In 65 121 human immunodeficiency virus–positive individuals from high-income countries, combined antiretroviral therapy (cART) reduced tuberculosis incidence by half. However, increases in tuberculosis incidence shortly after cART, especially in those >50 years old and those with CD4 cell counts <50 cells/μL, suggested unmasking immune reconstitution inflammatory syndrome.
Abstract
Background. The lower tuberculosis incidence reported in human immunodeficiency virus (HIV)–positive individuals receiving combined antiretroviral therapy (cART) is difficult to interpret causally. Furthermore, the role of unmasking immune reconstitution inflammatory syndrome (IRIS) is unclear. We aim to estimate the effect of cART on tuberculosis incidence in HIV-positive individuals in high-income countries.
Methods. The HIV-CAUSAL Collaboration consisted of 12 cohorts from the United States and Europe of HIV-positive, ART-naive, AIDS-free individuals aged ≥18 years with baseline CD4 cell count and HIV RNA levels followed up from 1996 through 2007. We estimated hazard ratios (HRs) for cART versus no cART, adjusted for time-varying CD4 cell count and HIV RNA level via inverse probability weighting.
Results. Of 65 121 individuals, 712 developed tuberculosis over 28 months of median follow-up (incidence, 3.0 cases per 1000 person-years). The HR for tuberculosis for cART versus no cART was 0.56 (95% confidence interval [CI], 0.44–0.72) overall, 1.04 (95% CI, 0.64–1.68) for individuals aged >50 years, and 1.46 (95% CI, 0.70–3.04) for people with a CD4 cell count of <50 cells/μL. Compared with people who had not started cART, HRs differed by time since cART initiation: 1.36 (95% CI, 0.98–1.89) for initiation <3 months ago and 0.44 (95% CI, 0.34–0.58) for initiation ≥3 months ago. Compared with people who had not initiated cART, HRs <3 months after cART initiation were 0.67 (95% CI, 0.38–1.18), 1.51 (95% CI, 0.98–2.31), and 3.20 (95% CI, 1.34–7.60) for people <35, 35–50, and >50 years old, respectively, and 2.30 (95% CI, 1.03–5.14) for people with a CD4 cell count of <50 cells/μL.
Conclusions. Tuberculosis incidence decreased after cART initiation but not among people >50 years old or with CD4 cell counts of <50 cells/μL. Despite an overall decrease in tuberculosis incidence, the increased rate during 3 months of ART suggests unmasking IRIS.
Tuberculosis is the most common AIDS-defining condition worldwide [1, 2]. The use of combined antiretroviral therapy (cART) against human immunodeficiency virus (HIV) is one of the strategies recommended by the World Health Organization (WHO) to prevent tuberculosis [1–3]. However, cART initiation may lead to immune reconstitution inflammatory syndrome (IRIS), a poorly understood immunological phenomenon [4, 5] that is particularly frequent in patients infected by Mycobacterium tuberculosis. IRIS may lead to either the paradoxical worsening of previously treated tuberculosis or the “unmasking” of subclinical tuberculosis for up to 3 months following cART initiation [4, 5]. Inflammatory signs of unmasked tuberculosis may range from mild to severe, the latter being IRIS in sensu stricto [5].
A randomized clinical trial in Haiti [6] and observational studies from low-income [7, 8], middle-income [9–11], and high-income [12–20] settings have found lower tuberculosis incidence among HIV-positive patients receiving cART. However, none of these studies differentiated between short- and long-term effects of cART, most included prevalent cART users, and all but 2 [8, 20] did not appropriately adjust for measured time-dependent confounders.
We estimated the effect of cART initiation on tuberculosis incidence in HIV-positive individuals in a large collaboration from Europe and the United States. To overcome some of the limitations of previous studies, we restricted our analyses to cART-naive patients and used inverse probability weighting to appropriately adjust for measured confounders. We present these estimates stratified by time since cART initiation, to explore short-term increases in tuberculosis incidence compatible with unmasking IRIS. For comparison purposes, we estimated the effect of cART on Pneumocystis jirovecii pneumonia (PCP), a condition for which cART has been shown to be protective [12] and for which IRIS is infrequent [5].
METHODS
Study Population
The HIV-CAUSAL Collaboration has been described elsewhere [21]. Briefly, the collaboration includes 12 prospective cohort studies from 6 European countries and the United States: UK CHIC (United Kingdom), UK Register of HIV Seroconverters (United Kingdom), ATHENA (the Netherlands), FHDH-ANRS CO4 (France), ANRS PRIMO (France), ANRS-SEROCO (France), SHCS (Switzerland), GEMES (Spain), PISCIS (Spain), CoRIS/CoRIS-MD (Spain), AMACS (Greece), and VACS-VC (United States). All cohorts are based on data collected for clinical purposes within healthcare systems with universal access to care.
The HIV-CAUSAL Collaboration includes 71743 HIV-positive antiretroviral-naive individuals aged ≥18 years. Analyses were restricted to those who (1) had adequate baseline data to adjust for confounding (having CD4 cell count and HIV RNA level measurements within 6 months of each other) and (2) had no diagnosis of an AIDS-defining illness (including tuberculosis) at baseline. In all, 188 patients were excluded because of condition 1 and 6434 because of condition 2, leaving 65 121 eligible patients.
An individual’s baseline was defined as the first time during 1996–2007 when all of the above criteria were met. An individual’s follow-up ended at tuberculosis diagnosis, death, 12 months after the most recent laboratory measurement, or cohort-specific administrative censoring (from December 2003 through September 2007), whichever occurred earliest. Our analyses were restricted to HIV-positive individuals who did not have a tuberculosis diagnosis and did not start cART during the first month after baseline.
Initiation of cART was defined as the date on which an individual initiated treatment with ≥3 antiretroviral drugs, 2 ritonavir-boosted protease inhibitors, or 1 nonnucleoside reverse-transcriptase inhibitor plus 1 boosted protease inhibitor. Alternative definitions of cART did not affect the overall effect estimate. PCP prophylaxis was generally initiated when the CD4 cell count dropped to <200 cells/μL, in accordance with international guidelines.
The primary outcome was tuberculosis diagnosis (pulmonary and extrapulmonary combined), although separate analyses for pulmonary tuberculosis and extrapulmonary tuberculosis were also conducted. Diagnostic procedures for tuberculosis in participating cohorts reflect standard clinical practice in Europe and the United States, with most diagnoses reported to be definite (generally defined as isolation of M. tuberculosis from a culture of any of the following clinical samples: sputum, bronchial washings, pleural fluid, lymph node, bone marrow aspirate, or cerebrospinal fluid) rather than presumptive (generally defined as clinical or radiological signs suggestive of tuberculosis, exclusion of other causes, and clinical response to tuberculosis treatment). Skin tests for M. tuberculosis infection were not systematically performed and isoniazid preventive treatment was not routinely implemented, reflecting clinical practice in most of Europe and the United States.
Statistical Methods
Incidence rates of tuberculosis were calculated as tuberculosis diagnoses per 1000 person-years. We estimated the average hazard ratio (HR) of tuberculosis for cART initiation versus noninitiation via a pooled logistic model for risk of tuberculosis at month m + 1 that included a time-varying indicator for ever use of cART through month m, month of follow-up m (restricted cubic splines with 5 knots), and the following baseline covariates: CD4 cell count (<50, 50–99, 100–199, 200–349, 350–499, or ≥500 cells/μL), HIV RNA level (<10 000, 10 000–100 000, or >100 000 copies/mL), sex, transmission group (heterosexual, men who have sex with men, injection drug users, or other/unknown), calendar year (1996–1998, 1999–2000, or 2001–2007), age (<35, 35–50, or >50 years), geographic origin (Western countries, sub-Saharan Africa, other countries, or unknown), time since HIV infection diagnosis (<3 or ≥3 months), and cohort. To estimate the HR as a function of time since cART initiation, we fit a separate model in which the indicator for cART use was replaced by a time-varying treatment variable with 3 categories: no cART initiation, <3 months after cART initiation, or ≥3 months after cART initiation. This cutoff was based in the consensus clinical case definition for tuberculosis-associated IRIS issued by the International Network for the Study of HIV-associated IRIS [22].
Because cART is more likely to be initiated in individuals with a low CD4 cell count and a high viral RNA level, estimates from the above regression models have to be adjusted for this time-dependent confounding by indication. To do so, one could add the time-varying confounders CD4 cell count and HIV RNA level as covariates in the logistic regression model. However, this standard approach may introduce bias because the confounders are affected by prior cART use and are on the causal pathway between cART and tuberculosis. We therefore used inverse probability weighting to adjust for measured time-dependent confounders that are affected by prior cART use. Formally, under the assumption that all time-varying predictors of both cART and tuberculosis were included in the analyses, our weighted model estimates the parameters of a marginal structural Cox model [23].
Each patient in the above logistic models received a time-varying weight inversely proportional to the probability of having their own observed history of cART initiation, as described elsewhere [24]. To estimate each patient’s probability of cART initiation in each month, we fit a pooled logistic model that included the covariates listed above for the outcome model plus the most recent measurement of the following time-varying covariates: CD4 cell count (restricted cubic splines with 6 knots), HIV RNA level (3 categories), AIDS (yes or no), and time since last laboratory measurement (5 categories). To better adjust for cohort-specific factors, the models also included separate product terms between cohort and time of follow-up, time-varying CD4 cell count, and HIV RNA level. Inverse probability weights were also computed to adjust for potential selection bias due to censoring by infrequent measurement. Both the cART initiation and censoring weights were stabilized and their product used to fit the weighted regression model. To avoid undue influence of outliers on the variance of the estimates, we truncated weights at a maximum value of 10. Truncation did not materially change the point estimates. The estimated weights used in the analyses had a mean of 1.04. We computed 95% confidence intervals (CIs) for the log HR of tuberculosis by adding and subtracting 1.96 times the standard error, where the standard error was the square root of a variance estimator that accounts for the weight-estimation procedure.
Several sensitivity analyses were also performed: (1) in addition to censoring individuals at 12 months without a laboratory measurement, we censored at 18 and 24 months after the last measurement; (2) the start of follow-up was delayed by 3 months to exclude early tuberculosis diagnoses (and thus ensure that only incident tuberculosis cases were included); (3) the weights were re-estimated by adding the rates of CD4 cell count and HIV RNA level change to the models; (4) the weights were re-estimated by lagging the CD4 cell count and HIV RNA level 7 or 14 days to ensure that cART initiation was predicted using prior laboratory measurements; and (5) additional inverse probability weights for censoring by death were estimated.
We fit stratum-specific weighted models to conduct subgroup analyses according to the following baseline characteristics: sex, age, HIV RNA level, CD4 cell count, transmission group, geographical origin, and year. For comparison purposes, we repeated the main analyses after replacing the outcome tuberculosis by PCP. We chose PCP because it is a frequent condition for which cART has been shown to be very effective and for which IRIS is infrequent. All analyses were conducted with SAS, version 9.2.
RESULTS
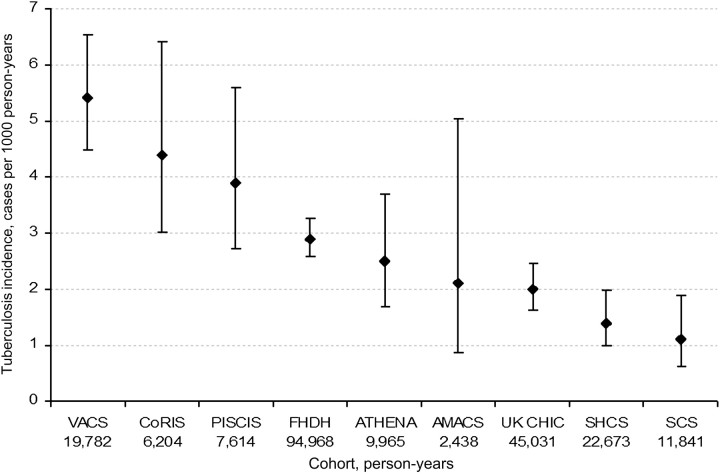
Our study included 65 121 HIV-positive individuals who met eligibility criteria from 1996 through 2007. The median follow-up was 28 months (interquartile range, 13–57 months). During follow-up, 712 individuals received a diagnosis of tuberculosis (493 had pulmonary and 296 had extrapulmonary tuberculosis) and 2275 individuals died. The overall tuberculosis incidence was 3.0 cases per 1000 person-years and varied by cohort (Figure 1).
Figure 1.
Tuberculosis incidence rates by cohort, HIV-CAUSAL Collaboration, 1996–2007. The countries (no. of eligible individuals) associated with each cohort are as follows: VACS, United States (7281; definite tuberculosis cases only are included); CoRIS, Spain (3198); PISCIS, Spain (2988); FHDH, France (26 089); ATHENA, Netherlands (4316); AMACS, Greece (750); UK CHIC, United Kingdom (13 183); SHCS, Switzerland (4510); and SCS, seroconverters from the UK Register of HIV Seroconverters in the United Kingdom, PRIMO/SEROCO in France, and GEMES in Spain (2806).
Tuberculosis incidence decreased with CD4 cell count and increased with viral load and age. Rates were similar for men and women, men who have sex with men had the lowest rates, and people of sub-Saharan African origin had about 4 times the rate of people born in Western countries (Table 1). Overall, the HR of tuberculosis for cART initiation versus no initiation was 0.56 (95% CI, 0.44–0.72). All HRs were <1 except for people whose baseline CD4 cell count was <50 cells/μL (HR, 1.46; 95% CI, 0.70–3.04) and for those aged >50 years (HR, 1.04; 95% CI, 0.64–1.68). The overall HR of pulmonary tuberculosis was 0.58 (95% CI, 0.44–0.77) and that of extrapulmonary tuberculosis was 0.45 (95% CI, 0.29–0.68).
Table 1.
Characteristics of Study Participants and Hazard Ratio of Tuberculosis for Combined Antiretroviral Therapy Initiation Versus No Initiation, HIV-CAUSAL Collaboration, 1996–2007
| Baseline Characteristic | Individuals, no. | Median Follow-up Time, mo (IQR) | Person-Years, no. | Tuberculosis Cases, no. | Tuberculosis Incidence, Cases Per 1000 Person-Years | cART Initiators, no. (%) | HR of Tuberculosisa (95% CI) |
| CD4 cell count, cells/μL | |||||||
| <50 | 2288 | 27 (11–59) | 7610 | 64 | 8.4 | 1809 (79) | 1.46 (0.70–3.04) |
| 50–99 | 2081 | 31 (13–64) | 7425 | 41 | 5.5 | 1712 (82) | 0.68 (0.28–1.63) |
| 100–199 | 6029 | 30 (13–62) | 21 298 | 104 | 4.9 | 4777 (79) | 0.39 (0.22–0.71) |
| 200–349 | 15 387 | 28 (13–58) | 52 974 | 199 | 3.8 | 10 340 (67) | 0.59 (0.37–0.94) |
| 350–499 | 16 759 | 28 (13–57) | 57 662 | 148 | 2.6 | 8133 (49) | 0.40 (0.26–0.63) |
| ≥500 | 22 577 | 26 (12–54) | 73 546 | 156 | 2.1 | 6972 (31) | 0.68 (0.43–1.08) |
| HIV RNA level, copies/mL | |||||||
| <10 000 | 20 479 | 25 (11–51) | 64 387 | 145 | 2.3 | 6877 (34) | 0.80 (0.48–1.35) |
| 10 000–100 000 | 29 870 | 28 (13–58) | 102 717 | 357 | 3.5 | 16, 252 (54) | 0.55 (0.40–0.75) |
| >100 000 | 14 772 | 31 (14–63) | 53 411 | 210 | 3.9 | 10 614 (72) | 0.51 (0.31–0.82) |
| Age, years | |||||||
| <35 | 28 522 | 29 (13–62) | 103 618 | 248 | 2.4 | 14 316 (50) | 0.33 (0.21–0.51) |
| 35–50 | 30 497 | 27 (12–54) | 96 774 | 368 | 3.8 | 15 759 (52) | 0.72 (0.53–1.00) |
| >50 | 6102 | 28 (12–56) | 20 123 | 96 | 4.8 | 3668 (60) | 1.04 (0.64–1.68) |
| Sex | |||||||
| Female | 16 157 | 27 (12–55) | 53 252 | 182 | 3.4 | 8526 (53) | 0.39 (0.24–0.63) |
| Male | 48 964 | 28 (13–58) | 167 263 | 530 | 3.2 | 25 217 (52) | 0.62 (0.46–0.83) |
| Transmission group | |||||||
| Heterosexual | 21 397 | 28 (13–56) | 71 923 | 284 | 3.9 | 11 718 (55) | 0.52 (0.36–0.77) |
| Men who have sex with men | 24 827 | 31 (14–63) | 91 643 | 95 | 1.0 | 12 591 (51) | 0.29 (0.13–0.61) |
| Injection drug users | 7175 | 24 (11–53) | 23 623 | 78 | 3.3 | 3314 (46) | 0.39 (0.18–0.86) |
| Other/unknown | 11 722 | 22 (11–47) | 33 326 | 255 | 7.7 | 6120 (53) | 0.89 (0.63–1.27) |
| Geographical origin | |||||||
| Western countries | 31 491 | 30 (14–62) | 114 999 | 206 | 1.8 | 16 235 (52) | 0.57 (0.35–0.91) |
| Sub-Saharan Africa | 8072 | 25 (12–50) | 23 799 | 169 | 7.1 | 4477 (55) | 0.50 (0.30–0.83) |
| Other countries | 4516 | 22 (11–45) | 12 264 | 51 | 4.2 | 2136 (47) | 0.81 (0.36–1.81) |
| Unknown | 21 042 | 27 (12–57) | 69 454 | 286 | 4.1 | 10 895 (52) | 0.61 (0.42–0.89) |
| Calendar period | |||||||
| 1996–1998 | 19 400 | 41 (16–106) | 95 665 | 258 | 2.7 | 11 109 (57) | 0.62 (0.40–0.98) |
| 1999–2000 | 8987 | 39 (15–83) | 36 192 | 134 | 3.7 | 5296 (59) | 0.53 (0.31–0.88) |
| 2001–2007 | 36 734 | 23 (11–42) | 88 658 | 320 | 3.6 | 17 338 (47) | 0.52 (0.37–0.74) |
| Overall | 65 121 | 28 (13–57) | 220 515 | 712 | 3.2 | 33 743 (52) | 0.56 (0.44–0.72) |
Abbreviations: cART, combined antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; IQR, interquartile range.
Estimated from a weighted pooled logistic model that included a time-varying indicator for ever use of cART, month of follow-up (restricted cubic splines with 5 knots), and the baseline covariates listed in the table. The inverse probability weights were a function of the baseline covariates and most recent measurement of the following time-varying covariates: CD4 cell count (restricted cubic splines with 6 knots), HIV RNA level (3 categories), AIDS (yes or no), and time since last laboratory measurement (5 categories).
The HRs for tuberculosis differed by time since cART initiation: 1.36 (95% CI, 0.98–1.89) <3 months after initiation and 0.44 (95% CI, 0.34–0.58) ≥3 months after initiation (Table 2). These estimates were similar for pulmonary or extrapulmonary tuberculosis: the HR for pulmonary tuberculosis was 1.31 (95% CI, 0.87–1.96) for <3 months and 0.47 (95% CI, 0.35–0.63) for ≥3 months, and the HR for extrapulmonary tuberculosis was 1.31 for <3 months (95% CI, 0.84–2.05) and 0.34 for ≥3 months (95% CI, 0.22–0.53). The corresponding estimates were similar when we excluded the cohort with the highest tuberculosis incidence (ie, VACS): 1.33 (95% CI, 0.91–1.94) for <3 months and 0.35 (95% CI, 0.24–0.49) for ≥3 months. The elevated tuberculosis risk <3 months after cART initiation was found in men with a baseline CD4 cell count of <50 cells/μL and HIV RNA load of <10 000 copies/mL. This early risk increased with age. Tuberculosis incidence was lower ≥3 months after cART initiation, compared with untreated individuals, in all subgroups except individuals with baseline CD4 cell count of <50 cells/μL (HR, 1.02; 95% CI, 0.44–2.36). Sensitivity analyses did not materially affect the estimates.
Table 2.
Hazard Ratio of Tuberculosis for Combined Antiretroviral Therapy Initiation Versus No Initiation by Time Since Initiation, HIV-CAUSAL Collaboration, 1996–2007
| No cART Initiation |
Time Since cART Initiation, <3 mo |
Time Since cART Initiation, ≥3 Mo |
||||
| Baseline Characteristic | Cases, no. | HRa | Cases, no. | HRa (95% CI) | Cases, no. | HRa (95% CI) |
| CD4 cell count, cells/μL | ||||||
| <50 | 14 | 1 (ref) | 15 | 2.30 (1.03–5.14) | 35 | 1.02 (0.44–2.36) |
| 50–99 | 10 | 1 (ref) | 8 | 1.17 (0.37–3.70) | 23 | 0.46 (0.22–0.99) |
| 100–199 | 37 | 1 (ref) | 14 | 0.94 (0.47–1.86) | 53 | 0.28 (0.15–0.54) |
| 200–349 | 88 | 1 (ref) | 21 | 1.50 (0.84–2.69) | 90 | 0.45 (0.27–0.75) |
| ≥350 | 195 | 1 (ref) | 10 | 0.70 (0.30–1.62) | 99 | 0.49 (0.35–0.70) |
| HIV RNA level, copies/mL | ||||||
| <10 000 | 92 | 1 (ref) | 8 | 2.05 (0.89–4.74) | 45 | 0.68 (0.39–1.17) |
| 10 000–100 000 | 173 | 1 (ref) | 26 | 1.27 (0.76–2.14) | 158 | 0.46 (0.33–0.64) |
| >100 000 | 79 | 1 (ref) | 34 | 1.10 (0.70–1.74) | 97 | 0.36 (0.20–0.62) |
| Age, years | ||||||
| <35 | 131 | 1 (ref) | 16 | 0.67 (0.38–1.18) | 101 | 0.29 (0.19–0.47) |
| 35–50 | 170 | 1 (ref) | 38 | 1.51 (0.98–2.31) | 160 | 0.59 (0.42–0.83) |
| >50 | 43 | 1 (ref) | 14 | 3.20 (1.34–7.60) | 39 | 0.59 (0.34–1.04) |
| Sex | ||||||
| Female | 96 | 1 (ref) | 11 | 0.65 (.33, 1.25) | 75 | 0.35 (0.21–0.59) |
| Male | 248 | 1 (ref) | 57 | 1.64 (1.13–2.37) | 225 | 0.47 (0.33–0.65) |
| Transmission group | ||||||
| Heterosexual | 138 | 1 (ref) | 30 | 1.35 (0.84–2.17) | 116 | 0.39 (0.26–0.60) |
| Men who have sex with men | 48 | 1 (ref) | 12 | 1.19 (0.46–3.12) | 35 | 0.20 (0.09–0.44) |
| Injection drug users | 41 | 1 (ref) | 7 | 1.23 (0.45–3.37) | 30 | 0.27 (0.13–0.60) |
| Other/unknown | 117 | 1 (ref) | 19 | 1.48 (0.85–2.56) | 119 | 0.79 (0.54–1.15) |
| Geographical origin | ||||||
| Western countries | 100 | 1 (ref) | 21 | 1.63 (0.86–3.10) | 85 | 0.42 (0.25–0.71) |
| Sub-Saharan Africa | 84 | 1 (ref) | 17 | 1.10 (0.55–2.17) | 68 | 0.39 (0.22–0.71) |
| Other countries | 26 | 1 (ref) | 6 | 1.70 (0.68–4.25) | 19 | 0.57 (0.23–1.42) |
| Unknown | 134 | 1 (ref) | 24 | 1.36 (0.83–2.22) | 128 | 0.52 (0.35–0.76) |
| Calendar period | ||||||
| 1996–1998 | 117 | 1 (ref) | 19 | 1.46 (0.81–2.64) | 122 | 0.53 (0.34–0.85) |
| 1999–2000 | 57 | 1 (ref) | 13 | 1.69 (0.77–3.71) | 64 | 0.37 (0.22–0.64) |
| 2001–2007 | 170 | 1 (ref) | 36 | 1.08 (0.69–1.69) | 114 | 0.41 (0.28–0.68) |
| Overall | 344 | 1 (ref) | 68 | 1.36 (0.98–1.89) | 300 | 0.44 (0.34–0.58) |
Abbreviations: cART, combined antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; ref, reference value.
Estimated from a weighted pooled logistic model that included a time-varying treatment variable with 3 categories (no cART initiation, <3 months after cART initiation, or ≥3 months after cART initiation), month of follow-up (restricted cubic splines with 5 knots), and the baseline covariates listed in the table. The inverse probability weights were a function of the baseline covariates and most recent measurement of the following time-varying covariates: CD4 cell count (restricted cubic splines with 6 knots), HIV RNA level (3 categories), AIDS (yes or no), and time since last laboratory measurement (5 categories).
During follow-up, 539 individuals received a diagnosis of PCP; the overall PCP incidence was 2.4 cases per 1000 person-years. The overall HR of PCP was 0.13 (95% CI, 0.09–0.20) for cART initiation versus no initiation; the HR varied by time since initiation within 3 months (HR, 0.48; 95% CI, 0.31–0.75) and after 3 months (HR, 0.10; 95% CI, 0.07–0.16), but not by age and CD4 cell count (Table 3). PCP prophylaxis was received by 24% of individuals; further adjustment for prophylaxis therapy did not materially change the results.
Table 3.
Hazard Ratio of Pneumocystis jirovecii Pneumonia for Combined Antiretroviral Therapy Initiation Versus No Initiation by Time Since Initiation and Selected Characteristics, HIV-CAUSAL Collaboration, 1996–2007
| No cART Initiation |
<3 Months Since cART Initiation |
≥3 Months After cART Initiation |
||||
| Baseline Characteristic | No. of Cases | HRa | No. of Cases | HRa (95% CI) | No. of Cases | HRa (95% CI) |
| CD4 cell count, cells/μL | ||||||
| <50 | 57 | 1 (ref) | 9 | 0.32 (0.15–0.67) | 38 | 0.30 (0.17–0.51) |
| 50–99 | 26 | 1 (ref) | 5 | 0.33 (0.12–0.92) | 31 | 0.18 (0.06–0.40) |
| 100–199 | 43 | 1 (ref) | 7 | 0.60 (0.22–1.64) | 40 | 0.19 (0.10–0.39) |
| 200–349 | 59 | 1 (ref) | 8 | 0.62 (0.25–1.58) | 46 | 0.06 (0.03–0.14) |
| ≥350 | 121 | 1 (ref) | 4 | 0.22 (0.07–0.65) | 45 | 0.16 (0.09–0.28) |
| HIV RNA level, copies/mL | ||||||
| <10 000 | 44 | 1 (ref) | 3 | 0.81 (0.25–2.67) | 23 | 0.31 (0.13–0.75) |
| 10 000–100,000 | 126 | 1 (ref) | 13 | 0.64 (0.31–1.31) | 74 | 0.07 (0.04–0.13) |
| >100 000 | 136 | 1 (ref) | 17 | 0.37 (0.20–0.67) | 103 | 0.15 (0.09–0.25) |
| Age, years | ||||||
| <35 | 96 | 1 (ref) | 9 | 0.43 (0.20–0.95) | 72 | 0.15 (0.08–0.29) |
| 35–50 | 166 | 1 (ref) | 21 | 0.47 (0.26–0.84) | 99 | 0.08 (0.05–0.14) |
| >50 | 44 | 1 (ref) | 3 | 0.51 (0.14–1.93) | 29 | 0.13 (0.05–0.32) |
| Sex | ||||||
| Female | 49 | 1 (ref) | 6 | 0.50 (0.17–1.46) | 27 | 0.04 (0.02–0.10) |
| Male | 257 | 1 (ref) | 27 | 0.48 (0.29–0.77) | 173 | 0.12 (0.08–0.19) |
| Overall | 306 | 1 (ref) | 33 | 0.48 (0.31–0.75) | 200 | 0.10 (0.07–0.16) |
Abbreviations: cART, combined antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; ref, reference value.
Estimated from a weighted pooled logistic model that included a time-varying treatment variable with 3 categories (no cART initiation, <3 months after cART initiation, or ≥3 months after cART initiation), month of follow-up (restricted cubic splines with 5 knots), and the baseline covariates. The inverse probability weights were a function of the baseline covariates and most recent measurement of the following time-varying covariates: CD4 cell count (restricted cubic splines with 6 knots), HIV RNA level (3 categories), AIDS (yes or no), and time since last laboratory measurement (5 categories).
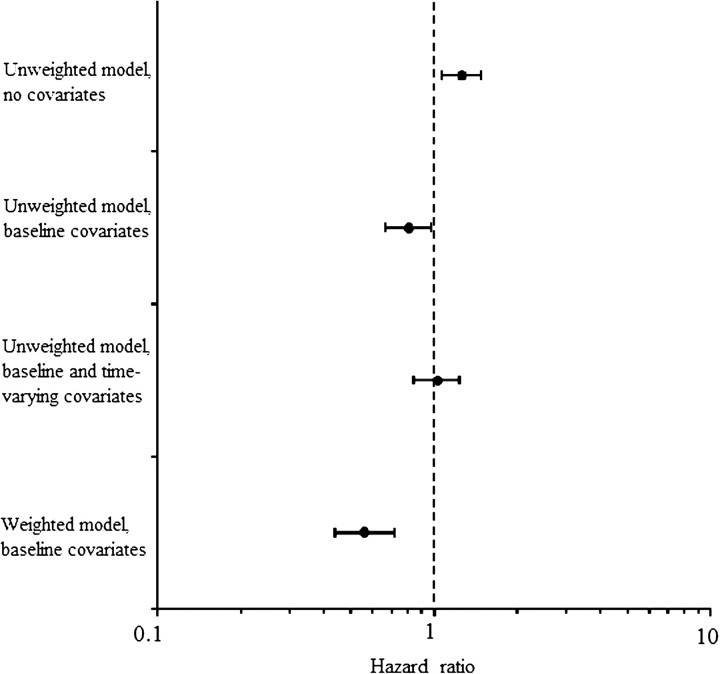
The probability of cART initiation was inversely related to CD4 cell count and directly related to HIV RNA levels. Compared with CD4 cell count of ≥500 cells/μL, the HR for cART initiation was 1.11, 2.7, 8.3, and 14.3 for CD4 cell count in the range 350–499, 200–349, 100–199, and <100 cells/μL, respectively. Compared with an HIV RNA level of <10000 copies/mL, the HR for cART initiation was 1.4 and 2.2 for HIV RNA levels of 10 000–100 000 and >100 000 copies/mL, respectively. Because CD4 cell count and HIV RNA level are affected by prior cART use, conventional methods for confounding adjustment are expected to result in biased estimates. As an illustration of this bias, Figure 2 shows the HR of tuberculosis from unweighted Cox models that adjusted for baseline covariates only (HR, 0.81; 95% CI, 0.67–0.97) and for both baseline and time-dependent covariates (HR, 1.03; 95% CI, 0.86–1.24).
Figure 2.
Hazard ratios of tuberculosis from conventional (unweighted) and inverse probability weighted models, HIV-CAUSAL Collaboration, 1996–2007.
DISCUSSION
This study shows a reduction of 44% in tuberculosis incidence among HIV-positive individuals who start cART in high-income countries. However, we found no evidence of reduction among those >50 years old or with CD4 cell counts <50 cells/μL. Despite the net lower risk of tuberculosis after cART initiation, tuberculosis incidence increased by 36% <3 months after cART initiation. This increased early risk of tuberculosis was higher in people with baseline CD4 cell counts of <50 cells/μL, in men, and in individuals ≥35 years old. An early increase in the risk of PCP among people receiving cART was not observed in any subgroup.
The reduction in tuberculosis incidence attributable to cART found in our study is closer to the null than most previously reported estimates from observational studies in other settings [7, 12–15, 19], possibly because of the selection criteria we imposed to avoid inadvertent classification of prevalent tuberculosis as incident tuberculosis. Individuals who received a diagnosis of tuberculosis (206 cases) within the first month of follow-up were excluded to prevent the inclusion of patients with prevalent tuberculosis cases who would have otherwise been assigned to the untreated group, overestimating the beneficial effect of cART. Indeed, including these cases resulted in HRs of tuberculosis of 1.02 (95% CI, 0.79–1.32) <3 months after cART initiation and 0.43 (95% CI, 0.34–0.54) ≥3 months after cART initiation, compared with no cART initiation.
Our study did not find a lower risk of tuberculosis after cART initiation among people with a baseline CD4 cell count of ≤50 cells/μL and those who were aged >50 years. This could be due to a higher tuberculosis incidence in the first 3 months following cART or to impaired immunological responses to M. tuberculosis in very immunosuppressed patients who start cART. We cannot distinguish between incident tuberculosis, unmasking tuberculosis IRIS, and unmasking tuberculosis without IRIS because individual tuberculosis diagnoses could not be assessed to confirm which individuals presented exaggerated inflammatory responses. However, the epidemiological pattern suggests that unmasking IRIS may be playing a role [4, 5, 25, 26]. A meta-analysis [5] found that the incidence of tuberculosis-associated paradoxical worsening IRIS is highest in individuals with a CD4 cell count <50 cells/μL, but data on unmasking IRIS are scarce. In contrast, a randomized trial in Haiti did not find an increased tuberculosis incidence after cART initiation, but all participants were screened for M. tuberculosis infection and started treatment with isoniazid if clinically indicated [6]. Lawn et al [27] have recently reported that an intensive screening strategy before cART initiation markedly decreased the incidence of tuberculosis and IRIS.
After the first three months, overall tuberculosis incidence was 56% lower in the people on cART than in people not on cART, although no decrease was observed in individuals with baseline CD4 cell counts <50 cells/μL. Our findings suggest that very immunosuppressed individuals cannot lower their overall risk of tuberculosis by taking cART for ≥3 months. These patients remain in a state of relevant immunodeficiency in spite of cART for longer periods and are therefore at higher risk of reactivating latent tuberculosis beyond 3 months after starting cART. Further follow-up of these cohorts will show whether the risk of tuberculosis does eventually drop with longer exposure to cART.
Tuberculosis incidence soon after cART initiation increased with age, especially in those aged >50 years. Although the prevalence of M. tuberculosis infection, and thus the risk of reactivating disease, increases with age [28], the large increase in tuberculosis incidence among individuals receiving cART was unexpected. One possible explanation is the existence of an age-associated IRIS: HIV infection induces a premature aging of the immune system, and people >50 years of age have poorer immunological responses to cART, which may contribute to the reactivation of latent tuberculosis [29].
The reduction in tuberculosis incidence attributable to cART may vary depending on the background risk of M. tuberculosis transmission, as people may develop tuberculosis following recent infection and/or reinfection in settings with high transmission rates [28, 30]. We do not have information on the background risk of tuberculosis transmission by cohort, and country tuberculosis rates are unlikely to be a good marker. However, despite different background risks of M. tuberculosis infection, the effect estimate of cART on tuberculosis incidence obtained from South Africa is similar to ours [8].
For comparison purposes we repeated the same analyses for PCP, a condition for which IRIS is uncommon [5]. We found a lower PCP incidence at all times after cART. The decreased risk of PCP during the first 3 months of cART contrasts with the increased risk of tuberculosis during the same period and supports the role of unmasking IRIS in the latter condition.
Our study has several limitations. First, cART was not randomly assigned, and thus the possibility of residual confounding cannot be ruled out. In particular, treatment initiation might have been prompted by unrecorded symptoms (eg, weight loss due to undiagnosed tuberculosis), which might partly explain the high risk of tuberculosis <3 months after cART initiation. To reduce this potential confounding, we excluded individuals with tuberculosis at or within 1 month of baseline. Although symptoms of undiagnosed tuberculosis might have prompted cART initiation in some individuals, the same applies to PCP symptoms because both conditions may have insidious presentations.
Our findings have implications for future research, clinical practice, and public health practice. Impaired immunological responses to M. tuberculosis in people starting cART at very low CD4 cell counts and those >50 years of age warrant further investigation. These data support the WHO STOP TB partnership statement that multiple interventions are needed to control tuberculosis in HIV-positive people. For cART to be useful in tuberculosis control, prompt HIV infection testing is needed so cART can be started when clinically indicated rather than at advanced immunodeficiency. Diagnosis and treatment of M. tuberculosis infection, as national and international guidelines recommend, should be reinforced. Even in countries with low tuberculosis transmission, intensified case finding remains essential; physicians should be aware of the potentially increased risk of developing tuberculosis after cART initiation.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support.
This work was supported by the National Institutes of Health (grants R01-AI073127 and U10-AA013566). The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Potential conflicts of interest.
Santiago Moreno has received travel grants, fees for speaking, and honoraria from various pharmaceutical companies, including Bristol-Myers Squibb, Gilead Sciences, Boehringer-Ingelheim, and Janssen Pharmaceutica. Inma Jarrín is employed by the Research Network on AIDS (RD06/006). Jonathan Sterne has received travel grants from GlaxoSmithKline and honoraria from Gilead Sciences. Caroline Sabin has received travel grants, fees for speaking, and honoraria from various pharmaceutical companies, including Bristol-Myers Squibb, Gilead Sciences, Boehringer-Ingelheim, Janssen Pharmaceutica, and Tibotec. Andrew Phillips has received fees for speaking and consultancy from various pharmaceutical companies, including Gilead Sciences, Bristol-Myers Squibb, Boehringer-Ingelheim, ViiV Healthcare, and Johnson & Johnson. Dominique Costagliola has received travel grants, consultancy fees, honoraria, or study grants from various pharmaceutical companies, including Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceutica, Merck-Sharp & Dohme-Chibret, Roche, and ViiV Healthcare. Sophie Abgrall has received travel grants or honoraria from pharmaceutical companies, including ViiV Healthcare, Abbott, Janssen-Cilag, Bristol-Myers Squibb, Gilead Sciences, and Boehringer-Ingelheim. Heiner C. Bucher has received travel grants, honoraria, and unrestricted research grants from various pharmaceutical companies, including GlaxoSmithKline, Bristol-Myers Squibb, Gilead Sciences, Roche, Abbott, Tibotec, and Boehringer-Ingelheim. Laurence Meyer has received honoraria from GlaxoSmithKline. The institution of Hansjakob Furrer has received payments for participation in advisory boards, unrestricted educational grants, and/or travel grants from Abbott, Bristol-Myers Squibb, ViiV Healthcare, Roche, Gilead Sciences, Merck-Sharp & Dohme, Boehringer-Ingelheim, Tibotec-Janssen, and ViiV Healthcare and research support from Gilead, Merck-Sharp & Dohme, and Roche. Giota Touloumi has received travel grants from GlaxoSmithKline and Abbott. Panagiotis Gargalianos has received travel grants from Pfizer and Bristol-Myers Squibb.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Appendix A. Writing Committee
Julia del Amo (1, 2), Santiago Moreno (3, 4), Heiner C. Bucher (5), Hansjakob Furrer (6), Roger Logan (7), Jonathan Sterne (8), Santiago Pérez-Hoyos (2, 9), Inma Jarrín (1, 2), Andrew Phillips (10), Sara Lodi (11), Ard van Sighem (12), Frank de Wolf (12), Caroline Sabin (10), Loveleen Bansi, (10), Amy Justice (13, 14), Joseph Goulet (14), José M. Miró (15, 16), Elena Ferrer (17), Laurence Meyer (18–20), Rémonie Seng (19, 20), Giota Toulomi (21), Panagiotis Gargalianos (22), Dominique Costagliola (23–25), Sophie Abgrall (24–26), and Miguel A. Hernán (7, 27).
Institutional affiliations: (1) CNE, Instituto de Salud Carlos III, Madrid, Spain; (2) CIBERESP, Instituto de Salud Carlos III, Madrid, Spain; (3) Ramón y Cajal Hospital, Madrid, Spain; (4) University of Alcalá de Henares, Madrid, Spain; (5) Basel Institute for Clinical Epidemiology and Biostatistics, University Hospital Basel, Switzerland; (6) Universitätsklinik für Infektiologie, Bern University Hospital and University of Bern, Switzerland; (6) Universitätsklinik für Infektiologie, Inselspital, Bern, Switzerland; (7) Harvard School of Public Health, Boston, Massachusetts; (8) Bristol University, United Kingdom; (9) Vall d’Hebron Research Institute, Barcelona, Spain; (10) University College London, United Kingdom; (11) Medical Research Council, London, United Kingdom; (12) Stichting HIV Monitoring, Amsterdam, the Netherlands; (13) Yale University School of Medicine, New Haven, Connecticut; (14) VA Connecticut Healthcare System, West Haven; (15) Hospital Clinic-IDIBAPS, Barcelona, Spain; (16) University of Barcelona, Spain; (17) Bellvitge-IDIBELL Hospital, University of Barcelona, L’Hospitalet de Llobregat, Spain; (18) Univ Paris Sud, UMR 1018, le Kremlin Bicêtre, France; (19) Inserm, UMR 1018, le Kremlin Bicêtre, France; (20) AP-HP, Hôpital de Bicêtre, Service de Santé Publique, le Kremlin Bicêtre, France; (21) Athens University Medical School, Athens, Greece; (22) General Hospital of Athens “G. Gennimatas,” Athens, Greece; (23) UPMC Univ Paris 06, UMR_S 943, Paris, France; (24) INSERM, UMR_S 943, Paris, France; (25) AP-HP, Hôpital Pitié-Salpétrière, Service des maladies infectieuses et tropicales, Paris, France; (26) Hôpital Avicenne, Service des maladies infectieuses et tropicales, Bobigny, France; (27) Harvard-MIT Division of Health Sciences and Technology, Boston, Massachusetts.
References
- 1.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–75. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 2. STOP TB Partnership. The global plan to stop TB 2006–2015. Available at: http://www.stoptb.org/. Accessed September 2011.
- 3.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis. 2010;10:489–98. doi: 10.1016/S1473-3099(10)70078-5. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 5.Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M IeDEA Southern and Central Africa. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–64. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 8.Fairall LR, Bachmann MO, Louwagie GM, et al. Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 9.Miranda A, Morgan M, Jamal L, et al. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLoS One. 2007;2:e826. doi: 10.1371/journal.pone.0000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–8. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santoro-Lopes G, Felix de Pinho A, Harrison LH, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34:543–6. doi: 10.1086/338641. [DOI] [PubMed] [Google Scholar]
- 12.Ledergerber B, Egger M, Erard V, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282:2220–6. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 13.Kirk O, Gatell JM, Mocroft A, et al. Infections with Mycobacterium tuberculosis and Mycobacterium avium among HIV-infected patients after the introduction of highly active antiretroviral therapy. EuroSIDA Study Group JD. Am J Respir Crit Care Med. 2000;162:865–72. doi: 10.1164/ajrccm.162.3.9908018. [DOI] [PubMed] [Google Scholar]
- 14.Girardi E, Antonucci G, Vanacore P, et al. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14:1985–91. doi: 10.1097/00002030-200009080-00015. [DOI] [PubMed] [Google Scholar]
- 15.Moreno S, Jarrin I, Iribarren JA, et al. Incidence and risk factors for tuberculosis in HIV-positive subjects by HAART status. Int J Tuberc Lung Dis. 2008;12:1393–400. [PubMed] [Google Scholar]
- 16.Abgrall S, Del Giudice P, Melica G, Costagliola D. FHDH-ANRS CO4. HIV-associated tuberculosis and immigration in a high-income country: incidence trends and risk factors in recent years. AIDS. 2010;24:763–71. doi: 10.1097/QAD.0b013e3283366747. [DOI] [PubMed] [Google Scholar]
- 17.Grant AD, Bansi L, Ainsworth J, et al. Tuberculosis among people with HIV infection in the United Kingdom: opportunities for prevention? AIDS. 2009;23:2507–15. doi: 10.1097/QAD.0b013e3283320dfd. [DOI] [PubMed] [Google Scholar]
- 18.Elzi L, Schlegel M, Weber R, et al. Reducing tuberculosis incidence by tuberculin skin testing, preventive treatment, and antiretroviral therapy in an area of low tuberculosis transmission. Clin Infect Dis. 2007;44:94–102. doi: 10.1086/510080. [DOI] [PubMed] [Google Scholar]
- 19.Jones JL, Hanson DL, Dworkin MS, et al. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4:1026–31. [PubMed] [Google Scholar]
- 20.Pettit AC, Jenkins CA, Stinnette SE, et al. Tuberculosis risk before and after highly active antiretroviral therapy initiation: does HAART increase the short-term TB risk in a low incidence TB setting? J Acquir Immune Defic Syndr. 2011;57:305–10. doi: 10.1097/QAI.0b013e3182182e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HIV-CAUSAL Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–37. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meintjes G, Lawn SD, Scano F, et al. for International Network for the Study of HIV-associated IRIS. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 24.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Lawn S, Wood R, Wilkinson R. Changing concepts of “latent tuberculosis infection” in patients living with HIV infection. Clin Dev Immunol. 2011;2011:980594. doi: 10.1155/2011/980594. Epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manabe YC, Breen R, Perti T, Girardi E, Sterling TR. Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: a disease spectrum after initiation of antiretroviral therapy. J Infect Dis. 2009;199:437–44. doi: 10.1086/595985. [DOI] [PubMed] [Google Scholar]
- 27.Lawn S, Kranzer K, Edwards D, McNally M, Bekker L, Wood R. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24:1323–8. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Styblo K. Epidemiology of tuberculosis. Selected papers. KNCV; 1991. 24:9–111. [Google Scholar]
- 29.Martin J, Volberding P. HIV and premature aging: a field still in its infancy. Ann Intern Med. 2010;153:477–9. doi: 10.7326/0003-4819-153-7-201010050-00013. [DOI] [PubMed] [Google Scholar]
- 30.Alland D, Kalkut GE, Moss AR, et al. Transmission of tuberculosis in New York City. N Engl J Med. 1994;330:1710–6. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]