Abstract
Purpose
Gelatinous drop-like corneal dystrophy (GDLD), also known as familial subepithelial corneal amyloidosis, is an autosomal recessive disorder that causes progressive corneal opacity due to accumulation of amyloid fibrils in the corneal stroma. Genetic analyses have revealed that a mutation in membrane component chromosome 1 surface marker 1 gene is responsible for GDLD. However, the mechanism of amyloid formation in the corneal stroma remains unclear. The present study attempted to reveal the role of advanced glycation end products (AGE) and d-amino acids in amyloid formation in GDLD.
Methods
Informed consent was obtained from five patients with GDLD, three patients with bullous keratopathy and three patients with interstitial keratitis and all the specimens were analysed. Localisation of amyloid fibrils was analysed using Congo-red and thioflavin T staining. In addition, the localisation of AGE (Nɛ-carboxy(methyl)-l-lysine, pyrraline and pentosidine) and d-β-aspartic acid-containing proteins, a major form of d-amino acid-containing proteins, was analysed immunohistochemically.
Results
In all GDLD specimens, strong immunoreactivity to AGE and d-β-aspartic acid-containing proteins was detected in the subepithelial amyloid-rich region. In contrast, amyloid fibrils, AGE, or d-amino acid-containing proteins were slightly detected in the corneal stroma of patients with bullous keratopathy and interstitial keratitis.
Conclusions
Abnormally accumulated proteins rich in AGE and d-β-aspartic acid co-localise in the amyloid lesions in GDLD. These results indicate that non-enzymatic post-translational modifications of proteins, including AGE formation and isomerisation of aspartyl residues, will be the cause as well as the result of amyloid fibril formations in GDLD.
Keywords: Advanced glycation end products, biochemistry, cornead-amino acids, d-β-aspartic acid, familial subepithelial corneal amyloidosis, GDLD, gelatinous drop-like corneal dystrophy, M1S1, Nɛ-(carboxy)methyl-l-lysin, optics and refraction, pathology, pentosidine, physiology, pyrraline, treatment surgery, tumour-associated calcium signal transducer 2 (TACSTD2)
Gelatinous drop-like corneal dystrophy (GDLD), also known as familial subepithelial corneal amyloidosis, is an autosomal recessive disorder first reported by Nakaizumi et al1 in 1914. From the histological viewpoint, dense accumulation of amyloid fibrils over the entire cornea leads to significant visual disturbances.2 3 Immunohistochemical and proteomic analyses have revealed that abnormal accumulation of lactoferrin and transforming growth factor, beta induced (TGFBI) is the cause of amyloid fibril formation.2 4 Recent advances in genetic analysis have revealed that mutations in the gene responsible for the membrane component chromosome 1 surface marker 1 (M1S1), also known as tumour-associated calcium signal transducer 2, are the underlying cause of GDLD.5 M1S1 was first reported as a tumour-associated antigen highly expressed in human trophoblast cells and epithelial carcinomas.6 7 Nakatsukasa et al8 recently reported that M1S1, in conjunction with occludin-7, serves as a corneal epithelium barrier. These results indicate that the loss of barrier function is a primary cause of GDLD. However, the mechanism underlying amyloid fibril formation and abnormal accumulation of lactoferrin and TGFBI in the cornea in GDLD remains unclear.
The molecular mechanisms underlying amyloid fibril formation are the focus of ‘folding diseases’, including Alzheimer's disease, Creutzfeldt–Jakob disease and amyloidosis.9 In these folding diseases, the misfolding of proteins is important for the development of amyloid fibril formation and abnormal accumulation of proteins. Recent studies have shown that non-enzymatic post-translational modifications of proteins are involved in the misfolding of proteins and the formation of amyloid fibrils.10 11 For example, the formation of advanced glycation end products (AGE)12 and the racemisation of amino acids and resultant d-amino acid-containing proteins are involved in the development of Alzheimer's disease.13 14 In this study, we focused on the development of AGE and d-amino acid-containing proteins as a potential cause of amyloid fibril formation in GDLD.
AGE are the final reaction product of reducing sugars and proteins.15 16 Reducing sugars, such as glucose and fructose, bind to proteins through Schiff base formation, followed by Amadori rearrangement, and turn into AGE after oxidation, dehydration and condensation. The formation of AGE occurs in the body and is involved in the development of diabetic complications and age-related disorders.15 16 Numerous products are generated from reducing sugars and proteins in the body. However, irrespective of the origin of the reducing sugars and proteins, the common molecular structures observed at the modification sites of AGE are Nɛ-(carboxy)methyl-l-lysine (CML), pentosidine, imidazolone and pyrraline.15 16 AGE tend to accumulate in the body because they generally show resistance to proteases. For the aforementioned reasons, AGE are detected in folding diseases, including Alzheimer's disease, atherosclerosis, age-related macular degeneration, pinguecula and climatic drop-like keratopathy.
One of the molecular mechanisms underlying amyloid fibril formation is the racemisation of amino acids in proteins and the resultant d-amino acid-containing proteins.13 14 Although proteins of all living organisms are composed exclusively of l-amino acids, biologically uncommon d-amino acids, that is, enantiomers of l-amino acids, have been recognised as the molecular basis for diseases related to ultraviolet irradiation and the ageing process.17 18 d-β-Aspartic acids were found in the lenses,17 19–22 teeth,17 23 24 bones,25 brains,26 skin,17 20 27 aortas,17 28 erythrocytes,29 lungs20 30 31 and ligaments of elderly donors.24 The presence of d-β-aspartic acid, a major form of d-amino acids, in aged tissues of the living body is considered to be a result of the racemisation of l-aspartic acid in proteins in metabolically inert tissues during one's lifetime. In addition, d-β-aspartyl residues in various proteins are considered not only an index of ultraviolet irradiation but also a useful marker of the ageing process. Furthermore, d-β-aspartic acid-containing proteins are involved in the abnormal accumulation of proteins in pinguecula,32 33 age-related macular degeneration21 33 and climatic drop-like keratopathy.33 34
Therefore, the formation of AGE and the racemisation of amino acids and the resultant d-amino acid-containing proteins are one of the molecular mechanisms underlying amyloid fibril formation. The present study was undertaken to reveal the contribution of d-amino acids and AGE to the pathogenesis of GDLD. To this end, the immunohistochemical localisation of d-β-Asp-containing proteins and AGE was investigated in surgical specimens obtained from patients with GDLD.
Materials and methods
Surgical cornea specimens with or without gelatinous drop-like dystrophy
Informed consent was obtained from all patients undergoing corneal surgery. Five corneas with GDLD from three patients (three men and two women, 59.2±14.3 years old) were obtained during penetrating keratoplasty. In addition, three corneas with bullous keratopathy from three patients (two men and one woman, 59.2±9.2 years old) and three corneas with interstitial keratitis (two men and one woman, 59.7±9.0 years old) were analysed. Haematoxylin-eosin staining and immunohistochemistry, described below, were performed on all surgical specimens.
Detection of amyloid fibrils and abnormal accumulation of proteins in corneas
Amyloid fibrils in corneal tissues were detected by Congo-red staining. Thioflavin T staining was used to detect abnormal accumulation of proteins in corneas. Thioflavin T is positive for amyloid as well as non-amyloid forms of proteins. After deparaffinisation and rehydration, the corneal specimens were stained with Meyer's haematoxylin solution for 10 min. After washing in running water, the specimens were treated with 0.02% thioflavin-T for 10 min. After washing again in running water for 10 min, the specimens were observed under a fluorescent microscope.
Antibodies against AGE
Monoclonal antibodies against AGE (CML, pentosidine and pyrraline) were purchased from Transgenic Co. Ltd. (Kumamoto, Japan).
Antibody against d-β-aspartic acid-containing peptides
The preparation and characterisation of the polyclonal antibody against d-β-aspartic acid-containing peptide have been described previously.35 The polyclonal antibody against the peptide, namely, Gly-Leu-d-β-Asp-Ala-Thr-Gly-Leu-d-β-Asp-Ala-Thr-Gly-Leu-d-β-Asp-Ala-Thr (anti-peptide 3R antibody), which corresponds to three repeats of positions 149–153 of human α-A-crystallin, was prepared and purified as previously described.35 This antibody clearly distinguishes the configuration of the aspartic residue; it reacts strongly with d-β-aspartic acid-containing peptides but does not react with l-α-aspartic acid-, l-β-aspartic acid-, or d-α-aspartic acid-containing peptides.35 36
Immunohistochemistry
Immunohistochemical localisation of AGE and d-β-aspartic acid-containing proteins was investigated according to previous reports.21 32 34 35 In brief, the surgical specimens were fixed with 4% paraformaldehyde (Sigma, St Louis, Missouri, USA) in 0.1 M phosphate buffer (pH 7.4) for 24 h. After embedding in paraffin, 4-μm thick sections of the samples were prepared. Following deparaffinisation, the sections were treated with monoclonal antibodies against AGE at a dilution of 1:250 or a polyclonal antibody against d-β-aspartic acid-containing peptides at a dilution of 1:500 in phosphate-buffered saline (PBS) containing 1% normal bovine serum albumin and kept at 4°C overnight. After washing the sections with PBS, the samples were treated with the reaction solution containing a secondary antibody labelled with horseradish peroxidase (Histofine Max-PO kit; Nichirei Co Ltd., Tokyo, Japan) and were kept for 30 min at room temperature. The sections were then incubated with diaminobenzidine (Sigma) in PBS. Finally, the sections were counterstained with haematoxylin. As a negative control, the primary antibody was replaced with normal rabbit serum IgG (1.0 μg/ml) diluted in PBS containing 1% bovine serum albumin (Sigma). The results of the immunohistochemistry were analysed and graded in a double-blind manner.
Results
Immunohistochemical localisation of AGE and d-β-aspartic acid-containing proteins in GDLD
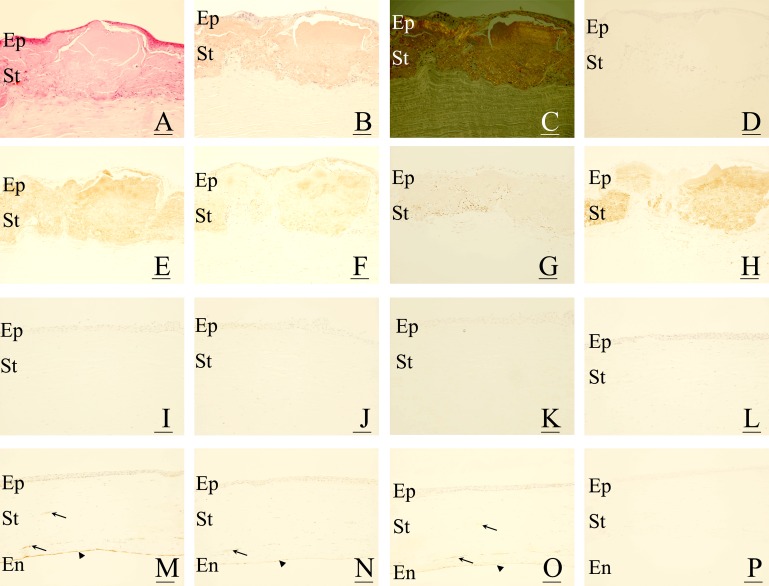
In corneal specimens with GDLD, abnormal accumulation of proteins was seen as eosinophilic lesions in the superficial layer of the corneal stroma (figure 1A). The lesion was stained with Congo-red (figure 1B), showing birefringence under a fluorescent microscope (figure 1C). No immunoreactivity was seen in corneas with GDLD in negative controls (figure 1D). In contrast, strong immunoreactivity to CML (figure 1E), moderate immunoreactivity to pyrraline (figure 1F) and weak immunoreactivity to pentosidine (figure 1G) were seen in the superficial layers of the corneal stroma. The localisation of AGE was consistent with amyloid fibrils. In addition, the intensity and the localisation of the immunoreactivity to AGE were almost identical in all GDLD specimens. Corneal endothelial cells were also positive for CML, pyrraline and pentosidine (data not shown). In all corneal specimens with GDLD, strong immunoreactivity to d-β-aspartic acid-containing peptides was detected in the superficial layers of the corneal stroma (figure 1H). The localisation of d-β-aspartic acid-containing proteins was consistent with amyloid fibrils.
Figure 1.
Immunohistochemical localisation of advanced glycation end products (AGE) and d-β-aspartic acid-containing proteins in gelatinous drop-like corneal dystrophy (GDLD). In corneas with GDLD (A–H), eosinophilic and abnormal accumulation of proteins is seen in the superficial layers (A). The accumulated proteins contain amyloid fibrils, which are positive for Congo-red staining (1B) with birefringence (C). The accumulated proteins are positive for AGE, including Nɛ-(carboxy)methyl-l-lysine (CML) (E), pyrraline (F) and pentosidine (G), and d-β-aspartic acid-containing proteins (H). No staining was observed in negative controls (D). In corneas with bullous keratopathy (I–L), no immunoreaction against AGE, including CML (I), pyrraline (J) and pentosidine (K), and d-β-aspartic acid-containing proteins (L), was seen in the corneal stroma. In corneas with interstitial keratitis (M–P), AGE, including CML (M), pyrraline (N) and pentosidine (O), were positive in blood vessels (arrows in M–O) as well as in the corneal endothelial cells (arrowheads in M–O). In contrast, no immunoreactivity against d-β-aspartic acid-containing proteins was seen in corneas with interstitial keratitis (P). For all figures, bar = 50 μm. En, corneal endothelium; Ep, corneal epithelium; St, corneal stroma.
Immunohistochemical localisation of AGE and d-β-aspartic acid-containing proteins in bullous keratopathy
In corneas with bullous keratopathy, no immunoreactivity was seen in CML (figure 1I), pyrraline (figure 1J), pentosidine (figure 1K), or d-β-aspartic acid-containing peptides (figure 1L).
Immunohistochemical localisation of AGE and d-β-aspartic acid-containing proteins in interstitial keratitis
In corneas with interstitial keratitis, strong immunoreactivity to CML (figure 1M) and moderate immunoreactivity to pyrraline (figure 1N) and pentosidine (figure 1O) was seen in the vascular endothelial cells (arrows). Note that corneal endothelial cells are also positive for CML, pyrraline and pentosidine (arrowheads). In contrast, no immunoreactivity to d-β-aspartic acid-containing proteins was seen in the cornea with interstitial keratitis (figure 1P).
The results of immunoreactivity to AGE and d-β-aspartic acid-containing proteins are summarised in table 1.
Table 1.
Summary of immunohistochemistry results
| AGE | d-β-Asp | |||
| CML | Pyrraline | Pentosidine | ||
| GDLD | ++ | + | ± | ++ |
| Bullous keratopathy | – | – | – | – |
| Interstitial keratitis | –* | –* | –* | – |
Advanced glycation end products (AGE) and d-β-aspartic acid-containing proteins (d-β-Asp) were positive in the corneal stroma of gelatinous drop-like corneal dystrophy (GDLD) specimens and were negative in the corneal stroma of specimens with bullous keratopathy and interstitial keratitis.
Positive only in the blood vessels.
CML, Nɛ-(carboxy)methyl-l-lysine.
Discussion
The present study focused on the formation of AGE and d-β-aspartic acid-containing proteins in the development of GDLD. The results indicate that the amyloid fibrils in GDLD contain AGE and d-amino acid-containing proteins. This implies that non-enzymatic post-translational modification of proteins, including the formation of AGE and d-β-aspartic acid, plays an important role in the development of GDLD.
The pathogenesis of GDLD includes the accumulation of amyloid fibrils in the superficial layers of the corneal stroma. To reveal the molecular mechanisms behind GDLD, we utilised an approach similar to that used for analysing other amyloid-forming diseases, including Alzheimer's disease and amyloidosis. In this context, the first approach is to detect the genes responsible for the disease. Tsujikawa et al5 reported that the mutations in the M1S1 gene on the second chromosome are responsible for GDLD; over 20 M1S1 mutations have been reported to cause GDLD. However, determination of the responsible genes is not sufficient to reveal the mechanisms underlying GDLD, because the proteins produced by the mutated genes are not always identical to the components of amyloid fibrils. In fact, M1S1 is not responsible for the formation of components of amyloid fibrils in GDLD.8
The second approach is to analyse the proteins constituting the amyloid fibrils using immunohistochemistry, immunoprecipitation and proteomics. The major component of amyloid fibrils in GDLD is lactoferrin with accessory components, including TGFBI, apolipoprotein J and amyloid AA.2 37 These results have led to the question of how mutations in the M1S1 gene result in the accumulation of lactoferrin in the corneal stroma. Kawasaki and Kinoshita2 propose that the mutated product of M1S1 aggravates the barrier function of the corneal epithelium because M1S1 is an important part of the barrier function, in combination with clusterin 1 and clusterin 7. However, it remains unclear how a hydrophilic protein such as lactoferrin turns into insoluble hydrophilic amyloid fibrils in GDLD.
The difficulties in analysing the molecular mechanism underlying GDLD are common with other amyloid-forming disorders, including Alzheimer's disease and amyloidosis. In most amyloid-forming diseases, genetic and proteomic analyses have revealed only a part of the molecular mechanisms of the disease. To solve these problems, non-enzymatic post-translational modifications of proteins, including AGE and d-amino acid-containing proteins, are often analysed. For example, formation of AGE and d-β-aspartic acids is reported in the senile plaques in Alzheimer's disease. In addition, racemisation of aspartic residue in Aβ1-40 results in the accelerated development of amyloid fibrils.13 14 These results indicate that post-translational modifications of proteins, including d-amino acid formation, are both the result and the cause of amyloid fibril development. In fact, we have found that structural changes of proteins greatly accelerate the amyloid fibril formation in corneal dystrophies.38 Taken together, the formation of AGE and d-β-aspartic acids would be causes of amyloid fibril formation in GDLD.
AGE formation and racemisation of amino acids in GDLD may be a new therapeutic target for the prevention and treatment of GDLD. For example, inhibitors of AGE formation, including aminoguanidine and pyridoxamine, are effective for the treatment of diabetic complications in experimental models.39 40 This suggests that these drugs are effective against AGE formation of lactoferrin in GDLD, thus inhibiting the formation of amyloid fibrils. In addition, alagebrium chloride (ALT-711) breaks down AGE in vivo and is effective for the treatment of arterial stiffness.41 Kinouchi et al42 have reported that d-aspartyl endopeptidase, especially when expressed in the liver, digests some of the d-amino acid-containing proteins. No data are available on whether ALT-711 and d-aspartyl endopeptidase degrade d-amino acid-containing lactoferrin in GDLD; however, the breakdown of AGE and d-amino acids containing lactoferrin may have a therapeutic effect.
The present study has shown that significant immunoreactivity to AGE and d-β-aspartic acid-containing proteins is detected in the superficial layers of the cornea in GDLD, where amyloid fibrils accumulate. However, the present study has not determined what types of proteins contain AGE and d-amino acids in GDLD. AGE and d-β-aspartic acid are not generated uniformly in proteins. For example, AGE formation is detected at 18 glycation sites and is detected in albumin derived from patients with diabetes.43 In cataracts, significant increases in d-β-aspartic acid formation are detected in Asp-58 and Asp-151, αA crystallin and Asp-36, and Asp-62 in αB crystallin.17 19 20 35 Therefore, further studies are required to detect the target of AGE and d-β-aspartic acid-containing proteins using proteomic and high-performance liquid chromatography analysis. Furthermore, these results will be helpful in the development of a GDLD model using synthetic peptides that have previously been reported in corneal dystrophies.
Footnotes
Contributors: YK organised the whole study. YT and MF analysed the pathological condition of the corneas. TO collected the corneas and performed keratoplasty. NF prepared the antibody to d-amino acids and organised the whole study.
Funding: This work is supported by the Ministry of Education, Science, Sports and Culture, grant for scientific research 21592216 (2009–2011) and 24592618 (2012–2015), Japan.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Ethics approval was provided by the University of Tokyo.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Nakaizumi G. A rare case of corneal dystrophy. Acta Soc Ophthal Jpn 1914;18:949–59 [Google Scholar]
- 2.Kawasaki S, Kinoshita S. Clinical and basic aspects of gelatinous drop-like corneal dystrophy. Dev Ophthalmol 2011;48:97–115 [DOI] [PubMed] [Google Scholar]
- 3.Weber FL, Babel J. Gelatinous drop-like dystrophy. A form of primary corneal amyloidosis. Arch Ophthalmol 1980;98:144–8 [DOI] [PubMed] [Google Scholar]
- 4.Akhtar S, Bron AJ, Qin X, et al. Gelatinous drop-like corneal dystrophy in a child with developmental delay: clinicopathological features and exclusion of the M1S1 gene. Eye (Lond) 2005;19:198–204 [DOI] [PubMed] [Google Scholar]
- 5.Tsujikawa M, Kurahashi H, Tanaka T, et al. Identification of the gene responsible for gelatinous drop-like corneal dystrophy. Nat Genet 1999;21:420–3 [DOI] [PubMed] [Google Scholar]
- 6.Linnenbach AJ, Wojcierowski J, Wu SA, et al. Sequence investigation of the major gastrointestinal tumor-associated antigen gene family, GA733. Proc Natl Acad Sci U S A 1989;86:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miotti S, Canevari S, Menard S, et al. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer 1987;39:297–303 [DOI] [PubMed] [Google Scholar]
- 8.Nakatsukasa M, Kawasaki S, Yamasaki K, et al. Tumor-associated calcium signal transducer 2 is required for the proper subcellular localization of claudin 1 and 7: implications in the pathogenesis of gelatinous drop-like corneal dystrophy. Am J Pathol 2010;177:1344–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makin OS, Serpell LC. Structures for amyloid fibrils. FEBS J 2005;272:5950–61 [DOI] [PubMed] [Google Scholar]
- 10.Benjannet S, Elagoz A, Wickham L, et al. Post-translational processing of β-secretase (β-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-β production. J Biol Chem 2001;276:10879–87 [DOI] [PubMed] [Google Scholar]
- 11.Broncel M, Falenski JA, Wagner SC, et al. How post-translational modifications influence amyloid formation: a systematic study of phosphorylation and glycosylation in model peptides. Chemistry 2010;16:7881–8 [DOI] [PubMed] [Google Scholar]
- 12.Vitek MP, Bhattacharya K, Glendening JM, et al. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci U S A 1994;91:4766–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubo T, Kumagae Y, Miller CA, et al. β-Amyloid racemized at the Ser26 residue in the brains of patients with Alzheimer disease: implications in the pathogenesis of Alzheimer disease. J Neuropathol Exp Neurol 2003;62:248–59 [DOI] [PubMed] [Google Scholar]
- 14.Tomiyama T, Asano S, Furiya Y, et al. Racemization of Asp23 residue affects the aggregation properties of Alzheimer amyloid β protein analogues. J Biol Chem 1994;269:10205–8 [PubMed] [Google Scholar]
- 15.Glenn JV, Stitt AW. The role of advanced glycation end products in retinal ageing and disease. Biochim Biophys Acta 2009;1790:1109–16 [DOI] [PubMed] [Google Scholar]
- 16.Stitt AW. The maillard reaction in eye diseases. Ann NY Acad Sci 2005;1043:582–97 [DOI] [PubMed] [Google Scholar]
- 17.Fujii N. D-Amino acid in elderly tissues. Biol Pharm Bull 2005;28:1585–9 [DOI] [PubMed] [Google Scholar]
- 18.Fujii N, Saito T. Homochirality and life. Chem Rec 2004;4:267–78 [DOI] [PubMed] [Google Scholar]
- 19.Fujii N, Harada K, Momose Y, et al. d-Amino acid formation induced by a chiral field within a human lens protein during aging. Biochem Biophys Res Commun 1999;263:322–6 [DOI] [PubMed] [Google Scholar]
- 20.Fujii N, Kaji Y, Nakamura T, et al. Collapse of homochirality of amino acids in proteins from various tissues during aging. Chem Biodivers 2010;7:1389–97 [DOI] [PubMed] [Google Scholar]
- 21.Kaji Y, Oshika T, Takazawa Y, et al. Localization of d-β-aspartic acid-containing proteins in human eyes. Invest Ophthalmol Vis Sci 2007;48:3923–7 [DOI] [PubMed] [Google Scholar]
- 22.Masters PM, Bada JL, Zigler JS., Jr Aspartic acid racemization in heavy molecular weight crystallins and water insoluble protein from normal human lenses and cataracts. Proc Natl Acad Sci U S A 1978;75:1204–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masters PM. Stereochemically altered noncollagenous protein from human dentin. Calcif Tissue Int 1983;35:43–7 [DOI] [PubMed] [Google Scholar]
- 24.Ritz-Timme S, Laumeier I, Collins M. Age estimation based on aspartic acid racemization in elastin from the yellow ligaments. Int J Leg Med 2003;117:96–101 [DOI] [PubMed] [Google Scholar]
- 25.Ohtani S. Estimation of age from dentin by utilizing the racemization of aspartic acid: influence of pH. Forensic Sci Int 1995;75:181–7 [DOI] [PubMed] [Google Scholar]
- 26.Shapira R, Chou CH. Differential racemization of aspartate and serine in human myelin basic protein. Biochem Biophys Res Commun 1987;146:1342–9 [DOI] [PubMed] [Google Scholar]
- 27.Mori Y, Aki K, Kuge K, et al. UV B-irradiation enhances the racemization and isomerization of aspartyl residues and production of Nɛ-carboxymethyl lysine (CML) in keratin of skin. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:3303–9 [DOI] [PubMed] [Google Scholar]
- 28.Powell JT, Vine N, Crossman M. On the accumulation of d-aspartate in elastin and other proteins of the ageing aorta. Atherosclerosis 1992;97:201–8 [DOI] [PubMed] [Google Scholar]
- 29.McFadden PN, Clarke S. Methylation at d-aspartyl residues in erythrocytes: possible step in the repair of aged membrane proteins. Proc Natl Acad Sci U S A 1982;79:2460–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motoie R, Fujii N, Tsunoda S, et al. Localization of d-β-aspartyl residue-containing proteins in various tissues. Int J Mol Sci 2009;10:1999–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro SD, Endicott SK, Province MA, et al. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of d-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 1991;87:1828–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaji Y, Oshika T, Okamoto F, et al. Immunohistochemical localisation of d-β-aspartic acid in pingueculae. Br J Ophthalmol 2009;93:974–6 [DOI] [PubMed] [Google Scholar]
- 33.Kaji Y, Oshika T, Takazawa Y, et al. Accumulation of d-β-aspartic acid-containing proteins in age-related ocular diseases. Chem Biodivers 2010;7:1364–70 [DOI] [PubMed] [Google Scholar]
- 34.Kaji Y, Oshika T, Takazawa Y, et al. Immunohistochemical localisation of d-β-aspartic acid-containing proteins in climatic droplet keratopathy. Br J Ophthalmol 2009;93:977–9 [DOI] [PubMed] [Google Scholar]
- 35.Fujii N, Shimo-Oka T, Ogiso M, et al. Localization of biologically uncommon d-β-aspartate-containing αA-crystallin in human eye lens. Mol Vis 2000;6:1–5 [PubMed] [Google Scholar]
- 36.Fujii N. d-Amino acids in living higher organisms. Orig Life Evol Biosph 2002;32:103–27 [DOI] [PubMed] [Google Scholar]
- 37.Akiya S, Furukawa H, Sakamoto H, et al. Histopathologic and immunohistochemical findings in gelatinous drop-like corneal dystrophy. Ophthalmic Res 1990;22:371–6 [DOI] [PubMed] [Google Scholar]
- 38.Ozawa D, Kaji Y, Yagi H, et al. Destruction of amyloid fibrils of keratoepithelin peptides by laser irradiation coupled with amyloid-specific thioflavin T. J Biol Chem 2010;286:10856–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YM, Steffes M, Donnelly T, et al. Prevention of cardiovascular and renal pathology of aging by the advanced glycation inhibitor aminoguanidine. Proc Natl Acad Sci U S A 1996;93:3902–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degenhardt TP, Alderson NL, Arrington DD, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int 2002;61:939–50 [DOI] [PubMed] [Google Scholar]
- 41.Little WC, Zile MR, Kitzman DW, et al. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail 2005;11:191–5 [DOI] [PubMed] [Google Scholar]
- 42.Kinouchi T, Nishio H, Nishiuchi Y, et al. Isolation and characterization of mammalian d-aspartyl endopeptidase. Amino Acids 2007;32:79–85 [DOI] [PubMed] [Google Scholar]
- 43.Frolov A, Hoffmann R. Identification and relative quantification of specific glycation sites in human serum albumin. Anal Bioanal Chem 2010;397:2349–56 [DOI] [PubMed] [Google Scholar]