Abstract
We randomized 115 children to trivalent inactivated influenza vaccine (TIV) or placebo. Over the following 9 months, TIV recipients had an increased risk of virologically-confirmed non-influenza infections (relative risk: 4.40; 95% confidence interval: 1.31-14.8). Being protected against influenza, TIV recipients may lack temporary non-specific immunity that protected against other respiratory viruses.
Influenza vaccination is effective in preventing influenza virus infection and associated morbidity among school-aged children [1, 2]. The potential for temporary nonspecific immunity between respiratory viruses after an infection and consequent interference at the population level between epidemics of these viruses has been hypothesized, with limited empirical evidence to date, mainly from ecological studies [3–15]. We investigated the incidence of acute upper respiratory tract infections (URTIs) associated with virologically confirmed respiratory virus infections in a randomized controlled trial of influenza vaccination.
METHODS
Recruitment and Follow-up of Participants
In a double-blind randomized controlled trial, we randomly allocated children aged 6–15 years to receive 2008–2009 seasonal trivalent influenza inactivated vaccine (TIV; 0.5 mL Vaxigrip; Sanofi Pasteur) or placebo [16]. Serum specimens were obtained from participants before vaccination from November through December 2008, a month after vaccination, in midstudy around April 2009, and at the end of the study from August through October 2009. Participants were followed up for illnesses through symptom diaries and telephone calls, and illness reports in any household member triggered home visits during which nasal and throat swab specimens (NTSs) were collected from all household members. We defined the follow-up period for each participant from 14 days after receipt of TIV or placebo to collection of midstudy serum samples as the winter season and from collection of midstudy samples through final serum sample obtainment as the summer season.
Proxy written informed consent was obtained for all participants from their parents or legal guardians, with additional written assent from those ≥8 years of age. The study protocol was approved by the Institutional Review Board of Hong Kong University.
Laboratory Methods
NTSs were tested for 19 respiratory viruses by the ResPlex II Plus multiplex array [17–19] and for influenza A and B by reverse-transcription polymerase chain reaction (RT-PCR) [16, 20] (Supplementary Appendix). We refer to infections determined by these assays as “confirmed” infections. Information on influenza serology is provided in the Supplementary Appendix .
Statistical Analysis
We defined an acute respiratory illness (ARI) determined by self-reported signs and symptoms as ≥2 of the following signs or symptoms: body temperature ≥37.8°C, headache, sore throat, cough, presence of phlegm, coryza, and myalgia [16]. We defined febrile acute respiratory illness (FARI) as body temperature ≥37.8°C plus cough or sore throat. Because duration of follow-up varied by participant, we estimated the incidence rates of ARI and FARI episodes and confirmed viral infections overall and during the winter and summer seasons and estimated the relative risk of these episodes for participants who received TIV versus placebo with use of the incidence rate ratio using Poisson regression (Supplementary Appendix). All statistical analyses were conducted using R, version 2.11.0 (R Development Core Team, Vienna, Austria). Data and syntax to reproduce these statistical analyses are available on the corresponding author's Web site.
RESULTS
Among the 115 participants who were followed up, the median duration of follow-up was 272 days (interquartile range, 264–285 days), with no statistically significant differences in age, sex, household size, or duration of follow-up between TIV and placebo recipients (Table 1). We identified 134 ARI episodes, of which 49 met the more stringent FARI case definition. Illnesses occurred throughout the study period (Supplementary Appendix Figure 1). There was no statistically significant difference in the risk of ARI or FARI between participants who received TIV and those who received placebo, either during winter or summer 2009 (Table 2).
Table 1.
Characteristics of Participants and Duration of Follow-up
| Characteristic | TIV (n = 69) | Placebo (n = 46) |
|---|---|---|
| Age group, No. (%) | ||
| 6–8 years | 19 (28) | 16 (35) |
| 9–11 years | 41 (59) | 27 (59) |
| 12–15 years | 9 (13) | 3 (7) |
| Female sex, No. (%) | 30 (43) | 23 (50) |
| Median duration of follow-up, days | 272 | 272 |
| Mean no. of individuals per household | 3.7 | 3.6 |
Abbreviation: TIV, trivalent inactivated influenza vaccine.
Table 2.
Incidence Rates of Acute Upper Respiratory Tract Infection Among 115 Participants Aged 6–15 Years Who Received Trivalent Inactivated Influenza Vaccine or Placebo
| TIV (n = 69) |
Placebo (n = 46) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Ratea | (95% CI) | Ratea | (95% CI) | Relative Risk (95% CI) | P Value | |
| Winter 2009 | |||||||
| ARIb episodes | 2080 | (1530–2830) | 2260 | (1550–3300) | 0.92 | (.57–1.50) | .74 |
| FARIb episodes | 609 | (346–1070) | 753 | (392–1450) | 0.81 | (.34–1.92) | .63 |
| Summer 2009 | |||||||
| ARIb episodes | 1510 | (1130–2020) | 1160 | (757–1780) | 1.30 | (.78–2.18) | .31 |
| FARIb episodes | 658 | (424–1020) | 442 | (221–884) | 1.49 | (.65–3.38) | .33 |
Abbreviations: ARI, acute respiratory illness; CI, confidence interval; FARI , febrile acute respiratory illness; TIV, trivalent inactivated influenza vaccine.
a Incidence rates were estimated as the number of ARI or FARI episodes per 1000 person-years of follow-up.
b ARI was defined as at least 2 of the following symptoms: body temperature ≥37.8°C, cough, sore throat, headache, runny nose, phlegm, and myalgia; FARI was defined as body temperature ≥37.8°C plus cough or sore throat.
We were able to collect 73 NTSs for testing from participants for 65 of 134 (49%) ARI episodes, which included 22 of 49 (45%) FARI episodes. The mean delay between ARI onset and collection of first NTS was 1.22 days, and 5% of NTSs were collected >3 days after illness onset, with no statistically significant differences between TIV and placebo recipients. We detected respiratory viruses in 32 of 65 NTSs (49%) collected during ARI episodes, which included 12 of 22 (55%) FARI episodes. We collected 85 NTSs from participants at times when one of their household contacts reported an acute URTI but the participants were not ill, and identified viruses in 3 of the specimens (4%), including influenza A (H3N2), coxsackie/echovirus, and coronavirus 229E.
There was no statistically significant difference in the risk of confirmed seasonal influenza infection between recipients of TIV or placebo, although the point estimate was consistent with protection in TIV recipients (relative risk [RR], 0.66; 95% confidence interval [CI], .13–3.27). TIV recipients had significantly lower risk of seasonal influenza infection based on serologic evidence (Supplementary Appendix). However, participants who received TIV had higher risk of ARI associated with confirmed noninfluenza respiratory virus infection (RR, 4.40; 95% CI, 1.31–14.8). Including 2 additional confirmed infections when participants did not report ARI, TIV recipients had higher risk of confirmed noninfluenza respiratory virus infection (RR, 3.46; 95% CI, 1.19–10.1). The majority of the noninfluenza respiratory virus detections were rhinoviruses and coxsackie/echoviruses, and the increased risk among TIV recipients was also statistically significant for these viruses (Table 3). Most respiratory virus detections occurred in March 2009, shortly after a period of peak seasonal influenza activity in February 2009 (Figure 1).
Table 3.
Incidence Rates of Respiratory Virus Detection by Reverse-Transcription Polymerase Chain Reaction and Multiplex Assay
| Variable | TIV (n = 69) |
Placebo (n = 46) |
P Value | ||||
|---|---|---|---|---|---|---|---|
| No. | Ratea | (95% CI) | No. | Ratea | (95% CI) | ||
| Any seasonal influenza | 3 | 58 | (19–180) | 3 | 88 | (28–270) | .61 |
| Seasonal influenza A (H1N1) | 2 | 39 | (10–160) | 2 | 59 | (15–240) | .68 |
| Seasonal influenza A (H3N2) | 1 | 19 | (3–140) | 0 | 0 | (0–88) | .31 |
| Seasonal influenza B | 0 | 0 | (0–58) | 1 | 29 | (4–210) | .17 |
| Pandemic influenza A (H1N1) | 3 | 58 | (19–180) | 0 | 0 | (0–88) | .08 |
| Any noninfluenza virusb | 20 | 390 | (250–600) | 3 | 88 | (28–270) | <.01 |
| Rhinovirus | 12 | 230 | (130–410) | 2 | 59 | (15–240) | .04 |
| Coxsackie/echovirus | 8 | 160 | (78–310) | 0 | 0 | (0–88) | <.01 |
| Other respiratory virusc | 5 | 97 | (40–230) | 1 | 29 | (4–210) | .22 |
| ARI episode with specimen collected but no virus detected | 19 | 369 | (235–578) | 14 | 412 | (244–696) | .75 |
| ARI episode with no specimen collected | 41 | 796 | (586–1080) | 28 | 824 | (569–1190) | .89 |
Incidence rates are from respiratory specimens collected from 115 participants aged 6–15 years who received trivalent influenza vaccine or placebo during 134 acute respiratory illness episodes.
Abbreviations: ARI, acute respiratory illness; CI, confidence interval; TIV, trivalent inactivated influenza vaccine.
a Incidence rates were estimated as the no. of virus detections or illness episodes per 1000 person-years of follow-up. ARI was defined as at least 2 of the following symptoms: body temperature ≥37.8°C, cough, sore throat, headache, runny nose, phlegm, and myalgia.
b In TIV recipients there were 4 detections with both rhinovirus and coxsackie/echovirus, and 1 detection with both coxsackie/echovirus and coronavirus NL63.
c Including positive detections of coronavirus, human metapneumovirus, parainfluenza, respiratory syncytial virus (RSV). The ResPlex II multiplex array tested for 19 virus targets including influenza types A and B (including 2009-H1N1), RSV types A and B, parainfluenza types 1–4, metapneumovirus, rhinovirus, coxsackievirus/echovirus, adenovirus types B and E, bocavirus, and coronavirus types NL63, HKU1, 229E, and OC43.
Figure 1.
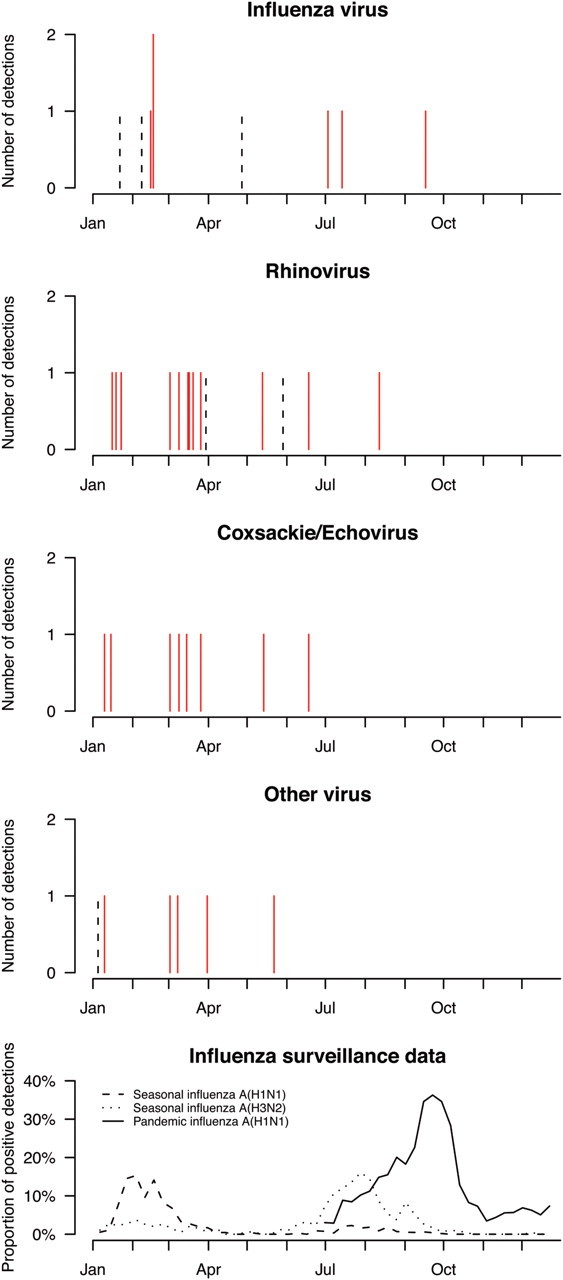
Timing of influenza and other respiratory virus detections in 115 participants aged 6–15 years (A–D), compared with local influenza surveillance data (E). Solid red bars indicate detections in 69 participants who received 2008–2009 trivalent inactivated influenza vaccine, and black dashed bars indicate detections in 46 participants who received placebo. The bottom panel shows local laboratory surveillance data on the proportion of influenza virus detections among specimens submitted to the Public Health Laboratory Service (PHLS). Less than 2% of PHLS specimens were positive for influenza B throughout the year. “Other viruses” included coronavirus, human metapneumovirus, parainfluenza, and respiratory syncytial virus.
DISCUSSION
In the prepandemic period of our study, we did not observe a statistically significant reduction in confirmed seasonal influenza virus infections in the TIV recipients (Table 3), although serological evidence (Supplementary Appendix) and point estimates of vaccine efficacy based on confirmed infections were consistent with protection of TIV recipients against the seasonal influenza viruses that circulated from January through March 2009 [16]. We identified a statistically significant increased risk of noninfluenza respiratory virus infection among TIV recipients (Table 3), including significant increases in the risk of rhinovirus and coxsackie/echovirus infection, which were most frequently detected in March 2009, immediately after the peak in seasonal influenza activity in February 2009 (Figure 1).
The increased risk of noninfluenza respiratory virus infection among TIV recipients could be an artefactual finding; for example, measurement bias could have resulted if participants were more likely to report their first ARI episode but less likely to report subsequent episodes, whereas there was no real difference in rhinovirus or other noninfluenza respiratory virus infections after the winter influenza season. The increased risk could also indicate a real effect. Receipt of TIV could increase influenza immunity at the expense of reduced immunity to noninfluenza respiratory viruses, by some unknown biological mechanism. Alternatively, our results could be explained by temporary nonspecific immunity after influenza virus infection, through the cell-mediated response or, more likely, the innate immune response to infection [21–23]. Participants who received TIV would have been protected against influenza in February 2009 but then would not have had heightened nonspecific immunity in the following weeks. They would then face a higher risk of certain other virus infections in March 2009, compared with placebo recipients (Figure 1). The duration of any temporary nonspecific immunity remains uncertain [13] but could be of the order of 2–4 weeks based on these observations. It is less likely that the interference observed here could be explained by reduced community exposures during convalescence (ie, behavioral rather than immunologic factors) [14].
The phenomenon of virus interference has been well known in virology for >60 years [24–27]. Ecological studies have reported phenomena potentially explained by viral interference [3–11]. Nonspecific immunity against noninfluenza respiratory viruses was reported in children for 1–2 weeks after receipt of live attenuated influenza vaccine [28]. Interference in respiratory and gastrointestinal infections has been reported after receipt of live oral poliovirus vaccine [29–32].
Our results are limited by the small sample size and the small number of confirmed infections. Despite this limitation, we were able to observe a statistically significant increased risk of confirmed noninfluenza respiratory virus infection among TIV recipients (Table 3). A negative association between serologic evidence of influenza infection and confirmed noninfluenza virus infection in winter 2009 was not statistically significant (odds ratio, 0.27; 95% CI, .01–2.05) (Supplementary Appendix). One must be cautious in interpreting serology in children who have received TIV [2, 33]. Finally, acute URTI incidence was based on self-report with regular telephone reminders, and we may have failed to identify some illnesses despite rigorous prospective follow-up.
Temporary nonspecific immunity leading to interference between epidemics of respiratory viruses could have important implications. First, as observed in our trial, TIV appeared to have poor efficacy against acute URTIs (Table 2), apparently because the protection against influenza virus infection conferred by TIV was offset by an increased risk of other respiratory virus infection (Table 3). Second, interference between respiratory viruses could suggest new approaches to mitigating epidemics [32]. Mass administration of live polio vaccine in children has been used to control enterovirus 71 epidemics [10, 31]. Finally, viral interference could bias estimates of influenza vaccine effectiveness in test-negative case-control studies (Supplementary Appendix) [2, 34–43]. One test-negative study reported an association between receipt of TIV and the risk of influenza-like illness associated with a noninfluenza virus [38].
Additional work is required to more fully characterize temporary nonspecific immunity overall and in specific groups, such as children. Animal studies [44–50] and volunteer adult human challenge studies [51] could provide useful evidence. Additional community-based observational cohort studies and community-based experimental studies, such as our vaccine trial, may be particularly suitable for investigating temporary nonspecific immunity, because most acute URTIs do not require medical attention.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Chan Kit Man, Calvin Cheng, Lai-Ming Ho, Ho Yuk Ling, Lam Yiu Pong, Tom Lui, Edward Ma, Loretta Mak, Gloria Ng, Joey Sin, Teresa So, Winnie Wai, Lan Wei, Jessica Wong, Eileen Yeung, and Jenny Yuen for research support and Sarah Cobey, Ed Goldstein, Heath Kelly, Nancy Leung, Marc Lipsitch, Ryosuke Omori, Mary Schooling, and Joe Wu for helpful discussions.
Financial support. This work was supported by the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant number AoE/M-12/06), the Hong Kong University Research Council Strategic Research Theme of Public Health, the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant number U54 GM088558), and the Research Fund for the Control of Infectious Disease, Food and Health Bureau, Government of the Hong Kong SAR (grant number PHE-2). The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Potential conflicts of interest. B. J. C. has received research funding from MedImmune. D. K. M. I. has received research funding from Roche. J. S. M. P. receives research funding from Crucell MV. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008;(2):CD004879. doi: 10.1002/14651858.CD004879.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 3.Glezen P, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 4.Glezen WP, Paredes A, Taber LH. Influenza in children. Relationship to other respiratory agents. JAMA. 1980;243:1345–9. doi: 10.1001/jama.243.13.1345. [DOI] [PubMed] [Google Scholar]
- 5.Anestad G. Interference between outbreaks of respiratory syncytial virus and influenza virus infection. Lancet. 1982;1:502. doi: 10.1016/s0140-6736(82)91466-0. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura N, Nishio H, Lee MJ, Uemura K. The clinical features of respiratory syncytial virus: lower respiratory tract infection after upper respiratory tract infection due to influenza virus. Pediatr Int. 2005;47:412–6. doi: 10.1111/j.1442-200x.2005.02099.x. [DOI] [PubMed] [Google Scholar]
- 7.Linde A, Rotzen-Ostlund M, Zweygberg-Wirgart B, Rubinova S, Brytting M. Does viral interference affect spread of influenza? Eurosurveillance. 2009 Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19354 . Accessed 18 April 2012. [PubMed] [Google Scholar]
- 8.Anestada G, Nordbo SA. Virus interference. Did rhinoviruses activity hamper the progress of the 2009 influenza A (H1N1) pandemic in Norway? Med Hypotheses. 2011;77:1132–4. doi: 10.1016/j.mehy.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Casalegno JS, Ottmann M, Duchamp MB, et al. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin Microbiol Infect. 2010;16:326–9. doi: 10.1111/j.1469-0691.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- 10.Berencsi G, Kapusinszky B, Rigo Z, Szomor K. Interference among viruses circulating and administered in Hungary from 1931 to 2008. Acta Microbiol Immunol Hung. 2010;57:73–86. doi: 10.1556/AMicr.57.2010.2.1. [DOI] [PubMed] [Google Scholar]
- 11.Mak GC, Wong AH, Ho WY, Lim W. The impact of pandemic influenza A (H1N1) 2009 on the circulation of respiratory viruses 2009–2011. Influenza Other Respi Viruses. 2012 doi: 10.1111/j.1750-2659.2011.00323.x. doi:10.1111/j.1750-2659.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Malanoski AP, Lin B, et al. Broad spectrum respiratory pathogen analysis of throat swabs from military recruits reveals interference between rhinoviruses and adenoviruses. Microb Ecol. 2010;59:623–34. doi: 10.1007/s00248-010-9636-3. [DOI] [PubMed] [Google Scholar]
- 13.Kelly H, Barry S, Laurie K, Mercer G. Seasonal influenza vaccination and the risk of infection with pandemic influenza: a possible illustration of non-specific temporary immunity following infection. Eurosurveillance. 2010 doi: 10.2807/ese.15.47.19722-en. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19722 . Accessed 18 April 2012. [DOI] [PubMed] [Google Scholar]
- 14.Rohani P, Green CJ, Mantilla-Beniers NB, Grenfell BT. Ecological interference between fatal diseases. Nature. 2003;422:885–8. doi: 10.1038/nature01542. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Halloran ME, Daniels MJ, Longini IM, Burke DS, Cummings DA. Modeling competing infectious pathogens from a Bayesian perspective: application to influenza studies with incomplete laboratory results. J Am Statist Assoc. 2010;105:1310–22. doi: 10.1198/jasa.2010.ap09581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowling BJ, Ng S, Ma ES, et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis. 2010;51:1370–9. doi: 10.1086/657311. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Swan DC, Smith SJ, et al. Simultaneous amplification and identification of 25 human papillomavirus types with Templex technology. J Clin Microbiol. 2006;44:4157–62. doi: 10.1128/JCM.01762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunstein J, Thomas E. Direct screening of clinical specimens for multiple respiratory pathogens using the Genaco Respiratory Panels 1 and 2. Diagn Mol Pathol. 2006;15:169–73. doi: 10.1097/01.pdm.0000210430.35340.53. [DOI] [PubMed] [Google Scholar]
- 19.Li H, McCormac MA, Estes RW, et al. Simultaneous detection and high-throughput identification of a panel of RNA viruses causing respiratory tract infections. J Clin Microbiol. 2007;45:2105–9. doi: 10.1128/JCM.00210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362:2175–84. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGill J, Heusel JW, Legge KL. Innate immune control and regulation of influenza virus infections. J Leukoc Biol. 2009;86:803–12. doi: 10.1189/jlb.0509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaitov MR, Laza-Stanca V, Edwards MR, et al. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–86. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 23.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–9. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy CE. Interference between St. Louis encephalitis virus and equine encephalomyelitis virus (Western Type) in the chick embryo. Science. 1944;99:517–8. doi: 10.1126/science.99.2582.517. [DOI] [PubMed] [Google Scholar]
- 25.Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science. 1943;98:87–9. doi: 10.1126/science.98.2534.87. [DOI] [PubMed] [Google Scholar]
- 26.Isaacs A, Lindenmann J. Virus interference I. The interferon. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1957;147:258–67. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 27.Lindenmann J. From interference to interferon: a brief historical introduction. Philos Trans R Soc Lond B Biol Sci. 1982;299:3–6. doi: 10.1098/rstb.1982.0101. [DOI] [PubMed] [Google Scholar]
- 28.Piedra PA, Gaglani MJ, Riggs M, et al. Live attenuated influenza vaccine, trivalent, is safe in healthy children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a community-based, nonrandomized, open-label trial. Pediatrics. 2005;116:e397–407. doi: 10.1542/peds.2004-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seppala E, Viskari H, Hoppu S, et al. Viral interference induced by live attenuated virus vaccine (OPV) can prevent otitis media. Vaccine. 2011;29:8615–8. doi: 10.1016/j.vaccine.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contreras G. Effect of the administration of oral poliovirus vaccine on infantile diarrhoea mortality. Vaccine. 1989;7:211–2. doi: 10.1016/0264-410x(89)90230-2. [DOI] [PubMed] [Google Scholar]
- 31.Shindarov LM, Chumakov MP, Voroshilova MK, et al. Epidemiological, clinical, and pathomorphological characteristics of epidemic poliomyelitis-like disease caused by enterovirus 71. J Hyg Epidemiol Microbiol Immunol. 1979;23:284–95. [PubMed] [Google Scholar]
- 32.Voroshilova MK. Potential use of nonpathogenic enteroviruses for control of human disease. Prog Med Virol. 1989;36:191–202. [PubMed] [Google Scholar]
- 33.Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis. 2011;203:1309–15. doi: 10.1093/infdis/jir015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skowronski DM, Gilbert M, Tweed SA, et al. Effectiveness of vaccine against medical consultation due to laboratory-confirmed influenza: results from a sentinel physician pilot project in British Columbia, 2004–2005. Can Commun Dis Rep. 2005;31:181–91. [PubMed] [Google Scholar]
- 35.Skowronski DM, Masaro C, Kwindt TL, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005–2006 season of dual A and B vaccine mismatch in Canada. Vaccine. 2007;25:2842–51. doi: 10.1016/j.vaccine.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Belongia EA, Kieke BA, Donahue JG, et al. Influenza vaccine effectiveness in Wisconsin during the 2007–08 season: comparison of interim and final results. Vaccine. 2011;29:6558–63. doi: 10.1016/j.vaccine.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Cheng AC, Kotsimbos T, Kelly HA, et al. Effectiveness of H1N1/09 monovalent and trivalent influenza vaccines against hospitalization with laboratory-confirmed H1N1/09 influenza in Australia: a test-negative case control study. Vaccine. 2011;29:7320–5. doi: 10.1016/j.vaccine.2011.07.087. [DOI] [PubMed] [Google Scholar]
- 38.Kelly H, Jacoby P, Dixon GA, et al. Vaccine effectiveness against laboratory-confirmed influenza in healthy young children: a case-control study. Pediatr Infect Dis J. 2011;30:107–11. doi: 10.1097/INF.0b013e318201811c. [DOI] [PubMed] [Google Scholar]
- 39.Skowronski DM, De Serres G, Crowcroft NS, et al. Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: four observational studies from Canada. PLoS Med. 2010;7(4):e1000258. doi: 10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kissling E, Valenciano M, Cohen JM, et al. I-MOVE Multi-Centre Case Control Study 2010–11: overall and stratified estimates of influenza vaccine effectiveness in Europe. PLoS One. 2011;6(11):e27622. doi: 10.1371/journal.pone.0027622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly HA, Grant KA, Fielding JE, et al. Pandemic influenza H1N1 2009 infection in Victoria, Australia: no evidence for harm or benefit following receipt of seasonal influenza vaccine in 2009. Vaccine. 2011;29:6419–26. doi: 10.1016/j.vaccine.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 42.Orenstein EW, De Serres G, Haber MJ, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–31. doi: 10.1093/ije/dym021. [DOI] [PubMed] [Google Scholar]
- 43.Fielding JE, Grant KA, Garcia K, Kelly HA. Effectiveness of seasonal influenza vaccine against pandemic (H1N1) 2009 virus, Australia, 2010. Emerg Infect Dis. 2011;17:1181–7. doi: 10.3201/eid1707.101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steel J, Staeheli P, Mubareka S, Garcia-Sastre A, Palese P, Lowen AC. Transmission of pandemic H1N1 influenza virus and impact of prior exposure to seasonal strains or interferon treatment. J Virol. 2010;84:21–6. doi: 10.1128/JVI.01732-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imai K, Nakamura K, Mase M, Tsukamoto K, Imada T, Yamaguchi S. Partial protection against challenge with the highly pathogenic H5N1 influenza virus isolated in Japan in chickens infected with the H9N2 influenza virus. Arch Virol. 2007;152:1395–400. doi: 10.1007/s00705-007-0953-x. [DOI] [PubMed] [Google Scholar]
- 46.Van Reeth K, Gregory V, Hay A, Pensaert M. Protection against a European H1N2 swine influenza virus in pigs previously infected with H1N1 and/or H3N2 subtypes. Vaccine. 2003;21:1375–81. doi: 10.1016/s0264-410x(02)00688-6. [DOI] [PubMed] [Google Scholar]
- 47.Kreijtz JH, Bodewes R, van den Brand JM, et al. Infection of mice with a human influenza A/H3N2 virus induces protective immunity against lethal infection with influenza A/H5N1 virus. Vaccine. 2009;27:4983–9. doi: 10.1016/j.vaccine.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 48.Domok I. Studies on the interaction between Coxsackie and poliomyelitis viruses. I. Simultaneous infection with B1 Coxsackie and Lansing poliomyelitis viruses in mice of different ages. Acta Microbiol Acad Sci Hung. 1957;4:183–95. [PubMed] [Google Scholar]
- 49.Domok I. Studies on the interaction between coxsackie and poliomyelitis viruses. III. The course of resistance to poliomyelitis virus induced by coxsackie B1 virus in young mice. Acta Virol. 1959;3:222–33. [PubMed] [Google Scholar]
- 50.Duffy CE, Jordan RT, Meyer HM., Jr. Effect of multiple inoculations on interference between St. Louis encephalitis virus and equine encephalomyelitis virus. Proc Soc Exp Biol Med. 1952;80:279–81. doi: 10.3181/00379727-80-19595. [DOI] [PubMed] [Google Scholar]
- 51.Keitel WA, Couch RB, Quarles JM, Cate TR, Baxter B, Maassab HF. Trivalent attenuated cold-adapted influenza virus vaccine: reduced viral shedding and serum antibody responses in susceptible adults. J Infect Dis. 1993;167:305–11. doi: 10.1093/infdis/167.2.305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


