Abstract
We used soft x-ray tomography (SXT) – a high-resolution, quantitative imaging technique – to measure cell size and organelle volumes in yeasts. Cell size is a key factor in initiating cell division in yeasts, whereas the number and volume of the organelles has a profound impact on the function and viability of a cell. Consequently, determining these cell parameters is fundamentally important in understanding yeast biology. SXT is well suited to this type of analysis. Specimens are imaged in a near-native state, and relatively large numbers of cells can be readily analyzed. In this study, we characterized haploid and diploid strains of Saccharomyces cerevisiae at each of the key stages in the cell cycle, and determined if there were relationships between cellular and organelle volumes. We then compared these results with SXT data obtained from Schizosaccharomyces pombe, the three main phenotypes displayed by the opportunistic yeast pathogen Candida albicans, and from a coff1-22 mutant strain of Saccharomyces cerevisiae. This comparison revealed that volumetric ratios were invariant irrespective of yeast strain, ploidy or morphology, leading to the conclusion these volumetric ratios are common in all yeasts.
Introduction
Cell size is a critical parameter in the regulation of cell division in yeasts (Bryan, et al., 2010; Conlon, et al., 2001; Johnston, et al., 1977; Jorgensen and Tyers, 2004; Umen, 2005). It has long been accepted that yeast cells must reach a minimum size before the process of cell division is initiated, and that the cells continue to grow as the cycle proceeds (Calvert and Dawes, 1984; Goranov, et al., 2009; Mitchison, 1957a; Mitchison, 1957b). As with all eukaryotes, yeast cells are compartmentalized into functionally distinct spaces by the creation of membrane-bound organelles. This sub-cellular arrangement maximizes both the complexity and the diversity of biochemical processes that can take place inside the cell (Fagarasanu, et al., 2007; Warren and Wickner, 1996). During the cell cycle organelle size must be precisely controlled, for both proper cellular function and correct organelle partitioning between mother and daughter prior to their separation since most organelles are inherited (Fagarasanu, et al., 2007; Warren and Wickner, 1996).
In the past it has been challenging to accurately measure the volume and density of organelles (Bryan, et al., 2010). Electron microscopy can only be used to image specimens that are less than 500 nm thick (Baumeister, et al., 1999). Consequently, even relatively small eukaryotic cells, such as yeasts, must be cut into sections 60 – 400 nm thick before they can be imaged. This requirement makes imaging all organelles in a eukaryotic cell technically challenging and very time consuming (Leis, et al., 2009). Fluorescence microscopy has also been widely applied to imaging organelles (Giepmans, et al., 2006; Tsien, 2005). However, this technique can only provide data on organelles that have been fluorescently stained, and only a small number of organelles can be stained before overlap occurs between the emission/excitation bands of different colored fluorescent probes. Moreover, in most cases it is difficult to accurately measure the actual volume of a labeled organelle based on its fluorescence emission signal. In particular, it is very difficult to determine the volume of irregularly shaped organelles (such as lipid bodies, mitochondria and vacuoles) using fluorescence. Over the past decade, soft x-ray tomography has emerged as a technique that is ideally suited to imaging and accurately quantifying organelles volumes (Gu, et al., 2007; Larabell and Le Gros, 2004a; Larabell and Le Gros, 2004b; Le Gros, et al., 2005; Leis, et al., 2009; McDermott, et al., 2009; Parkinson, et al., 2008; Schneider, et al., 2002; Schneider, et al., 2003; Weiss, et al., 2000)
Soft x-ray tomography has a number of highly advantageous characteristics in the context of imaging eukaryotic cells (McDermott, et al., 2009). Firstly, the short wavelength of the illuminating x-rays allows specimens to be imaged at high spatial resolution (Kirz, et al., 1995; Larabell and Le Gros, 2004a; McDermott, et al., 2009; Weiss, et al., 2000; Weiss, et al., 2001). Secondly, soft x-ray photons can penetrate and image fully hydrated specimens up to 15 μm thick (Attwood, 1999; Kirz, et al., 1995; Le Gros, et al., 2005; Schneider, 2003). Lastly, image contrast is obtained directly from the absorption of soft x-rays by the specimen, and therefore no potentially damaging specimen staining procedures are required (Le Gros, et al., 2005; McDermott, et al., 2009). In SXT the specimen-illuminating photons are in a region of the electromagnetic spectrum known as the ‘water window’. This lies between the K shell absorption edges of carbon (284 eV, λ=4.4 nm) and oxygen (543 eV, λ=2.3 nm) (Attwood, 1999; Kirz, et al., 1995; Le Gros, et al., 2005; McDermott, et al., 2009; Schmahl, et al., 1996; Weiss, et al., 2001)}. X-ray photons within this energy range are absorbed an order of magnitude more strongly by carbon- and nitrogen-containing biomolecules than by water (Le Gros, et al., 2005). This absorption adheres to Beer-Lambert’s Law, and is therefore linear with thickness (Attwood, 1999; Uchida, et al., 2009)}. Consequently, images produced by SXT are quantitative, with each biochemical component having a specific x-ray Linear Absorption Coefficient (LAC) (Le Gros, et al., 2005; Weiss and 2000). Therefore, organelles and other cell structures can be visualized directly, based on differences in biochemical composition and density. For example, dense lipid-rich structures absorb soft x-rays much more strongly than organelles with relatively high water content, such as vacuoles. This contrast mechanism is highly sensitive to subtle changes in LAC, for example the nucleolus can be very readily identified in a reconstruction of a yeast nucleus (Uchida, et al., 2009).
As with all tomographic imaging techniques, in SXT a number of projection images are collected and then used to calculate a volumetric reconstruction of the specimen (Leis, et al., 2009; McDermott, et al., 2009). In this work, projection images were collected using XM-2, a soft x-ray microscope dedicated to biological and biomedical imaging. The spatial resolution in images produced by XM-2 is determined by the zone plate optics (Attwood, 1999; Le Gros, et al., 2005; McDermott, et al., 2009). For all of the specimens imaged in this work the resolution was 50 nm. In the near future, incorporation of the latest generation of zone plates into XM-2 will increase the achievable spatial resolution to better than 20 nm (Chao, et al., 2005).
Yeasts have been a workhorse model eukaryotic cell for many years, in particular to study cell division and the effects of engineered mutations. Recently, much work has been carried out determining the genetic and molecular mechanisms of organelle inheritance in the budding yeast Saccharomyces cerevisiae (Umen, 2005; Zimmerberg and Kozlov, 2006). It is now generally accepted that the actin cytoskeleton and associated motors orchestrate organelle localization during cell division. However, details of the mechanisms that regulate organelle size in yeast during the cell cycle remain unclear in general, with the exception being regulation of nuclear size where there has been significant progress to date (Jorgensen, et al., 2007; Neumann and Nurse, 2007). In both budding and fission yeast the growth of the nucleus has been shown to be proportional to cell size (Jorgensen, et al., 2007; Neumann and Nurse, 2007). It has also been well established that ploidy has a direct bearing on cell size (Murray, et al., 1987). Therefore in this study we imaged both haploid and diploid strains of Saccharomyces cerevisiae to determine the effects of ploidy on the size of the cells and organelles and how cell and organelle size is controlled during the cell cycle. We observed a number of ratios between cell size and organelle volumes were conserved, irrespective of ploidy or stage in the cell cycle. We therefore compared data obtained from Saccharomyces cerevisiae with that from other strains of yeasts, including a strain of Candida albicans that undergoes phenotypic switching (Uchida, et al., 2009). In this way we examined these volumetric ratios in specimens across a range of yeast cell types and morphologies.
Materials and Methods
Strains, cell cultures, and growth conditions
Diploid: DDY1102, haploid: DDY904, and coff1-22 mutant: DDY1266 of S. cerevisiae strains were grown at 25°C to early log phase in YPD media. S. cerevisiae (ATCC 200060) was grown at 30°C to early log phase in YEPD media. C. albicans (ATCC 26555) was grown in YM media at 26°C for formation of yeas-like cells, at 30°C for formation of germ tubes, and at 37°C for hyphal form. S. pombe (strain #972 h) was grown at 30°C to early log phase in YES media. All media were supplemented with the appropriate amino acids.
Soft X-ray tomography
Projection images were collected using XM-2, the National Center for X-ray tomography soft x-ray microscope at the Advanced Light Source of Lawrence Berkeley National Laboratory. XM-2 was designed to investigate biological samples in their hydrated states. Specimens were simply transferred from the growth chamber, mounted in thin-walled glass capillary tubes and rapidly cryo-imobilized prior to being mounted in the cryogenic specimen rotation stage on the microscope (Le Gros, et al., 2005). During data collection, the cells were maintained in a stream of helium gas that had been cooled to liquid nitrogen temperatures (Le Gros, et al., 2005; McDermott, et al., 2009). Cooling the specimen allows collection of projection images while mitigating the effects of exposure to radiation. Each dataset (i.e., 90 projection images spanning a range of 180°) was collected using Fresnel zone plate based objective lens with a resolution of 50 nm (Larabell and Le Gros, 2004a). Exposure times for each projection image ranged from 150 to 300 msec. Projection images were manually aligned using fiducial markers on adjacent images using the IMOD software package (Kremer, et al., 1996). Tomographic reconstructions were calculated using the iterative reconstruction method (Mastronarde, 2005; Stayman and Fessler, 2004). The Amira software package (Mercury Computer Systems) was used to manually segment the reconstructed volumes, measure voxel values (i.e., absorption values in volume element of the reconstructed data) to calculate Linear Absorption Coefficients (LACs), and create the movies included as supplementary material.
Results
In the first instance we quantified the change in cell and organelle volumes during the cell cycle in Saccharomyces cerevisiae using soft x-ray tomography. These measurements were done in both haploid and diploid cells without pre-selecting cells at a certain stage of the cell cycle (for example, by using techniques such as Fluorescence Activated Cell Sorting (FACS) or centrifugal elutriation, or by chemically/biochemically inducing cell cycle arrest). Cells in log phase (OD=0.1-0.4) were mounted in glass capillary tubes and immediately cryo-immobilized; therefore, cells were imaged with minimal perturbation from their native-growth conditions. It has been well established from electron microscopy/tomographic imaging that use of chemical fixation agents leads to significant damage to cellular structures. In total we collected approximately 80 tomographic data sets. Since each field of view in the soft x-ray microscope used for this work is approximately 15 × 15 μm, between 1 and 6 yeast cells are imaged per data set, and as a result, more than 200 cells were analyzed in total.
Once the projection images were reconstructed the volumes were segmented to isolate individual cells and subsequently, their component organelles (Figure 1A). The reconstructed cells were segmented into discrete volumes based on the Linear Absorption Coefficients (LACs) (Figure 1B). For instance, volumes assigned as dense lipid bodies have an average LAC value of 0.55 μm-1, compared with more transmissive organelles such as nucleus, nucleolus, vacuole, and mitochondria that have typical LAC values of 0.26, 0.33, 0.22, and 0.36 μm-1 respectively. Assignment of organelle type to a particular segmented volume was guided by morphological characteristics established by other modalities. For example, the nucleus/nucleolus, mitochondria, and vacuoles have distinct and very recognizable morphologies. Once organelles from a number of cells had been segmented it was clear that the vacuoles could be categorized into one of five types, based on their morphology, internal structure and densities. The LAC values for these are shown in Figure 1C.
Figure 1.
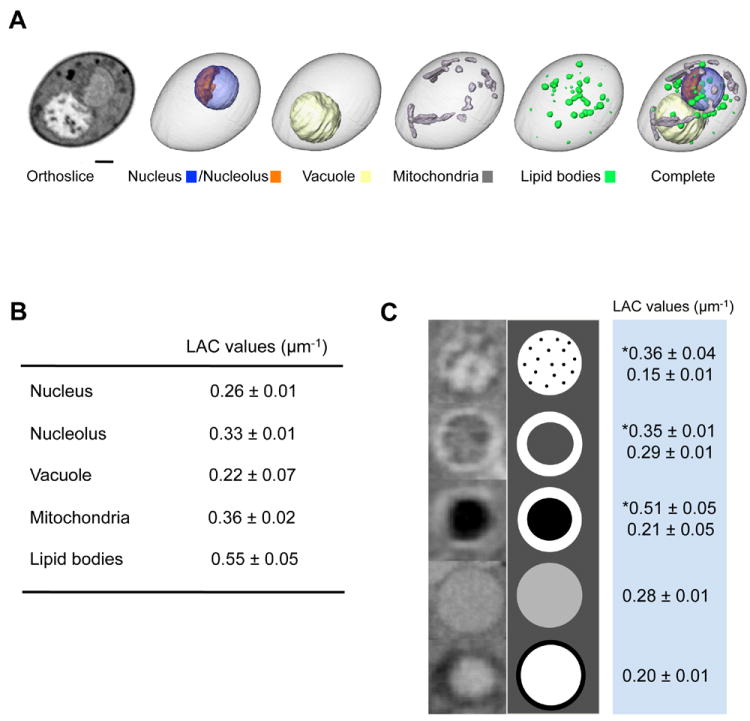
Segmentation of organelles based on Linear Absorption Coefficient (LAC) values. (A) A representative diploid cell shown in an orthoslice (i.e., a single slice of tomographic data) and individually segmented organelles; scale bar, 1 μm. (B) LAC values for each organelles. (C) Five different vacuolar compositions found in tomographic data (left), schematic views (middle), and LAC values (right).
Representative cells from each stage of the cell cycle are shown in Figure 2A. All the segmented cells were categorized into the appropriate phase of cell cycle based on the morphological state of cells and their organelles. It was assumed that these cells were actively going through the cell cycle until the instant they were cryo-immobilized. Using these segmented cells, we quantified cell (Figure 2B), cytosol (Figure 2C) and organelle (Figure 3) volumes, together with their surface areas (Figure 4). In haploid cells, the cell volumes were within the range 10 - 50 μm3 in G1, between 20 - 60 μm3 in S, 40 - 80 μm3 in G2, and between 60 – 100 μm3 in the M phase. In diploid cells, the cell volumes were within 20 - 60 μm3 in G1, within 30 - 80 μm3 in S, within 50 - 140 μm3 in G2, and within 70 – 140 μm3 in M phase. The minimum size requirement to be in G1 phase was observed to be 10 μm3 in haploid and 20 μm3 in diploid cells. A similar trend of cell volume distribution was also observed in diploid cells of another S. cerevisiae strain (ATCC200060) (Supplementary Figure 1). The average cytosolic volume (Figure 2C) was calculated by combining the cell wall/membrane volume with the total volume of segmented organelles, and then subtracting this combined volume from the total cell volume. On average, the nucleus occupied 6-11% of cell volume; the nucleolus occupied 20% of nucleus; vacuole(s) occupied 3-14% of cell; and mitochondria and lipid bodies occupied 1-2% of cell (Figure 3); therefore, the cytosol, other non-segmented organelles, large macromolecular complexes, such as the ribosomes, and the cytoskeleton occupy the remaining 70% of cell volume. The overall trend observed in the surface area of lipid bodies, mitochondria, vacuoles and nucleus mirrored that of the organelle volumes (Figure 4).
Figure 2.
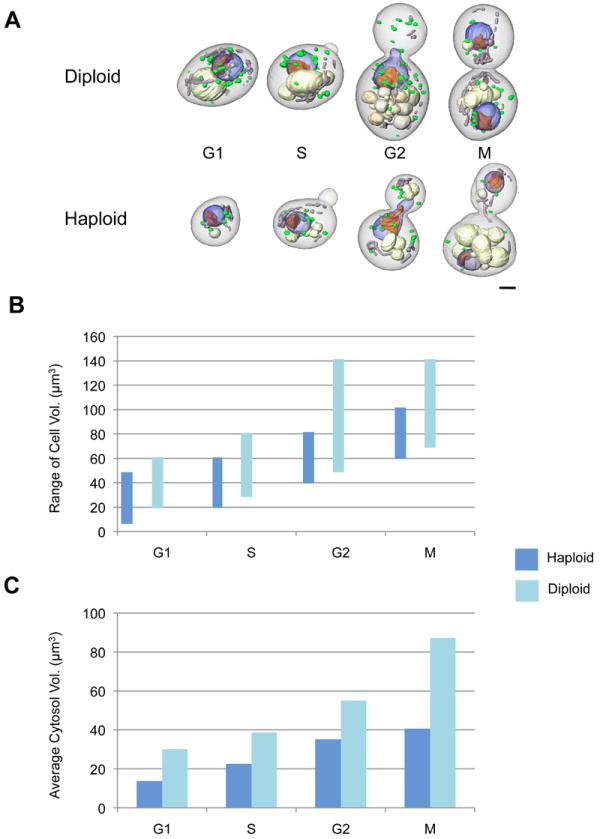
Measurement of cell volumes using segmented cells. (A) Representative haploid and diploid S. cerevisiae cells at the four main stages of cell cycle; scale bar, 1 μm. (B) Comparison of cell volumes in diploid and haploid cells. For this measurement, the total of 74 haploid cells and 80 diploid cells were analyzed. (C) Average cytosolic volume in diploid and haploid cells.
Figure 3.
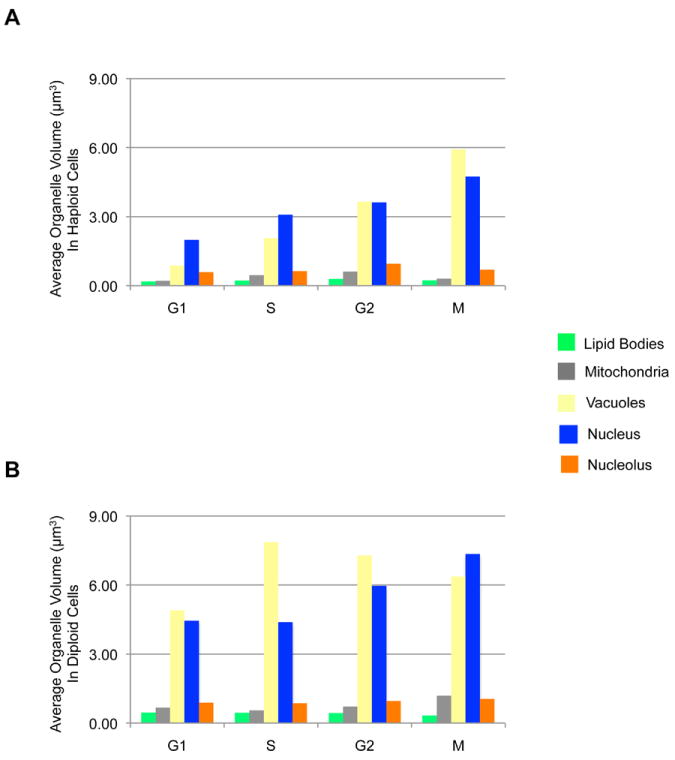
Average volume of organelles in haploid (A) and diploid (B) cells.
Figure 4.
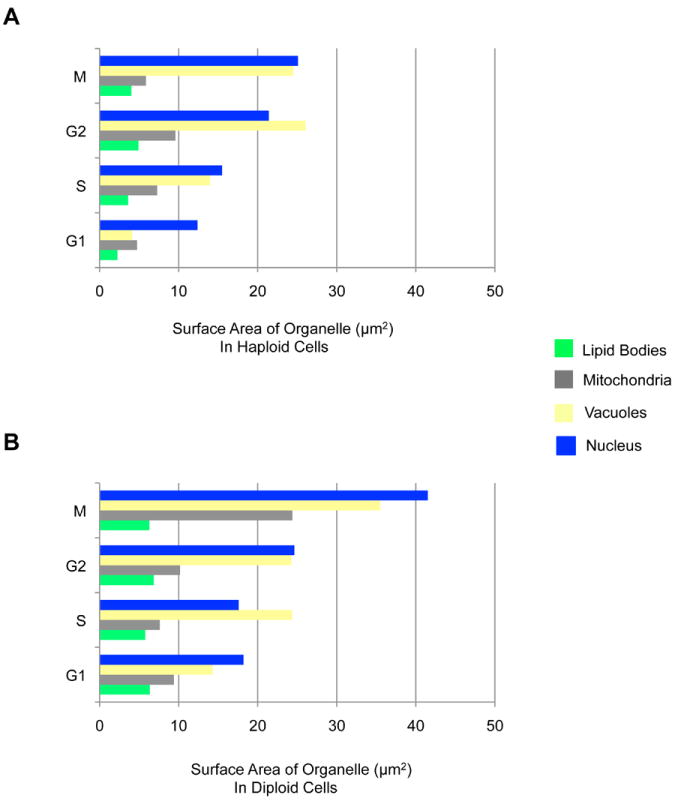
Average surface area of organelles in haploid (A) and diploid (B) cells.
We next examined how the major organelle volume correlates with the cell size as the cell cycle progresses (Figure 5A). In haploid cells, the ratio of total major organelle volume to cell volume (O/C ratio) was relatively constant through out the cell cycle (i.e., G1: 0.17, S: 0.18, G2: 0.18, M: 0.16). In diploid cells, the O/C ratio dropped slightly at M phase (i.e., 0.16) compared to the O/C ratios during G1 through G2 (i.e., G1: 0.22, S: 0.24, G2: 0.20); however, overall it appears that there is a linear correlation between cell size and major organelle volume in both haploid and diploid cells.
Figure 5.
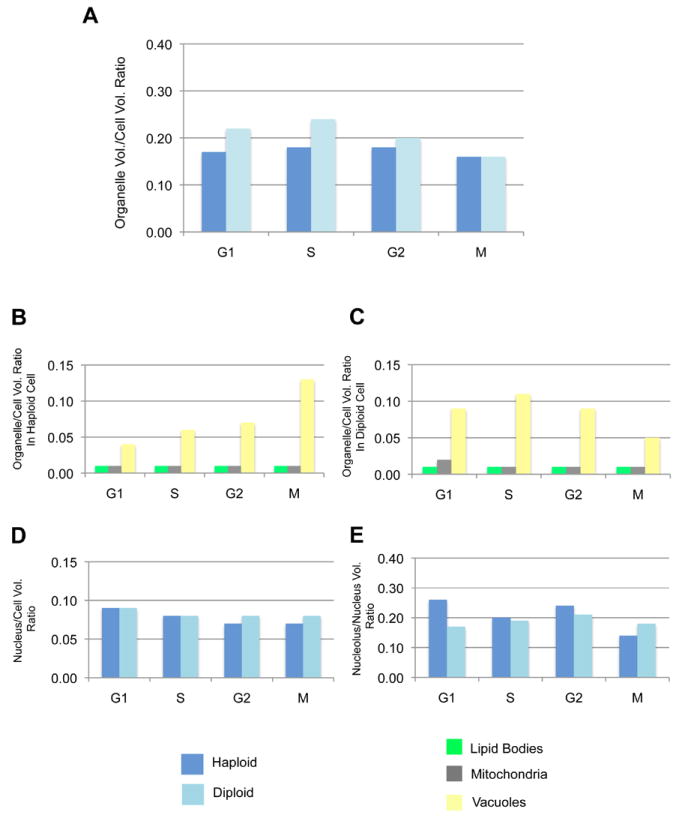
Analysis of organelle and cell size linkage during cell cycle. (A) The ratio of total organelle volume to cell volume (O/C ratio) in haploid and diploid cells. (B) The O/C ratio for lipid, mitochondria, and vacuoles in haploid cells. (C) The O/C ratio for lipid, mitochondria, and vacuoles in diploid cells. (D) The O/C ratio for nucleus in haploid and diploid cells. (E) The ratio of nucleolus volume to nucleus volume in haploid and diploid cells.
To determine if the O/C ratio is consistent for each organelle, we obtained the O/C ratio for lipid bodies, mitochondria, vacuoles (Figure 5B and C) and nucleus (Figure 5D). Interestingly, the O/C ratio for lipid bodies and mitochondria (i.e., ~ 0.01) was constant through out the cell cycle in both diploid and haploid cells. The O/C ratio for vacuoles was, however, much larger compared to those of lipid bodies and mitochondria. In haploid cells, the O/C ratio for vacuoles increased as the cell cycle proceeded (i.e., G1: 0.04, S: 0.06, G2: 0.07, M: 0.13). In contrast with the haploid cells, the O/C ratio for vacuoles in diploid cells showed a slight increase in ratio from G1 (0.09) to S (0.11) and then declined from S (0.11) to G2 (0.09) and M (0.05). Similar to the O/C ratio for lipid bodies and mitochondria, the O/C ratio for nucleus was fairly constant throughout the cell cycle in both haploid and diploid cells (i.e., 0.07 - 0.09). This result was similar to the ratio of nuclear to cell volume previously reported for both budding and fission yeast (Jorgensen, et al., 2007; Neumann and Nurse, 2007), in that the growth of nucleus was consistently proportional to the cell growth. We also observed that all organelles, except vacuoles, grew in direct proportion to cell size. Therefore, we conclude that the slight variation seen in the ratio of total organelle volume to cell volume (Figure 5A) was mainly due to the volume of vacuoles.
We quantified the ratio of nucleolus to nuclear volume (N/Nuc ratio) (Figure 5E). Similar to the ratio of nuclear to cell volumes, the N/Nuc ratio was relatively similar between haploid (0.14 – 0.26) and diploid (0.17 – 0.21) cells. In haploid cells, the N/Nuc ratio in G1, S, and G2 was, however, higher than those of diploid cells indicating that the nucleolus in these stages of haploid cells was slightly larger relative to the size of nucleus.
To better understand cell size control and organelle inheritance during bud emergence and formation of the daughter cell, we measured cell and organelle volumes in daughter and mother cells separately. The bud scar(s) on the cell surface was readily identified (an example of bud scars is shown in Supplementary Figure 2.). In this study, about 67% of cells had no bud scar, 14% of cells had 1 bud scar, 12% of cells had 2 bud scars, and less than 6% of cells had 3-7 bud scars. As shown in Figure 6A, there was a linear increase in the ratio of daughter to mother cell volume (D/M ratio) in both haploid and diploid cells, indicating that the size of both increases as the cell cycle progresses. In haploid cells, the D/M ratio of G2 was approximately 1.3 times larger (G2: 0.37) than those of diploid cells (G2: 0.29). We then compared the ratio of total organelle volume to cell volume (O/C ratio) in daughter and mother cells (Figure 6B). In haploid cells, the O/C ratio for mother cells was similar in S (0.17) and G2 (0.18), but the O/C ratio was increased by ~1.5 fold in M (0.28). In contrast, the O/C ratio for daughter cells was similar in G2 (0.11) and M (0.10), but decreased 10 fold in S (0.01). In diploid cells, the O/C ratio for mother cells was identical in S (0.22) and G2 (0.22), but was decreased by 1.4 fold in M (0.16); whereas the O/C ratio for daughter cells was identical in S (0.01) and G2 (0.01), but increased by 11 fold in M (0.11). Overall, the O/C ratios in daughter and mother cells were not as constant as those in the whole cells. In addition, it appears that the timing of organelle partitioning from the mother to the daughter cells was different in haploid and diploid cells.
Figure 6.
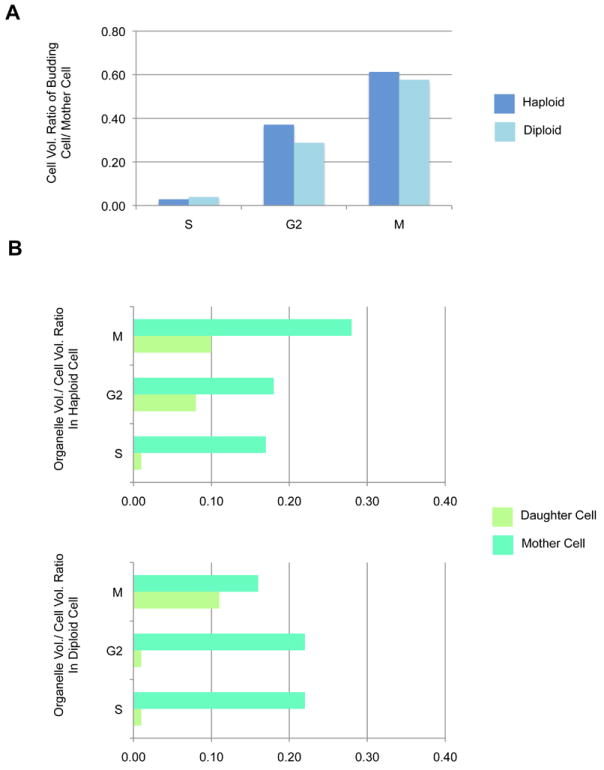
Analysis of organelle and cell size linkage in mother and daughter cells. (A) The ratio of budding/daughter cell volume to mother cell volume in haploid and diploid cells. The O/C ratio in haploid (B) and diploid (C) cells.
This work demonstrates that both haploid and diploid cells of S. cerevisiae maintain a constant ratio of total major organelle volume to cell volume throughout the cell cycle. To find out if this correlation was influenced by morphology, species, or mutation, we characterized Candida albicans (C. albicans), Schizosacchromyces pombe (S.pombe), and an S. cerevisiae mutant. Surprisingly, similar O/C ratios (i.e., 0.18 - 0.21) were observed in C. albicans that exhibited three different phenotypes (i.e., yeast like, germ tubes, and hypha), S. pombe cells at the different stages of cell cycle, and cofilin mutant cells (coff1-22) (data not shown). The ratio of nuclear to cell volume was also similar among all of these different yeasts (i.e., 0.05 -0.08). Therefore, it appears that maintaining the ratio of organelle volume to cell volume is important for the cells to grow and divide, and that these ratios are common over a wide range of yeast strains and morphologies.
In this study, vacuoles were observed to have different compositions (Figure 1C). These were verified by the linear absorption coefficient (LAC) values and categorized into five types. The type A vacuole had numerous small dense-inclusions. The type B and C vacuoles were similar to each other, in that both appeared to have a dense region that occupied the majority of the vacuole core; however, this portion was much more highly absorbing in type C vacuoles. The type D and E vacuoles were also similar to each other, but quite different from the type A – C vacuoles. Type D and E did not contain any inclusions or dense regions, but appeared to be mostly aqueous, although the type D vacuole had a slightly higher LAC value compared to that of the type E. Although we did not observe any strong correlation between the vacuole content morphology and its location in the cell or stage of the cell cycle, it seems likely that these patterns represent different functional processes. (Examples of their relative location in the cell are shown in Supplementary Figure 3.) It is known that vacuoles participate in various processes such as protein degradation, metabolic storage, ion homeostasis and osmo-regulation (Klionsky, et al., 1990), as do their counterpart in mammalian cells, lysosomes, and vacuoles in plant cells (Gieselmann and Braulke, 2009). In future work it will be important to determine the functional consequences of these differences in vacuolar compositions.
Discussion
Cell size and organelle volumes are enormously important physical characteristics that must be monitored and controlled in response to changing environmental conditions, phenotype, and the cell cycle. Soft x-ray tomography allows the internal architecture of cells in a near-native state to be imaged and quantified with high spatial resolution. The images produced by this technique have excellent natural contrast without requiring the cells to be first stained, or dehydrated. Moreover, compared to other high-resolution tomographic imaging techniques, SXT is capable of high throughput imaging. Since each reconstruction typically contains three to five yeast cells, this allows analysis of significant numbers of cells; for example, in this work more than 200 cells were reconstructed.
In this study we focused primarily on measuring cell size and the volumes of the major organelles in haploid and diploid strains of S. cerevisiae at the four key stages of the yeast cell cycle. We determined that the growth of the major organelles - with the notable exception of vacuoles – is strictly regulated in accordance with cell size. We observed the relative ratio of organelle to cell volumes to remain constant throughout the cell cycle. We also determined that the ratio of nucleolus to nuclear volume and ratio of nuclear to cell volumes were similar at all stages of the cell cycle. We expanded this study to include an examination of these ratios in S. pombe and C. albicans and found similar ratios to be maintained. Therefore, we conclude that in yeasts there are well defined optimal volumetric ratios, and since these were observed to be independent of strain, ploidy, and phenotype, these ratios are likely to be common to all yeast cells. We plan to extend this analysis in the future, with the goal of using mutant cells to improve our understanding of how cells control their size and that of their component organelles.
Supplementary Material
Suppl. Figure 1. Comparison of cell volumes in two strains of diploid cells: ATCC200060 and DDY1102.
Suppl. Figure 2. Bud scars on the surface of the mother cells shown within the bounded areas. Scale bar, 0.75 μm.
Suppl. Figure 3. Representative cells shown in orthoslice demonstrating the relative locations of vacuoles and their different components in the cell.
Suppl. Movie 1. A movie of the representative diploid and haploid cells from each phase of cell cycle.
Acknowledgments
We gratefully acknowledge Zenny Serrano for cell culture and expert technical assistance. This work was funded by the Department of Energy Office of Biological and Environmental Research Grant DE-AC02-05CH11231, the NIH National Center for Research Resources Grant RR019664 and NIH R01 50399 to DGD.
References
- Attwood DT. Soft x-rays and extreme ultraviolet radioation: principles and applications. Cambridge Uhiversity Press; 1999. [Google Scholar]
- Baumeister W, Grimm R, Walz J. Electron tomography of molecules and cells. Trends in Cell Biology. 1999;9:81–85. doi: 10.1016/s0962-8924(98)01423-8. [DOI] [PubMed] [Google Scholar]
- Bryan AK, Goranov A, Amon A, Manalis SR. Measurement of mass, density, and volume during the cell cycle of yeast. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:999–1004. doi: 10.1073/pnas.0901851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GR, Dawes IW. Cell-Size Control of Development in Saccharomyces-Cerevisiae. Nature. 1984;312:61–63. doi: 10.1038/312061a0. [DOI] [PubMed] [Google Scholar]
- Chao WL, Harteneck BD, Liddle JA, Anderson EH, Attwood DT. Soft X-ray microscopy at a spatial resolution better than 15nm. Nature. 2005;435:1210–1213. doi: 10.1038/nature03719. [DOI] [PubMed] [Google Scholar]
- Conlon IJ, Dunn GA, Mudge AW, Raff MC. Extracellular control of cell size. Nat Cell Biol. 2001;3:918–21. doi: 10.1038/ncb1001-918. [DOI] [PubMed] [Google Scholar]
- Fagarasanu A, Fagarasanu M, Rachubinski RA. Maintaining peroxisome populations: A story of division and inheritance. Annual Review of Cell and Developmental Biology. 2007;23:321–344. doi: 10.1146/annurev.cellbio.23.090506.123456. [DOI] [PubMed] [Google Scholar]
- Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. The Fluorescent Toolbox for Assessing Protein Location and Function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- Gieselmann V, Braulke T. Lysosomes Preface. Biochimica Et Biophysica Acta-Molecular Cell Research. 2009;1793:603–604. doi: 10.1016/j.bbamcr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Goranov AI, Cook M, Ricicova M, Ben-Ari G, Gonzalez C, Hansen C, Tyers M, Amon A. The rate of cell growth is governed by cell cycle stage. Genes Dev. 2009;23:1408–22. doi: 10.1101/gad.1777309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu WW, Etkin LD, Le Gros MA, Larabell CA. X-ray tomography of Schizosaccharomyces pombe. Differentiation. 2007;75:529–535. doi: 10.1111/j.1432-0436.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- Johnston GC, Pringle JR, Hartwell LH. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977;105:79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Jorgensen P, Edgington NP, Schneider BL, Rupes I, Tyers M, Futcher B. The size of the nucleus increases as yeast cells grow. Molecular Biology of the Cell. 2007;18:3523–3532. doi: 10.1091/mbc.E06-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P, Tyers M. How cells coordinate growth and division. Current Biology. 2004;14:R1014–R1027. doi: 10.1016/j.cub.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Kirz J, Jacobsen C, Howells M. Soft x-ray microscopes and their biological applications. Quarterly reviews of biophysics. 1995;28:33–130. doi: 10.1017/s0033583500003139. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Herman PK, Emr SD. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990;54:266–92. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. Journal of Structural Biology. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Larabell C, Le Gros M. Whole cell cryo X-ray tomography and protein localization at 50 micron resolution. Biophysical Journal. 2004a;86:185A–185A. [Google Scholar]
- Larabell CA, Le Gros MA. X-ray tomography generates 3-D reconstructions of the yeast, Saccharomyces cerevisiae, at 60-nm resolution. Molecular Biology of the Cell. 2004b;15:957–962. doi: 10.1091/mbc.E03-07-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros MA, McDermott G, Larabell CA. X-ray tomography of whole cells. Current Opinion in Structural Biology. 2005;15:593–600. doi: 10.1016/j.sbi.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Leis A, Rockel B, Andrees L, Baumeister W. Visualizing cells at the nanoscale. Trends in Biochemical Sciences. 2009;34:60–70. doi: 10.1016/j.tibs.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Fiducial Marker and Hybrid Alignment Methods for Single-and Double-Axis Tomography. In: Frank J, editor. Electron tomography : methods for three-dimensional visualization of structures in the cell. Springer; 2005. pp. 163–185. [Google Scholar]
- McDermott G, Le Gros MA, Knoechel CG, Uchida M, Larabell CA. Soft X-ray tomography and cryogenic light microscopy: the cool combination in cellular imaging. Trends Cell Biol. 2009;19:587–95. doi: 10.1016/j.tcb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison JM. The Growth of Single Cells. 1. Schizosaccharomyces Pombe. Experimental Cell Research. 1957a;13:244–262. doi: 10.1016/0014-4827(57)90005-8. [DOI] [PubMed] [Google Scholar]
- Mitchison JM. The growth of single cells. I. Schizosaccharomyces pombe. Exp Cell Res. 1957b;13:244–62. doi: 10.1016/0014-4827(57)90005-8. [DOI] [PubMed] [Google Scholar]
- Murray LE, Veinotdrebot LM, Hanicjoyce PJ, Singer RA, Johnston GC. Effect of Ploidy on the Critical Size for Cell-Proliferation of the Yeast Saccharomyces-Cerevisiae. Current Genetics. 1987;11:591–594. [Google Scholar]
- Neumann FR, Nurse P. Nuclear size control in fission yeast. Journal of Cell Biology. 2007;179:593–600. doi: 10.1083/jcb.200708054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DY, McDermott G, Etkin LD, Le Gros MA, Larabell CA. Quantitative 3-D imaging of eukaryotic cells using soft X-ray tomography. Journal of Structural Biology. 2008;162:380–386. doi: 10.1016/j.jsb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl G, Rudolph D, Schneider G, Thieme J, Schliebe T, Kaulich B, Hettwer M. Diffraction optics for x-ray imaging. Microelectronic Engineering. 1996;32:351–367. [Google Scholar]
- Schneider G. X-ray microscopy: methods and perspectives. Analytical & Bioanalytical Chemistry. 2003;376:558–561. doi: 10.1007/s00216-003-2007-x. [DOI] [PubMed] [Google Scholar]
- Schneider G, Anderson E, Vogt S, Knochel C, Weiss D, Legros M, Larabell C. Computed tomography of cryogenic cells. Surface Review & Letters. 2002;9:177–183. [Google Scholar]
- Schneider G, Denbeaux G, Anderson E, Pearson A, Bates W, Vogt S, Knochel C, Meyer MA, Zschech E. High resolution X-ray tomography with applications in biology and materials science. Journal de Physique IV. 2003;104:607–613. [Google Scholar]
- Stayman JW, Fessler JA. Compensation for nonuniform resolution using penalized-likelihood reconstruction in space-variant imaging systems. IEEE Transactions on Medical Imaging. 2004;23:269–84. doi: 10.1109/TMI.2003.823063. [DOI] [PubMed] [Google Scholar]
- Tsien RY. Building and breeding molecules to spy on cells and tumors. FEBS Lett. 2005;579:927–32. doi: 10.1016/j.febslet.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Uchida M, McDermott G, Wetzler M, Le Gros MA, Myllys M, Knoechel C, Barron AE, Larabell CA. Soft X-ray tomography of phenotypic switching and the cellular response to antifungal peptoids in Candida albicans. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906145106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umen JG. The elusive sizer. Current Opinion in Cell Biology. 2005;17:435–441. doi: 10.1016/j.ceb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Warren G, Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- Weiss D. Computed Tomography Based on Cryo X-ray Microscopic Images of Unsectioned Biological Specimens. Georg-August University of Göttingen; 2000. [Google Scholar]
- Weiss D, Schneider G, Niemann B, Guttmann P, Rudolph D, Schmahl G. Computed tomography of cryogenic biological specimens based on X-ray microscopic images. Ultramicroscopy. 2000;84:185–197. doi: 10.1016/s0304-3991(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Weiss D, Schneider G, Vogt S, Guttmann P, Niemann B, Rudolph D, Schmahl G. Tomographic imaging of biological specimens with the cryo transmission X-ray microscope. Nuclear Instruments & Methods in Physics Research Section A-Accelerators Spectrometers Detectors & Associated Equipment. 2001;467:1308–1311. [Google Scholar]
- Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nature Reviews Molecular Cell Biology. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1. Comparison of cell volumes in two strains of diploid cells: ATCC200060 and DDY1102.
Suppl. Figure 2. Bud scars on the surface of the mother cells shown within the bounded areas. Scale bar, 0.75 μm.
Suppl. Figure 3. Representative cells shown in orthoslice demonstrating the relative locations of vacuoles and their different components in the cell.
Suppl. Movie 1. A movie of the representative diploid and haploid cells from each phase of cell cycle.


