Our findings reveal that in 2004 the blaKPC-3 gene was present in a children’s long-term healthcare facility. This suggests pockets of Klebsiella pneumoniae carbapenemase–producing K. pneumoniae independent from sequence type 258 existing in locations not identified at that time.
Abstract
Background. Klebsiella pneumoniae isolates harboring the K. pneumoniae carbapenemase gene (blaKPC) are creating a significant healthcare threat in both acute and long-term care facilities (LTCFs). As part of a study conducted in 2004 to determine the risk of stool colonization with extended-spectrum cephalosporin-resistant gram-negative bacteria, 12 isolates of K. pneumoniae that exhibited nonsusceptibility to extended-spectrum cephalosporins were detected. All were gastrointestinal carriage isolates that were not associated with infection.
Methods. Reassessment of the carbapenem minimum inhibitory concentrations using revised 2011 Clinical Laboratory Standards Institute breakpoints uncovered carbapenem resistance. To further investigate, a DNA microarray assay, PCR-sequencing of bla genes, immunoblotting, repetitive-sequence-based PCR (rep-PCR) and multilocus sequence typing (MLST) were performed.
Results. The DNA microarray detected blaKPC in all 12 isolates, and blaKPC-3 was identified by PCR amplification and sequencing of the amplicon. In addition, a blaSHV-11 gene was detected in all isolates. Immunoblotting revealed “low-level” production of the K. pneumoniae carbapenemase, and rep-PCR indicated that all blaKPC-3-positive K. pneumoniae strains were genetically related (≥98% similar). According to MLST, all isolates belonged to sequence type 36. This sequence type has not been previously linked with blaKPC carriage. Plasmids from 3 representative isolates readily transferred the blaKPC-3 to Escherichia coli J-53 recipients.
Conclusions. Our findings reveal the “silent” dissemination of blaKPC-3 as part of Tn4401b on a mobile plasmid in Northeast Ohio nearly a decade ago and establish the first report, to our knowledge, of K. pneumoniae containing blaKPC-3 in an LTCF caring for neurologically impaired children and young adults.
Long-term care facilities (LTCFs) are essential components of healthcare delivery to many patients. Unfortunately, LTCFs are also recognized as “reservoirs of antibiotic resistance” [1]. In the past 3 decades numerous outbreaks of multidrug-resistant gram-negative and gram-positive organisms have been described in LTCFs [2, 3]. The spread of antibiotic-resistant pathogens transmitted from LTCFs to wider healthcare delivery systems that serve a large region is now appreciated as a major challenge in the design of effective infection control and antibiotic utilization strategies in the care of the elderly [4]. Among gram-negative bacteria, Escherichia coli– and Klebsiella pneumoniae–producing extended-spectrum β-lactamases (ESBLs), as well as carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa, are the most significant threats in this setting [5–10]. Especially concerning has been the national and global spread of carbapenem-resistant K. pneumoniae harboring blaKPC, belonging to sequence type (ST) 258 [7, 11, 12].
Although not as well documented as in adult patients, antibiotic-resistant gram-negative organisms are present in healthcare settings serving children, including pediatric LTCFs [13–16]. In a surveillance study examining the antibiotic susceptibility of normal flora of children residing in an LTCF in Cleveland, Ohio, nearly 40% of subjects were colonized with resistant bacteria, and >60% of organisms were resistant to >2 antibiotics tested [14]. Invasive devices were found to be a significant risk factor for colonization by resistant gram-negative bacteria [14].
Little is known about the spread of blaKPC harboring strains or whether the same risk factors are present in children and adults. Unfortunately, the clinical detection of blaKPC is undermined by heterogeneous expression of carbapenem resistance. Ertapenem minimum inhibitory concentrations (MICs) are the most sensitive for detection of K. pneumoniae carbapenemase (KPC) but may lack specificity, and therefore additional phenotypic tests (ie, modified Hodge test and boronic acid disk) have been devised [17–20]. MICs of carbapenems are dependent not only on the presence and the level of expression of blaKPC but also on changes in outer membrane proteins [7, 21, 22].
In this study we describe the “silent dissemination” and earliest report of KPC-producing K. pneumoniae in an LTCF caring for children and young adults with neurodevelopmental impairments. As part of a study conducted in 2004 to determine the risk of stool colonization by extended-spectrum cephalosporin-resistant gram-negative bacteria, we identified 12 strains of K. pneumoniae that exhibited nonsusceptibility to extended-spectrum cephalosporins. Reassessment of carbapenem MICs using recently revised breakpoints uncovered carbapenem resistance. Genetic analysis revealed that a single sequence type not previously reported to contain blaKPC had disseminated as early as 2004 in Northeast Ohio in this LTCF. Introduction of blaKPC into our region occurred before the description of the spread of ST 258, recognition of Tn4401, the KQ element, or the mobile genetic elements harboring this carbapenemase gene [23, 24].
MATERIALS AND METHODS
Setting and Selection of Strains
The study facility cares for 130 nonambulatory children and young adults with severe neurodevelopmental abnormalities. During the course of the study, the ages of the residents ranged between 5 and 47 years, with 81% distributed between ages 11 and 29 years.
The current investigation was derived from a larger study in which patient stool samples were collected between 1 July and 30 December 2004. The goal of the study was to evaluate the changes in gastrointestinal flora as a result of residence in the facility. Approximately 10-g portions of whole stool were collected from participating residents every 2 weeks by caregivers. Samples were placed in anaerobic containers (Anaerobic Systems) on site and then transported at ambient temperature to a research microbiology laboratory within 24 hours. The specimens were transferred to cryovials containing cooked meat medium (BBL) and glycerol, and stored at −70°C.
After an analysis of the epidemiology of antibiotic-resistant gram-negative organisms in a nearby pediatric intensive care unit revealed that colonization by resistant organisms was frequent in patients transferred from an LTCF [14], archived frozen specimens were assessed for the presence of bacteria expressing resistance to extended-spectrum cephalosporins. Samples from the first 50 subjects enrolled in the larger study were selected. Subjects were excluded if >2 successive samples were missing or the subject was hospitalized in an acute-care facility during the period of sample collection. The study was approved by the Institutional Review Board of University Hospitals Case Medical Center, and parents or legal guardians provided written informed consent.
Antimicrobial Susceptibility Testing
Single colonies of K. pneumoniae were recovered from frozen stocks, and MICs of 17 agents were determined using Sensititre GNXF trays (Trek Diagnostic Systems) as described elsewhere [18]. American Type Culture Collection control strains P. aeruginosa 27853 and E. coli 25922 were used as quality control strains for susceptibility testing. Twelve isolates of K. pneumoniae obtained were identified as nonsusceptible to third-generation cephalosporins using the then current National Committee for Clinical Laboratory Standards (NCCLS) criteria and were selected for study [25]. Carbapenem MIC results were reinterpreted according to criteria issued by the Clinical Laboratory Standards Institute (CLSI) in 2011 [26]. Breakpoints established by the US Food and Drug Administration were used to interpret MIC results for tigecycline (susceptible was defined as MIC ≤2 mg/L; resistant, as MIC ≥8 mg/L). In addition, modified Hodge tests (MHTs) with imipenem and meropenem were performed [27].
β-Lactamase Gene Screening Using a Microarray Detection System
The Check-Points ESBL/AmpC/KPC/NDM-1 assay (Check-MDR CT101; Check-Points) is a DNA low-density microarray testing method for detecting blaTEM, blaSHV, blaCTX-M, blaKPC, 6 additional groups of AmpCs (blaCMY-2-like, blaDHA, blaFOX, blaCMY-1-like/MOX, blaACC, and blaMIR/ACT), and the blaNDM-1 metallo-β-lactamase genes [28–30]. Briefly, genomic DNA was extracted from colonies of bacteria grown overnight using the DNeasy blood and tissue kit (Qiagen Sciences). Microarray assays were performed according to instructions of the manufacturer, generating templates of the target bla DNA sequence that are amplified, hybridized, and then detected by the instrument.
Polymerase Chain Reaction Confirmation of β-Lactamase Genes
Polymerase chain reaction (PCR) amplification of blaTEM, blaSHV, and blaKPC genes was achieved using established primers and amplified with a MJ Research Gradient Cycler Model PTC 225 (MJ Research) using thermocycling conditions adjusted to the primer melting temperatures [21, 22, 31]. In addition to bla genes, we tested for the presence of qnrA, qnrB, qnrC, aac(6′), rmtA, rmtB, rmtC, and rmtD genes by PCR using the primers and thermocycling conditions validated in other studies [23, 32]. Positive controls included well-characterized isolates in our collection [31, 32].
DNA Sequencing
Amplicons obtained using primers for blaKPC, blaSHV, and blaTEM were sequenced at a commercial sequencing facility (MCLAB). Additionally, the upstream sequence of blaKPC positive strains was also obtained by PCR amplification using primers described elsewhere [22, 24]. Sequence data were analyzed using Lasergene 7.2 software (DNAstar), and sequences were compared with BLAST online software (http://blast.ncbi.nlm.nih.gov), using the megablast algorithm.
Repetitive-Sequence-Based PCR
Genetic similarity among strains was investigated by repetitive-sequence-based PCR (rep-PCR) using the DiversiLab strain typing system (Bacterial BarCodes; bioMérieux), as described elsewhere [21]. DNA was isolated from strains using the MoBio UltraClean Microbial DNA Isolation Kit (MoBio), following the manufacturer’s instructions for gram-negative bacteria. Isolated DNA was amplified using the DiversiLab Klebsiella kit, and amplicons were analyzed on an Agilent 2100 Bioanalyzer (Agilent). Dendrograms and comparisons were generated by the DiversiLab Software (bioMérieux). Isolates with >95% similarity were considered of the same clone type. Strains from our collection were used as comparators [21].
Multilocus Sequence Typing
Multilocus sequence typing (MLST) was performed on all K. pneumoniae strains as described by Diancourt [33]. Sequences of 7 housekeeping genes (rpoB, gapA, mdh, pgi, phoE, infB, and tonB) were compared with the MLST database (http://www.pasteur.fr/recherche/genopole/PF8/mlst/).
Immunoblotting
Immunoblotting for assessment of KPC production was performed according to established methods [22].
Mating Experiments
To investigate whether there was transfer of bla genes, 3 representative blaKPC-3 producing strains susceptible to rifampin were selected for overnight culture in lysogeny broth (LB) with rifampin-resistant E. coli J-53. After overnight growth in LB, cells were plated on Mueller Hinton (MH) agar containing ampicillin (100 mg/L) and rifampin (100 mg/L). Colonies were replated on the same selective MH agar. These strains were screened by PCR for blaKPC, as described elsewhere [34].
RESULTS
The first 50 subjects were enrolled in the study between 1 July and 30 December 2004 and were considered for inclusion in the current analysis. Sixteen subjects were excluded owing to inadequate longitudinal sampling of specimens as defined in the Methods section, and 8 additional subjects were excluded owing to transfer to an acute care hospital during the period of observation. Among the remaining 26 residents, specimens were collected for a median of 11.5 weeks (range, 10–26 weeks). Stool specimens from 12 of the 26 subjects were positive for K. pneumoniae expressing resistance to extended-spectrum cephalosporins. In 4 of the 12 positive subjects the organism was cultivated from only 1 specimen, but in the remainder, excretion occurred over a median of 9 weeks (range, 4–26 weeks).
There were no characteristics distinguishing between residents whose specimens were K. pneumoniae positive and residents whose specimens were not; they were of similar age (median, 16.2 vs 19.9 years for those with positive vs those with negative specimens); similar proportions had been exposed to oral (64% vs 80%) or intravenous (28% vs 26%) antibiotics during the previous 12 months; and similar proportions had been hospitalized in the previous 12 months (35% vs 27%) (all P > .10). The residents carrying K. pneumoniae isolates were housed in all 5 of the facility’s pods, and all had been born in, and referred from, cities in Ohio.
The antimicrobial susceptibility testing results of the extended-spectrum cephalosporin-resistant K. pneumoniae isolates are summarized in Table 1. All 12 isolates were resistant to ceftazidime, cefotaxime, and aztreonam based on 2011 CLSI breakpoints. Reassessment of the carbapenem MICs using 2011 CLSI breakpoints revealed that all isolates were resistant to ertapenem and imipenem, 1 isolate was susceptible to meropenem, and 2 isolates were susceptible to doripenem [26]. The MHT was positive using imipenem and meropenem for all isolates, including the meropenem-susceptible strain (Kp-11). All strains were susceptible to cefepime, amikacin, colistin, and polymyxin B, and 9 were susceptible to gentamicin and tobramycin. Consistent with quinolone-prescribing practices in children, all isolates were susceptible to ciprofloxacin, whereas 2 strains were nonsusceptible to tigecycline (Kp-12 and Kp-17).
Table 1.
Antimicrobial Susceptibility Testing of Klebsiella pneumoniae Isolates Collected From a Long-term Care Facility
| MIC (mg/L) and Interpretation |
||||||||||||
| Antibiotic | Kp-4 | Kp-5 | Kp-7 | Kp-8 | Kp-9 | Kp-10 | Kp-11 | Kp-12 | Kp-14 | Kp-15 | Kp-16 | Kp-17 |
| Ciprofloxacin | ≤0.25 S | ≤0.25 S | ≤0.25 S | ≤0.25 S | ≤0.25 S | ≤0.25 S | ≤0.25 S | ≤0.25 S | ≤0.25 S | ≤0.25 S | ≤0.25 S | ≤0.25 S |
| Gentamicin | ≤1 S | ≤1 S | 8 I | ≤1 S | ≤1 S | >8 R | ≤1 S | ≤1 S | ≤1 S | ≤1 S | 8 I | ≤1 S |
| Amikacin | ≤4 S | ≤4 S | ≤4 S | ≤4 S | ≤4 S | ≤4 S | ≤4 S | ≤4 S | ≤4 S | ≤4 S | ≤4 S | ≤4 S |
| Tobramycin | ≤1 S | ≤1 S | 8 I | ≤1 S | ≤1 S | 8 I | ≤1 S | ≤1 S | ≤1 S | ≤1 S | 8 I | ≤1 S |
| Trimethoprim/sulfamethoxazole | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S | ≤0.5/9 S |
| Colistina | 0.5 S | 0.5 S | 0.5 S | 0.5 S | 1 S | 0.5 S | 0.5 S | 0.5 S | 0.5 S | 0.5 S | 0.5 S | 1 S |
| Polymyxin Ba | 0.5 S | 0.5 S | 2 S | 1 S | 1 S | 0.5 S | 1 S | 1 S | 1 S | 1 S | 1 S | 1 S |
| Tigecyclineb | 0.5 S | 0.5 S | 0.5 S | 0.5 S | 1 S | 0.5 S | ≤0.25 S | 4 I | 0.5 S | 0.5 S | 1 S | 4 I |
| Piperacillin/tazobactam | >64/4 R | >64/4 R | >64/4 R | >64/4 R | >64/4 R | >64/4 R | >64/4 R | >64/4 R | >64/4 R | >64/4 R | >64/4 R | >64/4 R |
| Ceftazidime | 16 R | 16 R | 16 R | 16 R | 16 R | 16 R | 16 R | >16 R | 16 R | 16 R | 16 R | >16 R |
| Cefotaxime | 8 R | 4 R | 4 R | 8 R | 8 R | 8 R | 8 R | 16 R | 4 R | 4 R | 8 R | 16 R |
| Cefepime | 4 S | 4 S | 4 S | 4 S | 4 S | 4 S | 8 S | 8 S | 4 S | 8 S | 8 S | 4 S |
| Aztreonam | >16 R | >16 R | >16 R | >16 R | >16 R | >16 R | >16 R | >16 R | >16 R | >16 R | >16 R | >16 R |
| Meropenem | 4 R | 2 I | 4 R | 8 R | 8 R | 8 R | ≤1 S | 4 R | 4 R | 4 R | 4 R | 4 R |
| Imipenem | 4 R | 4 R | 4 R | 4 R | 4 R | 4 R | 4 R | 4 R | 4 R | 4 R | 4 R | 4 R |
| Doripenem | 2 I | 2 I | >2 R | 1 S | >2 R | 2 I | 1 S | >2 R | 2 I | 2 I | 2 I | 2 I |
| Ertapenem | 4 R | 4 R | 4 R | 4 R | >4 R | 4 R | 4 R | 4 R | 2 R | >4 R | 4 R | >4 R |
| Modified Hodge testc | + | + | + | + | + | + | + | + | + | + | + | + |
Unless otherwise specified, susceptibility tests were interpreted according to 2011 Clinical Laboratory Standards Institute (CLSI) criteria. For aztreonam and ceftazidime, isolates were considered susceptible at minimum inhibitory concentrations (MICs) of ≤4 mg/L; intermediate at MICs of 8 mg/L; and resistant at ≥16 mg/L. For cefotaxime, meropenem, imipenem, and doripenem, ≤1 mg/L was considered susceptible; 2 mg/L, intermediate; and ≥4 mg/L, resistant. For ertapenem, ≤0.25 mg/L was considered susceptible; 0.5 mg/L, intermediate; and ≥1 mg/L, resistant.
Abbreviations: I, intermediate; MIC, minimum inhibitory concentration; R, resistant; S, susceptible.
CLSI breakpoints for Pseudomonas aeruginosa were applied.
Susceptibility tests were interpreted according to Food and Drug Administration criteria.
Performed with imipenem and meropenem, following CLSI recommendations. Plus signs indicate positive results.
PCR and Microarray Analysis
Using the Check-Points ESBL/AmpC/KPC/NDM-1 microarray and PCR/DNA sequencing, all the K. pneumoniae strains were found to contain blaKPC-3 and blaSHV-11 genes; blaTEM-1 was found in 3 isolates (Table 2). PCR amplification did not reveal qnrA, qnrB, qnrC, rmtA, rmtB, rmtC, rmtD, or aac(6′). These findings were consistent with the susceptibility results reported in Table 1.
Table 2.
Molecular Analysis of Klebsiella pneumoniae Collected From a Long-term Care Facility for Children and Young Adults
| Strain | MLST | blaKPCa | blaNDM-1 | blaAmpC | blaCTX-M | blaSHVb | blaTEMb |
| Kp-4 | 36 | + | − | − | − | WT | − |
| Kp-5 | 36 | + | − | − | − | WT | − |
| Kp-7 | 36 | + | − | − | − | WT | WT |
| Kp-8 | 36 | + | − | − | − | WT | − |
| Kp-9 | 36 | + | − | − | − | WT | − |
| Kp-10 | 36 | + | − | − | − | WT | WT |
| Kp-11 | 36 | + | − | − | − | WT | − |
| Kp-12 | 36 | + | − | − | − | WT | − |
| Kp-14 | 36 | + | − | − | − | WT | − |
| Kp-15 | 36 | + | − | − | − | WT | − |
| Kp-16 | 36 | + | − | − | − | WT | WT |
| Kp-17 | 36 | + | − | − | − | WT | − |
Plus signs indicate present. Minus signs indicate absent. Abbreviations: MLST, multilocus sequence typing: WT, wild type.
All blaKPC-3, as determined by sequencing.
WT indicates non-extended-spectrum β-lactamase blaSHV and blaTEM genes, which were confirmed by sequence analysis (ie, blaSHV-11 and blaTEM-1, respectively).
Rep-PCR and MLST
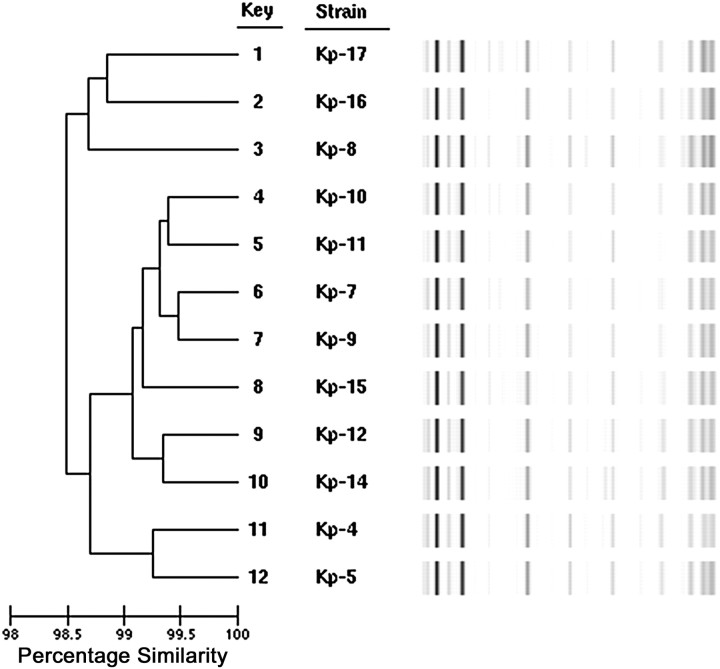
All strains of K. pneumoniae were found to be related (>98% similarity) by rep-PCR (Figure 1). Comparison with our nationwide collection of KPC strains showed that these strains were significantly different from any others we had previously examined (data not shown). As a result, we were prompted to perform MLST; all strains were identified as ST 36, a sequence type not previously associated with blaKPC.
Figure 1.
Dendrogram depicting >98% similarity and band patterns of Klebsiella pneumoniae carbapenemase–producing K. pneumoniae strains typed by repetitive-sequence-based polymerase chain reaction.
Immunoblotting
We confirmed the PCR results for KPC by performing an immunoblot with a highly specific antibody that recognizes KPC-2/3. Low-level expression was observed (data not shown).
Conjugation Experiments
Conjugation demonstrated transfer of blaKPC-3 on a mobile element from 3 isolates to E. coli J-53 recipients. The transfer of blaKPC-3 was verified by PCR and sequencing of the amplicon.
DISCUSSION
In this study we characterized isolates possessing blaKPC-3 dating back to 2004, which to the best of our knowledge represents the earliest detection of such a resistance determinant in an LTCF caring for children and young adults. This singular observation places the global epidemic of blaKPC spreading in healthcare facilities in an entirely new light. First, the patient population of LTCF’s caring for children typically is very different from residents in adult facilities, with the former predominantly composed of patients with significant neurological, muscular, and developmental disabilities but with few other comorbidities. Therefore, the epidemiologic characteristics of resistant microorganisms in pediatric facilities may differ significantly from those in institutions caring primarily for elderly adults. Few studies have been conducted in facilities caring for children, and unlike in older adults, we know little regarding the significance of blaKPC isolates in children in LTCFs and the long-term consequences of this colonization. This study draws attention to this concerning occurrence.
Second, all isolates were resistant to ceftazidime and cefotaxime, the basis for inclusion in the study. Phenotypic characterization showed all isolates were susceptible to cefepime, which initially led us to suspect that they might contain an AmpC β-lactamase. However, evidence of an AmpC was not detected. Therefore, this study not only confirms the difficulty in detecting KPC strains using previous CLSI break points [18, 25] but also indicates that the major pathway of dissemination of blaKPC in the time near its first detection may have been largely “silent.”
The implications of this pattern of spread for blaKPC genes are very important. The case of blaKPC dissemination in a manner that did not initially trigger detection by routine phenotypic testing may have been an important early selective advantage mechanism in which the bla gene sampled a variety of genetic backgrounds and range of sequence types that eventually led to a more permanent incorporation into the most advantageous genetic background. Moreover, ST 36 may have been a predecessor strain; a group of bacteria in which blaKPC resided for a while before eventually finding ST 258. As a point of reference, ST 36 and ST 258 are not closely related. They differ in 5 of the 7 housekeeping genes used to determine sequence type (http://www.pasteur.fr/recherche/genopole/PF8/mlst/).
Carbapenem breakpoint guidelines in 2004 did not identify these isolates as carbapenem resistant, even when examining the ertapenem MICs [25]. For instance, strain Kp-11 showed positive MHT, whereas MICs against ertapenem and meropenem were 4 and ≤1 mg/L, respectively. The revised 2011 CLSI criteria with lower breakpoints favor detection of carbapenem resistance [26].
Although we expected that susceptibility to quinolones would be preserved because children are not treated with these antimicrobials, blaKPC often coexists with quinolone-resistance determinants among K. pneumoniae isolated from adults [21]. Among these isolates, PCR did not reveal the presence of plasmid-mediated quinolone-resistance genes, or aminoglycoside-modifying enzyme genes. The presence of nonsusceptibility to tigecycline in 2 of the isolates was not anticipated, because tigecycline was approved for release by the US Food and Drug Administration in July 2005 after the collection of these isolates.
Upstream sequencing of blaKPC-3 revealed that this bla gene was located in Tn4401b in all strains. This variant does not contain the 100–base pair deletion described in Tn4401a. The level of KPC expression was examined by detecting the β-lactamase via immunoblotting. High-level KPC production was not observed, supporting the lower carbapenem MICs among these strains (data not shown) [22]. Our mating experiments suggested that transmission of a plasmid-bearing blaKPC-3 among different K. pneumoniae may also have occurred. Analysis of the genetic context of the plasmids in these strains is underway. The Check-Points microarray also confirmed the transfer of the blaKPC-3 gene into the J-53 E. coli recipients; however, the blaSHV-11 from isolates Kp-4, Kp-5, and Kp-11 did not transfer.
All KPC-producing strains were found to be closely related using molecular typing techniques that have been previously validated for K. pneumoniae, this suggested clonal relatedness. We have not yet determined whether this resistance gene or plasmid was introduced in the facility from a single source and disseminated or was introduced by multiple patients coming from referring hospitals.
It was also intriguing that MLST revealed the introduction of a new sequence type not known before to harbor blaKPC. Other studies have found blaKPC in STs 11, 14, 258, 277, 337, 338, 339, 340, and 588 [35]. To our knowledge, this is the first report of blaKPC-3 in a K. pneumoniae isolate of ST 36. This sequence type has been reported in Spain, Northern Europe, and Korea [35–38], and it is linked to blaCTX-M-15, blaSHV-11, qnrS1, blaOXA-1, and aac(6′)-Ib-cr genes. Our strains harbored a blaSHV-11 gene. In addition, 3 were positive for blaTEM-1 (Table 2). Because these bla genes are often found in mobile elements, the results are not unexpected.
Finally, the commercial microarray system used to analyze the isolates demonstrated 100% sensitivity and specificity for the detection of blaKPC, blaSHV, and blaTEM in this study. The analysis of the bla genes using this method was far more efficient than by conventional PCR. This assay gave us a “snapshot” of the β-lactamase background of antibiotic resistance in these gram-negative species. Evidence is accumulating that this microarray can offer real-time information potentially leading to the choice of appropriate antibiotic therapy.
In summary, this study reveals the silent dissemination of blaKPC-3 as part of Tn4401b in Northeast Ohio in 2004 and establishes the first report to our knowledge of K. pneumoniae producing KPC in pediatric LTCFs. An examination of the literature reveals that only 1 blaKPC containing E. coli isolate in Ohio was reported as part of the MYSTIC program’s 1999–2005 isolate survey and the SENTRY Antimicrobial Surveillance program from 2000 to 2004 did not report any blaKPC containing isolates from Ohio [39, 40]. Our findings are all the more striking because the organisms were discovered in relatively young patients, a population not previously identified as being at risk for harboring KPC-expressing organisms. The molecular background of these strains (all ST 36) suggests that a unique sequence type not previously reported to carry blaKPC was involved in the clonal spread. This report also highlights the importance of the revised 2011 CLSI guidelines for detecting carbapenem resistance mediated by blaKPC. We plan to perform a detailed molecular analysis of other enteric isolates and the mobile plasmids recovered from this survey to gain deeper insight into the transmission dynamics and genetic background. Most importantly, the findings of blaKPC on a mobile genetic element in an LTCF providing care to children and young adults with significant neurodevelopmental abnormalities points to an important area for enhanced infection control efforts.
Notes
Acknowledgment.
The authors would like to especially acknowledge the early contributions of Dr Michael Dul to this project.
Financial support.
This work was supported by the Veterans Affairs Merit Review Program, VISN 10 Geriatric Research Education and Clinical Center, Elan Pharmaceuticals, the National Institutes of Health (grant RO1 AI063517-07), and Steris Corporation (institutional grant to F. P.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bonomo RA. Multiple antibiotic-resistant bacteria in long-term-care facilities: an emerging problem in the practice of infectious diseases. Clin Infect Dis. 2000;31:1414–22. doi: 10.1086/317489. [DOI] [PubMed] [Google Scholar]
- 2.Lautenbach E, Marsicano R, Tolomeo P, et al. Epidemiology of antimicrobial resistance among gram-negative organisms recovered from patients in a multistate network of long-term care facilities. Infect Control Hosp Epidemiol. 2009;30:790–3. doi: 10.1086/599070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Fallon E, Pop-Vicas A, D'Agata E. The emerging threat of multidrug-resistant gram-negative organisms in long-term care facilities. J Gerontol A Biol Sci Med Sci. 2009;64:138–41. doi: 10.1093/gerona/gln020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother. 2010;65:1807–18. doi: 10.1093/jac/dkq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonomo RA, Donskey CJ, Blumer JL, et al. Cefotaxime-resistant bacteria colonizing older people admitted to an acute care hospital. J Am Geriatr Soc. 2003;51:519–22. doi: 10.1046/j.1532-5415.2003.51161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Medina T, Carmeli Y. The pivotal role of long-term care facilities in the epidemiology of Acinetobacter baumannii: another brick in the wall. Clin Infect Dis. 2010;50:1617–18. doi: 10.1086/652760. [DOI] [PubMed] [Google Scholar]
- 7.Endimiani A, Depasquale JM, Forero S, et al. Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J Antimicrob Chemother. 2009;64:1102–10. doi: 10.1093/jac/dkp327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson ND, Moland ES, Hong SG, et al. Surveillance of community-based reservoirs reveals the presence of CTX-M, imported AmpC, and OXA-30 beta-lactamases in urine isolates of Klebsiella pneumoniae and Escherichia coli in a U.S. community. Antimicrob Agents Chemother. 2008;52:3814–16. doi: 10.1128/AAC.00877-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolas-Chanoine MH, Jarlier V. Extended-spectrum beta-lactamases in long-term care facilities. Clin Microbiol Infect. 2008;14(Suppl 1):111–16. doi: 10.1111/j.1469-0691.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 10.Sengstock DM, Thyagarajan R, Apalara J, et al. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis. 2010;50:1611–16. doi: 10.1086/652759. [DOI] [PubMed] [Google Scholar]
- 11.Kitchel B, Rasheed JK, Patel JB, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother. 2009;53:3365–70. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–36. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 13.Dent A, Toltzis P. Descriptive and molecular epidemiology of gram-negative bacilli infections in the neonatal intensive care unit. Curr Opin Infect Dis. 2003;16:279–83. doi: 10.1097/00001432-200306000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Lidsky K, Hoyen C, Salvator A, Rice LB, Toltzis P. Antibiotic-resistant gram-negative organisms in pediatric chronic-care facilities. Clin Infect Dis. 2002;34:760–6. doi: 10.1086/338957. [DOI] [PubMed] [Google Scholar]
- 15.Toltzis P. Colonization with antibiotic-resistant gram-negative bacilli in the neonatal intensive care unit. Minerva Pediatr. 2003;55:385–93. [PubMed] [Google Scholar]
- 16.Toltzis P. Antibiotic-resistant gram-negative bacteria in hospitalized children. Clin Lab Med. 2004;24:363–80. doi: 10.1016/j.cll.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Doi Y, Potoski BA, Adams-Haduch JM, et al. Simple disk-based method for detection of Klebsiella pneumoniae carbapenemase-type beta-lactamase by use of a boronic acid compound. J Clin Microbiol. 2008;46:4083–6. doi: 10.1128/JCM.01408-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clin Microbiol. 2010;48:4417–25. doi: 10.1128/JCM.02458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGettigan SE, Andreacchio K, Edelstein PH. Specificity of ertapenem susceptibility screening for detection of Klebsiella pneumoniae carbapenemases. J Clin Microbiol. 2009;47:785–6. doi: 10.1128/JCM.02143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsakris A, Kristo I, Poulou A, et al. Evaluation of boronic acid disk tests for differentiating KPC-possessing Klebsiella pneumoniae isolates in the clinical laboratory. J Clin Microbiol. 2009;47:362–7. doi: 10.1128/JCM.01922-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother. 2009;63:427–37. doi: 10.1093/jac/dkn547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitchel B, Rasheed JK, Endimiani A, et al. Genetic factors associated with elevated carbapenem resistance in KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2010;54:4201–7. doi: 10.1128/AAC.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice LB, Carias LL, Hutton RA, et al. The KQ element, a complex genetic region conferring transferable resistance to carbapenems, aminoglycosides, and fluoroquinolones in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2008;52:3427–9. doi: 10.1128/AAC.00493-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naas T, Cuzon G, Villegas MV, et al. Genetic structures at the origin of acquisition of the beta-lactamase bla KPC gene. Antimicrob Agents Chemother. 2008;52:1257–63. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: ninth informational supplement. Wayne, PA: National Committee for Clinical Laboratory Standards; 1999. pp. M100–S9. [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI document. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. pp. M100–S21. [Google Scholar]
- 27.Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol. 2007;45:2723–5. doi: 10.1128/JCM.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endimiani A, Hujer AM, Hujer KM, et al. Evaluation of a commercial microarray system for detection of SHV-, TEM-, CTX-M-, and KPC-type beta-lactamase genes in gram-negative isolates. J Clin Microbiol. 2010;48:2618–22. doi: 10.1128/JCM.00568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naas T, Cuzon G, Bogaerts P, Glupczynski Y, Nordmann P. Evaluation of a DNA microarray (Check-MDR CT102) for the rapid detection of TEM, SHV and CTX-M extended-spectrum beta-lactamases (ESBLs), and of KPC, OXA-48, VIM, IMP, and NDM-1 carbapenemases. J Clin Microbiol. 2011;49:1608–13. doi: 10.1128/JCM.02607-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naas T, Cuzon G, Truong H, Bernabeu S, Nordmann P. Evaluation of a DNA microarray, the check-points ESBL/KPC array, for rapid detection of TEM, SHV, and CTX-M extended-spectrum beta-lactamases and KPC carbapenemases. Antimicrob Agents Chemother. 2010;54:3086–92. doi: 10.1128/AAC.01298-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hujer KM, Hujer AM, Hulten EA, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50:4114–23. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hujer KM, Hujer AM, Endimiani A, et al. Rapid determination of quinolone resistance in Acinetobacter spp. J Clin Microbiol. 2009;47:1436–42. doi: 10.1128/JCM.02380-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43:4178–82. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyen CM, Hujer AM, Hujer KM, et al. A clinical strain of Escherichia coli possessing CMY-2 plasmid-mediated amp C beta-lactamase: an emerging concern in pediatrics? Microb Drug Resist. 2002;8:329–33. doi: 10.1089/10766290260469598. [DOI] [PubMed] [Google Scholar]
- 35.Cuzon G, Naas T, Truong H, et al. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg Infect Dis. 2010;16:1349–56. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko KS, Yeom JS, Lee MY, Peck KR, Song JH. Clonal dissemination of extended-spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae isolates in a Korean hospital. J Korean Med Sci. 2008;23:53–60. doi: 10.3346/jkms.2008.23.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oteo J, Cuevas O, Lopez-Rodriguez I, et al. Emergence of CTX-M-15-producing Klebsiella pneumoniae of multilocus sequence types 1, 11, 14, 17, 20, 35 and 36 as pathogens and colonizers in newborns and adults. J Antimicrob Chemother. 2009;64:524–8. doi: 10.1093/jac/dkp211. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz E, Rojo-Bezares B, Saenz Y, et al. Outbreak caused by a multi-resistant Klebsiella pneumoniae strain of new sequence type ST341 carrying new genetic environments of aac(6′)-Ib-cr and qnrS1 genes in a neonatal intensive care unit in Spain. Int J Med Microbiol. 2010;300:464–9. doi: 10.1016/j.ijmm.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Deshpande LM, Jones RN, Fritsche TR, Sader HS. Occurrence and characterization of carbapenemase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program (2000–2004) Microb Drug Resist. 2006;12:223–30. doi: 10.1089/mdr.2006.12.223. [DOI] [PubMed] [Google Scholar]
- 40.Deshpande LM, Rhomberg PR, Sader HS, Jones RN. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States Medical Centers: report from the MYSTIC Program (1999–2005) Diagn Microbiol Infect Dis. 2006;56:367–72. doi: 10.1016/j.diagmicrobio.2006.07.004. [DOI] [PubMed] [Google Scholar]