprotective effect of both of these treatments. Previously, it has been reported that mTOR inhibition by rapamycin increases infarct size at 24 h post-I/R in vivo and accelerates cell death induced by oxidative stress in cardiomyocytes in vitro (12). In addition, mTOR activation by GSK-3β was shown to confer cardioprotection by inhibiting autophagy in I/R injury but not in ischemic injury alone (42). Consistent with this, we observed that after I/R injury, autophagy in the remote zone was suppressed in hearts from mTOR-Tg mice compared with WT mice. Like these previous reports, our results strongly suggest that the activation of mTOR signaling prevents myocardial injury in the acute phase of I/R injury.
In contrast, a previous report (4) demonstrated that mTOR inhibition by everolimus, a derivative of rapamycin, protected the heart in an in vivo MI model using an LAD permanent ligation without reperfusion. A recent report demonstrated that Tg mice with cardiac-specific overexpression of the Ras homolog enriched in brain GTPase Rheb, which binds directly to the mTOR catalytic domain and stimulates mTORC1 activity (1), exhibited an increase in infarct size after MI without reperfusion (34). The two types of cardiac injury, MI (without reperfusion) and I/R injury, induce cell death with different pathophysiological features (42); thus, activation of mTOR signaling may have different cardioprotective effects on these two types of injury. Comparing and contrasting the role of cardiac mTOR in different settings in future studies will be of great interest. Another previous study (16) demonstrated that in vivo treatment of mice with rapamycin conferred cardioprotection that was evident when the hearts were harvested and studied in a Langendorff perfusion model of I/R. This group also showed that pretreatment of adult mouse cardiomyocytes with rapamycin inhibited both necrosis and apoptosis after hypoxia-reoxygenation. The authors discussed the possibility that Akt activation was involved in the rescue effect of rapamycin in both these effects; however, they did not test this hypothesis in the study (16). Another study (11) showed that short (<12 h) but not long (24 h) rapamycin exposure of isolated cardiomyocytes enhanced Akt phosphorylation caused by insulin stimulation. These findings might reflect the activation of PI3K/Akt signaling by suppression of negative feedback inhibition of IRS-1 (10). Thus, studying mTOR signaling with rapamycin requires characterization of the feedback system in each setting.
To characterize cell death in I/R injury, we used Evans blue perfusion after I/R injury in ex vivo perfused hearts. Evans blue incorporation into cardiomyocytes indicates necrosis rather than apoptosis (27). In addition to biological assays for CK and LDH, the results with Evans blue incorporation strongly indicated that mTOR overexpression acts by suppressing necrosis rather than apoptosis in ex vivo I/R injury. In fact, our DNA laddering assay revealed no significant apoptosis after ex vivo I/R injury in either WT or mTOR-Tg mouse hearts. Consistent with our findings, a recent report (12) showed that rapamycin treatment did not affect apoptosis induced by hypoxia-reoxygenation in cardiomyocytes, whereas it increased necrosis in the setting. While the antinecrotic effect of mTOR has not been well characterized, many groups, including ours, have demonstrated significant effects of cardiac Akt on apoptosis (23). Consistent with our previous report (35), Akt, a substrate of mTORC2, was significantly activated at baseline in mTOR-Tg mice. Although apoptosis has been explored extensively in cardiac studies, recent findings strongly indicated that programmed necrosis plays an important role in cardiac diseases, including I/R injury (2, 29) (for a review, see Ref. 19). Since Akt and the substrates of Akt are considered suppressors of programmed necrosis (19), it is possible that mTOR activation could control programmed necrosis. Further investigation regarding the role of mTOR in necrosis is essential.
In the present study, we demonstrated that mTOR overexpression inhibited adverse LV remodeling in vivo, especially interstitial fibrosis. It has previously been shown that the magnitude of LV remodeling in patients is directly proportional to the initial infarct size after acute MI (26). Our results show that mTOR overexpression protected the heart during the acute phase after experimental I/R in vivo and during reperfusion after ex vivo transient ischemia. The cardioprotective effect of mTOR might account for the inhibition of LV remodeling after I/R in vivo. Of note, we have previously demonstrated that cardiac mTOR substantially inhibited the inflammatory response (35), which regulates fibrosis in LV remodeling (7). In the present study, we observed a decrease in secretion of IL-6, a decrease in expression of proinflammatory cytokines and chemokines, including IL-1β, IL-6, TNF-α, MCP-1, and MIP-1α, and an increase in the anti-inflammatory cytokine IL-10 in the hearts of mTOR-Tg mice subjected to ex vivo I/R injury. As previously reported, cytokine generation during ex vivo I/R injury is determined by cardiac damage (8). Therefore, we cannot conclude that mTOR overexpression directly regulates cytokine generation during reperfusion in this study. We (35) previously observed that mTOR overexpression suppressed LPS-induced IL-6 secretion from cardiomyocytes. Similar results were also seen during the ex vivo I/R injury. Since IL-6 is an important mediator of adverse LV remodeling after MI (18), the effects of mTOR overexpression on the inflammatory response might contribute to the inhibition of adverse LV remodeling in addition to the cardioprotective effect. Further experiments are required to determine if the anti-inflammatory effects of cardiac mTOR contribute to the inhibition of adverse LV remodeling after I/R injury.
In this study, while we found that cardiac mTOR is sufficient to protect the heart against I/R injury, we did not examine whether cardiac mTOR is necessary in the same setting. A previous report (43) using cardiac mTOR knockout mice showed that mTOR is necessary to preserve cardiac function in pathological hypertrophy but did not examine the response to ischemic injury. Since inflammation is a key contributor to the pathogenesis of LV remodeling in both I/R injury and pathological hypertrophy, it seems likely that cardiac mTOR is also necessary to prevent cardiac dysfunction after I/R injury. Studies using I/R models in mTOR knockout mice are necessary to verify this hypothesis.
In summary, we found that overexpression of cardiac mTOR was sufficient to protect the heart against I/R injury, accompanied by an inhibition of adverse LV remodeling. The results of our experiments in ex vivo perfused hearts from mTOR-Tg mice strongly suggest that mTOR overexpression suppressed cardiomyocyte necrosis in I/R injury and inhibited the inflammatory response during reperfusion. Since the majority of patients with acute coronary injury receive interventional therapy to reopen the coronary artery, it is important to understand the mechanisms underlying the cardioprotective effects of cardiac mTOR in I/R injury. A greater understanding of these mechanisms is likely to identify new targets for therapies that can maximize the benefits of reperfusion in acute MI.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-098423 (to T. Matsui) and HL-094677 (to A. Rosenzweig) and a Leducq Network for Research Excellence (to A. Rosenzweig).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.A., Y.K., and T.M. conception and design of research; T.A., Y.K., C.-Y.X., B.T.I., M.T., and T.M. performed experiments; T.A., Y.K., and T.M. analyzed data; T.A., Y.K., M.S.-C., K.H., and T.M. interpreted results of experiments; T.A., Y.K., and T.M. prepared figures; T.A., Y.K., and T.M. drafted manuscript; T.A., Y.K., C.-Y.X., M.S.-C., A.R., K.H., and T.M. edited and revised manuscript; T.A., Y.K., C.-Y.X., B.T.I., M.T., M.S.-C., A.R., K.H., and T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Hiroko Aoyagi for technical assistance.
Present address of Y. Kusakari: Department of Cell Physiology, Jikei University School of Medicine, Tokyo, Japan.
REFERENCES
- 1. Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene 25: 6361–6372, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Burchfield JS, Dong JW, Sakata Y, Gao F, Tzeng HP, Topkara VK, Entman ML, Sivasubramanian N, Mann DL. The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Circ Heart Fail 3: 157–164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol 54: 2435–2446, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Cittadini A, Monti MG, Petrillo V, Esposito G, Imparato G, Luciani A, Urciuolo F, Bobbio E, Natale CF, Sacca L, Netti PA. Complementary therapeutic effects of dual delivery of insulin-like growth factor-1 and vascular endothelial growth factor by gelatin microspheres in experimental heart failure. Eur J Heart Fail 13: 1264–1274, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol 2011 [DOI] [PubMed] [Google Scholar]
- 7. Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res 58: 88–111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gurevitch J, Frolkis I, Yuhas Y, Paz Y, Matsa M, Mohr R, Yakirevich V. Tumor necrosis factor-α is released from the isolated heart undergoing ischemia and reperfusion. J Am Coll Cardiol 28: 247–252, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci 30: 35–42, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Harston RK, McKillop JC, Moschella PC, Van Laer A, Quinones LS, Baicu CF, Balasubramanian S, Zile MR, Kuppuswamy D. Rapamycin treatment augments both protein ubiquitination and Akt activation in pressure-overloaded rat myocardium. Am J Physiol Heart Circ Physiol 300: H1696–H1706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hernandez G, Lal H, Fidalgo M, Guerrero A, Zalvide J, Force T, Pombo CM. A novel cardioprotective p38-MAPK/mTOR pathway. Exp Cell Res 317: 2938–2949, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127: 125–137, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res 89: 1191–1198, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J 19: 5720–5728, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol 41: 256–264, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kis A, Yellon DM, Baxter GF. Second window of protection following myocardial preconditioning: an essential role for PI3 kinase and p70S6 kinase. J Mol Cell Cardiol 35: 1063–1071, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Kobara M, Noda K, Kitamura M, Okamoto A, Shiraishi T, Toba H, Matsubara H, Nakata T. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc Res 87: 424–430, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Kung G, Konstantinidis K, Kitsis RN. Programmed necrosis, not apoptosis, in the heart. Circ Res 108: 1017–1036, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Kusakari Y, Xiao CY, Himes N, Kinsella SD, Takahashi M, Rosenzweig A, Matsui T. Myocyte injury along myofibers in left ventricular remodeling after myocardial infarction. Interact Cardiovasc Thorac Surg 9: 951–955, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation 100: 2373–2379, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277: 22896–22901, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol 38: 63–71, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335., 2001 [DOI] [PubMed] [Google Scholar]
- 25. Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion. Roles of AMP-activated protein kinase and beclin 1 in mediating autophagy. Circ Res 100: 914–922, 2007 [DOI] [PubMed] [Google Scholar]
- 26. McKay RG, Pfeffer MA, Pasternak RC, Markis JE, Come PC, Nakao S, Alderman JD, Ferguson JJ, Safian RD, Grossman W. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation 74: 693–702, 1986 [DOI] [PubMed] [Google Scholar]
- 27. Miller DL, Li P, Dou C, Armstrong WF, Gordon D. Evans blue staining of cardiomyocytes induced by myocardial contrast echocardiography in rats: evidence for necrosis instead of apoptosis. Ultrasound Med Biol 33: 1988–1996, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings B, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest 115: 2128–2138, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. N Engl J Med 357: 1631–1638, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Okumura H, Nagaya N, Itoh T, Okano I, Hino J, Mori K, Tsukamoto Y, Ishibashi-Ueda H, Miwa S, Tambara K, Toyokuni S, Yutani C, Kangawa K. Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation 109: 242–248, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Prasad A, Stone GW, Aymong E, Zimetbaum PJ, McLaughlin M, Mehran R, Garcia E, Tcheng JE, Cox DA, Grines CL, Gersh BJ. Impact of ST-segment resolution after primary angioplasty on outcomes after myocardial infarction in elderly patients: an analysis from the CADILLAC trial. Am Heart J 147: 669–675, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 125: 1134–1146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol 299: C1256–C1266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sotiropoulos KB, Clermont A, Yasuda Y, Rask-Madsen C, Mastumoto M, Takahashi J, Della Vecchia K, Kondo T, Aiello LP, King GL. Adipose-specific effect of rosiglitazone on vascular permeability and protein kinase C activation: novel mechanism for PPARγ agonist's effects on edema and weight gain. FASEB J 20: 1203–1205, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev 79: 215–262, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Vilahur G, Juan-Babot O, Pena E, Onate B, Casani L, Badimon L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol 50: 522–533, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72: 19–44, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 97: 276–281, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of GSK-3β during myocardial ischemia and ischemia/reperfusion. Circ Res 109: 502–511, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang D, Contu R, Latronico MV, Zhang JL, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest 120: 2805–2816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H-ATPase. Science 334: 678–683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]