We identified baseline (before chemotherapy) and early nonbaseline prognostic factors for neutropenia-related invasive aspergillosis in myeloma patients. Higher baseline platelet count and creatinine clearance rate and normalization of serum galactomannan level ≤7 days after the first positive serum Aspergillus galactomannan index were associated with better outcome.
Abstract
Background. Invasive aspergillosis (IA) is a life-threatening infection for immunocompromised patients. Improvement in IA outcome has been hampered by lack of early prognostic factors, namely, those available before starting chemotherapy (baseline) or early in the course of IA (nonbaseline). We hypothesized that prognostic factors can be identified before chemotherapy, ≤7 days from the first positive serum Aspergillus galactomannan index (s-GMI).
Methods. We analyzed 98 patients with multiple myeloma who developed neutropenia-related IA and had a positive s-GMI. Three response criteria were used: kinetics of s-GMI, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) definitions, and 6-week survival. Baseline and nonbaseline variables were analyzed separately.
Results. Independent response predictors at baseline were a platelet count ≥65,000 platelets/mm3 (odds ratio [OR], 1.009; 95% confidence interval [CI], 1.001–1.017; P = .03) by s-GMI kinetics, and a platelet count ≥65,000 platelets/mm3 (OR, 1.009; 95% CI, 1.002–1.017; P = .01) and a creatinine clearance rate ≥53 mL/min (OR, 1.024; 95% CI, 1.006–1.042; P = .009) by EORTC/MSG criteria, with response rates of 83% and 28% when both variables were above or below these cutoffs, respectively (P < .001). Only baseline creatinine clearance rate ≥53 mL/min predicted 6-week survival (P = .003). Normalization of the s-GMI ≤7 days after the first positive s-GMI and neutrophil recovery were the nonbaseline factors associated with positive outcomes.
Conclusions. Two simple, inexpensive to measure, widely available, and routinely collected prechemotherapy values, platelet count and creatinine clearance rate, predict IA outcome and stratify patients into low-, intermediate-, and high-risk categories, while early evaluation of s-GMI allows timely treatment modification. These findings may improve patient outcomes by optimizing management strategies for this serious infection and may prove valuable in designing clinical trials of interventions to improve IA outcomes.
Invasive aspergillosis (IA) is the leading invasive mycosis in patients with hematological malignancies and remains associated with unfavorable outcomes [1]. Various prognostic factors have been identified, including extent of the infection, persistent neutropenia, and use of systemic corticosteroids [2–17]. However, most of these variables are available only after the diagnosis of IA and cannot be successfully applied toward early intervention or treatment modification [18].
Identification of prognostic factors that are available before starting chemotherapy or early during the disease course would improve our ability to individualize antifungal strategies [18] and to design clinical trials of IA, a challenging task when applying conventional criteria, particularly when Aspergillus-specific laboratory markers are not included in the primary study end point [18]. Indeed, a recently completed large randomized clinical trial enrolled 454 patients at 165 multinational sites over approximately 3 years but failed to meet the primary end point for superiority of combination therapy over monotherapy [19]. This likely occurred because the study's primary end point, 6-week all-cause mortality, was not Aspergillus-specific and mortality can be caused by several non-IA related factors, such as graft-versus-host disease and others [18].
Galactomannan is a cell wall component of Aspergillus species that is released by growing hyphae during active aspergillosis. The serum Aspergillus galactomannan index (s-GMI) is an excellent surrogate marker for the diagnosis of IA in patients with hematological cancers [18], and s-GMI positivity precedes the overt manifestations of IA [20]. Furthermore, we and others have shown that s-GMI serves as a valid surrogate end point for clinical outcome, with a strong correlation between normalization of s-GMI and treatment success and survival [21–25]. We therefore hypothesized that simple and objective early prognostic factors for IA can be identified before commencing the chemotherapy course that precedes IA development and can be detected early during the disease course by using s-GMI, an Aspergillus-specific marker.
PATIENTS AND METHODS
We conducted a retrospective study of all episodes of IA in patients with multiple myeloma cared for at the Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, between January 2003 and December 2009. The study was approved by the Institutional Review Board. Evaluation included a review of medical records and radiologic tests, including computerized axial tomography of sinuses and lungs.
One hundred and sixteen episodes of IA were diagnosed during the study period, as previously reported [26]. For this analysis, we focused on patients who developed IA during neutropenia. Ninety-eight episodes were evaluable after exclusion of 12 that developed in nonneutropenic settings, 4 that were recurrent, and 2 during which s-GMI was negative. Patients were managed according to predefined standards of care, as described elsewhere [26], including use of fluconazole prophylaxis and serial measurement of s-GMI (≥3 times/week) during periods at risk (ie, following myelosuppressive chemotherapy or receipt of a melphalan-based conditioning regimen for autologous hematopoietic stem cell transplantation [MEL-ASCT]). Mould-active agents (voriconazole or liposomal amphotericin B) were used at the first positive s-GMI and confirmation of IA on the basis of host, clinical, and radiologic criteria. Cases were classified as proven or probable, according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) definitions [27], or as probable, without prespecified radiologic findings [26].
Three response criteria were used to examine prognostic factors: response according to s-GMI kinetics, with survival, as previously defined [21]; the EORTC/MSG response definitions [28]; and 6-week survival regardless of cause of death. Response by s-GMI kinetics was defined as survival and repeatedly negative s-GMI for ≥2 weeks after the first negative s-GMI in the absence of new extrapulmonary lesions of IA, while failure was defined as persistently positive s-GMI. Death within the 14-day period of negative s-GMI was also considered failure, unless aspergillosis could not be documented at autopsy.
We examined potential predictors immediately prior to starting the chemotherapy regimen after which IA was diagnosed (baseline) and potential predictors after IA was diagnosed (nonbaseline). Baseline variables included sex, age, body surface area (BSA), body mass index (BMI; defined as the weight in kilograms divided by the square of the height in meters), myeloma status, treatment (chemotherapy or MEL-ASCT), number of prior ASCTs, cumulative corticosteroid doses (prednisone equivalent) and receipt of other immunosuppressants (60 days and 30 days before diagnosis of IA, respectively), white blood cell (WBC) count, absolute neutrophil count (ANC), absolute lymphocyte count, platelet count, absolute CD4 cell count, creatinine clearance rate, serum bilirubin level, cytomegalovirus (CMV) serologic status, and iron overload, defined as increased marrow iron stores [29].
Nonbaseline variables included ANC at diagnosis of IA; CMV reactivation, defined as ≥600 copies/mL by plasma quantitative polymerase chain reaction; site of IA (lung, sinuses, other); value of first positive s-GMI; normalization of s-GMI ≤7 days from the first positive s-GMI; duration of neutropenia (ANC, <500 neutrophils/mm3); neutrophil recovery (ANC, ≥500 neutrophils/mm3 on 3 consecutive days); and cumulative corticosteroid doses given ≤6 weeks after diagnosis.
Separate statistical analyses for baseline and nonbaseline variables were conducted. Univariate analysis was performed using the Fisher or χ2 test (as appropriate) for categorical variables and the Wilcoxon test for continuous variables. Variables with a P value <.1 were entered in a multivariate logistic regression analysis. Receiver operating characteristic (ROC) curves were used to determine the best cutoff for the continuous variables that were significant by multivariate analysis. Kaplan-Meyer curves were constructed and compared using the log-rank test. All tests were 2-tailed, and P values <.05 were considered statistically significant.
RESULTS
The characteristics of 98 evaluable cases of IA are shown in Table 1. Antineoplastic treatments were myeloablative MEL-ASCT and myelosuppressive chemotherapy in 50 and 48 patients, respectively. Aspergillosis was classified as proven in 5 cases and probable in 93; 61 case were probable by EORTC/MSG definitions [27], and 32 were probable without prespecified radiologic findings [26].
Table 1.
Characteristics of 98 Patients With Multiple Myeloma and Neutropenia-Related Invasive Aspergillosis Defined at Baseline and After the Diagnosis of Aspergillosis
| Characteristic | Value |
|---|---|
| At baselinea | |
| Sex, male:female | 53:45 |
| Age, years | 61 (38–81) |
| Body surface area, m2 | 1.81 (1.30–2.55) |
| Bone mass index | 25.60 (16.20–43.37) |
| Active myeloma | 81 (83) |
| Antineoplastic treatment | |
| ASCT | 50 (51) |
| Chemotherapy | 48 (49) |
| Prior ASCT | 1 (0–4) |
| Months from diagnosis of underlying disease to IA | 24 (0–186) |
| Days from chemotherapy to diagnosis of IA | 11 (0–32) |
| Receipt of corticosteroids | 94 (96) |
| Cumulative prednisone equivalent dose in mg | 1150 (0–7590) |
| Other immunosuppressive therapiesb | 17 (17) |
| WBC count, cells/mm3 | 3365 (10–26,610) |
| ANC, neutrophils/mm3 | 2178 (0–22,314) |
| ALC, lymphocytes/mm3 | 375 (0–4554) |
| Platelet count, ×103 platelets/mm3 | 73 (7–345) |
| Serum level of uninvolved Ig, mg/dL | 222 (10–985) |
| Absolute CD4 cell, count/mm3 | 189 (19–1192) |
| Creatinine clearance rate, mL/min | 60 (2–171) |
| Serum bilirubin level, mg/dL | 0.6 (0.2–1.6) |
| Cytomegalovirus seropositive | 65 (66) |
| At diagnosis of aspergillosis | |
| ANC, neutrophils/mm3 | 14 (0–10,900) |
| Days with ANC <500 neutrophils/mm3 | 10 (1–54) |
| Days with ANC <100 neutrophils/mm3 | 7 (1–27) |
| s-GMI tests, No. | 21 (4–64) |
| Cytomegalovirus reactivationc | 14/65 (21) |
| Concomitant respiratory viral infectiond | 12 (12) |
| Classification of aspergillosis | |
| Proven | 5 (5) |
| Probable | 61 (652) |
| Probable without prespecified radiologic findingse | 32 (33) |
| Antifungal treatment | |
| None | 6 (6) |
| Voriconazole | 55 (56) |
| Liposomal amphotericin B | 25 (26) |
| Otherf | 12 (12) |
Data are No. or proportion (%) of patients or median (range).
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; ASCT, autologous stem cell transplantation; IA, invasive aspergillosis; Ig, immunoglobulin; RSV, respiratory syncytial virus; s-GMI, serum Aspergillus galactomannan index; WBC, white blood cell.
a Baseline refers to variables measured immediately prior to starting the antineoplastic regimen after which IA was diagnosed.
b Other immunosuppressive therapies included sirolimus (in 12 patients) and alemtuzumab, rituximab, fludarabine, etanercept, and sirolimus + rituximab (in 1 each).
c Defined as >600 copies per mL of plasma by quantitative polymerase chain reaction.
d Concomitant viral infections included RSV (in 5 patients), influenza B virus (in 2), and RSV + influenza B virus, parainfluenza virus type 3, influenza A virus, metapneumovirus, and RSV + parainfluenza type 3 (in 1 each).
e As defined in [26].
f Other treatments included voriconazole + liposomal amphotericin B (in 4 patients), voriconazole + micafungin (in 2), micafungin (in 3), anidulafungin (in 2), and voriconazole + anidulafungin or voriconazole + placebo (in 1).
Factors Associated With Response, as Defined by s-GMI Kinetics
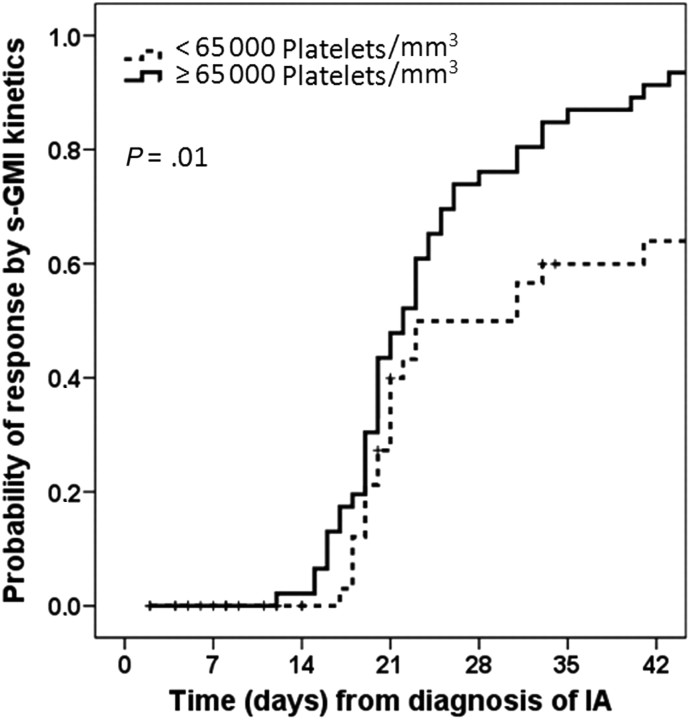
Response defined on the basis of s-GMI kinetics was observed in 72 of 97 patients (74.2%) but could not be assessed in 1 patient (lost to follow-up). Baseline predictors of response by univariate analysis were higher BSA, BMI, and platelet count; lower serum bilirubin level; adequate or low marrow iron stores; and controlled myeloma (Table 2). Higher platelet count was the only significant prognostic variable by multivariate analysis (odds ratio [OR], 1.009; 95% confidence interval [CI], 1.001–1.017; P = .03). The best cutoff for platelet count was 65,000 platelets/mm3, with a sensitivity of 62% and a specificity of 72%. A Kaplan-Meier curve (Figure 1) shows a 91% probability of response at 6 weeks for patients with a baseline platelet count ≥65,000 platelets/mm3, compared with only 68% for patients with a lower count (P = .01).
Table 2.
Univariate Analysis of Predictive Outcome Variables in 98 Patients With Multiple Myeloma Who Developed Neutropenia-Related Aspergillosis
| Variable | Outcomea |
P | |
|---|---|---|---|
| Favorable | Unfavorable | ||
| Response based on s-GMI kineticsb | n = 72 | n = 25 | |
| At baseline | |||
| Body surface area, m2 | 1.89 (1.30–2.35) | 1.72 (1.40–2.55) | .03 |
| Body mass index | 26.15 (16.37–43.37) | 23.20 (16.20–42.48) | .04 |
| Active myeloma | 56 (77.8) | 24 (96.0) | .04 |
| Platelet count, ×103 platelets/mm3 | 95.5 (13–345) | 39 (7–258) | .005 |
| Serum bilirubin level, mg/dL | 0.6 (0.3–1.6) | 0.8 (0.3–2.2) | .01 |
| Iron overload | 32/68 (47.1) | 16 (72.7) | .04 |
| After diagnosis of aspergillosis | |||
| Normalization of s-GMIc | 34 (47.2) | 3 (12.0) | .002 |
| Neutrophil recoveryd | 71 (98.6) | 16 (64.0) | <.001 |
| Response based on the EORTC/MSG definitionse | n = 59 | n = 34 | |
| At baseline | |||
| Body mass index | 26.24 (16.37–43.37) | 23.25 (16.20–42.48) | .03 |
| Active myeloma | 44 (76.4) | 33 (97.1) | .006 |
| Platelet count, ×103 platelets/mm3 | 102 (13–345) | 44,5 (7–258) | .003 |
| Serum bilirubin level, mg/dL | 0.6 (0.3–1.0) | 0.7 (0.3–2.2) | .007 |
| Creatinine clearance rate, mL/min | 62 (2–171) | 51 (7–86) | .004 |
| After diagnosis of aspergillosis | |||
| ANC, neutrophils/mm3f | 50 (0–10,900) | 10 (0–5000) | .01 |
| Normalization of s-GMIc | 29 (49.2) | 7 (20.6) | .006 |
| Neutrophil recoveryd | 59 (100) | 24 (70.6) | <.001 |
| Response based on 6-week survival | n = 67 | n = 31 | |
| At baseline | |||
| Body mass index | 26.05 (16.37–43.37) | 23.20 (16.20–42.48) | .04 |
| Active myeloma | 50 (74.6) | 31 (100) | .002 |
| Previous ASCTs, No. | 1 (0–3) | 2 (0–4) | .02 |
| Receipt of other immunosuppressive agents | 7 (10.4) | 10 (32.3) | .008 |
| WBC count, cells/mm3 | 3840 (20–26,610) | 2510 (10–18,100) | .03 |
| Platelet count, ×103 platelets/mm3 | 102 (13–345) | 42 (7–258) | .001 |
| Serum bilirubin level, mg/dL | 0.6 (0.3–2.2) | 0.7 (0.3–1.6) | .02 |
| Creatinine clearance rate, mL/min | 62 (2–171) | 50 (7–85) | .001 |
| After diagnosis of aspergillosis | |||
| ANC, neutrophils/mm3f | 80 (0–10,900) | 10 (0–5000) | .01 |
| Normalization of s-GMIc | 30 (45.5) | 7 (22.6) | .03 |
| Neutrophil recoveryd | 67 (100) | 21 (67.7) | <.001 |
Data are No. or proportion (%) of patients or median (range). Outcomes were examined according to the kinetics of serum Aspergillus galactomannan, the EORTC/MSG response definitions, and 6-week survival. Variables were defined at baseline and after the diagnosis of aspergillosis.
Abbreviations: ANC, absolute neutrophil count; ASCT, autologous stem cell transplantation; EORTC/MSG, European Organization for Research and Treatment of Cancer/Mycosis Study Group; IA, invasive aspergillosis; s-GMI, serum Aspergillus galactomannan index; WBC, white blood cell.
a Favorable and unfavorable response outcomes are success and failure, respectively, for s-GMI kinetics and EORTC/MSG definitions; favorable and unfavorable outcomes are survived and died, respectively, for 6-week survival.
b Response by s-GMI kinetics was not assessed in 1 patient, who was lost to follow-up.
c Within 7 days after the first positive s-GMI.
d Defined as an ANC of ≥500 neutrophils/mm3 on 3 consecutive days.
e Response by EORTC/MSG was not assessed in 5 patients, who were not available for response assessment at 6 weeks.
f At diagnosis of IA.
Figure 1.
Probability of aspergillosis response by the kinetics of serum Aspergillus galactomannan in patients with multiple myeloma according to their baseline platelet counts. Abbreviations: IA, invasive aspergillosis; s-GMI, serum Aspergillus galactomannan index.
Normalization of s-GMI within 7 days and neutrophil recovery were the only 2 nonbaseline variables that predicted good outcome by univariate analysis (Table 2) and by multivariate analyses (normalization of s-GMI: OR, 5.356 [95% CI, 1.316–21.901; P = .02]; neutrophil recovery: OR, 32.734 [95% CI, 3.630–295.164; P = .002] ) (Table 3).
Table 3.
Multivariate Analysis of Outcome Predictors in 98 Patients With Multiple Myeloma Who Developed Neutropenia-Related Aspergillosis
| Variable | Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Response based on s-GMI kinetics | |||
| Baseline | |||
| Platelet count (per 1000 platelets/mm3) | 1.009 | 1.001–1.017 | .03 |
| After diagnosis of aspergillosis | |||
| Neutrophil recoverya | 32.734 | 3.630–295.164 | .002 |
| s-GMI normalization ≤7 days from diagnosis of IA | 5.356 | 1.316–21.901 | .02 |
| Response based on the EORTC/MSG definitions | |||
| Baseline | |||
| Platelet count (per 1000 platelets/mm3) | 1.009 | 1.002–1.017 | .01 |
| Creatinine clearance rate (per mL/min) | 1.024 | 1.006–1.042 | .009 |
| After diagnosis of aspergillosis | |||
| s-GMI normalization ≤7 days from diagnosis of IA | 2.900 | 1.009–8.333 | .048 |
| Response based on 6-week survival | |||
| Baseline | |||
| Creatinine clearance rate (per mL/min) | 1.031 | 1.010–1.052 | .003 |
| After diagnosis of aspergillosis | NS | ||
Outcomes were examined according to the kinetics of serum Aspergillus galactomannan, the EORTC/MSG response definitions, and 6-week survival. Variables were defined at baseline and after the diagnosis of aspergillosis.
Abbreviations: IA, invasive aspergillosis; EORTC/MSG, European Organization for Research and Treatment of Cancer/Mycosis Study Group; NS, not significant; s-GMI, serum Aspergillus galactomannan index.
a Defined as an ANC of ≥500 neutrophils/mm3 on 3 consecutive days.
Factors Associated With Response, as Defined by EORTC/MSG Criteria
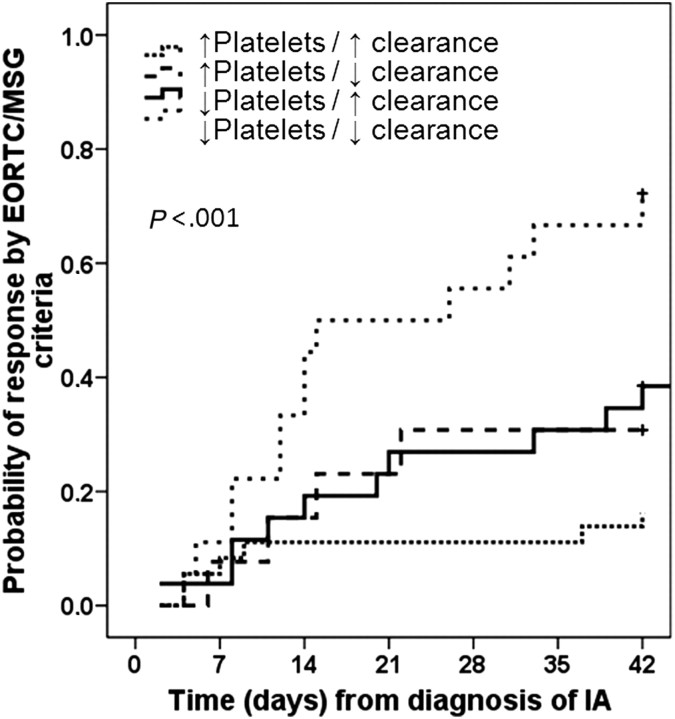
Response was observed in 59 of 93 cases (63.4%). Assessment was not possible in 5 patients (lost to follow-up before the 6-week time point). Baseline variables associated with response by univariate analysis were higher BMI, platelet count, and creatinine clearance rate; lower serum bilirubin level; and controlled myeloma (Table 2). Higher baseline platelet count (OR, 1.009; 95% CI, 1.002–1.017; P = .01) and higher baseline creatinine clearance rate (OR, 1.024; 95% CI, 1.006–1.042; P = .009) were independent predictors by multivariate analysis (Table 3). The cutoffs for platelet count and creatinine clearance rate were 65,000 platelets/mm3 (sensitivity 66%, specificity 71%) and 53 mL/min (sensitivity 76%, specificity 53%), respectively. The probability of response was 83% when both variables were above cutoffs, 69% when platelet levels were ≥65,000 platelets/mm3 but the creatinine clearance rate was <53 mL/min, and 61% when the creatinine clearance rate was ≥53 mL/min but platelet levels were <65,000 platelets/mm3. When both variables were below cutoffs, the probability of response was only 28% (P < .001) (Figure 2).
Figure 2.
Probability of aspergillosis response by the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORCT-MSG) criteria in patients with multiple myeloma according to their baseline platelet count and baseline creatinine clearance rate. Abbreviations: IA, invasive aspergillosis; ↑ platelet, baseline platelet count ≥65,000 platelets/mm3; ↓ platelet, baseline platelet count <65,000 platelets/mm3; ↑ clearance, baseline creatinine clearance rate ≥53 mL/min; ↓ clearance, baseline creatinine clearance rate <53 mL/min.
Higher ANC at diagnosis of IA, neutrophil recovery, and normalization of s-GMI within 7 days were the only nonbaseline variables significantly associated with response by univariate analysis (Table 2), while only normalization of s-GMI within 7 days (OR, 2.900; 95% CI, 1.009–8.333; P = .048) remained significant by multivariate analysis (Table 3).
Factors Associated With 6-Week Survival
Sixty-seven patients (68.4%) were alive at 6 weeks. Baseline predictors of survival by univariate analysis included higher BMI, WBC, platelet counts, and creatinine clearance rate; lower serum bilirubin level; lower number of prior ASCTs; nonreceipt of immunosuppressants other than corticosteroids; and controlled myeloma (Table 2). Only higher creatinine clearance rate (OR, 1.031; 95% CI, 1.010–1.052; P = .003) remained significant on multivariate analysis with a cutoff of 53 mL/min (sensitivity 78%, specificity 58%) (Table 3).
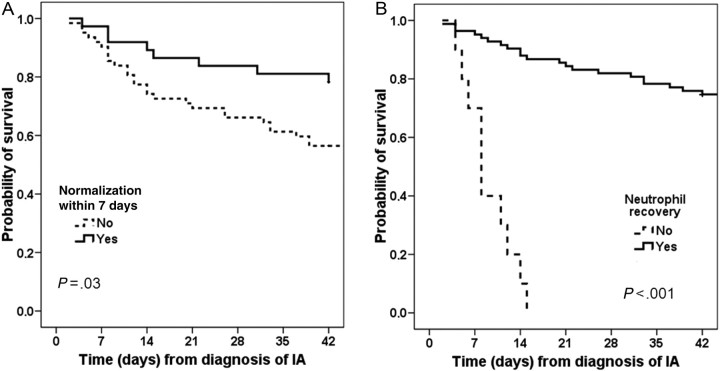
Higher ANC at diagnosis of IA, s-GMI normalization within 7 days, and neutrophil recovery were associated with 6-week survival by univariate analysis (Table 2) but not by multivariate analysis (Table 3). Figure 3 shows the Kaplan-Meier survival curves according to normalization of s-GMI ≤7 days after the first positive s-GMI and neutrophil recovery.
Figure 3.
Probability of 6-week survival according to (A) normalization of serum Aspergillus galactomannan index ≤7 days after the first positive serum Aspergillus galactomannan index or (B) neutrophil recovery. Abbreviations: IA, invasive aspergillosis.
DISCUSSION
To our knowledge, our study is the first to examine the significance of baseline prechemotherapy prognostic factors for neutropenia-related aspergillosis and to do so using platelet count and renal function, 2 simple, inexpensive to measure, widely available, and routinely collected data. Two nonbaseline variables, normalization of s-GMI ≤7 days after the first positive s-GMI and neutrophil recovery, were also identified as independent prognostic factors for IA.
That platelet count independently predicted the outcome of IA should not be surprising. Indeed, platelet counts are good indicators of bone marrow reserve and hence the likelihood of timely neutrophil recovery; platelet counts independently predict the ability to mobilize autologous peripheral blood stem cells [30] and hematological recovery following high-dose chemotherapy and ASCT [31] and are used to plan dosage schedules for antineoplastic therapies [32, 33]. The protective effect of higher platelet counts may also result from the ability of platelets to interfere with the virulence of Aspergillus species [34] and to act synergistically with antifungal agents against these pathogens [35].
Several chemotherapeutic agents, including intravenous MEL used in MEL-ASCT, are renally excreted, and MEL-treated patients with renal failure have higher risk for severe oral mucositis as a result of higher drug exposure [36]. Higher MEL exposure is also known to result in more severe and prolonged myelosuppression and immunosuppression [37]. Our finding that patients with renal dysfunction experienced worse outcomes is in keeping with these reports [36, 37] and with our prior analysis identifying elevated serum creatinine level as an independent risk factor for severe oral mucositis following MEL-ASCT [38]. The importance of creatinine clearance rate as a prognostic factor for IA in settings other than multiple myeloma deserves further investigation.
Fifteen studies examined the prognostic factors for IA among patients with hematologic cancers (Table 4). Unfortunately, most variables evaluated were not obtained at baseline and hence cannot be applied towards early intervention, therapy modification, or stratification in clinical trials [18].
Table 4.
Prognostic Factors for Invasive Aspergillosis Among Patients With Hematologic Cancers: Findings of Our Current Study in the Context of Prior Reports
| Author, Year | Episodes of IA, No. | Setting | Treatment | Factors Associated With Poor Outcome |
||
|---|---|---|---|---|---|---|
| At Baseline | Nonbaseline, Before IA | Nonbaseline, After IA | ||||
| Present study | 98 | Multiple myeloma with neutropenia | Chemotherapya and ASCT | Platelet count <65,000 platelets/mm3, creatinine clearance rate <53 mL/min | None | Positive s-GMI after 7 days from first positive s-GMI, persistent neutropenia |
| Acute leukemia receiving conventional chemotherapy | ||||||
| Ribrag, 1993 [2] | 21 | Acute leukemia | Chemotherapya | Leukemia not in remission | None | None |
| Pagano, 2010 [14] | 140 | AML | Chemotherapya | Leukemia not in remission | None | Persistent neutropenia |
| Allo-SCT | ||||||
| Ribaud, 1999 [3] | 27 | Hem Ca | Allo-SCT | None | Cumulative prednisone dose ≥7 mg/kg 1 week before IA | GVHD acute (grade ≥2) or extensive chronic |
| Cordonnier, 2006 [6] | 51 | Hem Ca | Allo-SCT (41), ASCT (10) | Age 12–35 years (vs <12, 36–47, and >47 years) | None | Diffuse (vs localized) lung infiltrates, pleural effusion, receipt of ≥2 mg/kg of corticosteroids at IA |
| Upton, 2007 [7] | 405 | Hem Ca | Allo-SCT (391), ASCT (24) | Pulmonary dysfunction, HLA-mismatch, NMA regimen | Total bilirubin level >6.5 mg/dL and creatinine level >2.5 mg/dL ≤7 days before IA diagnosis; 75% of patients had late IA | Late IA (>40 days after SCT), neutropenia at diagnosis of IA, receipt of ≥2 mg/kg of prednisone 1 week after IA diagnosis, disseminated IA |
| Mikulska, 2009 [17] | 45 | Hem Ca | Allo-SCT | Conditioning without ATG | None | Receipt of corticosteroids, low platelet count, low serum IgA level or creatinine level >1.5 mg/dL |
| Baddley, 2010 [12] | 642 | Various diseases | Allo-SCT (337), ASCT (78), SOT (227) | None | Neutropenia ≤30 days before IA diagnosis | Renal insufficiency, liver insufficiency or receipt of corticosteroids, late IA (>30 days after SCT), proven IA |
| Allo-SCT and conventional chemotherapy | ||||||
| Yeghen, 2000 [4] | 87 | Hem Ca | Allo-SCT (32), ASCT (4), chemotherapya (51) | Relapsed malignancy | None | Diffuse (vs localized) lung infiltrates |
| Subira, 2002 [5] | 41 | Hem Ca | Allo-SCT (12), ASCT (3), chemotherapya (24) | Allo-SCT | Persistent neutropenia | |
| Gallien, 2008 [8] | 34 | Hem Ca (26), HIV infection (7), diabetes mellitus (1) | Allo-SCT (18), ASCT (2), chemotherapya (5), others (9) | None | Neutropenia for ≥10 days ≤60 days before IA diagnosis | None |
| Nivoix, 2008 [9] | 289 | Mixed (192 Hem Ca), nonmalignant conditions (21), others | Allo-SCT (41), SOT (10), others | Allo-SCT, prior noninfectious respiratory disease | None | Progression of underlying cancer, ≥0.2 mg/kg of corticosteroids on day of IA diagnosis, disseminated IA, diffuse lung involvement, proven or probable IA (vs possible), creatinine clearance rate <60 mL/min, neutropenia (<500 neutrophils/mm3) ≤4 days after start of IA treatment |
| Parody, 2009 [10] | 130 | Hem Ca | Allo-SCT (49), chemotherapya (81) | Alternative donor for allo-SCT group | None | Disseminated IA, organ impairment, severe cytopenias, and receipt of ≥2 mg/kg/day of corticosteroids at IA diagnosis |
| Reuter, 2009 [11] | 212 | Hem Ca | Allo- and ASCT (49), chemotherapya (163) | None | None | Extrapulmonary disease, duration of neutropenia |
| Koo, 2010 [22] | 93 | Mixed population (58 Hem Ca), solid tumor (6), others | Allo-SCT (34), SOT (11), others | Receipt of 0.3 mg/kg/day prednisone for >3 weeks ≤90 days before IA | None | High s-GMI at IA diagnosis, low s-GMI decayb |
| Ramos, 2011 [16] | 44 | Hem Ca | Allo-SCT (140), ASCT (32), chemotherapya (277) | None | None | Persistent neutropenia, ICU admission, therapy with agents other than antimold azoles |
Variables were defined at baseline (before chemotherapy); nonbaseline, before IA (ie, after the start of chemotherapy but before IA diagnosis); and nonbaseline, after IA (ie, after IA diagnosis).
Abbreviations: Allo-SCT, allogeneic SCT; AML, acute myelogenous leukemia; ASCT, autologous SCT; ATG, antithymocyte globulins; CNS, central nervous system; GVHD, graft-versus-host disease; Hem Ca, hematologic cancer; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; IA, invasive aspergillosis; ICU, intensive care unit; IgA, immunoglobulin A; NMA, nonmyeloablative; SCT, stem cell transplantation; s-GMI, serum Aspergillus galactomannan index; SOT, solid organ transplantation.
a Myelosuppressive but not myeloablative.
b Decay calculated by dividing the difference between baseline s-GMI and s-GMI obtained around 1 week after the first positive s-GMI.
To better appreciate the reported prognostic factors for IA, we classified them as baseline (prechemotherapy) and nonbaseline factors, the latter further classified as nonbaseline, pre-IA (ie, after chemotherapy but before IA diagnosis) and nonbaseline, post-IA (after IA diagnosis).
Two studies of patients with acute leukemia treated with nonmyeloablative chemotherapy identified nonremission status as the only predictor of poor outcome [2, 14], while 3 of 5 studies of allogeneic SCT [3, 6, 7, 12, 17] identified pulmonary dysfunction, HLA-mismatched transplantation and nonmyeloablative conditioning [7], conditioning without antithymocyte globulin [17], and, surprisingly, younger age [6]. Five of 8 studies that evaluated patients with different underlying diseases and therapies, including SCT [4, 5, 8–11, 16], did not identify baseline prognostic factors, while 3 suggested that relapsed malignancy [4], prior noninfectious respiratory disease [9], and receipt of 0.3 mg/kg/day of prednisone for >3 weeks ≤90 days before IA [22] negatively affected outcome. The large variation in early outcome predictors among these studies is likely the result of large heterogeneity in underlying diseases, therapeutic modalities, diagnostic methods, and antifungal strategies [2–17].
Nonbaseline pre-IA prognostic factors were identified in 4 of 15 studies, including neutropenia ≤30 or ≤60 days before IA [8, 12], serum bilirubin level of >6.5 mg/dL and serum creatinine level of >2.5 mg/dL [7], and prednisone dose of ≥7 mg/kg ≤1 week before IA diagnosis [3]. However, the common occurrence of these events and their timing in relation to IA limit their clinical usefulness.
Several post-IA factors were associated with poor outcomes, including extensive pulmonary or disseminated IA [4, 6, 7, 9–11], corticosteroids [6, 7, 9, 10, 12, 17], neutropenia (at diagnosis of IA or duration of persistent neutropenia) [5, 7, 11, 16], thrombocytopenia [17], renal [9, 12, 17] and other organ dysfunction [10, 12, 16], and relapsed malignancy [4, 9]. Severe graft-versus-host disease negatively impacted outcome in one study [3]. Unfortunately, these post-IA variables have limited clinical application because they are considered “too little, too late” [18].
Only 3 studies evaluated the prognostic significance of s-GMI. Positive s-GMI at diagnosis did not impact outcome in one study [8] but was associated with lower 12-week survival by univariate but not multivariate analysis in another [9], while higher s-GMI at diagnosis of IA independently predicted poor 6- and 12-week survival in a third, in which the rate of s-GMI decay ≤7 days after the first positive s-GMI was significantly associated with outcome [22]. Although we could not confirm a prognostic significance of a high s-GMI at diagnosis, the superior outcomes we observed among our patients whose s-GMI normalized ≤7 days are in agreement with this report [22] and with others showing strong correlation between declining s-GMI values and positive outcomes [21–25].
Our results have important clinical and research implications. Stratification of patients into low-, intermediate-, or high-risk categories according to baseline platelet count and creatinine clearance rate may allow risk-adapted strategies to IA [18] with low risk are patients best managed with diagnostic-driven, preemptive s-GMI-guided therapy while mould-active prophylaxis is reserved for patients at high risk of failure to pre-emptive therapy. Managing patients at intermediate risk can be individualized depending on the rapid availability of s-GMI results and other local considerations.
We previously reported that response based on s-GMI kinetics correlated well with the EORTC/MSG response criteria but were more objective and allowed earlier response classification (3 weeks) than the EORTC/MSG 6-week time point [21]. We now show that clinicians can use s-GMI to assess response at an even earlier time point, ≤7 days after the first positive s-GMI.
Because most patients with myeloma receive high doses of corticosteroids, usually in fixed predefined doses, and because of the narrow age distribution of this population [39], we could have missed a potential association between outcomes and older age or cumulative corticosteroid doses [3, 7, 12, 22]. Our focus on a homogeneous population (same underlying disease, care at a single institution with standardized antineoplastic therapies, and infectious disease management) represents an important strength because it eliminates the confounding variables associated with heterogeneous populations with different diseases and therapies. Nonetheless, the role of baseline platelet count and creatinine clearance rate as prognostic factors should be confirmed in other patient populations.
We conclude that platelet count and renal function, 2 simple, inexpensive to measure, widely available, and routinely collected laboratory data, can predict the outcome of neutropenia-related IA, even before the start of chemotherapy, and that the evaluation of s-GMI ≤7 days after the first positive s-GMI provides clinicians with the opportunity to modify therapy early during the course of IA.
Notes
Financial support. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil (grant 1051-09-1 to S. A. N.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (grants 200425/2009-9 and 301025/2008-8 to M. N.), and National Cancer Institute, National Institutes of Health (grant 5P01CA055819-16, 2010 to E. A.)
Potential conflicts of interest. M. N. has consulted for and has been on the speakers’ bureau for Astellas, Merck, and Pfizer. E. A. has consulted for Astellas. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgements section.
References
- 1.Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91:1068–75. [PubMed] [Google Scholar]
- 2.Ribrag V, Dreyfus F, Venot A, Leblong V, Lanore JJ, Varet B. Prognostic factors of invasive pulmonary aspergillosis in leukemic patients. Leuk Lymphoma. 1993;10:317–21. doi: 10.3109/10428199309148554. [DOI] [PubMed] [Google Scholar]
- 3.Ribaud P, Chastang C, Latge JP, et al. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin Infect Dis. 1999;28:322–30. doi: 10.1086/515116. [DOI] [PubMed] [Google Scholar]
- 4.Yeghen T, Kibbler CC, Prentice HG, et al. Management of invasive pulmonary aspergillosis in hematology patients: a review of 87 consecutive cases at a single institution. Clin Infect Dis. 2000;31:859–68. doi: 10.1086/318133. [DOI] [PubMed] [Google Scholar]
- 5.Subira M, Martino R, Franquet T, et al. Invasive pulmonary aspergillosis in patients with hematologic malignancies: survival and prognostic factors. Haematologica. 2002;87:528–34. [PubMed] [Google Scholar]
- 6.Cordonnier C, Ribaud P, Herbrecht R, et al. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin Infect Dis. 2006;42:955–63. doi: 10.1086/500934. [DOI] [PubMed] [Google Scholar]
- 7.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–40. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 8.Gallien S, Fournier S, Porcher R, et al. Therapeutic outcome and prognostic factors of invasive aspergillosis in an infectious disease department: a review of 34 cases. Infection. 2008;36:533–8. doi: 10.1007/s15010-008-7375-x. [DOI] [PubMed] [Google Scholar]
- 9.Nivoix Y, Velten M, Letscher-Bru V, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47:1176–84. doi: 10.1086/592255. [DOI] [PubMed] [Google Scholar]
- 10.Parody R, Martino R, Sanchez F, Subira M, Hidalgo A, Sierra J. Predicting survival in adults with invasive aspergillosis during therapy for hematological malignancies or after hematopoietic stem cell transplantation: single-center analysis and validation of the Seattle, French, and Strasbourg prognostic indexes. Am J Hematol. 2009;84:571–8. doi: 10.1002/ajh.21488. [DOI] [PubMed] [Google Scholar]
- 11.Reuter S, Kern W, Zenz C, Kern P. Prognostic factors for invasive aspergillosis in patients with haematological malignancies. Scand J Infect Dis. 2009;41:483–90. doi: 10.1080/00365540902856529. [DOI] [PubMed] [Google Scholar]
- 12.Baddley JW, Andes DR, Marr KA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;50:1559–67. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12:220–9. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 14.Pagano L, Caira M, Candoni A, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010;95:644–50. doi: 10.3324/haematol.2009.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, Jiang EL, Yang DL, et al. Risk factors and prognosis of invasive fungal infections in allogeneic stem cell transplantation recipients: a single-institution experience. Transpl Infect Dis. 2010;12:316–21. doi: 10.1111/j.1399-3062.2010.00497.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramos ER, Jiang Y, Hachem R, Kassis C, Kontoyiannis DP, Raad I. Outcome analysis of invasive aspergillosis in hematologic malignancy and hematopoietic stem cell transplant patients: the role of novel antimold azoles. Oncologist. 2011;16:1049–60. doi: 10.1634/theoncologist.2010-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikulska M, Raiola AM, Bruno B, et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant. 2009;44:361–70. doi: 10.1038/bmt.2009.39. [DOI] [PubMed] [Google Scholar]
- 18.Anaissie EJ. Trial design for mold-active agents: time to break the mold—aspergillosis in neutropenic adults. Clin Infect Dis. 2007;44:1298–306. doi: 10.1086/514352. [DOI] [PubMed] [Google Scholar]
- 19.Marr KA, Schlamm H, Rottinghaus ST, et al. A randomised, double-blind study of combination antifungal therapy with voriconazole and anidulafungin versus voriconazole monotherapy for primary treatment of invasive aspergillosis. LB 2812. 2012 22nd European Congress of Clinical Microbiology and Infectious Diseases, London. [Google Scholar]
- 20.Maertens J, Van EJ, Verhaegen J, Verbeken E, Verschakelen J, Boogaerts M. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis. 2002;186:1297–306. doi: 10.1086/343804. [DOI] [PubMed] [Google Scholar]
- 21.Nouer SA, Nucci M, Kumar NS, Grazziutti M, Barlogie B, Anaissie E. Earlier response assessment in invasive aspergillosis based on the kinetics of serum Aspergillus galactomannan: proposal for a new definition. Clin Infect Dis. 2011;53:671–6. doi: 10.1093/cid/cir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo S, Bryar JM, Baden LR, Marty FM. Prognostic features of galactomannan antigenemia in galactomannan-positive invasive aspergillosis. J Clin Microbiol. 2010;48:1255–60. doi: 10.1128/JCM.02281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maertens J, Buve K, Theunissen K, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer. 2009;115:355–62. doi: 10.1002/cncr.24022. [DOI] [PubMed] [Google Scholar]
- 24.Miceli MH, Grazziutti ML, Woods G, et al. Strong correlation between serum Aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin Infect Dis. 2008;46:1412–22. doi: 10.1086/528714. [DOI] [PubMed] [Google Scholar]
- 25.Woods G, Miceli MH, Grazziutti ML, Zhao W, Barlogie B, Anaissie E. Serum Aspergillus galactomannan antigen values strongly correlate with outcome of invasive aspergillosis: a study of 56 patients with hematologic cancer. Cancer. 2007;110:830–4. doi: 10.1002/cncr.22863. [DOI] [PubMed] [Google Scholar]
- 26.Nucci M, Nouer SA, Grazziutti M, Kumar NS, Barlogie B, Anaissie E. Probable invasive aspergillosis without prespecified radiologic findings: proposal for inclusion of a new category of aspergillosis and implications for studying novel therapies. Clin Infect Dis. 2010;51:1273–80. doi: 10.1086/657065. [DOI] [PubMed] [Google Scholar]
- 27.de PB, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47:674–83. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miceli MH, Dong L, Grazziutti ML, et al. Iron overload is a major risk factor for severe infection after autologous stem cell transplantation: a study of 367 myeloma patients. Bone Marrow Transplant. 2006;37:857–64. doi: 10.1038/sj.bmt.1705340. [DOI] [PubMed] [Google Scholar]
- 30.Ozkurt ZN, Yegin ZA, Suyani E, et al. Factors affecting stem cell mobilization for autologous hematopoietic stem cell transplantation. J Clin Apher. 2010;25:280–6. doi: 10.1002/jca.20246. [DOI] [PubMed] [Google Scholar]
- 31.Nieboer P, de Vries EG, Vellenga E, et al. Factors influencing haematological recovery following high-dose chemotherapy and peripheral stem-cell transplantation for haematological malignancies; 1-year analysis. Eur J Cancer. 2004;40:1199–207. doi: 10.1016/j.ejca.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 32.Jacene HA, Filice R, Kasecamp W, Wahl RL. Comparison of 90Y-ibritumomab tiuxetan and 131I-tositumomab in clinical practice. J Nucl Med. 2007;48:1767–76. doi: 10.2967/jnumed.107.043489. [DOI] [PubMed] [Google Scholar]
- 33.Israel VK, Jiang C, Muggia FM, et al. Intraperitoneal 5-fluoro-2'-deoxyuridine (FUDR) and (S)-leucovorin for disease predominantly confined to the peritoneal cavity: a pharmacokinetic and toxicity study. Cancer Chemother Pharmacol. 1995;37:32–8. doi: 10.1007/BF00685626. [DOI] [PubMed] [Google Scholar]
- 34.Perkhofer S, Kehrel BE, Dierich MP, et al. Human platelets attenuate Aspergillus species via granule-dependent mechanisms. J Infect Dis. 2008;198:1243–6. doi: 10.1086/591458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkhofer S, Trappl K, Striessnig B, Nussbaumer W, Lass-Florl C. Platelets enhance activity of antimycotic substances against non-Aspergillus fumigatus Aspergillus species in vitro. Med Mycol. 2011;49:157–66. doi: 10.3109/13693786.2010.510150. [DOI] [PubMed] [Google Scholar]
- 36.Kergueris MF, Milpied N, Moreau P, Harousseau JL, Larousse C. Pharmacokinetics of high-dose melphalan in adults: influence of renal function. Anticancer Res. 1994;14:2379–82. [PubMed] [Google Scholar]
- 37.Cornwell GG, III, Pajak TF, McIntyre OR, Kochwa S, Dosik H. Influence of renal failure on myelosuppressive effects of melphalan: Cancer and Leukemia Group B experience. Cancer Treat Rep. 1982;66:475–81. [PubMed] [Google Scholar]
- 38.Grazziutti ML, Dong L, Miceli MH, et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant. 2006;38:501–6. doi: 10.1038/sj.bmt.1705471. [DOI] [PubMed] [Google Scholar]
- 39.Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of multiple myeloma during the past 5 decades: stable incidence rates for all age groups in the population but rapidly changing age distribution in the clinic. Mayo Clin Proc. 2010;85:225–30. doi: 10.4065/mcp.2009.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]