Abstract
Cardiac interstitial fibrillar collagen accumulation, such as that associated with chronic pressure overload (PO), has been shown to impair left ventricular diastolic function. Therefore, insight into cellular mechanisms that mediate excessive collagen deposition in the myocardium is pivotal to this important area of research. Collagen is secreted as a soluble procollagen molecule with NH2- and COOH (C)-terminal propeptides. Cleavage of these propeptides is required for collagen incorporation to insoluble collagen fibrils. The C-procollagen proteinase, bone morphogenic protein 1, cleaves the C-propeptide of procollagen. Procollagen C-endopeptidase enhancer (PCOLCE) 2, an enhancer of bone morphogenic protein-1 activity in vitro, is expressed at high levels in the myocardium. However, whether the absence of PCOLCE2 affects collagen content at baseline or after PO induced by transverse aortic constriction (TAC) has never been examined. Accordingly, in vivo procollagen processing and deposition were examined in wild-type (WT) and PCOLCE2-null mice. No significant differences in collagen content or myocardial stiffness were detected in non-TAC (control) PCOLCE2-null versus WT mice. After TAC-induced PO, PCOLCE2-null hearts demonstrated a lesser collagen content (PCOLCE2-null TAC collagen volume fraction, 0.41% ± 0.07 vs. WT TAC, 1.2% ± 0.3) and lower muscle stiffness compared with WT PO hearts [PCOLCE2-null myocardial stiffness (β), 0.041 ± 0.002 vs. WT myocardial stiffness, 0.065 ± 0.001]. In addition, in vitro, PCOLCE2-null cardiac fibroblasts exhibited reductions in efficiency of C-propeptide cleavage, as demonstrated by increases in procollagen α1(I) and decreased levels of processed collagen α1(I) versus WT cardiac fibroblasts. Hence, PCOLCE2 is required for efficient procollagen processing and deposition of fibrillar collagen in the PO myocardium. These results support a critical role for procollagen processing in the regulation of collagen deposition in the heart.
Keywords: procollagen, procollagen C-proteinase enhancer-2, fibrosis, hypertrophy
collagen type i is the most prevalent fibrillar collagen in the cardiac interstitium (5). Increases in myocardial collagen content have been shown to impair cardiac function specifically by causing an increase in ventricular passive diastolic stiffness (9, 27). Pressure overload (PO) hypertrophy, such as that seen in individuals with hypertension, is frequently associated with increases in ventricular collagen content (9, 27). Increases in both size and number of collagen fibers in the heart have been demonstrated in human biopsy and in animal models of PO hypertrophy (2, 8, 21). However, a more complete understanding of cellular mechanisms that govern collagen deposition and accumulation in hypertrophic myocardium is needed.
Collagen is secreted as a soluble procollagen molecule with an NH2- (N) and a COOH (C)-terminal propeptide attached (18). Removal of these propeptides is considered essential for efficient incorporation of soluble collagen into insoluble collagen fibrils. Particularly, enzymatic cleavage of the C-terminal propeptide by bone morphogenic protein (BMP) 1 is required for collagen fibril incorporation into insoluble collagen fibrils (4, 13, 15). Cardiac expression of BMP-1 increases in response to fibrotic deposition of collagen (17). Two enhancer proteins, procollagen C-endopeptidase enhancer [PCOLCE/procollagen C-proteinase enhancer (PCPE)] 1 and 2, have been identified that enhance the catalytic activity of BMP-1 for fibrillar procollagens, in vitro (20, 22). Both PCOLCE1 and 2 were found to be expressed in the heart, however, cardiac levels of PCOLCE2 were greater than that of PCOLCE1, and, furthermore, heart tissue demonstrated the highest level of expression of PCOLCE2 of the tissues that were sampled (20). To date, no studies addressing the functional significance of cardiac expression of PCOLCE2 in vivo have been reported.
Previous studies have suggested that the regulation of procollagen processing in the heart is an important point of control in normal tissue and particularly in response to increased collagen deposition such as that seen in hearts with PO hypertrophy (1, 2). To test the functional relevance of PCOLCE2 in hypertrophic deposition of collagen I, PCOLCE2-null mice were subjected to transverse aortic constriction (TAC) for 4 wk and evaluated for differences in levels of collagen versus wild-type (WT) mice. We hypothesized that the absence of PCOLCE2 would result in decreased amounts of interstitial collagen in hypertrophic myocardium along with associated decreases in muscle stiffness.
MATERIAL AND METHODS
Animals and protocols.
PCOLCE-2 null (C57Bl6) mice were a kind gift of Drs. Conrad Bleul and Christiane Happe (Max-Planck Institute, Freiburg, Germany) (12). For in vivo studies, 25 PCOLCE2 null and 25 WT age-matched (3–5 mo, ∼equal representation of male and female banded) mice were subjected to PO induced by TAC. Fifteen PCOLCE2-null TAC (7 female and 8 male) mice and 10 WT TAC (4 male and 6 female) mice survived to 4 wk (Fig. 1). Ten mice of each genotype (5 males and 5 females) served as non-TAC controls. Each of the four groups of mice were divided into two subsets to undergo three experimental protocols at the time of harvest: 1) five left ventricles from each group were used for hydroxyproline analysis, and 2) the remaining five hearts from each group were used to 2a) assess papillary muscle function and 2b) assess collagen volume fraction (CVF) by histochemical analysis. All procedures performed were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee in accordance with National Institutes of Health (NIH) guidelines.
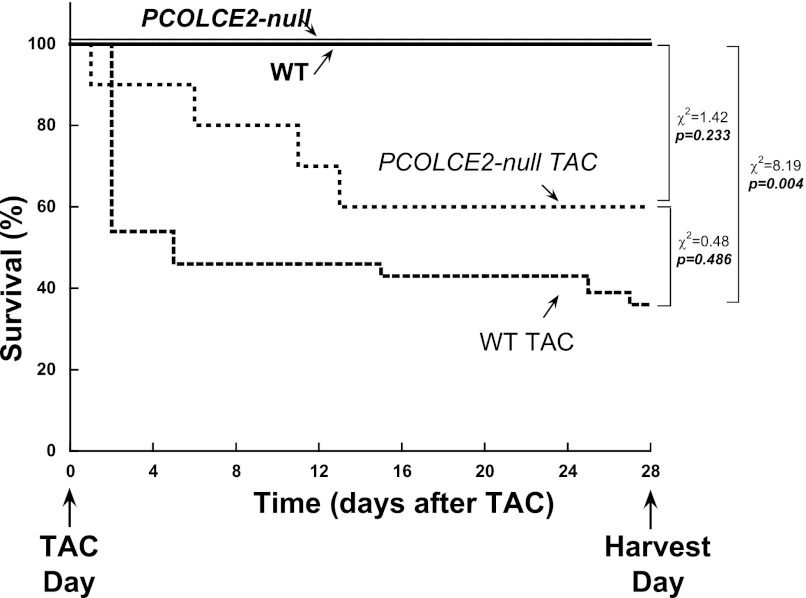
Fig. 1.
Survival curves of wild-type (WT) and procollagen C-endopeptidase enhancer (PCOLCE2)-null transverse aortic constriction (TAC) mice. WT and PCOLCE2-null mice both demonstrated ∼50% mortality in response to pressure overload generated by 4 wk of TAC. Although higher numbers of PCOLCE2-null mice subjected to pressure overload survived to 28 days vs. WT mice, the differences did not reach statistical significance.
Echocardiography.
Mice from each genotype underwent echocardiography at baseline and after 4 wk of TAC to examine in vivo left ventricular (LV) structure and function using a 40-MHz transducer and Vevo 770 (Visualsonics, Toronto, Canada) (2). Three to six beats were averaged for each measurement. LV dimension and wall thickness were measured at end diastole and end systole using the American Society of Echocardiography criteria. Mean wall thickness was calculated as the average of the interventricular septal wall thickness and LV posterior wall thickness. LV mass was calculated using the formula: LV mass = {1.05·[(IVSd + LVPWd + LVIDd)3 − (LVID)3]}·0.8, where IVSd is interventricular septal wall thickness in diastole, LVPWd is LV posterior wall thickness in diastole, and LVIDd is LV internal dimension (LVID) in diastole.
LV mass was normalized to body weight (ratio of LV weight to body weight) and tibial length (ratio of LV weight to tibial length). LV end-diastolic volume (LVEDV) and end-systolic volume (LVESV) were determined using the Simpson's method of discs. Ejection fraction (EF, %) was calculated as: EF = 100·(LVEDV − LVESV)/LVEDV.
Transverse aortic constriction.
TAC was carried out as previously described (2). Briefly, mice were initially anesthetized using inhalation of isoflurane (in a chamber containing 5% isoflurane vapors) until a surgical anesthetic plane was established. A small (5 mm) skin incision was made in the neck region, and the mouse was orotracheally intubated and connected to a rodent ventilator. Buprenorphine (0.05 mg/kg sc) was injected for analgesia. A horizontal incision of 5 mm was made at the level of the suprasternal notch. Transverse arch banding was performed by placing a 7.0 silk suture over a 27-gauge needle, causing complete occlusion of the aorta. The needle was then removed, restoring the aortic lumen but leaving a severely stenotic aortic orifice. A drainage tube was placed in the incision, air was evacuated via the drainage tube, the drainage tube was then removed, and the incision was closed. The mouse was then extubated and allowed to recover under veterinary care on a warming blanket in a container supplemented with oxygen.
Immunohistochemistry.
Hearts from non-TAC and TAC mice of each genotype were bisected, embedded in paraffin, and sectioned on a microtome. Sections were stained with Picrosirius red (PSR) to detect collagen fibers and viewed with polarized light under dark field optics to detect birifringence of collagen fibers. Quantitative analysis of PSR-stained images captured with polarized light were performed in WT control (n = 5), WT TAC (n = 5), PCOLCE2 null control (n = 5), and PCOLCE2 null TAC (n = 5) mice. Five fields chosen at random from each cardiac mouse section were scanned using Sigma Scan software (Aspire Software International, Ashburn VA). Fields with large blood vessels were excluded from the analysis. Areas from the subendocardial to subepicardial space were examined. The epicardial surface (collagen capsule) was excluded. CVF was calculated as the area stained by PSR divided by the total area of interest using Sigma scan software (2).
Hydroxyproline analysis.
Five LVs each from non-TAC and 4-wk TAC hearts of each genotype were used for hydroxyproline analysis (24). Frozen LV tissue was lyophilized, weighed (dry weight), pulverized, resuspended in 1 M NaCl with protease inhibitors, tumbled overnight at 4°C, and centrifuged. The supernatant then contained the NaCl-soluble collagen (i.e., non-cross-linked collagen); the pellet contained the NaCl-insoluble collagen (fully mature cross-linked fibrillar collagen). Each fraction was processed separately using the method described below. Each fractionation underwent acid hydrolysis in 6 N HCl for 18 h at 120°C, followed by neutralization (to pH 7) with 4 N NaOH. Chloramine T was added to each sample and incubated for 20 min. Samples in Ehrlich's reagent, consisting of 60% perchloric acid, 15 ml 1-propanol, and 3.75 g p-dimethyl-amino-benzaldehyde in 25 ml, were incubated at 60°C for 20 min. Absorbance at 558λ was read on a spectrophotometer. Amounts of collagen in each sample were quantified as microgram hydroxyproline per milligram dry weight LV.
Papillary muscle preparation and myocardial function measurements.
Mice (5 mice of each genotype, non-TAC and 4-wk TAC) were anesthetized and given 200 U of heparin intraperitoneally, and then the LV was isolated, the aorta was cannulated, the LV was perfused with 2,3-butanedione monoxime, and the papillary muscle was isolated. The methods used to isolate and study murine papillary muscle were previously described (2). Briefly, passive diastolic stiffness was examined in two ways: 1) define rest stress at maximal muscle length (Lmax) and 2) perform a muscle stretch at a very slow stretch rate (1 mm/min) beginning from near slack length (very lightly preloaded muscle at 0.1 g) to a muscle length of 15% greater than that at slack length (equivalent to Lmax preload). The myocardial stress versus strain relationship during this muscle stretch was used to calculate the passive stiffness constant, β, as Stress = Ae(βStrain) + C, where A and C are constant coefficients. Myocardial stress was calculated from muscle force divided by muscle cross-sectional area, and the strain was calculated as (L − L0)/L0, where L is muscle length during stretch and L0 is muscle length at 0.1-g preload.
Fibroblast isolation and immunoblot analysis.
For in vitro studies, 12 WT and 12 PCOLCE2-null mice were used to generate three independent primary cardiac fibroblast cultures. Fibroblasts were isolated as previously described (10). Protein isolated from detergent (1% deoxycholate) extractions of cell layers grown for 2 days in the presence of ascorbate were separated by SDS-PAGE and transferred to nitrocellulose. Equal amounts of protein were loaded according to bicinchoninic acid assay of detergent-soluble protein in cell layers. Procollagen α1(I) (unprocessed, N- and C-propeptides retained), pC collagen α1(I) (partially processed, C-propeptide retained, N-propeptide removed), and collagen α1(I) (fully processed, both propeptides removed) were detected using primary antibodies against murine anti-collagen I (MD Biosciences, St. Paul, MN) and anti-PCOLCE-2 (GeneTex, Irvine, CA), followed by appropriate secondary antibody conjugated to horseradish peroxidase and chemiluminescent reagent. Quantification of protein bands from scanned images was performed using NIH ImageJ software program.
Statistical analysis.
Data are means ± SE in table and figures. Echocardiography data obtained at baseline were comparable for the four experimental groups. Therefore, all comparisons in this study were made at the 4-wk time point between WT, WT TAC, PCOLCE2-null, and PCOLCE2-null TAC. Differences between these four experimental groups were determined using one-way analysis of variance followed by Tukey testing of pairwise analysis. A P value <0.05 was considered significant. The survival data presented in Fig. 1 used a standard Kaplan-Meier analysis and differences in dichotomous variables were determined by χ2 test. P < 0.05 was considered significant. The authors had full access to, and take full responsibility for, the integrity of the data.
RESULTS
PCOLCE2-null mice.
PCOLCE2-null mice were found to be fertile and appeared grossly phenotypically normal and indistinguishable from WT mice (6, 12). No significant differences in body weight, heart weight, or tibia length were found in adult PCOLCE2-null mice compared with age-matched WT animals (3–5 mo of age). PSR-stained sections of hearts from WT and PCOLCE2-null mice revealed no apparent differences in cardiac interstitial collagen in the absence of PCOLCE2 expression (Fig. 2, top left and bottom left). No change in structure or function as detected by echocardiography and gravimetric measurements were detected in PCOLCE2-null mice versus that of WT mice (control, Table 1).
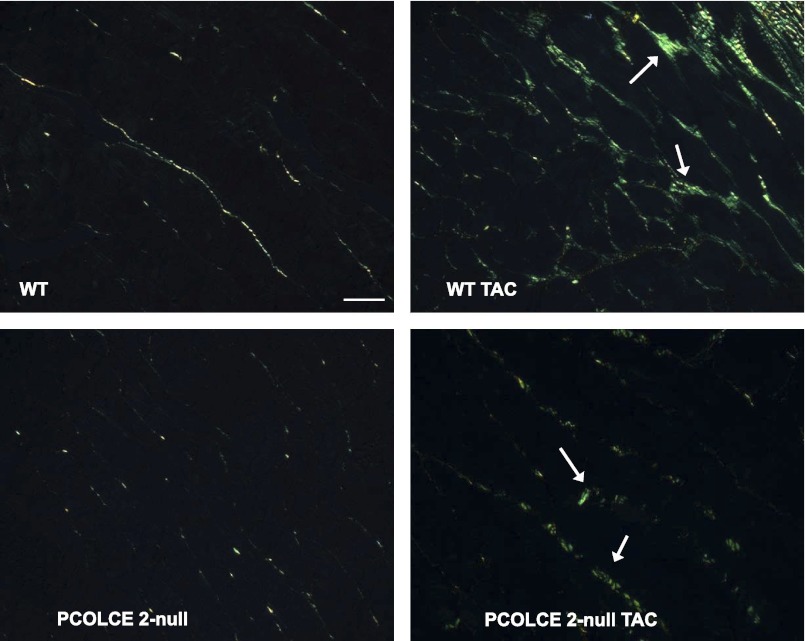
Fig. 2.
Histochemistry of fibrillar collagen in PCOLCE2-null hearts. Picrosirius red-stained section of left ventricle (LV) from WT (top left), WT TAC (top right), PCOLCE2-null (bottom left), and PCOLCE2-null TAC (bottom right) mice revealed apparent decreases in interstitial collagen deposition in response to TAC. Arrows indicate insoluble collagen. Bar = 20 μm.
Table 1.
Effects of 4-wk TAC on LV structure and function in PCOLCE2-null vs. WT mice
| Control |
TAC |
|||
|---|---|---|---|---|
| WT | PCOLCE2-null | WT | PCOLCE2-null | |
| Age, mo | 4 ± 1 | 4 ± 1 | 5 ± 1 | 5 ± 1 |
| BW, g | 29 ± 2 | 38 ± 4 | 28 ± 1 | 34 ± 5 |
| LV mass, mg | 84 ± 9 | 115 ± 11 | 175 ± 12* | 176 ± 12* |
| LV/BW, mg/g | 2.9 ± 0.1 | 3.1 ± 0.1 | 6.3 ± 0.4* | 5.4 ± 0.4* |
| AOPG. mmHg | 0 | 0 | 97 ± 3* | 97 ± 2* |
| LV EDV, μl | 58 ± 3 | 64 ± 5 | 62 ± 4 | 61 ± 6 |
| LV EF, % | 62 ± 2 | 62 ± 2 | 62 ± 4 | 60 ± 3 |
| Wall thickness, mm | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1* | 1.1 ± 0.1* |
| Heart rate, beats/min | 552 ± 15 | 571 ± 8 | 594 ± 11 | 527 ± 9 |
Values are means ± SE.
AOPG, aortic pressure gradient across the transverse aortic constriction (TAC); BW, body weight; EDV, end-diastolic volume; EF, ejection fraction; LV, left ventricular; PCOLCE2, procollagen C-endopeptidase enhancer 2; WT, wild-type.
P < 0.05 vs. corresponding baseline control. No significant differences were found between genotypes at baseline or TAC. LV mass, AOPG, LV EDV, and LV EF were quantified from data collected by echocardiography.
Pressure overload.
PCOLCE2-null mice were found to exhibit a similar incidence of mortality, ∼50%, in response to PO as that of WT mice (∼50%, Fig. 1). The myocardium of WT mice underwent significant hypertrophic growth. LV weight versus body weight (ratio of LV to BW) was significantly increased after TAC versus non-TAC control WT (Table 1). No significant differences in ejection fraction and end-diastolic volume were found in WT TAC versus non-TAC control WT (2). Likewise, PCOLCE2-null TAC mice had comparable amounts of hypertrophic growth versus non-TAC PCOLCE2-null mice as well as no significant differences in ejection fraction or end-diastolic volume (Table 1).
Collagen accumulation in PO myocardium.
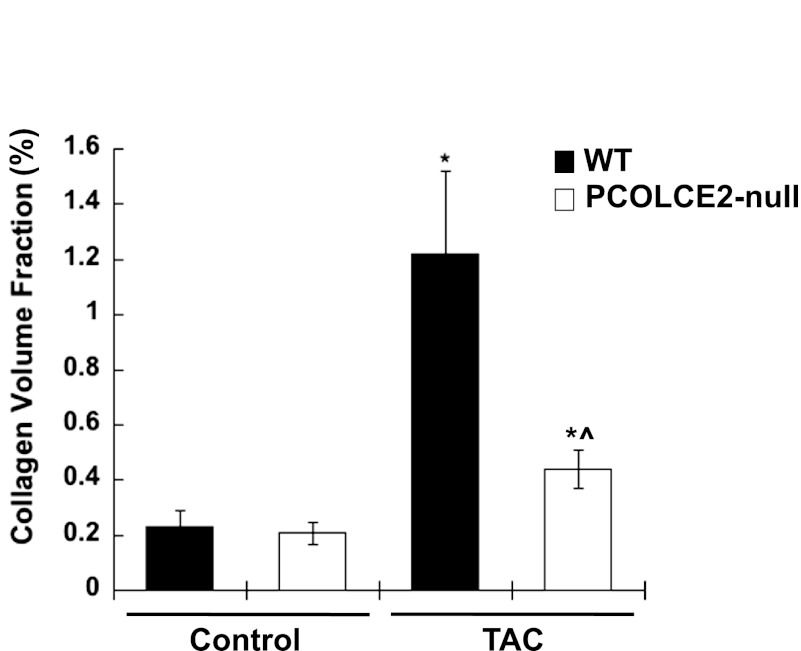
Differences in levels of collagen in control PCOLCE2-null versus control WT cardiac tissue were not detected by either CVF measurements or by hydroxyproline analysis (Figs. 3 and 4). However, in mice subjected to TAC, WT mice developed significant fibrosis indicated by a ∼sixfold increase in CVF (Fig. 3). Similar increases in CVFs in WT TAC mice were observed in our previous studies (2). However, in the absence of PCOLCE2 expression, decreased amounts of fibrillar collagen were evident in hearts with PO versus that of WT TAC. CVF revealed only a ∼twofold increase in collagenous interstitial extracellular matrix (ECM) in PO myocardium in the absence of PCOLCE2-null expression. Decreases in interstitial collagen as well as perivascular fibrosis were apparent in PCOLCE2-null hearts (Fig. 2).
Fig. 3.
Reductions in collagen volume fractions (CVFs) in PCOLCE2-null TAC hearts: morphological quantification of CVF from Picrosirius red-stained sections of WT (black bars) and PCOLCE2-null (white bars) LV with and without TAC demonstrated significant increases in CVF in WT TAC mice, whereas PCOLCE2-null TAC showed less of an increase in CVF vs. WT TAC hearts. No differences in CVF in control WT vs. PCOLCE2-null hearts were found. Error bars = means ± SE. *P < 0.05 vs. corresponding non-TAC control; ^P < 0.05 vs. WT TAC.
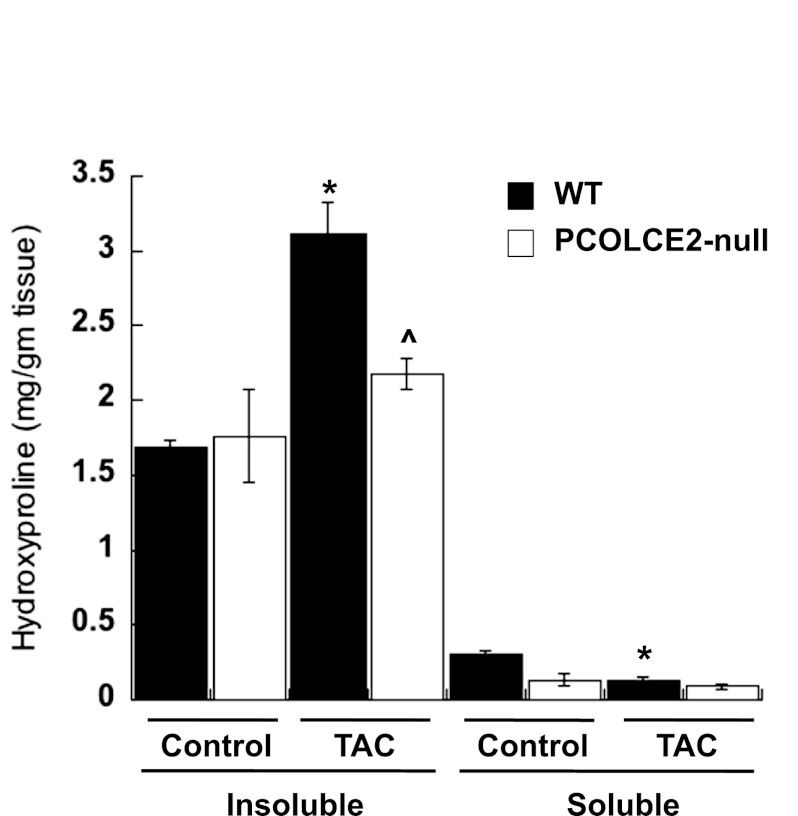
Fig. 4.
Decreased insoluble collagen incorporation in PCOLCE2-null TAC hearts. Hydroxyproline analysis of WT (black bars) and PCOLCE2-null (white bars) LV with and without TAC showed increased amounts of insoluble collagen in WT TAC LV vs. WT control, whereas no differences in insoluble collagen in PCOLCE2-null TAC vs. control LV were detected. In addition, levels of soluble collagen were significantly reduced in WT TAC vs. control WT LV, whereas there was no detectable decrease in levels of soluble collagen in PCOLCE2-null LV. No significant differences in levels of soluble collagen between WT and PCOLCE2-null control hearts were found (P = 0.09). Error bars = means ± SE. *P < 0.001 vs. corresponding control; ^P < 0.005 vs. WT TAC.
Hydroxyproline analysis of soluble and insoluble cardiac collagen in WT and PCOLCE2-null TAC mice also showed decreased amounts of insoluble collagen in PCOLCE2-null TAC versus that of WT TAC mice (Fig. 4). No differences in levels of insoluble collagen as measured by hydroxyproline analysis were detected in control PCOLCE2-null hearts compared with WT hearts. In response to PO, levels of soluble collagen were found to decrease significantly in WT mice (Fig. 4). Interestingly, soluble cardiac collagen levels were slightly decreased in PCOLCE2-null control versus WT control tissue; however, a significant decrease in soluble collagen was not detected in PCOLCE2-null mice in response to TAC.
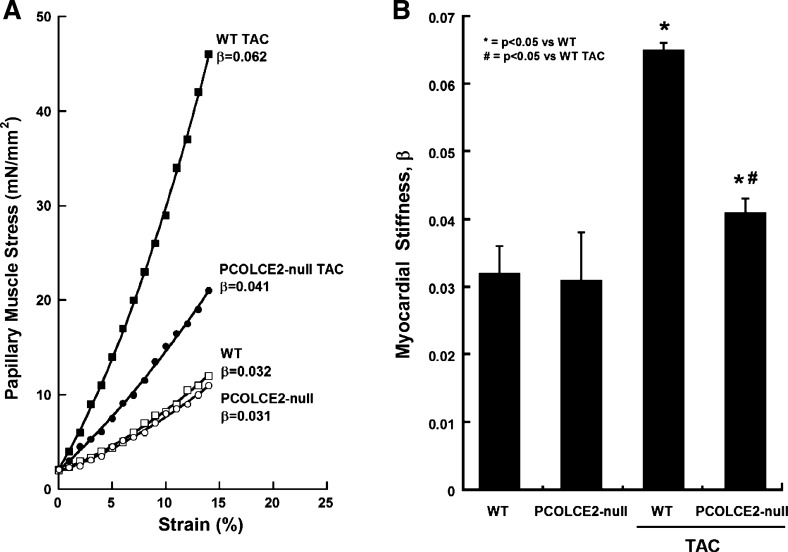
Myocardial passive stiffness.
Representative results from each group of mice are shown in Fig. 5A. WT mice subjected to TAC demonstrated notable increases in stiffness compared with WT control, whereas the increases in stiffness demonstrated by PCOLCE2-null TAC mice were significantly less than that of WT TAC and not significantly increased from PCOLCE2 control. No differences in stiffness were detected in control PCOLCE2-null mice versus control WT mice. Pooled data generated from papillary muscles taken from five mice per group demonstrated significantly higher levels of stiffness in WT TAC compared with WT control and compared with PCOLCE2-null TAC mice (Fig. 5B). Hence, in line with insoluble collagen accumulation, PCOLCE2 was demonstrated to be necessary for the development of robust collagen deposition and associated increases in muscle stiffness of PO myocardium.
Fig. 5.
Reductions in diastolic stiffness in TAC papillary muscle of PCOLCE2-null mice. A: examples of the passive diastolic myocardial stress vs. strain curves for the 4 groups of animals studied: control WT, control PCOLCE2-null, WT TAC, and PCOLCE2-null TAC. B: values (means ± SE) of the passive stiffness constant, β, for the 4 groups of animals studied: WT (control), PCOLCE2-null (control), WT TAC, and PCOLCE2-null TAC. *P < 0.05 vs. corresponding non-TAC control, #P < 0.05 vs. WT TAC.
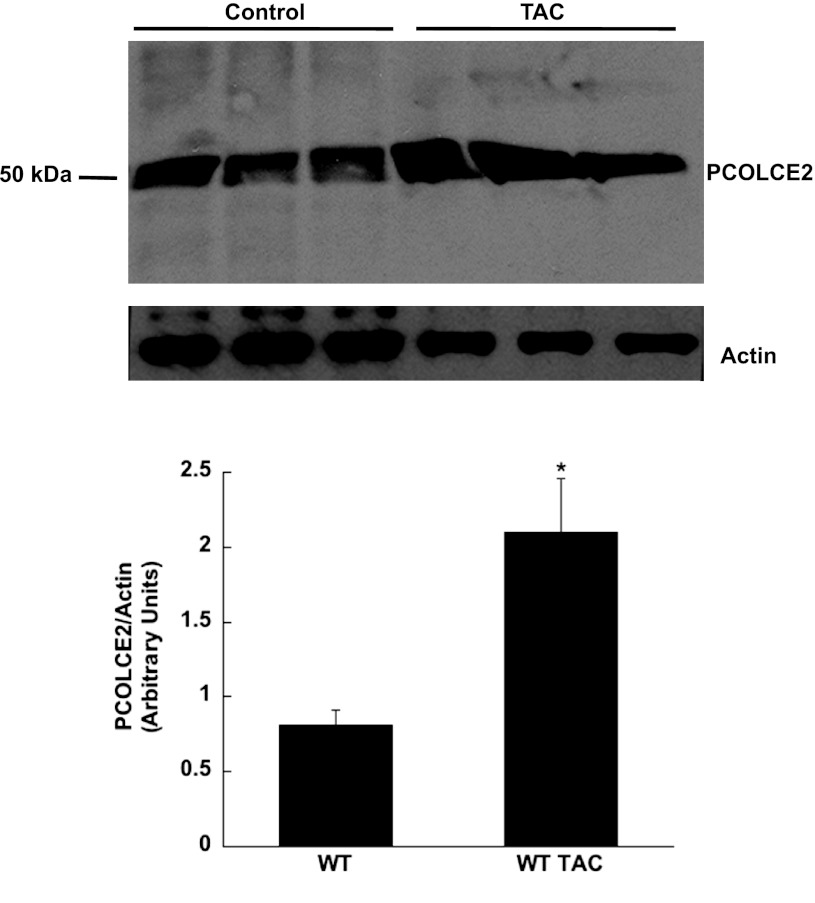
Increased PCOLCE-2 in PO myocardium.
Levels of PCOLCE-2 were increased in WT TAC myocardium compared with control. Shown in Fig. 6, top, are Western blot analysis of protein extracted from three separate WT control and WT TAC hearts. Quantification of the protein bands is shown in Fig. 6, bottom. PCOLCE-2 levels were found to significantly increase approximately twofold in TAC hearts.
Fig. 6.
Levels of PCOLCE-2 are increased in TAC hearts. Top: protein extracted from control and TAC hearts were separated by SDS-PAGE and probed with anti-PCOLCE-2 antibodies. Bottom: quantification of protein bands shown in A. *P < 0.05 vs. control.
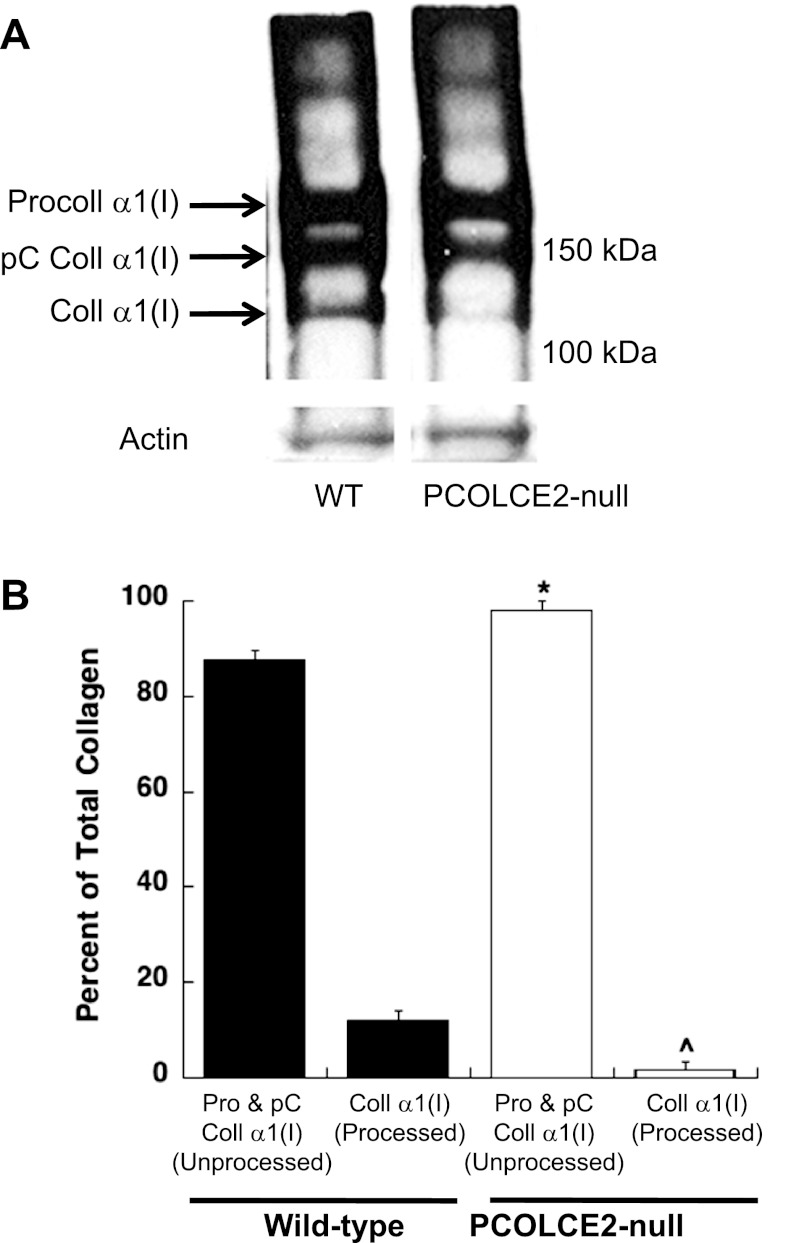
Procollagen processing by PCOLCE-2-null cardiac fibroblasts.
As shown in Fig. 7A, immunoblot analysis of collagen produced by WT cardiac fibroblasts showed the presence of procollagen α1(I), the processing intermediate pC collagen α1(I) (procollagen with the N-propeptide removed), and collagen α1(I) (both propeptides removed). As PCOLCE2 has been shown to enhance C-propeptide proteinase activity, procollagen α1(I) and pC collagen α1(I) are predicted to be present at higher levels in PCOLCE2-null cells. Immunoblot analysis revealed that PCOLCE2-null cells did, in fact, exhibit higher levels of procollagen and pC collagen α1(I) relative to that of WT cells (Fig. 7A). Quantification from three separate primary cell isolations is shown in Fig. 7B, demonstrating a consistent decrease in procollagen processing by cardiac fibroblasts in the absence of PCOLCE2 expression. We conclude that PCOLCE2 enhances procollagen processing in cardiac fibroblasts and that lack of PCOLCE2 reduces cardiac collagen deposition in PO hearts.
Fig. 7.
Significant reductions in procollagen processing by PCOLCE2-null cardiac fibroblasts. A: procollagen processing by primary cardiac fibroblasts isolated from WT and PCOLCE2-null mice was evaluated by Western blot analysis of proteins soluble in detergent from fibroblast cell layers (see materials and methods). Noncontiguous lanes from a single gel are shown. Procoll, procollagen; pC, C-propeptide; Coll, collagen. B: quantification of 3 separate cardiac fibroblast preparations revealed a consistent decrease in procollagen processing in the absence of PCOLCE2-null (white bars) vs. WT (black bars) cells demonstrated by increased amounts of unprocessed, procollagen α1(I)/pC collagen α1(I) and decreased amounts of processed, collagen α1(I). Pro, procollagen. Error bars = means ± SE. *P < 0.05 vs. WT procollagen α1(I); ^P < 0.05 vs. WT collagen α1(I).
DISCUSSION
Cardiac PCOLCE2.
PCOLCE2 is predicted to enhance the cleavage of procollagen I by C-propeptide proteinases such as BMP-1 in tissues and has been shown to mediate such activity in vitro (20). Greater relative expression of PCOLCE2 in hearts suggested an important role for this enhancer activity in cardiac interstitium (20). However, to date no published effects of the functional significance of cardiac PCOLCE2 expression have been reported. Although expression of PCOLCE2 did not appear to significantly affect baseline levels of collagen in the cardiac interstitium, we report here a notable decrease in collagen accumulation in response to PO hypertrophy in PCOLCE2-null TAC versus WT TAC mice. The decrease in collagen accumulation was accompanied by significant reductions in papillary muscle stiffness in PCOLCE2-null TAC compared with WT TAC hearts. Thus we conclude that PCOLCE2 is an important determinant of procollagen processing in PO myocardium and that the absence of PCOLCE2 results in reduced collagen content accompanied by diminished ventricular stiffness in response to PO hypertrophy.
Decreased procollagen processing by PCOLCE2-null cardiac fibroblasts.
We hypothesized that decreases in cardiac collagen in PO myocardium of PCOLCE2-null mice resulted from an incomplete processing of procollagen and thus decreased deposition of collagen to insoluble ECM. To determine whether differences in procollagen processing by cardiac fibroblasts occurred in the absence of PCOLCE2, primary cardiac fibroblasts from WT and PCOLCE2-null mice were monitored for procollagen processing in vitro. We have found that procollagen and procollagen intermediates are not present at detectable levels in cardiac tissue, whereas procollagen processing by cardiac fibroblasts in culture is less efficient and thus facilitates analyses of procollagen processing events (9). In fact, a consistent decrease in procollagen processing was observed in PCOLCE2-null cardiac fibroblasts compared with WT. Nonetheless, procollagen C-proteinase activity was apparent in PCOLCE2-null cardiac fibroblasts. As PCOLCE1 was also found to be expressed in heart and in cardiac fibroblasts, this enhancer might compensate to a limited extent for the absence of PCOLCE2 in cardiac fibroblasts and tissues of PCOLCE2-null mice (16, 19). In addition, secreted frizzled-related protein 2 has also been implicated in the enhancement of procollagen C-propeptide proteinase activity in the heart, although this activity is somewhat controversial (11, 17, 23). We hypothesize that given the importance of procollagen processing in fibril formation, a highly tissue-specific process, the profile of procollagen enhancer proteins facilitates tissue-specific procollagen processing and fibril assembly. However, research into specific functional differences in PCPE activity is just emerging. Nonetheless, clearly a lack of PCOLCE2 resulted in decreased insoluble collagen content in response to PO in PCOLCE2-null versus WT hearts.
Phenotypic alterations in PCOLCE2-null mice.
To date, differences in levels of collagen in PCOLCE2-null tissues have not been previously reported. Francone et al. (6), however, did report a phenotypic difference in lipid profiles from PCOLCE2-null mice versus that of WT. PCOLCE-2 was recently reported to affect proteolytic processing of pro-apolipoprotein (Apo) A-I, the major protein component of high-density lipoprotein, through enhancement of N-terminal cleavage of ApoA-I by BMP-1, the same enzyme responsible for procollagen C-proteinase activity. Accordingly significant differences in plasma ApoA-I levels were found in PCOLCE2-null versus WT mice. The results from PCOLCE2-null mice confirmed previously reported associations between polymorphisms in the human PCOLCE-2 gene and serum high-density lipoprotein levels (26). We predict that differences found in collagen deposition in myocardium in response to PO were based primarily in procollagen processing as changes in circulating lipid profiles is not anticipated to significantly reduce collagen deposition in response to PO.
Procollagen postsynthetic processing in cardiac interstitium.
Procollagen postsynthetic processing is a potential point of regulation in tissues as soluble procollagen must be processed by removal of the C- and N-terminal propeptides before efficient incorporation of monomeric collagen into insoluble ECM. Once incorporated, collagen is stabilized by the formation of cross-links. We have previously shown that the expression of the matricellular secreted protein acidic and rich in cysteine (SPARC), a collagen-binding protein implicated in postsynthetic processing, was necessary for insoluble collagen incorporation in response to TAC (2). Other transgenic null mice with abrogation of matricellular protein expression, such as thrombospondins (TSP) 1 and 4, have also demonstrated phenotypic alterations in myocardial function in response to TAC (7, 25). In both the case of TSP-1−/− and TSP-4−/− mice, alterations in collagen accumulation and ventricular function were found, although cellular mechanisms of action of TSPs in terms of procollagen processing have yet to be characterized. Lysyl oxidase, the enzyme responsible for modification of lysyl residues that form collagen cross-links, was likewise shown to be a critical factor in modulating diastolic function in pigs (14).
The fact that greater levels of soluble collagen were not detected in PCOLCE-2-null TAC hearts was unexpected. For example, SPARC-null hearts demonstrated increased levels of soluble collagen in response to TAC (2). However, whether the C-propeptide is removed extracellularly or intracellularly in cardiac fibroblasts is not currently established. Canty et al. (3) has shown that C-propeptide processing can occur in intracellular vesicles before release of collagen molecules to the extracellular space. Possibly, inefficient intracellular processing of the C-propeptide in the absence of PCOLCE-2 resulted in increased intracellular procollagen degradation that would not necessarily affect pools of soluble collagen extracted in 1 M NaCl. However, the decrease observed in levels of insoluble collagen in PCOLCE-2-null hearts strongly suggested that inefficient processing of the C-propeptide of procollagen I leads to decreases in insoluble collagen accumulation. Whether efficient removal of the N-terminal propeptide of procollagen is also a determinant of collagen deposition in response to PO remains to be assessed and will likely be the subject of future studies.
Conclusions.
The decrease observed in collagen content in the absence of PCOLCE2 expression in PO myocardium illustrates the importance of procollagen postsynthetic processing in the regulation of collagen accumulation in the heart. Hence, factors that participate in the regulation of postsynthetic procollagen processing are potential markers to monitor collagen accumulation or perhaps to serve as targets to alleviate fibrotic deposition of myocardial collagen.
GRANTS
This study was supported by the Research Service of the Department of Veterans Affairs (to M. R. Zile and A. D. Bradshaw) and National Institutes of Health Grants PO1-HL-48788 (to M. R. Zile), HL-094517 (to A. D. Bradshaw), and P20-RR-017696 (to A. D. Bradshaw).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.F.B., Y.Z., L.R., M.R.Z., and A.D.B. conception and design of research; C.F.B., Y.Z., A.O.V.L., L.R., and A.D.B. performed experiments; C.F.B., Y.Z., A.O.V.L., L.R., and A.D.B. analyzed data; C.F.B., L.R., M.R.Z., and A.D.B. interpreted results of experiments; C.F.B. and A.D.B. prepared figures; C.F.B., M.R.Z., and A.D.B. edited and revised manuscript; C.F.B., M.R.Z., and A.D.B. approved final version of manuscript; A.D.B. drafted manuscript.
REFERENCES
- 1.Bishop JE, Rhodes S, Laurent GJ, Low RB, Stirewalt WS. The regulation of collagen deposition in the hypertrophying heart. Ann NY Acad Sci 752: 236–239, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation 119: 269–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol 165: 553–563, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, Wilcox W, Krakow D, Cohn DH, Reardon W, Byers PH, Lapiere CM, Prockop DJ, Nusgens BV. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet 65: 308–317, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eghbali M, Eghbali M, Robinson TF, Seifter S, Blumenfeld OO. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc Res 23: 723–729, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Francone OL, Ishida BY, de la Llera-Moya M, Royer L, Happe C, Zhu J, Chalkey RJ, Schaefer P, Cox C, Burlingame A, Kane JP, Rothblat GH. Disruption of the murine procollagen C-proteinase enhancer 2 gene causes accumulation of pro-apoA-I and increased HDL levels. J Lipid Res 52: 1974–1983, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frolova EG, Sopko N, Blech L, Popovic ZB, Li J, Vasanji A, Drumm C, Krukovets I, Jain MK, Penn MS, Plow EF, Stenina OI. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J 2012. February 23 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaasch WH, Delorey DE, St, John Sutton MG, Zile MR. Patterns of structural and functional remodeling of the left ventricle in chronic heart failure. Am J Cardiol 102: 459–462, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Gaasch WH, Zile MR. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med 55: 373–394, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Harris BS, Zhang Y, Card L, Rivera LB, Brekken RA, Bradshaw AD. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am J Physiol Heart Circ Physiol 301: H841–H847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He W, Zhang L, Ni A, Zhang Z, Mirotsou M, Mao L, Pratt RE, Dzau VJ. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci USA 107: 21110–21115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinzel K, Bleul CC. The Foxn1-dependent transcripts PCOLCE2 and mPPP1R16B are not required for normal thymopoiesis. Eur J Immunol 37: 2562–2571, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol 26: 508–523, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato S, Spinale FG, Tanaka R, Johnson W, Cooper Gt, Zile MR. Inhibition of collagen cross-linking: effects on fibrillar collagen and ventricular diastolic function. Am J Physiol Heart Circ Physiol 269: H863–H868, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science 271: 360–362, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Kessler-Icekson G, Schlesinger H, Freimann S, Kessler E. Expression of procollagen C-proteinase enhancer-1 in the remodeling rat heart is stimulated by aldosterone. Int J Biochem Cell Biol 38: 358–365, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, Yamada C, Liu TC, Huang G, Basson CT, Kispert A, Greenspan DS, Sato TN. Secreted frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 11: 46–55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64: 403–434, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Shalitin N, Schlesinger H, Levy MJ, Kessler E, Kessler-Icekson G. Expression of procollagen C-proteinase enhancer in cultured rat heart fibroblasts: evidence for co-regulation with type I collagen. J Cell Biochem 90: 397–407, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Steiglitz BM, Keene DR, Greenspan DS. PCOLCE2 encodes a functional procollagen C-proteinase enhancer (PCPE2) that is a collagen-binding protein differing in distribution of expression and post-translational modification from the previously described PCPE1. J Biol Chem 277: 49820–49830, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Stroud JD, Baicu CF, Barnes MA, Spinale FG, Zile MR. Viscoelastic properties of pressure overload hypertrophied myocardium: effect of serine protease treatment. Am J Physiol Heart Circ Physiol 282: H2324–H2335, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Takahara K, Kessler E, Biniaminov L, Brusel M, Eddy RL, Jani-Sait S, Shows TB, Greenspan DS. Type I procollagen COOH-terminal proteinase enhancer protein: identification, primary structure, and chromosomal localization of the cognate human gene (PCOLCE). J Biol Chem 269: 26280–26285, 1994 [PubMed] [Google Scholar]
- 23.von Marschall Z, Fisher LW. Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1). Matrix Biol 29: 295–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93: 440–447, 1961 [DOI] [PubMed] [Google Scholar]
- 25.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 58: 902–911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Gardner J, Pullinger CR, Kane JP, Thompson JF, Francone OL. Regulation of apoAI processing by procollagen C-proteinase enhancer-2 and bone morphogenetic protein-1. J Lipid Res 50: 1330–1339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350: 1953–1959, 2004 [DOI] [PubMed] [Google Scholar]