In cross-sectional analysis of 287 human immunodeficiency virus–infected persons with high hepatitis C virus prevalence, liver fibrosis defined by elastography was associated with higher CD4+ T-cell percentages relative to absolute CD4+ number; this discordance was most apparent in persons with marked lymphopenia.
Abstract
Background. Cirrhosis of the liver can induce splenic sequestration of peripheral blood cells, recently suggested to reduce the number but not percentage of circulating CD4+ T cells in persons uninfected with human immunodeficiency virus (HIV). We investigated whether earlier stages of liver fibrosis prior to cirrhosis were associated with discordance between CD4 count (CD4N) and CD4 percentage (CD4%) in HIV-infected patients.
Methods. In cross-sectional analysis of 287 HIV-infected participants of the AIDS Linked to the Intravenous Experience cohort, we evaluated CD4N, CD4%, and transient elastography staging of liver fibrosis. High CD4+ lymphocyte discordance was defined as higher CD4% relative to CD4N based on accepted clinical cutoffs; multivariable logistic regression was used to determine covariates associated with discordance.
Results. Of 287 participants, 99 (34.4%) had high CD4+ discordance, which increased to 76% of 114 participants with marked lymphopenia (total lymphocyte count [TLC] ≤1200 cells/μL). In multivariable analysis, the odds of having high CD4+ discordance was increased in persons with significant liver fibrosis compared to those without fibrosis (odds ratio, 1.69; 95% confidence interval [CI], .95–2.96); the odds ratio of discordance increased to 2.66 (95% CI, 1.11–6.40) among the subset of participants with TLC ≤1200 cells/μL. The odds for discordance associated with cirrhosis were of similar magnitude as those observed with significant fibrosis.
Conclusions. In HIV-infected persons, liver fibrosis is associated with discordant peripheral CD4+ lymphocyte results, especially in the setting of marked lymphopenia. Clinicians should also consider CD4% when interpreting absolute CD4+ counts of HIV-infected persons with known or suspected liver disease, particularly if TLC is <1200 cells/μL.
INTRODUCTION
The number of circulating CD4+ T lymphocytes (CD4N) in patients with human immunodeficiency virus (HIV) has well-validated predictive value for assessing HIV disease stage, predicting progression to clinical AIDS and AIDS-related death, determining antiretroviral treatment eligibility, and monitoring response to therapy [1–4]. However, despite widespread reliance on CD4N, debate persists as to whether CD4N or CD4+ T-lymphocyte percentage (CD4%) is the most appropriate HIV disease marker [5–8]. Of particular concern, substantial discordance between CD4N and CD4% has recently been reported in patients with liver cirrhosis. Among 60 HIV-seronegative adults with biopsy-proven liver cirrhosis, McGovern et al [9] demonstrated that 65% had low CD4N, yet 95% had normal CD4%. The authors hypothesized that global splenic sequestration of blood cell lines, including lymphocytes, occurred secondary to portal hypertension.
If discordance between CD4N and CD4% also occurs in HIV-infected persons with liver disease, reliance of clinicians on the lower CD4N relative to the CD4% could result in variation in timing of initiation of highly active antiretroviral therapy (HAART) or interpretation of HAART responses. These concerns have heightened discussion regarding the appropriateness of updating HIV treatment guidelines for persons with cirrhosis [10, 11]. Further, it remains unknown what degree of liver disease may be associated with discordance. To help in resolving these issues, systematic evaluation of CD4N and CD4% concordance among HIV-infected persons with standardized assessment of underlying liver disease is needed. In the current study, we evaluated the prevalence and identified correlates of discordance between CD4N and CD4% among a predominantly black population of HIV-infected injection drug users (IDUs) with a high prevalence of hepatitis C virus (HCV) infection. Importantly, we used transient elastography to systematically ascertain the severity of liver fibrosis or cirrhosis among all study participants.
METHODS
Selection and Description of Participants
The AIDS Linked to the Intravenous Experience (ALIVE) cohort was designed to identify predictors of HIV acquisition and to study the natural history of HIV infection among IDUs, as described in detail previously [12]. In brief, HIV-infected and uninfected IDUs were enrolled through community-based recruitment in Baltimore, Maryland, in 1988–1989. Eligibility criteria included age of ≥18 years and history of intravenous drug use. Subsequently, participants were followed with collection of detailed behavioral and clinical data and laboratory testing performed at 6-month study visits. Additional participants were recruited in 1994–1995, 1998, and 2005–2008 using a similar recruitment strategy and eligibility criteria. For the current analysis, ALIVE participants were included if they were HIV-infected with available data for CD4N, CD4%, HAART status, and transient elastography from the same study visit (Table 1; N = 287).
Table 1.
Characteristics of Study Participants
| Characteristic | N = 287 |
|---|---|
| Demographic | |
| Age, years | 47.5 (42.4–51.9) |
| Female | 102 (35.5%) |
| Black | 270 (94.1%) |
| Behavioral | |
| Injection drug usea | 123 (42.9%) |
| Alcohol usea | |
| None | 138 (48.1%) |
| Weekly | 125 (43.6%) |
| Daily | 24 (8.4%) |
| HIV-related | |
| CD4N | 282 (147–463) |
| CD4% | 22 (14–30) |
| HIV RNA | 1020 (400–32 000) |
| HIV RNA >400 copies/mL | 156 (54.6%) |
| Recent HAART usea | 134 (46.7%) |
| Hematologic | |
| Platelet count | 219 (180–259) |
| TLC | 1316 (952–1850) |
| Liver-related | |
| AST (IU/mL) | 39 (29–59) |
| ALT (IU/mL) | 31 (21–47) |
| HCV Ab positive | 269 (93.7%) |
| HCV RNA (log10 IU/mL)b | 6.55 (6.01–7.08) |
| HBsAg positive | 14 (4.9%) |
| Liver stiffness (kPa) | 7.25 (5.40–10.6) |
All values are No. (%) for dichotomous variables or median (interquartile range) for continuous variables.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CD4N, CD4+ T-cell lymphocyte count; CD4%, CD4+ T-cell lymphocyte percentage; HAART, highly active antiretroviral therapy; HBsAg, hepatitis B virus surface antigen; HCV Ab, hepatitis C virus antibody; HIV, human immunodeficiency virus; IU, international units; kPa, kilopascal; TLC, total lymphocyte count.
a In the preceding 6 months.
b HCV RNA testing available for 136 (51%) participants.
Description of Data
Demographic and behavioral information was obtained from ALIVE study visits. The definition of self-reported recent HAART use has been explained in detail elsewhere [13]; in brief, HAART was defined as receipt of 1 of the following antiretroviral combinations in the preceding 6 months: (1) protease inhibitor (PI) + 2 nucleoside reverse transcriptase inhibitors (NRTIs), (2) nonnucleoside reverse transcriptase inhibitor (NNRTI) + 2 NRTIs, (3) abacavir in combination with at least 2 other NRTIs, or (4) PI + NNRTI + NRTI.
Laboratory Testing
Measurements of T-cell subsets were obtained using heparinized whole blood stained with monoclonal antibodies using a modified whole blood method [14, 15]. Measurements of CD4% and white blood cells were determined by standard flow cytometry. CD4N, the product of the total lymphocyte count (TLC) and the CD4%, was determined from the flow cytometry and automated complete blood cell count and differential results [4]. HIV antibody, HCV antibody, HCV RNA, hepatitis B virus (HBV) surface antigen, and liver enzyme testing (aspartate aminotransferase and alanine aminotransferase) was performed using standard assays as described elsewhere [4, 12, 16–18].
Liver Fibrosis Assessment
Transient elastography (FibroScan; Echosens, Paris, France) was routinely performed at ALIVE study visits to calculate liver stiffness measurement (LSM) reflective of the degree of liver fibrosis and expressed in kilopascals (kPa) [19]. LSM scores ≥9.3 kPa were considered indicative of the presence of significant liver fibrosis (equivalent to Metavir score ≥F2) and ≥12.3 kPa indicative of liver cirrhosis (equivalent to Metavir score F4) based on prior elastography validation studies performed in this population [20].
Statistical Analysis
To determine concordance or discordance between CD4N and CD4%, we used previously published categorizations consistently used in prior studies among HIV-infected populations (see x- and y-axes, Table 2) [3,6,21,22]. Concordance was defined as agreement of the CD4% relative to 5 categories of CD4N. Subsequently, high discordance was defined as when the CD4% was in a higher category relative to the expected CD4N, and conversely, low discordance was defined to occur when the CD4% category was lower than the expected CD4N. A percentile-based method of defining discordance was also explored whereby individuals were categorized into 10 groups of CD4N; an individual was then labeled as high discordant if the CD4% was above the 90th percentile of CD4% in that group, and classified as low discordant if the CD4% was below the 10th percentile of CD4% in that group. However, this method for determining discordance yielded qualitatively similar results (data not shown).
Table 2.
Concordance and Discordance Between CD4 Cell Number and Percentage Categories for HIV-Infected ALIVE Study Participants
| CD4N (cells/μL) | CD4% |
Total | ||||
|---|---|---|---|---|---|---|
| 0–7 | 8–14 | 15–21 | 21–28 | >28 | ||
| 0–50 | 17 | 0 | 0 | 0 | 0 | 17 |
| 51–200 | 11 | 39 | 22 | 8 | 2 | 82 |
| 201–350 | 1 | 11 | 29 | 30 | 14 | 85 |
| 351–500 | 0 | 0 | 8 | 12 | 23 | 43 |
| >500 | 0 | 0 | 4 | 19 | 37 | 60 |
| Total | 29 | 50 | 63 | 69 | 76 | 287 |
All values are No. of subjects. Bolded type represents concordant participants [(CD4N = CD4%) = 134 (47%)]. Nonbolded type indicates discordant participants; high discordance (CD4% > CD4N) = 99 (34%) and low discordance (CD4% < CD4N) = 54 (19%).
Abbreviations: ALIVE, AIDS Linked to the Intravenous Experience; CD4N, CD4+ T-cell lymphocyte count; CD4%, CD4+ T-cell lymphocyte percentage.
Student t test and χ2 tests were used to evaluate differences in demographic and clinical characteristics between participants with and without significant liver fibrosis (LSM ≥9.3 kPa). Logistic regression was used to determine significant associations between individual covariates and discordance between CD4N and CD4% in the univariate analysis (P < .10). Discordance was defined as either high (CD4% > CD4N) or low (CD4N > CD4%) and predictors of each type were analyzed independently. Analyses were conducted with LSM dichotomized at 9.3 kPa and 12.3 kPa, and as a categorical variable including both cutpoints. In order to account for the effect of HAART on HIV viral load, HAART and viral load were analyzed as separate variables and also as a combined variable. Subset analyses were conducted among patients with CD4N ≤/>350 cells/μL and TLC of ≤/>1200 cells/μL. The CD4N cutpoint represents the level recommended for initiating (rather than considering) HAART in the United States, whereas the TLC cutpoint was previously used in World Health Organization guidelines for HIV treatment in resource-limited countries [23].
Stepwise procedures were utilized to construct multivariable logistic regression models to predict the odds of discordance. Multivariable analyses began with all variables identified in the univariate analysis using a cutoff significance level of P < .10. Demographic factors (age, sex, and race) were forced into models as they are known confounders of CD4N and CD4% [24–27]. Pearson test was used to evaluate goodness-of-fit, and variance inflation factors were examined for evidence of collinearity. Analyses were conducted using Stata software, version 10.0 (StataCorp, College Station, TX).
RESULTS
Study Participants
The median age of participants was 47.5 years; 94.1% were black, and 64.5% were males (Table 1). Approximately 43% of participants reported injection drug use in the past 6 months, while a little more than half of participants reported recent alcohol consumption. Overall, 54.6% had detectable HIV RNA and 46.7% had received HAART within the preceding 6 months; the median CD4N was 282 cells/μL and median CD4% was 22%. Significant liver fibrosis, defined as LSM ≥9.3 kPa, was present in 90 (31.4%) participants, although few had elevated liver enzymes. No statistically significant differences in demographic or behavioral variables were noted between those with or without liver fibrosis (data not shown).
CD4N and CD4% Distribution
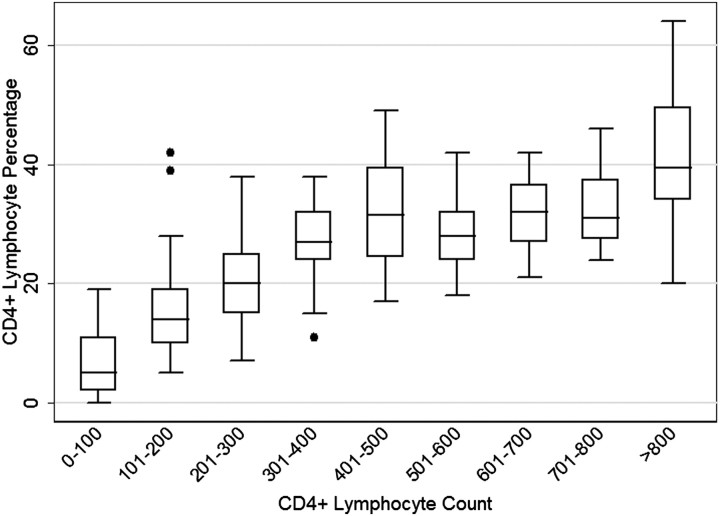
In examination of the distribution of CD4% across categories of CD4N (Figure 1), at CD4 cell counts ≤350 cells/μL, there was a positive correlation between CD4N and CD4% measurements (r = 0.73). This effect diminished with CD4+ counts >350 cells/μL (r = 0.35).
Figure 1.
Boxplots of the distribution of CD4 cell percentages by absolute CD4 cell counts among human immunodeficiency virus–infected participants. The box displays the interquartile range (depicting the 25th and 75th percentiles) surrounding the median value. The whiskers represent values falling within 1.5 times the 25th and 75th percentiles and outliers (solid dots) depict values that fall outside this range.
CD4N and CD4% Concordance and Discordance
Concordance and discordance between CD4N and CD4% measurements were also examined within previously established categories (Table 2). Of 287 participants, 134 (46.7%) had concordant measures, 99 (34.4%) were classified as high discordant (ie, CD4% > CD4N), and 54 (18.8%) were classified as low discordant (ie, CD4% < CD4N).
Correlates of high discordance were examined by univariate and multivariable logistic regression (Table 3). Demographic, behavioral, and most clinical parameters were not strongly associated with high discordance. There was a suggestion that increasing alcohol consumption may increase the odds of high discordance, although with limited numbers the estimates were imprecise. In univariate and multivariable analysis adjusting for age, sex, and race, the presence of liver fibrosis was associated with an increased likelihood of high discordance (Table 3, model 1), although this did not achieve statistical significance. Of note, elastography-defined cirrhosis (LSM ≥12.3 kPa) was not more strongly associated with high discordance than was the presence of significant liver fibrosis (LSM ≥9.3 kPa; data not shown).
Table 3.
Demographic, Behavioral, and Clinical Correlates of Participants With High Discordance Compared to Concordant Participants
| Characteristic | Univariate analysis |
Multivariable analysis |
|
|---|---|---|---|
| Crude OR (95% CI) |
Model 1 |
Model 2 |
|
| All |
TLC ≤1200 cells/μL |
||
| AOR (95% CI) |
AOR (95% CI) |
||
| N = 287 | n = 233 | n = 114 | |
| Demographic | |||
| Age (per y) | 0.98 (.95–1.02) | 0.98 (.94–1.02) | 1.03 (.96–1.10) |
| Female | 0.85 (.50–1.46) | 0.80 (.45–1.41) | 0.88 (.38–2.03) |
| Black | 0.47 (.16–1.36) | 0.52 (.17–1.56) | …a |
| Behavioral | |||
| Any injection drug useb | 0.85 (.50–1.43) | … | … |
| Alcohol useb | |||
| None | Ref | … | … |
| Weekly | 1.35 (.78–2.35) | … | … |
| Daily | 1.82 (.71–4.70) | … | … |
| Hematologic | |||
| Platelets | 0.87 (.6–1.27) | … | … |
| HIV-related | |||
| HIV RNA >400 copies/mL | 0.94 (.55–1.58) | … | … |
| HAART useb | 0.95 (.56–1.27) | … | … |
| Liver-related | |||
| AST | 1.00 (1.00–1.01) | … | … |
| ALT | 1.00 (.99–1.01) | … | … |
| HCV Ab positive | 0.98 (.33–2.93) | … | … |
| HBsAg positive | 1.13 (.34–3.83) | … | … |
| LSM ≥9.3 kPa | 1.62 (.93–2.84) | 1.68 (.95–2.96) | 2.66 (1.11–6.40) |
High discordance: where CD4 cell percentage measures high relative to the absolute CD4 cell count. There were 134 persons in the concordant group and 99 in the high discordance group.
Abbreviations: ALT, alanine aminotransferase; AOR, adjusted odds ratio; AST, aspartate aminotransferase; CI, confidence interval; HAART, highly active antiretroviral therapy; HBsAg, hepatitis B virus surface antigen; HCV Ab, hepatitis C virus antibody; HIV, human immunodeficiency virus; LSM, liver stiffness measurement by elastography; OR, odds ratio; TLC, total lymphocyte count.
a Dropped due to inestimability as all patients in this group were black.
b In the preceding 6 months.
High discordance of CD4% relative to CD4N was noted to be extremely common among participants with TLC <1200 cells/μL (76%) compared to those with TLC values above this level (24%, P < .001). Among the subset of participants with TLC <1200 cells/μL (Table 3, model 2), liver fibrosis was strongly associated with high discordance (odds ratio [OR], 2.66; 95% confidence interval [CI], 1.11–6.40). In contrast, there were no significant predictors of high discordance in participants with TLC ≥1200 cells/μL. Similarly, no differences were found between those with lower versus higher CD4N, dichotomized at 350 cells/μL.
Predictors of low discordance (CD4% low relative to CD4N) were also examined. After adjusting for age, sex, race, and alcohol use, recent HAART use was found to significantly increase the odds of low discordance (OR, 2.53; 95% CI, 1.27–5.06). In this model, weekly alcohol use was associated with reduced odds of low discordance (OR, 0.40; 95% CI, .19–.83; P < .05). No other factors were significantly associated with low discordance nor were there qualitative differences found in subgroup analyses by TLC and CD4 cell count (data not shown).
DISCUSSION
Accurate evaluation of immune status in HIV-infected persons is critical to assessing HIV disease progression, for determining when to initiate therapy, and for monitoring therapeutic response. In this study of well-characterized HIV-infected IDUs with a high prevalence of HCV coinfection, we identified a high degree of discordance between CD4N and CD4%. The use of noninvasive transient elastography afforded us the opportunity to systematically ascertain liver fibrosis on all patients, obviating the reliance on liver biopsy, which has limited patient acceptance. Previously, transient elastography has been validated to perform well as an indicator of liver fibrosis and cirrhosis in both our and other study populations [20, 28, 29]. A recent study by Montes et al [30] demonstrated decreased CD4N associated with hepatic stiffness as measured by transient elastography. Our study extends the earlier findings of McGovern et al [9] that demonstrated low CDN but normal CD4% in HIV-negative patients with biopsy-proven cirrhosis to include HIV-infected persons with less advanced liver disease. We found that participants with significant liver fibrosis (LSM ≥9.3 kPa) were more likely to have higher CD4% than what would be expected based on the CD4N. Notably, this association was strongest in those with substantial lymphopenia, as characterized by TLC <1200 cells/μL. In total, these data suggest that liver disease–related lymphopenia may contribute to CD4N/% discordance.
Prior studies have yielded conflicting results regarding the use of CD4N over CD4% as a prognostic indicator in HIV infection. Indeed, some studies conducted early in the epidemic found a greater prognostic value for CD4% as compared with CD4N [5, 8]. In 2005, Hulgan et al [6] reported that CD4% served as a useful indicator of when to begin treatment in patients with CD4N >350 lymphocytes/μL and more recently demonstrated that CD4% and CD4N were independent predictors of disease progression in patients initiating HAART [7]. In 2009, Guiguet et al [31] showed CD4% to be predictive of clinical progression independent of CD4N and viral load. However, Gebo et al [21] found that absolute CD4N was “the more important measure of immune status” and would be “preferred over the CD4% for making treatment decisions in HIV-infected adults.”
In our study, more than three-fourths of persons with marked lymphopenia (TLC <1200 cells/μL) had higher CD4% relative to the CD4N. The etiology of lymphopenia in our study participants may be multifactorial, with contributions from HIV infection, alcohol, and other factors in addition to liver disease. Progressive HIV infection leads eventually to loss of T-cell homeostasis and declines in the number of circulating CD4 and CD8 lymphocytes; TLC also declines concurrently with these changes [32]. In resource-limited countries, TLC serves as a useful marker of HIV disease progression [23]; to our knowledge, these data have not been evaluated in relation to liver fibrosis. Natural history data in US populations also documented that TLC declines are predictive of development of AIDS [33], again without regard to the presence of liver disease. Among treated patients, antiretrovirals and other drugs commonly used in HIV-infected patients may be associated with lymphopenia. Alcohol use may directly contribute to lymphopenia even in the absence of alcohol-related liver disease [34]. Acute bacterial infections may induce lymphopenia [35]. Liver fibrosis in HCV-infected patients has been associated with lower CD4N [36], although HCV infection has also been reported to contribute to decreased CD4N through pathways independent of hepatic mechanisms, such as via increased apoptosis [37]. Notably, chronic HCV infection does not appear to substantially impact TLC counts [38]. In addition, both HCV coinfection [39] and injection drug use [40] have been associated with poorer immune reconstitution following initiation of HAART, though these studies did not routinely assess liver disease severity and these effects remain controversial [41].
Irrespective of the potential contribution of these other factors, liver disease likely remains a primary underlying cause of lymphopenia and associated alterations in CD4 lymphocyte numbers in our HIV/HCV-coinfected participants. We report an increase in CD4% to CD4N discordance associated with liver fibrosis. Lymphopenia and perturbations of T-cell subsets have been well described in patients with liver cirrhosis [42–44]. Even prior to the AIDS epidemic, persons with hemophilia were recognized to have persistent lymphopenia, which was strongly associated with advanced liver disease and liver-related mortality [45]. Similarly, in HIV-uninfected persons with hemophilia at high risk for HIV infection, CD4+ lymphopenia was attributed primarily to the total lymphopenia caused by underlying liver disease [46].
In most prior studies, CD4+ lymphopenia has been described primarily in advanced liver disease, generally in the setting of portal hypertension and cirrhosis [9, 42, 44]. The primary underlying mechanism has been suggested to involve splenic sequestration of cell lines. However, we did not observe greater CD4%/CD4N discordance among persons with cirrhosis compared to those with any liver fibrosis (corresponding to Metavir fibrosis stage F2), although these analyses were limited by smaller numbers of cirrhotic patients. Although splenic sequestration may occur to some degree at earlier stages of fibrosis, these data suggest that additional explanations for the mechanism of CD4% to CD4N discordance should be evaluated.
From a clinical standpoint, the utility of using CD4% vs CD4N for HIV treatment–related decisions remains unclear. Recognizing that multiple factors can influence white blood cell counts or lymphocyte percentages, it is not surprising that CD4% demonstrates less variability from test to test than does CD4N [6]. Our data suggest that consideration of CD4% may be appropriate among persons with liver fibrosis, especially with significant lymphopenia. Antiretroviral treatment guidelines now indicate that CD4% would be the most appropriate parameter to evaluate when factors such as acute infections, splenectomy, or medications are likely to influence white blood cell counts and differentials [47]. From a practical standpoint, our findings suggest a relatively simple approach could be for clinicians to evaluate CD4% in addition to CD4N whenever a patient's TLC is <1200 cells/μL. For HAART initiation, consideration of CD4% in persons with liver disease may be of reduced importance, as the relatively lower CD4N may actually lead to these persons being started on therapy earlier relative to persons without liver disease. This would be consistent with the recent evolution of treatment recommendations that suggest consideration of earlier treatment in HIV-infected persons coinfected with HBV or HCV [2, 47].
From a research standpoint, it should be recognized that systematic differences in assessment of immune status may occur based on the presence of liver disease, which is highly correlated to hepatitis coinfection. For example, in trials where CD4N-guided therapy resulted in antiretroviral treatment interruptions [48], it is possible that coinfected persons might actually have had even higher CD4% values at interruption relative to persons without coinfection. Further, CD4N/% discordance occurring selectively in hepatitis-coinfected patients may confound interpretation of similar rates of opportunistic disease or of increased risk for nonopportunistic disease reported in coinfected compared to HIV-monoinfected patients [49, 50].
Our study had some limitations. With a larger sample size, we might have observed a statistically significant effect of liver fibrosis on CD4%/CD4N discordance among all participants, not just those with TLC <1200 cells/μL. We also may have seen a stronger effect among those with cirrhosis compared to those with significant fibrosis. Our study represents a single urban site consisting primarily of HIV/HCV-coinfected black IDUs. As such, it may not be generalizable to other populations. However, our population is largely reflective of the HIV/HCV-coinfected population in the United States. Finally, this is a cross-sectional study, so correlation to clinical outcomes such as progression to AIDS or mortality was not possible.
In summary, we found that liver fibrosis was associated with a higher CD4% relative to the CD4N in HIV-infected persons, especially when TLC <1200 cells/μL. Based on these data, clinicians may need to consider incorporating CD4% into the decision-making process of providing appropriate care to HIV-infected persons with underlying liver fibrosis. Notably, TLC <1200 cells/μL could provide a useful and readily available marker to prompt evaluation of CD4%. To definitively address these issues, future studies should directly compare the prognostic value of CD4N and CD4% in predicting clinical outcomes in populations with a range of severity of liver disease. Elastography or other noninvasive fibrosis markers may allow such studies to be performed on a scale large enough to allow conclusive determinations regarding use of CD4N and CD4%.
Notes
Acknowledgments. We thank the ALIVE study participants and study staff for their important contributions to this ongoing work.
Financial support. This work was supported by the National Institute on Drug Abuse (R01 DA16078, R01 DA12568, and R01 DA04334) and supported in part by the American Cancer Society (MRSG-07-284-01-CCE to G. D. K.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fahey J, Taylor J, Detels R, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–72. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society—USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 3.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Vlahov D, Graham N, Hoover D, et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: plasma viral load and CD4+ cell count. JAMA. 1998;279:35–40. doi: 10.1001/jama.279.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Burcham J, Marmor M, Dubin N, et al. CD4 percent is the best predictor of development of AIDS in a cohort of HIV-infected homosexual men. AIDS. 1991;5:365–72. doi: 10.1097/00002030-199104000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hulgan T, Raffanti S, Kheshti A, et al. CD4 lymphocyte percentage predicts disease progression in HIV-infected patients initiating highly active antiretroviral therapy with CD4 lymphocyte counts >350 lymphocytes/mm3. J Infect Dis. 2005;192:950–7. doi: 10.1086/432955. [DOI] [PubMed] [Google Scholar]
- 7.Hulgan T, Shepherd BE, Raffanti SP, et al. Absolute count and percentage of CD4+ lymphocytes are independent predictors of disease progression in HIV-infected persons initiating highly active antiretroviral therapy. J Infect Dis. 2007;195:425–31. doi: 10.1086/510536. [DOI] [PubMed] [Google Scholar]
- 8.Taylor JM, Fahey JL, Detels R, Giorgi JV. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to use. J Acquir Immune Defic Syndr. 1989;2:114–24. [PubMed] [Google Scholar]
- 9.McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431–7. doi: 10.1086/509580. [DOI] [PubMed] [Google Scholar]
- 10.Bongiovanni M, Gori A, Lepri AC, et al. Is the CD4 cell percentage a better marker of immunosuppression than the absolute CD4 cell count in HIV-infected patients with cirrhosis? Clin Infect Dis. 2007;45:650–3. doi: 10.1086/520025. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi RT. Cirrhosis is associated with low CD4+ T cell counts: implications for HIV-infected patients with liver disease. Clin Infect Dis. 2007;44:438–40. doi: 10.1086/510682. [DOI] [PubMed] [Google Scholar]
- 12.Vlahov D, Anthony JC, Munoz A, Margolik J, Celetano D, Solomon L. The ALIVE study: a longitudinal study of HIV-1 infection in intravenous drug users: description of methods. J Drug Issues. 1991;21:759–76. [PubMed] [Google Scholar]
- 13.Mehta SH, Lucas G, Astemborski J, Kirk GD, Vlahov D, Galai N. Early immunologic and virologic responses to highly active antiretroviral therapy and subsequent disease progression among HIV-infected injection drug users. AIDS Care. 2007;19:637–45. doi: 10.1080/09540120701235644. [DOI] [PubMed] [Google Scholar]
- 14.Giorgi JV, Cheng HL, Margolick JB, et al. The Multicenter AIDS Cohort Study Group. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the multicenter AIDS cohort study experience. Clin Immunol Immunopathol. 1990;55:173–86. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman RA, Kung PC, Hansen WP, Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci USA. 1980;77:4914–7. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inglesby TV, Rai R, Astemborski J, et al. A prospective, community-based evaluation of liver enzymes in individuals with hepatitis C after drug use. Hepatology. 1999;29:590–6. doi: 10.1002/hep.510290219. [DOI] [PubMed] [Google Scholar]
- 17.Levine OS, Vlahov D, Koehler J, Cohn S, Spronk AM, Nelson KE. Seroepidemiology of hepatitis B virus in a population of injecting drug users. Association with drug injection patterns. Am J Epidemiol. 1995;142:331–41. doi: 10.1093/oxfordjournals.aje.a117639. [DOI] [PubMed] [Google Scholar]
- 18.McOmish F, Yap PL, Dow BC, et al. Geographical distribution of hepatitis C virus genotypes in blood donors: an international collaborative survey. J Clin Microbiol. 1994;32:884–92. doi: 10.1128/jcm.32.4.884-892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 20.Kirk GD, Astemborski J, Mehta SH, et al. Assessment of liver fibrosis by transient elastography in persons with hepatitis C virus infection or HIV-hepatitis C virus coinfection. Clin Infect Dis. 2009;48:963–72. doi: 10.1086/597350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebo KA, Gallant JE, Keruly JC, Moore RD. Absolute CD4 vs. CD4 percentage for predicting the risk of opportunistic illness in HIV infection. J Acquir Immune Defic Syndr. 2004;36:1028–33. doi: 10.1097/00126334-200408150-00005. [DOI] [PubMed] [Google Scholar]
- 22.Masur H, Ognibene FP, Yarchoan R, et al. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989;111:223–31. doi: 10.7326/0003-4819-111-3-223. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Scaling up antiretroviral therapy in resource-limited settings. Guidelines for a public health approach. Executive Summary. April 2002 Geneva, Switzerland. [PubMed] [Google Scholar]
- 24.Babiker AG, Peto T, Porter K, Walker AS, Darbyshire JH. Age as a determinant of survival in HIV infection. J Clin Epi. 2001;54:S16–21. doi: 10.1016/s0895-4356(01)00456-5. [DOI] [PubMed] [Google Scholar]
- 25.Chaisson RE, Fuchs E, Stanton D, et al. Racial heterogeneity of HIV antigenemia in persons with HIV infection. AIDS. 1991;5:177–80. doi: 10.1097/00002030-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, May M, Chene G. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 27.Umeh OC, Currier JS. Sex differences in HIV: natural history, pharmacokinetics, and drug toxicity. Curr Infect Dis Rep. 2005;7:73–8. doi: 10.1007/s11908-005-0026-9. [DOI] [PubMed] [Google Scholar]
- 28.De Ledinghen V, Douvin C, Kettaneh A, et al. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–9. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen-Khac E, Dominique C. Noninvasive diagnosis of liver fibrosis by ultrasonic transient elastography (FibroScan) Eur J Gastroenterol Hepatol. 2006;18:1321–5. doi: 10.1097/01.meg.0000243884.55562.37. [DOI] [PubMed] [Google Scholar]
- 30.Montes ML, Castro JM, Gonzalez J, San Jose Valdes B, Arribas JR. [Relationship between hepatic fibrosis measured by transient elastography and peripheral blood CD4+ lymphocyte values.] Enferm Infecc Microbiol Clin. 2008;26:561–3. doi: 10.1157/13128273. [DOI] [PubMed] [Google Scholar]
- 31.Guiguet M, Kendjo E, Carcelain G, et al. CD4+ T-cell percentage is an independent predictor of clinical progression in AIDS-free antiretroviral-naive patients with CD4+ T-cell counts >200 cells/mm3. Antivir Ther. 2009;14:451–7. doi: 10.1177/135965350901400311. [DOI] [PubMed] [Google Scholar]
- 32.Margolick JB, Muñoz A, Donnenberg AD, et al. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. Nat Med. 1995;1:674–80. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 33.Lau B, Gange SJ, Phair JP, Riddler SA, Detels R, Margolick JB. Use of total lymphocyte count and hemoglobin concentration for monitoring progression of HIV infection. J Acquir Immune Defic Syndr. 2005;39:620–5. [PubMed] [Google Scholar]
- 34.Liu YK. Effects of alcohol on granulocytes and lymphocytes. Semin Hematol. 1980;17:130–6. [PubMed] [Google Scholar]
- 35.Proust J, Rosenzweig P, Debouzy C, Moulias R. Lymphopenia induced by acute bacterial infections in the elderly: a sign of age-related immune dysfunction of major prognostic significance. Gerontology. 1985;31:178–85. doi: 10.1159/000212700. [DOI] [PubMed] [Google Scholar]
- 36.Rashkin S, Rouster S, Goodman ZD, Sherman KE. T-helper cells and liver fibrosis in hepatitis C virus-monoinfected patients. J Viral Hepat. 2010;17:222–6. doi: 10.1111/j.1365-2893.2009.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korner C, Kramer B, Schulte D, et al. Effects of HCV co-infection on apoptosis of CD4+ T-cells in HIV-positive patients. Clin Sci (Lond) 2009;116:861–70. doi: 10.1042/CS20080532. [DOI] [PubMed] [Google Scholar]
- 38.Streiff MB, Mehta S, Thomas DL. Peripheral blood count abnormalities among patients with hepatitis C in the United States. Hepatology. 2002;35:947–52. doi: 10.1053/jhep.2002.32486. [DOI] [PubMed] [Google Scholar]
- 39.Miller MF, Haley C, Koziel MJ, Rowley CF. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis. 2005;41:713–20. doi: 10.1086/432618. [DOI] [PubMed] [Google Scholar]
- 40.Dronda F, Zamora J, Moreno S, et al. CD4 cell recovery during successful antiretroviral therapy in naive HIV-infected patients: the role of intravenous drug use. AIDS. 2004;18:2210–2. doi: 10.1097/00002030-200411050-00018. [DOI] [PubMed] [Google Scholar]
- 41.Yacisin K, Maida I, Rios MJ, Soriano V, Nunez M. Hepatitis C virus coinfection does not affect CD4 restoration in HIV-infected patients after initiation of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:935–40. doi: 10.1089/aid.2008.0069. [DOI] [PubMed] [Google Scholar]
- 42.Maradona Hidalgo JA, Arribas Castrillo JM, Rodrigo Saez L. [Alcoholic liver cirrhosis, portal hypertension, and behavior of different lymphocyte populations.] Med Clin (Barc) 1981;76:211–3. [PubMed] [Google Scholar]
- 43.Perrin D, Bignon JD, Beaujard E, Cheneau ML. [Populations of circulating T lymphocytes in patients with alcoholic cirrhosis] Gastroenterol Clin Biol. 1984;8:907–10. [PubMed] [Google Scholar]
- 44.Muller C, Wolf H, Gottlicher J, Eibl MM. Helper-inducer and suppressor-inducer lymphocyte subsets in alcoholic cirrhosis. Scand J Gastroenterol. 1991;26:295–301. doi: 10.3109/00365529109025045. [DOI] [PubMed] [Google Scholar]
- 45.Eyster ME, Whitehurst DA, Catalano PM, et al. Long-term follow-up of hemophiliacs with lymphocytopenia or thrombocytopenia. Blood. 1985;66:1317–20. [PubMed] [Google Scholar]
- 46.O'Brien TR, Diamondstone L, Fried MW, et al. Idiopathic CD4+ T-lymphocytopenia in HIV seronegative men with hemophilia and sex partners of HIV seropositive men. Am J Hematol. 1995;49:201–6. doi: 10.1002/ajh.2830490305. [DOI] [PubMed] [Google Scholar]
- 47.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 14 October 2011; 1–167. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 10 November 2011. [Google Scholar]
- 48.El-Sadr WM, Grund B, Neuhaus J, et al. SMART Study Group. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med. 2008;149:289–99. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- 49.Wyles DL. Hepatitis virus coinfection in the strategic management of antiretroviral therapy (SMART) study: a marker for non-liver, non-opportunistic disease mortality. Clin Infect Dis. 2008;47:1476–8. doi: 10.1086/593103. [DOI] [PubMed] [Google Scholar]
- 50.Tebaldi E, Peters L, Neuhaus J, et al. Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the strategic management of antiretroviral therapy (SMART) study. Clin Infect Dis. 2008;47:1468–75. doi: 10.1086/593102. [DOI] [PubMed] [Google Scholar]