Abstract
The isolated retrograde-perfused Langendorff heart and the isolated ejecting heart have, over many decades, resulted in fundamental discoveries that form the underpinnings of our current understanding of the biology and physiology of the heart. These two experimental methodologies have proven invaluable in studying pharmacological effects on myocardial function, metabolism, and vascular reactivity and in the investigation of clinically relevant disease states such as ischemia-reperfusion injury, diabetes, obesity, and heart failure. With the advent of the genomics era, the isolated mouse heart preparation has gained prominence as an ex vivo research tool for investigators studying the impact of gene modification in the intact heart. This review summarizes the historical development of the isolated heart and provides a practical guide for the establishment of the Langendorff and ejecting heart preparations with a particular emphasis on the murine heart. In addition, current applications and novel methods of recording cardiovascular parameters in the isolated heart preparation will be discussed. With continued advances in methodological recordings, the isolated mouse heart preparation will remain physiologically relevant for the foreseeable future, serving as an integral bridge between in vitro assays and in vivo approaches.
Keywords: mouse, isolated heart, optical recording
this article is part of a collection on Historical Perspectives in Cardiovascular Physiology. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
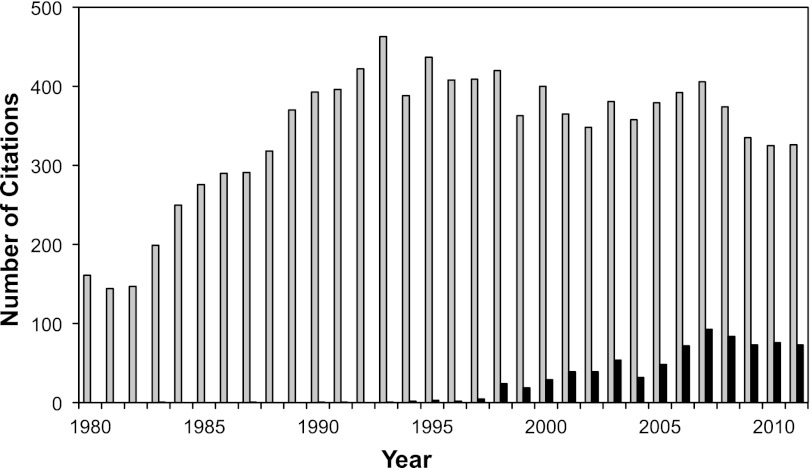
It has been over a century since the inception of the retrograde-perfused heart, according to Oskar Langendorff and almost half a century since James Neely and Henry Morgan developed the ejecting heart model, better known as the “working heart.” To this day, these methodologies continue to be stalwart investigational tools in cardiovascular and pharmacological research. Charting the number of publications per year over the last 30 years (Fig. 1), we see a dramatic rise from the 1980s to the 1990s with the number of publications remaining relatively steady in the past decade. The longevity of these isolated heart preparations stem from their relative simplicity and experimental reproducibility and the ability to investigate myocardial responses in the absence of confounding peripheral neurohormonal factors or other organs in the intact animal. This is particularly relevant when studying ischemia-reperfusion injury or pharmacological interventions in the heart that could trigger noncardiac stress responses or prolonged arrhythmias and even cardiac arrest that would be lethal in the whole animal. The advantages and limitations of the isolated heart methodologies, particularly for the Langendorff-perfused rat heart, and basic instructions on how to establish these preparations are reviewed elsewhere (9, 70, 71). The ability to genetically manipulate and rapidly breed the mouse and the relatively low cost of animal housing have made the mouse the experimental model of choice for gene targeting studies. In this regard, the National Institutes of Health-initiated Knockout Mouse Project (KOMP) aims to generate a resource comprised of mouse embryonic stem cells containing a null mutation in every gene in the mouse genome (49). With the increasing availability of genetically modified mice for cardiovascular research, the isolated mouse heart preparation deserves special consideration and will be the subject of this review. Furthermore, current state-of-the-art methodologies in measuring cardiovascular parameters using the isolated mouse heart will be reviewed.
Fig. 1.
Number of PubMed citations per year, since 1980, that have used an isolated heart preparation (total citations, gray bars). PubMed citations using the mouse heart (black bars) have grown steadily since the late 1990s. The query searched for keywords: isolated perfused heart, Langendorff (some obvious keywords such as lung, liver, kidney, etc., were excluded).
Historical Perspective
The isolated frog heart.
The evolution of the isolated heart model was a concerted effort beginning in 1866 with the publication of the isolated perfused frog heart preparation by Carl Ludwig and Elias Cyon at the Physiological Institute at the University of Leipzig (17, 77). At that time, the frog heart was considered ideal for experimentation; it is a relatively uncomplicated organ containing only one ventricle and no coronary vasculature (the highly trabeculated frog ventricle enables efficient diffusion of gasses, nutrients, and metabolites). As originally described, the frog heart was excised and cannulas were inserted into the aorta and inferior vena cava and the heart perfused with rabbit serum. Diastolic filling via the vena cava was achieved by hydrostatic pressure, and the serum was ejected from the ventricle through the aorta and recirculated via glass tubes back to the vena cava.
The simplicity of the isolated frog heart preparation pioneered by Ludwig belies its importance as many fundamental discoveries in cardiac function were made using this technique. In the early 1880s, Sydney Ringer published a series of reports establishing the importance of perfusate electrolytes: calcium, potassium, and sodium in maintaining contraction in the isolated heart. His discoveries led to the emergence of the formulaic “Ringer's solution,” which served as a precursor for subsequent modified saline solutions such as Tyrode and Krebs-Henseleit buffers (KHBs, commonly used for retrograde heart perfusion, see below), media solutions for in vitro cell culture studies, and clinical cardioplegia and intravenous saline solutions (57). In 1895, Otto Frank used an improved frog heart preparation and showed that increased filling of the frog heart was accompanied by increased isovolumetric pressures. This finding was elaborated almost 20 years later by Ernest Starling who used an isolated dog heart-lung preparation, previously developed by H. Newell Martin (53), to show that end-diastolic volume regulates the work of the heart (37). Although Otto Frank and Ernest Starling are largely credited for describing the Frank-Starling relationship, there is ample evidence that Carl Ludwig and his disciples at the Leipzig Physiological Institute made the initial observations of the Frank-Starling mechanism in the frog heart decades before their more acclaimed counterparts (37, 78). In 1921, Otto Loewi published a clever experiment involving two frog hearts. The first heart was isolated with its nerve supply intact and electrically stimulated at the vagus causing the heart to slow down. The perfusate ejected from the first heart was used to perfuse a second denervated heart, causing the second heart to also slow down. He used this same preparation to demonstrate that stimulation of the sympathetic nerve caused both hearts to speed up. These observations formed the basis of parasympathetic and sympathetic chemical neurotransmission for which Otto Loewi shared the Nobel prize in 1936 with Henry Dale (66).
The Langendorff heart.
In 1895, Oskar Langendorff made the next major breakthrough in the evolution of the isolated heart preparation by using mammalian hearts (mainly cats, but also rabbits and dogs). Langendorff made a key modification from the isolated frog heart model by introducing the concept of retrograde perfusion where the ascending aorta was cannulated and the serum perfusate delivered under constant hydrostatic pressure to the aortic root (45). This reverse perfusion forces the aortic valves to close and the perfusate is shunted to the coronary ostia, thus perfusing the coronary vasculature of the heart. Venous return eventually drains via the coronary sinus into the right atrium, and the effluent is ejected from the right ventricle out the severed pulmonary artery and allowed to drip from the heart. Langendorff measured cardiac function by suturing one end of a thread to the apex of the heart and the other end to a mechanical recording device to measure isometric contractions along the long axis of the heart (45). This method of retrograde perfusion of the isolated mammalian heart became widely known as the Langendorff heart preparation and revolutionized research in mammalian heart physiology and biology (70, 77). Langendorff himself made the seminal discovery that the coronary circulation was essential in maintaining mammalian heart function and confirmed previous fundamental discoveries made in the frog heart concerning the negative chronotropic effect of vagal nerve stimulation and administration of a muscarinic agonist, as well as positive chronotropic effect of atropine (45). In 1904, Gottlieb and Magnus modified the Langendorff method by introducing the isovolumic contracting method (26), where a small balloon inserted into the left ventricle (LV) was filled with fluid to measure isovolumic pressures. A further modification to the Langendorff model was made in 1939 by Katz et al. who used a constant flow retrograde perfusion method to measure changes in perfusion pressure and thus determine vascular resistance (38).
The ejecting heart.
Even though the Langendorff-perfused isolated heart is beating, the LV chamber is essentially empty and the preparation is considered nonworking since no perfusate is ejected from the heart. James Neely and Howard Morgan made the next major modification in the isolated heart model and in 1967 described an isolated rat heart preparation that performed physiologically relevant mechanical work (60). This preparation is commonly referred to as the “working heart,” although the more appropriate term is the ejecting heart as the Langendorff heart is also “working.” In this model, the aorta of a rat heart was attached via a cannula to an aortic outflow line and initially perfused in the Langendorff mode via a sidearm to the aortic line. A second cannula was inserted into the left atrium, and heart work was initiated by clamping the retrograde perfusion line while simultaneously unclamping the atrial inflow and aortic outflow lines. The atrial inflow line delivered perfusate at a constant preload hydrostatic pressure via the left atrium to the LV, and as the LV fills and contracts, perfusate is ejected out the aortic outflow line against a constant afterload hydrostatic pressure. Myocardial perfusion is achieved in a more physiological manner; during the course of ventricular relaxation, the aortic hydrostatic pressure leads to orthograde perfusion of the coronary vasculature of the heart. This method allowed ventricular preload and afterload to be accurately controlled, and Neely and Morgan were able to show that pressure development correlated well with oxygen consumption over a wide range of loading conditions (60).
Considerations Before Constructing an Isolated Mouse Heart Apparatus
Depending on specific experimental requirements, certain parameters need to be carefully considered by any investigator contemplating isolated mouse heart perfusion studies. Langendorff or ejecting heart, constant perfusion or constant flow, isometric force or isovolumic pressure, and buffer perfused or erythrocyte perfused: each has its own advantages and limitations that must be taken into account. The diminutive size and fragility of the mouse heart and its high heart rate pose additional challenges to the investigator. It is crucial to recognize and address all these factors so the investigator can get the most out of the selected mouse heart preparation.
Langendorff perfusion.
The Langendorff apparatus is the simplest and most widely employed preparation and, depending on the mode of perfusion (constant pressure or constant flow), is well suited for studying pharmacological interventions on myocardial function, electrical conduction, vascular reactivity, endothelial and smooth muscle function (12), as well as studies on myocardial ischemia and ischemic syndromes such as stunning and preconditioning (22, 43, 74).
With constant pressure perfusion, the heart maintains the ability to autoregulate coronary vascular tone, which is important during ischemia-reperfusion injury experiments when perfusion to part of the vascular bed is restricted (or eliminated in the case of permanent coronary ligation). In this scenario, constant flow during reperfusion maintained at the same rate before ischemia would force a greater volume of perfusate through the compromised vascular bed, thus shearing and potentially damaging the coronary arteries. Conversely, autoregulatory mechanisms that strive to increase coronary flow under increased workload conditions (e.g., inotropic challenge) are overridden with constant flow, which carries a risk of developing low-grade ischemia. The advantage of constant flow perfusion (with a high-fidelity peristaltic pump) is that precise and reproducible degrees of low flow can be induced to study the effect of low-flow ischemia in the heart (3). Constant flow is also particularly well suited for studying the effect of vasoactive substances on coronary vasomotor tone; coronary pressure is a sensitive parameter that is easily monitored, and the coronary vascular resistance (an index of coronary vascular tone) is derived from this measurement using Ohm's law (3, 21). It is possible and preferable to construct a Langendorff apparatus that incorporates both elements of constant pressure and flow perfusion; this would provide greater flexibility in experimental design and even allow the perfuser to readily switch between these two modes within a single experimental protocol.
Contractile force can be measured in the Langendorff-perfused mouse heart by attaching a hook with a suture through the apex of the heart and attaching the other end of the suture to an isometric force transducer, as originally described by Langendorff (45). Tension is applied to the suture, and changes in contractions along the longitudinal axis of the heart can be measured; a preload can be applied by means of a traction device, and length-tension curves can be obtained by incremental increases in traction. The mouse heart is a delicate organ, however, and there is potential for apical damage and tearing at the site of hook placement. A more widely employed method is the measurement of isovolumic pressures in the mouse heart. This is achieved by inserting a small balloon (attached to a fluid-filled catheter connected to a pressure transducer) into the LV. The balloon is inflated with fluid to fill the ventricular chamber, and as the heart contracts, pressure is transmitted via the balloon through the catheter to the pressure transducer and indexes of ventricular performance can be measured. Moreover, the volume of the balloon can be incrementally adjusted to obtain Frank-Starling curves. The main advantage of this method is that performance of the entire LV can be assessed. Also, hearts can be KCl arrested and the balloon adjusted at a constant end-diastolic volume or pressure and formalin fixed for histology or infarct size analysis (23, 34). The disadvantage is the added complexity in constructing the very small balloons of similar size and the difficulty of balloon insertion into the LV of the mouse.
Ejecting heart.
The isolated ejecting heart apparatus is more complicated than its Langendorff counterpart, requiring additional components for setup and is technically more demanding since it necessitates an extra cannulation step in the heart. The main advantage of the ejecting heart is that it performs physiological relevant mechanical work, with ventricular filling at a constant preload via the left atrium and ventricular ejection against an afterload via the aorta. An additional advantage is that preload and afterload can be varied over a wide range, thus the effect of pharmacological agents on the heart under various physiological loading conditions can be readily ascertained. The ejecting heart preparation is the model of choice for metabolic utilization studies where the metabolism of individual substrates under physiological conditions of energy demand can be readily interrogated (5, 7, 8). In addition, cardiac pressure-volume relations can be readily assessed in the ejecting heart using a microconductance catheter (27).
Crystalloid perfusion.
KHB was modified from Ringer's solution and used by Hans Krebs to perfuse liver tissue in experiments that ultimately led to the discovery of the urea cycle (40). KHB is the most widely employed crystalloid bicarbonate buffer for isolated organ perfusion studies including the heart (see below for formulation). Glucose is typically used as the only metabolic substrate in KHB, owing to the heart's efficiency in extracting energy from almost any fuel source. It is generally accepted that in the normal in vivo heart, fatty acids are the preferred metabolic fuel, and this has been corroborated in the isolated normoxic rat heart (51). However, studies in the isolated ejecting mouse heart have been conflicting with reports of fatty acids oxidation ranging from 40–70% of total oxidative metabolism (1, 8, 46). Despite the importance of fatty acids, they are generally omitted from KHB because of their poor solubility in aqueous solutions and the frothing that occurs when using gas dispersion tubes to oxygenate the buffer. For this same reason, albumin is also omitted, which contributes to the low oncotic pressure of KHB. Thus crystalloid-perfused hearts are prone to tissue edema, especially in prolonged experiments or ischemia-reperfusion studies (76). While the elevated glucose concentration in KHB is necessary to maintain the mouse high heart rate (and high-energy demand), it is important to recognize that when studying metabolic diseases such as diabetes, alternate metabolic substrates such as lactate and/or pyruvate should be considered to supplant the excess glucose (75). A further limitation of KHB is its low oxygen-carrying capacity; however, this is mitigated by vigorous gassing of the perfusate at high 95% O2, 5% CO2 to obtain a Po2 > 500 mmHg which is enough to adequately oxygenate the heart. The low oncotic pressure and oxygen-carrying capacity of KHB results in high coronary flow rates (>15 ml·min−1·g−1 heart wet wt) in Langendorff-perfused hearts that is several times greater than what is physiologically normal (11). This severely limits the coronary reserve, estimated at 0.5- to twofold increase in baseline coronary flow in the KHB-perfused mouse heart compared with a fivefold increase in the in vivo mouse heart (11, 28, 64). Despite these limitations, KHB perfusion remains a practical and useful method and the preparation is fairly stable with ∼10% decrease in mouse myocardial function per hour (30, 72).
Erythrocyte perfusion.
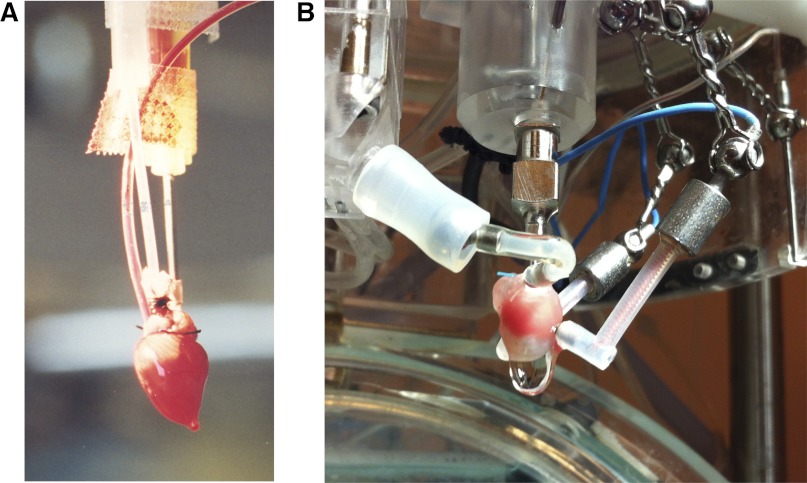
Isolated heart preparations perfused with solutions supplemented with erythrocytes offer the advantage of being extremely stable as they obviate many of the limitations of crystalloid buffers. Bovine red blood cells have an ∼6-μm diameter similar to that of the mouse (2), thus in terms of quantity the use of fresh cow blood is the most practical source for obtaining erythrocytes. Fresh cow blood is centrifuged, and the supernatant and layer of white cells are removed to obtained packed erythrocytes. The erythrocytes are reconstituted with KHB, and since erythrocytes have a high oxygen carrying capacity, vigorous gassing of the perfusate is not required. This enables supplementation of the perfusate with palmitic acid as a source of free fatty acid and albumin to raise the oncotic pressure of the perfusate (13, 23, 47). The erythrocytes are reconstituted with the modified KHB to obtain a final erythrocyte perfusate at a hematocrit of 40%. The main advantage of erythrocyte perfusion is an extremely stable preparation (<5% decrease in myocardial function per hour) with significantly less edema, resulting in an improved functional performance. Coronary flows in erythrocyte-perfused rabbit hearts are near physiological and better preserved following ischemic injury, and the coronary flow reserve is fourfold greater compared with KHB-perfused hearts (20, 63). Furthermore, recycling of the erythrocyte perfusate, which increases the risk of hemolysis, is generally not necessary in the isolated mouse heart because of the small volume of total perfusate required for a typical experiment. The investigator needs to ensure that the perfusion circuit does not contain any glass components since this will accelerate erythrocyte hemolysis. While clearly superior, the additional expense and time-consuming nature of preparing the erythrocyte perfusate and the technical challenge of clearly viewing the bloody heart during cannulation and instrumentation (see Fig. 4A) have dissuaded many investigators from adopting this perfusion technique.
Fig. 4.
A: isolated isovolumically contracting mouse heart erythrocyte perfused in the Langendorff mode. The aorta was cannulated with a 20-gauge (G) blunt-ended needle (right of picture). A balloon was inserted into the LV and connected via tubing (middle of picture) to a pressure transducer. The pulmonary artery was cannulated with polyethylene tubing (blood-filled tubing, left of picture) to collect the coronary effluent. B: isolated mouse ejecting heart with crystalloid perfusion (the LV was infarcted as evidenced by the whitish pallor of the lower portion of the heart). The aorta was cannulated with an 18-G blunt-ended steel needle (center of picture); a custom-made water-jacketed windkessel can be seen just above the aortic cannula. The preload line including 18-G atrial cannula is shown to the left of the picture; the preload line is attached to a ball-and-socket joint to allow freedom of movement in all planes. Shown to the right of the picture are two epicardial pacing wires held in place with ball-and-socket joints.
The advantages and limitations of the isolated heart preparations and the various parameters discussed are summarized in Table 1. It should be cautioned that even with careful preparation, the most challenging aspect of the isolated heart preparation is the small size of the mouse heart, and it may take the inexperienced perfuser some time to properly cannulate the heart and even longer to obtain reproducible data.
Table 1.
Summary of isolated heart perfusion modes and parameters and their advantages and limitations
| Advantages | Limitations | Type of Studies/Measurements | |
|---|---|---|---|
| Langendorff | Relatively simple setup | Nonphysiological work | Pharmacological intervention, ischemia-reperfusion injury, vascular reactivity, electrical conduction |
| Constant pressure | Autoregulatory mechanisms intact, allowing for perfusion to match the demands of the heart | Vascular reactivity may be difficult to assess due to inherent low coronary flows | Pharmacological interventions, ischemia-reperfusion injury |
| Constant flow | Precise induction of low-grade ischemia; vasomotor tone is derived from the coronary pressure | Autoregulation overridden, risk of ischemia with increased metabolic demand or vascular damage following reperfusion injury | Low-flow ischemia, vascular reactivity |
| Isometric | Simple instrumentation and very reproducible | Cardiac performance only measured in the long axis of the heart | Length-tension relationship |
| Isovolumic | Measurement of overall myocardial performance | Reproducibility of balloons and difficulty of balloon insertion in the left ventricle | Pressure-volume relationship (Frank-Starling curves) |
| Ejecting heart | Physiological relevant work; preload and afterload can be varied | Complicated setup and technically challenging | Pharmacological interventions, metabolic studies, loading conditions |
| KHB perfused | Simple preparation of Krebs-Henseleit buffer perfusate | Susceptible to tissue edema due to high flow rates and low oncotic pressure of Krebs-Henseleit buffer | |
| Erythrocyte perfused | More physiologically relevant perfusate, very stable preparation | Time-consuming perfusate preparation; Requires access to local slaughterhouse |
Preparation of Perfusate
Krebs-Henseleit buffer.
The typical KHB formulation for the mouse heart preparation consists of (in mM) 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 24.0 NaHCO3, 1.8 CaCl2, and 11.1 glucose. It should be noted that the free calcium ion concentration in mouse whole blood is ∼1.35 mM, thus the typical mouse KHB calcium concentration used by most investigators will result in a hypercontractile state. Only high-quality ultrapure water (Milli-Q) for preparation of all buffers should be used. It is best to prepare a stock solution of KHB (5× or 10×) without glucose (to avoid bacterial growth) and store at 4°C. On the day of the experiment, prepare 1× KHB from the stock solution, add and dissolve glucose, and filter using a 0.2-μm vacuum filter system (Corning). Warm final buffer perfusate on a hot plate to 37°C; place a glass gas dispenser in the buffer and gas with 95% O2-5% CO2 to yield a Po2 > 500 mmHg (pH 7.4).
Erythrocyte perfusate.
Fresh cow blood is typically collected at a local slaughterhouse in a vessel containing sodium heparin (15,000 U/l) and immediately placed on ice (13, 23, 47). The blood is filtered (200-μm mesh filter) and centrifuged at 5°C at 1,000 g for 15 min. The supernatant is aspirated along with the white buffy coat layer, and the packed erythrocytes are reconstituted with KHB at a 1:1 ratio. The centrifugation and reconstitution steps are repeated three more times, and the final resuspension step is omitted to obtain packed erythrocytes that are essentially white blood cell and platelet free. If the cells are to be used the following day, the packed erythrocytes can be stored at 4°C until use (unused erythrocytes should be discarded after 4 days of initial washing). The KHB for the erythrocyte perfusate is supplemented with palmitic acid, albumin, and lactic acid. Thus the modified KHB contains (in mM) 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 26.6 NaHCO3, 2.0 CaCl2, 5.5 glucose, and 1.0 lactate, supplemented with 0.4 palmitic acid and 4 g% bovine serum albumin. The bovine serum albumin is first dissolved in warm KHB, followed by palmitic acid, and the packed erythrocytes are reconstituted with the modified KHB to obtain the final erythrocyte perfusate at a hematocrit of 40%. Gentamicin (0.2 mg/dl) is added to the perfusate to retard bacterial growth. If possible, the erythrocyte perfusate should be checked with an electrolyte analyzer and ionic composition adjusted as needed. The erythrocyte perfusate is warmed to 37°C and equilibrated with a gas mixture consisting of 20% O2-3% CO2-77% N2 (see below for details) to yield a Po2 of 140–160 mmHg (pH 7.4). Care should be taken to avoid blood/erythrocyte contact with glassware during the preparation process and during circulation of the erythrocyte perfusate at the time of experiment since this will accelerate erythrocyte hemolysis.
Mouse Langendorff Apparatus
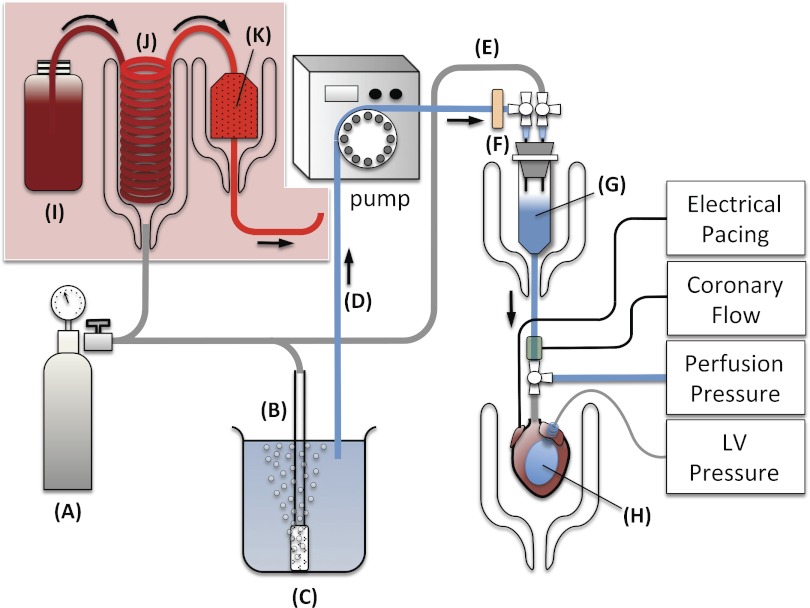
There are adequate to very good commercially available turnkey Langendorff perfusion systems designed specifically for the mouse heart (ADInstruments, Harvard Apparatus, Radnoti). If the investigator is constrained by budget, an apparatus can be constructed at modest cost, requiring at a minimum the following hardware: a water bath circulator, water-jacketed glassware for the heart and compliance chamber, a peristaltic pump, two pressure transducers, and a data acquisition system (e.g., PowerLab, ADInstruments). While there are various iterations of the Langendorff apparatus, the assembly that will be described will incorporate both the constant pressure and constant flow modes of Langendorff perfusion (Fig. 2). To accommodate crystalloid and erythrocyte buffer perfusion, the perfusion circuit will be designed so that there is no contact with glass during circulation of the perfusate. This arrangement will provide the greatest experimental flexibility to the investigator.
Fig. 2.
Langendorff apparatus: 95% O2-5% CO2 tank (A), gas dispersion tube (B), Krebs-Henseleit buffer (KHB) reservoir (C), perfusate line (D), pressure line (E), filter disc (F), compliance chamber (G), balloon (H), erythrocyte perfusate (I), oxygenator (J), and blood filter (K). The KHB reservoir is warmed on a hot plate to 37°C, and the compliance chamber and heart chamber are maintained at 37°C by circulating warm water through the water-jacketed chambers. The heart is electrically paced via platinum electrodes placed on the epicardial surface of the right ventricle. Coronary flow is measured by placing an in-line flow probe in the aortic perfusion line. Coronary perfusion pressure is measured via a sidearm connected to a pressure transducer. Left ventricular (LV) pressure is measured via a balloon inserted in the LV chamber and connected via polyethylene tubing to a pressure transducer. An additional sidearm can be added to the aortic perfusion line for infusion of pharmacological agents. For erythrocyte perfusion, tank A will be a gas mixture of 20% O2-3% CO2-77% N2, and B and C will be replaced by I–K.
KHB, placed in a reservoir and warmed on a hotplate to 37°C, is oxygenated via a glass tube gas disperser with a gas mixture of 95% O2-5% CO2. A peristaltic pump is used to draw the oxygenated perfusate via silicone tubing through a 0.2-μm disc filter to remove any microparticulates. The key component of the system is a compliance chamber, consisting of an inverted 30-ml plastic syringe tube with the plunger removed and sealed at the top with a rubber stopper; two 16-gauge (G) needles pierce the top of the rubber stopper and are each connected to a three-way stopcock. One of the stopcocks is connected to the perfusate line, and the other stopcock is connected to a pressure line connected to a pressure regulator, which regulates the pressure from the same 95% O2-5% CO2 gas tank used to oxygenate the perfusate. The perfusate enters the compliance chamber at a constant flow (the stopcock from the pressure line is closed). The compliance chamber (warmed to 37°C inside a water-jacketed chamber) is partially filled with perfusate, and this acts to dampen the pressure oscillations caused by the peristaltic pump and also serves as an effective bubble trap. The perfusate exits from the bottom of the compliance chamber and enters a four-way stopcock connected to the aortic perfusion cannula. To measure real-time coronary perfusion pressure, the sidearm of the four-way stopcock is attached via pressure tubing to a pressure transducer.
The aortic cannula consists of a 20-G blunt-ended needle with a groove machined into the distal end of the needle to secure the aorta to the cannula. Once the heart is cannulated and instrumented, coronary flow can be measured by timed collections of the coronary effluent; more practically, coronary flow can be measured in real time by placing an in-line flow probe directly above the four-way stopcock connected to the perfusion cannula. The cannulated heart is immersed in a water-jacketed KHB bath and warmed to 37°C. The mode of perfusion can be switched from constant flow to constant pressure by opening the stopcock from the pressure line to the compliance chamber. Care should be taken to initially set the pressure regulator so the pressure line is at low pressure, the coronary perfusion pressure can be monitored in real-time, and pressure from the regulator gradually increased to a constant coronary perfusion pressure of 80 mmHg. The perfusate level in the compliance chamber should be carefully monitored at first to ascertain emptying or filling and the pump flow adjusted accordingly (with experience, the perfuser will learn the normal perfusion parameters for the system and only minor adjustments in pump flow will be needed throughout the experiment). The apparatus can be switched back to constant flow by simply turning off the pressure line of the compliance chamber.
For erythrocyte perfusion, an additional heat-jacketed reservoir and chamber are needed (Fig. 2). An oxygenator is fashioned from highly gas-permeable silicone tubing wound into a coil and placed in the interior of the heat-jacketed reservoir. The peristaltic pump draws the nonoxygenated erythrocyte perfusate from a plastic container through the oxygenator. Continual flow of a gas mixture composed of 20% O2-3% CO2-77% N2 is administered to the interior of the water-jacketed reservoir, thereby warming and allowing rapid diffusion of gases into the erythrocyte perfusate circulating through the oxygenator. The oxygenated erythrocyte perfusate (Po2 of 140–160 mmHg, pH 7.4) is filtered and warmed as it passes through an in-line 40-μm blood filter (Pall) placed inside a heat-jacketed water chamber before entering the compliance chamber. Shown in Fig. 4A is an isolated mouse heart that is erythrocyte perfused in the Langendorff mode.
Mouse Ejecting Heart Apparatus
The assembly of an ejecting heart apparatus able to accommodate both crystalloid and erythrocyte perfusion will be described to again provide greater experimental flexibility to the investigator. The first step in establishing the mouse ejecting heart preparation is to incorporate the Langendorff apparatus, which is already described in detail (Fig. 2). Additional components are an atrial reservoir connected to a preload line (consisting of flexible silicone tubing connected to an atrial cannula) for diastolic filling of the heart and an afterload line against which the ventricle ejects. A second peristaltic pump is also required for recirculation of the perfusate; alternatively, a second pump head could be attached to the peristaltic pump.
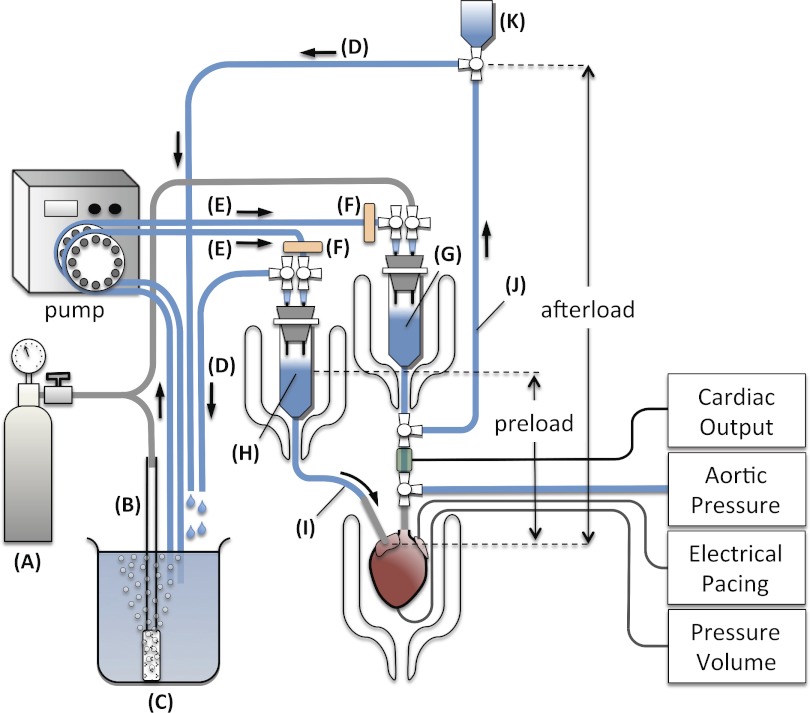
In the ejecting heart mode, perfusate is delivered from the atrial reservoir via the preload line to the left atrium, and then the LV, where it is ejected antegrade out through the aorta into the afterload line; hence, cardiac output flows through both the preload and afterload lines (Fig. 3). Cardiac output in the conscious mouse ranges from ∼20 ml/min to a high of 26 ml/min with volume loading (35). In the ex vivo buffer-perfused ejecting mouse heart, cardiac output ranges from 8–19 ml/min at preloads of 10–25 mmHg and an afterload of 50 mmHg and can be as high as 22 ml/min with increased calcium concentration (19, 27, 46). To accommodate these high flow rates, it is critical that the minimal bore size in the preload line, i.e., the atrial cannula can deliver perfusate from the atrial reservoir at a high enough rate so as not to become rate limiting at the expected maximal cardiac output of the heart. Given that diastolic filling time is approximately half of the cardiac cycle, the atrial inflow rate must be at least twice the expected maximal cardiac output. Reported atrial cannula bore diameters range from 0.64 to 1.14 mm (20–16 G); an 18-G (inner diameter, 0.95 mm) cannula has an estimated flow rate of ∼40 ml/min, which can accommodate a cardiac output up to 20 ml/min, which is more than adequate for most experiments. Shown in Fig. 4B is an isolated mouse heart with the left atrium cannulated using an 18-G blunt-ended needle with a groove machined into the distal end (for securing the atria to the cannula). To facilitate atrial cannulation, it is important that the preload line is flexible, and this can be accomplished by clamping the preload line (atrial cannula and tubing) to a ball-and-socket joint that allows freedom of movement in all planes (Fig. 4B, left).
Fig. 3.
Ejecting heart apparatus: 95% O2-5% CO2 tank (A), gas dispersion tube (B), KHB reservoir (C), recirculation line (D), perfusate line (E), filter disc (F), windkessel (G), atrial reservoir (H), preload line (I), afterload line (J), and overflow reservoir (K). The atrial reservoir, windkessel, and heart chamber are maintained at 37°C by circulating warm water through the water-jacketed chambers. The heart is electrically paced via platinum electrodes placed on the epicardial surface of the right ventricle. Cardiac output is measured by placing an in-line flow probe in the afterload line. Aortic pressure is measured via a sidearm connected to a pressure transducer. An additional in-line flow probe and pressure tranducer sidearm can be placed in the preload line to measure atrial flow rate and atrial pressure. LV pressures and volumes can be simultaneously measured via a 1.4-Fr high-fidelity transducer inserted into the apex of the LV and sutured. Cardiac preload and afterload can be adjusted by varying the heights of the atrial reservoir and overflow reservoir.
Likewise, the aortic cannula must be able to accommodate maximal cardiac output without or minimally restricting flow (low aortic impedance). This requires the aortic cannula bore to be at least as large as the aorta itself, which makes aortic cannulation all the more difficult (with Langendorff perfusion, the aortic cannula only needs to be large enough to adequately perfuse the coronaries). Typical aortic cannula bore sizes reported for the ejecting mouse heart range between 0.92 and 0.97 mm (19, 27, 46). De Windt and colleagues (19) reported in a ejecting mouse heart model that the optimal impedance characteristics for the aortic cannula is one with an inner diameter of >0.79 mm and a length of <4.1 mm. Figure 4B shows the isolated mouse heart cannulated at the aorta with a blunt-ended 18-G steel cannula (inner diameter of 0.95 mm) with the length cut to 4 mm and a groove machined into the distal end to secure the aorta to the cannula. The afterload line consists of silicone tubing connected at one end to a sidearm of a four-way stopcock positioned right below the compliance chamber (Fig. 3). This compliance chamber will later work as a windkessel to simulate the elastic compliance of the major arteries (62). The other end of the silicone tubing is connected to an open reservoir positioned at an initial afterload height of 50 cmH2O. To prime the afterload line, warm oxygenated KHB perfusate is pumped through a 0.2-μm disc filter into the partially filled compliance chamber (the stopcock from the pressure line is closed), and as the perfusate exits the compliance chamber, it is redirected by the four-way stopcock up the aortic line. As the top reservoir fills, the aortic perfusate overflows where it is recirculated via silicone tubing to the oxygenated KHB reservoir. Once the aortic line is primed, the perfusate is redirected by the four-way stopcock to the aortic pressure cannula. The preload line consists of the atrial cannula connected to silicone tubing which is connected at the other end to an atrial reservoir (constructed in the same way as the compliance chamber). Oxygenated KHB is pumped through a 0.2 μm disc filter into the open atrial reservoir; the left atrial cannula is primed with perfusate and is turned off. The perfusate level in the atrial reservoir is set at a predetermined height by drawing off excess perfusate via silicone tubing which is recirculated back to the KHB reservoir.
For erythrocyte perfusion, two additional oxygenator and filtering setups are required as shown in Fig. 2. One is needed before the compliance chamber as described in the Langendorff perfusion setup. The second one is needed before the atrial reservoir for oxygenation of the erythrocyte perfusate supplying the left atrium of the heart.
Harvesting, Cannulation, and Instrumentation of the Mouse Heart
Before anesthesia and surgery, it is prudent to administer heparin (10,000 U/kg) to the mouse to reduce any risk of thrombus formation in the coronary vasculature or blood coagulation during harvesting of the heart. There are several means of inducing anesthesia in the mouse, and the investigator should follow their institutional guidelines as to the most appropriate procedure for anesthesia. Following the onset of deep anesthesia, ascertained by loss of the pedal pain withdrawal reflex, the mouse is placed in a supine position. A transabdominal incision is made, and the diaphragm is cut to expose the thoracic cavity. A thoracotomy is performed by cutting bilaterally across the ribs, and the thoracic cage is deflected back to expose the heart. The pericardium is removed, and curved forceps are used to gently grasp the aorta, vena cava, and pulmonary vessels. The vessels are gently lifted by the forceps to hold up the heart and in one motion the vessels are excised below the forceps, and the heart is immediately transferred to a dissection dish containing ice-cold KHB solution to arrest the heart. This procedure is preferred since even gentle cradling and lifting the mouse heart between ones fingers could result in contusion injury. Unlike hearts from larger animals, the mouse heart needs to be trimmed to gain clear access to the aortic root; this can be achieved in under a minute by an experienced perfuser. The aorta is gently held by forceps to expose the inner lumen and the heart rapidly transferred to the aortic perfusion cannula with perfusate dripping to minimize the risk of introducing air into the heart. The aorta is carefully slipped over the end of the perfusion cannula and temporarily held with a bulldog clip, then securely sutured to the groove of the cannula using 5-0 silk. An alternative and perhaps easier method of mouse aortic cannulation is to place a dish containing ice-cold KHB over a stereomicroscope and position the tip of the aortic cannula (connected to a syringe primed with KHB) at an angle below the surface level of the buffer. The trimmed heart is then transferred to the dish with the cannula, and under magnification, the aorta is cannulated and secured with a suture. The cannulated heart is quickly detached from the syringe and connected to the dripping perfusion end of the Langendorff apparatus. Once perfused with warm perfusate, the heart will immediately beat and the coronary perfusion pressure can be set to 80 mmHg, and excess cardiac and noncardiac tissue can be trimmed away.
Langendorff instrumentation.
The balloon (isovolumic) method of LV pressure assessment is the most widely employed technique for measuring LV performance and will be described here for the mouse heart.
Given the diminutive size of the mouse heart, constructing balloons for mouse LV pressure determination poses unique challenges to the investigator. The LV balloon should have the following characteristics: 1) appropriately sized for the mouse heart, the balloon should be inflated to greater than the size of the stretched mouse ventricular lumen without itself contributing to the LV pressure; 2) highly flexible to follow the contours of the ventricular lumen; 3) highly compliant and thin to efficiently and accurately transmit LV pressures to the fluid in the balloon; and 4) high linear frequency response of the pressure measurement system (balloon and transducer including tubing and attachments) to ensure faithful recording of LV pressures. Commercial latex balloons or the use of condom tips should be avoided as they do not meet all these criteria. It is recommended that the investigator construct their own balloons using ultrathin plastic film from commercial food wrap (Saran wrap or cling film) since this material has been shown to be quite satisfactory for making mouse balloons (72). A 30-mm-diameter disc cutout from ultrathin film is wrapped around polyethylene (PE)-50 tubing (13, 23, 47) or a blunt-ended 21-G needle (72) and secured with a 5-0 silk suture (the balloon should be securely sutured close to the tip of the tubing or needle). The tubing or needle is connected to a syringe filled with degassed (boiled) ultrapure water, and the balloon is inflated to double its volume to check for leaks and to stretch it, thereby making it less elastic and more compliant. The balloon is connected via PE tubing to a pressure transducer, and care should be taken to remove any air bubbles in the pressure recording system (balloon, transducer, and tubing attachment) to avoid damping of the pressure signal.
Following initial Langendorff perfusion of the heart at 80-mmHg constant pressure, an incision is made in the left atrial appendage, and a short cannula (PE-50, heat flanged at the end) is passed via the mitral valve and pierced through the apex of the LV to vent Thebesian drainage. Without this, fluid will accumulate over time in the LV and distort the LV pressure readings. The balloon is compacted by first inflating with fluid and gradually emptying it while rolling the balloon into a point. The pointed balloon is inserted through the atrial appendage via the mitral valve into the LV and secured to the atrial appendage. The heart is paced by positioning platinum electrodes on the epicardial surface of the right ventricle. The pulmonary artery can be cannulated (PE-50 tubing, see Fig. 4A) to collect the coronary effluent for biochemical analysis or for measurement of coronary flow. Following instrumentation, the heart is immersed in a temperature-controlled KHB bath. The balloon is inflated with degassed water to give an end-diastolic pressure of 5–10 mmHg, and LV pressures are recorded via a pressure transducer; alternatively, a 1.4-Fr high-fidelity microtip transducer (Millar) can be advanced via the balloon tubing and positioned inside the balloon (47). It is critical that the temperature of the heart is maintained at or close to 37°C throughout the experiment. This can be achieved by placing thermistors in the thermostatically controlled KHB bath and the perfusion line entering the heart to monitor and maintain temperature at 37°C. Alternatively, a thin thermistor can be positioned inside the right ventricle during instrumentation to directly monitor the temperature of the heart. The heart should be allowed to equilibrate for 15 min before experimentation.
Ejecting heart instrumentation.
The heart is initially cannulated and perfused in the Langendorff mode at a constant perfusion pressure of 80 mmHg, as previously described. The atrial cannula (connected via tubing to the atrial reservoir) is clamped and held by a ball-and-socket joint that allows freedom of movement in all planes (Fig. 4B, left). The atrial cannula is allowed to drip with perfusate, and the left atrium is cannulated through one of the orifices of the pulmonary veins and sutured to the groove of the cannula. Care should be taken in tying off the remaining vessels so that there are no leaks; the atria will bulge once all vessels are tied off, and the atrial line should be turned off. To simultaneously measure LV pressures and volumes, a small apical stab is made and the tip of a 1.4-Fr high-fidelity transducer (Millar) is advanced into the LV chamber and carefully secured via a purse-string suture. Platinum electrodes can be positioned on the epicardial surface of the right ventricle to pace the heart. The heart is immersed in the KHB bath, and the temperature of the heart is maintained at 37°C as previously described. The heart is allowed to equilibrate 15 min in the Langendorff perfusion mode. The height of the atrial reservoir is set at an initial preload height of 10 cmH2O, and the afterload height is set at 50 cmH2O.
The ejecting heart mode is initiated by turning off the pressure and perfusate lines in the compliance chamber and turning on the four-way stopcock at the bottom of the compliance chamber to all three lines (the compliance chamber, afterload line, and aortic perfusion line). At this time, the heart is still being perfused in the Langendorff mode by the afterload line. The atrial preload line is quickly turned on to allow filling of the LV via the atria, and the LV will now start to pump the perfusate antegrade out the aorta against the afterload line and the overflow aortic perfusate is recirculated. The closed compliance chamber now acts as a windkessel where the volume of air simulates the elastic compliance of the aorta and its major arteries by converting the pulsating flow from the aorta into a more even flow and contributing to the propulsion of flow through the vascular system (62). Without the windkessel the ventricle ejects against the rigid cannula and tubing, which is energetically more demanding and will result in eventual failure of the heart.
New Methods
The Langendorff and ejecting heart preparations are extremely versatile research tools and have proven invaluable in characterizing myocardial function, metabolism, and vascular reactivity in physiological, pathological, and pharmacological investigations. Specific applications, such as ischemia-reperfusion injury, infarct size measurement, myocyte isolation, and measurements of metabolites using nuclear magnetic resonance and electron paramagnetic resonance have been adequately reviewed elsewhere (9, 33, 41, 70); instead, we will highlight more recent optical technologies in the isolated heart preparation.
Optical mapping.
The development of optical mapping systems and voltage-sensitive fluorescent dyes to record transmembrane potentials in Langendorff-perfused hearts have had an important impact on the study of the patterns of electrical activity in the heart (24, 32). While the small size and rapid basal heart rate pose limitations of the mouse as a model for human electrophysiology, the unique advantage of the mouse model to manipulate any gene involved in excitation-contraction (E-C) coupling have led to a greater understanding of cardiac arrhythmia mechanisms. Using optical mapping of Langendorff-perfused mouse hearts loaded with the voltage-sensitive dye 4-β-[2-(di-n-butylamino)-6-naphthylvinyl]pyridinium (di-4-ANEPPS), Baker et al. showed that deficiency in the slowly inactivating potassium current resulted in prolongation of action potential duration, altered restitution kinetics, and greater gradients of refractoriness from apex to base, which promoted sustained reentrant ventricular tachycardia (4); Eloff et al. showed a slowing in conduction velocity in mouse hearts deficient in the gap junction protein connexin 43 (25); and Baudenbacher et al. showed that mouse hearts with increased myofilament calcium sensitivity were more susceptible to cardiac arrhythmia (6). One limitation of conventional voltage-sensitive dyes, e.g., di-4-ANEPPS, is that their blue-green excitation light is strongly absorbed by blood, thus making them unsuitable for erythrocyte perfusion studies. To overcome this problem, near-infrared (IR) voltage-sensitive fluorescent dyes were developed with spectral characteristics that are optimized for erythrocyte-perfused hearts (55).
Multiparametric optical mapping.
Intracellular calcium flux is another critical determinant of E-C coupling, and Choi et al. loaded Langendorff-perfused guinea pig hearts with fluorescent dyes RH237 and Rhod-2 to simultaneously record transmembrane voltage and intracellular calcium transients, respectively (15). This multiparametric optical mapping method has been adapted by London et al. to show that both action potential prolongation and calcium handling abnormalities contribute to arrhythmias in a TNF-α transgenic mouse model of heart failure (44, 50). The ability to efficiently synthesize fluorescent dyes has led to significant improvements in the design of voltage- and calcium-sensitive dyes and has also opened the door for optical imaging of other (patho)physiological parameters including nitric oxide, mitochondrial membrane potential (Δψm), glucose uptake, and oxidative stress (10, 52, 58, 61).
A major constraint of conventional (multiparametric) optical mapping systems is their inherent sensitivity to motion artifacts, thus the fluorescent signals are recorded from nonbeating Langendorff hearts that have been immobilized by use of E-C uncouplers, such as 2,3-butanedione monoxime, blebbistatin, or cytochalasin D (67). These uncouplers (2,3-butanedione monoxime, in particular) could have significant electrophysiological side effects. To circumvent the use of E-C uncouplers, Valverde et al. used a suction-sealed fiber-optic holder to attenuate motion related fluorescence artifacts and restrict optical illumination to a small regional area on the myocardium (73). Using this approach, they were able to load fluorescent dyes Rhod-2, Mag-fluo-4, and di-8-ANEPPS to assess regional cytosolic calcium, sarcoplasmic reticulum calcium, and transmembrane potential, respectively, in isovolumically contracting Langendorff-perfused mouse heart (73).
Two-photon excitation microscopy.
Optical imaging spatially averages surface fluorescence recordings, thus responses of individual cells in the heart are obscured. Two-photon excitation (TPE) microscopy allows for real-time imaging of dynamic events in single cardiomyocytes within the intact heart at subcellular scale resolution and greater tissue depth (69). Rubart et al. loaded Langendorff-perfused mouse hearts with fluorescent calcium indicators Rhod-2 or fura-2 and, using TPE microscopy, were able to record highly synchronized intracellular calcium transients among neighboring cardiomyocytes at depths ≤ 100 μm below the epicardial surface (65). Matsumoto-Ida et al. loaded Langendorff-perfused rat hearts with tetramethylrhodamine ethyl ester, a fluorescent Δψm indicator, and using TPE microscopy, were able to demonstrate widespread loss of Δψm (a harbinger of cardiomyocyte death) in individual cardiomyocytes when hearts were subjected to ischemia-reperfusion injury (56). Davidson et al. performed multiphoton imaging of Langendorff-perfused mouse hearts expressing a calcium-sensitive reporter loaded with tetramethylrhodamine methyl ester and were able to resolve calcium transients, Δψm, and NAD(P)H autofluorescence (a readout for redox status) at submyocyte resolution (18). Using this technique in hearts subjected to hypoxia and reoxygenation, they were able to show at the onset of reoxygenation loss of Δψm in cardiomyocytes and that this was preceded by oxidative stress and calcium overload in these cardiomyocytes (18).
As in conventional optical mapping, TPE imaging is highly sensitive to motion artifacts and requires immobilization of the perfused heart using E-C uncouplers to prevent out of plane focusing of the regions of interest (69).
Second harmonic generation.
Second harmonic generation (SHG) imaging uses two-photon microscopy to excite and detect the backscatter of intrinsic optical signals in biological specimens, without the need of exogenous fluorescent probes, and has most effectively been used to image ordered protein assemblies (such as collagen fibers) in cells and tissues (14). SHG imaging in conjunction with TPE fluorescence microscopy could present a unique approach to study the impact of collagen deposition on electrical conduction, mitochondrial function, redox status, etc., in the post-myocardial infarction remodeled heart. As proof of concept, Scherschel and Rubart combined SHG imaging and TPE microscopy in a Langendorff-perfused infarcted mouse heart and were able to visualize collagen fibers and simultaneously record intracellular calcium transients in neighboring cardiomyocytes in the infarct border zone (69). An exciting new development in SHG imaging has been the ability to directly visualize sarcomeres in skeletal muscle fibers of live mice and humans (48). Since TPE imaging of the heart relies on the use of E-C contraction uncouplers to minimize motion artifacts, the SHG technique could in principle readily be applied to the isolated perfused immobilized heart to visualize cardiac sarcomeres, in situ.
Near-IR spectroscopy.
Near-IR light has the unique characteristic of deep tissue penetration (measured in millimeters), and the spectral region encompasses electronic transitions of myoglobin (and hemoglobin, which has a near-IR spectrum essentially identical to that of myoglobin) and cytochrome-c oxidase (16). Since the spectra of myoglobin and hemoglobin are sensitive to oxygenation and cytochrome-c spectrum is sensitive to its redox status, near-IR spectroscopy can yield information concerning both tissue oxygenation (intracellular Po2) and mitochondrial metabolism. The method involves placement of a small fiber-optic light guide (connected to a near-IR spectrometer) on the surface of an isolated heart to record absorbance changes of oxygenated/deoxygenated myoglobin and cytochrome-c redox state and has been used in Langendorff-perfused as well as isolated ejecting heart preparations (29, 31, 36, 42, 54, 68). The development of fluorescent dyes that exhibit strong excitation and fluorescence in the near-IR spectral range has expanded the imaging capabilities of this technique (39). Munch et al. have used Rhod800, a near-IR dye, in Langendorff-perfused rat hearts as a deposition flow tracer to assess regional myocardial blood flow (59), and Matiukas et al. have developed near-IR voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused hearts (55).
Conclusions
With the advent of genetically engineered mice, the isolated heart preparation (with its broad spectrum of physiological, biochemical, and morphological measurements) has become a powerful research tool in providing insight into the genetic mechanism of cardiovascular physiology and disease. With continual advances in optical and other methodological recordings, the isolated mouse heart preparation has become indispensable, serving as a physiologically relevant bridge between the study of intracellular signaling pathways using in vitro assays and the impact on the intact animal using in vivo approaches.
GRANTS
C. C. Lim is funded by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-095813 and a Stahlman grant from Vanderbilt University Medical Center. R. Liao is funded by NHLBI Grants HL-088533, HL-086967, HL-093148, and HL-099073. B. K. Podesser is supported by funds from the Ludwig Boltzmann Gesellschaft, Austria.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.L., B.K.P., and C.C.L. edited and revised manuscript; R.L., B.K.P., and C.C.L. approved final version of manuscript; B.K.P. and C.C.L. drafted manuscript.
REFERENCES
- 1. Aasum E, Hafstad AD, Larsen TS. Changes in substrate metabolism in isolated mouse hearts following ischemia-reperfusion. Mol Cell Biochem 249: 97– 103, 2003 [PubMed] [Google Scholar]
- 2. Albritton E. Standard Values in Blood. Philadelphia: Saunders, 1952 [Google Scholar]
- 3. Assayag P, Charlemagne D, Marty I, de Leiris J, Lompre AM, Boucher F, Valere PE, Lortet S, Swynghedauw B, Besse S. Effects of sustained low-flow ischemia on myocardial function and calcium-regulating proteins in adult and senescent rat hearts. Cardiovasc Res 38: 169– 180, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Baker LC, London B, Choi BR, Koren G, Salama G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ Res 86: 396– 407, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Barr RL, Lopaschuk GD. Methodology for measuring in vitro/ex vivo cardiac energy metabolism. J Pharmacol Toxicol Methods 43: 141– 152, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest 118: 3893– 3903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belke DD, Larsen TS, Gibbs EM, Severson DL. Glucose metabolism in perfused mouse hearts overexpressing human GLUT-4 glucose transporter. Am J Physiol Endocrinol Metab 280: E420– E427, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Belke DD, Larsen TS, Lopaschuk GD, Severson DL. Glucose and fatty acid metabolism in the isolated working mouse heart. Am J Physiol Regul Integr Comp Physiol 277: R1210– R1217, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol 50: 940– 950, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Biary N, Xie C, Kauffman J, Akar FG. Biophysical properties and functional consequences of reactive oxygen species (ROS)-induced ROS release in intact myocardium. J Physiol 589: 5167– 5179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bratkovsky S, Aasum E, Birkeland CH, Riemersma RA, Myhre ES, Larsen TS. Measurement of coronary flow reserve in isolated hearts from mice. Acta Physiol Scand 181: 167– 172, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Broadley KJ. An analysis of the coronary vascular responses to catecholamines, using a modified Langendorff heart preparation. Br J Pharmacol 40: 617– 629, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brooks WW, Apstein CS. Effect of treppe on isovolumic function in the isolated blood-perfused mouse heart. J Mol Cell Cardiol 28: 1817– 1822, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc 7: 654– 669, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi BR, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol 529: 171– 188, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooper CE, Springett R. Measurement of cytochrome oxidase and mitochondrial energetics by near-infrared spectroscopy. Philos Trans R Soc Lond B Biol Sci 352: 669– 676, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cyon E. Uber den Einfluss der Temperaturanderungen auf Zahl, Dauer und Starke der Herzschlage. Berichte uber die Verhandlungen der Koniglich Sachsischen Gesllschaft der Wissenschaften zu Leipzig. Mathematisch-Physische Classe 18: 256– 306, 1866 [Google Scholar]
- 18. Davidson SM, Yellon DM, Murphy MP, Duchen MR. Slow calcium waves and redox changes precede mitochondrial permeability transition pore opening in the intact heart during hypoxia and reoxygenation. Cardiovasc Res 93: 445– 453, 2012 [DOI] [PubMed] [Google Scholar]
- 19. De Windt LJ, Willems J, Reneman RS, Van der Vusse GJ, Arts T, Van Bilsen M. An improved isolated, left ventricular ejecting, murine heart model. Functional and metabolic evaluation. Pflügers Arch 437: 182– 190, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Deng Q, Scicli AG, Lawton C, Silverman NA. Coronary flow reserve after ischemia and reperfusion of the isolated heart. Divergent results with crystalloid versus blood perfusion. J Thorac Cardiovasc Surg 109: 466– 472, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Dijkman MA, Heslinga JW, Sipkema P, Westerhof N. Perfusion-induced changes in cardiac contractility and oxygen consumption are not endothelium-dependent. Cardiovasc Res 33: 593– 600, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Downey J, Yellon D. The biology of preconditioning. In: Stunning, Hibernation and Preconditioning. Clinical Pathophysiology of Myocardial Ischemial. Philadelphia: Raven, 1997, p. 105–119 [Google Scholar]
- 23. Eberli FR, Sam F, Ngoy S, Apstein CS, Colucci WS. Left-ventricular structural and functional remodeling in the mouse after myocardial infarction: assessment with the isovolumetrically-contracting Langendorff heart. J Mol Cell Cardiol 30: 1443– 1447, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Efimov IR, Nikolski VP, Salama G. Optical imaging of the heart. Circ Res 95: 21– 33, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res 51: 681– 690, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Gottlieb R, Magnus R. Digitalis und Herzabeit. Nach Versuchen an uberlebenden Warmbluterherzen. Path Pharamakol 51: 30– 63, 1904 [Google Scholar]
- 27. Grieve DJ, Cave AC, Byrne JA, Layland J, Shah AM. Analysis of ex vivo left ventricular pressure-volume relations in the isolated murine ejecting heart. Exp Physiol 89: 573– 582, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffet GE. Effects of isoflurane on coronary blood flow velocity in young, old and ApoE−/− mice measured by Doppler ultrasound. Ultrasound Med Biol 33: 512– 521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hassinen IE, Hiltunen JK, Takala TE. Reflectance spectrophotometric monitoring of the isolated perfused heart as a method of measuring the oxidation-reduction state of cytochromes and oxygenation of myoglobin. Cardiovasc Res 15: 86– 91, 1981 [DOI] [PubMed] [Google Scholar]
- 30. Headrick JP, Peart J, Hack B, Flood A, Matherne GP. Functional properties and responses to ischaemia-reperfusion in Langendorff perfused mouse heart. Exp Physiol 86: 703– 716, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Heineman FW, Kupriyanov VV, Marshall R, Fralix TA, Balaban RS. Myocardial oxygenation in the isolated working rabbit heart as a function of work. Am J Physiol Heart Circ Physiol 262: H255– H267, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Herron TJ, Lee P, Jalife J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ Res 110: 609– 623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ingwall JS. Transgenesis and cardiac energetics: new insights into cardiac metabolism. J Mol Cell Cardiol 37: 613– 623, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Jain M, Liao R, Podesser BK, Ngoy S, Apstein CS, Eberli FR. Influence of gender on the response to hemodynamic overload after myocardial infarction. Am J Physiol Heart Circ Physiol 283: H2544– H2550, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Janssen B, Debets J, Leenders P, Smits J. Chronic measurement of cardiac output in conscious mice. Am J Physiol Regul Integr Comp Physiol 282: R928– R935, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Jilkina O, Kuzio B, Rendell J, Xiang B, Kupriyanov VV. K+ transport and energetics in Kir6.2−/− mouse hearts assessed by 87Rb and 31P magnetic resonance and optical spectroscopy. J Mol Cell Cardiol 41: 893– 901, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Katz AM. Ernest Henry Starling, his predecessors, and the “Law of the Heart”. Circulation 106: 2986– 2992, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Katz G, Pain W, Tiller P. A new method for coronary perfusion of the mammalian heart. Arch Int Pharmacodyn 61: 109– 112, 1939 [Google Scholar]
- 39. Klohs J, Wunder A, Licha K. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res Cardiol 103: 144– 151, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Krebs HA, Henseleit K. Untersuchungen uber die Harnstoffbildung im Tierkoerper. Hoppe-Seyler's Zeitschrift fur Physiol Chemie 210: 33– 66, 1932 [Google Scholar]
- 41. Kuppusamy P, Zweier JL. Cardiac applications of EPR imaging. NMR Biomed 17: 226– 239, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Kupriyanov VV, Shaw RA, Xiang B, Mantsch H, Deslauriers R. Oxygen regulation of energy metabolism in isolated pig hearts: a near-IR spectroscopy study. J Mol Cell Cardiol 29: 2431– 2439, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Kusuoka H, Porterfield JK, Weisman HF, Weisfeldt ML, Marban E. Pathophysiology and pathogenesis of stunned myocardium. Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest 79: 950– 961, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lang D, Sulkin M, Lou Q, Efimov IR. Optical mapping of action potentials and calcium transients in the mouse heart. J Vis Exp 55: 3275, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Langendorff O. Untersuchungen am uberlebenden Saugethierherzen. Pflügers Arch 61: 291– 332, 1895 [Google Scholar]
- 46. Larsen TS, Belke DD, Sas R, Giles WR, Severson DL, Lopaschuk GD, Tyberg JV. The isolated working mouse heart: methodological considerations. Pflügers Arch 437: 979– 985, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Lim CC, Liao R, Varma N, Apstein CS. Impaired lusitropy-frequency in the aging mouse: role of Ca2+-handling proteins and effects of isoproterenol. Am J Physiol Heart Circ Physiol 277: H2083– H2090, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Llewellyn ME, Barretto RP, Delp SL, Schnitzer MJ. Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans. Nature 454: 784– 788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lloyd KC. A knockout mouse resource for the biomedical research community. Ann NY Acad Sci 1245: 24– 26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. London B, Baker LC, Lee JS, Shusterman V, Choi BR, Kubota T, McTiernan CF, Feldman AM, Salama G. Calcium-dependent arrhythmias in transgenic mice with heart failure. Am J Physiol Heart Circ Physiol 284: H431– H441, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Lopaschuk GD, Barr RL. Measurements of fatty acid and carbohydrate metabolism in the isolated working rat heart. Mol Cell Biochem 172: 137– 147, 1997 [PubMed] [Google Scholar]
- 52. Lyon AR, Joudrey PJ, Jin D, Nass RD, Aon MA, O'Rourke B, Akar FG. Optical imaging of mitochondrial function uncovers actively propagating waves of mitochondrial membrane potential collapse across intact heart. J Mol Cell Cardiol 49: 565– 575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin H. The direct influence of gradual variations of temperature upon the rate of beat of the dog's heart. Phil Trans R Soc Lond 174: 663– 688, 1883 [Google Scholar]
- 54. Masuda K, Truscott K, Lin PC, Kreutzer U, Chung Y, Sriram R, Jue T. Determination of myoglobin concentration in blood-perfused tissue. Eur J Appl Physiol 104: 41– 48, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei MD, Watras J, Loew LM. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm 4: 1441– 1451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matsumoto-Ida M, Akao M, Takeda T, Kato M, Kita T. Real-time 2-photon imaging of mitochondrial function in perfused rat hearts subjected to ischemia/reperfusion. Circulation 114: 1497– 1503, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Miller DJ. Sydney Ringer; physiological saline, calcium and the contraction of the heart. J Physiol 555: 585– 587, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Montaigne D, Marechal X, Baccouch R, Modine T, Preau S, Zannis K, Marchetti P, Lancel S, Neviere R. Stabilization of mitochondrial membrane potential prevents doxorubicin-induced cardiotoxicity in isolated rat heart. Toxicol Appl Pharmacol 244: 300– 307, 2010 [DOI] [PubMed] [Google Scholar]
- 59. Munch G, McKay S, Gussakovsky E, Kuzio B, Kupriyanov VV, Jilkina O. Rhodamine 800 as a near-infrared fluorescent deposition flow tracer in rodent hearts. J Biomed Opt 16: 065001, 2011 [DOI] [PubMed] [Google Scholar]
- 60. Neely JR, Liebermeister H, Battersby EJ, Morgan HE. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol 212: 804– 814, 1967 [DOI] [PubMed] [Google Scholar]
- 61. Patel VH, Brack KE, Coote JH, Ng GA. A novel method of measuring nitric-oxide-dependent fluorescence using 4,5-diaminofluorescein (DAF-2) in the isolated Langendorff-perfused rabbit heart. Pflügers Arch 456: 635– 645, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Podesser B, Hausleithner V, Seitelberger R, Wollenek G, Wolner E, Steiert H. New developments in the isolated working heart: a comparison of neonatal, immature, and adult rabbits after sixty minutes of ischemia in respect to hemodynamic and biochemical parameters. J Pharmac Toxicol Methods 30: 189– 196, 1993 [DOI] [PubMed] [Google Scholar]
- 63. Podesser BK, Hallstrom S, Schima H, Huber L, Weisser J, Kroner A, Furst W, Wolner E. The erythrocyte-perfused “working heart” model: hemodynamic and metabolic performance in comparison to crystalloid perfused hearts. J Pharmac Toxicol Methods 41: 9– 15, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Reichelt ME, Willems L, Hack BA, Peart JN, Headrick JP. Cardiac and coronary function in the Langendorff-perfused mouse heart model. Exp Physiol 94: 54– 70, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Rubart M, Wang E, Dunn KW, Field LJ. Two-photon molecular excitation imaging of Ca2+ transients in Langendorff-perfused mouse hearts. Am J Physiol Cell Physiol 284: C1654– C1668, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Rubin RP. A brief history of great discoveries in pharmacology: in celebration of the centennial anniversary of the founding of the American Society of Pharmacology and Experimental Therapeutics. Pharmacol Rev 59: 289– 359, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Salama G, Hwang SM. Simultaneous optical mapping of intracellular free calcium and action potentials from Langendorff perfused hearts. In: Current protocols in Cytometry. Chap. 12, Unit 12 17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schenkman KA, Arakaki LS, Ciesielski WA, Beard DA. Optical spectroscopy demonstrates elevated intracellular oxygenation in an endotoxic model of sepsis in the perfused heart. Shock 27: 695– 700, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Scherschel JA, Rubart M. Cardiovascular imaging using two-photon microscopy. Microsc Microanal 14: 492– 506, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff—still viable in the new millennium. J Pharmac Toxicol Methods 55: 113– 126, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Sutherland FJ, Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res 41: 613– 627, 2000 [DOI] [PubMed] [Google Scholar]
- 72. Sutherland FJ, Shattock MJ, Baker KE, Hearse DJ. Mouse isolated perfused heart: characteristics and cautions. Clin Exp Pharmacol Physiol 30: 867– 878, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Valverde CA, Kornyeyev D, Ferreiro M, Petrosky AD, Mattiazzi A, Escobar AL. Transient Ca2+ depletion of the sarcoplasmic reticulum at the onset of reperfusion. Cardiovasc Res 85: 671– 680, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Verdouw PD, van den Doel MA, de Zeeuw S, Duncker DJ. Animal models in the study of myocardial ischaemia and ischaemic syndromes. Cardiovasc Res 39: 121– 135, 1998 [DOI] [PubMed] [Google Scholar]
- 75. Vincent G, Khairallah M, Bouchard B, Des Rosiers C. Metabolic phenotyping of the diseased rat heart using 13C-substrates and ex vivo perfusion in the working mode. Mol Cell Biochem 242: 89– 99, 2003 [PubMed] [Google Scholar]
- 76. Vogel WM, Cerel AW, Apstein CS. Post-ischemic cardiac chamber stiffness and coronary vasomotion: the role of edema and effects of dextran. J Mol Cell Cardiol 18: 1207– 1218, 1986 [DOI] [PubMed] [Google Scholar]
- 77. Zimmer HG. The isolated perfused heart and its pioneers. News Physiol Sci 13: 203– 210, 1998 [DOI] [PubMed] [Google Scholar]
- 78. Zimmer HG. Who discovered the Frank-Starling mechanism? News Physiol Sci 17: 181– 184, 2002 [DOI] [PubMed] [Google Scholar]