Human immunodeficiency virus (HIV)–infected African Americans (AAs) may have a higher risk of cardiovascular complications. Our study suggests that vitamin D deficiency Is independently associated with silent coronary artery disease (CAD) in HIV-infected AAs without symptoms/clinical evidence of CAD. Further longitudinal studies are needed.
Abstract
Background. Growing evidence suggests that vitamin D deficiency Is associated with clinical coronary artery disease (CAD). The relationship between vitamin D deficiency and subclinical CAD in HIV-infected individuals is not well-characterized.
Methods. Computed tomographic (CT) coronary angiography was performed using contrast-enhanced 64-slice multidetector CT imaging, and vitamin D levels and the presence of traditional and novel risk factor for CAD were obtained in 674 HIV-infected African American (AA) participants aged 25–54 years in Baltimore, MD, without symptoms/clinical evidence of CAD.
Results. The prevalence of vitamin D deficiency (25-hydroxy vitamin D <10 ng/mL) was 20.0% (95% confidence interval [CI], 16.9–23.1). Significant (≥50%) coronary stenosis was present in 64 (9.5%) of 674 participants. Multiple logistic regression analysis revealed that male gender (adjusted odds ratio [OR], 2.19; 95% CI, 1.17–4.10), diastolic BP ≥85 mmHg (adjusted OR: 1.94, 95% CI: 1.02 –3.68), low-density lipoprotein cholesterol ≥100 mg/dL (adjusted OR, 1.95; 95% CI, 1.13–3.36), cocaine use for ≥15 years (adjusted OR, 1.77; 95% CI, 1.01–3.10), use of antiretroviral therapies for ≥6 months (adjusted OR, 2.26; 95% CI, 1.17–4.36), year of enrollment after 2005 (adjusted ORs for 2006–2007, 2008–2009, and 2010 were 0.32 [95% CI, 0.13–0.76], 0.26 [95% CI, 0.12–0.56], and 0.32 (95% CI, 0.15–0.65], respectively), and vitamin D deficiency (adjusted OR, 2.28; 95% CI, 1.23–4.21) were independently associated with significant coronary stenosis.
Conclusions. Both vitamin D deficiency and silent CAD are prevalent in HIV-infected AAs. In addition to management of traditional CAD risk factors and substance abuse, vitamin D deficiency should be evaluated in HIV-infected AAs. These data support the conduct of a prospective trial of vitamin D in this high-risk patient population.
INTRODUCTION
African Americans (AAs) with human immunodeficiency virus (HIV) infection are at increased risk for coronary artery disease (CAD). This may be related, in part, to the metabolic effects associated with the long-term use of antiretroviral therapies (ARTs), particularly as HIV infection is increasingly a chronic, manageable disease [1–8]. In addition, AAs have the highest overall CAD mortality rate of any ethnic group [9]. Although many traditional risk factors are more prevalent in AAs, traditional risk factors alone do not entirely explain their increased associated CAD risk.
Recent evidence suggests that vitamin D (VD) deficiency is associated with an increased risk for clinical and subclinical CAD [10–13] and it is well known that VD deficiency is highly prevalent in AAs [14]. We therefore investigated the prevalence of VD deficiency in HIV-infected AAs without known, or symptoms for, CAD, and whether VD deficiency was associated with the presence of significant coronary stenosis on computed tomographic (CT) coronary angiography. The objective of this study, therefore, was to test the hypothesis that VD deficiency is independently associated with increased prevalence of subclinical CAD in AAs with HIV infection, using baseline cross-sectional data from a cohort of AA participants in Baltimore, MD.
METHODS
Study Participants
Between August 2003 and June 2011, 674 HIV-infected AA participants from Baltimore, MD, were enrolled in an observational study investigating the effects of antiretroviral regimens on subclinical atherosclerosis. The goal of the overall study was to investigate the association of HIV infection, cocaine abuse, ART, and other factors which might explain the risk for subclinical atherosclerosis in AAs with HIV infection.
Inclusion criteria were ≥25 years of age, AA race, and HIV infection. Exclusion criteria were (1) any evidence of clinical CAD, (2) any symptoms believed to be related to CAD, (3) glomerular filtration rate <60 mL/min/1.73 m2, (4) known allergy to the contrast used for the CT, and (5) pregnancy.
A medical chart review was used to confirm information on medical history and medications that was provided by the subjects. Interviews regarding sociodemographics and drug-use behaviors were conducted; blood pressure (BP) measurement and contrast-enhanced coronary CT angiography (CTA) were performed; and lipid profiles, VD, and high-sensitivity C-reactive protein (hsCRP) levels obtained.
The Johns Hopkins Medicine Institutional Review Board approved the study protocol and consent form, and all study participants provided written informed consent. All procedures used in this study were in accordance with institutional guidelines.
MAIN PROCEDURES
Vitamin D Measurement
Sera were collected, centrifuged and stored at −70°C until analyzed. Serum 25-hydroxy VD was determined by a direct, competitive chemiluminescence immunoassay (DiaSorin, Stillwater, MN) [15]. The level of detection for 25-hydroxy VD was <4 ng/mL. This method accurately measures D2 and D3 and is reported as a total 25-hydroxy VD. The reference range is 32–100 ng/mL. This study identifies VD deficiency according to the Framingham Offspring Study as serum 25-hydroxy VD <10 ng/mL.
Coronary CT Angiography With a 64 Slice Siemens Multidetector CT Scanner
A noncontrast multidetector CT (MDCT) scan was performed on a Sensation 64 Cardiac Siemens Medical Solutions scanner (Erlangen, Germany) to determine the coronary artery calcium score with a sequential scan of 3-mm slices with prospective electrocardiogram (ECG) triggering, 30 × 0.6-mm detector collimation, and tube current 135 mAs at 120 kV. Subsequently, coronary CTA was performed on the same equipment using 80 mL iso-osmolar contrast agent (320 mg iodine/mL) injected at 4–5 mL/s. Imaging was performed with retrospective ECG gating, 32 × 0.6-mm detector collimation with flying focal spot to give effective detector collimation of 64 × 0.6 mm, 330 ms gantry rotation, 850 mAs, and 120 kV. Subsequently, 0.75-mm-thick axial slices were reconstructed at 0.4-mm intervals with B25 kernel using a half-scan reconstruction algorithm with resulting temporal resolution of 165–185 ms. Ten reconstructions were done through the cardiac cycle at 10% increments in the R-R interval. If needed, patients received metoprolol prior to the scan to achieve a heart rate <65/minute.
Regions of interest were placed over each of the coronary arteries with a threshold for pixels of greater than 130 Hounsfield units for determining calcified plaque. Coronary vessels were assessed for patency and stenoses using 3-D visualization tools after the axial images were reviewed for determination of anatomy, quality of the study, and appearance of the vessels.
One reviewer (E. K. F), blinded to the participants’ risk factor profiles, independently evaluated the contrast-enhanced MDCT scans by examining the axial slices, curved multiplanar reformations, and thin-slab maximum intensity projections. The coronary artery tree was segmented according to the modified American Heart Association classification, and the segments were investigated for plaque and luminal narrowing. The coronary arteries were divided into proximal, mid, and distal segments, with each segment investigated for luminal narrowing. Plaques were classified as calcified or noncalcified, and the degree of stenosis was classified as <50% or ≥50% diameter stenosis. Diameter stenosis ≥50% was defined as significant coronary stenosis.
Statistical Analysis
Statistical analysis was performed with SAS software (SAS 9.3, SAS Institute, Cary, NC). All continuous parameters were summarized by medians and interquartile ranges (IQRs), and all categorical parameters were summarized as proportions. To compare between-group differences in demographic and clinical characteristics, lipid profiles, drug-use behaviors, and other factors, the nonparametric Wilcoxon 2-sample test was used for continuous variables and the Fisher exact test was employed for categorical variables.
Univariate logistic regression models were first fitted to evaluate the crude association between the presence of significant coronary stenosis and each of the factors—including age, sex, total serum cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, serum triglycerides, VD, hsCRP, cigarette smoking, alcohol use, glucose level, BP, body mass index, CD4 lymphocyte cell count, HIV RNA quantification, ARTs, cocaine use, and Framingham risk score [16], individually. Duration of cocaine use was categorized as dichotomous (≥15 years vs otherwise). ARTs were categorized on the basis of exposure to 3 classes—nucleoside reverse-transcriptase inhibitors (NRTIs), nonnucleoside reverse-transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs)—and the duration of ART use. Duration of ART use was categorized as dichotomous (ART naive or use for <6 months; use for ≥6 month). Year of ART initiation was categorized as never initiated, before 1996, 1996–2003, 2004–2007, and 2008–2010. Year of enrollment was categorized as 2003–2005, 2006–2007, 2008–2009, and 2010. Those factors that were significant at the P < .15 level in the univariate models were put into the multiple logistic regression models to identify the ones independently associated with the presence of significant coronary stenosis. Those variables that ceased to make significant contributions to the models based on these 2 criteria were deleted in a stagewise manner and a new model was refitted. This process of eliminating, refitting, and verifying continued until all of the variables included were statistically significant, yielding a final model [17]. The P values reported are 2-sided. A P value <.05 indicated statistical significance.
RESULTS
General Characteristics
The general and clinical characteristics of the study participants by the presence of significant coronary stenosis are presented in Table 1. Of the 674 participants in this study, 427 (63.4%) were males. The median age (with IQR) was 46 (41–51) years.
Table 1.
Characteristics of Study Participants by the Presence of Significant Coronary Stenosisa
| Total | Significant Coronary Stenosis |
|||
|---|---|---|---|---|
| Characteristic | (N = 674) | No (N = 610) | Yes (N = 64) | P value |
| Age (year) | 46 (41–51) | 46 (40–51) | 49 (46–54) | <.0001 |
| Male (%) | 63.4 | 62.0 | 76.6 | .02 |
| Family history of CAD (%) | 23.6 | 22.8 | 31.3 | .13 |
| Cocaine use (%) | 47.8 | 46.6 | 59.4 | .05 |
| Cocaine use >15 years | 38.1 | 36.9 | 50.0 | .04 |
| Cigarette smoking (%) | 83.4 | 82.6 | 90.6 | .10 |
| Alcohol use (%) | 86.4 | 86.2 | 87.5 | .78 |
| hsCRP ≥2 mg/dL (%) | 47.4 | 47.1 | 50.0 | .66 |
| hsCRP (mg/dL) | 1.8 (0.6–4.8) | 1.8 (0.6–4.8) | 2.1 (0.6–5.0) | .55 |
| Serum 25-hydroxy VD (ng/mL) | 17 (11–26) | 17.5 (11–26) | 14.5 (8–24) | .06 |
| VD deficiency (%) | 19.9 | 18.7 | 31.3 | .01 |
| Systolic BP (mm Hg) | 117 (107–128) | 116 (107–127) | 121 (111–129) | .08 |
| Diastolic BP (mm Hg) | 73 (67–82) | 73 (66–81) | 76 (69–85) | .08 |
| Glucose (mg/dL) | 85 (78–92) | 85 (78–92) | 86 (78–93) | .40 |
| BMI | 24.8 (22.0–29.1) | 24.8 (22.0–29.2) | 24.9 (21.7–27.7) | .26 |
| Baseline CD4 (cells/µL) | 329 (172–527) | 329 (172–527) | 319 (196–593) | .93 |
| Baseline viral load (copies/mL) | 11838 (643–64967) | 12000 (716–69131) | 8870 (494–32730) | .53 |
| Total cholesterol (mg/dL) | 163 (140–192) | 162 (139–191) | 170 (155–210) | .003 |
| LDL-C (mg/dL) | 86 (67–108) | 85 (66–106) | 98 (73–114) | .005 |
| HDL-C (mg/dL) | 51 (39–62) | 50(39–62) | 52 (36–63) | .69 |
| Triglycerides (mg/dL) | 105 (74–151) | 103 (72–148) | 132 (88–182) | .008 |
| Year of enrollment (%) | .0008 | |||
| 2003–2005 | 13.4 | 11.6 | 29.7 | |
| 2006–2007 | 16.5 | 16.6 | 15.6 | |
| 2008–2009 | 32.2 | 33.1 | 23.4 | |
| 2010–2011 | 38.0 | 38.7 | 31.3 | |
| Year of ART initiation (%) | .10 | |||
| Never initiated | 22.1 | 22.8 | 15.6 | |
| Before 1996 | 10.5 | 10.0 | 15.6 | |
| 1996–2003 | 30.9 | 30.5 | 34.3 | |
| 2004–2007 | 22.1 | 21.5 | 28.1 | |
| 2008–2010 | 14.4 | 15.2 | 6.3 | |
| NRTI use (month) | 17.9 (0.0–66.0) | 17.4 (0.0–65.0) | 25.0 (5.6–96.0) | .06 |
| NNRTI use (month) | 0 (0.0–12.0) | 0.0 (0.0–12.0) | 0.0 (0.0–16.5) | .79 |
| PI use (month) | 5.5 (0.0–48.0) | 5.0 (0.0–48.0) | 5.9 (0.0–48.5) | .70 |
| ART use (month) | 25.0 (0.9–82.0) | 24.0 (0.5–80.0) | 37.5 (11.0–98.0) | .07 |
| Framingham risk score | 4.0 (2.0–7.0) | 4.0 (2.0–7.0) | 7.0 (4.0–8.0) | <.0001 |
| Framingham score <10.0 (%) | 86.8 | 87.5 | 79.7 | .08 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index (kg/m2); BP, blood pressure; CAD, coronary artery disease; CD4, CD4 cell count; glucose, fasting glucose; hsCRP, high-sensitivity C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; VD, vitamin D; viral load, HIV RNA quantification.
a Median (interquartile range) for continuous variables, proportion (%) for categorical variables.
The median VD level was 17.0 ng/dL (IQR, 11.0–26.0). The prevalence rate of VD deficiency (serum 25-hydroxy VD <10 ng/mL) was 20.0% (95% CI, 16.9–23.1).
The median total cholesterol level was 163 mg/dL (IQR, 140–192). The median LDL cholesterol level was 86 mg/dL (IQR, 67–108). The median HDL cholesterol level was 51 mg/dL (IQR, 39–62). The median triglycerides level was 105 mg/dL (IQR, 74–151). The median systolic BP was 117 mm Hg (IQR, 107–128), and the median diastolic BP was 73 mm Hg (IQR, 67–82). Eighty-three percent were cigarette smokers. According to the Framingham risk score algorithm, 585 (86.8%) of the 674 participants (355 of the 427 men and 230 of the 247 women) had low risk of CAD, while 51 (79.7%) of the 64 participants (38 of the 49 men and 13 of the 15 women) with significant coronary stenosis had low risk of CAD [16].
There were significant (or borderline significant) differences between those with and without significant coronary artery stenoses not only in traditional risk factors, including age, gender, cigarette smoking, systolic and diastolic BP, total cholesterol, LDL cholesterol, triglycerides, and Framingham risk score, but also in VD deficiency, cocaine use, duration of exposure to NRTIs, and duration of exposure to ARTs. There were no significant differences in hsCRP, CD4 lymphocyte cell count, and plasma viral load levels between those with and those without significant coronary stenosis (Table 1).
Prevalence of Significant Coronary Stenosis
The overall prevalence of the presence of significant coronary stenosis was 9.5% (95% CI, 7.4–12.0). The prevalence of VD deficiency was 19.9% (95% CI, 16.9–23.1).
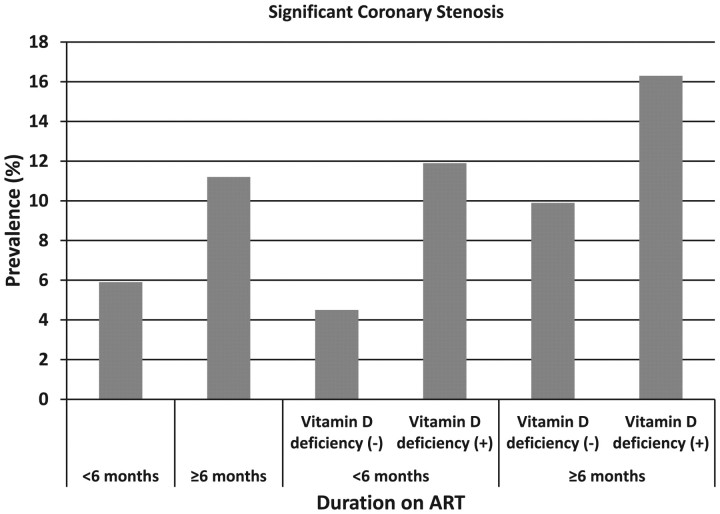
The prevalences of significant coronary stenosis were 5.9% and 11.2% in those who used ARTs for <6 months and who used ARTs for ≥6 months, respectively (Figure 1). The prevalences were 4.5%, 11.9%, 9.9%, and 16.3% in those who had used ART for <6 months and were not VD deficient, who had used ART for <6 months and were VD deficient, who had used ART for ≥6 months and were not VD deficient, and who had used ART for ≥6 months and were VD deficient, respectively (Figure 1).
Figure 1.
Prevalence of significant coronary stenosis by duration of antiretroviral therapy (ART) use and vitamin D deficiency status. The prevalences of significant coronary stenosis were 5.9% (13/219) and 11.2% (51/455) in those who used ARTs for <6 months and who used ARTs for ≥6 months, respectively. The prevalences were 4.5% (8/177), 11.9% (5/42), 9.9% (36/363), and 16.3% (15/92) in those who had used ART for <6 months and were not vitamin D deficient, who had used ART for <6 months and were vitamin D deficient, who had used ART for ≥6 months and were not vitamin D deficient, and who had used ART for ≥6 months and were vitamin D deficient, respectively. Abbreviation: ART, antiretroviral therapy.
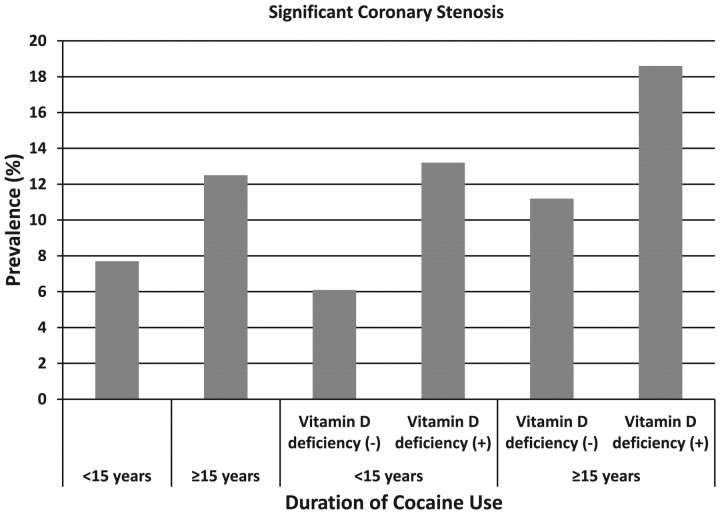
The prevalences of significant coronary stenosis were 7.7% and 12.5% in those who had never used cocaine or used cocaine for <15 years, and who used cocaine for ≥15 years, respectively (Figure 2). The prevalences were 6.1%, 13.2%, 11.2%, and 18.6% in those who had never used cocaine or used cocaine for <15 years and were not VD deficient, who had never used cocaine or used cocaine for <15 years and were VD deficient, who had used cocaine for ≥15 years and were not VD deficient, and who had used cocaine for ≥15 years and were VD deficient, respectively (Figure 2).
Figure 2.
Prevalence of significant coronary stenosis by duration of cocaine use and vitamin D deficiency status. The prevalences of significant coronary stenosis were 7.7% (32/417) and 12.5% (32/257) in those who had never used cocaine or used cocaine for <15 years and who used cocaine for ≥15 years, respectively. The prevalences were 6.1% (20/326), 13.2% (12/91), 11.2% (24/214), and 18.6% (8/43) in those who had never used cocaine or used cocaine for <15 years and were not vitamin D deficient, who had never used cocaine or used cocaine for <15 years and were vitamin D deficient, who had used cocaine for ≥15 years and were not vitamin D deficient, and who had used cocaine for ≥15 years and were vitamin D deficient, respectively.
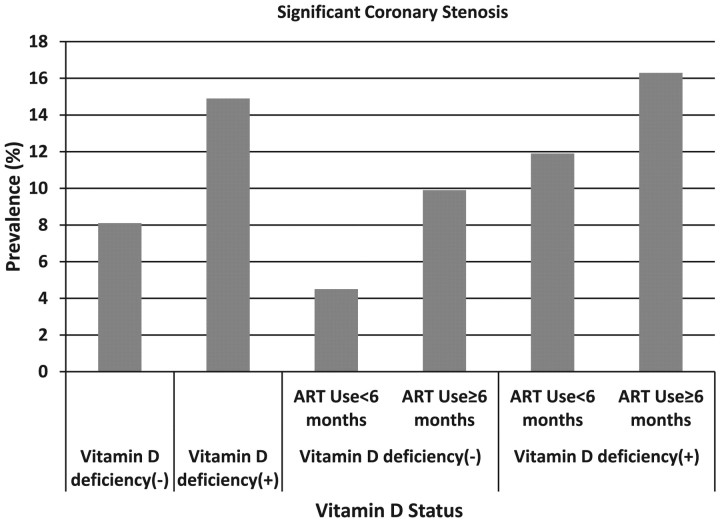
The prevalences were 8.1%, and 14.9% in those who had had not VD deficiency and who had VD deficiency, respectively (Figure 3). The prevalences were 4.5%, 9.9%, 11.9%, and 16.3% in those who had never used ART or used ART for <6 months and were not VD deficient, who had used ART for ≥6 months and were not VD deficient, who had never used ART or used ART for <6 months and were VD deficient, and who had used ART for ≥6 months and were VD deficient, respectively (Figure 3).
Figure 3.
Prevalence of significant coronary stenosis by vitamin D deficiency status and duration of antiretroviral therapy (ART) use. The prevalences were 8.1% (44/540) and 14.9% (20/134) in those who were not vitamin D deficient and who were vitamin D deficient, respectively. The prevalences were 4.5% (8/177), 9.9% (36/363), 11.9% (5/42), and 16.3% (15/92) for those who had never used ART or used ART for <6 months and were not vitamin D deficient, who had used ART for ≥6 months and were not vitamin D deficient, who had never used ART or used ART for <6 months and were vitamin D deficient, and who had used ART for ≥6 months and were vitamin D deficient, respectively. Abbreviation: ART, antiretroviral therapy.
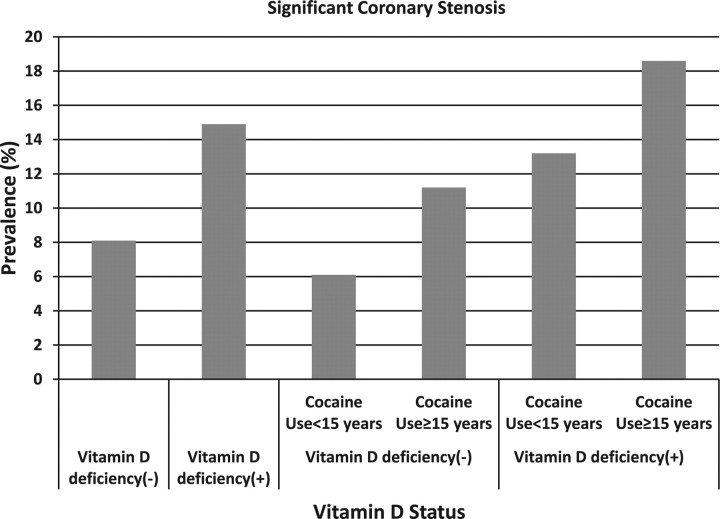
The prevalences were 6.1%, 11.2%, 13.2%, and 18.6% in those who had never used cocaine or used cocaine for <15 years and were not VD deficient, who had used cocaine for ≥15 years and were not VD deficient, who had never used cocaine or used cocaine for <15 years and were VD deficient, and who had used cocaine for ≥15 years and were VD deficient, respectively (Figure 4).
Figure 4.
Prevalence of significant coronary stenosis by vitamin D deficiency status and duration of cocaine use. The prevalences were 8.1% (44/540) and 14.9% (20/134) in those who did not have vitamin D deficiency and who had vitamin D deficiency, respectively. The prevalences were 6.1% (20/326), 11.2% (24/214), 13.2% (12/91), and 18.6% (8/43) in those who had never used cocaine or used cocaine for <15 years and were not vitamin D deficient, who had used cocaine for ≥15 years and were not vitamin D deficient, who had never used cocaine or used cocaine for <15 years and were vitamin D deficient, and who had used cocaine for ≥15 years and were vitamin D deficient, respectively.
Factors Associated With the Presence of Significant Coronary Stenosis
By univariate logistic regression analyses, traditional risk factors associated with the presence of significant coronary stenosis included age ≥50 years, male gender, diastolic BP ≥85 mm Hg, total cholesterol ≥160 mg/dL, serum LDL cholesterol concentration ≥100 mg/dL, triglycerides ≥130 mg/dL, and Framingham risk score ≥10%. Nontraditional risk factors associated with the presence of significant coronary stenosis included cocaine use for ≥15 years, exposure to any NRTIs for ≥6 months, exposure to any ARTs for ≥6 months, year of ART initiation, year of enrollment, and VD deficiency.
The final model indicated that a significant risk of the presence of significant stenosis was associated with previously described traditional risk factors, including male gender (adjusted OR, 2.19; 95% CI, 1.17–4.10), diastolic BP ≥85 mm Hg (adjusted OR, 1.94; 95% CI, 1.02 –3.68), and LDL cholesterol concentration ≥100 mg/dL (adjusted OR, 1.95; 95% CI, 1.13–3.36). The analysis also showed that cocaine use for ≥15 years (adjusted OR, 1.77; 95% CI, 1.01–3.10), use of ARTs for ≥6 months (adjusted OR, 2.26; 95% CI, 1.17–4.36), year of enrollment after 2005 (adjusted ORs for 2006–2007, 2008–2009, and 2010 were 0.32 [95% CI, 0.13–0.76], 0.26 [95% CI, 0.12–0.56], and 0.32, respectively [95% CI, 0.15–0.65]), and VD deficiency [adjusted OR, 2.28; 95% CI, 1.23–4.21] were independently associated with the presence of significant coronary stenosis (Table 2). Interactions between VD deficiency and other factors were not statistically significant in the multiple logistic regression models.
Table 2.
Demographic, Laboratory, and Clinical Factors in Relation to the Presence of Significant Coronary Stenosis, Logistic Regression Analysisa
| Subclinical CAD |
||
|---|---|---|
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) |
| Age | ||
| <50 years | 1.00 | … |
| ≥50 years | 1.93 (1.15–2.93) | … |
| Sex | ||
| Female | 1.00 | 1.00 |
| Male | 2.01 (1.10–3.66) | 2.19 (1.17–4.10) |
| Family history of CAD | ||
| No | 1.00 | … |
| Yes | 1.54 (0.88–2.70) | … |
| Cigarette smoking | ||
| Never | 1.00 | … |
| Ever | 2.03 (0.86–4.83) | … |
| Alcohol use | ||
| No | 1.00 | … |
| Yes | 1.12 (0.52–2.43) | … |
| Cocaine use | ||
| Never | 1.00 | … |
| Ever | 1.68 (0.99–2.83) | … |
| Duration of cocaine use | ||
| <15 years | 1.00 | 1.00 |
| ≥15 years | 1.71 (1.02–2.87) | 1.77 (1.01–3.10) |
| hsCRP >2 mg/dL | ||
| No | 1.00 | … |
| Yes | 1.12 (0.67–1.88) | … |
| Vitamin D deficiency | ||
| No | 1.00 | 1.00 |
| Yes | 1.98 (1.12–3.49) | 2.28 (1.23–4.21) |
| Systolic BP | ||
| <130 mm Hg | 1.00 | … |
| ≥130 mm Hg | 1.16 (0.62–2.16) | … |
| Diastolic BP (mm Hg) | ||
| <85 mm Hg | 1.00 | 1.00 |
| ≥85 mm Hg | 1.81 (0.99–3.32) | 1.94 (1.02–3.68) |
| Fasting glucose | ||
| <85 mg/dL | 1.00 | … |
| ≥85 mg/dL | 1.20 (0.72–2.01) | … |
| BMI | ||
| <24 kg/m2 | 1.00 | … |
| ≥24 kg/m2 | 0.80 (0.48–1.35) | … |
| Baseline CD4 count | ||
| <350 cells/µL | 1.00 | … |
| ≥350 cells/µL | 1.02 (0.39–2.70) | … |
| Baseline viral load | ||
| ≥400 copies/mL | 1.00 | … |
| <400 copies/mL | 1.26 (0.39–4.09) | … |
| Total cholesterol (mg/dL) | ||
| <160 mg/dL | 1.00 | … |
| ≥160 mg/dL | 1.61 (0.94–2.74) | … |
| LDL-C | ||
| <100 mg/dL | 1.00 | 1.00 |
| ≥100 mg/dL | 1.89 (1.12–3.18) | 1.95 (1.13–3.36) |
| HDL-C | ||
| <50 mg/dL | 1.00 | … |
| ≥50 mg/dL | 1.17 (0.69–1.96) | … |
| Triglycerides (mg/dL) | ||
| <130 mg/dL | 1.00 | … |
| ≥130 mg/dL | 2.02 (1.20–3.39) | … |
| Year of ART initiation | ||
| Never initiated | 1.00 | … |
| Before 1996 | 2.28 (0.90–5.76) | … |
| 1996–2003 | 1.64 (0.75–3.58) | … |
| 2004–2007 | 1.91 (0.85–4.29) | … |
| 2008–2010 | 0.60 (0.18–1.96) | … |
| Year of enrollment | ||
| 2003–2005 | 1.00 | 1.00 |
| 2006–2007 | 0.37 (0.16–0.84) | 0.32 (0.13–0.76) |
| 2008–2009 | 0.28 (0.13–0.58) | 0.26 (0.12–0.56) |
| 2010–2011 | 0.43 (0.16–0.63) | 0.32 (0.15–0.65) |
| NRTI use | ||
| <6 months | 1.00 | … |
| ≥6 months | 1.92 (1.06–3.46) | … |
| NNRTI use (month) | ||
| <6 months | 1.00 | … |
| 6 months | 1.08 (0.61–1.92) | … |
| PI use (month) | ||
| <6 months | 1.00 | … |
| ≥6 months | 1.02 (0.61–1.70) | … |
| ART use (month) | ||
| <6 months | 1.00 | 1.00 |
| ≥6 months | 1.79 (0.97–3.30) | 2.26 (1.17–4.36) |
| Framingham score ≥10.0 (%) | ||
| No | 1.00 | … |
| Yes | 1.79 (0.93–3.45) | … |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index (kg/m2); BP, blood pressure; CAD, coronary artery disease; CD4, CD4 cell count; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; OR, odds ratio; viral load, HIV RNA quantification; VD, vitamin D; VD deficiency, serum 25-hydroxy VD <10 ng/mL.
aCrude OR (95%CI) was based on univariate logistic analysis, adjusted OR (95%CI) was based on a final logistic model.
DISCUSSION
There are several main findings of our study. The overall prevalence rate of significant coronary stenosis in this population was 9.5% (95% CI, 7.4–12.0). This rate is high, considering that almost 90% of the population was at low risk based on the Framingham risk score. Even among those who had significant coronary stenosis, the median Framingham risk score was only 7.0% with an IQR of 4.0%–8.0%, suggesting nontraditional risk factor(s) may play important roles in this patient population. The study found that among cardiovascularly asymptomatic AAs with HIV infection, VD deficiency was independently associated with a greater than 2-fold increase in the risk of the presence of significant coronary stenosis.
This study also found that both cocaine use and ART use were associated with subclinical CAD. These results are consistent with our previous observations [18–29]. Since the enrollment period for this cross-sectional analysis was long (from 2003 through 2011), the ART medications changed considerably during this period. To minimize the impact of calendar time of enrollment on the relationship between ART use and the outcome, we adjusted for year of enrollment in the multiple logistic regression analysis. Thus, our finding suggesting that duration of ART use is associated with the presence of significant stenosis may not be influenced by changes in medications.
Humans obtain most VD from cutaneous synthesis following ultraviolet B light [30]. The prevalence of VD deficiency is much higher in AAs than other racial/ethnic groups possibly due to multiple factors, including increased pigmentation in darker skin, which limits cutaneous VD synthesis [12, 30, 31].
Although growing evidence suggests that VD deficiency may be associated with clinical CAD [12, 31, 32], the relationship between VD deficiency and subclinical CAD has not been extensively investigated yet. Our findings suggest that VD deficiency is independently associated with the presence of significant coronary stenosis. To our knowledge, this is the first study to examine whether VD deficiency is associated with silent CAD in cardiovascularly asymptomatic AA adults with HIV infection.
New evidence links VD deficiency and endothelial dysfunction, suggesting one responsible mechanism linking deficiency with CAD [33–35]. A recently published animal study reported that early-life VD deficiency is associated with endothelial vasodilator dysfunction as well as elevated BP [36]. A clinical study testing the hypothesis that vascular endothelial function, assessed by endothelium-dependent dilation, is related to serum VD status among middle-aged and older adults without clinical disease concluded that lower serum 25-hydroxy VD status is associated with vascular endothelial dysfunction among healthy middle-aged/older adults [37]. To explore the mechanism in terms of whether VD deficiency influences coronary endothelial dysfunction, the authors used an accurate noninvasive test for assessment of coronary endothelial function–positron emission tomography (PET) [38]. The maximum stress flow represents the full capacity of a given microcirculatory vascular bed and is determined by endothelial function [39, 40]. Seven HIV-infected participants in this study population (4 with VD deficiency and 3 without, aged 25–50 years, systolic BP <130 mm Hg, diastolic BP <85 mm Hg, normal lipid levels, and mean Framingham risk score = 3.8%) were included in this PET study. The results show that although the rest flow was similar in these 2 groups, the maximum stress flow in the VD deficiency group was significantly lower than that in the non-VD deficiency group (P = .034), suggesting that VD deficiency may be associated with coronary endothelial dysfunction (unpublished data).
A recent publication reported an inverse relationship between circulating 25-hydroxy VD and aorta and carotid calcified plaque in AAs, and that this relationship between 25-hydroxy VD and subclinical atherosclerosis in AAs was unique [41]. There are important differences between the 2 studies. Most importantly, the Freedman's study population all had diabetes and included patients with clinical CAD and other clinical manifestations of atherosclerotic disease. It is also likely that some of their participants may have taken VD supplements [42]. The calcium scores also indicate a markedly different patient population, and the data are derived from a study population in which 80% of study subjects had a mean coronary calcification score of 642. These important differences deserve further investigation, including the conduct of longitudinal studies to examine the relationship between circulating 25-hydroxy VD levels, subclinical atherosclerosis, and subsequent events in AAs without clinically overt disease [42].
This study has several limitations. First, because all of the study participants were AAs and were not a random sample of the people with HIV infection, the results should be interpreted with caution. Second, since the majority of participants were smokers, we could not perform stratified analyses to investigate the independent effect of cigarette smoking and the joint effect of cigarette smoking and cocaine use on the outcome. Third, although the overall investigation is a cohort study, the data presented here are cross-sectional only. Causality cannot be determined with a cross-sectional study. Fourth, due to the nature of cross-sectional design, some hidden confounding factors, such as socioeconomic status, were not adjusted for. Fifth, since the association between VD deficiency and significant stenosis may not be causal, it is possible that VD deficiency is a marker of low socioeconomic status and/or an adverse lifestyle that are responsible for the outcome. Furthermore, since this study was performed in AAs living in inner-city Baltimore, where cocaine use is often intertwined with other drug addictions, the effects of these drugs (or multiple-drug interactions) on CAD could not completely be controlled for by statistical analyses.
Despite its limitations, this study's findings of a high rate of silent CAD in cardiovascularly asymptomatic HIV-infected AAs have disturbing but important implications for the early prevention of CAD and the management of clinically silent CAD in this population. Since the prevalences of smoking and cocaine use are extremely high in this population, our results further underscore the need for aggressive interventions directed toward these important risk factors. As compared with identifying and successfully managing some traditional risk factors for CAD, including cigarette smoking cessation and maintaining optimal BP control, VD deficiency may be much easier to screen for and treat. Thus, the confirmation of such an association could have significant implications for improving patient care and lowering CAD in HIV-infected AAs.
Notes
Acknowledgments. We thank the study participants for their contributions.
Financial support. This work was supported by grants from the National Institute on Drug Abuse, National Institutes of Health (NIH R01-DA12777, DA25524, and DA15020).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–30. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 2.Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–73. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Vergis EN, Paterson DL, Wagener MM, Swindells S, Singh N. Dyslipidaemia in HIV-infected patients: association with adherence to potent antiretroviral therapy. Int J STD AIDS. 2001;12:463–8. doi: 10.1258/0956462011923507. [DOI] [PubMed] [Google Scholar]
- 4.Galli M, Ridolfo AL, Adorni F, et al. Body habitus changes and metabolic alterations in protease inhibitor-naive HIV-1-infected patients treated with two nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2002;29:21–31. doi: 10.1097/00126334-200201010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Friis-Møller N, Weber R, Reiss P, et al. Cardiovascular risk factors in HIV patients—association with antiretroviral therapy: results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 6.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 7.The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2004. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 8.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–10. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 9.Clark LT, Ferdinand KC, Flack JM, et al. Coronary heart disease in African Americans. Heart Dis. 2001;3:97–108. doi: 10.1097/00132580-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Camargo CA., Jr Vitamin D and cardiovascular disease time for large randomized trials. J Am Coll Cardiol. 2011;58:1442–4. doi: 10.1016/j.jacc.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–56. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai H, Fishman E, Gerstenblith G, et al. Vitamin D deficiency is associated with significant coronary stenoses in asymptomatic African American chronic cocaine users. Int J Cardiol. 2011 Feb 2 doi: 10.1016/j.ijcard.2011.01.032. [Epub ahead of print]. PMID:21295360 [PubMed—as supplied by publisher] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris SS. Does vitamin D deficiency contribute to increased rates of cardiovascular disease and type 2 diabetes in African Americans? Am J Clin Nutr. 2011;93(suppl):1175S–8S. doi: 10.3945/ajcn.110.003491. [DOI] [PubMed] [Google Scholar]
- 15.Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical validation of the 25(OH) vitamin D assay for the Liaison automated analyzer. Clin Biochem. 2004;37:867–74. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989. ISBN: 0-471-61553–6. [Google Scholar]
- 18.Lai S, Fishman EK, Lai H, et al. Long-term cocaine use and antiretroviral therapy are associated with silent coronary artery disease in African Americans with HIV infection who have no cardiovascular symptoms. Clin Infect Dis. 2008;46:600–10. doi: 10.1086/526782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng Q, Lima JAC, Lai H, et al. Coronary artery calcification, atherogenic lipid changes and increased erythrocyte volume in HIV-1 infected participants treated with protease inhibitors. American Heart J. 2002;144:642–8. doi: 10.1067/mhj.2002.125009. [DOI] [PubMed] [Google Scholar]
- 20.Meng Q, Lima JAC, Lai H, et al. Use of HIV protease inhibitors Is associated with left ventricular morphologic changes and diastolic dysfunction. J Acquir Immune Defic Syndr. 2002;30:306–10. doi: 10.1097/00126334-200207010-00006. [DOI] [PubMed] [Google Scholar]
- 21.Lai S, Lai H, Meng Q, et al. Effect of cocaine use on coronary calcification among black adults in Baltimore. Am J Cardiol. 2002;90:326–8. doi: 10.1016/s0002-9149(02)02475-x. [DOI] [PubMed] [Google Scholar]
- 22.Meng Q, Lima JAC, Lai H, et al. Elevated C- reactive protein levels are associated with endothelial dysfunction in chronic cocaine users. Int J Cardiol. 2003;88:191–8. doi: 10.1016/s0167-5273(02)00394-7. [DOI] [PubMed] [Google Scholar]
- 23.Lai S, Lai H, Celentano DD, et al. Factors associated with accelerated atherosclerosis in HIV-1–infected persons treated with protease inhibitors. AIDS Patient Care STDS. 2003;17:211–9. doi: 10.1089/108729103321655863. [DOI] [PubMed] [Google Scholar]
- 24.Tong W, Lima JAC, Lai H, Celentano DD, Dai S, Lai S. Relation of coronary artery calcium to left ventricular mass in African Americans. Am J Cardiol. 2004;93:490–2. doi: 10.1016/j.amjcard.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 25.Tong W, Lima JA, Meng Q, Flynn E, Lai S. Long-term cocaine use and cardiac diastolic dysfunction: an echocardiographic study. Int J Cardiol. 2004;97:25–8. doi: 10.1016/j.ijcard.2003.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Lai S, Lima JAC, Lai H, et al. HIV-1 infection, cocaine, and coronary calcification. Arch Intern Med. 2005;165:690–5. doi: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 27.Meng QY, DU JF, Lai H, Lai SH. Coronary artery calcification is associated with atherogenic lipid changes, cardiac dysfunction and morphologic abnormalities in HIV-1 infected black adults. Chin Med J (Engl) 2005;118:412–4. [PubMed] [Google Scholar]
- 28.Ren S, Tong W, Lai H, Osman NF, Pannu H, Lai S. Effect of long-term cocaine use on regional left ventricular function as determined by magnetic resonance imaging. Am J Cardiol. 2006;97:1085–8. doi: 10.1016/j.amjcard.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 29.Lai S, Bartlett J, Lai H, et al. Long-term combination antiretroviral therapy is associated with the risk of coronary plaques in African Americans with HIV infection. AIDS Patient Care STDs. 2009;23:815–24. doi: 10.1089/apc.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 31.Gelfand JM, Cree BA, McElroy J, et al. Vitamin D in African Americans with multiple sclerosis. Neurology. 2011;76:1824–30. doi: 10.1212/WNL.0b013e31821cccf5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxy vitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–60. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 33.Reddy Vanga S, Good M, Howard PA, Vacek JL. Role of vitamin D in cardiovascular health. Am J Cardiol. 2010;106:798–805. doi: 10.1016/j.amjcard.2010.04.042. Epub 2010 Aug 1. [DOI] [PubMed] [Google Scholar]
- 34.Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–30. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 35.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–5. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 36.Tare M, Emmett SJ, Coleman HA, et al. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol. 2011 Aug 1 doi: 10.1113/jphysiol.2011.214726. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxy vitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–9. doi: 10.1161/HYPERTENSIONAHA.110.160929. Epub 2010 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–9. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 39.Dayanikli F, Grambow D, Muzik O, Moscan L, Rubenfire M, Schwaiger M. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation. 1994;90:808–17. doi: 10.1161/01.cir.90.2.808. [DOI] [PubMed] [Google Scholar]
- 40.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using N-13-ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–42. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 41.Freedman BI, Wagenknecht LE, Hairston KG, et al. Vitamin D, adiposity, and calcified atherosclerotic plaque in African-Americans. J Clin Endocrinol Metab. 2010;95:1076–83. doi: 10.1210/jc.2009-1797. Epub 2010 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollis BW. Short-term and long-term consequences and concerns regarding valid assessment of vitamin D deficiency: comparison of recent food supplementation and clinical guidance reports. Curr Opin Clin Nutr Metab Care. 2011;14:598–604. doi: 10.1097/MCO.0b013e32834be798. [DOI] [PubMed] [Google Scholar]