Abstract
The oxygen dependence of respiration in striated muscle in situ was studied by measuring the rate of decrease of interstitial Po2 [oxygen disappearance curve (ODC)] following rapid arrest of blood flow by pneumatic tissue compression, which ejected red blood cells from the muscle vessels and made the ODC independent from oxygen bound to hemoglobin. After the contribution of photo-consumption of oxygen by the method was evaluated and accounted for, the corrected ODCs were converted into the Po2 dependence of oxygen consumption, V̇o2, proportional to the rate of Po2 decrease. Fitting equations obtained from a model of heterogeneous intracellular Po2 were applied to recover the parameters describing respiration in muscle fibers, with a predicted sigmoidal shape for the dependence of V̇o2 on Po2. This curve consists of two regions connected by the point for critical Po2 of the cell (i.e., Po2 at the sarcolemma when the center of the cell becomes anoxic). The critical Po2 was below the Po2 for half-maximal respiratory rate (P50) for the cells. In six muscles at rest, the rate of oxygen consumption was 139 ± 6 nl O2/cm3·s and mitochondrial P50 was k = 10.5 ± 0.8 mmHg. The range of Po2 values inside the muscle fibers was found to be 4–5 mmHg at the critical Po2. The oxygen dependence of respiration can be studied in thin muscles under different experimental conditions. In resting muscle, the critical Po2 was substantially lower than the interstitial Po2 of 53 ± 2 mmHg, a finding that indicates that V̇o2 under this circumstance is independent of oxygen supply and is discordant with the conventional hypothesis of metabolic regulation of the oxygen supply to tissue.
Keywords: skeletal muscle, respiratory rate, interstitial Po2, oxygen disappearance curve, phosphorescence quenching method, cell Po2 gradient
the coordination of oxygen demand and supply in skeletal muscle and in the heart is carried out by a mechanism not yet completely understood. Current cardiovascular texts propose the century-old hypothesis of metabolic control of capillary blood flow as the accepted theory of local autoregulation (review Ref. 38). According to this hypothesis, a decline of oxygen delivery leads to a decrease of intracellular Po2, an evoked release of a metabolic vasodilator into the extracellular space, the dilation of arterioles, and, eventually, an increase in the flow velocity of blood and number of perfused capillaries. A key aspect of this model is the oxygen dependence of cellular metabolism, making the mitochondria or entire cell sensitive to an inadequate oxygen supply (11).
The oxygen dependence of respiration for isolated mitochondria and cells is represented by a hyperbolic curve empirically described by Hill's equation (Eq. 10) (29, 39, 46, 59, 60) with the parameters Vm (maximal respiratory rate), Hill coefficient = 1 to 1.4, and P50 (Po2 corresponding to a V̇o2 of one-half Vm). The curve shows the relative independence of the rate of respiration at high Po2, and a strong dependence at low Po2, while the point of transition of the two portions of the curve defines the critical Po2 Pcrit.
It was shown in early studies (10, 22, 25, 29, 56) that the oxygen consumption of isolated mitochondria and cells remains relatively independent of the Po2 in their environment over a wide range of Po2. A suspension of isolated mitochondria is insensitive to Po2 elevation >1 mmHg (10, 13, 14, 40, 60). Suspensions of isolated resting muscle cells show values of apparent P50 higher than those in mitochondria, yet much lower than the Po2 in venous blood, which is approximately equilibrated with the tissue around capillaries (2, 14, 24, 26, 35, 39). Thus, based on measurements made on isolated mitochondria and myocytes, mitochondria may not serve as oxygen sensors monitoring the physiological Po2 level in tissue because of their low critical Po2 (54).
Oxygen consumption by mitochondria depends on the rate of biochemical processes and the availability of substrates and oxygen (7, 10, 13, 14, 60). At the cellular level, the oxygen dependence of respiration is modulated by the cellular functional state and its capacity for oxygen transport. Diffusivity and solubility of oxygen, in concert with cell size and the spatial distribution of mitochondria, appear to be additional determinants of the oxygen dependence of respiration (2, 4, 21, 24, 37, 48, 53).
On the tissue/organ level, the external control of cell respiration (via contraction) and microcirculatory control of oxygen delivery appear in addition to the existing mechanisms of regulation at the levels of individual mitochondria and cells. It is also suggested that inhibiting cytochrome c oxidase with nitric oxide produces the contribution of intercellular regulation of muscle respiration by all tissue cells including the vascular endothelium (8, 12, 42). Thus the factors affecting the oxygen dependence of respiration in the tissue lead to a set of parameters Vm, P50, and Pcrit different from those obtained in isolated cells and mitochondria. The importance of the study of oxygen dependence of respiration in situ was well formulated by Wilson (54): “The oxygen dependence of cellular oxidative phosphorylation remains highly controversial. Quantitative knowledge of that dependence is critical for understanding of not only cellular biochemistry but also a wide range of physiological functions that help to regulate both metabolism and the oxygen delivery system. Is mitochondrial oxidative phosphorylation dependent on the oxygen pressures in normal tissues?” To answer this question, new approaches for the study of the oxygen dependence of respiration in living muscle in situ have been sought.
With the introduction of the polarographic method for measuring oxygen in tissues, it became possible to record the disappearance of oxygen caused by a momentary stoppage of blood flow. The interpretation of these curves was aimed at obtaining information on the rate of tissue respiration and its dependence on oxygen tension in the tissue (9, 34). The method was not widely used because of the complexity of accounting for the contribution of oxygenated blood and the limitations associated with the microelectrode technique of measuring oxygen.
The invention of the phosphorescence quenching method (PQM) paved the way for the measurement of Po2 in microscopic volumes of various organs (52, 61). Now one can record separate measurements of oxygen in the microvessels (3, 49, 62), in the interstitial fluid (44, 50, 58), and within individual muscle cells (45). Interstitial oxygen tension takes on an intermediate value between the intracapillary and intracellular Po2, reflecting the current balance between rates of delivery and consumption of oxygen by muscle fibers. Furthermore, the interstitial oxygen tension is the Po2 on the surface of muscle cells, representing the boundary condition for the diffusion of oxygen into the cell. The critical Po2 of skeletal muscle in situ was determined for the first time in 1999 by recording the fall in interstitial Po2 caused by the rapid arrest of blood flow (36). As a criterion for the critical oxygen tension, workers used an increase of NADH fluorescence and a sharp change in the rate of decline in interstitial Po2. In normally perfused resting muscle, the authors reported venular Po2 of 17.7 mmHg and a 3-mmHg Po2 decrease to the interstitial Po2 of 14.6 mmHg. Interstitial critical Po2 as defined by the two different criteria mentioned above was found to be in the range of 2.4–2.9 mmHg, which was slightly higher than that in isolated muscle fibers.
In our present work, we develop this approach by improving the quality of the Po2 measurements through reducing the artifact of oxygen consumption caused by the phosphorescence quenching method in a stationary fluid. Correction for the artifacts is done when calculating the oxygen disappearance curve (ODC) recorded in the interstitium. We also present a model for the interpretation of the dynamics of the interstitial Po2 decline due to oxygen consumption by muscle fibers to develop a new fitting model for the analysis of experimental data on the oxygen dependence of respiration in muscle.
METHODS
In this study, we propose a method for the analysis of the ODCs in the interstitium of a thin skeletal muscle produced by the rapid pneumatic compression of the tissue. The measuring procedure for Po2 and V̇o2 in a muscle using the phosphorescent oxygen probe loaded into the interstitial space has been published before (18). However, previously we used only the initial part of an ODC to evaluate the respiration rate in the spinotrapezius muscle. In our present work, we have developed an approach for analysis of the entire ODC to determine the oxygen dependency of respiration of the muscle fibers in situ.
A thin planar muscle prepared for intravital microscopy (1) was placed between a thermo-stabilized sapphire plate and a gas barrier film. The interstitial space of the muscle was loaded with an albumin bound phosphorescent oxygen probe. Blood flow in the muscle was interrupted by rapidly inflating a bag of transparent film attached to the objective lens. Also, the removal of red blood cells (RBCs) from the muscle in the measuring volume was achieved and confirmed by microscopic observation. For the Po2 measurements, a brief light pulse (laser 532 nm, 15-ns duration, 1 pulse/s) was used to excite the probe inside a tissue disk of radius 300 μm.
In the following analysis, we use the flash number, n, as the independent variable instead of time. The index n = 0 denotes the variable before the onset of compression. The first flash after compression is denoted by n = 1. Under the conditions described above, the interstitial Po2 = P0 for normal blood flow in capillaries. Then, the rapid compression of the muscle removes RBCs from the vessels, leaving only physically dissolved oxygen in the tissue. From that moment, the interstitial Po2 inside the illuminated tissue disk is measured, thus forming the ODC data set (Pn; see Fig. 1).
Fig. 1.
A typical oxygen disappearance curve (ODC) as an average of 5 curves recorded at different sites in the same muscle. Po2 values correspond to those measured in the interstitial fluid using phosphorescence quenching microscopy and thus represent Po2 on the surface of muscle fibers at the measurement site. Pn, Po2 outside the illuminated tissue disk at the moment of the nth flash.
The rate of Po2 change inside the sampled volume (Pn′) depends on three components: first, the metabolic or cellular oxygen consumption component (Vn) which is the subject of interest; second, the photo-consumption by the method itself (KPn); and third, the diffusion oxygen inflow from the surrounding tissue, proportional to the Po2 difference Z(pn − Pn) at the boundary of the illuminated region. Here pn is the Po2 outside the illuminated tissue disk at the moment of the nth flash, and the parameters K and Z are empirical coefficients of oxygen photo-consumption and inflow, respectively, which can be evaluated by fitting the experimental test data to the equations that follow. To account for all the factors influencing the measured rate of Po2 decrease, consider the equation:
| (1) |
The data set (Pn) is obtained from the experimental ODC, and the rate of Po2 drop (Pn′) can be calculated by differentiating the ODC. The goal is to evaluate the rate of tissue respiration V̇o2 from the metabolic component Vn, which is calculated for a flash rate F = 1 Hz and the oxygen solubility in the muscle [α = 39 nl O2/(cm3·mmHg); Ref. 30] as:
| (2) |
We can simplify Eq. 1 for the case when the metabolic component is absent (Vn = 0), for example, in a sample of dead tissue excised after the experiment (18). The ODC recorded in the sample under the same conditions of measurements as in vivo can be used for the evaluation of the coefficients K and Z and verification of the validity of assumptions underlying the model. In that case, the tissue outside the illuminated disk remains saturated with oxygen at an initial steady state Po2 of pn = P0.
The solution of Eq. 1 under these conditions predicts an exponential decline of Po2:
| (3) |
In the presence of oxygen inflow across the boundary of the illuminated region of tissue, the ODC approaches an asymptotic Po2 (Pa) formed by equilibrium between the processes of oxygen photo-consumption and inflow:
| (4) |
When oxygen inflow is negligible, as in the case of an excitation area much larger than the area of detection, the Po2 asymptotically approaches zero and Eq. 3 is transformed into:
| (5) |
In our previous work (18), we have shown that Eq. 3 is a good fitting model of the ODC in nonrespiring tissue. In this special test, we have determined values for the coefficients K (= 4.1·10−3) and Z (= 1.5·10−3) for correction of measurements made in situ. These coefficients are dimensionless; however, since we have omitted the flash rate 1 Hz in the equations for simplicity, the dimension appears as [s−1].
Oxygen dependence of respiratory rate (V̇o2 vs. Po2) for muscle fibers in situ. In our present work, we employed the phosphorescent probe distributed in muscle interstitial (extracellular and extravascular) space. Rapid (∼0.1-s pressure elevation) application of external pressure to the tissue expels the RBCs from the vessels and makes the ODC independent of hemoglobin. That experimental situation opens the opportunity to recover the dependence of muscle V̇o2 on Po2 in the interstitial space, i.e., on the surfaces of the muscle fibers. In that case, the entire ODC from P0 to near zero Po2 level has to be analyzed. With rising flash number, n, the difference between external and internal Po2 (pn − Pn) increases, so the contribution of oxygen inflow must be taken into account.
Following tissue compression Po2 in the tissue outside the illuminated spot decreases only due to tissue respiration (no photo-consumption), so that the rate of Po2 change is:
| (6) |
If coefficient Z is not small enough to ignore the oxygen inflow, then the Vn can be obtained through the iterative calculation:
| (7) |
| (8) |
Analysis of an ODC can be greatly simplified if the inflow contribution is negligible and the time course of the oxygen consumption rate after occlusion can then be expressed as:
| (9) |
This is possible when the illuminated spot is much larger than the region of detection but is not always acceptable because of the intention to avoid light exposure of adjacent sites in case of subsequent multiple measurements in the same muscle. Our data were collected in the presence of oxygen inflow, so that is why Eq. 8 was used in the analysis.
The obtained Vn are separated from the artifacts and can be converted into V̇o2 according to Eq. 2. A plot of (V̇o2)n vs. (Pn) values at sequential flashes represents the oxygen dependence of respiration for muscle fibers in situ (see Fig. 5), which can be fit with a sigmoid curve, described by Hill's equation, to evaluate the parameters Vm, maximal respiration rate for a collection of muscle fibers (hereafter the symbol V is used to designate the rate of oxygen consumption); P50, oxygen tension for half-maximal respiration rate; and a, Hill coefficient:
Fig. 5.
Typical plot of the oxygen dependency for respiration of the rat spinotrapezius muscle. Data set was transformed from the ODC shown in Fig. 1. Parameters estimated by fitting these data are: VM = 138.8 nl O2/(cm3·s), k = 9.4 mmHg, and Δ = 3.0 and 3.3 mmHg. Po2 values plotted on the horizontal axis correspond to interstitial Po2 on the surfaces of the group of muscle fibers at the site of these measurements.
| (10) |
However, this empirical approach gives only a limited understanding of the dependence of the rate of cell respiration on oxygen level. For that purpose, we developed a model to relate interstitial Po2 and the rate of mitochondrial respiration per unit volume of the cell.
Interpretation of the oxygen dependence curves. Since the oxygen probe is distributed in the interstitial space, it reports the Po2 on the surface of muscle fibers, at the sarcolemma, both during steady state and during the transient conditions of the ODC. Thus the curve relating respiration to the Po2 on the surface of muscle cells can be analyzed using an appropriate model. That model should take into account the respiratory dependence of microscopic intracellular volumes (related to the functional activity of mitochondria) and the Po2 gradient in cells produced by the transport resistance due to diffusion.
Our model is based on the assumption that all oxygen sinks (mitochondria) in the muscle fibers are identical to each other in their respiratory properties, which means they obey a hyperbolic equation (39, 40, 53, 60), written below in a normalized form:
| (11) |
where v is the local specific oxygen consumption (by an elementary volume); VM is the maximal volume-specific O2 consumption, which is the same for the entire tissue (Vm) and for the elementary volumes inside the cells (VM), so that we can set Vm = VM; p is the local intracellular Po2; and k is the local Po2 corresponding to the half-maximal respiration rate (i.e., P50 for mitochondria). We have attempted to explain the origin of the sigmoidal oxygen dependence of muscle cell respiration (Eq. 10) on the basis of the hyperbolic oxygen dependence of mitochondrial respiration (Eq. 11) and the intracellular gradient of Po2. In a generalized muscle fiber (Fig. 2), the elementary volumes of the cell are depicted by concentric isobars. However, all these sinks in the muscle cells are localized in tissue volumes under different local oxygen tension p that creates heterogeneity in oxygen consumption rates inside the cell.
Fig. 2.
Cross-section of a generalized muscle fiber. For the current interstitial oxygen tension at the surface of the muscle fiber, P, the total respiration rate by the cell is V, and the core (i.e., center) Po2 is Pc. For an isobaric fraction of the cellular volume, f, the local Po2 is p and the local consumption rate is v. Intracellular Po2 range, P − Pc, is Δ.
In our experiments, the parallel changes in interstitial Po2 and V̇o2 (P and V) were determined as values obtained at the sarcolemma (Fig. 2). There is no requirement of any special shape (circular, hexagonal, etc.) of the fiber cross-section; it can be quite natural. The only assumption is the existence of a Po2 gradient inside the muscle fibers, expressed as the difference, Δ = P − Pc between the surface (i.e., interstitial) Po2 = P and Po2 = Pc in the center of the fiber, i.e., the point in the fiber with the lowest Po2.
A fraction of tissue volume f, having a given Po2 = p (isobaric volumes), also has the same respiration rate v (Fig. 2). As a first approximation we can consider the distribution f(p) to be a Uniform (or Rectangular, Fig. 3) distribution having a width Δ = P − Pc and a density f = 1/Δ, meaning that the total volume is equal to unity and the probability density function can be applied to represent the tissue volume distribution as a function of Po2. This approach also will allow us to define the first and second moments of this distribution, yielding its mean value and width. Our aim is the recovery of information on the properties of intracellular respiration by determining best fit parameters for experimental data points using the equations generated by the model.
Fig. 3.
Uniform distribution of the tissue volume as a function of p (variable intracellular Po2) presented for 3 distinct situations. For normoxic conditions, the distribution for all elementary volumes of cells have p > 0; at the critical Po2, the distribution is characterized by the condition Pc = 0; while for the hypoxic case, the width of the distribution of the respiring volume of tissue is reduced (anoxic part of volume is shaded).
The Uniform distribution of the intracellular volume based on Po2, with density f = 1/Δ, is presented in the diagrams of Fig. 3. Interstitial Po2 = P is the right border of the cellular volume distribution on the oxygen tension p having width Δ. There are three possible physiological situations in the muscle fiber: 1) normoxia, P > Δ; 2) critical Po2, P = Δ; and 3) hypoxia, P < Δ. When P > Δ all isobaric volumes f in a cell have Po2 > 0 and participate in oxygen consumption. When P = Δ, Pc = 0 and v = 0 at the center of the fiber; this value of P is known as “critical.” For P < Δ, some deep volumes presented by the shaded region left of zero Po2 are excluded from respiration. Since negative Po2 values are impossible, that part of the cell volume also has Po2 = 0 and total V is the sum (or integral, see Eq. 12) of the oxygen consumption rates only in volumes having Po2 > 0.
The total oxygen consumption rate V is the sum of respiratory rates, v, of the isobaric volumes multiplied by their volume fractions (f = 1/Δ). Generally, using Eq. 11, the total oxygen consumption rate normalized to the maximal rate VM can be written as:
| (12) |
This expression can be presented in a form convenient for integration:
| (13) |
The limits of integration of Eq. 13 are different for each of the situations shown in Fig. 3, and the solutions for V are also different. The consumption curve (i.e., V as a function of P) for a generalized muscle fiber or tissue consists of two different regions that correspond to two different interstitial Po2 conditions:
| (14) |
| (15) |
The line formed by the points separating the two regions of V(P), that is V for P = Δ (middle plot), is described by the equation for the critical Po2:
| (16) |
The equations obtained for the normoxic and hypoxic ranges (Eqs. 14 and 15) of interstitial Po2 can be used as fitting models for the analysis of experimental curves on the oxygen dependence of respiration, while Eq. 16 may be applied for accurate evaluation of the critical Po2.
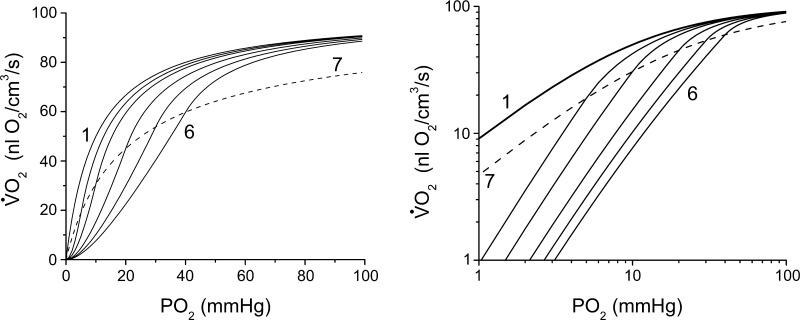
Equations 14–16 make it possible to predict the behavior of the oxygen dependence of cellular respiration for different ranges of the intracellular Po2 gradient and oxygen demand. The set of theoretical curves generated for different Δ are shown in Fig. 4. The curves are calculated for a set of parameters [VM = 100 nl O2/(cm3·s), k = 10 mmHg, and Δ = 0, 5, 10, 20, 30, 40 mmHg] to demonstrate the effect of an intracellular oxygen gradient on the oxygen dependency of respiration. The first curve (Fig. 4, curve 1) is the oxygen dependence for mitochondria described by Eq. 11. This is the same relationship for a whole cell in the absence of an oxygen gradient due to intracellular diffusion resistance. When the different contributions of the diffusion resistance occur, the Po2 difference between the sarcolemma and core (Fig. 4, curves 2–6, Δ = 5–40 mmHg) leads to a sigmoidal appearance of the cellular respiration dependence on Po2. This connection allows us to determine the parameters for the mitochondrial respiratory dependency on oxygen from the observed experimental oxygen dependency of oxygen consumption for whole cells. Each of the five solid curves (2–6) consists of two regions, a normoxic region described by V1 and a hypoxic region described by V2, according to Eqs. 14 and 15, respectively. The dashed line (curve 7) corresponds to the situation (critical Po2) described by V3 (Eq. 16), indicating the points separating the normoxic and hypoxic regions of the curves. The same curves plotted as a double-logarithmic plot (Fig. 4, right) demonstrate that the hypoxic regions are transformed into straight lines, which turn into hyperbolic lines above the dashed line 7, corresponding to the critical dependence, V3. Curve 1 represents the case when there is no Po2 difference between the cellular surface and the core, for example, in the case of zero diffusion resistance or a very thin cell. An increase in diffusion resistance or thickness of the cells leads to a proportional shift in curve 7 to the right. The same effect is caused by an increase in k, which reflects a greater oxygen dependence of mitochondrial respiration.
Fig. 4.
Theoretical curves for the oxygen dependency of respiration generated for a set of parameters [VM = 100 nl O2/(cm3·s), k = 10 mmHg, and Δ = 0, 5, 10, 20, 30, and 40 mmHg]. Left: curve 1, with Δ = 0, represents the mitochondrial Po2 dependence according to Eq. 11 without any diffusional resistance and Po2 gradients. The five other solid curves (2 through 6) represent the Po2 dependencies for Po2 differences between the cell surface and its core of 5–40 mmHg. Each curve consists of 2 regions, the normoxic region described by V1 and the hypoxic region described by V2, according to Eqs. 14 and 15, respectively. Dashed line (curve 7) corresponds to the critical Po2 curve, V3 (Eq. 16), indicating the points which separate the normoxic and hypoxic regions of the curves. Right: same curves plotted on a double-logarithmic plot to demonstrate that the hypoxic regions are straight lines, which turn into hyperbolic lines above the threshold line 7 for critical dependency of oxygen consumption on Po2, V3.
Animal experiments.
The experimental protocol followed for these measurements was previously published in detail (18). All procedures were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. Six female Sprague-Dawley rats were initially anesthetized with a mixture of ketamine/acepromazine (72/3 mg/kg ip). Once femoral vein access was obtained, the animals received supplemental anesthesia as a continuous intravenous infusion of alfaxalone acetate (Alfaxan, Schering-Plough Animal Health, Welwyn Garden City, UK; ∼0.1 mg/kg/min). At the termination of an experiment, Euthasol (150 mg/kg iv, pentobarbital component; Delmarva, Midlothian, VA) was administered while the animal was under a surgical plane of anesthesia. The spinotrapezius muscle was used for measurement of interstitial Po2 and the surgical preparation was similar to the original description by Bailey et al. (1) and Gray (19). The muscle was placed on a thermo-stabilized (37°C) pedestal of the animal platform (17). The muscle was covered with gas barrier plastic film (Saran, Dow Corning, Midland, MI). An objective-mounted film airbag connected to a pressure controller allowed organ compression at 130 mmHg, which rapidly squeezed blood out of microvessels in the thin spinotrapezius muscle (15). Circular regions of muscle 600 μm in diameter and containing no large microvessels were selected for V̇o2 measurements. The Po2 was sampled once a second during 200 s of Po2 data collection in a reactive hyperemia-type protocol. Before rapid airbag inflation, the interstitial Po2 at normal tissue perfusion (i.e., baseline) was recorded for 30 s. This was followed by 90 s of muscle compression to arrest blood flow, after which the airbag was deflated for the remainder of the recording period (i.e., 80 s). This protocol was repeated at 3–11 different sites around the muscle, with 5- to 10-min intervals between measurements. Preparation quality and viability were confirmed by a return of interstitial Po2 to baseline between consecutive measurements. The measurement of Po2 with PQM has been described in detail previously (18). Respiration rates, Vn, were calculated according to Eq. 8. Each ODC was differentiated using a five-point differentiation smoothing function, after checking that this procedure had no effect on the fitting analysis. The Levenberg-Marquardt algorithm was used for Po2 calculations to fit the multiple phosphorescence decays (one Po2 value per second for 200 s) with a program put together using the LabView software platform (National Instruments, Austin, TX). Statistical calculations and parameter fitting were made with the Origin 7.0 software package. All data are presented as means ± SE (number of measurements).
RESULTS
The oxygen disappearance curves were recorded at 34 sites in 6 spinotrapezius muscles with measurements at 3–11 sites per muscle. Curves obtained in the same muscle were aligned (time base “correction”) and averaged (see Fig. 1, as an example). Measures described previously were taken to reduce the artifact of oxygen photo-consumption, and its contribution at the normal interstitial Po2 was 0.6%. The effect of oxygen inflow into the detection area was noticeable at the lowest Po2 (accounting for 3.5% of the Po2 change). Equation 8 was used, along with these measured values, to correct the oxygen disappearance curves. The resulting corrected curves were used to calculate the dependence of oxygen consumption on Po2, which was then plotted and fit with Hill's equation (Eq. 10). The parameters recovered for the total data set were as follows: Vm = 120.9 ± 7.7 nl O2/(cm3·s); P50 = 11.1 ± 0.9 mmHg; and the exponent a = 2.0 ± 0.1.
For further analysis of the oxygen dependency of respiration, we used fitting Eqs. 14 and 15 (see Fig. 5) to estimate the intracellular Po2 range Δ, VM, and k . The parameters VM , k, and Δ1 were determined first for the normoxic region of the curve (Eq. 14), which comprises most of its length; then the hypoxic region of the curve was fit (Eq. 15) at fixed VM and k taken from the first procedure, to make a second estimation of the Po2 range, Δ2. An example of such an analysis is shown in Fig. 5 (the same data set as in Fig. 1), where most of the points belong to the normoxic region of the curve described by Eq. 14 and the low Po2 segment was fit with Eq. 15. A double-logarithmic plot facilitates finding the point of separation between the two regions of the overall curve; it could also be calculated using Eq. 16.
The set of curves averaged for each muscle was homogeneous, but the range in maximal and minimal VM and k among the muscles was twofold (see Table 1). The average difference between the intracellular Po2 ranges calculated with Eqs. 14 and 15 (i.e., Δ1 and Δ2) was within 1 mmHg, and these data sets are well correlated (R = 0.87; p = 0.025). A high correlation was also found between VM and k (R = 0.94; p = 0.0055), while the other parameter sets showed no significant correlation. It follows from the derivation of Eq. 16 that the critical Po2 is equal to Δ, and, for the value obtained for Δ of 4–5 mmHg, the corresponding critical oxygen consumption is 21.2–25.2 nl O2/(cm3·s).
Table 1.
Parameter estimation for oxygen dependency of respiration in six spinotrapezius muscles in situ
| Muscle No. | NODC | P0, mmHg | VM, nl O2/cm3·s | k, mmHg | Δ1, mmHg | Δ2, mmHg |
|---|---|---|---|---|---|---|
| 1 | 6 | 69.1 ± 1.3 | 138.8 | 9.41 | 3.27 | 3.02 |
| 2 | 6 | 33.1 ± 3.8 | 111.5 | 7.95 | 3.89 | 3.15 |
| 3 | 11 | 55.9 ± 4.4 | 107.6 | 6.46 | 6.67 | 4.78 |
| 4 | 3 | 59.8 ± 1.4 | 167.7 | 10.79 | 6.63 | 7.05 |
| 5 | 3 | 49.2 ± 1.0 | 209.8 | 19.26 | 4.23 | 3.10 |
| 6 | 5 | 48.5 ± 1.4 | 182.1 | 18.06 | 4.52 | 3.42 |
| Means ± SE | 34 | 52.9 ± 2.0 | 139.1 ± 6.1 | 10.5 ± 0.8 | 5.0 ± 0.2 | 4.0 ± 0.2 |
Best-fit parameters of oxygen consumption and Po2 gradients in six spinotrapezius muscles, evaluated with Eqs. 14 and 15. Weighted means for 34 ODCs are shown.
NODC, number of sites in the same muscle used for averaging the oxygen disappearance curves (ODCs); P0, interstitial Po2 measured just before the onset of tissue compression; Vm, maximal respiratory rate; k, mitochondrial Po2 corresponding to half-maximal oxygen consumption; Δ1 and Δ2, intracellular Po2 range evaluated from Eqs. 14 and 15, respectively.
DISCUSSION
Further improvement of the optical technique (PQM) to measure Po2 in living organs, including corrections for instrumental artifacts and incorporation of several significant technical innovations, made it possible to update the study of tissue respiration in situ previously made by Richmond et al. (36). To eliminate the intravascular phosphorescence signal, the oxygen probe was loaded directly by diffusion into the intercellular space of the thin muscle. To eliminate the influence of intravascular oxygen, the flow arrest was performed by pneumatic compression of the muscle, which squeezed RBCs out of the microvessels. The pressure in the air bag rapidly rose to a level above the systolic blood pressure and extrusion of blood from the compressed muscle was monitored with video microscopy. The diameter of the measuring area was increased to 600 μm (vs. 20 μm in Ref. 36) to include the interstitial space around 10 muscle fibers and make the diameter of the measuring volume similar to its depth. The larger sampling volume allowed us to reduce the excitation energy density and flash rate to 1.8 pJ/μm2 and F = 1 Hz (vs. 31 pJ/μm2 and 50 Hz in Ref. 36) and provided a phosphorescence decay signal with signal-to-noise ratio good enough for analysis of individual decays.
These technical improvements significantly reduced the photo-consumption of oxygen by this method (16, 18). That is why the interstitial Po2 in our experiments was significantly higher at rest: 53 mmHg vs. 15 mmHg in the study by Richmond et al. (36). Similar values of interstitial Po2 in skeletal muscles have been reported by other workers. Recent studies (57, 58) of interstitial oxygenation with the PQM using new oxygen probes found that the peak of the histogram of interstitial Po2 in mouse skeletal muscle corresponded to 41 mmHg. The interstitial Po2 measured near 1st, 2nd, and 3rd order arterioles in rat cremaster muscle varied between 51–29 mmHg (43). In the rat diaphragm muscle, average microvascular Po2 was normally ∼50 mmHg, which may also indicate similar Po2 in the interstitium (32). Peri-arteriolar Po2 for 2A arterioles in cat muscle, measured with a microelectrode, was found to be 52–40 mmHg, depending on the Po2 of the superfusate (6). In the rat spinotrapezius muscle, the Po2 values obtained with a microelectrode in the vicinity of venules were close to 50 mmHg (27). It should be noted that the reference volume of a polarographic electrode is not limited to the interstitial space but also includes the intracellular content having a lower Po2 than that in the interstitium. The PQM also opened the possibility to localize Po2 measurements in a selected compartment: intravascular, interstitial, or intracellular (23, 58).
Recording the ODCs in a stationary interstitial fluid requires a series of tens of light pulses, so the artifact of accumulated photo-consumption should be considered and corrected for. To accomplish this, a mathematical model of oxygen measurements in a microscopic volume of muscle was formulated and the contribution of photo-consumption and diffusional inflow of oxygen was determined and used to correct the data. In future experiments, the analysis can be simplified by increasing the size of the excitation area compared with the area of signal detection, which will make the contribution of oxygen inflow negligible.
Corrected data on the metabolic component of ODCs were converted to respiration rates and plotted against the corresponding values of Po2, thus forming a scatter plot of oxygen dependency of muscle fiber respiration in situ. The data obtained were well approximated by Hill's equation (Eq. 10), which was used to determine the parameters Vm = 120.9 nl O2/(cm3·s), P50 = 11.1 mmHg, and the exponent a = 2.0. The sigmoidal curve describing the oxygen dependency of respiration does not contain a specific point indicating the critical Po2 associated with the appearance of an anoxic core in muscle fibers. This fact limits the usefulness of an empirical fitting model and points out the need for finding an analytical description of the oxygen dependence of cell respiration, based on knowledge of oxygen uptake by mitochondria and the intracellular oxygen gradient created by the diffusional influx of oxygen into a cell.
There are a number of studies on mathematical modeling of oxygen diffusion combined with its consumption within a tissue slice or a given cell geometry. These models are aimed at finding the shape of the Po2 profile in a flat sheet, sphere, or circular cylinder. The oxygen dependency of respiration is assumed to be constant (20) or possess a specific Michaelis-Menten (MM) kinetics (28, 33). The latter possibility (Eq. 11) is a good representation for the kinetics of mitochondrial respiration (53) sometimes being used with the caveat of “pseudo” MM kinetics. Many of the published models are presented in the form of numerical solutions and/or are applicable only to ideal geometric forms, which reduce their practical value for the analysis of experimental results. For this purpose, it is necessary to find a quantitative explanation, relating the properties of mitochondrial respiration (pseudo-MM kinetics) with a heterogeneous distribution of intracellular oxygen, which leads to a sigmoidal curve describing the collective oxygen dependency.
We have presented a curve of the collective oxygen dependency as a product of the oxygen consumption kinetics of individual oxygen sinks (pseudo-MM) and the cell volume distribution on the basis of Po2 isobars, given by a simple probability density function. This approach allowed us to describe the heterogeneity of the oxygen distribution inside a cell with two parameters: the Po2 on the cell surface P and the width of the intracellular Po2 distribution Δ, which arose from the combined diffusion and chemical reaction inside the cell. P and Δ have relatively straightforward physiological meanings and they can be converted into statistical moments of the intracellular Po2 distribution. We aimed to obtain fitting functions (Eqs. 14–16) that could be applied to experimental data to recover the parameters P and Δ and predict the shape of the oxygen dependency for oxygen consumption in a skeletal muscle. This approach has the potential to be extended to form a histogram-like model, in which several Uniform distributions with different weighting coefficients can be recovered by fitting the experimental points of the ODC. The first attempts at direct measurements of Po2 distributions within cardiomyocytes (31) showed that the distribution of mitochondrial Po2 may depend on the fraction of oxygen in the inspired gas mixture, and, therefore, knowledge of the characteristics of this distribution are necessary for understanding the functional state of cells.
To establish the validity of the Uniform distribution to describe the heterogeneity of intracellular Po2, let us compare the radial profiles of Po2 in the case of a muscle fiber in the form of a circular cylinder of radius R. As a simple example, we consider the conventional case of constant, uniform oxygen consumption V̇o2 and p(r) > 0 throughout the fiber. For this situation, the radial dependence of Po2 is:
| (17) |
where Do2 is the diffusion coefficient and α is the solubility of oxygen. From this equation, the volume fraction of the fiber contained within radius r is related to Po2 at this radius by
| (18) |
where the Po2 at the center of the fiber (r = 0) is Pc = P − V̇o2R2/4Do2α. The Po2 volume density function, f(p), for this situation is given by its definition, f(p) = 4α Do2/V̇o2R2. Note that the right hand side of this equation is 1/(P − Pc) or 1/Δ. This is exactly the value of f(p) used for the Uniform distribution in Eq. 12. For the MM kinetics used to describe the Po2 dependence of mitochondrial oxygen consumption in our model (Eq. 11), the Po2 profile will still be parabolic to a good approximation and thus the Uniform distribution given by f(p) = 1/Δ will be appropriate.
A parabolic profile is the typical result for oxygen diffusion/consumption in a cylindrical fiber (20) and has been repeatedly confirmed in experiments on isolated muscle cells (47, 48). However, the observation of a parabolic Po2 profile does not necessarily require correspondence with Hill's model (20) in which the oxygen consumption by elementary cell volumes is independent of the Po2. The diffusion coefficient Do2 can be calculated according to Hill's model as (4, 20, 45):
| (19) |
Calculations based on the values of parameters at the critical Po2 (Table 1) gives Do2 = 0.25·10−6 cm2/s, which is much smaller than literature values (2, 5, 30). An explanation of this discrepancy lies in the inapplicability of Hill's model to the situation in real cells in which respiration is dependent on oxygen tension over wide limits (53, 56). This wider Po2 dependency range is described by Wilson et al. (53, 56) such that changes in the concentrations of various intracellular metabolic factors work together to maintain a relatively constant oxygen consumption in the face of decreasing Po2. However, below a critical Po2, changes in the concentrations of these substances are not able to work together to maintain oxygen consumption and it begins to fall. An additional factor to consider is the significant difference between the shape of muscle cells and a circular cylinder. Replacing the square of the radius by the cross-sectional area (45, 51) in the calculation of the diffusion coefficient using Hill's model is incorrect.
It should be noted that the proposed model is shape-independent and based on the assumption of intracellular heterogeneity in Po2, which can be described by a Uniform distribution defined by the two parameters P and Δ. Mathematical solutions of the model formulated by Eq. 12 for the three situations of cellular oxygenation–normoxic, hypoxic, and critical–are represented by the sigmoidal composite curve consisting of two regions connected at the point of critical Po2. On a double-logarithmic plot, the low Po2 region (Eq. 15) appears as a straight line in contrast to the hyperbolic region (Eq. 14; Fig. 4, right). Remarkably, this property of the oxygen dependence curves was discovered earlier and used to determine the critical Po2 in experiments with isolated muscle cells (4). The resulting Eqs. 14–16 do not have a formal resemblance to Hill's equation, although the resulting sigmoidal curves are obviously similar to it but depend only on the difference of Po2 between the surface and center of the muscle fibers (Fig. 4). By accounting for the oxygen dependence of mitochondrial respiration, we obtained a description of their collective effect at the cellular level, which somewhat changes the understanding of critical Po2 and the oxygen dependency of cellular respiration. The actual critical Po2, corresponding to zero Po2 at the cell core, can be even lower than P50 for small Δ, although the oxygen dependence of respiration extends to much higher Po2 (see Fig. 5).
The parameters recovered by fitting the experimental oxygen dependency curves (i.e., V̇o2 vs. Po2) with Eqs. 14–16 are presented in Table 1. Relatively small differences were observed in the asymptotic values of VM from the two models we considered (121 nl O2/cm3·s from Eq. 10 and 139 nl O2/cm3·s from Eqs. 14–16). Practically no differences were found between the P50 = 11.1 mmHg obtained for muscle fibers using Hill's equation (Eq. 10) and k = 10.5 mmHg for mitochondrial respiration. It is well known that the P50 for coupled isolated mitochondria under a sufficient concentration of ATP is ∼0.5–1 mmHg, while in the presence of an uncoupler, P50 is <0.03 mmHg (14, 60). It has also been shown that diffusion limitations approximately double the value of P50 in isolated cells (26, 39, 53). The oxygen dependence of respiration in isolated mitochondria and cells is usually studied with vigorous stirring to reduce the contribution of diffusion resistance (14, 60). For cells in organs and tissues, convective effects are limited to blood flow through nearby microvessels, while both interstitial fluid and sarcoplasm are essentially stationary in a resting striated muscle. There is a possibility that the P50 value is dependent on the diffusional resistance to oxygen transport between the capillary to mitochondria, and this may be part of the explanation as to why the oxygen dependence of respiration extends to >30 mmHg (53, 55). The question of the extent to which diffusion of oxygen determines the oxygen dependence of cellular respiration in situ is extremely important, but poorly understood.
The Po2 difference, Δ, and critical Po2 estimated with Eqs. 14 and 15 yielded close results and all six pairs of values are well correlated. In this regard, one may consider the possible distortion of the curve of oxygen dependency through interference caused by the presence of myoglobin. Due to the very low P50 for myoglobin (2.39 mmHg at 37°C and pH = 7.0; Ref. 41), it is highly saturated at normal Po2, so that the effect on oxygen dependency should occur only at low Po2. If the effect of myoglobin is not negligible, then the difference in the observed values of Δ1 and Δ2 would be expected to be significant, but they are not (Table 1). The final resolution of this issue will require experiments in which the influence of muscle myoglobin has been eliminated; however, close agreement between Δ1 and Δ2 indicates the marginal impact of myoglobin in the spinotrapezius muscle.
The definitions of critical Po2 are different for mitochondria and cells. The contribution of diffusion resistance to Pcrit in isolated mitochondria is negligible due to their small size, while for the whole cell it can be the determining factor at a high level of metabolism. In a muscle, a sharp increase in NADH fluorescence reports mitochondrial anoxia, while an abrupt change in the rate of decline of extracellular Po2 corresponds to Pcrit for the myocytes (35, 36). Critical oxygen tension in the cells of the spinotrapezius muscle was measured in isolated cells and in situ, and Pcrit in isolated cells was 1.25 mmHg, somewhat lower than the in situ value of 2.9 mmHg (35, 36). According to our data, a Pcrit of 4 to 5 mmHg is close to these values but too low for involvement of the critical Po2 in oxygen sensing by resting myocytes. However, due to diffusion limitations, the oxygen dependency of respiration extends to the range of physiological oxygen pressure in the interstitium (53, 56) or 53 mmHg in the present study. It should be noted that the sensitivity of the respiratory rate to oxygen is small at this Po2, but it may increase with increasing intracellular differences of Po2 (right shift in Fig. 4) caused by an augmentation in metabolic activity or cell diameter.
Taking advantage of the range of variability of the parameters obtained in six muscles, we assessed the connections among them and found that VM and k are strongly correlated. This correlation indicates a self-similarity of the oxygen dependence curves for various rates of metabolism. In that case, curves with different VM are located to the right of the line passing through the origin with а slope equal to the diffusion resistance of the cell (V̇o2/Po2). Given the small number of muscles studied, this relationship can be considered only hypothetically possible. Later, this phenomenon can be studied with greater precision, considering the ability of muscles to increase their maximum oxygen consumption many fold. In the proposed model, VM is assumed to be the same for different values of Δ, which simplifies the analysis but limits its applicability. Clearly, a significant increase in Δ is the result of increased respiration rate, and, in the analysis of future experiments with stimulated oxygen consumption, the physical relationship between VM and Δ will be taken into account.
In conclusion, we have developed an approach to study the oxygen dependence of respiration in a skeletal muscle in situ, using Po2 measurements in interstitial fluid made with phosphorescence quenching microscopy and rapid pneumatic compression of the tissue. The metabolic component of the oxygen disappearance curve was used to construct a plot of oxygen dependency of cell respiration, which was analyzed using a model for oxygen consumption developed for the situation of heterogeneous Po2. The model predicted a number of properties for the oxygen dependence of cellular respiration associated with the existence of a respiratory-induced Po2 gradient in cells: 1) the dependence has a sigmoidal shape with an increasing rightward P50 shift with increasing intracellular Po2 gradient; 2) the dependence is described by two different functions, which represent normoxic and hypoxic regions of the model, the graphs of which are connected at the point for the critical Po2 of the cell; and 3) at physiological values of the intracellular Po2 gradient, the critical Po2 for the cells is below their P50.
Above the critical Po2 or critical oxygen delivery, as usually understood, most published studies demonstrate that oxygen consumption is independent of oxygen delivery. Our analysis showed that, although the critical cellular Po2 is much lower than the physiological oxygen tension in the interstitium for resting muscle, the oxygen dependency of cellular respiration may reach high Po2 values. To what extent the respiratory oxygen dependency of muscle fibers determines their ability to serve as oxygen sensors in the regulation of oxygen delivery can be established in future experiments applying this novel method to the situation of enhanced oxygen consumption caused by muscle stimulation and uncoupling of oxidative phosphorylation.
GRANTS
This research is supported by National Heart, Lung, and Blood Institute Grants HL-18292 and HL-79087.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S.G. conception and design of research; A.S.G. performed experiments; A.S.G. analyzed data; A.S.G. and R.N.P. interpreted results of experiments; A.S.G. prepared figures; A.S.G. drafted manuscript; A.S.G. and R.N.P. edited and revised manuscript; A.S.G. and R.N.P. approved final version of manuscript.
REFERENCES
- 1.Bailey JK, Kindig CA, Behnke BJ, Musch TI, Schmid-Schoenbein GW, Poole DC. Spinotrapezius muscle microcirculatory function: effects of surgical exteriorization. Am J Physiol Heart Circ Physiol 279: H3131–H3137, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Baranov VI, Belichenko VM, Shoshenko CA. Oxygen diffusion coefficient in isolated chicken red and white skeletal muscle fibers in ontogenesis. Microvasc Res 60: 168–176, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC. Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol 133: 229–239, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Belichenko VM, Baranov VI, Novosel'tsev SV, Shoshenko KA. [Coefficient of oxygen diffusion in fibers of the skeletal muscles]. Aviakosm Ekolog Med 36: 31–38, 2002 [PubMed] [Google Scholar]
- 5.Bentley TB, Meng H, Pittman RN. Temperature dependence of oxygen diffusion and consumption in mammalian striated muscle. Am J Physiol Heart Circ Physiol 264: H1825–H1830, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Boegehold MA, Johnson PC. Periarteriolar and tissue Po2 during sympathetic escape in skeletal muscle. Am J Physiol Heart Circ Physiol 254: H929–H936, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Brown GC. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J 284: 1–13, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356: 295–298, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Buerk DG, Nair PK, Bridges EW, Hanley TR. Interpretation of oxygen disappearance curves measured in blood perfused tissues. Adv Exp Med Biol 200: 151–161, 1986 [DOI] [PubMed] [Google Scholar]
- 10.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217: 383–393, 1955 [PubMed] [Google Scholar]
- 11.Chandel NS, Schumacker PT. Cellular oxygen sensing by mitochondria: old questions, new insight. J Appl Physiol 88: 1880–1889, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption. II. Molecular mechanism and tissue physiology. Am J Physiol Cell Physiol 292: C1993–C2003, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Gnaiger E. Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir Physiol 128: 277–297, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Gnaiger E, Steinlechner-Maran R, Mendez G, Eberl T, Margreiter R. Control of mitochondrial and cellular respiration by oxygen. J Bioenerg Biomembr 27: 583–596, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Golub AS, Barker MC, Pittman RN. Po2 profiles near arterioles and tissue oxygen consumption in rat mesentery. Am J Physiol Heart Circ Physiol 293: H1097–H1106, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Golub AS, Pittman RN. Po2 measurements in the microcirculation using phosphorescence quenching microscopy at high magnification. Am J Physiol Heart Circ Physiol 294: H2905–H2916, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Golub AS, Pittman RN. Thermostatic animal platform for intravital microscopy of thin tissues. Microvasc Res 66: 213–217, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Golub AS, Tevald MA, Pittman RN. Phosphorescence quenching microrespirometry of skeletal muscle in situ. Am J Physiol Heart Circ Physiol 300: H135–H143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray SD. Rat spinotrapezius muscle preparation for microscopic observation of the terminal vascular bed. Microvasc Res 5: 395–400, 1973 [DOI] [PubMed] [Google Scholar]
- 20.Hill AV. The diffusion of oxygen and lactic acid through tissues. Proc Royal Soc London 104: 39–96, 1928 [Google Scholar]
- 21.Hill AV. On the time required for diffusion and its relation to processes in muscle. Proc Royal Soc London 135: 446–453, 1948 [Google Scholar]
- 22.Hill DK. Oxygen tension and the respiration of resting frog's muscle. J Physiol 107: 479–495, 1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan MC. Phosphorescence quenching method for measurement of intracellular Po2 in isolated skeletal muscle fibers. J Appl Physiol 86: 720–724, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Jones DP, Kennedy FG. Analysis of intracellular oxygenation of isolated adult cardiac myocytes. Am J Physiol Cell Physiol 250: C384–C390, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Kempner W. Effect of oxygen tension on cellular metabolism. J Cell Comp Physiol 10: 339–363, 1937 [Google Scholar]
- 26.Kennedy FG, Jones DP. Oxygen dependence of mitochondrial function in isolated rat cardiac myocytes. Am J Physiol Cell Physiol 250: C374–C383, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Lash JM, Bohlen HG. Perivascular and tissue Po2 in contracting rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 252: H1192–H1202, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Lin SH. Oxygen diffusion in a spherical cell with nonlinear oxygen uptake kinetics. J Theor Biol 60: 449–457, 1976 [DOI] [PubMed] [Google Scholar]
- 29.Longmuir IS. Respiration rate of rat-liver cells at low oxygen concentrations. Biochem J 65: 378–382, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahler M, Louy C, Homsher E, Peskoff A. Reappraisal of diffusion, solubility, and consumption of oxygen in frog skeletal muscle, with applications to muscle energy balance. J Gen Physiol 86: 105–134, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mik EG, Ince C, Eerbeek O, Heinen A, Stap J, Hooibrink B, Schumacher CA, Balestra GM, Johannes T, Beek JF, Nieuwenhuis AF, van Horssen P, Spaan JA, Zuurbier CJ. Mitochondrial oxygen tension within the heart. J Mol Cell Cardiol 46: 943–951, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Poole DC, Wagner PD, Wilson DF. Diaphragm microvascular plasma Po2 measured in vivo. J Appl Physiol 79: 2050–2057, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Popel AS. Diffusion in tissue slices with metabolism obeying Michaelis-Menten kinetics. J Theor Biol 80: 325–332, 1979 [DOI] [PubMed] [Google Scholar]
- 34.Reneau DD, Halsey JH., Jr Interpretation of oxygen disappearance rates in brain cortex following total ischaemia. Adv Exp Med Biol 94: 189–198, 1977 [DOI] [PubMed] [Google Scholar]
- 35.Richmond KN, Burnite S, Lynch RM. Oxygen sensitivity of mitochondrial metabolic state in isolated skeletal and cardiac myocytes. Am J Physiol Cell Physiol 273: C1613–C1622, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Richmond KN, Shonat RD, Lynch RM, Johnson PC. Critical Po2 of skeletal muscle in vivo. Am J Physiol Heart Circ Physiol 277: H1831–H1840, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Robiolio M, Rumsey WL, Wilson DF. Oxygen diffusion and mitochondrial respiration in neuroblastoma cells. Am J Physiol Cell Physiol 256: C1207–C1213, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol 97: 384–392, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem 265: 15392–15402, 1990 [PubMed] [Google Scholar]
- 40.Scandurra FM, Gnaiger E. Cell respiration under hypoxia: facts and artefacts in mitochondrial oxygen kinetics. Adv Exp Med Biol 662: 7–25, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Schenkman KA, Marble DR, Burns DH, Feigl EO. Myoglobin oxygen dissociation by multiwavelength spectroscopy. J Appl Physiol 82: 86–92, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Shen W, Hintze TH, Wolin MS. Nitric oxide. An important signaling mechanism between vascular endothelium and parenchymal cells in the regulation of oxygen consumption. Circulation 92: 3505–3512, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Shibata M, Ichioka S, Ando J, Kamiya A. Microvascular and interstitial Po2 measurements in rat skeletal muscle by phosphorescence quenching. J Appl Physiol 91: 321–327, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Smith LM, Golub AS, Pittman RN. Interstitial PO(2) determination by phosphorescence quenching microscopy. Microcirculation 9: 389–395, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Stary CM, Hogan MC. Effect of varied extracellular Po2 on muscle performance in Xenopus single skeletal muscle fibers. J Appl Physiol 86: 1812–1816, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Steinlechner-Maran R, Eberl T, Kunc M, Margreiter R, Gnaiger E. Oxygen dependence of respiration in coupled and uncoupled endothelial cells. Am J Physiol Cell Physiol 271: C2053–C2061, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Takahashi E, Asano K. Mitochondrial respiratory control can compensate for intracellular O2 gradients in cardiomyocytes at low Po2. Am J Physiol Heart Circ Physiol 283: H871–H878, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Takahashi E, Doi K. Impact of diffusional oxygen transport on oxidative metabolism in the heart. Jpn J Physiol 48: 243–252, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Torres Filho IP, Intaglietta M. Microvessel Po2 measurements by phosphorescence decay method. Am J Physiol Heart Circ Physiol 265: H1434–H1438, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Torres Filho IP, Leunig M, Yuan F, Intaglietta M, Jain RK. Noninvasive measurement of microvascular and interstitial oxygen profiles in a human tumor in SCID mice. Proc Natl Acad Sci USA 91: 2081–2085, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Laarse WJ, des Tombe AL, van Beek-Harmsen BJ, Lee-de Groot MB, Jaspers RT. Krogh's diffusion coefficient for oxygen in isolated Xenopus skeletal muscle fibers and rat myocardial trabeculae at maximum rates of oxygen consumption. J Appl Physiol 99: 2173–2180, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Vanderkooi JM, Maniara G, Green TJ, Wilson DF. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem 262: 5476–5482, 1987 [PubMed] [Google Scholar]
- 53.Wilson DF. Contribution of diffusion to the oxygen dependence of energy metabolism in cells. Experientia 46: 1160–1162, 1990 [DOI] [PubMed] [Google Scholar]
- 54.Wilson DF. Quantifying the role of oxygen pressure in tissue function. Am J Physiol Heart Circ Physiol 294: H11–H13, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Wilson DF, Erecinska M. Effect of oxygen concentration on cellular metabolism. Chest 88: 229S–232S, 1985 [DOI] [PubMed] [Google Scholar]
- 56.Wilson DF, Erecinska M, Drown C, Silver IA. The oxygen dependence of cellular energy metabolism. Arch Biochem Biophys 195: 485–493, 1979 [DOI] [PubMed] [Google Scholar]
- 57.Wilson DF, Lee WM, Makonnen S, Apreleva S, Vinogradov SA. Oxygen pressures in the interstitial space of skeletal muscle and tumors in vivo. Adv Exp Med Biol 614: 53–62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson DF, Lee WM, Makonnen S, Finikova O, Apreleva S, Vinogradov SA. Oxygen pressures in the interstitial space and their relationship to those in the blood plasma in resting skeletal muscle. J Appl Physiol 101: 1648–1656, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Wilson DF, Owen CS, Erecinska M. Quantitative dependence of mitochondrial oxidative phosphorylation on oxygen concentration: a mathematical model. Arch Biochem Biophys 195: 494–504, 1979 [DOI] [PubMed] [Google Scholar]
- 60.Wilson DF, Rumsey WL, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem 263: 2712–2718, 1988 [PubMed] [Google Scholar]
- 61.Wilson DF, Vanderkooi JM, Green TJ, Maniara G, DeFeo SP, Bloomgarden DC. A versatile and sensitive method for measuring oxygen. Adv Exp Med Biol 215: 71–77, 1987 [DOI] [PubMed] [Google Scholar]
- 62.Zheng L, Golub AS, Pittman RN. Determination of Po2 and its heterogeneity in single capillaries. Am J Physiol Heart Circ Physiol 271: H365–H372, 1996 [DOI] [PubMed] [Google Scholar]