Abstract
Alveolar hypoxia produces a rapid and widespread systemic inflammation in rats. The inflammation is initiated by the release into the circulation of monocyte chemoattractant protein-1 (MCP-1) from alveolar macrophages (AMO) activated by the low alveolar Po2. Circulating MCP-1 induces mast cell (MC) degranulation with renin release and activation of the local renin-angiotensin system, leading to microvascular leukocyte recruitment and increased vascular permeability. We investigated the effect of dexamethasone, a synthetic anti-inflammatory glucocorticoid, on the development of the systemic inflammation of alveolar hypoxia and its site(s) of action in the inflammatory cascade. The inflammatory steps investigated were the activation of primary cultures of AMO by hypoxia, the degranulation of MCs by MCP-1 in the mesentery microcirculation of rats, and the effect of angiotensin II (ANG II) on the leukocyte/endothelial interface of the mesentery microcirculation. Dexamethasone prevented the mesentery inflammation in conscious rats breathing 10% O2 for 4 h by acting in all key steps of the inflammatory cascade. Dexamethasone: 1) blocked the hypoxia-induced AMO activation and the release of MCP-1 and abolished the increase in plasma MCP-1 of conscious, hypoxic rats; 2) prevented the MCP-1-induced degranulation of mesentery perivascular MCs and reduced the number of peritoneal MCs, and 3) blocked the leukocyte-endothelial adherence and the extravasation of albumin induced by topical ANG II in the mesentery. The effect at each site was sufficient to prevent the AMO-initiated inflammation of hypoxia. These results may explain the effectiveness of dexamethasone in the treatment of the systemic effects of alveolar hypoxia.
Keywords: alveolar macrophages, mast cells
alveolar hypoxia occurs when inspired Po2 is reduced, as in altitude, or in pulmonary diseases presenting regional or global hypoventilation. Rats and mice exposed to low inspired Po2 develop a widespread systemic inflammation within minutes of the onset of hypoxia. This response is characterized by generation of reactive oxygen species (ROS) (35, 42), degranulation of perivascular mast cells (MCs) (36), rolling, adherence, and emigration of leukocytes to the perivascular space, and increased vascular permeability (43, 44). The systemic inflammation has been observed in the mesentery (3, 4, 35, 36, 42–44), skeletal muscle (6, 11, 12, 26, 32), and pial microcirculations (23). The similar characteristics of these responses reflect common underlying mechanisms in these diverse microvascular beds.
A substantial body of evidence indicates that the inflammation is not initiated by low systemic tissue Po2 but by a mediator released from alveolar macrophages (AMO) activated by the low alveolar Po2 (3, 4, 11). The possible role of an AMO-borne circulating mediator as the trigger of inflammation in systemic microvascular beds was suggested by the observation that the inflammation occurs only when alveolar Po2 is reduced, independent of the systemic microvascular Po2 (6, 32), and that topical application of plasma from hypoxic rats and of supernatant of hypoxic AMO induces inflammation in tissues of normoxic animals (6, 11, 26). The AMO-borne mediator, which has been identified as monocyte chemoattractant protein-1 (MCP-1), a chemokine of the CC family, also known as CCL2, is transported by the circulation and activates MCs (3), which release renin and activate the local renin-angiotensin system (RAS) system (5). Angiotensin II (ANG II) generated as a result of the RAS activation initiates the microvascular phase of the inflammatory response (4, 5, 12). The systemic inflammation is abrogated by depletion of AMO (11), stabilization of MCs (36), and blockade of the RAS (12), as well as by administration of antioxidants and nitric oxide donors (42, 43).
Numerous links between hypoxia and inflammation have been demonstrated. At the cellular level, it is now clear that, in addition to reduced tissue Po2, inflammatory agents and ROS are capable of promoting the stabilization of hypoxia-inducible factors and thereby influence the expression of genes that control O2 delivery and uptake (31, 38). At the organism level, acute alveolar hypoxia in humans or laboratory animals is frequently associated with systemic effects that present an inflammatory component: examples are the illnesses of high altitude such as acute mountain sickness and high-altitude cerebral edema (1, 13, 14, 16, 19) and the multiple organ failure secondary to atelectasis (18), acute lung injury (29, 37), and pulmonary contusion (27). Conversely, the edema and tissue swelling of inflammation leads to impaired O2 delivery and tissue hypoxia. Accordingly, the mechanisms responsible for the inflammation of hypoxia, the factors that modify it, and the role of inflammation on the overall mechanisms of adaptation to hypoxia constitute an important and growing research subject.
Dexamethasone is a synthetic glucocorticoid used clinically for its anti-inflammatory properties and, more specifically, to reduce associated edema. Hence, dexamethasone is highly effective in the treatment of acute diseases of high altitude, in particular high-altitude cerebral edema (20). In addition, recent studies also show that dexamethasone improves exercise capacity and pulmonary gas exchange in subjects prone to the development of high-altitude pulmonary edema (10). The present experiments were designed to determine the effect of dexamethasone on the systemic inflammation of alveolar hypoxia and to investigate the possible site(s) of the systemic inflammatory cascade at which dexamethasone may act. Dexamethasone influences AMO and MC function, and has anti-inflammatory effects in various systemic microvascular beds; accordingly, we reasoned that it may act at different sites of the inflammatory cascade of hypoxia. The results show that dexamethasone acts in all key steps of the inflammation and that its effect on each site is sufficient to abrogate the systemic inflammation initiated by activation of AMO.
METHODS
The Animal Care and Use Committee of the University of Kansas Medical Center, an institution accredited by the American Association for Accreditation of Laboratory Animal Care, approved all procedures.
Dexamethasone Pretreatment
Dexamethasone (0.1 mg/kg) was administered subcutaneously 24 h before the experiments to male Sprague-Dawley rats; a second dose was given 30 min before the initiation of the experiments. Untreated controls were given vehicle with the same schedule.
Primary Cultures of AMO
AMO were harvested by bronchoalveolar lavage (BAL) from dexamethasone-treated and control rats anesthetized with pentobarbital sodium, 40 mg/kg ip. Catheters were placed in the jugular vein (PE-50) and in the trachea (PE-240). After euthanasia with an overdose of pentobarbital sodium (150 mg/kg iv), BAL was carried out as described previously (11). The fluid collected was centrifuged at 1,500 rpm for 10 min, the cell pellet was resuspended in 2 ml DMEM with 10% serum, plated in a sterile flask and placed in an incubator at 37°C, and equilibrated with 5% CO2 in air for 45 min. The supernatant was discarded and replaced with serum-free DMEM equilibrated with 15% O2-5% CO2-80% N2. After 30 min, the equilibrating gas mixture was replaced with a hypoxic gas mixture consisting of 5% O2-5% CO2-90% N2. These gas mixtures provide Po2 values that approximate those to which AMO are exposed in vivo during normoxia and during 10% O2 breathing, respectively. The cultures were gassed via a needle placed through the cap of the culture dish and connected to the gas source. With the use of this approach, steady-state Po2 values are reached within 2–3 min of equilibration (4).
An electrochemical detection system (Apollo 4000; World Precision Instruments, Sarasota, FL) was used to determine H2O2 concentration in the supernatant, as described before (4). At the end of the hypoxic period, a 0.2-ml sample of supernatant was obtained for MCP-1 concentration measurement with enzyme-linked immunosorbent assay (ELISA) (SABiosciences, Frederick, MD). At the end of the experiment, the supernatant was removed, and 2 ml of 0.4% Trypan blue was added to the culture and mixed for 2 min. Photographs of five different areas of the culture, containing ∼200 cells each, were obtained 30 min after Trypan blue addition. Cell viability expressed as the percentage of cells excluding Trypan blue exceeded 98% in all experiments.
Peritoneal Lavage
Peritoneal MCs and macrophages were harvested by peritoneal lavage of control and dexamethasone-treated rats anesthetized with ketamine, 40 mg/kg, and atropine, 5 mg/kg im (4). Before the lavage, a catheter was placed in the jugular vein to obtain a blood sample for determination of plasma concentration of stem cell factor (SCF, c-kit ligand) using an ELISA kit (Ray Biotech, Norcross, GA), after which the animals were killed with 150 mg/kg pentobarbital sodium intravenously. After lavage, MCs were separated from macrophages by differential centrifugation using a Percoll solution. MCs isolated essentially had no contamination with macrophages. The cells were counted using a hemocytometer.
Bright-Field Intravital Microscopy
The procedures for intravital microscopy of the mesentery have been described in detail before (43, 44). Briefly, male Sprague-Dawley rats (225–250 g) were anesthetized with ketamine, 40 mg/kg, and atropine, 5 mg/kg im. PE-50 catheters were placed in the jugular vein and carotid artery for injection of solutions and measurement of arterial blood pressure and heart rate. The abdomen was opened via a midline incision, and the ileocecal portion of the intestine was gently drawn out, exteriorized, and mounted on a transparent plastic stage. The intestinal loop was covered with Saran wrap to prevent drying of the tissue and to minimize the effect of ambient oxygen on the mesenteric microcirculation. The Saran wrap cover was briefly lifted when solutions were applied topically to the mesentery. The animals were covered with a thermal blanket to maintain rectal temperature at 37°C. Postcapillary venules of 20–40 μm diameter were selected for microscopic observation. Adherent leukocytes were defined as those remaining stationary for at least 30 s. Leukocyte-endothelial adherence was expressed as the number of adherent leukocytes per 100 μm vessel. Ruthenium red, 5 mg/100 ml, was used to detect degranulation of MCs. MC degranulation intensity (MCDI) was estimated using image analysis (AnaliSYS software Soft Imaging, Lakewood, CO) and expressed in arbitrary units. At least five MC were analyzed in each experiment. At the end of the experiment, the rats were killed with pentobarbital sodium (150 mg/kg iv).
Measurement of Extravasation of Fluorescent Albumin
Rats were prepared for intravital microscopy of the mesentery circulation as described above. After a suitable venule was identified by bright-field microscopy, fluorescein isothiocyanate (FITC)-labeled bovine albumin was injected intravenously (50 mg/kg), and images were obtained under fluorescence microscopy. To minimize photo bleaching, fluorescence recording was <15 s in any given area. Fluorescence from FITC-albumin (excitation wavelength 420–490 nm, emission wavelength 520 nm) was detected with an F-view SIS camera. Images were analyzed for intravascular fluorescence intensity in an area including the full width of the vessel and 100 μm in length, avoiding areas with underlying vessels. Extravascular fluorescence was measured on both sides of the 100-μm venule segment. The same section of the microcirculation was analyzed in all the images of a given experiment. The magnitude of albumin extravasation was estimated by the ratio of the extravascular-to-intravascular fluorescence.
Exposure of Conscious Rats to Hypoxia
Dexamethasone-treated and untreated rats, 250–300 g, were anesthetized with ketamine, 40 mg/kg, and atropine, 5 mg/kg im. PE-50 catheters were placed in the carotid artery and the external jugular vein, tunneled subcutaneously, exteriorized at the back of the neck, cut at a length of ∼2.5 cm, and flame-sealed. Four to 6 h after recovery from anesthesia, an arterial blood sample was obtained under normoxic conditions, after which the animals were placed in a Lucite chamber where either room air or 10% O2-90% N2 were circulated. Blood samples were obtained at 5, 30, and 60 min of hypoxia. Blood removed was replaced with donor blood. Arterial blood pressure and heart rate and chamber O2 concentration were recorded continuously. At the end of the experiment, the animals were killed with pentobarbital sodium, 150 mg /kg iv.
In a separate series of experiments, dexamethasone-treated and untreated conscious rats were placed in the hypoxic chamber at 10% O2. After 4 h of exposure to hypoxia, the animals were anesthetized with ketamine, 40 mg/kg, and atropine, 5 mg/kg im and prepared for intravital microscopy of the mesenteric microcirculation as described above. The animals breathed 10% O2-90% N2 throughout surgery and the entire experimental procedure. An additional group of untreated animals was placed in the chamber for 4 h under normoxic conditions (FiO2 = 0.21).
Statistics
Data are presented as means ± SE. Each experiment served as its own control, with the data after a given treatment compared with the corresponding average control. Average control was the mean of all the values observed in the control period. Significance was established with a t-test for paired samples. Intergroup comparisons were carried out with a one-way ANOVA followed by Bonferroni tests for multiple comparisons. A P value <0.05 was considered to indicate a significant difference.
RESULTS
Effect of Dexamethasone on the Inflammatory Response of the Mesenteric Microcirculation to Alveolar Hypoxia
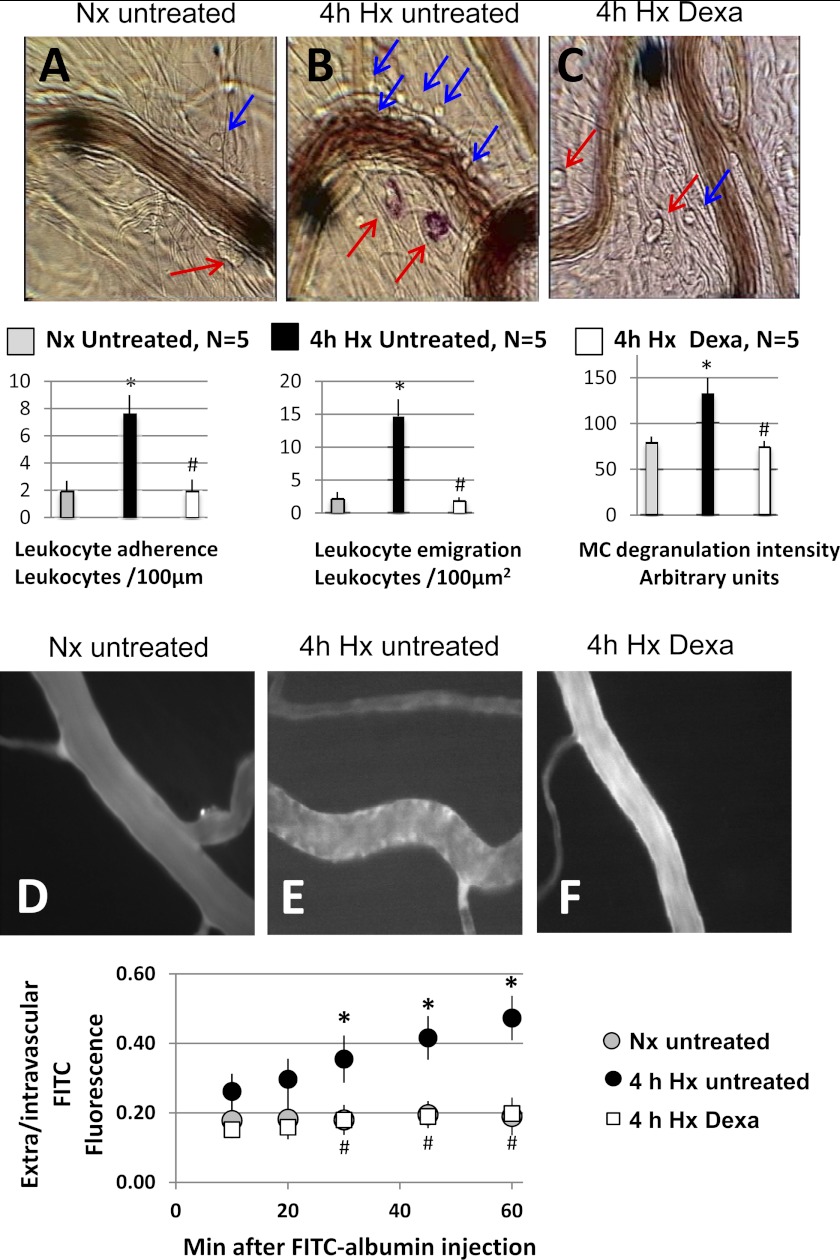
Figure 1 shows representative bright-field (Fig. 1, A–C) and fluorescence (Fig. 1, D–F) photomicrographs of the mesentery microcirculation of normoxic untreated animals and untreated and dexamethasone-treated animals that had been exposed to 4 h of hypoxia in the conscious state. The hypoxic animals were prepared for intravital microscopy while they continued to breathe 10% O2. The bar graphs under the bright-field photomicrographs show average values of inflammatory markers as follows: leukocyte-endothelial adherence, leukocyte emigration, and MCDI, obtained in five rats of each group. The graph below photomicrographs D–F shows the time course of the changes in extravascular-to-intravascular FITC fluorescence intensity ratio. The untreated rats show the typical response of the mesentery microcirculation to alveolar hypoxia (compare Fig. 1A with 1B): increased leukocyte-endothelial adherence, emigration of leukocytes to the perivascular fluid, degranulation of MCs evidenced by uptake of ruthenium red, and increased extravasation of FITC-labeled albumin (compare Fig. 1D with 1E), demonstrated by the continued increase in the extravascular-to-intravascular ratio of fluorescence intensity, documented for 1 h after FITC-albumin injection. It is clear that dexamethasone substantially attenuates the expression of all inflammatory markers generated by exposure to hypoxia for 4 h.
Fig. 1.
Top: representative bright-field photomicrographs of mesentery postcapillary veins of an untreated rat breathing room air (A) and of an untreated rat (B) and a dexamethasone-treated rat (C) after 4 h of breathing 10% O2 in the conscious state. Blue arrows indicate adherent or emigrated leukocytes, and red arrows indicate mast cells (MCs). The red color of MCs due to uptake of ruthenium red indicates degranulation. The large solid black circles are used to align the optical Doppler velocimeter and occasionally are moved to obtain a better image of the leukocyte-endothelial interface for photographs. The bar graphs below the bright-field images are average data (n = 5/group) of leukocyte-endothelial adherence (left), leukocyte emigration (center), and MC degranulation intensity (right). Bright-field data were obtained 30 min after the postsurgery stabilization period, with the animals in the hypoxia groups breathing 10% O2 continuously. D–F: representative fluorescence images of mesenteric postcapillary venules of an untreated rat breathing room air (D), and of an untreated (E) and a dexamethasone-treated rat (F) after 4 h of breathing 10% O2 in the conscious state. The graph below the fluorescence images depicts the time course of the extravascular-to-intravascular fluorescein isothiocyanate (FITC)-albumin fluorescence intensity ratio after iv injection of the dye in the three groups of rats. Data are mean ± SE of 5 rats in each group. *P < 0.05, 4 h hypoxia (Hx) untreated vs. normoxia (Nx) untreated. #P < 0.05, 4 h Hx Dexa vs. 4 h Hx untreated.
Effect of dexamethasone on the response of AMO to hypoxia.
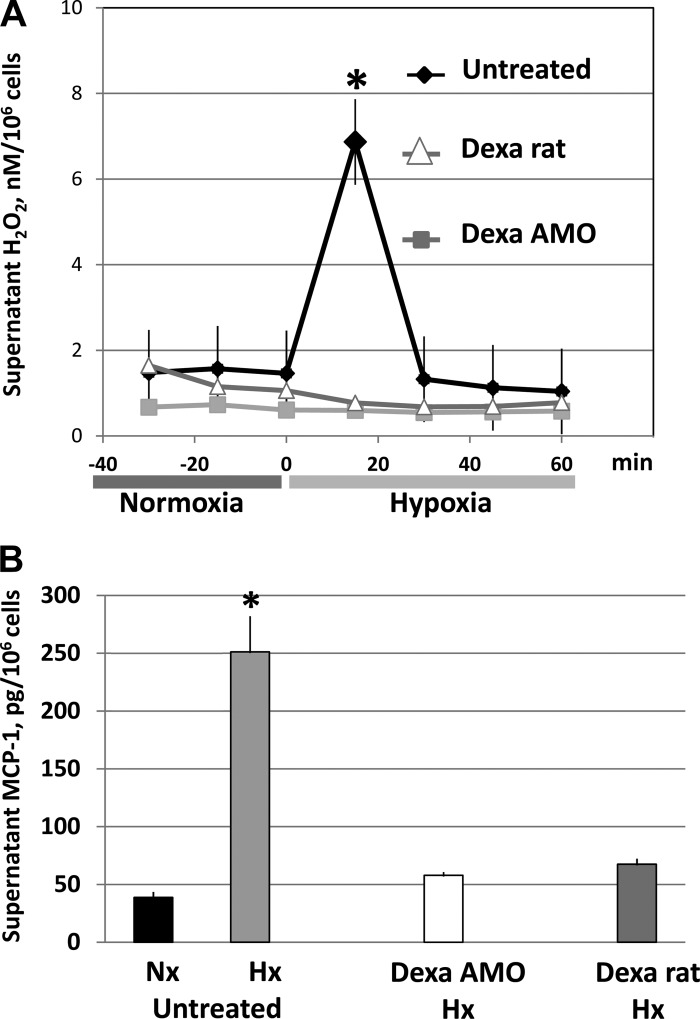
As expected from previous data (3, 4), AMO obtained from untreated rats showed a rapid and transitory release of H2O2 after 15 min of exposure to 5% O2 (Fig. 2A). This response is the consequence of the generation of superoxide that characterizes the respiratory burst produced by AMO activation (21): H2O2 generated from the dismutation of superoxide produced during the burst is released into the supernatant. Release of H2O2 did not occur when Po2 was lowered in cultures obtained from rats pretreated with dexamethasone or in cultures obtained from untreated rats to which dexamethasone (1 μM) was added to the supernatant before lowering Po2. Each set of data represents the average of five individual primary cell cultures.
Fig. 2.
Effect of dexamethasone (Dexa) on the hypoxia-induced release of H2O2 (A) and monocyte chemoattractant protein (MCP)-1 (B) by primary cultures of alveolar macrophages (AMO). Cultures were equilibrated with 15% O2-5%CO2-balance N2 (normoxia) and 5% O2-5% CO2-balance N2 (hypoxia). Samples for supernatant MCP-1 concentration were obtained at the end of the normoxic period in the untreated group and at 15 min of hypoxia in the untreated, Dexa AMO, and Dexa rat groups. Dexa rats, AMO harvested from rats treated with dexamethasone; Dexa AMO, AMO from untreated rats to which dexamethasone (1 μM) was added to the supernatant 30 min before the experiment. Data are means ± SE of 5 primary cultures in each group. *P < 0.05 vs. corresponding average H2O2 concentration obtained during normoxia (A) and vs. untreated Nx sample (B).
The activation of AMO by hypoxia resulted in the expected release of MCP-1 into the supernatant (Fig. 2B and Ref. 3). The inhibitory effect of dexamethasone on AMO activation by hypoxia was reflected in abrogation of the release of MCP-1. This was the case in the cultures obtained from dexamethasone-treated rats as well as in the AMO cultures treated with dexamethasone in vitro, in neither of which MCP-1 concentration differed significantly from the normoxic untreated control.
Effects of dexamethasone on plasma MCP-1 concentration during hypoxia.
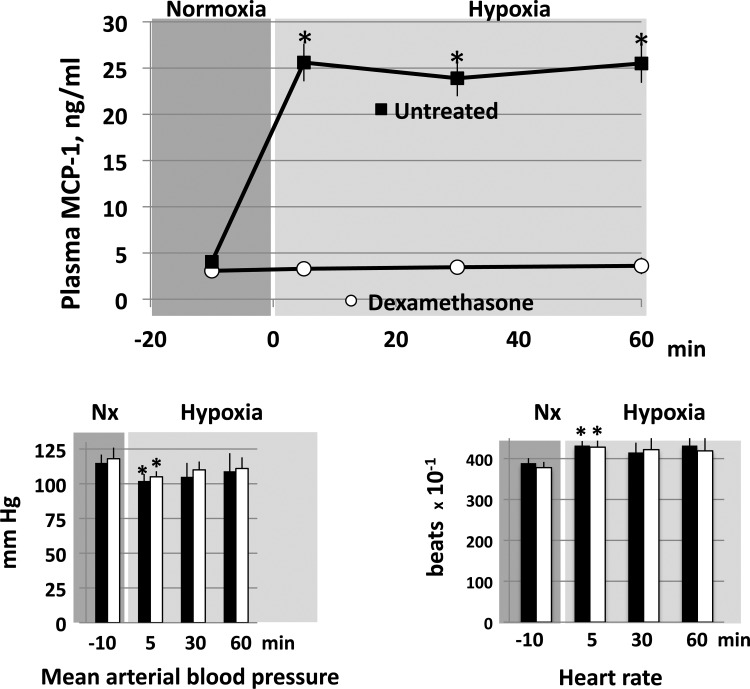
Figure 3 shows that alveolar hypoxia induced the expected (3) rapid and sustained rise in plasma concentration of MCP-1 in the untreated rats (n = 5). Dexamethasone completely inhibited this response: plasma MCP-1 remained at prehypoxic levels throughout the 60 min of exposure to hypoxia (n = 5). Mean arterial blood pressure and heart rate of the untreated rats showed the typical response of conscious rats to this level of hypoxia (12): a modest decrease in blood pressure accompanied by an increase in heart rate; both were significantly different from their respective controls only at 5 min of hypoxia. There was no difference between the responses of untreated and dexamethasone-treated rats.
Fig. 3.
Plasma MCP-1 concentration (top) and mean arterial blood pressure and heart rate (bottom) of conscious rats before and during 10% O2 breathing. Data are means ± SE of 5 untreated and 5 dexamethasone-treated rats. *P < 0.05 vs. corresponding normoxic control.
Effect of dexamethasone on the response of the normoxic mesenteric microcirculation to MCP-1.
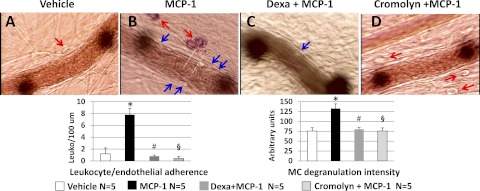
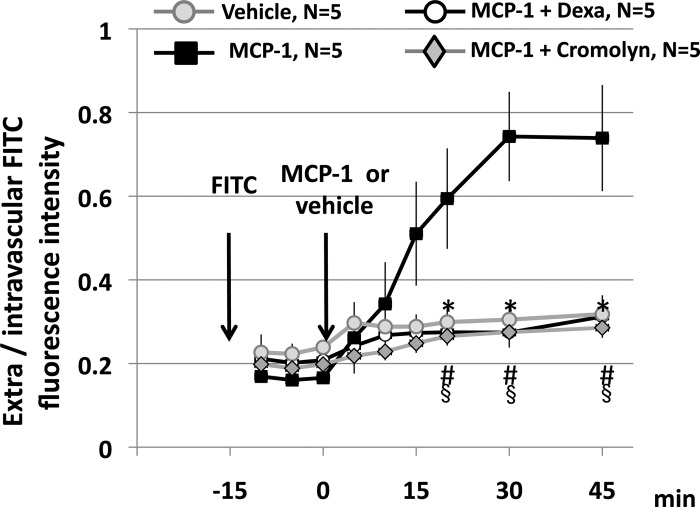
Topical application of MCP-1 (30 ng/ml) to the mesentery of normoxic untreated rats produced MC degranulation, evidenced by the uptake of ruthenium red, and leukocyte-endothelial adherence (compare with the effect of vehicle, Fig. 4A). These effects of MCP-1 were prevented by dexamethasone (Fig. 4C). Administration of the MC stabilizer cromolyn (8-mg bolus followed by 0.15 mg/ml, 2 ml/h iv) to previously untreated rats prevented the effects of topical MCP-1 on MC degranulation as well as the increase in leukocyte-endothelial adherence (Fig. 4D).
Fig. 4.
Representative photomicrographs of mesentery postcapillary venules of untreated rats receiving vehicle (A) or MCP-1, 30 ng/ml (B). C: dexamethasone-treated rat receiving MCP-1. D: effect of MCP-1 on a rat pretreated with the MC stabilizer cromolyn 30 min before MCP-1. The images were obtained ∼30 min after topical application of MCP-1 (30 ng/ml) or vehicle on the mesentery. Red arrows point to MCs, and blue arrows point to adherent leukocytes (Leuko). MC degranulation in the untreated rat is evidenced by the red coloration produced by ruthenium red uptake. No MCs were identified in the image of the dexamethasone-treated rat. Cromolyn prevents the MC degranulation and leukocyte-endothelial adherence produced by topical MCP-1 applied after a 30-min control period. The data depicted in the bar graphs were obtained 30 min after administration of MCP-1 or vehicle. Data are means ± SE of 5 rats in each group. *P < 0.05, MCP-1 vs. vehicle. #P < 0.05, MCP-1 + Dexa vs. MCP-1. §P < 0.05, MCP-1 + cromolyn vs. MCP-1.
Topical application of MCP-1 to untreated rats produced a significant increase in the extravasation of FITC-albumin, as shown by the progressive increase in the extravascular-to-intravascular ratio of fluorescence intensity above the values observed before MCP-1 application (Fig. 5). In contrast, data of untreated rats administered vehicle show that extravasation of FITC-albumin after 1 h of injection is minimal, as shown by the lack of significant difference between the initial and final values of extravascular-to-intravascular fluorescence intensity ratio in this group. As with the inflammatory markers shown in Fig. 4, dexamethasone prevented the extravasation of albumin that follows topical application of MCP-1. When MCP-1 was applied in control rats pretreated with cromolyn, no increase in FITC-albumin was observed. This, together with the data of Fig. 4, show that dexamethasone prevents the activation of MCs by MCP-1. Furthermore, the finding that MCP-1 has no inflammatory effects in rats treated with the MC stabilizer cromolyn suggests that MCP-1 has no direct effects downstream of MCs.
Fig. 5.
Effect of MCP-1 on the extravasation of FITC-albumin. The arrows indicate the times of iv injection of FITC-albumin and the topical administration of MCP-1 or vehicle. Data are means ± SE of 5 rats in each group. *P < 0.05, vehicle vs. MCP-1. #P < 0.05, MCP-1 + Dexa vs. MCP-1. §P < 0.05, MCP-1 + cromolyn vs. MCP-1.
Effect of Dexamethasone on Peritoneal MC Number and on the Plasma Concentration of Soluble DCF
In the course of these experiments, it became apparent that the number of MCs visualized in the microcirculation was lower in the dexamethasone-treated rats. This was confirmed by counting MC recovered by peritoneal lavage, which showed a significantly lower number of peritoneal MC, but not of peritoneal macrophages, in the dexamethasone-treated rats (Table 1).
Table 1.
Effect of dexamethasone on peritoneal mast cell and macrophage number and on plasma levels of soluble SCF
| n | Untreated | Dexamethasone | |
|---|---|---|---|
| PMO, 106 cells/rat | 5 | 15.60 ± 1.39 | 15.25 ± 1.65 |
| MC, 106 cells/rat | 5 | 1.34 ± 0.08 | 0.40 ± 0.06* |
| Plasma SCF, pg/ml | 3 | 424.8 ± 52.3 | 160.1 ± 13.9* |
Values are means ± SE; n, no. of rats in each group.
SCF, stem cell factor; PMO, peritoneal macrophages; MC, peritoneal mast cells.
P < 0.05 vs. untreated.
The plasma concentration of SCF, a cytokine required for MC development, was significantly reduced in the dexamethasone-treated rats (Table 1).
Effect of Dexamethasone on the Response of the Mesentery to Topical ANG II.
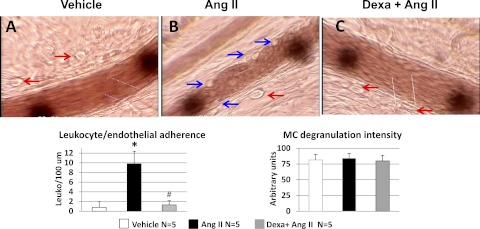
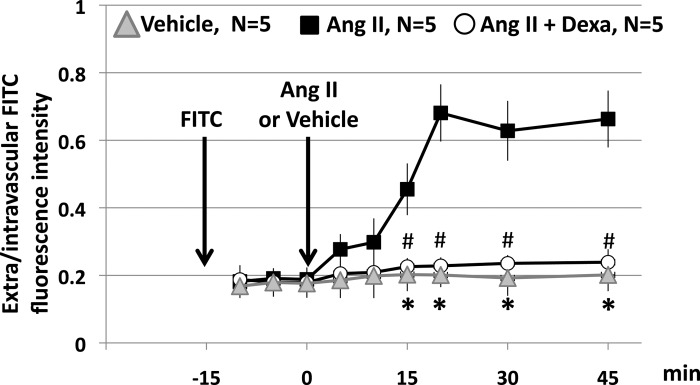
Figure 6 shows photomicrograph obtained 30 min after topical application of vehicle (Fig. 6A) or ANG II (10 nM) to the mesentery of untreated (Fig. 6B) and dexamethasone-treated (Fig. 6C) rats: the typical response to ANG II, leukocyte-endothelial adherence in the absence of MC degranulation, (4) is evident in the untreated rats. Dexamethasone completely blocked the response to ANG II. Figure 7 shows that dexamethasone also prevents the progressive increase in FITC-albumin that follows topical ANG II.
Fig. 6.
Representative photomicrographs of mesentery postcapillary venules of an untreated rat given vehicle (A) or ANG II, 10 nM (B) and of a dexamethasone-treated rat given ANG II (C). Images were obtained ∼30 min after the topical administration of ANG II or vehicle. Vehicle and ANG II were applied after a 30-min control period. The red arrows point to MC, and the blue arrows point to adherent leukocytes. Notice the adherence of leukocytes in the absence of MC degranulation produced by ANG II in the untreated rat. The data depicted in the bar graph were obtained 30 min after application of ANG II or vehicle. Data are means ± SE of 5 rats/group. *P < 0.05, vehicle vs. ANG II. #P < 0.05, Dexa + ANG II vs. ANG II.
Fig. 7.
Effect of dexamethasone in the ANG II-induced increase in FITC-albumin extravasation. The vertical arrows indicate the times of injection of FITC-albumin and the topical administration of ANG II (10 nM) or vehicle. Data are means ± SE of 5 rats in each group. *P < 0.05, vehicle vs. ANG II. # P < 0.05, ANG II + Dexa vs. ANG II.
DISCUSSION
The results show that pretreatment with dexamethasone prevents the inflammatory response of the mesenteric microcirculation to alveolar hypoxia. This is due to inhibitory effects of dexamethasone at each of the key steps of the inflammatory cascade: AMO, MCs, and the leukocyte-endothelial interface. Furthermore, the data show that the effect at each site is sufficient to block the inflammatory response.
Experimental Design
To determine the effects of dexamethasone on the systemic inflammation of alveolar hypoxia, we exposed conscious rats to 10% inspired O2 for 4 h, using a dexamethasone dose comparable to that used in humans (20). While degranulation of MCs by circulating AMO-borne MCP-1 is evident within minutes of the reduction of alveolar Po2 (36), some inflammatory markers such as leukocyte emigration and increased vascular permeability develop with a slower time course (43). Exposing the animals to hypoxia for 4 h ensured the full expression of all inflammatory markers in the untreated rats and provided an adequate background to evaluate the effects of dexamethasone.
The inspired Po2 used in these experiments is equivalent to that of an altitude of 5,500 meters. This value was chosen because the response of rats to this fairly severe degree of hypoxia has been studied extensively (3, 4, 6, 11, 12, 32, 35. 36, 42–44). In addition, since the incidence of altitude illnesses increases with altitude, we reasoned that it would be important to determine the effectiveness of dexamethasone in conditions in which there is a high likelihood of these diseases. While the systemic inflammatory response to this level of hypoxia has been well characterized, there is less information on the systemic effects of more moderate levels of hypoxia, such as those that may be seen in patients with pulmonary disease. Reducing Po2 from normoxic values to ∼65 and to ∼5 Torr produced increasing release of H2O2 in isolated AMO cultures (4). This suggests that AMO are activated at relatively mild levels of alveolar hypoxia and that the intensity of their response is Po2-dependent, although it is not known if the graded release of H2O2 is accompanied by proportionate releases of MCP-1. Similarly, a Po2-dependent increase in microvascular ROS fluorescence intensity, leukocyte-endothelial adherence, and MC degranulation was observed in the mesentery of rats breathing hypoxic O2 mixtures that result in alveolar Po2 values of ∼70, 40, and 30 Torr, respectively (35). Nevertheless, while this evidence suggests that the AMO-initiated systemic inflammation may develop at Po2 levels closer to those seen in pulmonary disease, the present results are applicable only to the relatively severe degree of hypoxia used in these experiments. It is reasonable to assume, however, that, if dexamethasone is effective at low Po2 levels, it would also be effective in less severe hypoxia.
The mesenteric microcirculation was studied because the effects of hypoxia have been well characterized in this microvascular bed (3, 4, 35, 36, 42–44); in addition, exposure of the mesentery is a relatively simple procedure that can be accomplished in a few minutes, thereby limiting the effects of prolonged surgery and anesthesia. The inflammatory responses of mesentery, skeletal muscle, and pial microcirculation have common characteristics such as MC degranulation and RAS activation (3, 4, 6, 11, 12, 23, 26, 32, 35, 36 42–44). Thus, data in the mesentery are likely to be a reasonable representation of an inflammatory response initiated by a circulating mediator activating MCs, a cell type ubiquitously distributed throughout the organism.
Pretreatment rather than dexamethasone administration after the onset of hypoxia was done because the inflammation involves several linked processes with different time courses. Given the rapid initiation of the inflammation, the timing of the treatment would introduce another variable in the experimental design. Administration of dexamethasone 24 h before the onset of hypoxia avoided these possible drawbacks. While this approach does not mimic the clinical use of dexamethasone, it does provide useful information on the mechanism of action of this agent.
A major objective of the research was to determine the possible sites of action of dexamethasone in the inflammatory cascade. This was investigated by determining the effect of dexamethasone on the response of each key site to its specific stimulus. Previous work has shown that AMO are activated by low Po2 (3, 4), whereas MCs and the leukocyte-endothelial interface do not respond to a reduction of local Po2 (4, 6, 32). Rather, MCs are activated by circulating AMO-borne MCP-1 (3), and the inflammatory responses at the leukocyte-endothelial interface are the result of ANG II (4, 12) generated by the release of renin from MCs (5).
Finally, it should be acknowledged that, while this experimental design is appropriate to address the questions asked, it is not directed to provide information on the cellular signal transduction pathways affected by dexamethasone. The results obtained provide an overall view of the effects of dexamethasone on the inflammatory cascade at the intact organism level and represent a necessary initial step in the elucidation of the mechanisms of action of dexamethasone in the inflammation of hypoxia.
Activation of AMO by Hypoxia
The results clearly show that dexamethasone prevents the development of the systemic inflammation of hypoxia by acting at each of the key sites of the inflammatory cascade. Dexamethasone prevented the respiratory burst and release of MCP-1 that follows exposure of AMO to hypoxia (Fig. 2). The role of dexamethasone and other glucocorticoids in attenuating the activation of AMO by specific effectors has long been known and is thought to explain the beneficial effects of these agents in some pulmonary diseases such as asthma (2, 7). Dexamethasone inhibits the in vivo and in vitro release of several cytokines and chemokines, including MCP-1 induced by activation of AMO by LPS and other inflammatory agents (2, 24). Our results extend these observations by showing that dexamethasone blocks the effects of AMO activation and release of MCP-1 elicited by a different stimulus, in this case reduced Po2. It should be noted that the response of AMO to hypoxia appears to differ from that elicited by other stimuli such as lipopolysaccharide (LPS), at least within the time frame of the development of the systemic inflammation of alveolar hypoxia: previous results show that from 16 inflammatory mediators investigated, MCP-1 was the only agent released from AMO within minutes of the reduction of Po2 (4).
Dexamethasone had the same effect whether it was administered to the animals or to cultures of AMO harvested from untreated rats (Fig. 2, A and B). Previous studies also showed similar in vivo and in vitro effects of dexamethasone in reducing AMO phagocytic activity and LPS-induced release of tumor necrosis factor-α (24).
As a result of the inhibition of AMO activation by dexamethasone, plasma MCP-1 of the treated rats failed to increase during hypoxia (Fig. 3). The main source of the plasma MCP-1 increase, at least during the first 30 min of hypoxia, appears to be AMO, since this increase is eliminated by AMO depletion with intratracheal clodronate liposome administration (3). After 60 min of hypoxia, however, plasma MCP-1 concentration increases moderately in AMO-depleted rats, suggesting the participation of sources of MCP-1 in addition to AMO. In the present experiments, plasma MCP-1 of the dexamethasone-treated rats failed to increase during the entire 60 min of hypoxia, implying that dexamethasone also blocks the release of MCP-1 from non-AMO sources.
These results provide additional support to the critical role of AMO activation in the initiation of the inflammation and show that the effect of dexamethasone on AMO is sufficient to prevent the systemic inflammation of hypoxia. Depletion of AMO prevents the hypoxia-induced inflammation in skeletal muscle (11) and mesentery (3, 4); dexamethasone has a similar effect, in this case by functionally eliminating the role of AMO in the initiation of the systemic inflammation.
Response of MCs to MCP-1
The second key step in the inflammatory cascade of alveolar hypoxia is the degranulation of MCs by AMO-borne MCP-1. Local tissue hypoxia does not produce MC degranulation if alveolar Po2 is maintained at normal values (6, 32); similarly, reduction of medium Po2 does not activate MC in vitro (4). MCP-1 administered to normoxic rats replicates the microvascular inflammatory response to alveolar hypoxia (Figs. 4 and 5 and Ref. 3); conversely, blockade of MCP-1 receptors with RS-102895 prevents the development of the microvascular inflammation in hypoxic rats (3). Accordingly, MCP-1 is the appropriate agent to investigate the effects of dexamethasone at this step of the cascade. Prevention of MCP-1-induced MC degranulation by dexamethasone was accompanied by inhibition of the downstream responses, leukocyte-endothelial adherence (Fig. 4), and vascular extravasation of albumin (Fig. 5). These responses highlight the central role of MC activation in the AMO-initiated inflammation and are consistent with previous observations that stabilization of MCs with cromolyn blocks all the downstream inflammatory responses to alveolar hypoxia and that MC secretagogues replicate the microvascular inflammation of hypoxia (36).
The data on the effects of the MC stabilizer cromolyn provide additional evidence concerning the role of MCP-1 in the systemic inflammation of hypoxia. Cromolyn prevented MC degranulation by MCP-1, as well as the downstream inflammatory responses that follow the MC degranulation (Figs. 4 and 5). This shows that the effects of MCP-1 on leukocyte-endothelial adherence and in albumin extravasation are mediated by its effect on MCs, rather than by a direct action of MCP-1 on the leukocyte-endothelial interface. These results support previous findings that, although MCP-1 induces MC degranulation and P-selectin-mediated leukocyte/endothelial interactions in the cremaster microcirculation, it does not induce expression of P-selectin in endothelial cells (41).
In addition to preventing the activation of MCs by MCP-1, dexamethasone produced a substantial decrease in the number of harvested peritoneal MCs. The extent of MC depletion observed here is similar to that found before within analogous time frames (34). It is likely that the effects on MC number and on MC activation by MCP-1 are both due to the well-known inhibition by dexamethasone of the synthesis of SCF, a cytokine necessary for MC development and function that regulates proliferation, differentiation, adhesion, and release of inflammatory mediators by MC (30). SCF synthesis is rapidly inhibited by dexamethasone and other glucocorticoids (8). MC depletion was accompanied by a decrease in the plasma concentration of the soluble form of SCF to <50% of the value observed in the untreated rats (Table 1). The decrease in circulating SCF points to a systemic reduction of this agent, and is consistent with observations of dexamethasone-induced MC depletion in various organs (8, 30, 34). Given the central role of MC activation in the inflammatory response, it is likely that the effect of dexamethasone observed in the mesentery extends to other organs where the inflammation of hypoxia is expressed. Because MC stabilization with cromolyn completely blocks the AMO-initiated inflammation (36), the effect of dexamethasone at this step is likely to be sufficient by itself to prevent the inflammation.
Response of the Leukocyte-Endothelial Interface to ANG II
The final step in the inflammatory cascade is the increase in leukocyte-endothelial interactions and in vascular permeability produced by ANG II. Degranulation of MCs by MCP-1 leads to release of MC renin and angiotensin-converting enzyme, activation of the local RAS, and generation of ANG II (5), which acts downstream of MCs to induce microvascular inflammation (Fig. 6 and Refs. 3, 12, and 28). On the other hand, local hypoxia in the absence of alveolar hypoxia fails to elicit increased leukocyte-endothelial interactions (6, 32). In view of these facts, ANG II is the agent of choice to investigate the effect of dexamethasone on this step of the inflammatory cascade.
Dexamethasone prevented the increases in leukocyte-endothelial adherence and in vascular permeability produced by topical ANG II (Figs. 6 and 7). ANG II participates in virtually all the steps of the inflammatory response, including rolling, adhesion, and extravasation of leukocytes as well as increase in vascular permeability (22). The increased leukocyte-endothelial adhesive interactions produced by ANG II in the mesentery are attenuated by fucoidan and anti-P-selectin monoclonal antibody (28), indicating an involvement of P-selectin in this phenomenon. Dexamethasone attenuates the expression of P-selectin in systemic inflammation (45), suggesting a possible mechanism for dexamethasone in hypoxia. A possible role of P-selectin in the systemic effects of altitude hypoxia is suggested by the elevated levels of soluble P-selectin in individuals acutely exposed to altitude (19).
The effects of ANG II on vascular permeability may in part depend on the release of inflammatory agents by adherent leukocytes; in addition, ANG II directly increases vascular permeability, at least in part by increasing the expression of vascular endothelial growth factor (VEGF), a potent permeability factor (46). Dexamethasone reduces VEGF expression, suggesting a possible mechanism for its effect of ANG II- mediated increase in vascular permeability (9, 17).
While it is clear that dexamethasone blocks the systemic inflammation that develops at the onset of hypoxia, it should be kept in mind that alveolar hypoxia is a complex stimulus; in addition to the phenomena described here, additional mechanisms with different time courses, including changes in gene expression, contribute to the overall response to hypoxia. Whether dexamethasone influences these responses and alters the adaptation of intact organisms to more prolonged periods of hypoxia should be the subject of further research.
The present study highlights the extrapulmonary effects of AMO activation. While most known effects of AMO stimulation occur within the lung, evidence of systemic inflammatory effects resulting from substances released by AMO has been accumulating within the past few years. Examples are the bone marrow effects of cytokines released by AMO in idiopathic pulmonary fibrosis (39) or in response to exposure to particulate matter (15). Cytokines released from AMO after particle deposition in the lung are thought to act on the bone marrow to produce the release of platelets and leukocytes into the systemic circulation and stimulate the release of acute- phase proteins (40), leading to systemic endothelial dysfunction and inflammation of characteristics similar to that described here (25). It has been suggested that overspill of inflammatory mediators from the lung contribute to systemic inflammation in pulmonary diseases, including chronic obstructive pulmonary disease (33). This suggests that activation of AMO by various stimuli may represent a mechanism underlying some of the systemic inflammatory processes observed in various pulmonary pathologies. The findings of this study suggest that the effects of dexamethasone and other glucocorticoids in pulmonary diseases involving AMO may extend to the systemic effects of these conditions.
GRANTS
This work was supported by NIH R01 HL-39443 (N. C. Gonzalez). J. Chao was recipient of a Pre-Doctoral Fellowship from the American Heart Association, Midwest Affiliate. Z. Viets was a recipient of a Biomedical Research Scholarship, University of Kansas Medical Center. P. Donham was a Fellow of the Frontiers in Physiology, American Physiological Society.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.C., J.G.W., and N.C.G. conception and design of research; J.C., Z.V., and P.D. performed experiments; J.C., Z.V., P.D., and N.C.G. analyzed data; J.C., Z.V., P.D., J.G.W., and N.C.G. interpreted results of experiments; J.C., Z.V., and N.C.G. prepared figures; J.C. drafted manuscript; J.C., Z.V., P.D., J.G.W., and N.C.G. edited and revised manuscript; J.C., Z.V., P.D., J.G.W., and N.C.G. approved final version of manuscript.
ACKNOWLEDGMENTS
Current address for J. Chao: Department of Surgery, University of Nebraska Medical Center, Omaha, NE 69198–5850.
REFERENCES
- 1.Beidleman BA, Muza SR, Fulco CS, Cymerman A, Staab JE, Sawka MN, Lewis SF, Skrinar GS. White blood cell, and hormonal responses to 4300 m altitude before and after intermittent altitude exposure. Clin Sci (Lond) 111: 163–169, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, Chung KF. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax 63: 784–790, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Chao J, Donham P, van Rooijen N, Wood JG, Gonzalez NC. Monocyte chemoattractant protein-1 released from alveolar macrophages mediates the systemic inflammation of acute alveolar hypoxia. Am J Respir Cell Mol Biol 45: 53–61, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao J, Wood JG, Blanco VG, Gonzalez NC. The systemic inflammation of alveolar hypoxia is initiated by alveolar macrophage-borne mediator(s). Am J Respir Cell Mol Biol 41: 573–582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao J, Blanco G, Wood JG, Gonzalez NC. Renin released from mast cells activated by circulating MCP-1 initiates the microvascular phase of the systemic inflammation of hypoxia. Am J Physiol Heart Circ Physiol 301: H2264–H2270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dix R, Orth T, Allen J, Wood JG, Gonzalez NC. Activation of mast cells by systemic hypoxia, but not by local hypoxia, mediates increased leukocyte-endothelial adherence in cremaster venules. J Appl Physiol 95: 2495–2502, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Fiel SB, Vincken W. Systemic corticosteroid therapy for acute asthma exacerbations J. Asthma 43: 321–331, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Finotto S, Mekori YA, Metcalfe DD. Glucocorticoids decrease tissue mast cell number by reducing the production of the c-kit ligand, stem cell factor, by resident cells: in vitro and in vivo evidence in murine systems. J Clin Invest 99: 1721–1728, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Pharmacol 411: 231–243, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Fischler M, Maggiorini M, Dorschner L, Debrunner J, Bernheim A, Kiencke S, Mairbaurl H, Bloch KE, Naeije R, Brunner-La Rocca HP. Dexamethasone but not tadalafil improves exercise capacity in adults prone to high-altitude pulmonary edema. Am J Respir Crit Care Med 180: 346–352, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez NC, Allen J, Blanco VG, Schmidt EJ, van Rooijen N, Wood JG. Alveolar macrophages are necessary for the systemic inflammation of acute alveolar hypoxia. J Appl Physiol 103: 1386–1394, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez NC, Allen J, Schmidt EJ, Casillan AJ, Orth T, Wood JG. Role of the renin-angiotensin system in the systemic microvascular inflammation of alveolar hypoxia. Am J Physiol Heart Circ Physiol 292: H2285–H2294, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hartmann G, Tschop M, Fischer R, Bidlingmaier C, Riepl R, Tschop K, Hautmann H, Endres S, Toepfer M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 12: 246–252, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Himadri P, Kumari SS, Chitharanjan M, Dhananjay S. Role of oxidative stress, and inflammation in hypoxia-induced cerebral edema: a molecular approach. High Alt Med Biol 11: 231–244, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Ishi H, Hayashi H, Hogg JC, Fujii T, Goto Y, Sakamoto N, Mukae H, Vincent R, van Eeden SF. Alveolar macrophage-epithelial cell interaction following exposure to atmospheric particles induces the release of mediators involved in monocyte mobilization and recruitment (Abstract). Respir Res 6: 87, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalpana S, Dhananjay S, Lilly G, Rai Sam M. Cobalt chloride attenuates hypobaric hypoxia-induced vascular leakage in rat brain: molecular mechanisms of action of cobalt chloride. Toxicol Applied Pharmacol 231: 354–363, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Lee JM, Park JS, Jo SA, Kim YO, Kim CW, Jo I. Dexamethasone coordinately regulates angiopoietin-1 and VEGF: A mechanism of glucocorticoid-induced stabilization of the blood-brain barrier. Biochem Biophys Res Commun 371: 243–248, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kisala JM, Ayala A, Stephan RN, Chaudry IH. A model of pulmonary atelectasis in rats: activation of alveolar macrophage and cytokine release. Am J Physiol Regul Integr Comp Physiol 264: R610–R614, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Lehmann T, Mairbaurl H, Pleisch B, Maggiorini M, Bartsch P, Reinhart WH. Platelet count and function at high altitude and in high-altitude pulmonary edema. J Appl Physiol 100: 690–694, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Luks AM, Swenson ER. Medication and dosage considerations in the prophylaxis and treatment of high-altitude illness. Chest 133: 744–755, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, Ma GF, Konttinen YT. Regulation of macrophage activation. Cell Mol Life Sci 60: 2334–2346, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 29: 367–374, 2008 [DOI] [PubMed] [Google Scholar]
- 23.McDonald J, Gonzalez NC, Wood JG. Mast cell degranulation promotes the cerebral microvascular inflammatory response to hypoxia (Abstrac). FASEB J 17: A1282, 2003 [Google Scholar]
- 24.Nakamura Y, Murai T, Ogawa Y. Effect of in vitro and in vivo administration of dexamethasone on rat macrophage functions: comparison between alveolar and peritoneal macrophages. Eur Respir J 9: 301–306, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Nurkiewicz TR, Porter DW, Barger M, Milecchia L, Rao KM, Marvar PJ, Hubbs AF, Castronova V, Boegehold MA. Systemic microvascular dysfunction, and inflammation after pulmonary particulate matter exposure. Environ Health Perspect 114: 412–419, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orth T, Allen JA, Wood JG, Gonzalez NC. Plasma from conscious hypoxic rats stimulates leukocyte-endotelial interaction in normoxic cremaster venules J. Appl Physiol 99: 290–297, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Perl M, Gebhard F, Bruckner UB, Ayala A, Braumuller S, Buttner C, Kinzl L, Knoferl MW. Pulmonary contusion causes impairment of macrophage and lymphocyte immune functions and increases mortality associated with a subsequent septic challenge. Crit Care Med 33: 1351–1358, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Piqueras L, Kubes P, Alvarez A, O'Connor E, Issekutz AC, Esplugues JV, Sanz MJ. Angiotensin II induces leukocyte-endothelial cell interactions in vivo via AT1, and AT2 receptor mediated P-selectin upregulation. Circulation 102: 2118–2123, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 288: L3–L15, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol 533: 327–340, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem 275: 26765–26771, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Shah S, Allen J, Wood JG, Gonzalez NC. Dissociation between skeletal muscle microvascular Po2 and hypoxia-induced microvascular inflammation. J Appl Physiol 94: 2323–2329, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Sinden NJ, Stockley RA. Systemic inflammation, and comorbidity in COPD: a result of overspill of inflammatory mediators from the lungs? Review of the evidence. Thorax 65: 930–936, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Soda K, Kawabori S, Kanai N, Bienenstock J, Perdue MH. Steroid-induced depletion of mucosal mast cells and eosinophils in intestine of athymic nude rats. Int Arch Allergy Immunol 101: 39–46, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Steiner DRS, Gonzalez NC, Wood JG. Interactions between reactive O2 species generation and nitric oxide in the microvascular response to systemic hypoxia. J Appl Physiol 93: 1411–1418, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Steiner DR, Gonzalez NC, Wood JG. Mast cells mediate the microvascular inflammatory response to systemic hypoxia. J Appl Physiol 94: 325–334, 2003 [DOI] [PubMed] [Google Scholar]
- 37.St. John RC, Mizer LA, Kindt GC, Weisbrode SE, Moore SA, Dorinsky PM. Acid aspiration-induced acute lung injury causes leukocyte-dependent systemic organ injury. J Appl Physiol 74: 1994–2003, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol Lond 586: 4055–4059, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsantes A, Stergios T, Papadhimitriou SI, Bonovas S, Kavalierou L, Vaipoulos G, Meletis I. Suboptimal erythropoietic response to hypoxemia in idiopathic pulmonary fibrosis. Chest 124: 548–553, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fuji T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic in- flammatory response induced by particulate matter air pollutants. Am J Respir Crit Care Med 164: 826–830, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Wan MX, Wang Y, Liu Q, Schramm R, Thorlacius H. CC chemokines induce P-selectin-dependent neutrophil rolling and recruitment in vivo: intermediary role of mast cells. Br J Pharmacol 138: 698–706, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood JG, Johnson JS, Mattioli LF, Gonzalez NC. Systemic hypoxia promotes leukocyte-endothelial adherence via reactive oxidant generation. J Appl Physiol 87: 1734–1740, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Wood JG, Johnson JS, Mattioli LF, Gonzalez NC. Systemic hypoxia increases leukocyte emigration and vascular permeability in conscious rats. J Appl Physiol 89: 1561–1568, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Wood JG, Mattioli LF, Gonzalez NC. Hypoxia causes leukocyte adherence to mesenteric venules in nonacclimatized, but not in acclimatized, rats. J Appl Physiol 87: 873–881 1999 [DOI] [PubMed] [Google Scholar]
- 45.Xiping Z, Jun F, Jie Z, Bingyan Y, Jing M, Wei Z, Jing Y, Penghui J, Wenqin Y, Ninnin Z, Jiao H. Influence of dexamethasone on the expression levels of P-selectin in multiple organs of rats with severe acute pancreatitis. Inflamm Res 59: 31–39, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Zhao Q, Ishibashi M, Hiasa K, Takeshita A, Egashira K. Essential role of vascular endothelial growth factor in angiotensin II-induced vascular inflammation and remodeling. Hypertension 44: 264–270, 2004 [DOI] [PubMed] [Google Scholar]