Abstract
The sympathetic nervous system, leptin, and renin-angiotensin system (RAS) have been implicated in obesity-associated hypertension. There is increasing evidence for the presence of both leptin and angiotensin II receptors in several key brain cardiovascular and metabolic control regions. We tested the hypothesis that the brain RAS plays a facilitatory role in the sympathetic nerve responses to leptin. In rats, intracerebroventricular (ICV) administration of losartan (5 μg) selectively inhibited increases in renal and brown adipose tissue (BAT) sympathetic nerve activity (SNA) produced by leptin (10 μg ICV) but did not reduce the SNA responses to corticotrophin-releasing factor (CRF) or the melanocortin receptor agonist MTII. In mice with deletion of angiotensin II type-1a receptors (AT1aR−/−), increases in renal and BAT SNA induced by leptin (2 μg ICV) were impaired whereas SNA responses to MTII were preserved. Decreases in food intake and body weight with ICV leptin did not differ in AT1aR−/− vs. AT1aR+/+ mice. ICV leptin in rats increased AT1aR and angiotensin-converting enzyme (ACE) mRNA in the subfornical organ and AT1aR mRNA in the arcuate nucleus, suggesting leptin-induced upregulation of the brain RAS in specific brain regions. To evaluate the role of de novo production of brain angiotensin II in SNA responses to leptin, we treated rats with captopril (12.5 μg ICV). Captopril attenuated leptin effects on renal and BAT SNA. In conclusion, these studies provide evidence that the brain RAS selectively facilitates renal and BAT sympathetic nerve responses to leptin while sparing effects on food intake.
Keywords: kidney, brown adipose tissue, losartan, captopril, angiotensin-converting enzyme, angiotensin II type-1a receptor deletion
hypertension is a frequent consequence of obesity (14, 22, 29, 33). Circulating levels of leptin, an adipocyte-derived hormone that acts on the brain, are elevated in animal models of diet-induced obesity (45) and in many obese humans (9, 16). In addition to decreasing food intake, leptin increases sympathetic nerve activity (SNA) to thermogenic brown adipose tissue (BAT) and to nonthermogenic tissues such as the kidney (13, 26). Leptin-induced sympathetic excitation has been implicated in obesity-induced hypertension (1, 6, 10, 41, 42, 45).
The renin-angiotensin system (RAS) has also been implicated in the pathogenesis of obesity-induced hypertension (15, 21, 52), but the sites and mechanisms of the prohypertensive actions of the RAS in obesity are unclear. In addition to the classic circulating RAS, there are tissue-specific RAS including, among others, adipose tissue (15, 52) and brain (11, 19, 20, 37) RAS. The adipose RAS has been implicated in obesity hypertension through an increase in adipose tissue angiotensinogen expression, which promotes adipose tissue development and increases circulating ANG II (4, 5, 15). The brain RAS also contributes to the regulation of SNA and arterial pressure (11, 19, 20, 37) and therefore might be involved in obesity-induced hypertension.
There is evidence for both angiotensin and leptin receptors in several brain regions involved in regulation of SNA. For example, there are angiotensin (ANG) II type 1 receptors (AT1R) in the arcuate nucleus (ARC) (2, 36), a prominent site of leptin action. There is also evidence for leptin receptors and signaling in other brain regions, such as the subfornical organ (SFO) (47–49) and nucleus tractus solitarii (3, 17, 25, 32) that are involved in angiotensin signaling.
Additionally, there is evidence for a facilitatory peripheral leptin-angiotensin interaction (7, 27). Plasma and lung angiotensin-converting enzyme (ACE) activity and mRNA are decreased in leptin-deficient, ob/ob mice (27). Administration of leptin increases systemic ACE activity (27). In ob/ob mice, leptin increases ANG II and augments the depressor response to ACE inhibition (27). In addition, Cassis et al. (7) demonstrated that ANG II produced by adipocytes increases leptin release from adipocytes. Similarly, RAS inhibition by chronic aliskiren (50), captopril (54), enalapril (46), or candesartan (57), or global genetic knockout of ACE (28) or AT2 receptors (56) reduces circulating leptin levels.
Based on evidence for both angiotensin and leptin receptors in several brain regions involved in the regulation of SNA and for a peripheral facilitatory leptin-angiotensin interaction, we tested in rats and mice the hypothesis that the brain RAS facilitates sympathetic nerve responses to leptin, i.e., the concept of a brain leptin-RAS interaction in the regulation of SNA. We evaluated effects of cerebroventricular administration of losartan or captopril on SNA responses to leptin in rats and also compared responses to cerebroventricular administration of leptin in ANG II type 1a receptor knockout vs. wild-type mice.
METHODS
Animals
Male Sprague-Dawley rats (Harlan Laboratories) aged 14 to 16 wk with body weights between 450 and 525 g, and male ANG II type 1a receptor knockout (AT1aR−/−) and wild-type homozygous (AT1aR+/+ or AT1aR+/−) mice aged 12 to 18 wk with body weights between 18 and 32 g were studied. AT1aR knockout mice and wild-type controls were obtained from two sources: 1) JAX Laboratories where the AT1aR−/− were bred on a C57Bl/6J background and wild-type mice consisted of C57Bl/6J nonlittermate controls, and 2) the University of Iowa Transgenic Facility where the AT1aR−/− mice on a C57BL/6 background were initially crossed with 129SvJ strain mice and then maintained by +/− X +/− breeding. Experimental mice were AT1aR−/−. Control mice consisted of homozygous wild-type littermates. Mice were genotyped by PCR using genomic DNA obtained from the tail (primers: AT1 forward: 5′-GCA TCA TCT TTG TGG TGG G-3′; AT1 reverse: 5′-ATC AGC ACA TCC AGG AAT G-3′; AT1 neo: 5′-TGC CGA GAA AGT ATC CAT CAT GGC TGA TGC-3′). Animals were housed in a temperature-controlled room with a 12:12-h light-dark cycle. Standard laboratory chow and tap water were provided ad libitum. All studies and procedures were approved by the University of Iowa Animal Research Committee. All studies were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Experimental Preparations and Protocols
Lateral cerebroventricular cannulation.
For the placement of a lateral cerebroventricular (ICV) cannula, rats or mice were anesthetized with intraperitoneal ketamine (91 mg/kg) and xylazine (9.1 mg/kg). In rats, under aseptic conditions and anesthesia, the head was then placed in a stereotaxic frame (David Kopf Instruments) and a stainless steel 23-gauge, 22-mm long cannula was implanted into the left lateral cerebral ventricle using coordinates based on the atlas of Paxinos and Watson (38): −0.3-mm caudal, −1.4-mm lateral, and −5.7- to −6.1-mm ventral relative to the bregma. In mice, a stainless steel 25-gauge, 9-mm long cannula was implanted into the left lateral cerebral ventricle under the following coordinates: −0.3-mm caudal, −1.0-mm lateral, and −3.0-mm ventral relative to the bregma (18, 44). The cannula was anchored in place with a stainless steel machine screw and dental cement. A stylet was inserted to seal the cannula until use. After 7 days of recovery, animals were prepared for the procedures and protocols described below.
Effects of losartan or captopril on sympathetic nerve responses to ICV leptin in rats.
After recovery from ICV cannulation, rats were anesthetized with intraperitoneal urethane (1.5 g/kg; Sigma-Aldrich Biochemical). Anesthesia was maintained with urethane, administered via a right femoral venous catheter as needed. Arterial pressure and heart rate were monitored with a catheter inserted into the tail artery. The trachea was cannulated and each rat was allowed to breathe oxygen-enriched air spontaneously. Rectal temperature was maintained at 37.5°C using a temperature controlled surgical table and lamp. As described previously (32), multifiber recordings of SNA were obtained from a nerve to the left kidney. In some experiments, we also obtained recordings of SNA to interscapular BAT. The left kidney was exposed through a retroperitoneal flank incision. With the use of a dissecting microscope, a renal nerve was carefully dissected free and placed on a bipolar 36-gauge platinum-iridium electrode. In experiments that included recordings of BAT SNA, interscapular BAT was exposed through a nape incision. A nerve fiber innervating BAT was identified and placed on the bipolar electrode. When optimal recordings of renal and BAT SNA were obtained, the respective nerves were covered and secured with silicone gel for recordings of SNA to kidney and BAT, respectively. The electrodes on the nerves to BAT and kidney were connected to two separate high-impedance probes (HIP-511; Grass Instruments), and each nerve signal was amplified to 105 times and filtered at low and high frequency cutoffs of 100 and 1,000 Hz, respectively, with a Grass P5 AC preamplifier. The filtered, amplified nerve signals were routed to an oscilloscope (model 54501A; Hewlett-Packard) for monitoring the visual quality of the sympathetic nerve recording, to an analog digital converter (ADInstruments) for continuous counting of spikes that exceed threshold set above baseline noise, and finally to a Maclab (model 8s; ADInstruments) for continuous acquisition of the blood pressure, heart rate, and simultaneous renal and BAT SNA for storage and later analysis on a Mac computer (MacBook Pro). The following protocols were performed.
1) To determine the efficacy and time course of losartan-induced AT1R blockade, we measured the arterial pressure response to ICV ANG II (50 ng; Sigma-Aldrich Biochemical) before and sequentially for 60 min after ICV losartan (5 μg; Sigma-Aldrich Biochemical). As described in results, these studies revealed that the pressor response to ICV ANG II was blocked 15 min after losartan but restored at 60 min. Accordingly, in the following protocol, leptin was administered ICV 15 min after losartan. In addition, we performed another protocol in which leptin or vehicle was administered ICV 60 min after ICV losartan at a time when losartan no longer produced blockade of AT1R. To determine the time course of captopril blockade of brain ANG II production, we measured arterial pressure responses to repeated injections of ANG I (50 ng; Sigma-Aldrich Biochemical) and ANG II (50 ng) intravenously over 5 h in rats given captopril (12.5 μg ICV) or vehicle.
2) The effects of ICV losartan or captopril on SNA responses to ICV leptin were studied. After allowing ≥15 min for a stable baseline, vehicle (2 μl saline) or the AT1R blocker losartan (5 μg) was injected ICV. Another cohort of rats received ICV captopril (12.5 μg; Sigma-Aldrich Biochemical) instead of losartan. Fifteen minutes later, leptin (10 μg; R&D Systems) or vehicle was injected ICV as a bolus. Renal and BAT SNA (measured as spikes/second), arterial pressure, and heart rate were simultaneously recorded at baseline, during the ICV injections (vehicle, losartan, or captopril, followed by vehicle or leptin), and throughout the next 4 h. Animals were euthanized with an overdose of urethane after 4 h of recording. Similar experiments were performed with ICV injection of corticotropin-releasing factor (CRF; 5 μg; Phoenix Pharmaceuticals) or with ICV injection of the melanocortin receptor agonist MTII (0.62 ug; Phoenix Pharmaceuticals) instead of leptin to test the specificity of the effects of losartan on sympathetic responsiveness.
3) To determine if ICV administration of losartan blocked SNA responses to systemic as well as central neural administration of leptin, we studied the effects of ICV losartan (5 μg) or vehicle on the renal SNA responses to intravenous bolus injection of leptin (0.75 μg/g body wt) in anesthetized rats.
Sympathetic nerve responses to ICV leptin in AT1aR−/− and AT1aR±/± mice.
After 7 days of recovery from ICV cannulation, mice were anesthetized with ketamine/xylazine and instrumented for measurement of arterial pressure and heart rate (left carotid artery catheter) and for maintenance of anesthesia (right jugular vein catheter) with intravenous α-chloralose (initial dose: 25 mg/kg, sustaining dose: 6 mg·kg−1·h−1; MP Biomedicals). Body temperature was maintained at 37.5°C with a temperature controlled surgical table and lamp. The left kidney was exposed retroperitoneally through a flank incision, and mice were prepared for multifiber recording of renal SNA. In another experiment, in a separate group of mice, we obtained recordings of SNA to interscapular BAT. With the use of a dissecting microscope, a renal nerve was carefully dissected free and placed on a bipolar 36-gauge platinum-iridium electrode. In separate experiments, using a dissecting microscope, a nerve fiber innervating interscapular BAT was exposed through an incision and was placed on the bipolar electrode. When an optimal recording of renal or BAT SNA was obtained, the electrode was covered with silicone gel (Kwik-Sil; World Precision Instruments). The amplification, filtering, recording, and analysis of the nerve signal were identical to those described above in the rat. Renal or BAT SNA (measured as spikes/second), arterial pressure, and heart rate were recorded at baseline and for 4 h after ICV injection of leptin (2 μg) in AT1aR−/− and AT1aR+/+ mice. In separate groups of knockout and wild-type mice, we evaluated renal SNA responses to the melanocortin agonist MTII (2 μg ICV; Phoenix Pharmaceuticals) to test the specificity of the effects of AT1aR deletion on sympathetic responsiveness.
Effects of ICV leptin administration on food intake and body weight.
Body weight and 24 h food intake were measured in AT1aR−/− and AT1aR+/+ single-housed mice after an ICV bolus injection of leptin (2 μg/2 μl) or vehicle (2 μl) daily. An ICV cannula was implanted 1 wk before the study. Measurements of food intake and body weight were performed daily at 4 PM followed by leptin or vehicle ICV injection. At the end of the study, blood was collected from the tail of AT1aR−/− and AT1aR+/+ mice in heparinized tubes. Plasma was obtained after being centrifuged for 20 min at 2,000 g. Leptin levels were measured using an ELISA kit (R&D Systems) following the manufacturer's protocol.
Effects of ICV leptin on brain AT1aR and ACE mRNA expression in rats.
In separate experiments, rats were implanted with a lateral cerebroventricular cannula and were allowed to recover for 1 wk. Each rat was then anesthetized with urethane (1.5 g/kg IP) in the same manner as described above. The rats then underwent ICV injection of either leptin (10 μg) or vehicle (2 μl). Four hours later, each animal was euthanized with urethane and the brain was quickly removed, frozen in liquid nitrogen, and stored at −80°C. The frozen brain was cut into 300-μm coronal sections, and the paraventricular nucleus (PVN) and SFO were punched using a 15-gauge needle centered over the PVN and SFO as described previously (53). To dissect the ARC, the median eminence was first removed from a frozen brain with a scalpel under a stereo magnifier. The ARC bilaterally was then dissected from the caudal part of the hypothalamus. For quantitative PCR, total RNA was isolated from rat ARC, SFO, and PVN by TRIzol method (according to the manufacturer's protocol, Invitrogen). Total RNA, 1 μg, from each brain region was treated by DNase I. First-strand cDNA was synthesized with SuperScript III reverse transcriptase (Invitrogen), and cDNA was diluted 10 times for quantitative PCR. Real-time quantitative PCR was performed using TaqMan Gene Expression Assays (Applied Biosystems) to compare the expression level of two selected genes. The three genes selected and the associated TaqMan assay identifiers were as follows: Agtr1a (#Rn01435427_A1), ACE (#Rn00061094_A1), and GAPDH (Rn99999916_s1, used as a control). PCR reactions were performed in separate wells of a 96-well plate. Thermal cycling consisted of an initial incubation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min in an iCycler (Bio-Rad). Data were analyzed using iCycler software to determine Ct values. ΔCt values were generated by subtracting the Ct value of GAPDH from the Ct value for the gene of interest. Fold change relative to the corresponding control groups was calculated using the ΔΔCt method (31).
Data analysis.
Results are expressed as means ± SE. SNA, measured in spikes per second, is expressed as percent change from baseline. Data were analyzed using independent Student t-tests or two-way repeated-measures ANOVA followed by post hoc analyses using Bonferroni multiple-comparisons procedures when main effects reached significance. A value of P ≤ 0.05 was considered significant.
RESULTS
Effects of ICV Losartan on Pressor Responses to ICV ANG II in Rats
We evaluated the efficacy and time course of losartan blockade on arterial pressure responses to ANG II (50 ng/2 μl ICV). The pressor response to ICV ANG II was virtually abolished (P < 0.05) 15 min after losartan (5 μg ICV) but was restored to control levels 60 min after losartan. ANG II-induced increases in mean arterial pressure (MAP) before and 15 and 60 min after ICV losartan were 18 ± 2, 0 ± 1, and 17 ± 1 mmHg (n = 6), respectively. These results demonstrated that losartan (5 μg ICV) produced effective but short-lived blockade of brain AT1R.
Effects of Losartan on SNA Responses to Leptin, CRF, and MTII in Rats
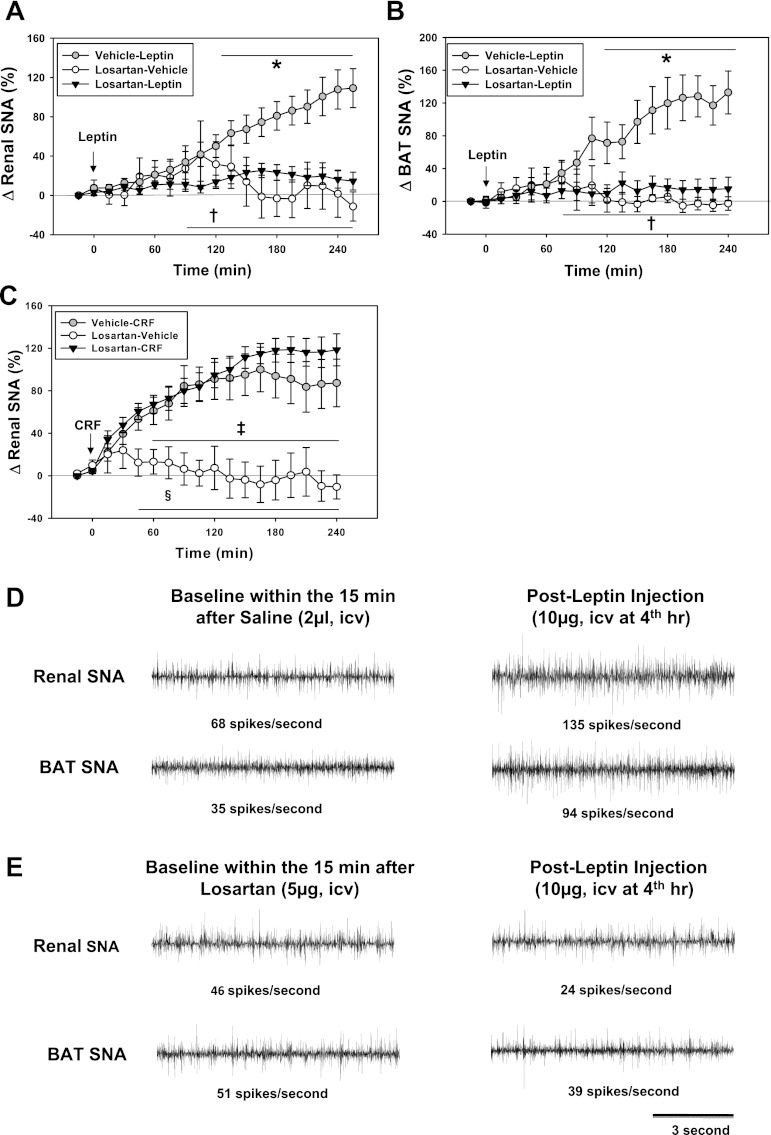
We examined effects of losartan (5 μg ICV) on sympathetic nerve responses to leptin (administered 15 min after losartan). As shown in Fig. 1A, losartan (5 μg ICV) significantly attenuated renal SNA responses to ICV leptin (10 μg; Fig. 1, A, D, and E). ICV losartan also significantly attenuated SNA to BAT (Fig. 1, B, D, and E). The attenuation of leptin-induced sympathetic activation by losartan appeared selective as treatment with losartan (5 μg ICV) did not attenuate renal SNA responses to ICV administration of CRF (Fig. 1C) or to ICV MTII. Increases in renal SNA with ICV MTII were 128 ± 47% 4 h after vehicle (n = 6) and 116 ± 31% 4 h after losartan (P < 0.05, compared with baseline; n = 6, each). We also performed experiments to determine if losartan would attenuate the SNA responses to leptin administered 60 min (rather than 15 min) after losartan, at a time when losartan does not block the pressor responses to ANG II (see above). Losartan did not attenuate the renal SNA responses to leptin given 60 min after losartan. In these experiments, increases in renal SNA 4 h after leptin were 52 ± 14% (n = 12) after losartan and 38 ± 12 (n = 7) after vehicle.
Fig. 1.
Effects of losartan intracerebroventricular (ICV; 5 μg) on sympathetic nerve activity (SNA) responses to leptin (10 μg) or corticotrophin-releasing factor (CRF; 5 μg) in rats. Leptin or CRF was given 15 min after losartan or vehicle. A: changes in renal SNA in response to leptin after vehicle (n = 21) or losartan (n = 25). Renal SNA after losartan alone (n = 6) is also shown. B: changes in brown adipose tissue (BAT) SNA in response to leptin ICV (vehicle-leptin, n = 15; losartan-leptin, n = 18; and losartan-vehicle, n = 6). C: renal SNA responses to CRF in rats after 4 h (vehicle-CRF, n = 7; losartan-CRF, n = 7; and losartan-vehicle, n = 7). D and E: segments of original recordings of renal (D and E, top) and BAT SNA (D and E, bottom) at baseline and 4 h after leptin (10 μg ICV). *P < 0.05 (losartan-leptin vs. vehicle-leptin); †P < 0.05 (losartan-vehicle vs. vehicle-leptin). ‡P < 0.05 (vehicle-CRF vs. losartan-vehicle); §P < 0.05 (losartan-CRF vs. losartan-vehicle).
Acute injection of leptin (10 μg ICV; Table 1) or CRF (5 μg ICV) did not significantly change arterial pressure or heart rate after either vehicle or losartan.
Table 1.
Time course of hemodynamic responses after ICV leptin in rats pretreated with losartan
| Time After Treatment, min |
|||||
|---|---|---|---|---|---|
| Pretreatment/Treatment | Baseline | 60 | 120 | 180 | 240 |
| MAP, mmHg | |||||
| Saline/leptin (21) | 96 ± 4 | 95 ± 3 | 98 ± 3 | 95 ± 4 | 94 ± 4 |
| Losartan/leptin (25) | 98 ± 8 | 100 ± 5 | 106 ± 4 | 104 ± 4 | 99 ± 5 |
| Losartan/saline (6) | 92 ± 5 | 100 ± 8 | 90 ± 7 | 98 ± 8 | 93 ± 5 |
| HR, beats/min | |||||
| Saline/leptin (21) | 351 ± 12 | 356 ± 11 | 384 ± 20 | 380 ± 11 | 396 ± 23 |
| Losartan/leptin (25) | 378 ± 7 | 368 ± 6 | 382 ± 8 | 384 ± 6 | 387 ± 9 |
| Losartan/saline (6) | 356 ± 11 | 381 ± 17 | 382 ± 9 | 375 ± 10 | 370 ± 13 |
Values are means ± SE; nos. in parenthesis equal no. of rats. MAP, mean arterial pressure; HR, heart rate.
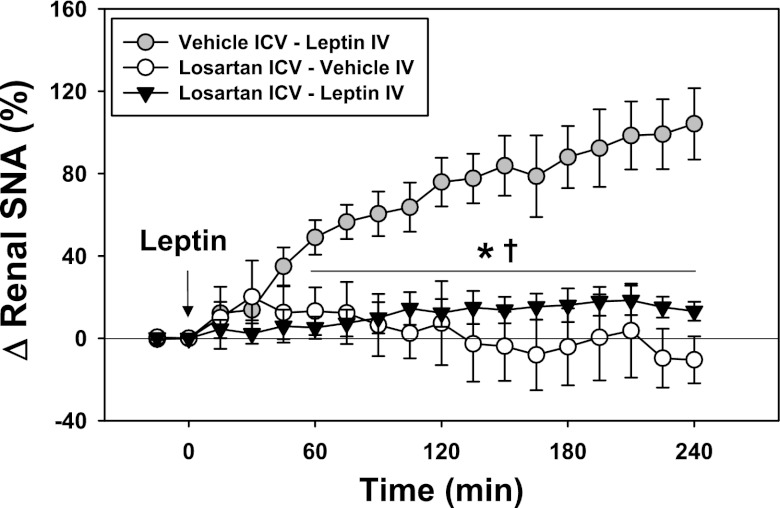
As shown in Fig. 2, ICV losartan (5 μg) also attenuated renal SNA responses to intravenous administration of leptin (0.75 μg/g body wt).
Fig. 2.
Effects of ICV administration of losartan (5 μg) or vehicle on renal SNA responses to systemic administration of leptin (0.75 μg/g body wt IV; n = 5, each group). *P < 0.05 (losartan-leptin vs. vehicle-leptin); †P < 0.05 (losartan-vehicle vs. vehicle-leptin).
SNA Responses to Leptin in AT1aR Knockout Mice
The findings described above with losartan prompted us to study mice with deletion of AT1aR to 1) focus on AT1aR since losartan does not distinguish between AT1aR vs. AT1bR; 2) test for a brain leptin-angiotensin interaction in another species; and 3) study the leptin-angiotensin interaction using genetic deletion instead of pharmacologic blockade of angiotensin receptors.
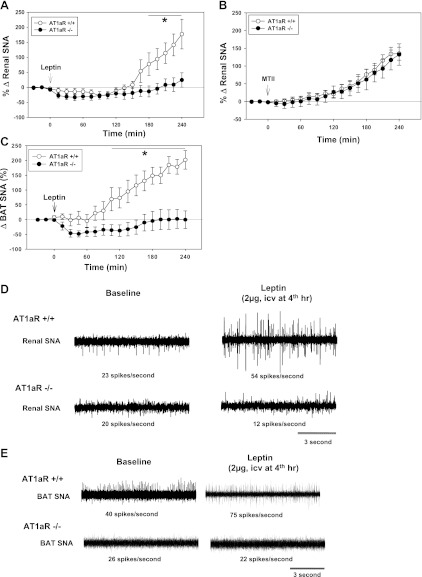
Increases in renal SNA induced by leptin (2 μg ICV) were significantly less (P < 0.05) in AT1aR−/− than in wild-type AT1aR+/+ control mice (Fig. 3, A and D). The renal SNA responses to leptin in the knockout mice from two different sources with two different genetic backgrounds did not differ significantly. The changes in renal SNA 4 h after leptin were +22 ± 20% in AT1aR−/− mice on a C57Bl/6J background (n = 7) vs. +28 ± 29% in AT1aR−/− on a C57 X 129 mixed genetic background (n = 3).
Fig. 3.
A: changes in renal SNA in response to leptin (2 μg ICV) in angiotensin II type-1a receptor knockout (AT1aR−/−) mice (n = 10) compared with wild-type homozygous (AT1aR+/+) mice (n = 14). B: changes in renal SNA in response to the melanocortin receptor agonist MTII (2 μg ICV) in AT1aR−/− mice (n = 12) and AT1aR+/+ mice (n = 18). C: BAT SNA responses to leptin (2 μg ICV) in AT1aR−/− (n = 7) vs. AT1aR+/+ mice (n = 7). D and E: segments of original recordings of the effects of leptin (2 ug ICV) on renal (D) and BAT (E) SNA in AT1aR+/+ and in AT1aR−/− mice. *P < 0.05 (AT1aR−/− vs. AT1aR+/+ mice).
The attenuation of leptin-induced increases in renal SNA in AT1aR−/− mice could not be explained by lack of sympathetic responsiveness, because as shown in Fig. 3B increases in renal SNA induced by MTII (2 μg ICV) did not differ significantly in AT1aR−/− vs. AT1aR+/+ mice.
The effects of AT1aR deletion on BAT SNA responses to leptin, 2 μg ICV (Fig. 3C), were similar to effects on renal SNA. In wild-type mice, leptin increased BAT SNA, but there was no increase in BAT SNA after leptin treatment in AT1aR−/− (Fig. 3E).
As reported previously (8, 30), baseline MAP before administration of leptin was lower (P < 0.05) in the anesthetized AT1aR−/− mice than in AT1aR+/+ mice (Table 2). MAP declined (P < 0.05) during 4 h of the experiments in both AT1aR+/+ and AT1aR−/− mice, but the relative decreases from baseline did not differ between wild-type vs. knockout or between treatments (Table 2). There were no significant changes in heart rate after leptin in either the anesthetized AT1aR−/− mice or in AT1aR+/+ mice (Table 2).
Table 2.
Time course of hemodynamic responses after ICV leptin in AT1aR+/+ and AT1aR−/− mice
| Time After Leptin, min |
|||||
|---|---|---|---|---|---|
| Baseline | 60 | 120 | 180 | 240 | |
| MAP, mmHg | |||||
| AT1aR+/+ (16) | 76 ± 4 | 61 ± 3† | 58 ± 3† | 58 ± 4† | 53 ± 4† |
| AT1aR−/− (12) | 53 ± 2* | 47 ± 2* | 48 ± 3* | 47 ± 3* | 40 ± 3*† |
| HR, beats/min | |||||
| AT1aR+/+ (16) | 283 ± 10 | 323 ± 17 | 370 ± 17† | 438 ± 20† | 494 ± 14† |
| AT1aR−/− (12) | 283 ± 19 | 318 ± 19 | 399 ± 12† | 459 ± 13† | 499 ± 1† |
Values are means ± SE; nos. in parenthesis equal no. of rats.
P < 0.05 for ANG II type 1a receptor knockout (AT1aR−/−) vs. wild-type homozygous (AT1aR+/+) mice.
P < 0.05 vs. baseline.
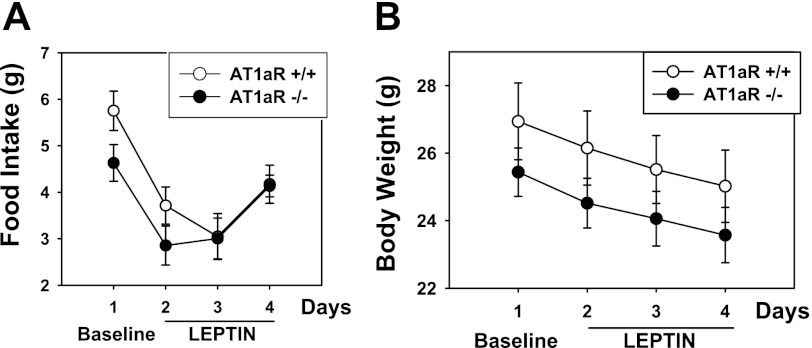
Effects of AT1aR Deletion on Food Intake and Body Weight Responses to Leptin
Baseline food intake tended to be higher in AT1aR wild-type compared with knockout mice (Fig. 4A), but the difference was not significant. As reported by others (30), baseline body weight did not differ significantly in AT1aR−/− and AT1aR+/+ mice (Fig. 4B). In separate groups of mice, baseline plasma leptin levels were not different in AT1aR−/− mice (7.1 ± 0.9 ng/ml; n = 10) and AT1aR+/+ mice (7.3 ± 1.4 ng/ml; n = 10). Leptin was injected for 3 consecutive days ICV (2 μg daily) followed by measurement of food intake and body weight. The leptin-induced decreases in food intake in AT1aR−/− and AT1aR+/+ mice (Fig. 4A; P < 0.05 in both groups) did not differ in the two groups. The leptin-induced decreases in body weight during administration of leptin ICV (Fig. 4B; P < 0.05 in both groups) also did not differ in the two groups.
Fig. 4.
Food intake (A) and body weight (B) in AT1aR−/− (n = 5) and AT1aR+/+ (n = 7) mice at baseline (day 1) and with daily ICV injections of leptin (2 μg; days 2–4). Leptin produced a significant decrease (P ≤ 0.05) in food intake and in body weight in both AT1aR−/− and AT1aR±/±, but there was no significant difference in these responses between AT1aR−/− and AT1aR±/±.
Effects of Leptin on AT1aR and ACE Expression in Brain Regions
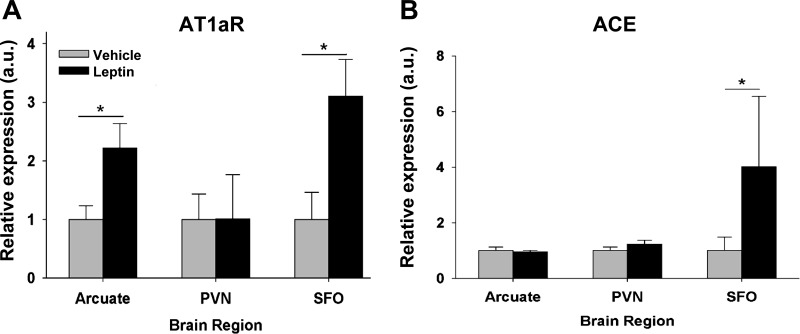
In rats, 4 h after ICV administration of leptin (10 μg), there were significant increases in AT1aR mRNA (Fig. 5A) and ACE mRNA (Fig. 5B) expression in the SFO. Leptin increased AT1aR mRNA but not ACE mRNA in the ARC (Fig. 5, A and B, P < 0.05) and did not increase either AT1aR or ACE mRNA in the PVN (Fig. 5, A and B).
Fig. 5.
Relative expression of AT1aR (A) and angiotensin-converting enzyme (ACE; B) mRNA in arcuate nucleus, paraventricular nucleus (PVN), and subfornical organ (SFO) of Sprague-Dawley rats 4 h after ICV injection of leptin (10 μg) or vehicle (n = 6 each group). *P ≤ 0.05 (vehicle vs. leptin).
Effects of Captopril on SNA Responses to Leptin or CRF in Rats
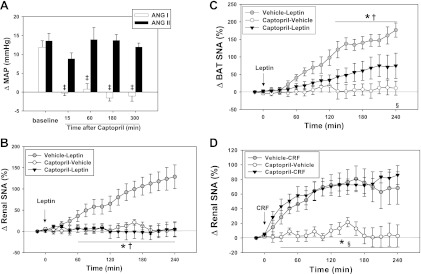
Because leptin increased ACE mRNA in the SFO, we wanted to determine if inhibition of brain ACE with ICV captopril would affect SNA responses to leptin. We verified the efficacy and time course of ACE inhibition by measuring blood pressure responses to repeated injections of ANG I and ANG II before and during 6 h after captopril (12.5 μg ICV). Captopril in this dose blocked (P < 0.05) the effect of ANG I (50 ng) on blood pressure from 15 min to 6 h after captopril (Fig. 6A) but did not block increases in blood pressure with ANG II (50 ng).
Fig. 6.
A: changes in mean arterial pressure (MAP) after intravenous administration of angiotensin I (ANG I; 50 ng; n = 4) or ANG II (50 ng; n = 4) at baseline and for 15 to 300 min after captopril (12.5 μg ICV) in rats. Effects of captopril (12.5 μg ICV) on renal (B) and BAT (C) SNA responses to leptin (10 μg ICV) in rats (n = 6 each group). D: renal SNA responses to CRF in rats after captopril (12.5 μg ICV: after 4 h, n = 6 each group). ‡P < 0.05 (vs. baseline); *P < 0.05 (vehicle-leptin vs. captopril-leptin); †P < 0.05 (vehicle-leptin vs. captopril-vehicle); §P < 0.05 (captopril-leptin vs. captopril-vehicle). For D: *P < 0.05 (vehicle-CRF vs. captopril-vehicle); §P < 0.05 (captopril-CRF vs. captopril-vehicle).
Inhibition of ACE by injection of captopril (12.5 μg ICV) attenuated renal and BAT SNA responses to ICV leptin (Fig. 6, B and C). CRF-induced increases in renal and BAT SNA were not altered by captopril (Fig. 6D).
Acute injection of leptin (10 μg ICV) or CRF (5 μg ICV) did not significantly change arterial pressure or heart rate after either vehicle or captopril (Table 3).
Table 3.
Time course of hemodynamic responses after ICV leptin in rats pretreated with captopril
| Time After Treatment, min |
|||||
|---|---|---|---|---|---|
| Pretreatment/Treatment | Baseline | 60 | 120 | 180 | 240 |
| MAP, mmHg | |||||
| Saline/leptin (6) | 94 ± 3 | 100 ± 4 | 102 ± 5 | 100 ± 4 | 97 ± 5 |
| Captopril/leptin (6) | 100 ± 6 | 94 ± 3 | 96 ± 3 | 96 ± 6 | 96 ± 6 |
| Captopril/saline (6) | 105 ± 11 | 114 ± 8 | 113 ± 9 | 114 ± 11 | 110 ± 11 |
| HR, beats/min | |||||
| Saline/leptin (6) | 378 ± 19 | 386 ± 5 | 391 ± 9 | 393 ± 11 | 398 ± 10 |
| Captopril/leptin (6) | 397 ± 9 | 377 ± 5 | 393 ± 14 | 392 ± 5 | 409 ± 8 |
| Captopril/saline (6) | 367 ± 11 | 370 ± 11 | 385 ± 13 | 391 ± 13 | 402 ± 18 |
Values are means ± SE; nos. in parenthesis equal no. of rats.
DISCUSSION
There are three major findings in this study. First, deletion of AT1aR in mice or pharmacologic blockade of brain AT1R in rats attenuated leptin-induced increases in renal and BAT SNA but did not alter SNA responses to other sympathoexcitatory stimuli such as melanocortin receptor stimulation or CRF, suggesting selectivity of the brain RAS-leptin interaction in the regulation of SNA. Second, deletion of AT1aR did not attenuate leptin-induced decreases in food intake or body weight. Third, cerebroventricular administration of captopril attenuated leptin effects on renal and BAT SNA in rats, suggesting that production of ANG II within the brain contributes to the interaction between the brain RAS and leptin. These studies support the concept of a brain leptin-RAS interaction in which the brain RAS facilitates leptin-induced increases in SNA while sparing effects of leptin on feeding behavior.
Our studies do not permit definitive insight into the molecular mechanisms or the site(s) of the brain RAS-leptin interaction. The measurements of mRNA suggest two possible mechanisms for the brain leptin-RAS interaction. The first is a leptin-induced upregulation of brain AT1aR. This is suggested by the leptin-induced increases in AT1aR mRNA in the ARC and SFO, both sites of leptin action. The second is a leptin-induced increase in ACE with de novo synthesis of brain ANG II acting on AT1aR. We observed a leptin-induced increase in ACE mRNA in the SFO. We also observed that ICV captopril attenuated SNA responses to ICV leptin. These findings suggest that a leptin-induced increase in ANG II formation in the brain participates in the RAS-leptin interaction.
There are several possible brain sites for the RAS-leptin interaction. We (23) have recently shown that leptin receptors in the ARC contribute importantly to leptin-induced increases in renal SNA and blood pressure, and there is increasing evidence for functional AT1R in the ARC (2, 36). Thus the ARC is a possible site of the RAS-leptin interaction. In addition to leptin actions in the ARC, there is mounting evidence for a distributed brain network of leptin action (17). This network includes the nucleus tractus solitarii (3, 17, 25, 32), the SFO (47, 48), and the ventromedial and dorsomedial hypothalamic nuclei (14, 35), all of which are involved in neurohumoral control of the circulation and angiotensin action. These brain regions, therefore, all represent potential sites of the brain RAS-leptin interaction.
Mice with global deletion of AT1aR had attenuation of the SNA responses to leptin but preservation of food intake and body weight responses to leptin. The normal leptin-induced decreases in food intake and body weight in the AT1aR−/− mice are consistent with data indicating that these mice do not have increased adiposity (30). In a broader context, this disconnect between the food intake and renal SNA responses to leptin is consistent with previous studies from our laboratories (10, 44, 45) and others (1) for selectivity of leptin actions. We (34, 44, 45) previously reported that diet-induced obese mice and two models of murine monogenic obesity have preservation of renal SNA responses to leptin despite partial responses or resistance to leptin-induced decreases in food intake and body weight. This selectivity of leptin resistance has implications for a potential role of leptin in obesity-induced hypertension (34). In the present study, blockade of the brain RAS produced opposite effects on SNA and food intake, i.e., attenuation of renal SNA response to leptin with preservation of the food intake responses. We submit that this dissociation of the SNA and food intake responses to leptin with disruption of the brain RAS is potentially of greater relevance than would be a uniform effect of RAS blockade on SNA and food intake to leptin.
Others have reported that delivery of ANG II specifically to the brain results in increased energy expenditure, with variable effects on food intake. Porter et al. demonstrated evidence for elevated metabolic rate in both young (39) and adult (40) rats during chronic ICV infusion of ANG II. De Kloet et al. (12) recently reported that chronic ICV administration of ANG II in rats augmented energy expenditure and sympathetic activity to BAT. The infusion of ANG II also reduced food intake, and this correlated with increased hypothalamic agouti-related protein, proopiomelanocortin, adrenocorticotropic hormone, and thyrotropin-releasing hormone. Yoshida et al. (55) also recently documented anorexic effects of ANG II administered either to the subcutaneous space or ICV, and these effects were associated with suppression of hypothalamic neuropeptide Y and orexin expression. In contrast, Grobe et al. (19) recently reported differential effects of the brain RAS on food intake and SNA. In that study, “sRA” mice with transgenic brain-specific overexpression of the RAS had a dramatic elevation of BAT SNA and energy expenditure that was blocked by propranolol. The sRA mice exhibited no consistent reduction in total daily food intake. Further, with a 20% reduction in body mass, food intake expressed per body weight was substantially increased in the sRA mice. We (18) have also demonstrated that chronic DOCA-salt treatment in C57BL/6J mice results in an elevation in resting metabolic rate that is dependent on brain AT1 activation but DOCA-salt treatment had no effect on food intake. Thus, while several investigators have demonstrated that the brain RAS reliably increases thermogenic energy expenditure, there is conflicting evidence on the effect of the brain RAS on ingestive behavior.
Our studies were quite different from the above-mentioned studies, as we focused on a leptin-brain RAS interaction and not solely on the brain RAS. Nevertheless, our finding that deletion of AT1aR attenuated the BAT SNA responses to leptin seems consistent with the studies of Porter et al. (39, 40), de Kloet et al. (12), and Grobe et al. (18, 19). The surprising finding is that we did not observe an attenuation of the body weight effects of leptin in the AT1aR knockout mice given that deletion or blockade of AT1R blunted leptin-induced increases in BAT SNA. Since sympathetically mediated thermogenic metabolism contributes to the weight-reducing action of leptin, one might expect that blunting the effects of leptin on BAT SNA by disruption of the brain RAS would reduce the body weight effects of leptin. We did not, however, observe attenuation of the body weight response to leptin treatment in AT1aR−/− vs. AT1aR+/+ mice. We cannot explain these observations except to suggest that other mechanisms might have compensated for the metabolic effects of attenuation of leptin-induced increases in BAT SNA. For example, we studied mice with global deletion of AT1aR. Deletion of peripheral AT1aR that are known to have a role in adipose tissue differentiation might have offset the metabolic effect of an attenuation of leptin-induced decreases in BAT SNA. Similarly, we cannot discount possible alterations in thermoregulatory behaviors (burrowing, saliva spreading, and huddling) and physical activity in the mice, which could confound interpretation of body mass data.
Our data support a selective interaction between the brain RAS and leptin. Blockade of brain AT1R in rats abolished SNA responses to leptin but did not attenuate CRF or MTII induced increases in SNA. Similarly, deletion of AT1aR in mice virtually abolished SNA responses to leptin but spared SNA responses to melanocortin receptor stimulation with MTII. The renal SNA and cardiovascular responses to leptin are mediated substantially through stimulation of melanocortin four receptors (43, 51). The findings that disruption of AT1aR attenuated SNA responses to leptin but not to MTII in mice and that losartan blocked SNA responses to leptin but not to MTII or CRF in rats suggest an unexpected level of selectivity in the brain RAS interaction that appears to involve SNA responses to leptin but not to melanocortin receptor agonists or CRF.
As expected (8, 30), the anesthetized AT1aR knockout mice had lower blood pressure than their wild-type controls, raising the possibility that the lower blood pressure may have produced a nonspecific depression of sympathetic responsiveness. This seems unlikely because another sympathoexcitatory stimulus, namely MTII, produced increases in renal SNA that were similar in the AT1aR knockout and wild-type mice.
Another possible interpretation of our results is that the leptin-induced increases in SNA are baroreceptor mediated and that blockade or deletion of AT1R attenuates these baroreflex-mediated increases in SNA. There are two lines of evidence against this explanation. First, arterial pressure did not decrease with leptin after losartan, captopril, or vehicle in rats. Second, blockade of AT1R in rats or deletion of AT1aR in mice attenuated BAT as well as renal SNA responses to leptin. We previously reported that baroreceptor reflexes do not mediate or modulate leptin-induced increases in BAT SNA (24). These lines of evidence indicate that the effect of AT1aR blockade or deletion on the SNA responses to leptin cannot be attributed to attenuation of a normal baroreflex increase in SNA.
Our experiments involved measurement of SNA responses to acute administration of leptin. Whether these findings on a leptin-RAS interaction in regulation of SNA can be reproduced with chronic leptin treatment in unanesthetized animals remains to be determined. In addition, chronic studies are necessary to determine if blockade or deletion of brain AT1R attenuates the effects of chronic administration of leptin on arterial pressure in unanesthetized rodents.
Perspectives
The present studies provide evidence for a selective brain leptin-RAS interaction in regulation of SNA. Deletion or pharmacologic blockade of brain AT1R attenuated renal and BAT sympathetic nerve responses to ICV administration of leptin without blocking responses to other sympathoexcitatory stimuli. In addition, global deletion of AT1aR did not affect leptin-induced decreases in food intake or body weight. These findings suggest that the brain RAS facilitates leptin-induced increases in SNA while sparing effects of leptin on feeding behavior. This study does not delineate the significance of this brain leptin-RAS interaction in terms of regulation of arterial pressure in lean or obese mice or during increases in circulating leptin. In a broader sense, the study adds to the evidence that there is selectivity, not uniformity, in the modulation of leptin actions.
GRANTS
This work was supported by the National Institutes of Health Program Project Grants PO1-HL-084207 (to A. L. Mark, K. Rahmouni, and C. D. Sigmund) and HL-061446 (to C. D. Sigmund) and National Institutes of Health Pathway to Independence K99/R00 Award HL-098276 (J. L. Grobe), by research endowed chairs and funds from the Roy J. and Lucille A. Carver Trust (to A. L. Mark and C. D. Sigmund), by American Diabetes Association award 1-11-BS-127 (to K. Rahmouni), and by the American Heart Association Midwest Affiliate Postdoctoral Fellowship 11POST5610024 (to A. M. Hilzendeger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.M.H., D.A.M., L.B., X.L., K.R., C.D.S., and A.L.M. conception and design of research; A.M.H., D.A.M., L.B., and X.L. performed experiments; A.M.H., D.A.M., L.B., D.D., X.L., J.L.G., K.R., C.D.S., and A.L.M. analyzed data; A.M.H., D.A.M., D.D., X.L., J.L.G., K.R., C.D.S., and A.L.M. interpreted results of experiments; A.M.H., D.A.M., J.L.G., and A.L.M. prepared figures; A.M.H. and A.L.M. drafted manuscript; A.M.H., D.A.M., X.L., J.L.G., K.R., C.D.S., and A.L.M. edited and revised manuscript; A.M.H., D.A.M., L.B., D.D., X.L., J.L.G., K.R., C.D.S., and A.L.M. approved final version of manuscript.
ACKNOWLEDGEMENTS
We acknowledge the technical assistance of Yang Yu, Shunguang Wei, Zhihau Zhang, and Khristofor Agassandian in the dissection of the brain regions for the molecular studies. We thank Norma Sinclair, Patricia Yarolem, and JoAnn Schwarting from the Transgenic Animal Facility for genotyping of AT1aR-deficient mice.
REFERENCES
- 1. Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest 105: 1243–1252, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arakawa H, Chitravanshi VC, Sapru HN. The hypothalamic arcuate nucleus: a new site of cardiovascular action of angiotensin-(1–12) and angiotensin II. Am J Physiol Heart Circ Physiol 300: H951–H960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension 54: 1001–1008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol 287: R943–R949, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Boustany CM, Brown DR, Randall DC, Cassis LA. AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 289: R181–R186, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 39: 496–501, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Cassis LA, English VL, Bharadwaj K, Boustany CM. Differential effects of local versus systemic angiotensin II in the regulation of leptin release from adipocytes. Endocrinology 145: 169–174, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Chen D, La Greca L, Head GA, Walther T, Mayorov DN. Blood pressure reactivity to emotional stress is reduced in AT1A-receptor knockout mice on normal, but not high salt intake. Hypertens Res 32: 559–564, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Considine RV, Caro JF. Leptin and the regulation of body weight. Int J Biochem Cell Biol 29: 1255–1272, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes 51: 439–442, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Davisson RL, Yang G, Beltz TG, Cassell MD, Johnson AK, Sigmund CD. The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circ Res 83: 1047–1058, 1998 [DOI] [PubMed] [Google Scholar]
- 12. de Kloet AD, Krause EG, Scott KA, Foster MT, Herman JP, Sakai RR, Seeley RJ, Woods SC. Central angiotensin II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab 301: E1081–E1091, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes 46: 2040–2043, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Dyer AR, Elliott P, Shipley M, Stamler R, Stamler J. Body mass index and associations of sodium and potassium with blood pressure in INTERSALT. Hypertension 23: 729–736, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med 79: 21–29, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Friedman JM. Obesity: Causes and control of excess body fat. Nature 459: 340–342, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity 14, Suppl 5: 216S–221S, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology (Bethesda) 23: 187–193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall JE. The kidney, hypertension, obesity. Hypertension 41: 625–633, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res 108: 808–812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hausberg M, Morgan DA, Chapleau MA, Sivitz WI, Mark AL, Haynes WG. Differential modulation of leptin-induced sympathoexcitation by baroreflex activation. J Hypertens 20: 1633–1641, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hilzendeger AM, Morais RL, Todiras M, Plehm R, da Costa Goncalves A, Qadri F, Araujo RC, Gross V, Nakaie CR, Casarini DE, Carmona AK, Bader M, Pesquero JB. Leptin regulates ACE activity in mice. J Mol Med 88: 899–907, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Jayasooriya AP, Mathai ML, Walker LL, Begg DP, Denton DA, Cameron-Smith D, Egan GF, McKinley MJ, Rodger PD, Sinclair AJ, Wark JD, Weisinger HS, Jois M, Weisinger RS. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci USA 105: 6531–6536, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kannel WB, Brand N, Skinner JJ, Jr, Dawber TR, McNamara PM. The relation of adiposity to blood pressure and development of hypertension. The Framingham Study. Ann Intern Med 67: 48–59, 1967 [DOI] [PubMed] [Google Scholar]
- 30. Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, Horiuchi M, Hirata Y, Ogawa Y. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology 146: 3481–3489, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension 53: 375–380, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. State-of-the-art-lecture: Obesity-induced hypertension: new concepts from the emerging biology of obesity. Hypertension 33: 537–541, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Mark AL, Correia ML, Rahmouni K, Haynes WG. Selective leptin resistance: a new concept in leptin physiology with cardiovascular implications. J Hypertens 20: 1245–1250, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension 42: 488–493, 2003 [DOI] [PubMed] [Google Scholar]
- 36. McKinley MJ, Allen AM, Clevers J, Paxinos G, Mendelsohn FA. Angiotensin receptor binding in human hypothalamus: autoradiographic localization. Brain Res 420: 375–379, 1987 [DOI] [PubMed] [Google Scholar]
- 37. Morimoto S, Cassell MD, Sigmund CD. Glia- and neuron-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem 277: 33235–33241, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed). San Diego, CA: Academic, 1998 [Google Scholar]
- 39. Porter JP, Anderson JM, Robison RJ, Phillips AC. Effect of central angiotensin II on body weight gain in young rats. Brain Res 959: 20–28, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Porter JP, Potratz KR. Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am J Physiol Regul Integr Comp Physiol 287: R422–R428, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 55: 862–868, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest 118: 1458–1467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 23: 5998–6004, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rahmouni K, Haynes WG, Morgan DA, Mark AL. Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension 39: 486–490, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54: 2012–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Santos EL, de Picoli Souza K, Guimaraes PB, Reis FC, Silva SM, Costa-Neto CM, Luz J, Pesquero JB. Effect of angiotensin converting enzyme inhibitor enalapril on body weight and composition in young rats. Int Immunopharmacol 8: 247–253, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA, Ferguson AV. The subfornical organ: a central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol 296: R512–R520, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Smith PM, Ferguson AV. Cardiovascular actions of leptin in the subfornical organ are abolished by diet-induced obesity. J Neuroendocrinol 24: 504–510, 2012 [DOI] [PubMed] [Google Scholar]
- 49. Smith PM, Ferguson AV. Circulating signals as critical regulators of autonomic state–central roles for the subfornical organ. Am J Physiol Regul Integr Comp Physiol 299: R405–R415, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Stucchi P, Cano V, Ruiz-Gayo M, Fernandez-Alfonso MS. Aliskiren reduces body-weight gain, adiposity and plasma leptin during diet-induced obesity. Br J Pharmacol 158: 771–778, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension 48: 58–64, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol 302: 111–117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wei SG, Zhang ZH, Yu Y, Felder RB. Systemically administered tempol reduces neuronal activity in paraventricular nucleus of hypothalamus and rostral ventrolateral medulla in rats. J Hypertens 27: 543–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weisinger RS, Stanley TK, Begg DP, Weisinger HS, Spark KJ, Jois M. Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6J mice maintained on a high fat diet. Physiol Behav 98: 192–197, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Yoshida T, Semprun-Prieto L, Wainford RD, Sukhanov S, Kapusta DR, Delafontaine P. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology 153: 1411–1420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yvan-Charvet L, Massiera F, Lamande N, Ailhaud G, Teboul M, Moustaid-Moussa N, Gasc JM, Quignard-Boulange A. Deficiency of angiotensin type 2 receptor rescues obesity but not hypertension induced by overexpression of angiotensinogen in adipose tissue. Endocrinology 150: 1421–1428, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Zorad S, Dou JT, Benicky J, Hutanu D, Tybitanclova K, Zhou J, Saavedra JM. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma. Eur J Pharmacol 552: 112–122, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]