Abstract
Prolonged use of linezolid was temporally and mechanistically associated with rhabdomyolysis in a patient being treated for extensively drug-resistant tuberculosis. Careful laboratory monitoring of such subjects is important during prolonged linezolid therapy.
Linezolid (Zyvox), an oxazolidinone antibiotic that is widely used for gram-positive infections, is increasingly being used for highly drug-resistant tuberculosis, although it is not approved for this use by any regulatory body. In case reports and series, linezolid seems to be useful for the treatment of both multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis. These reports have revealed that certain side effects are common with the long-term use of linezolid, especially optic and peripheral neurologic and hematologic toxicities [1]. To better understand the clinical usefulness and toxicity of linezolid treatment for patients with tuberculosis, we are performing a prospective, institutional review board–approved study, entitled “A Phase IIa, Randomized, Two-Arm, Open-label, Clinical Trial of the Efficacy of Linezolid Combined with Antituberculous Therapy in Subjects with Extensively Drug-Resistant (XDR) Pulmonary Tuberculosis” (ClinicalTrials.gov registry number NCT00727844). All subjects receive and sign an informed consent document prior to enrollment. In this study, subjects are randomly allocated to receive 600 mg linezolid daily, either immediately or following a 2-month delay, until sputum smear conversion or therapy is received for 4 months, whichever comes first. Subjects then undergo a second randomization in which one group continues to receive 600 mg of linezolid daily and the other group receives therapy deescalated to a dose of 300 mg daily. Linezolid treatment is continued for 18–24 months after culture conversion. Here, we describe a case of recurrent rhabdomyolysis related to the use of linezolid in a subject enrolled in this trial.
CASE REPORT
This subject was a 37-year-old male with a >3-year history of chronic, sputum smear–positive XDR tuberculosis. He initiated linezolid 600 mg daily per the study protocol in June 2010. This dose was well tolerated, and after sputum smear conversion he was randomized, on day 70 of therapy, to receive a reduced dose of 300 mg linezolid daily. His companion tuberculosis drugs, which he had taken since November 2007, included cycloserine, prothionamide, clarithromycin, and levofloxacin. He had no significant comorbid illnesses, and his only other medications were pyridoxine and ranitidine.
On day 76 of therapy, as part of his routine blood chemistry monitoring, he was found to have an elevated aspartate aminotransferase (AST) level of 280 IU/L (normal range, 8–38 IU/L). He was asymptomatic with regard to this abnormal laboratory finding, experiencing no abdominal pain, fever, nausea, or other symptoms. Results of all other laboratory tests, including those measuring complete blood count, alanine aminotransferase (ALT) level, total bilirubin level, and alkaline phosphatase level, were normal. Prothionamide and linezolid treatments were both considered potential causes of the elevated AST level and were discontinued that same day. His other tuberculosis medications were continued without interruption. His AST level quickly normalized, and linezolid therapy was restarted (without prothionamide) on day 84 of therapy. His AST level remained normal for >2 months, leading to the conclusion that the elevated AST level was likely a result of prothionamide-induced hepatotoxicity [2]. On day 155 of therapy, the subject experienced another elevation of the AST level, to 411 IU/L. In addition, the ALT level was mildly elevated, to 60 IU/L (normal range, 4–44 IU/L), and the creatine kinase (CK) level was markedly elevated, to 18 511 IU/L (normal range, 32–294 IU/L). All tuberculosis drugs were stopped, findings of liver ultrasonography were normal, serologic testing for acute and chronic hepatitis A and B virus infections did not reveal any evidence of coinfection, and electrocardiogram findings were normal. Lactate levels fluctuated throughout both events and were mildly elevated (between 1.4 and 2.6 mM). At this point, the subject was given a diagnosis of rhabdomyolysis and was hydrated to prevent kidney damage. A urinary dipstick test done on day 161 of therapy revealed occult blood; however, on subsequent microscopy, only 1–2 red blood cells per high power field were observed, implying the presence of myoglobinuria. On further examination, 100% of the isoenzyme of CK in serum was found to be of skeletal muscle origin. He denied any recent ethanol use or muscle injury, although he was exercising regularly. After this diagnosis, stored plasma obtained during his previous episode of elevated transaminase levels, on day 79 of therapy, was analyzed, and CK levels were found to have also been elevated (to 2960 IU/L), indicating that the prior event was also an episode of rhabdomyolysis. After linezolid treatment was stopped (on day 155 of therapy), his abnormal laboratory values normalized, and his mitochondrial function rebounded to levels slightly higher than they were initially. Linezolid therapy was restarted on day 176, and the subject was warned to avoid excessive physical exertion. The subject is under close follow-up and to date is tolerating linezolid.
DISCUSSION
This subject's recurring rhabdomyolysis was likely to have been due to linezolid-induced myopathy. This is supported by the timeline of events and their resolution with relationship to linezolid use, by the mechanistic plausibility of the association, and by prior reports in the literature. According to the adverse drug reaction probability scale devised by Naranjo et al [3], this adverse drug reaction had a score of 7, making linezolid the probable agent responsible for this event. In addition, there was a previous case report of an elevated CK level in a patient taking linezolid [4]. This subject was also taking levofloxacin, which is also known to cause rhabdomyolysis [5]. While levofloxacin use may have been a contributing factor, it should be noted that during the initial episode of elevated AST and CK levels, levofloxacin therapy was continued, and levels of both enzymes normalized despite ongoing levofloxacin use. This subject had also been receiving levofloxacin for >3 years prior to these events, making it a less likely cause. His ability to tolerate rechallenge with linezolid the second time may be related to the combined effects of his previous regimen, to decreases in physical exertion, or to both factors but is consistent with maintenance of substantial mitochondrial function.
POTENTIAL DRUG-DRUG INTERACTION WITH CLARITHROMYCIN
Linezolid is not directly metabolized by any cytochrome P450 isoform, but some speculate that it is a substrate of P-glycoprotein (P-gp), a transmembrane transporter expressed in various body tissues [1, 2]. The role of this transporter is to mediate efflux of xenobiotics across anatomic barriers, such as from within enterocytes back into the intestinal lumen. Rifampicin is a known inducer of P-gp that has been shown to decrease linezolid levels up to 35%, suggesting that linezolid is a substrate for this transporter [6]. This subject was taking clarithromycin, which is known to inhibit P-gp [7] and has been reported to increase linezolid levels by 50%. A single case report has suggested that clarithromycin alone can induce rhabdomyolysis [8]. However, in this case the more likely cause was linezolid, owing to the timing and history of extended use of clarithromycin. However, it should be noted that the ratio of basolateral-to-apical efflux to apical-to-basolateral efflux of 1.1 observed in Madin Darby canine kidney cells transfected with the human multidrug resistance 1 gene suggests that linezolid may not be a substrate of P-gp (Pfizer, unpublished data). Thus, the contribution of clarithromycin to the observed toxicity remains unclear. Plasma levels of linezolid in this subject were not significantly higher than those in other subjects in this study, with peak concentrations of 17.6 µg/mL and 9.3 µg/mL associated with receipt of 600 mg and 300 mg linezolid, respectively, during clarithromycin coadministration.
Linezolid, along with other drugs (or certain foods), may lead to serotonin syndrome and thereby cause rhabdomyolysis by inducing a state of neuromuscular excitement that can cause muscle damage [9]. This syndrome includes muscle rigidity, hyperreflexia, hypertension, and agitation, none of which were present in this subject. However, even in the absence of serotonergic agents, the Korean diet often includes fermented fish, cabbage (kimchi), and soybean paste, all of which may contain tyramine, a biogenic amine that could contribute to a serotonin syndrome [10].
POTENTIAL MECHANISM
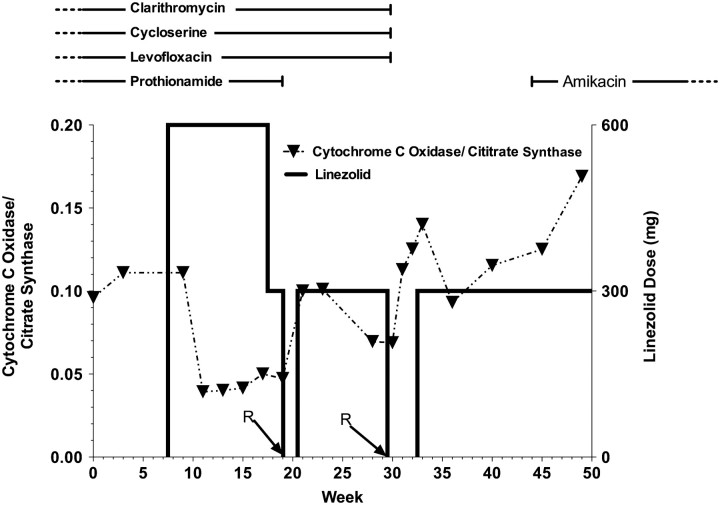
Rhabdomyolysis has been associated with inborn errors in the mitochondrial fatty acid β-oxidation pathway [11], and drugs such as nucleoside analogs inhibit mitochondrial function [12]. There is significant homology between the mitochondrial ribosome and the bacterial ribosome, which is the target of linezolid [13]. Decreases in mitochondrial function have previously been shown to be associated with linezolid use, and a single episode of myositis has been reported with minimal detail [14]. We monitored mitochondrial function during therapy and at the time of the adverse events by measuring the ratio of mitochondrially synthesized cytochrome c oxidase to cytoplasmically synthesized citrate synthase, as previously described [15]. A decrease in this ratio is considered to represent decreased mitochondrial protein synthesis per mitochondrial mass. In this case, there was a decrease in this ratio after the initiation of treatment, and the decrease dissipated each time linezolid therapy was withdrawn. While these data are only from a single patient, they are evidence that this event was correlated with linezolid-induced inhibition of mitochondrial protein synthesis (Figure 1). An analysis of this assay and its relationship to other observed adverse events in the full cohort is ongoing and will be reported when complete.
Figure 1.
The temporal sequence of events for this subject following study enrollment. An arrow and the letter R indicate the 2 episodes of rhabdomyolysis prior to week 20 and week 30. The dark horizontal line with reference to the right axis indicates the linezolid dose throughout, and the ratio of the chromosomally encoded cytochrome c oxidase to the mitochondrial citrate synthase is indicated by a dotted line with triangles denoting the measured values. The horizontal lines at the top indicate concurrent tuberculosis therapies and their timing, with a vertical hash indicating stop or start and a dashed line indicating prior or continuing therapy.
CONCLUSION
In individuals receiving long-term linezolid therapy, rhabdomyolysis is possible and should be considered in the differential diagnosis of elevated AST or CK levels. There was a decrease in mitochondrial protein synthesis that was dose related and coincided temporally with rhabdomyolysis. In this case, linezolid-induced inhibition of mitochondrial protein synthesis may be the mechanistic cause of this event and may be a means of predicting toxicity and the dose-related degree of this toxicity.
Notes
Acknowledgments. We thank the subjects participating in this study; the physicians, nurses, and other staff at the National Masan Tuberculosis Hospital, for their support in this study and patient care; Pfizer, for kindly donating the study drug; and the safety monitoring committee (Barbara Seaworth, Jae-Joon Yim, and Debra Benator), for their guidance in this study.
Financial support. This study was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by the Korean Ministry of Health and Welfare through the Korean Centers for Disease Control.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest. 2008;134:187–92. doi: 10.1378/chest.07-1988. [DOI] [PubMed] [Google Scholar]
- 2.Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirology. 2006;11:699–707. doi: 10.1111/j.1440-1843.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- 3.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 4.Allison GW, Perla RJ, Belliveau PP, Angelis SM. Elevated creatine phosphokinase levels associated with linezolid therapy. Am J Health Syst Pharm. 2009;66:1097–100. doi: 10.2146/ajhp080228. [DOI] [PubMed] [Google Scholar]
- 5.Hsiao SH, Chang CM, Tsao CJ, Lee YY, Hsu MY, Wu TJ. Acute rhabdomyolysis associated with ofloxacin/levofloxacin therapy. Ann Pharmacother. 2005;39:146–9. doi: 10.1345/aph.1E285. [DOI] [PubMed] [Google Scholar]
- 6.McKee EE, Ferguson M, Bentley AT, Marks TA. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob Agents Chemother. 2006;50:2042–9. doi: 10.1128/AAC.01411-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolhuis MS, van Altena R, Uges DR, van der Werf TS, Kosterink JG, Alffenaar JW. Clarithromycin significantly increases linezolid serum concentrations. Antimicrob Agents Chemother. 2010;54:5418–9. doi: 10.1128/AAC.00757-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brener ZZ, Bilik I, Khorets B, Winchester JF, Bergman M. Rhabdomyolysis following clarithromycin monotherapy. Am J Med Sci. 2009;338:78. doi: 10.1097/MAJ.0b013e31819e221f. [DOI] [PubMed] [Google Scholar]
- 9.Packer S, Berman SA. Serotonin syndrome precipitated by the monoamine oxidase inhibitor linezolid. Am J Psychiatry. 2007;164:346–7. doi: 10.1176/ajp.2007.164.2.346b. [DOI] [PubMed] [Google Scholar]
- 10.Mah JH, Kim YJ, No HK, Hwang HJ. Determination of biogenic amines in kimchi, Korean traditional fermented vegetable products. Food Sci Biotechnol. 2004;13:826–9. [Google Scholar]
- 11.Kompare M, Rizzo WB. Mitochondrial fatty-acid oxidation disorders. Semin Pediatr Neurol. 2008;15:140–9. doi: 10.1016/j.spen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Scruggs ER, Naylor AJD. Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology. 2008;82:83–8. doi: 10.1159/000134943. [DOI] [PubMed] [Google Scholar]
- 13.Nagiec EE, Wu LP, Swaney SM, et al. Oxazolidinones inhibit cellular proliferation via inhibition of mitochondrial protein synthesis. Antimicrob Agents Chemother. 2005;49:3896–902. doi: 10.1128/AAC.49.9.3896-3902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beekmann SE, Gilbert DN, Polgreen PM. Toxicity of extended courses of linezolid: results of an Infectious Diseases Society of America Emerging Infections Network survey. Diagn Microbiol Infect Dis. 2008;62:407–10. doi: 10.1016/j.diagmicrobio.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Garrabou G, Soriano A, Lopez S, et al. Reversible inhibition of mitochondrial protein synthesis during linezolid-related hyperlactatemia. Antimicrob Agents Chemother. 2007;51:962–7. doi: 10.1128/AAC.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]