This meta-analysis demonstrates significant increases in mortality and noncures with tigecycline versus comparator antibiotics. Tigecycline should not be used as the principal agent to treat serious infections for which other active antibiotics are available.
Abstract
Background. On the basis of noninferiority trials, tigecycline received Food and Drug Administration (FDA) approval in 2005. In 2010, the FDA warned in a safety communication that tigecycline was associated with an increased risk of death.
Methods. PubMed, EMBASE, Scopus, and ClinicalTrials.gov were searched using the terms “tigecycline” and “randomized controlled trial (RCT)” through April 2011. Excess deaths and noncure rates for both approved and nonapproved indications were examined using meta-analysis.
Results. Ten published and 3 unpublished studies met inclusion criteria (N = 7434). No significant heterogeneity was seen for mortality (I2 = 0%; P = .99) or noncure rates (I2 = 25%; P = .19). Across randomized controlled trials, tigecycline was associated with increased mortality (risk difference [RD], 0.7%; 95% confidence interval [CI], 0.1%–1.2%; P = .01) and noncure rates (RD, 2.9%; 95% CI, 0.6%–5.2%; P = .01). Effects were not isolated to type of infection or comparator antibiotic regimen, and the impact on survival remained significant when limited to trials of approved indications (I2 = 0%; RD, 0.6%; P = .04). A pooled analysis of the 5 trials completed by early 2005 before tigecycline was approved would have demonstrated a similar harmful effect of tigecycline on survival (I2 = 0%; RD, 0.7%; P = .06).
Conclusions. Pooling noninferiority studies to examine survival may help ensure the safety and efficacy of new antibiotics. The association of tigecycline with excess deaths and noncure includes indications for which it is approved and marketed. Tigecycline cannot be relied on in serious infections.
INTRODUCTION
The emergence of antibiotic-resistant organisms has made the development of new antimicrobial agents a top priority. Tigecycline was designed to meet this need [1] with in vitro activity against methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus species, and penicillin-resistant Streptococcus pneumoniae, but not against Proteus species and Pseudomonas aeruginosa [2]. On 28 January 2005, the Food and Drug Administration (FDA) granted it priority review status, 6 months later approving its use as monotherapy in complicated intra-abdominal and skin and skin structure infections. In 2009, tigecycline also received approval for community-acquired pneumonia. These approvals were based on noninferiority trials with modified intention-to-treat designs using microbiological and infection cure rates as primary end points [3–15]. A recent subgroup analysis also reported that tigecycline was noninferior in bloodstream infections [16–18].
Despite repeated findings of noninferiority, the FDA released a warning and updated the drug label in September 2010, citing an increased mortality risk associated with tigecycline, compared with active comparator antibiotics [19]. Pooling across 13 trials of approved and unapproved indications, tigecycline had an overall adjusted mortality rate of 4% compared with 3% for the comparator group, a relative mortality increase of 33%. The FDA safety communication emphasized that the “risk was most clearly seen in patients with hospital acquired pneumonia” for which tigecycline is not approved, but acknowledged that excess mortality was also seen in patients treated for approved indications. Although the cause of excess deaths could not be determined, it was postulated that most deaths were secondary to progression of infection [19]. Of note, even predating the FDA warning, the efficacy of tigecycline in severe infections had been repeatedly questioned [2, 18, 20, 21].
Contrary to the 2010 FDA evaluation, 2 meta-analyses published this year failed to show that tigecycline significantly increased mortality. One evaluated only a subset of available data (8 of 13 studies) [22], and 1, using all studies [23], did not include mortality data for 1 trial [7] and used underreported mortality results for another [8]. In contrast, a third recent meta-analysis supported the FDA evaluation but had methodological issues that may raise doubts about validity [24]. Although Tasina et al [23] and Yahav et al [24] reached different conclusions, both excluded unpublished but available data on osteomyelitis cure rates among patients with diabetic foot infections [8] and included a phase II study in which tigecycline served as the active comparator for an experimental quinolone [25]. Furthermore, Yahav et al [24] incorrectly extracted cure rates for 2 studies [9, 11]. Finally, FDA analyses in both 2005 and 2010 [19, 26] included 6 deaths (2 tigecycline and 4 control) that were, perhaps more properly, recorded as survivors in published reports [9, 11] and by Pfizer (personal written communication). Classifying these patients who died late after a prespecified reporting period ended as deaths made tigecycline appear to be slightly less harmful. Subtracting published data [10, 12] from the aggregated FDA analysis [26], Yahav et al [24] also mistakenly assigned these putative survivors, reclassified as deaths, to the wrong trial [6].
Thus, it is not entirely clear that tigecycline is inferior to comparator antibiotics and whether its indications should be further restricted. Several guidelines, including ones endorsed by the Infectious Diseases Society of America, have supported using tigecycline monotherapy in selected infections [27–30]. As noted, the FDA safety communication [19] and the 3 recent meta-analyses [22–24] are split evenly on whether tigecycline increased mortality because of the examination of nonidentical RCT results. Here, mortality and cure rates were reexamined using all available data from RCTs of tigecycline. Discrepancies among previous analyses and both published and unpublished data sources were adjudicated by direct written communication with Pfizer, the manufacturer of tigecycline.
METHODS
Literature Search
PubMed, EMBASE, Scopus, and Web of Science databases were searched through April 2011 to identify studies randomizing patients to treatment with tigecycline or active comparator antibiotics and recording cure rates and mortality. To find unpublished studies, Clinicaltrials.gov, Cochrane Central Register of Controlled Trials, Micromedex and Gateways to Clinical Trials, Cochrane Database of Abstracts of Reviews of Effects, and Current Controlled Trials were also searched. Studies were excluded if they were not in English, suspended, still enrolling patients, or presented pooled or subgroup data from already included studies.
Data Collection
Two authors independently reviewed included studies (PP and JS). A third author (CN) resolved discrepancies. Mortality rates and noncure rates were selected as end points of interest. Additional data from selected trials were collected (Figure 1 and Table 1).
Figure 1.
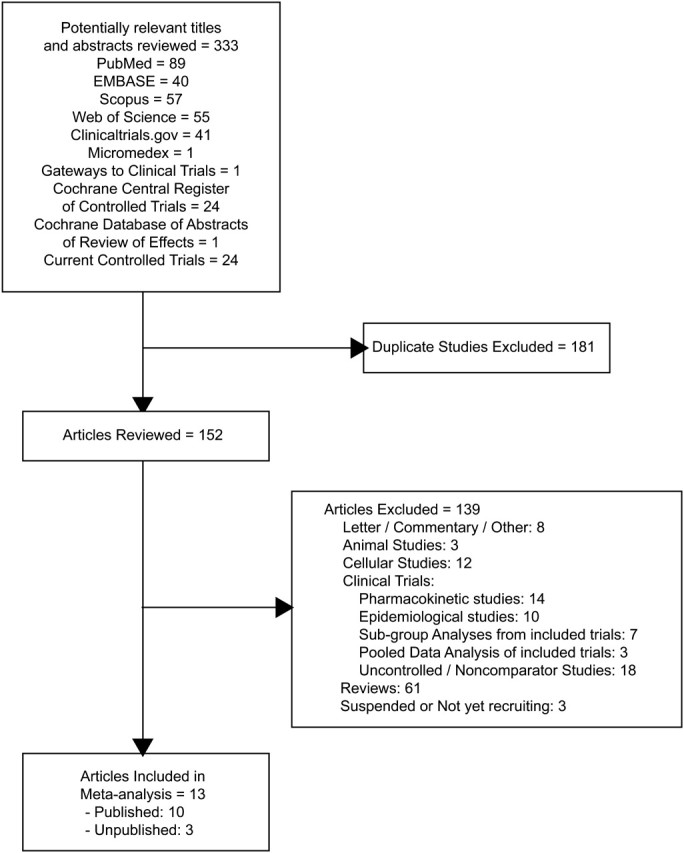
Study selection.
Table 1.
Study Characteristics
| Condition Treated | Author (Reference) | Year Published | Dates of Enrollment | Study Type | Tigecycline Regimen (Intravenous) | Comparator Antibiotic Regimen (Intravenous) | Study End Point Test of Cure in Daysa,b,c |
|---|---|---|---|---|---|---|---|
| Chen (5) | 2010 | 11/05–12/06d | Open label | Tigecycline 100 mg × 1, then 50 mg q12h | Imipenem/cilastatin 500 mg/500 mg q6h | 12–37 | |
| Oliva (12) | 2005 | 11/02–08/04 | Double blinded | Tigecycline 100 mg × 1, then 50 mg q12h | Imipenem/cilastatin 500 mg/500 mg q6h | 14–35 | |
| Complicated intra-abdominal infections | Fomin (10) | 2005 | 11/02–05/04 | Double blinded | Tigecycline 100 mg × 1, then 50 mg q12h | Imipenem/cilastatin 500 mg/500 mg q6h | 14–35 |
| NCT00230971 (6) | Unpublished | 11/05–07/08 | Open label | Tigecycline 100 mg × 1, then 50 mg q12h | Ceftriaxone 2 g daily and metronidazole 1–2 g daily in divided doses | 10–28 | |
| Towfigh (15) | 2010 | 09/05–02/08 | Open label | Tigecycline 100 mg × 1, then 50 mg q12h | Ceftriaxone 2 g daily and metronidazole 1–2 g daily in divided doses | 10–21 | |
| Diabetic foot infection | NCT00366249 (8) | Unpublished | 01/07–02/09 | Double blinded | Tigecycline 150 mg daily | Ertapenem 1 g daily±Vancomycin | >12 |
| Community acquired pneumonia | Tanaseanu (14) | 2009 | 01/04–01/05d | Double blinded | Tigecycline 100 mg × 1, then 50 mg q12h | Levofloxacin 500 mg daily or q12h | 10–21 |
| Bergallo (3) | 2009 | 06/03–07/05d | Double blinded | Tigecycline 100 mg × 1, then 50 mg q12he | Levofloxacin 500 mg dailye | 7–23 | |
| MRSA/VRE infection | Florescu (9) MRSA | 2008 | 11/03–08/05d | Double blinded | Tigecycline 100 mg × 1, then 50 mg q12h | Vancomycin 1 g q12h for MRSAf | 12–37 |
| Florescu (9) VRE | 2008 | 11/03–08/05d | Double blinded | Tigecycline 100 mg × 1, then 50 mg q12h | Linezolid 600 mg q12h for VREf | 12–37 | |
| Hospital-acquired pneumonia | Freire (11) | 2010 | 03/04–12/06d | Double blinded | Tigecycline 100 mg × 1, then 50 mg q12h±Ceftazidime 2 g q8h if P. aeruginosa infection suspected±aminoglycoside | Imipenem/cilastatin 500 mg–1 g q8h±Vancomycin 1 g q12h if MRSA infection suspected±aminoglycoside | 10–21 |
| Breedt (4) | 2005 | 11/02–12/03d | Double blinded | Tigecycline 100 mg × 1, then 50 mg q12h | Vancomycin 1 g q12h and aztreonam 2 g q12h | 12–92 | |
| Complicated skin and skin structure infections | Sacchidanand (13) | 2005 | No dates included in text | Double blinded | Tigecycline 100 mg × 1, then 50 mg q12h | Vancomycin 1 g q12h and aztreonam 2 g q12h | 12–92 |
| NCT00368537 (7) | Unpublished | 09/06–09/08d | Open label | Tigecycline 100 mg × 1, then 50 mg q12h | Ampicillin-sulbactam 1.5–3 g q6h or amoxicillin-clavulanate 1.2 g q6–8h w/vancomycin 1 g q12h or teicoplanin 400 mg × 1 then 200 mg daily added if concern for MRSA | 10–28 |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus.
a Range of days following completion of antibiotics at which point Test of Cure evaluation was carried out.
b In the clinically modified intention-to-treat population (c-mITT) noncures were evaluated at the test of cure visit (TOC) and included any death as well as any change in treatment.
c Mortality was reported in the modified intention-to-treat population (mITT) if it occurred from enrollment in the study until the time of the TOC visit.
d Study text did not include whether dates provided reflected duration of data collection or dates of patient enrollment.
e At hospital discharge, patients could switch to open-label oral Levofloxacin therapy to complete a regimen not to exceed 14 days.
f Patients with suspected polymicrobial infection containing gram-negative bacteria could receive nonstudy antibacterial agents or irrigants.
Statistical Analysis
Overall mortality rates from modified intention-to-treat (mITT) populations and noncure rates from clinical mITT (c-mITT) populations were used for our primary analysis (Table 2). Mortality and noncure data for 3 unpublished studies [6–8] were retrieved from Clinicaltrials.gov and Pfizer (personal written communication). For NCT00366249 [8], noncure data were pooled for patients with and without osteomyelitis. Two of the 13 studies did not report c-mITT data, and therefore, clinically evaluable (CE) [7, 15] data were used for c-mITT analysis. Separate secondary analyses were conducted using CE or microbiologically evaluable (ME) data only. The effect of tigecycline (versus comparator) treatment was assessed using the risk difference (RD) of death or noncure. Heterogeneity among studies was assessed using the Q statistic and I2 value [31]. Overall treatment effects were estimated using random-effects models (Comprehensive Meta-analysis, version 2; http://www.metaanalysis.com/), and potential confounders were investigated by metaregression [32]. Publication bias was evaluated using funnel plots and Egger's regression [33], which could have limited power [34]. Fisher exact test compared the proportions of patients with persistent bacteremia using SAS, version 9.2 (SAS Institute).
Table 2.
Populations Studied
| Group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ITTa |
mITTa |
Clinical Modified Intentiona to Treat (c-mITT) |
CEa |
ME |
|||||||
| Condition Treated | Author (Reference) | Tigecycline | Comparator | Tigecycline | Comparator | Tigecycline | Comparator | Tigecycline | Comparator | Tigecycline | Comparator |
| Complicated intra-abdominal infections | Chen (5) | 99 | 104 | 97 | 102 | 97 | 98 | 77 | 87 | 52 | 48 |
| Oliva (12) | 417 | 417 | 413 | 412 | 408 | 399 | 341 | 351 | 247 | 255 | |
| Fomin (10) | 409 | 415 | 404 | 413 | 393 | 401 | 344 | 346 | 265 | 258 | |
| NCT00230971 (6) | 235 | 238 | 232 | 235 | NR | NR | 198 | 189 | 119 | 108 | |
| Towfigh (15) | 237 | 236 | 236 | 231 | 228 | 220 | 189 | 187 | 138 | 137 | |
| Diabetic foot infection | NCT00366249 (8) | NR | NR | 553 | 508 | 529 | 499 | 446 | 429 | 316 | 317 |
| Community acquired pneumonia | Tanaseanu (14) | 220 | 214 | 216 | 212 | 203 | 200 | 144 | 136 | 91 | 86 |
| Bergallo (3) | 212 | 213 | 208 | 210 | 191 | 203 | 138 | 156 | 75 | 93 | |
| MRSA/VRE infection | Florescu (9) MRSA | 118 | 39 | 117 | 39 | 100 | 33 | 86 | 31 | 86 | 31 |
| Florescu (9) VRE | 11 | 4 | 11 | 4 | 8 | 3 | 3 | 3 | 3 | 3 | |
| Hospital-acquired pneumonia | Freire (11) | 474 | 471 | 467 | 467 | 440 | 429 | 268 | 243 | 194 | 189 |
| Complicated skin and skin structure infections | Breedt (4) | 275 | 271 | 274 | 269 | 261 | 259 | 223 | 213 | 164 | 148 |
| Sacchidanand (13) | 295 | 288 | 292 | 281 | 277 | 260 | 199 | 198 | 115 | 113 | |
| NCT00368537 (7) | NR | NR | 268b | 263b | NR | NR | 209b | 196b | NR | NR | |
Abbreviations: CE, clinically evaluable; c-mITT, clinically modified intention-to-treat; ITT, intention-to-treat; ME, microbiologically evaluable; mITT, modified intention-to-treat; MRSA, methicillin-resistant Staphylococcus aureus; NR, not reported; VRE, vancomycin-resistant Enterococcus.
a All trials used the same mITT protocol in which all patients meeting inclusion/exclusion criteria formed the ITT cohort, but only those receiving at least 1 dose of study drug were included in the mITT cohort. Patients in the mITT cohort meeting minimal criteria for the target infection became the c-mITT cohort. The c-mITT cohort was further narrowed to a microbiologically m-mITT that included c-mITT patients with a confirmed bacterial isolate at baseline, and a CE group that included c-mITT patients meeting criteria as both evaluable and having a test of cure visit. Patients in the CE group with a susceptible bacterial isolate at presentation comprised the ME group.
b Data obtained from Pfizer and Clinicaltrials.gov.
RESULTS
Study Characteristics
Thirteen RCTs met inclusion criteria: 10 published and 3 unpublished (Table 1). Patients were enrolled from November 2002 through February 2009. Nine studies were double-blind and 4 open-label (Table 1). Five trials studied intra-abdominal infections [5, 6, 10, 12, 15], 3 studied skin and skin structure infections [4, 7, 13], 2 studied community-acquired and 1 hospital-acquired pneumonia [3, 11, 14], 1 studied diabetic foot infection [8], and 1 studied methicillin-resistant S. aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections [9]. These 13 studies enrolled 172–1061 patients each, for a total of 7434 (mITT cohort).
Mortality and Noncure Rates
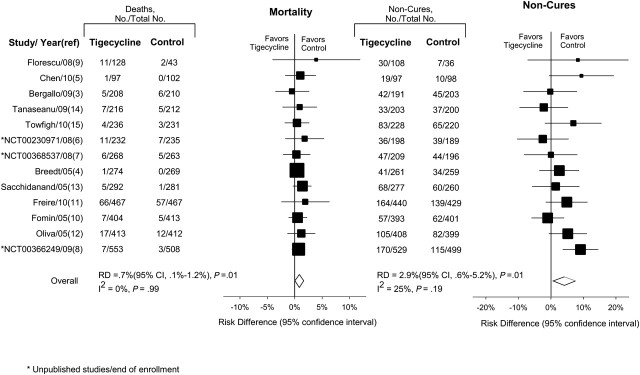
Combining the 13 studies, 148 deaths occurred among 3788 patients treated with tigecycline (4% mortality), and 106 deaths occurred among 3646 patients treated with comparator antibiotics (3% mortality). There was no significant heterogeneity among these studies for mortality (I2 = 0%; P = .99). Furthermore, no evidence of publication bias was detected by Eggers regression test (data not shown) [33]. For tigecycline treatment, overall mortality rate (mITT cohort) was increased, compared with comparator antibiotics (RD, 0.7%; 95% confidence interval [CI], .1–1.2; P = .01) (Figure 2).
Figure 2.
Mortality and noncure rates using RD. The size of data markers is proportional to the inverse variance of each point estimate. RR, another commonly used summary statistic, was examined to test the consistency of the above findings. Tigecycline versus comparator antibiotics was associated with a significant 30% increase in mortality rates (RR, 1.30; 95% CI, 1.02–1.65; P = .04) and a significant 12% increase in c-mITT noncure (RR, 1.12; 95% CI, 1.02–1.23; P = .02). Abbreviations: CI, confidence interval; RD, risk difference; RR, relative risk.
For noncure rates in the c-mITT population across studies (Table 2), both heterogeneity (I2 = 25%; P = .19) and the Eggers regression test [33] evaluating publication bias were nonsignificant (data not shown). Aggregated data from all available studies demonstrated significantly higher noncure rates with tigecycline, compared with comparator antibiotics (RD, 2.9%; 95% CI, .6–5.2; P = .01).
Subgroup Analysis
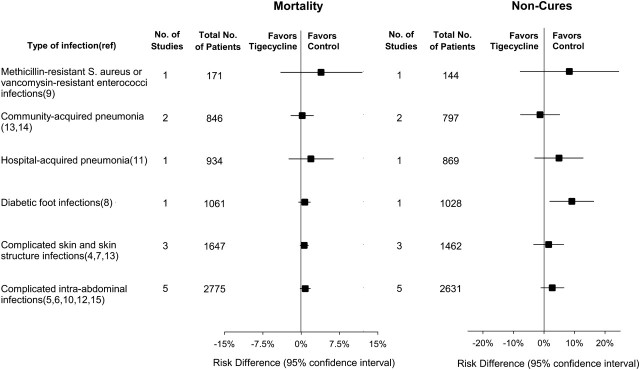
Figure 3 shows the mortality RD for different infection types. For each type, mortality was numerically increased with tigecycline treatment relative to comparator antibiotics, consistent with the overall mortality effect (Figure 3). There was no significant effect (P = .94) of infection type on the mortality increase associated with tigecycline.
Figure 3.
Subgroup analysis (types of infections). The actual increase in mortality and P value by type of infection using the RD are as follows: across the 5 intra-abdominal infection studies, RD was 0.8% (P = .13); across the 3 skin and skin structure studies, RD was 0.6% (P = .14); across the 2 community-acquired pneumonia studies, RD was 0.2% (P = .86); in the 1 diabetic foot infection study, RD was 0.7% (P = .25); in the 1 hospital-acquired pneumonia study, RD was 1.9% (P = .38); and in the 1 study of MSRA and VRE infections, RD was 3.9% (P = .33). Infection-specific results for noncure are as follows: across the 5 intra-abdominal infection studies, RD was 2.7% (P = .16); across the 3 skin and skin structure studies, RD was 1.5% (P = .54); across the 2 community-acquired pneumonia studies, RD was -1.3% (P = .70); in the 1 diabetic foot infection study, RD was 9.1% (P = .01); in the 1 hospital-acquired pneumonia study, RD was 4.9% (P = .23); and in the 1 study of MRSA and VRE infections, RD was 8.3% (P = .31). For comparison, a subgroup analysis examining another summary statistic, the RR of death and noncure for each of 5 indications showed the following: for complicated skin and skin structure infections, mortality RR was 1.72 (P = .28) and noncure RR was 1.02 (P = .76); for complicated intra-abdominal infections, mortality RR was 1.48 (P = .11) and noncure RR was 1.15 (P = .07); for diabetic foot infections, mortality RR was 2.14 (P = .27) and noncure RR was 1.39 (P = .001); for community-acquired pneumonia, mortality RR was 1.08 (P = .85) and noncure RR was 0.94 (P = .67); for hospital-acquired pneumonia, mortality RR was 1.15 (P = .13) and noncure RR was 1.15 (P = .15);and for drug-resistant pathogen infections (MRSA and VRE), mortality RR was 1.85 (P = .41) and noncure RR was 1.43 (P = .34).
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; RD, risk difference; VRE, vancomycin-resistant Enterococcus.
In each type of infection but 1 (community-acquired pneumonia), the c-mITT noncure rate was numerically increased with tigecycline treatment, compared with comparators, consistent with the overall increase in noncure rates (Figure 3). Type of infection had no significant effect (P = .36) on the higher noncure rates seen with tigecycline. Variously defined subpopulations were also evaluated across the 13 trials for noncure, including CE- and ME-only data. Both subgroups had increased noncure rates (RD, 2.1%; 95% CI, −.4 to 4.7; P = .10 and RD, 1.4%; 95% CI, −1.4 to 4.2; P = .32, respectively), consistent with the overall increase in noncures for tigecycline seen in the c-mITT analysis, but did not reach statistical significance, possibly because of smaller sample size (Table 2).
With use of metaregression, the number of patients in a trial did not significantly alter the magnitude of tigecycline-associated increases in mITT mortality rates or c-mITT noncure rates (P = .95 and P = .13, respectively; data not shown). Furthermore, there was also no significant effect of trial design on tigecycline-associated increased risks comparing double-blind with open-label studies for mortality (P = .96); RD, 0.7% (95% CI, .1–1.3) P = .02 vs RD, 0.7% [95% CI, −.6 to 2.0] P = .29, respectively, and for c-mITT noncures: (P = .97); RD, 2.9% (95% CI, .2–5.7) P = .04 vs RD, 2.8% (95% CI, −2.1 to 7.7) P = .26, respectively.
Approved and Nonapproved Indications
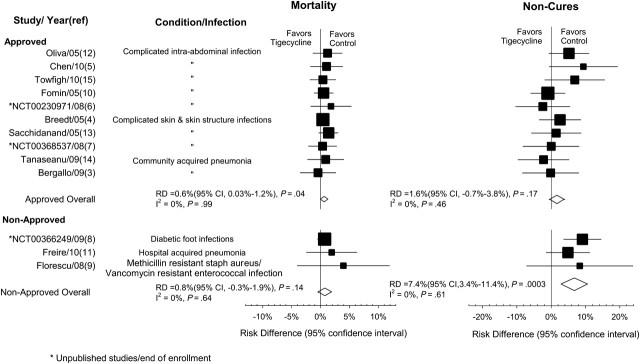
Mortality showed no significant heterogeneity among the 3 studies [8, 9, 11] for nonapproved (I2 = 0%; P = .64) or the 10 studies [3–7, 10, 12–15] for approved indications (I2 = 0%; P = .99). Excess mortality with tigecycline was similar (P = .78) comparing RCTs for non–FDA-approved indications (RD, 0.8%; 95% CI, −.3 to 1.9; P = .14) with RCTs for indications that eventually received FDA approval (RD, 0.6%; 95% CI, .03–1.2; P = .04) (Figure 4).
Figure 4.
Subgroup analysis (approved vs nonapproved indications). Abbreviations: CI, confidence interval; RD, risk difference.
For noncure rates, no significant heterogeneity was seen among trials for either nonapproved (I2 = 0%; P = .61) or approved (I2 = 0%; P = .46) indications. For the 3 nonapproved indications, the noncure RD was 7.4% (95% CI, 3.4–11.4; P = .0003). Among the 10 trials for approved indications, the RD was 1.6% (95% CI, −.7 to 3.8; P = .17). The noncure rate difference for FDA-approved indications was smaller than the overall estimate and much lower than in trials for nonapproved indications (P = .01), but still favored comparator regimens over tigecycline.
Cumulative Mortality and c-mITT Noncures
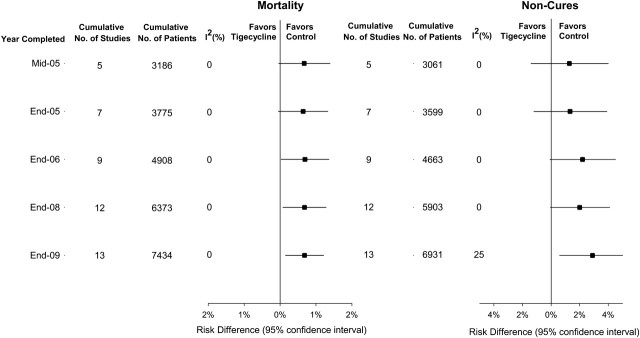
Figure 5 displays the cumulative mortality and c-mITT noncure RDs by the year studies completed enrollment. In June 2005, when tigecycline received regulatory approval, 5 trials had completed enrollment (I2 = 0%). The cumulative RD for death for these trials was 0.7% (P = .06). Nine trials were completed before 2007 (I2 = 0%), and the cumulative increased risk of death associated with tigecycline had become more apparent (RD, 0.7%; P = .04). Additional trial data had little effect on the cumulative RD for death. By 2009, after 12 trials (I2 = 0%), c-mITT noncure differences approached significance, favoring comparator antibiotics over tigecycline (RD, 2.0%; P = .055).
Figure 5.
Cumulative mortality and cumulative noncure rate analyses for the tigecycline noninferiority trials identified in this meta-analysis from mid-2005 (the date when tigecycline received FDA approval as monotherapy for complicated intra-abdominal and skin infections) through the end of 2009 (the date by which the last study in this meta-analysis was completed).
DISCUSSION
Thirteen RCTs, 10 published and 3 unpublished (9 double-blinded, 4 open labeled), met inclusion/exclusion criteria for this meta-analysis. Tigecycline versus active comparators demonstrated a significant increase in mortality and noncure rates. Overall, tigecycline was associated with a 0.7% absolute or 30% relative increase in mortality and a 2.9% absolute or 12% relative increase in noncure rates. In subgroup analysis, this effect was independent of infection type, trial design, and study size. Of importance, unfavorable outcomes were similarly associated with tigecycline therapy for both FDA-approved and nonapproved indications.
Although the underlying cause of excess death in trials of tigecycline is uncertain, the corresponding elevated risk of noncure suggests the possibility of inadequate antimicrobial activity. Tigecycline is primarily a bacteriostatic agent with nonlinear protein binding and a very large volume of distribution ranging from 5 to 10 L/kg [2]. The standard dosing regimen (100 mg load, followed by 50 mg every 12 hours) produces a maximum steady-state serum concentration of only 0.6 μg/mL [35]. Although tissue-to-serum area under the curve concentrations are routinely ≥2-fold [35–37], serum levels are quite low relative to commonly used sensitivity breakpoints (generally ≤0.5 µg/mL, but up to ≤2.0 µg/mL depending on bacteria type) [2]. In severe infections with high bacteremia risk, low serum levels combined with bacteriostatic rather than bactericidal activity may lead to an unfavorable microbiological response. This is supported by data from a subgroup analysis of bacteremic patients. Gardiner et al. [16] found that 9 of 91 tigecycline-treated bacteremic patients had persistent bacteremia defined as positive blood culture results for >24 hours after initiation of antibiotics. In comparison, only 1 of 79 comparator-treated patients had persistent bacteremia. This decrease in bacterial clearance with tigecycline, compared with comparators, is statistically significant (P = .02, Fischer exact test). Notwithstanding evidence of inadequate antimicrobial activity, direct drug toxicity or other mechanisms cannot be completely eliminated as possible contributors to the excess mortality observed with tigecycline.
Before its June 2005 approval, tigecycline passed multiple tests for noninferiority in well-designed clinical trials. How can a pooled analysis including these same studies, most suggesting equivalence, now provide clear evidence that tigecycline is inferior and increases risk of death? All tigecycline RCTs used a prespecified noninferiority margin of 15% for test of cure [3–15], but data from historical cohorts suggest that this criterion may not be ideal for all infections [38]. Relatively wide equivalence margins decrease sample size and, therefore, the time and cost of performing comparator trials [39, 40]. However, the larger studies required by a smaller noninferiority margin might well have revealed the harmful impact of tigecycline on survival sooner and possibly prevented its approval for indications for which it is ill suited.
The FDA Statistical Review and Evaluation of tigecycline in 2005 included 5 clinical trials (1 incomplete) encompassing 3 different indications [26]. A meta-analysis of this data would have demonstrated a mortality RD of 0.7% (95% CI, −.1 to 1.4; P = .07), very similar overall results to the current analysis. Note for comparison that our cumulative analysis for mid-2005 (Figure 5) excludes the ongoing trial [14] but includes another study [7] that had completed enrollment in January 2005. Among the 4 completed trials analyzed in the FDA Statistical Review [26], tigecycline was associated with 62% (126 of 202) of the infectious severe adverse events, whereas comparator antibiotics accounted for only 38% (76 of 202). Some individual infectious severe adverse events reached or approached significance (tigecycline vs comparator), as follows: sepsis (12 of 1383 vs 3 of 1375; P = .03), abnormal wound healing (23 of 817 vs 11 of 825; P = .04), pneumonia (9 of 817 vs 3 of 825; P = .09), and infection (24 of 1383 vs 14 of 1375; P = .14). Nonetheless, on the basis of the New Drug Application in 2005, the medical reviewer for the FDA could not conclusively link the excess deaths to a clear lack of treatment effect or other properties of tigecycline [41]. The reviewer noted further that “because these studies were not powered to detect a difference in death rates between the 2 treatment arms, it cannot be concluded from this information alone that a true difference does not exist.” By the end of 2006, the cumulative analysis in Figure 5 indicates a statistically significant increase in mortality associated with tigecycline. By 2010, a significant increase in both mortality and noncure rates became evident (Figure 4). As shown here, the increase in mortality associated with tigecycline is also significant when limited to indications for which the antibiotic has received regulatory approval.
An expedited review and approval process is essential to address critical therapeutic needs, including the development of new anti-infective agents to combat multidrug-resistant organisms. Furthermore, noninferiority trials are often the only ethical approach to evaluate new antimicrobial agents for serious conditions that have existing safe and effective therapeutic options. Although surrogate and composite primary end points have become standard to evaluate antibiotics, well-defined and reliable end points, such as mortality, are still closely examined for harmful trends. The FDA was aware of consistent increases in mortality and infectious complications across tigecycline studies in 2005, but was concerned about the hazards of aggregating data from different types of infection [26, 41]. Even given this decision, convening a public Anti-Infective Advisory Committee meeting in 2005 and closely reexamining mortality annually by meta-analysis might have provided additional opportunities to recognize the association of tigecycline with excess deaths. By 2010, however, mounting evidence favoring comparators over tigecycline led the FDA to issue a Safety Communication warning of excess deaths based on meta-analysis.
Tigecycline studies were conducted in 5 different infectious processes using a variety of active comparator antibiotics. It is known that meta-analyses of pooled results from trials evaluating different types of disease processes may provide misleading results despite statistical evidence of homogeneity [42]. Furthermore, subsequent large randomized trials may fail to confirm findings from properly performed meta-analyses [40, 42–44]. Similar to most meta-analyses, patient-level data were not available. Our cumulative analysis (Figure 5) could increase the false-positive rate [45]. Several studies allowed discretionary addition of specific antibiotics to tigecycline, but assessment of efficacy as monotherapy or combined with other agents was not possible. Nonetheless, all 13 RCTs evaluated here share identical intention-to-treat study designs and similar patient populations, and target serious bacterial infections. Of note, comparator regimens were not uniformly first-line antibiotics for the indication under study, which if anything, should have biased trials in favor of tigecycline. A forest plot of all evaluated studies (Figure 2) reveals that tigecycline was associated with a numerically higher mortality in 12 of 13 studies. Although each indication lacks adequate sample size to reach significance individually, the consistency of excess deaths associated with tigecycline is compelling. Moreover, mortality is a highly objective and troubling outcome that precludes second chances for adjusting therapy.
This meta-analysis of 10 published and 3 unpublished randomized controlled trials of tigecycline demonstrated a significant overall increase in mortality and noncure rates. A sensitivity analysis shows that these findings apply to indications for which tigecycline has received FDA approval. The apparent weakness of tigecycline as an antimicrobial agent may partly be explained by its bacteriostatic action and low serum levels, which result in higher noncure rates and delayed clearance of bacteremia. Initially meeting prespecified noninferiority criteria in individual clinical trials, but subsequently finding increased mortality and noncure rates overall, underscores the limitations and pitfalls of the noninferiority study design.
Ultimately, it may be difficult to justify a noninferiority margin of any size when evaluating therapies for conditions that can result in death or irreversible morbidity. In such situations, an analysis of mortality is always warranted and may support approval or heighten concern, as is the case for tigecycline. Furthermore, this experience with tigecycline illustrates that in vitro minimum inhibitory concentrations and animal studies [2] may not always predict equivalency in clinical trials. Finally, it is important to indicate that, after approval, tigecycline was used as an active comparator for an investigational agent [25]. Erroneously designating an agent as noninferior and, therefore, suitable as an active comparator could obscure harm from new antibiotics and lead to inappropriate approvals. Clearly, tigecycline should not be used when other effective antibiotic choices are available. Using tigecycline in life-threatening infections for which there are few or no alternative agents may be justifiable, but is only supported by anecdotal evidence.
Notes
Acknowledgments. We thank Judith Welsh, a librarian, for her help with the literature search; Kelly Byrne and Juli Maltagliati, for editing and formatting the manuscript; Dr. Dong Wang, Dr. Steve Solomon, and Jing Feng, for creating tables and figures; and Dr. Henry Masur and Dr. Harvey Klein, for reviewing the manuscript.
Financial support. This work was supported by intramural National Institutes of Health funds. The work by the authors was done as part of US government–funded research; however, the opinions expressed are not necessarily those of the National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zhanel GG, Homenuik K, Nichol K, et al. The glycylcyclines: a comparative review with the tetracyclines. Drugs. 2004;64:63–88. doi: 10.2165/00003495-200464010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Noskin GA. Tigecycline: a new glycylcycline for treatment of serious infections. Clin Infect Dis. 2005;41(suppl 5):S303–14. doi: 10.1086/431672. [DOI] [PubMed] [Google Scholar]
- 3.Bergallo C, Jasovich A, Teglia O, et al. Safety and efficacy of intravenous tigecycline in treatment of community-acquired pneumonia: results from a double-blind randomized phase 3 comparison study with levofloxacin. Diagn Microbiol Infect Dis. 2009;63:52–61. doi: 10.1016/j.diagmicrobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Breedt J, Teras J, Gardovskis J, et al. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother. 2005;49:4658–66. doi: 10.1128/AAC.49.11.4658-4666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Wu J, Zhang Y, et al. Efficacy and safety of tigecycline monotherapy vs. imipenem/cilastatin in Chinese patients with complicated intra-abdominal infections: a randomized controlled trial. BMC Infect Dis. 2010;10:217. doi: 10.1186/1471-2334-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinicaltrials.gov. Study NCT00230971. Study comparing tigecycline versus ceftriaxone sodium plus metronidazole in complicated intra-abdominal infection (cIAI) [cited 3 August 2011]. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00230971?term=00230971&rank=1 . [Google Scholar]
- 7.Clinicaltrials.gov. Study NCT00368537. Study comparing the safety and efficacy of tigecycline with ampicillin-sulbactam or amoxicillin-clavulanate to treat skin infections. [cited 3 August 2011]. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00368537?term=00368537&rank=1 . [Google Scholar]
- 8.Clinicaltrials.gov. Study NCT00366249. Study evaluating the safety and efficacy of a once-daily dose of tigecycline vs. ertapenem in diabetic foot infections (DFI) with a substudy in patients with diabetic foot infections complicated by osteomyelitis. [cited 3 August 2011]. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00366249?term=00366249&rank=1 . [Google Scholar]
- 9.Florescu I, Beuran M, Dimov R, et al. Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a Phase 3, multicentre, double-blind, randomized study. J Antimicrob Chemother. 2008;62(suppl 1):i17–28. doi: 10.1093/jac/dkn250. [DOI] [PubMed] [Google Scholar]
- 10.Fomin P, Beuran M, Gradauskas A, et al. Tigecycline is efficacious in the treatment of complicated intra-abdominal infections. Int J Surg. 2005;3:35–47. doi: 10.1016/j.ijsu.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Freire AT, Melnyk V, Kim MJ, et al. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis. 2010;68:140–51. doi: 10.1016/j.diagmicrobio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Oliva ME, Rekha A, Yellin A, et al. A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [Study ID Numbers: 3074A1-301-WW; ClinicalTrials.gov Identifier: NCT00081744] BMC Infect Dis. 2005;5:88. doi: 10.1186/1471-2334-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacchidanand S, Penn RL, Embil JM, et al. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: Results from a phase 3, randomized, double-blind trial. Int J Infect Dis. 2005;9:251–61. doi: 10.1016/j.ijid.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Tanaseanu C, Milutinovic S, Calistru PI, et al. Efficacy and safety of tigecycline versus levofloxacin for community-acquired pneumonia. BMC Pulm Med. 2009;9:44. doi: 10.1186/1471-2466-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towfigh S, Pasternak J, Poirier A, Leister H, Babinchak T. A multicentre, open-label, randomized comparative study of tigecycline versus ceftriaxone sodium plus metronidazole for the treatment of hospitalized subjects with complicated intra-abdominal infections. Clin Microbiol Infect. 2010;16:1274–81. doi: 10.1111/j.1469-0691.2010.03122.x. [DOI] [PubMed] [Google Scholar]
- 16.Gardiner D, Dukart G, Cooper A, Babinchak T. Safety and efficacy of intravenous tigecycline in subjects with secondary bacteremia: pooled results from 8 phase III clinical trials. Clin Infect Dis. 2010;50:229–38. doi: 10.1086/648720. [DOI] [PubMed] [Google Scholar]
- 17.Gardiner DF, Babinchak T, McGovern P. Correspondence: reply to Tarchini. Clin Infect Dis. 2010;51:868. [Google Scholar]
- 18.Tarchini G. Tigecycline and bacteremia–the dangers of post hoc analysis of pooled data. Clin Infect Dis. 2010;51:867–8. doi: 10.1086/656289. author reply 8. [DOI] [PubMed] [Google Scholar]
- 19.FDA Drug Safety Communication. Increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. 2010 September 1, [cited 3 August 2011]; Available at: http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm . [Google Scholar]
- 20.Falagas ME, Metaxas EI. Tigecycline for the treatment of patients with community-acquired pneumonia requiring hospitalization. Expert Rev Anti Infect Ther. 2009;7:913–23. doi: 10.1586/eri.09.73. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Cabrera E, Jimenez-Mejias ME, Gil Navarro MV, et al. Superinfection during treatment of nosocomial infections with tigecycline. Eur J Clin Microbiol Infect Dis. 2010;29:867–71. doi: 10.1007/s10096-010-0942-y. [DOI] [PubMed] [Google Scholar]
- 22.Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother. 2011;55:1162–72. doi: 10.1128/AAC.01402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis. 2011;11:834–44. doi: 10.1016/S1473-3099(11)70177-3. [DOI] [PubMed] [Google Scholar]
- 24.Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66:1963–71. doi: 10.1093/jac/dkr242. [DOI] [PubMed] [Google Scholar]
- 25.O'Riordan W, Surber J, Manos P, et al. Results of a phase 2 study comparing two doses of delafloxacin to tigecycline in adults with complicated skin and skin-structure infections. Clin Microbiol Infect. 2009;15(suppl 4):S519. doi: 10.1016/j.ijid.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services Food and Drug Administration. Statistical Review and Evaluation: Clinical Studies NDA/Serial Number: 021821 Tigecycline. 2005 [cited 3 August 2011]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021821Orig1s000StatR.pdf . Accessed 23 April 2012. [Google Scholar]
- 27.Brink AJ, Bizos D, Boffard KD, et al. Guideline: appropriate use of tigecycline. S Afr Med J. 2010;100(6 Pt 2):388–94. doi: 10.7196/samj.4109. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 29.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–64. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 30.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.Sutton A, Abrams K, Jones D, Sheldon T, Song F. Methods for meta-analysis in medical research. New York: John Wiley; 2000. [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 35.Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis. 2005;41(suppl 5):S333–40. doi: 10.1086/431674. [DOI] [PubMed] [Google Scholar]
- 36.Boucher HW. Challenges in anti-infective development in the era of bad bugs, no drugs: a regulatory perspective using the example of bloodstream infection as an indication. Clin Infect Dis. 2010;50(suppl 1):S4–9. doi: 10.1086/647937. [DOI] [PubMed] [Google Scholar]
- 37.Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006;58:1221–9. doi: 10.1093/jac/dkl403. [DOI] [PubMed] [Google Scholar]
- 38.Fleming TR, Powers JH. Issues in noninferiority trials: the evidence in community-acquired pneumonia. Clin Infect Dis. 2008;47(suppl 3):S108–20. doi: 10.1086/591390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garattini S, Bertele V. Non-inferiority trials are unethical because they disregard patients' interests. Lancet. 2007;370:1875–7. doi: 10.1016/S0140-6736(07)61604-3. [DOI] [PubMed] [Google Scholar]
- 40.Powers JH. Noninferiority and equivalence trials: deciphering ‘similarity’ of medical interventions. Stat Med. 2008;27:343–52. doi: 10.1002/sim.3138. [DOI] [PubMed] [Google Scholar]
- 41.US Department of Health and Human Services Food and Drug Administration. Medical Review and Evaluation: Clinical Studies NDA/Serial Number: 021821 Tigecycline. 2005 [cited 3 August 2011]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021821Orig1s000MedR.pdf . Accessed on 23 April 2012. [Google Scholar]
- 42.Bailar JC., 3rd The promise and problems of meta-analysis. N Engl J Med. 1997;337:559–61. doi: 10.1056/NEJM199708213370810. [DOI] [PubMed] [Google Scholar]
- 43.Powers JH. Increasing the efficiency of clinical trials of antimicrobials: the scientific basis of substantial evidence of effectiveness of drugs. Clin Infect Dis. 2007;45(Suppl 2):S153–62. doi: 10.1086/519253. [DOI] [PubMed] [Google Scholar]
- 44.Rovers MM, Glasziou P, Appelman CL, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet. 2006;368:1429–35. doi: 10.1016/S0140-6736(06)69606-2. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JP, Whitehead A, Simmonds M. Sequential methods for random-effects meta-analysis. Stat Med. 2011;30:903–21. doi: 10.1002/sim.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]