Abstract
Near-infrared spectroscopy (NIRS) is a well-known method used to measure muscle oxygenation and hemodynamics in vivo. The application of arterial occlusions allows for the assessment of muscle oxygen consumption (mV̇o2) using NIRS. The aim of this study was to measure skeletal muscle mitochondrial capacity using blood volume-corrected NIRS signals that represent oxygenated hemoglobin/myoglobin (O2Hb) and deoxygenated hemoglobin/myoglobin (HHb). We also assessed the reliability and reproducibility of NIRS measurements of resting oxygen consumption and mitochondrial capacity. Twenty-four subjects, including four with chronic spinal cord injury, were tested using either the vastus lateralis or gastrocnemius muscles. Ten healthy, able-bodied subjects were tested on two occasions within a period of 7 days to assess the reliability and reproducibility. NIRS signals were corrected for blood volume changes using three different methods. Resting oxygen consumption had a mean coefficient of variation (CV) of 2.4% (range 1–32%). The recovery of oxygen consumption (mV̇o2) after electrical stimulation at 4 Hz was fit to an exponential curve, which represents mitochondrial capacity. The time constant for the recovery of mV̇o2 was reproducible with a mean CV of 10% (range 1–22%) only when correcting for blood volume changes. We also examined the effects of adipose tissue thickness on measurements of mV̇o2. We found the mV̇o2 measurements using absolute units to be influenced by adipose tissue thickness (ATT), and this relationship was removed when an ischemic calibration was performed, supporting its use to compare mV̇o2 between individuals of varying ATT. In conclusion, in vivo oxidative capacity can be assessed using blood volume-corrected NIRS signals with a high degree of reliability and reproducibility.
Keywords: mitochondrial capacity, electrical stimulation, oxidative metabolism
measuring skeletal muscle oxidative metabolism has been important in understanding muscle function in health and disease (19, 23). Noninvasive methodologies have enhanced the study of mitochondrial function, particularly in human participants. The primary noninvasive method of measuring mitochondrial function has been magnetic resonance spectroscopy (MRS), and in particular the use of the kinetics of phosphocreatine (PCr) resynthesis after exercise as a direct assessment of mitochondrial capacity (30, 35). However, MRS is a costly technique that requires large, expensive equipment and a high level of technical expertise, making measurement difficult. Near-infrared spectroscopy (NIRS) provides a noninvasive measure of muscle oxygenation (9, 10, 20, 31). Commercially available NIRS devices typically provide information about the relative changes in oxygenated hemoglobin/myoglobin (O2Hb), deoxygenated hemoglobin/myoglobin (HHb), and total hemoglobin or blood volume (tHb). Compared with MRS, these NIRS devices are much smaller, less expensive, portable, and easier to use, making them more practically useful for a clinical setting.
NIRS measurement of skeletal muscle oxygen consumption has been done previously using both venous and arterial occlusions (2, 18, 28, 36). However, a number of researchers have suggested that during occlusions there is a blood volume change that could confound the slope measurements for oxygen consumption (4, 38). Blood volume changes during occlusions can mask changes in the NIRS signals due to oxygen consumption. Some researchers have suggested that the deoxygenated hemoglobin/myoglobin (HHb) signal is less sensitive to blood volume changes (5, 17). Motobe and colleagues (36) first described a method for measuring mitochondrial capacity using transient arterial occlusions and NIRS. In this study, participants performed short-duration handgrip exercise followed by repeated transient arterial occlusions to measure muscle oxygen consumption (mV̇o2) (see Figure 1A of Ref. 36). The repeated arterial occlusions were fit to a monoexponential curve and the time constant was calculated as a measure of muscle mitochondrial function (see Figure 1B of Ref. 36). After a closer look at Figure 1A of Ref. 36, there is an obvious blood volume (tHb) change during the arterial occlusions, which has an influence on the measurement of mV̇o2. In order for the arterial occlusion method of measuring mV̇o2 to be valid, the NIRS signal should reflect a symmetrical change in O2Hb and HHb, and therefore no change in tHb (tHb = O2Hb + HHb). The dissociation of oxygen molecules from oxyhemoglobin/myoglobin, which supplies the oxygen required for oxidative phosphorylation, should be reflected in equal and opposite changes in the NIRS signals for O2Hb and HHb.
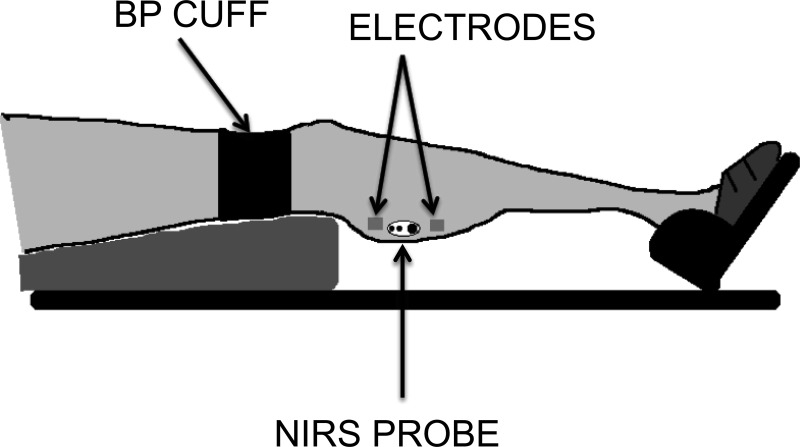
Fig. 1.
Experimental setup for measurements on the gastrocnemius muscle. A similar set up was used for measurements on the vastus lateralis muscle. BP, blood pressure; NIRS, near-infrared spectroscopy.
In this paper, we provide detailed methods for blood volume corrections, which allow for the reliable assessment of skeletal muscle mitochondrial function using NIRS. We have applied a blood volume correction to data from a variety of subjects including healthy college-aged participants (both recreationally active and sedentary) and clinical populations such as individuals with spinal cord injury. We also assessed the reliability and test-retest reproducibility of our measurements in young, healthy individuals.
MATERIALS AND METHODS
Subjects
Twenty-four subjects (14 male, 10 female) were tested in this study. Subjects were chosen to represent a wide variety of muscle oxidative capacity and included college-aged endurance-trained and sedentary, as well as four individuals with chronic spinal cord injury (SCI). Ten healthy, young subjects (6 male, 4 female) were tested on two separate days over a period of 1 wk to assess the reliability and reproducibility of our measurements. The study was conducted with the approval of the Institutional Review Board at the University of Georgia (Athens, GA), and all subjects gave written, informed consent before testing.
Experimental Protocol
Each subject was placed on a padded table, supine, with both legs extended (0° of flexion). The right foot was placed into a home-built isometric exercise device to limit motion artifact in the NIRS signal. The foot was strapped firmly to the exercise device using nonelastic Velcro straps proximal to the base of the fifth digit, and the knee was supported. The NIRS optode was placed at the level of the largest circumference of the muscle of interest (medial gastrocnemius or vastus lateralis) and secured with Velcro straps and biadhesive tape. Four aluminum foil electrodes attached to a Theratouch 4.7 stimulator (Rich-mar, Inola, OK) were placed on the skin, two proximal and two distal to the NIRS optode (Fig. 1). A blood pressure cuff (Hokanson E20 cuff inflator; Bellevue, WA) was placed proximal to the NIRS optode.
Testing occurred on one visit to the laboratory. Subjects who participated in the reproducibility study were tested on a second occasion 3–7 days after the first session. Adipose tissue thickness (ATT) was measured at the site of the NIRS optode using B-mode ultrasound (LOGIQ e; GE HealthCare). Subjects were instructed not to consume caffeine or tobacco on the day of the test or to use alcohol or perform moderate or heavy physical activity for at least 24 h before the test.
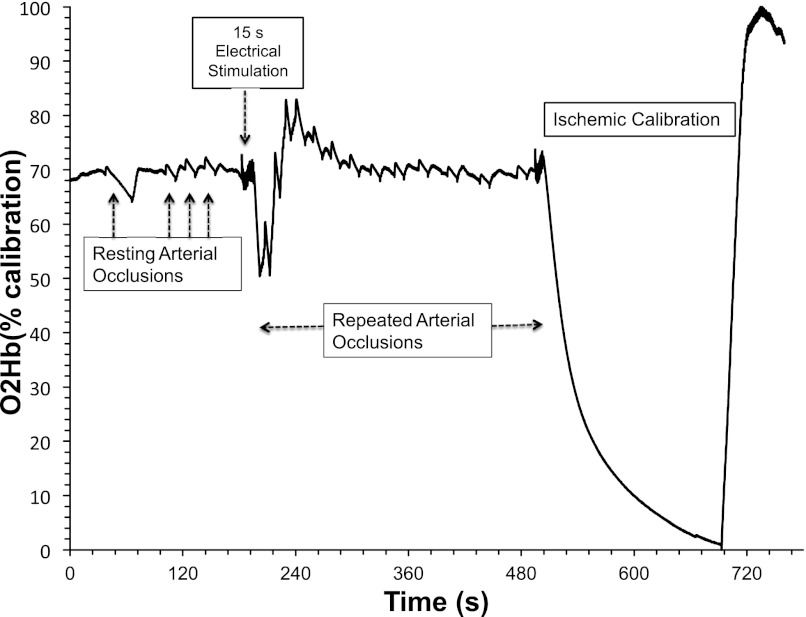
The test protocol consisted of a baseline measurement of muscle oxygenation, followed by inflation of a blood pressure cuff (250–300 mmHg) for the measurement of resting mV̇o2. To increase mV̇o2, 15 s of continuous electrical stimulation at 4 Hz was applied to the muscle. Previous studies have shown short-duration exercise produces similar initial rates of phosphocreatine resynthesis (40). The current intensity was adjusted for each individual to produce twitch contractions at the maximal tolerable level. Pilot experiments suggested that small differences in stimulation level did not influence measurements of metabolic rate (data not reported). Immediately following the electrical stimulation, a series of 10–18 brief (5–10 s) arterial occlusions were applied to measure the rate of recovery of mV̇o2 back to resting levels. To maximize our ability to measure the recovery of mV̇o2 while minimizing the discomfort to participants, the duration between arterial occlusions begins at 5 s and extends to 20 s by the end of the repeated occlusions (i.e., 5 s for cuffs 1–5, 10 s for cuffs 6–10, 15–20 s for cuffs 11–15). Finally, to normalize the NIRS signal, a 3- to 5-min arterial occlusion was applied to completely deoxygenate the tissue under the optode (i.e., 0% oxygenation), and the peak hyperemic response upon release of the cuff was used to indicate 100% oxygenation (Fig. 2). A brief 5-s electrical stimulation period was performed prior to this 3- to 5-min arterial occlusion to increase metabolic rate, thereby minimizing the duration of the arterial occlusion and the discomfort imposed on the participants.
Fig. 2.
Muscle oxygenated hemoglobin/myoglobin (O2Hb; as a percentage of the ischemic calibration) during rest, resting arterial occlusions, and a 15-s electrical stimulation exercise followed by a series of transient arterial occlusions after exercise. The final 3–5 min are an ischemic calibration used to determine a relative concentration.
NIRS Device
NIRS signals were obtained using a continuous-wave NIRS device (Oxymon MK III, Artinis Medical Systems), which consists of two channels (2 equivalent pulsed light sources, 2 avalanche photodiode detectors, shielding from ambient light), and uses intensity-modulated light at a frequency of 1 MHz, and laser diodes at three wavelengths (905, 850, and 770 nm) corresponding to the absorption wavelengths of oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb), with an autosensing power supply (∼40 W at 110–240 V). The probe was set for two source-detector separation distances after measurement of adipose tissue thickness. The NIRS data were collected at 10 Hz.
Calculation of Muscle Oxygen Consumption
mV̇o2 was calculated as the slope of change in O2Hb and HHb during the arterial occlusion using simple linear regression. mV̇o2 was calculated in absolute units using a differential pathlength factor (DPF) of 4, as suggested by the manufacturer. mV̇o2 was also expressed as a percentage of the ischemic calibration per unit time. This measurement was made at rest and repeated a number of times after exercise. The postexercise repeated measurements of mV̇o2 were fit to a monoexponential curve according to the formula below:
| (1) |
For this equation, y represents relative mV̇o2 during the arterial occlusion, End is the mV̇o2 immediately after the cessation of exercise, Delta is the change in mV̇o2 from rest to end exercise, and Tc is the fitting time constant.
Correction for Blood Volume
NIRS data were analyzed using custom-written routines for Matlab v. 7.13.0.564 (The Mathworks, Natick, MA). The calculation of a blood volume correction factor (β) is based on the assumption that during an arterial occlusion, changes in O2Hb and HHb occur with a 1:1 ratio that represents mitochondrial oxygen consumption only (no arterial oxygen delivery or venous return of deoxygenated blood), thus making the area under the NIRS probe a closed system. The following equation can be used to conceptualize the blood volume change:
| (2) |
The change in NIRS signals (both HHb and O2Hb) during an arterial occlusion occurs due to a metabolic consumption of oxygen and a blood volume flux from the redistribution of heme between high-pressure arteries/arterioles and low-pressure veins/venules. Therefore, accurate quantification of mV̇o2 requires either 1) no change in blood volume, or 2) the removal of blood volume change from the NIRS signal (i.e., mV̇o2 = ΔNIRSSignal − Δblood volume).
To correct NIRS signals for changes in blood volume, the blood change must be proportioned into oxygenated and deoxygenated sources. The equation below describes the calculation for this correction factor:
| (3) |
For this equation, β is the blood volume correction factor, t represents time, O2Hb is the oxygenated hemoglobin/myoglobin signal, and HHb is the deoxygenated hemoglobin/myoglobin signal. β is a nondirectional factor that represents the proportionality of the blood volume change (values range from 0 to 1).
We used three approaches to determine β.
Method 1.
This method calculates a single β for each arterial occlusion. The proportion of blood volume change attributed to the O2Hb signal (β) is calculated for each data point during an arterial occlusion. Next, the average β is calculated for these data points. Once β is obtained, it is applied to each individual data point using Eqs. 4 and 5 below. A single correction factor (β) is applied to each individual data point over the course of the arterial occlusion.
Method 2.
This method calculates a β for each data point and can account for small changes in the proportionality of the blood volume change throughout a cuff. Each data point is then corrected using its corresponding β according to Eqs. 4 and 5 below.
Method 3.
This method calculates a global β and assumes this value does not change throughout the test. This global β is then applied to each arterial occlusion. To calculate β, a postprocessing correction was performed using an iterative least-squares error method for obtaining β (using Nelder-Mead algorithm from Matlab's fminsearch function). This routine searches for the β that minimized the sum of squared residuals (a residual being the difference between O2Hb and HHb signals).
Once β was defined, its application to the raw data is shown below:
| (4) |
| (5) |
where O2Hbc and HHbc are the corrected oxygenated and deoxygenated hemoglobin/myoglobin signals, respectively; tHb if the arithmetic sum of the uncorrected O2Hb and HHb (the blood volume signal from the NIRS device); and β is the blood volume correction factor. In Eq. 4, the raw O2Hb signal is corrected by subtracting the proportion of the blood volume [tHb × (1 − β)] change attributed to O2Hb, thus producing a corrected O2Hb signal that represents the O2Hb signal minus the oxygenated blood volume change. Similarly, in Eq. 5, the raw HHb signal is corrected by subtracting the proportion of blood volume [tHb × β] change attributed to HHb, thus producing a corrected HHb signal that represents the HHb signal minus the deoxygenated blood volume change.
Statistical Analysis
Data are presented as means ± SD. Test-retest reliability was analyzed using coefficient of variation (CV) and intraclass correlation coefficients (ICC). Relative reliability is related to an individual maintaining his/her position within a sample for repeated measurements (1). We assess this type of reliability with the ICC, which indicates error in measurements as a proportion of the total variance in scores. ICC analysis was done using a downloadable spreadsheet (22). The absolute reliability can be described as the degree to which repeated measurements vary for individuals. This was performed by calculating the CV for each subject, which is the SD of measurements recorded during both tests divided by the mean of the two tests. CV was expressed as a percentage. Statistical analyses were performed using SPSS 19.0 (IBM, Armonk, NY). The relationship between two variables was analyzed by least-squares regression analysis. Significance was accepted when P < 0.05.
RESULTS
All subjects were able to complete testing with no adverse events. The physical characteristics of participants are shown in Table 1.
Table 1.
Physical characteristics of participants
| Muscle Tested | Height, cm | Weight, kg | Age, yr | Sex, M/F | ATT, mm |
|---|---|---|---|---|---|
| Medial gastrocnemius (n = 10) | 172.8 ± 6.9 | 67.5 ± 7.7 | 23.3 ± 3.0 | 6 M/4 F | 6.0 ± 1.6 |
| Vastus lateralis (n = 14) | 175.2 ± 12.8 | 71.2 ± 16.0 | 35.5 ± 15.4 | 8 M/6 F | 8.0 ± 2.8 |
Values are expressed as means ± SD. M, men; F, women; ATT, adipose tissue thickness.
Blood Volume Correction
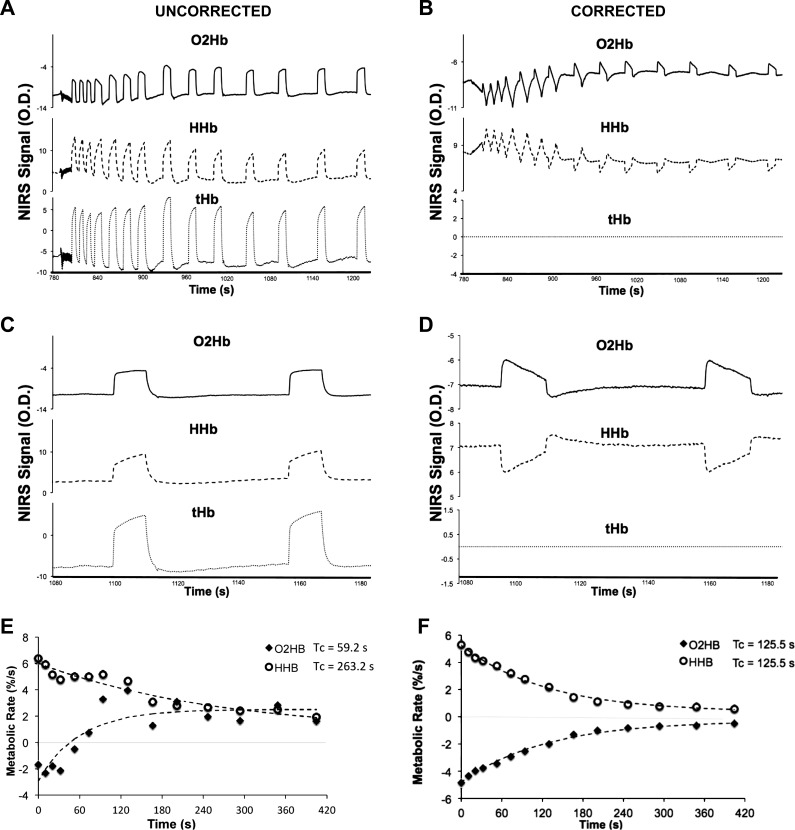
All data were analyzed using the three approaches described above for correction of the blood volume influence on the NIRS signal. There were no differences in resting, postexercise mV̇o2, or the recovery time constant using the three methods (P = 0.39). All three methods produced similar results (R2Method1.Method2 = 0.99; R2Method1.Method3 = 0.98; R2Method2.Method3 = 0.96). While all three approaches produced similar results, correcting each data point (method 2) for its corresponding blood volume change was the most robust method and produced the most reliable results. Therefore, the remainder of the results was presented using method 2 (individualized β's for each arterial occlusion). The application of the blood volume correction can be seen in Fig. 3.
Fig. 3.
Postexercise arterial occlusion data for the oxygenated hemoglobin/myoglobin (O2Hb), deoxygenated hemoglobin/myoglobin (HHb), and the blood volume (tHb) signals for one individual uncorrected (A) and with a blood volume correction (B). Magnification of the final two cuffs for uncorrected (C) and corrected (D) signals are also shown. Monoexponential recovery curves are also shown for uncorrected (E) and corrected (F) data. The correction used was the individualized βi approach (see materials and methods for description).
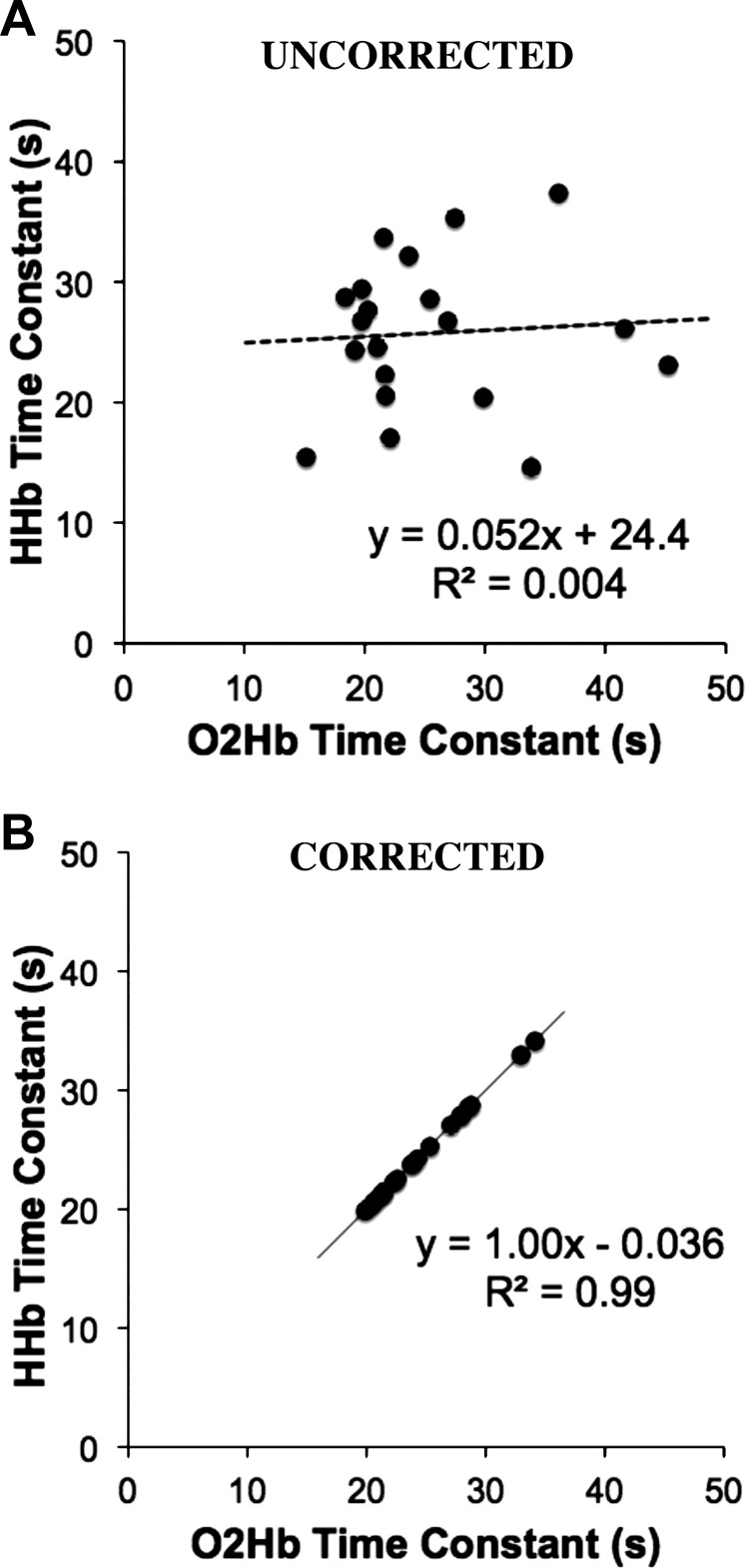
Without correcting for blood volume, there is an unequal change in the O2Hb and HHb signals during arterial occlusions in nearly all of the tests. We found no relationship between the recovery time constants for the O2Hb and HHb signals (P = 0.78) without the blood volume correction (Fig. 4A). When correcting for blood volume, there is a strong relationship between the O2Hb and HHb signals (P < 0.001) as shown in Fig. 4B. We did find the HHb signal was less susceptible to blood volume changes than the O2Hb signal in most cases. Because blood volume changes mask changes in oxygen consumption, the raw data, uncorrected for blood volume, were excluded from the remainder of the analysis. The average β for all subjects and all test days was 0.52 ± 0.21 (mean ± SD). The blood volume correction (β) was variable between individuals (CV = 40.1%). However, β was consistent between cuffs, and is not influenced by the metabolic rate of the tissue (Fig. 5). The mean CV between cuffs within a single test was 7.5% (range = 0.3–26%). β (within subjects) was more variable between test days 1 and 2 (CV = 34%). Figure 5 shows the group average β over the time for a resting arterial occlusion and the first end-exercise arterial occlusion. Although β was variable between subjects, all individuals presented similar responses: no change in β over the first 3 s followed by a gradual rise in β during the final 1 s of occlusion (Fig. 5). Within-subject β from the resting occlusions was not statistically different from end-exercise β for both test 1 and test 2 (P = 0.414 and P = 0.486, respectively). Resting β was not statistically different for participants between test 1 and test 2 (P = 0.823). Similarly, end-exercise β was not statistically different for participants between test 1 and test 2 (P = 0.805).
Fig. 4.
Comparisons between the recovery time constants for the oxygenated hemoglobin/myoglobin (O2Hb) and deoxygenated hemoglobin/myoglobin (HHb) signals without (A) and with (B) the blood volume correction.
Fig. 5.
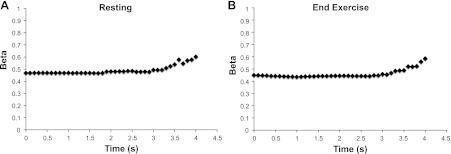
Blood volume correction factor (β) calculated for each individual data point over the course a resting arterial occlusion (A) and the first end-exercise arterial occlusion (B). Slope measurements of oxygen consumption were made over the first 3 s. Data presented are the mean changes in β for all individuals and all tests. Error bars were omitted for visual presentation, as β was variable between individuals. Despite individual differences the change in β over time was similar between individuals for resting cuffs (low metabolic rate) and end-exercise cuffs (high metabolic rate).
Reproducibility
The reproducibility of the NIRS measurements for resting mV̇o2 and the end-exercise recovery of mV̇o2 was investigated by testing 10 individuals on two separate occasions. All values for resting mV̇o2 and the recovery of mV̇o2 as well as the CV and ICC are shown in Table 2.
Table 2.
Resting and end-exercise oxygen consumption from NIRS
| Day 1 (n = 10) | Day 2 (n = 10) | Mean CV, % | ICC (95% CI) | |
|---|---|---|---|---|
| Resting mV̇o2, %/s | ||||
| O2Hb | ||||
| Uncorrected | 0.25 ± 0.11 | 0.24 ± 0.17 | 31.4 | −0.44 |
| Corrected | 0.28 ± 0.08 | 0.28 ± 0.11 | 2.4 | 0.19 |
| HHb | ||||
| Uncorrected | 0.32 ± 0.11 | 0.33 ± 0.12 | 10.7 | 0.08 |
| Corrected | 0.28 ± 0.08 | 0.28 ± 0.11 | 2.4 | 0.19 |
| End-exercise recovery | ||||
| O2Hb Tc, s | ||||
| Uncorrected | 24.3 ± 8.2 | 23.7 ± 6.1 | 21.0 | 0.14 |
| Corrected | 24.5 ± 4.0 | 22.1 ± 2.1 | 10.6 | 0.68 |
| HHb Tc, s | ||||
| Uncorrected | 28.3 ± 7.6 | 23.3 ± 7.6 | 20.6 | 0.57 |
| Corrected | 24.5 ± 4.0 | 22.1 ± 2.1 | 10.6 | 0.67 |
Values are expressed as means ± SD. Resting muscle oxygen consumption (mV̇o2) is expressed as a percentage of the ischemic calibration per second. The end-exercise recovery of mV̇o2 is expressed as the time constant (Tc) of the exponential fit. NIRS, near-infrared spectroscopy; CV, coefficient of variation; ICC, intraclass correlation coefficient; 95% CI, 95% lower and upper confidence intervals.
Resting oxygen consumption.
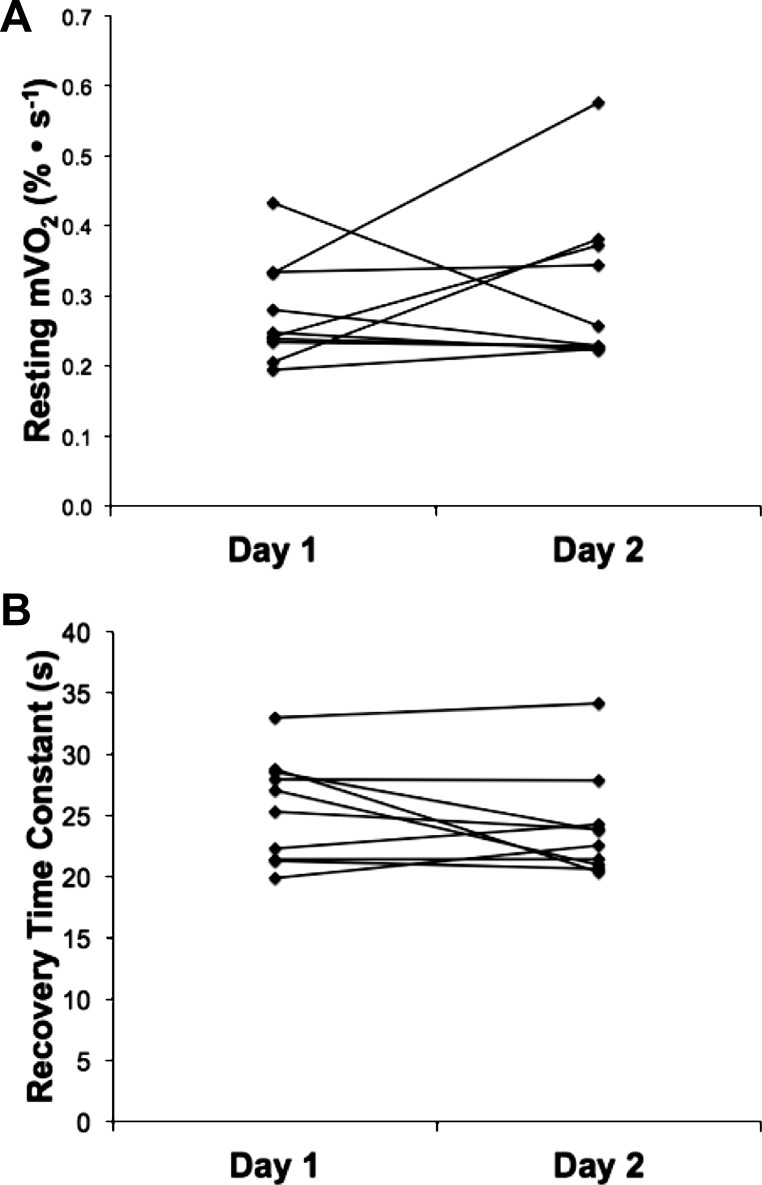
Resting oxygen consumption was measured by NIRS during an arterial occlusion (∼270 mmHg). Resting mV̇o2 of all participants from day 1 and day 2 is shown in Fig. 6A. The mean within-subject CV for comparison between days was 2.4% (range 1–32%). There were no differences in resting oxygen consumption between the vastus lateralis and gastrocnemius muscles. We also compared the reliability and reproducibility of resting mV̇o2 from a single arterial occlusion (∼30 s) with the average of three 10-s arterial occlusions (see Fig. 2). Averaging the three short resting cuffs decreased the within-subjects CV compared with the single cuff (CV = 16% vs. 20%, for the average of 3 measurements and single measurement, respectively). This suggests that averaging three short arterial occlusions for the measurement of resting oxygen consumption produces a modest reduction in the variability. We saw no evidence for short repeated cuffs influencing resting metabolic rate.
Fig. 6.
Reproducibility results for resting muscle oxygen consumption (mV̇o2) (A) and the postexercise recovery time constant of muscle oxygen consumption (B) for day 1 and day 2. Data are presented for each individual that completed two test sessions.
End-exercise recovery of mV̇o2.
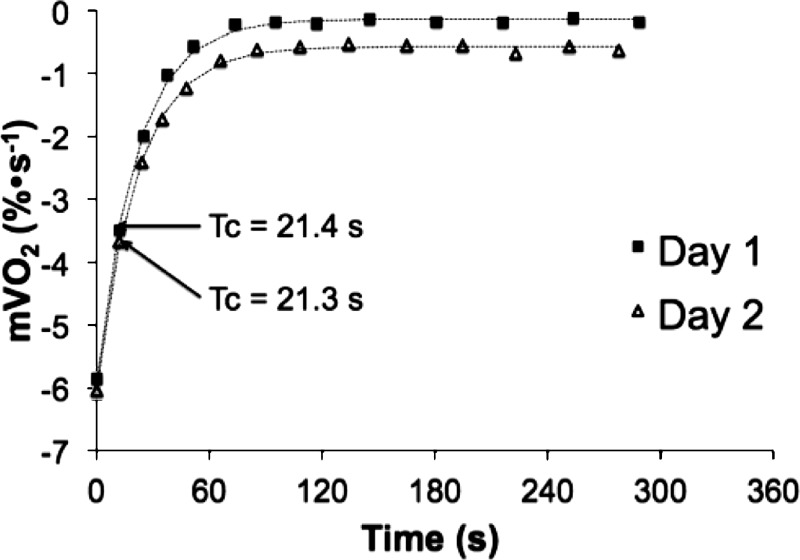
End-exercise recovery of mV̇o2 was measured by NIRS using brief (5–10 s) repeated arterial occlusions (∼270 mmHg). The time constant for the recovery of mV̇o2 following electrical stimulation was 24.5 ± 4.0 s (CV = 16%) for day 1 and 22.1 ± 2.1 s (CV = 10%) for day 2. Recovery mV̇o2 time constants of all participants from day 1 and day 2 are shown in Fig. 6B. Representative mV̇o2 recovery curves from single subject for day 1 and day 2 are shown in Fig. 7. The mean coefficient of variation between day 1 and day 2 was 10.6% (Table 2).
Fig. 7.
Sample recovery curves for a healthy, able-bodied subject from day 1 and day 2. Raw, blood volume-corrected data are represented by solid squares (day 1) and open triangles (day 2).
Influence of Depth
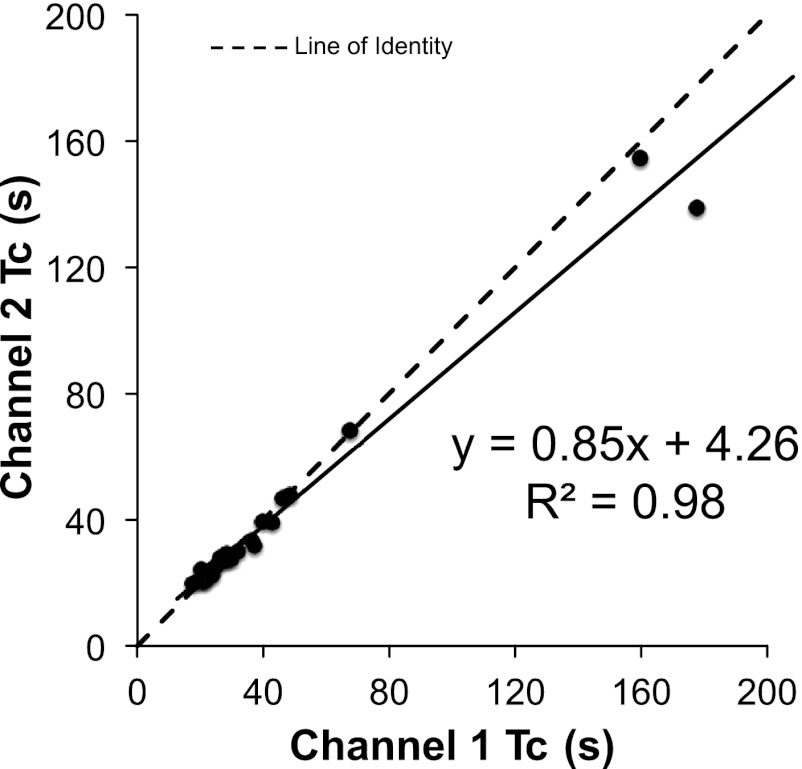
All NIRS testing was performed using two interoptode distances ranging from 3 to 4.5 cm. We compared resting mV̇o2 measured at the shallow vs. deep NIRS signals and found good agreement between channels (r = 0.91, P < 0.001) only when values were expressed as a percentage of the ischemic calibration. Using a differential pathlength factor (DPF) of 4, as recommended by the manufacturer, the relationship between shallow and deep penetration depths is weaker but still significant (r = 0.68, P < 0.001). Figure 8 illustrates the agreement between penetration depths for the corrected recovery time constants. There were no significant differences in mV̇o2 between penetration depths (P = 0.21).
Fig. 8.
Comparisons between the NIRS interoptode distances for blood volume-corrected recovery time constants (Tc).
Effects of ATT on mV̇o2
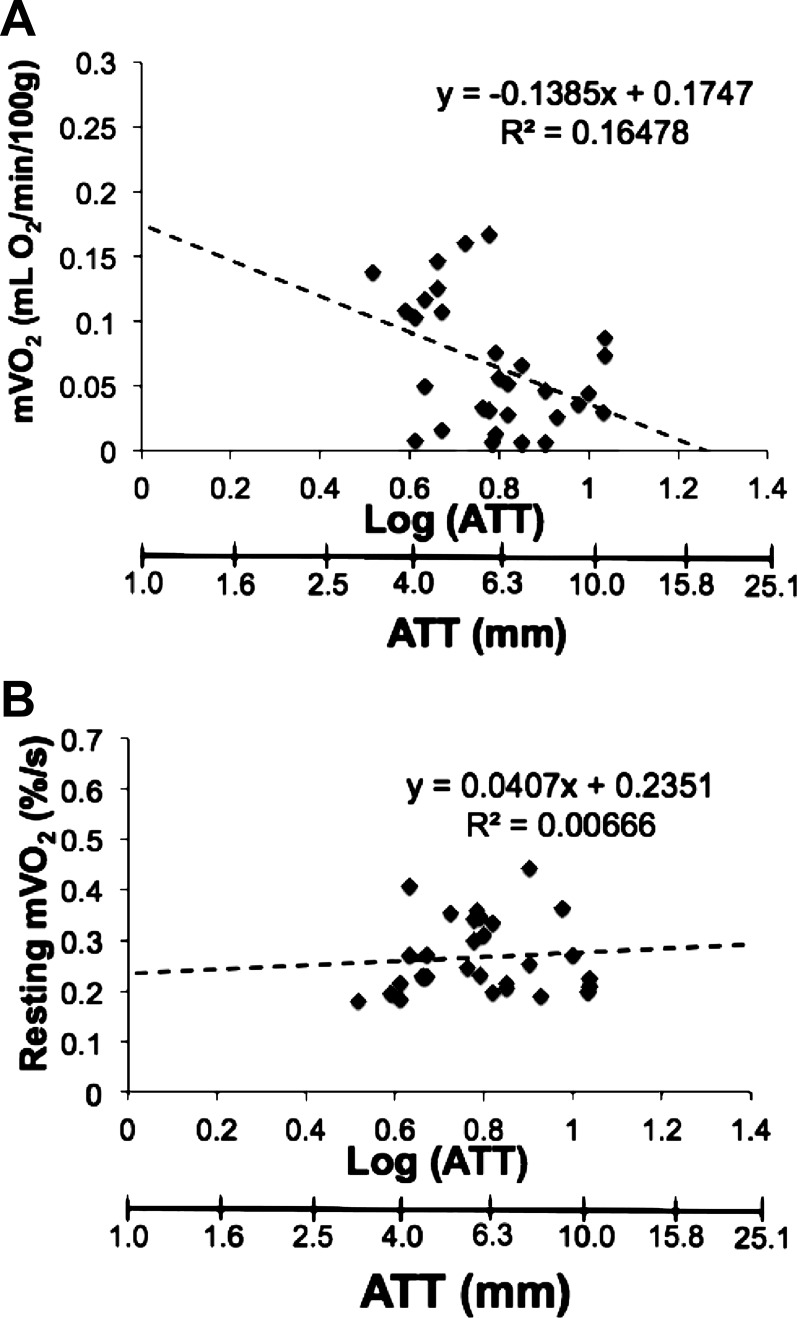
ATT was 6.0 ± 1.6 mm on top of the medial gastrocnemius and 8.0 ± 2.8 mm on top of the vastus lateralis muscle. ATT ranged from 3.3 to 12.0 mm in our participants. Resting mV̇o2 was expressed two ways: using the recommended DPF of 4 to calculate absolute concentration changes and as a percentage of the ischemic calibration. The influence of ATT on mV̇o2 for both methods is shown in Fig. 9. There was a significant relationship between ATT and mV̇o2 (r = 0.41, P = 0.026) calculated using the DPF method (Fig. 9A). This relationship was no longer existent (r = 0.006, P = 0.668) when expressing resting mV̇o2 as a percentage of the ischemic calibration (Fig. 9B).
Fig. 9.
Relationship between resting mV̇o2 and adipose tissue thickness (ATT). A: calculation of resting mV̇o2 in absolute units using a differential pathlength factor (DPF) = 4. B: calculation of mV̇o2 as a percentage of the ischemic calibration. Data represent resting metabolic rate from all subjects tested and all test days.
DISCUSSION
The primary finding of this study was the demonstration of methods to correct NIRS signals to account for changes in blood volume. The three different methods of blood volume correction resulted in consistent and reliable NIRS-based signals of skeletal muscle O2Hb and HHb during arterial occlusion measurements of oxidative metabolism. The appearance of blood volume changes in the NIRS signal, even with arterial and venous occlusion, has been reported by others (5, 8, 13, 17, 25). The data from our raw, uncorrected signals support these volume effects. Our data also support the idea that the HHb signal is less susceptible to changes in blood volume (17). While less influenced by blood volume shifts with occlusion, the uncorrected HHb signal resulted in recovery time constants that were twice as variable when compared with the blood volume-corrected HHb time constants. This suggests that the blood volume correction is needed to accurately detect oxygen consumption measurements for all the signals coming from the NIRS devices.
The exact cause of blood volume changes during arterial occlusions is currently unknown. Blood volume changes could be attributed to movement of heme concentrations from larger vessels (undetectable by NIRS) to microvascular beds detected by NIRS (31). Since the NIRS probe is measuring distally to the arterial occlusion in the vascular tree, there is still a redistribution of heme under the NIRS probe between the high-pressure arterial and low-pressure venous systems. The blood volume correction factor (β) was consistent between cuffs and was not influenced by the metabolic rate of the tissue. While the correction factor value was variable between subjects, all subjects had similar changes in β over time. The β did not change over the first 3 s of arterial occlusion (where the slope measurements for oxygen consumption were made), followed by a slight increase during the final 1–2 s of the occlusion. In most cases seen in this study the proportion of heme was primarily oxygenated heme, suggesting the influx of detectable signal was coming from the arterial side of the vascular tree. The effect of correcting NIRS signals for blood volume changes on measurements of oxygen consumption varies depending on both the magnitude and composition of the blood volume change. In most cases, the uncorrected data have large blood volume changes, primarily oxygenated heme, compared with the change due to metabolism. Therefore removing the blood volume change reverses the direction of the O2Hb signal during the arterial occlusions, thus allowing for the measurement of oxygen consumption. In some cases, the blood volume change is smaller than seen in Fig. 3, and as a result correcting for blood volume has a small effect on the measured slopes. Because the correction factor to account for blood volume was more variable between participants and test sessions, we suggest that the correction method needs to be applied to each set of experiments. Furthermore, because the proportion of blood volume change can occur over time, we feel that correcting each data point in the O2Hb and HHb NIRS signals (method 2) produces the most accurate results.
In this study, resting oxygen consumption was more variable than the postexercise recovery of oxygen consumption. The variability of resting measurements of muscle metabolism in this study was consistent with previous studies. Van Beekvelt et al. (38) measured resting mV̇o2 using venous and arterial occlusion methods, and reported similar between-subjects reliability (CV = 33%) to our results (CV = 30% and 39% for day 1 and day 2, respectively). Hamaoka et al. (18) reported resting muscle oxygen consumption as a percentage change in the O2Hb signal of five health male subjects to be 0.39%/s, which is slightly higher than our average resting mV̇o2 of 0.28%/s.
In this study, we provide evidence that blood volume-corrected NIRS measurements for the recovery rate of mV̇o2 after electrical stimulation exhibit good reliability. We reported a mean CV of 10% (range 1–22%) between day 1 and day 2. Our results are comparable to coefficients of variation reported for phosphocreatine recovery kinetics (27, 33, 40). The time constant for recovery of mV̇o2 measured in the gastrocnemius muscle also agrees with some reported values for phosphocreatine recovery (16, 21), but is faster than other reports (15, 32, 33, 37). One possible explanation for this difference is the individuals included in the reproducibility portion of this study were aerobically conditioned. Endurance-trained individuals have faster phosphocreatine recovery rates compared with their sedentary or less aerobically fit counterparts (26, 34).
There are a number of potentially confounding factors for the quantification of continuous-wave NIRS signals including unknown optical pathlength, absorption, and scattering coefficients, as well as the influence of ATT. The development of frequency- and time-domain NIRS devices has enabled the continuous measurement of the pathlength and absorption/scattering coefficients (3, 10–12, 14, 41). However, the influence of ATT on NIRS signals still exists even with more advanced NIRS devices (24). As in the case of this study, and others using continuous-wave NIRS, ATT will influence the NIRS signal as the estimated depth of penetration is one-half the interoptode distance. The differential pathlength factor (DPF), which corrects for the scattering of photons in tissue, has been applied to continuous-wave NIRS for calculation of absolute concentration changes (6, 7). With the large range of ATT of our participants (3.3–12.0 mm), we found a significant relationship between mV̇o2 calculated using the DPF method and ATT, consistent with previous studies (39). This suggests that the DPF may be different between individuals and/or locations of measurement within an individual, thereby supporting the need to measure DPF. However, previous studies have shown that ATT influences muscle deoxygenation kinetics even when accounting for differences in pathlength (24). With the application of an ischemic calibration, the mV̇o2 expressed as a percentage was no longer influenced by ATT (Fig. 9B). The ischemic calibration scales NIRS signals according to the maximal “physiological” range. Thus our results support the use of a physiological ischemic calibration for the calculation of muscle oxygen saturation and consumption when comparing between individuals.
This study used two interoptode separation distances. This enabled us to compare measurements of muscle oxygen consumption between interoptode distances, to account for the potential effect of penetration depth on the metabolic measurements. The depth of muscle activation with electrical stimulation was unknown in this study, which could mean that some inactive tissue is contributing to NIRS signals. We did not find a significant influence of optode distance (sample depth) on muscle metabolic measurements, suggesting that the measurements of mitochondrial function can be compared between people with different ATT thicknesses and intramuscular fat percentages, as long as an ischemic calibration is performed.
In this study, the calculation of mV̇o2 in absolute units was influenced by adipose tissue and skin overlying the muscle. Therefore, we chose to use an ischemic calibration to report our measurements of mV̇o2 as a percentage change per unit time. This was a limitation in our study. To convert these relative units to absolute units of oxygen consumption (mM O2/time) assumptions would need to be made regarding muscle hematocrit, myoglobin concentration, and the contribution of myoglobin/hemoglobin to the NIRS signals. Some researchers have suggested hemoglobin to be the main contributor to the NIRS signal (8). In contrast, Marcinek et al. (29) reported that myoglobin contributes ∼80% to the NIRS signal using wavelength shift analysis. Future studies are needed to test these assumptions and calibrations to convert data from percent calibration to units of oxygen consumption (in mM O2).
In conclusion, we measured mitochondrial capacity using NIRS and assessed the reliability and reproducibility of resting muscle oxygen consumption and the recovery rate of muscle oxygen consumption after exercise. A blood volume correction has been developed and applied to arterial occlusion measurements of oxygen consumption. Without correcting for blood volume changes, the metabolic exchange between O2Hb and HHb may be masked by blood changes under the NIRS probe. We demonstrate three methods for correcting blood volume changes. This study was performed using a continuous-wave NIRS system. The need to account for heme signals with different oxygen saturations moving into the NIRS-detectable tissue area should apply to time and frequency domain NIRS systems; however, future studies will be needed to test the method with those types of devices. In addition, more studies need to be conducted to explore differences in blood volume changes between individuals, and to accurately quantify NIRS measurements of oxygen consumption. Furthermore, comparisons also need to be made to established methods such as magnetic resonance spectroscopy.
GRANTS
This study was funded in part by National Institutes of Health Grant HD-039676.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.E.R., M.L.E., and K.K.M. conception and design of research; T.E.R., M.L.E., J.T.B., and H.-J.Y. performed experiments; T.E.R. analyzed data; T.E.R., M.L.E., J.T.B., H.-J.Y., and K.K.M. interpreted results of experiments; T.E.R. prepared figures; T.E.R. drafted manuscript; T.E.R., M.L.E., J.T.B., H.-J.Y., and K.K.M. edited and revised manuscript; T.E.R., M.L.E., J.T.B., H.-J.Y., and K.K.M. approved final version of manuscript.
REFERENCES
- 1. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med 26: 217–238, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Binzoni T, Cooper CE, Wittekind AL, Beneke R, Elwell CE, Van De Ville D, Leung TS. A new method to measure local oxygen consumption in human skeletal muscle during dynamic exercise using near-infrared spectroscopy. Physiol Measurement 31: 1257–1269, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Boone J, Koppo K, Barstow TJ, Bouckaert J. Pattern of deoxy[Hb+Mb] during ramp cycle exercise: influence of aerobic fitness status. Eur J Appl Physiol 105: 851–859, 2009 [DOI] [PubMed] [Google Scholar]
- 4. De Blasi RA, Almenrader N, Aurisicchio P, Ferrari M. Comparison of two methods of measuring forearm oxygen consumption (mV̇o2) by near infrared spectroscopy. J Biomed Optics 2: 171–175, 1997 [DOI] [PubMed] [Google Scholar]
- 5. DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95: 113–120, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Duncan A, Meek JH, Clemence M, Elwell CE, Fallon P, Tyszczuk L, Cope M, Delpy DT. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res 39: 889–894, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol 40: 295–304, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Ferrari M, Binzoni T, Quaresima V. Oxidative metabolism in muscle. Philos Trans R Soc Lond B Biol Sci 352: 677–683, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrari M, Mathalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Transact A Math Phys Eng Sci 369: 4577–4590, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29: 463–487, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Ferreira LF, Hueber DM, Barstow TJ. Effects of assuming constant optical scattering on measurements of muscle oxygenation by near-infrared spectroscopy during exercise. J Appl Physiol 102: 358–367, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Ferreira LF, Koga S, Barstow TJ. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J Appl Physiol 103: 1999–2004, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ. Effects of pedal frequency on estimated muscle microvascular O2 extraction. Eur J Appl Physiol 96: 558–563, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Ferreira LF, Townsend DK, Lutjemeier BJ, Barstow TJ. Muscle capillary blood flow kinetics estimated from pulmonary O2 uptake and near-infrared spectroscopy. J Appl Physiol 98: 1820–1828, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Forbes SC, Paganini AT, Slade JM, Towse TF, Meyer RA. Phosphocreatine recovery kinetics following low- and high-intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am J Physiol Regul Integr Comp Physiol 296: R161–R170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forbes SC, Slade JM, Francis RM, Meyer RA. Comparison of oxidative capacity among leg muscles in humans using gated 31P 2-D chemical shift imaging. NMR Biomed 22: 1063–1071, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 95: 149–158, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Hamaoka T, Iwane H, Shimomitsu T, Katsumura T, Murase N, Nishio S, Osada T, Kurosawa Y, Chance B. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol 81: 1410–1417, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Hamaoka T, McCully KK, Niwayama M, Chance B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: recent developments. Philos Transact A Math Phys Eng Sci 369: 4591–4604, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Optics 12: 062105–062105, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol 86: 2013–2018, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Hopkins WG. Reliability from consecutive pairs of trials. In: A Nice View of Statistics of Statics. http://sportsci.org/resource/stats/xrely.xls. [Jan 10, 2011, 2011]
- 23. Kent-Braun JA, Miller RG, Weiner MW. Human skeletal muscle metabolism in health and disease: utility of magnetic resonance spectroscopy. Exerc Sport Sci Rev 23: 305–348, 1995 [PubMed] [Google Scholar]
- 24. Koga S, Poole DC, Fukuoka Y, Ferreira LF, Kondo N, Ohmae E, Barstow TJ. Methodological validation of the dynamic heterogeneity of muscle deoxygenation within the quadriceps during cycle exercise. Am J Physiol Regul Integr Comp Physiol 301: R534–R541, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Kowalchuk JM, Rossiter HB, Ward SA, Whipp BJ. The effect of resistive breathing on leg muscle oxygenation using near-infrared spectroscopy during exercise in men. Exp Physiol 87: 601–611, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. In vivo oxidative capacity varies with muscle and training status in young adults. J Appl Physiol 107: 873–879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Layec G, Bringard A, Le Fur Y, Vilmen C, Micallef JP, Perrey S, Cozzone PJ, Bendahan D. Reproducibility assessment of metabolic variables characterizing muscle energetics in vivo: a 31P-MRS study. Magn Reson Med 62: 840–854, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Malagoni AM, Felisatti M, Mandini S, Mascoli F, Manfredini R, Basaglia N, Zamboni P, Manfredini F. Resting muscle oxygen consumption by near-infrared spectroscopy in peripheral arterial disease: a parameter to be considered in a clinical setting? Angiology 61: 530–536, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Marcinek DJ, Amara CE, Matz K, Conley KE, Schenkman KA. Wavelength shift analysis: a simple method to determine the contribution of hemoglobin and myoglobin to in vivo optical spectra. Appl Spectrosc 61: 665–669, 2007 [DOI] [PubMed] [Google Scholar]
- 30. McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol 75: 813–819, 1993 [DOI] [PubMed] [Google Scholar]
- 31. McCully KK, Hamaoka T. Near-infrared spectroscopy: what can it tell us about oxygen saturation in skeletal muscle? Exerc Sport Sci Rev 28: 123–127, 2000 [PubMed] [Google Scholar]
- 32. McCully KK, Iotti S, Kendrick K, Wang Z, Posner JD, Leigh J, Jr, Chance B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol 77: 5–10, 1994 [DOI] [PubMed] [Google Scholar]
- 33. McCully KK, Turner TN, Langley J, Zhao Q. The reproducibility of measurements of intramuscular magnesium concentrations and muscle oxidative capacity using 31P MRS. Dynamic Med 8: 5–5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mccully KK, Vandenborne K, Demeirleir K, Posner JD, Leigh JS. Muscle metabolism in track athletes, using 31P magnetic resonance spectroscopy. Can J Physiol Pharm 70: 1353–1359, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol 254: C548–C553, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Motobe M, Murase N, Osada T, Homma T, Ueda C, Nagasawa T, Kitahara A, Ichimura S, Kurosawa Y, Katsumura T, Hoshika A, Hamaoka T. Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn Med 3: 2, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Slade JM, Towse TF, Delano MC, Wiseman RW, Meyer RA. A gated 31P NMR method for the estimation of phosphocreatine recovery time and contractile ATP cost in human muscle. NMR Biomed 19: 573–580, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG. Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J Appl Physiol 90: 511–519, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Van Beekvelt MCP, Borghuis MS, Engelen BGM, Wevers RA, Colier WN. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin Sci 101: 21–28, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Walter G, Vandenborne K, McCully KK, Leigh JS. Noninvasive measurement of phosphocreatine recovery kinetics in single human muscles. Am J Physiol Cell Physiol 272: C525–C534, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Wolf M, Ferrari M, Quaresima V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J Biomed Optics 12: 062104, 2007 [DOI] [PubMed] [Google Scholar]