Abstract
High attack rates and the ability of Staphylococcus aureus to develop resistance to all antibiotics in medical practice heightens the urgency for vaccine development. S. aureus causes many disease syndromes, including invasive disease, pneumonia, and skin and soft tissue infections. It remains unclear whether a single vaccine could protect against all of these. Vaccine composition is also challenging. Active immunization with conjugated types 5 and 8 capsular polysaccharides, an iron scavenging protein, isdB, and passive immunization against clumping factor A and lipoteichoic acid have all proven unsuccessful in clinical trials. Many experts advocate an approach using multiple antigens and have suggested that the right combination of antigens has not yet been identified. Others advocate that a successful vaccine will require antigens that work by multiple immunologic mechanisms. Targeting staphylococcal protein A and stimulating the T-helper 17 lymphocyte pathway have each received recent attention as alternative approaches to vaccination in addition to the more traditional identification of opsonophagocytic antibodies. Many questions remain as to how to successfully formulate a successful vaccine and to whom it should be deployed.
PROBLEM OVERVIEW
Staphylococcus aureus is the most commonly isolated human bacterial pathogen and is an important cause of skin and soft tissue infections (SSTIs), pneumonia, and invasive infections [1]. An epidemic of S. aureus infections with onset in the 1990s has intensified interest regarding this important pathogen [2]. In the United States, this epidemic has been driven by the serial emergence of 2 new S. aureus genetic backgrounds, USA400 and USA300, circulating in the community, predominantly as methicillin-resistant clones, so-called community-associated methicillin-resistant S. aureus (CA-MRSA) [3].
Available data suggest that S. aureus infections now constitute a public health imperative. Klevens et al [4] estimated that invasive MRSA infections occurred at a rate of 31.8/100 000 per year and were responsible for the death of 18 650 patients (mortality rate: 6.3/100 000) in the United States in 2005. Liu et al [5] found that 1 in 316 people in San Francisco sought medical care for an MRSA infection in a recent year [5]. At Fort Benning, GA, it was recently estimated that the attack rate for medically attended MRSA infections was >35/1000 per year [6]. These data suggest an urgent need for improved strategies for control and prevention of S. aureus infections. They contrast with the much lower 2009 Centers for Disease Control and Prevention (CDC) case estimates of 0.28/100 000 with 0.04/100 000 mortality from meningococcal disease and 14.3/100 000 cases of invasive pneumococcal disease with a mortality rate of 1.6/100 000.
ANTIBIOTIC RESISTANCE: A TALE OF REMARKABLE VERSATILITY
Resistant strains of S. aureus have been identified for every antibiotic introduced into clinical practice [7].
Resistance to vancomycin [8, 9], linezolid [10, 11], daptomycin [12], and mupirocin [13] have all been identified as clinical concerns. This continuing saga of antimicrobial resistance in S. aureus and the slowing of the development of new antimicrobials is reminiscent of similar clinical concerns with Haemophilus influenzae type b, where resistance to ampicillin and chloramphenicol, and S. pneumoniae, where resistance to penicillin, sounded clinical alarms that infections caused by these important pathogens had become increasingly difficult to treat. In both instances, the deployment of effective vaccination muffled many increasing concerns.
CHANGING THE DEFINITION OF THE POPULATION AT RISK
If a vaccine against S. aureus were available, to whom would it be targeted? Prior to the late 1990s, MRSA infections occurred almost exclusively among patients with known exposure to the healthcare setting. However, the epidemic of CA-MRSA infections in the United States has required a redefinition of the risk factors for MRSA disease. The major change is that otherwise healthy individuals in the community are now at risk for MRSA infections [2]. Children, incarcerated populations, poor, homeless, young adults, military personnel in boot camps, day-care center contacts, household contacts, Pacific Islanders in Hawaii, Native Americans in Alaska, athletes (particularly those engaging in contact sports), patients with cystic fibrosis, and patients infected by human immunodeficiency virus (HIV) have all been affected, as have individuals who travel to or from the United States [2]. Many have been slow to grasp this profound change in MRSA epidemiology. Complexity has been added by several factors: HA-MRSA strains still circulate in the healthcare environment, although their transmission rate has decreased, likely due to improved infection control measures [14]. HA-MRSA isolates can sometimes be isolated from individuals in the community, especially adults; moreover, the new CA-MRSA strains have been detected in healthcare environments such as hospitals. They can also be transmitted among community members who have healthcare risk factors. Also, methicillin-susceptible isolates of similar genetic background to CA-MRSA isolates also circulate in the community. Importantly, neither the term MRSA or methicillin-susceptible S. aureus designate a specific “strain” of S. aureus, and their use sometimes oversimplifies the complexity of the problem. Driven primarily by a large increase in USA300 infections, S. aureus infections have increased dramatically in the last 10–15 years. Therefore, a successful vaccine, the formulation of which has eluded researchers for many years, must be able to prevent disease caused by strains from a broad range of genetic backgrounds that possess a range of virulence factors and manifest in multiple clinical presentations.
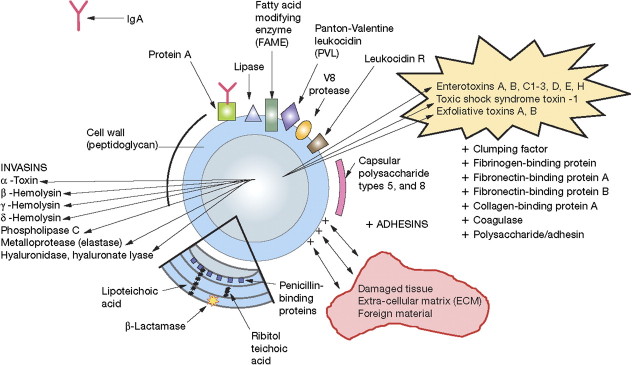
Figure 1.
Selected Staphylococcus aureus virulence factors. Abbreviation: Ig, immunoglobin.
THE TARGET POPULATION
The increase in the clinical burden of S. aureus disease associated with the recent epidemic and the occurrence of many infections among previously healthy individuals has prompted discussions about whether there is a need for a universal immunization strategy or a niche-based one. Until now, development of a vaccine has proceeded slowly, in part because its use might be limited to the subpopulation at highest risk for invasive disease. Thus, active and passive immunization trials to date have been targeted to prevent, for example, bacteremia among dialysis patients [15], infections among neonates, or postoperative mediastinitis among patients undergoing elective cardiac surgery and sternotomy.
Why then contemplate a universal vaccine strategy? The attack rate for invasive S. aureus disease is high enough to justify a universal approach. Both children and adults are at high risk [4]. If a single vaccine were effective against invasive disease, pneumonia, and SSTIs, the justification would be even greater. Such a vaccine could be conveniently integrated into the schedule for administration in the first year of life. Scheduled visits for immunizations during the first 6 months of life exist already into which a new vaccine could be incorporated. A goal would be to combine an effective S. aureus vaccine with other vaccine antigens such as a combination diphtheria/acellular pertussis/tetanus vaccine to make a new, combination vaccine. It is important to emphasize that, until the mid-1990s, identifying the subset of the population at risk for invasive S. aureus disease was relatively simple. However, the advent of the CA-MRSA epidemic has forced redefinition of the “at-risk” population. In advance of definitive definition of the “new” risk factors for CA-MRSA, one could argue that the entire population, given the correct exposure, should be considered at risk.
Other attempts to target subsets of the population at highest risk for disease have been unsuccessful. For example, hepatitis B vaccine became available in 1982 and initially was recommended only for certain high-risk subpopulations. However, this strategy failed to decrease the incidence of hepatitis B in the United States [16]. In contrast, a universal immunization strategy with 3 doses administered in early childhood has produced a marked decline in the incidence of hepatitis B in the United States [17, 18].
WHAT S. AUREUS INFECTIOUS SYNDROMES SHOULD A VACCINE PREVENT?
An important issue concerns the expectations of the vaccine, should one be identified. S. aureus is a species well adapted to its human host. Most often, it is a commensal bacterium that can asymptomatically colonize the nasopharynx, presumably by adhering to the nasal mucosa. The recent recognition that some S. aureus genetic backgrounds preferentially colonize the throat [19] (R. Daum et al, unpublished), rectum, or another site does not change the concept. Although it may be true that when bacteremia occurs, a colonizing, genetically identical strain can often be found in the nasopharynx [20], most colonized individuals do not become bacteremic. Furthermore, many individuals develop clinically apparent infection in the absence of detectable colonization.
Many patients with an SSTI do not seek medical attention. Despite this, SSTIs constitute the most frequent medically attended clinical illnesses, and constitute a major healthcare burden as many seek acute care in an emergency room or another facility. A few patients with an SSTI require hospitalization for major surgical drainage and/or intravenous antimicrobial therapy.
More serious invasive disease occurs in several settings. Otherwise healthy individuals in the community have sustained severe, sometimes overwhelming infection. A few patients have been described with overwhelming sepsis with an illness whose tempo resembles meningococcemia [21, 22, 23]. Others have had serious illness with a less fulminating course; examples include necrotizing pneumonia [24], osteomyelitis [25], and necrotizing fasciitis [26].
In addition to these disease syndromes occurring among otherwise healthy individuals, S. aureus causes serious infections among patients with underlying diseases such as diabetes mellitus and HIV. Additionally, it is well known that patients with indwelling intravenous access lines, or patients who receive hemodialysis, particularly when the integrity of the integument has been violated, are at high risk for S. aureus infections.
An ongoing discussion, then, concerns which clinical scenarios should be vaccine-preventable. Asymptomatic carriage, SSTI, pneumonia, or invasive disease are among the possibilities. Eradication of asymptomatic S. aureus carriage is probably not the appropriate primary goal for vaccine efficacy as many colonizing S. aureus genetic backgrounds are rarely isolated from medically attended disease. Prevention of SSTIs and pneumonia, on the other hand, would be desirable. Available evidence, however, from animal models suggests that the pathophysiology of these distinct clinical syndromes differs from each other and may require different immunomodulary strategies for prevention. Invasive disease associated with bloodstream infection is the obvious target for vaccine efficacy. However, even here, it must be borne in mind that a bloodstream infection complicating an indwelling venous catheter most likely has different immunopathophysiology than a bloodstream infection in an otherwise healthy patient.
VACCINE DEVELOPMENT EFFORTS TO DATE
How then to make the ideal S. aureus vaccine? It is increasingly clear that this will not be simple. There are several relevant considerations. It has been traditional to expect a vaccine to induce protective antibodies that will protect against infection caused by the target pathogen. One task, then, is to identify which is/are the protective antigens and which are the protective antibodies. S. aureus poses a challenge in this regard as many functions necessary for microbial pathogenicity, ranging from host cell adherence to iron scavenging (Figure), employ redundant microbial machinery; pathogenicity may not be interfered with if only 1 of several genes performing a similar function is targeted.
Cell Surface Polysaccharides as Vaccine Candidates
Prior to the advent of epidemic USA300 disease in the United States, the identification of a polysaccharide capsule on the surface of most clinical S. aureus isolates suggested that they might prove to be effective vaccine antigens, particularly if conjugated to a protein carrier. After all, this approach proved successful in the development of vaccines directed against the type b capsular polysaccharide of H. influenzae, multiple capsular polysaccharides of S. pneumoniae, and the capsular polysaccharides of serotypes A, C, W-135, and Y of Neisseria meningitidis. For S. aureus, 2 capsular types (5 and 8) comprised a majority of examined clinical isolate collections. Therefore, the polysaccharides from these 2 types were individually conjugated to a protein carrier, Pseudomonas exotoxoid A. Efforts to show protection in animal models were hampered by variable capsule expression during growth and variable strategies for experimental challenge. Nevertheless, data were sufficiently encouraging to prompt a clinical trial sponsored by NABI Biopharmaceuticals. The target population was dialysis patients in northern California, and the clinical endpoint chosen for efficacy analysis was bacteremia.
The vaccine was well tolerated. The efficacy estimates from this trial provided a confusing picture. No significant efficacy was observed 2–20 weeks after immunization. Data analyzed at the 30- and 40-week postimmunization time points revealed modest efficacy (63%; 95% confidence interval [CI], 14%–86%, and 57%; 95% CI, 10%–81%, respectively). However, efficacy dissipated at the 50-, 54-, or 91-week postimmunization time points. At these times, the vaccine efficacy estimates were 25%–26% and insignificantly different from zero. Nevertheless, the investigators interpreted these data to indicate that the vaccine had provided some protection for about 40 weeks after immunization [15].
The geometric mean serum anticapsular antibody concentration among the vaccinees was 80 μg/mL, a value vastly exceeding concentrations believed to be protective against H. influenzae type b and S. pneumoniae. Additionally, there were no discernible differences between the peak antibody levels in bacteremic and nonbacteremic vaccinees. Moreover, the antibody concentrations peaked 6 weeks after immunization and declined thereafter, although the postulated significant protection was not observed until many weeks later.
About 20% of the blood isolates could not be assigned to type 5 or type 8 [15]. Although NABI claimed that these strains belonged to “serotype 336,” this has subsequently been shown to be wall teichoic acid [27], present in both capsular-positive and -negative isolates.
A second randomized placebo-controlled clinical trial with this bivalent capsular polysaccharide-protein conjugate vaccine was conducted at ∼200 sites in the United States, also among dialysis recipients. Efficacy was evaluated 3–35 weeks postimmunization. This trial produced a clearer result in that there was no decrease in types 5 and 8 S. aureus bacteremia among vaccinees (http://www.bizjournals.com/southflorida/stories/2005/10/31/daily27.html, accessed November 2011).
Why did the types 5 and 8 conjugated capsular polysaccharide vaccine prove unsuccessful? There are several possible explanations. Most simply, the capsule may not be the protective antigen. Unlike the case with H. influenzae type b and S. pneumoniae, where it was clear that circulating anticapsular antibody was protective, no such demonstration has been made for S. aureus. Second, many strains are unencapsulated. A relatively high frequency of isolates lacking the types 5 and 8 capsule was found in the clinical trial among dialysis patients. Importantly, USA300, the genetic background most responsible for the CA-MRSA outbreak currently affecting the United States, does not elaborate either the type 5 or type 8 capsule [29]. Third, it is possible, although unlikely, that the capsules are protective antigens but that dialysis patients were too immunocompromised to be protected against S. aureus bacteremia by this antibody-mediated approach.
Another carbohydrate antigen, poly-N-acetylglucosamine (PNAG), is a surface polymer produced by a variety of bacterial species, including S. aureus and S. epidermidis [30]. PNAG is an adhesin that facilitates bacterial cell-to-cell contact in biofilms [31]. Its biosynthesis is controlled by a locus called icaADBC [32]. PNAG exists in acetylated and deacetylated forms [33]. The latter is retained on the staphylococcal cell surface [33, 34]. Interestingly, antibodies to the acetylated and the deacetylated forms differ in their biologic activity. Only antibodies to the deacetylated PNAG are opsonic [35]. When rabbits were immunized with a deacetylated conjugate PNAG vaccine, their serum, passively administered to mice, evoked a modest decrease in bacteremia inoculated with 1 S. aureus strain. Serum samples raised to a nondeacetylated PNAG conjugate did not decrease bacteremia in the 2 strains tested. Thus, deacetylated PNAG (dPNAG) but not PNAG may be a protective antigen against S. aureus invasive disease [36]. A monoclonal antibody prepared against dPNAG has been prepared for a clinical trial, although the target population has not yet been identified.
S. aureus Proteins as Vaccine Candidates
Efforts are underway to search for other protective antigens. Kuklin et al [37] at Merck identified the Fe-scavenging, cell wall–anchored protein isdB as a potential vaccine candidate. It was identified because patients with S. aureus infections had a brisk antibody response to it upon convalescence. The protein proved immunogenic in mice and rhesus monkeys. A phase II/III trial among adults scheduled for elective cardiac surgery was underway at multiple medical centers with an endpoint of protection against S. aureus mediastinitis. However, enrollment was halted in April 2011 for reasons that have not yet been made public (Table 1).
Table 1.
S. aureus Vaccines Receiving Clinical Evaluation As of November 2011
| Enrolling or about to enroll | |||
| Manufacturer | Vaccine | Type of Study | Composition of Vaccine |
| Novartis | 4 component | Phase I-Adult | Not publicly disclosed. Components are all proteins |
| Pfizer | 4 component | Phase-I-Adult | Conjugated capsular polysacharides, types 5 and 8, Clumping factor A, Manganese transporter C |
| Novadigm | recombinant protein | Phase-I-Adult | rA13p-N |
| GSK | 4 component | Phase-I-Adult | Not publicly disclosed |
| Enrollment Complete | |||
| Merck | 1 component | Phase II/III eficacy | isdB1 |
| Biosynexus | Passive monoclonal antibody | Phase IIb/III eficacy | anti-lipoteichoic acid antibody2 |
Enrollment halted 2011 by recommendation of the Independent Data Monitoring Committee.
No significant decrease in ‘staphyloccal sepisis’ among very low birth weight neonates.
Adhesins such as clumping factor A (ClfA) have also received attention as possible protective antigens. ClfA binds to fibrinogen, blood clots, damaged epithelium, and platelets, often important early targets in S. aureus pathogenesis. It has been hypothesized that antibody limiting S. aureus from performing these tasks might negatively impact its invasiveness. Accordingly, Veronate, an immunoglobulin preparation made from a pool of high-titer anti-ClfA serum samples, was administered to neonates, a high-risk population for invasive S. aureus disease. Despite some promise from an initial trial [38], a phase III trial aimed at preventing S. aureus bacteremia among neonates was not successful [38]. Additionally, a monoclonal anticlumping factor A antibody called tefibazumab (Aurexis) (http://en/wikipedia.org/wiki/Tefibazumab, accessed November 2011) was given to adults with S. aureus bacteremia with some demonstrated efficacy against relapse and complications of bacteremia [39]. The next steps for Aurexis have not yet been made public.
Other protein antigens have received preclinical evaluation. The antigens include the Panton-Valentine leukocidin [40], α-hemolysin [41], and a vaccine containing isdA, isdB, SdrD, and SdrE [42].
Other Vaccine Candidate Antigens
The notion that lipoteichoic acid might generate protective antibody has received recent attention. Indeed, a monoclonal antibody against lipoteichoic acid called pagibaximab was in clinical trial among neonates with the stated goal of preventing S. aureus and coagulase negative staphylococcal bacteremia. Enrollment was completed in 2010, but “no significant decrease in staphylococcal sepsis” was found.
Other vaccines are under study in early clinical trials sponsored by Pfizer, Novartis, Novadigm, and GSK. Their composition, if publicly revealed, is presented in the Table.
WHAT ABOUT LOW-LEVEL ANTIBODIES FOUND IN HUMAN SERUM SAMPLE SURVEYS?
A poorly understood conundrum concerns the presence of “low-level” antibody to many staphylococcal proteins in nonimmune human serum. Antibodies to putative protective antigens such as the Panton-Valentine leukocidin, α-toxin (α-hemolysin), or ClfA and many other ‘virulence factors’ are widely prevalent. Their role is not clear. Some have suggested that these antibodies do not have the necessary functional activity for protection. Others have suggested that low-level antibodies such as these cannot overcome ineffectual protein A binding. Still others have suggested that there is differential susceptibility to S. aureus infection and individuals with low-level antibodies targeted to protective antigens are not the population at risk.
DEALING WITH PROTEIN A
Protein A is a 40–60 kDa signature cell-wall protein encoded by the spa gene. It is synthesized by nearly all S. aureus isolates and binds to the Fc region of human immunoglobin (Ig) G1 and IgG2 with high affinity as well as mouse IgG2a and IgG2b. Binding of protein A to the Fc γ region of the immunoglobulin molecule through interaction with the heavy chain results in the wrong orientation (with respect to physiologic antibody function), and thus protein A could interfere with the role of antibody in opsonization and phagocytosis. Protein A may therefore protect S. aureus from antibody attack. Protein A has also been termed a B-cell superantigen [43], because it also binds to VH3 B-cell receptors and limits their antibody manufacturing capacity. Furthermore, marginal zone B cells and B-1 cells undergo, preferentially, induced cell death with supraclonal depletion and immune tolerance upon exposure to protein A. Thus, protein A may limit the efficacy of a vaccine that depends on opsonophagocytic antibody production. Protein A might also interfere with vaccine efficacy by virtue of B-cell binding and interfering with B-cell function.
Kim et al [44] recently engineered a mutant protein A molecule that had mutations targeted to the immunoglobulin binding site and the VH3 B-cell binding site. This mutant protein A SpAKKAA molecule does not bind the Fc fragment of immunoglobulins or trigger B-cell apoptosis. Immunization of mice with SpAKKAA elicited antibodies that neutralized immunoglobulin-binding activities of protein A, provided some protection in a mouse model of bacteremic S. aureus infection, and augmented immunogenicity of unrelated antigens.
THE ROLE OF T CELLS
A new chapter in S. aureus vaccine development was recently opened by the recognition that lymphocyte-mediated pathways play a role in immunity to S. aureus infections. Several observations increase the plausibility of this idea. Spellberg et al [45] found that interferon γ (IFN-γ)–deficient mice were hypersusceptible to infection when S. aureus was inoculated intravenously [45–47]. Others found that mice deficient in interleukin 17A (IL-17A) and F (IL-17F) developed spontaneous S. aureus skin infections [48, 49]. Hence, it may be possible to induce memory T cells that are capable of increasing phagocyte recruitment to sites of infection and facilitate clearance of S. aureus from tissues. In support of this, a novel vaccine strategy against S. aureus has been based on the immunologic activity of a candidal adhesin rAls3p. A recombinant N-terminus vaccine molecule, rAls3p-N, is under investigation as a putative vaccine candidate [45–47]. The rAls3p-N vaccine induced high antibody concentrations, but these antibodies were not protective when used to passively immunize against S. aureus intravenous challenge [45]. Serum concentrations of anti-rAls3p-N antibodies in individual mice did not correlate with the risk of death from S. aureus infection [47]. Furthermore, the vaccine was equally effective in B-cell–deficient and wild-type mice but had no efficacy in T-cell–deficient mice [45]. Adoptive transfer of immune B220+ B cells did not transfer protection, but transfer of CD4+ T cells did. The vaccine was ineffective in IFN-γ– and IL-17A–deficient mice and in gp91phox-/- mice that are unable to produce superoxide [47]. Cross-adoptive transfer experiments confirmed that functional phagocytes were operative in vaccine-mediated protection at the downstream effector stage. Additionally, vaccination increased the recruitment and activation of phagocytes at sites of tissue infection in mice, and cytokines produced by vaccine-primed lymphocyte improved the ability of phagocytes to kill S. aureus. Hence, the rAls3p-N vaccine demonstrates that it is feasible to induce a protective immune response in mice against S. aureus in the absence of induction of protective antibodies and by inducing a protective T-helper 1 (Th1)/Th17 response [50–52].
These observations do not preclude development of a humoral-based vaccine against S. aureus. Rather, they suggest that cell-mediated vaccines merit additional focus and raise the possibility of combining antigens that stimulate humoral and cellular responses against S. aureus. Indeed, this strategy may be the most likely to result in a protective vaccine against S. aureus.
CONCLUSION
How should a S. aureus vaccine be formulated? The traditional approach of identifying the correct antigen or antigen combination and relying on the production of protective antibody has come under recent scrutiny and may need to be modified. Although many experts believe that multiple vaccine antigens should be included in a vaccine formulation to prevent S. aureus infections, this view is usually advocated to mean that multiple antigens that elicit multiple antibodies that together offer protection will be required.
What about capitalizing on new concepts recently learned? Perhaps the notion that multiple antigens will be required can be reformulated to mean that multiple immunologic approaches will be required, such as the blunting of interference from protein A and the stimulation of the Th17 pathway. It is possible, then, that the optimal strategy should employ multiple antigens that work by multiple immunologic mechanisms. Whether such an approach can be explored and would prove superior to those currently under investigation will require additional study.
How could an efficacy/effectiveness trial be designed? This is not an easy question, and a full discussion is beyond the scope of this review. Examples of possible relevant target populations include (1) military recruits, (2) household contacts of index patients, and (3) patients with a single episode of S. aureus infection who have a high rate of recurrent disease. All 3 of these populations have relative ease of ascertainment and suitably high attack rates, thus allowing calculation of a feasible sample size.
Notes
Acknowledgments.
We are grateful to Michael David, Chris Montgomery, and Jean Lee for critical review of the manuscript.
Financial support.
This work was supported by grants R01AI40481, R01AI067584, R01CI000373, and U01-CI000384 to R. S. D., and R01 AI072052 to B. S. The University of Chicago has received grant support from Clorox, Pfizer, Sage Products, and Sanofi Pasteur on R. S. D.’s behalf. LABiomed has received support from Gilead, Astellas, Novartis, and Cubist on B. S.’s behalf.
Potential conflicts of interest.
R. S. D. has served on paid advisory boards for Clorox, Sanofi Pasteur, GlaxoSmithKline, Pfizer, Cerexa, and Astellas/Theravance. R. S. D. and B. S. both serve on the Scientific Advisory Board of Sevident, Inc. B. S. is a shareholder in Novadigm Therapeutics, Inc., and has consulted for Pfizer, Basilea, The Medicines Company, Novartis, Achaogen, Trius, GlaxoSmithKline, Eisai, Anacor, Zimek, Theravance, and Meiji.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–87. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford S, Boyle-Vavra S, Daum R. Community associated methicillin-resistant Staphylococcus aureus. In: Hooper DC, Scheld WM, Hughes JM, editors. Emerging infections 7. 2007. pp. 153–79. [Google Scholar]
- 4.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Graber CJ, Karr M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clin Infect Dis. 2008;46:1637–46. doi: 10.1086/587893. [DOI] [PubMed] [Google Scholar]
- 6.Morrison-Rodriguez SM, Pacha LA, Patrick JE, Jordan NN. Community-associated methicillin-resistant Staphylococcus aureus infections at an Army training installation. Epidemiol Infect. 2010;138:721–9. doi: 10.1017/S0950268810000142. [DOI] [PubMed] [Google Scholar]
- 7.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–41. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howden BP, Davies JK, Johnson PD, Stinear TP, ML G. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Périchon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Ch. 2009;53:4580–7. doi: 10.1128/AAC.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke JB, Hilgers M, Shaw KJ. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700) Antimicrob Agents Ch. 2009;53:5265–74. doi: 10.1128/AAC.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendes RE, Deshpande LM, Castanheira M, DiPersio J, Saubolle MA, Jones RN. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob Agents Ch. 2008;52:2244–6. doi: 10.1128/AAC.00231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–65. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 13.Cadilla A, David MZ, Daum RS, Boyle-Vavra S. Association of high-level mupirocin resistance and multidrug-resistant methicillin-resistant Staphylococcus aureus at an academic center in the Midwestern United States. J Clin Micro. 2010;49:95–100. doi: 10.1128/JCM.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallen AJ, Mu Y, Bulens S, et al. Health care–associated invasive MRSA infections, 2005–2008. JAMA. 2010;304:641–8. doi: 10.1001/jama.2010.1115. [DOI] [PubMed] [Google Scholar]
- 15.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro CN, HS M. Hepatitis B epidemiology and prevention. Epidemiol Rev. 1990;12:221–7. doi: 10.1093/oxfordjournals.epirev.a036055. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. A Comprehensive immunization strategy to eliminate transmission of hepatitis B Virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of infants, children and adolescents. MMWR Recomm Rep. 2005;54:1–23. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus—United States. MMWR Morb Mortal Wkly Rep. 1981;30:557–9. [PubMed] [Google Scholar]
- 19.Lee CJ, Sankaran S, Mukherjee DV, et al. Staphylococcus aureus oropharyngeal carriage in a prison population. Clin Infect Dis. 2011;52:775–8. doi: 10.1093/cid/cir026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia: Study Group. N Engl J Med. 2001;344:11–6. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 21.Adem P, Montgomery C, Husain A, et al. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. New Engl J Med. 2005;353:1245–51. doi: 10.1056/NEJMoa044194. [DOI] [PubMed] [Google Scholar]
- 22.Mongkolrattanothai K, Boyle S, Kahana MD, Daum RS. Severe Staphylococcus aureus infections caused by clonally related community-acquired methicillin-susceptible and methicillin-resistant isolates. Clin Infect Dis. 2003;37:1050–8. doi: 10.1086/378277. [DOI] [PubMed] [Google Scholar]
- 23.Kravitz GR, Dries DJ, Peterson ML, Schlievert PM. Purpura fulminans due to Staphylococcus aureus. Clin Infect Dis. 2005;40:941–7. doi: 10.1086/428573. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez BE, Hulten KG, Dishop MK, et al. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis. 2005;41:583–90. doi: 10.1086/432475. [DOI] [PubMed] [Google Scholar]
- 25.Bocchini CE, Hulten KG, Mason EO, Jr, Gonzalez BE, Hammerman WA, Kaplan SL. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006;117:433–40. doi: 10.1542/peds.2005-0566. [DOI] [PubMed] [Google Scholar]
- 26.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 27.Verdier I, Durand G, Bes M, et al. Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. 2007;45:725–9. doi: 10.1128/JCM.01572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery CP, Boyle-Vavra S, Adem P, et al. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis. 2008;198:561–70. doi: 10.1086/590157. [DOI] [PubMed] [Google Scholar]
- 29.Maira-Litran T, Kropec A, Abeygunawardana C, et al. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect Immun. 2002;70:4433–40. doi: 10.1128/IAI.70.8.4433-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. The intercellular adhesin (ica) locus is present in Staphylococcus aureus and required for biofilm formation. Infect Immun. 1999;67:5427–33. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heilman C, Schweitzer O, Gerke C, Schnell NF, Nichols WW, Götz F. Molecular basis of intercellular adhesion in the biofilm forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–91. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 32.Vuong C, Kocianova S, Voyich JM, et al. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion and virulence. J Biol Chem. 2004;279:54881–6. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- 33.Cerca N, Jefferson K, Maira-Litran T, et al. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect Immun. 2007;75:3401–13. doi: 10.1128/IAI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly-Quintos C, Kropec A, Briggs S, Ordonez CL, Goldmann DA, Pier GB. The role of epitope specificity in the human opsonic antibody response to the staphylococcal surface polysaccharide poly N-acetyl glucosamine. J Infect Dis. 2005;192:2012–9. doi: 10.1086/497604. [DOI] [PubMed] [Google Scholar]
- 35.Maira-Litran T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect Immun. 2005;73:6752–62. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuklin NA, Clark DJ, Secore S, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74:2215–23. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufman D. Veronate (Inhibitex) Curr Opin Investig Drugs. 2006;7:172–9. [PubMed] [Google Scholar]
- 38.Brown EL, Dumitrescu O, Thomas D, et al. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infec. 2009;15:156–64. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–94. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci USA. 2006;103:16942–7. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodyear CS, Silverman GJ. B cell superantigens: a microbe’s answer to innate-like B cells and natural antibodies. Springer Sem Immun. 2005;26:463–84. doi: 10.1007/s00281-004-0190-2. [DOI] [PubMed] [Google Scholar]
- 42.Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med. 2010;207:1863–70. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spellberg B, Ibrahim AS, Yeaman MR, et al. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun. 2008;76:4574–80. doi: 10.1128/IAI.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin L, Ibrahim AS, Baquir B, Avanesian V, Fu Y, Spellberg B. Immunological surrogate marker of rAls3p-N vaccine-induced protection against Staphylococcus aureus. FEMS Immunol Med Mic. 2009;55:293–5. doi: 10.1111/j.1574-695X.2008.00531.x. Epub 2009 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishigame H, Kakuta S, Nagai T, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–19. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Cho JS, Pietras EM, Garcia NC, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–3. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pachl J, Svoboda P, Jacobs F, et al. Mycograb Invasive Candidiasis Study Group: A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis. 2006;42:1404–13. doi: 10.1086/503428. [DOI] [PubMed] [Google Scholar]
- 49.Torosantucci A, Bromuro C, Chiani P, et al. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torosantucci A, Chiani P, Bromuro C, et al. Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS One. 2009;4:e5392. doi: 10.1371/journal.pone.0005392. Epub 2009 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]