Abstract
The beneficial pharmacological effects of flavonoids such as chemo-prevention against cancer, aging and heart diseases are severely limited due to their extensive in vivo glucuronidation by UGTs. UGTs showed regiospecificity (i.e. position preference) in the glucuronidation of the flavonoids based on substrate’s chemical structure. In this paper, glucuronide(s) of 36 flavones and flavonols were generated using an in vitro glucuronidation reaction. UPLC/MS/MS was used to confirm the degree (mono- or di-) of glucuronidation in flavonoids with up to four hydroxyl group. UV spectra of flavonoids and their respective mono-O-glucuronides were generated using UPLC with an online diode-arrayed detector. Analysis of the extent of shift in spectra of glucuronides in Band I and Band II regions as reflected by changes in λmax value was used to identify the position of glucuronidation. The data showed that glucuronidation of 3- and 4’-hydroxyl resulted in Band I λmax hypsochromic shift (or blue shift) of 13–30 nm and 5–10 nm, respectively. And glucuronidation of 5-hydroxyl group caused Band II λmax hypsochromic shift of 5–10 nm. In contrast, glucuronidation of 7-hydroxyl group did not cause any λmax change in Band I or II λmax whereas glucuronidation of 6-hydroxyl group did not cause predictable changes in λmax values. The paper demonstrated for the first time that a rapid and robust analysis method using λmax changes in online UV spectra can be used to pinpoint region-specific glucuronidation of flavones and flavonols with hydroxyl groups at 4’, 3, 5, and/or 7 position(s).
Keywords: flavonoids, glucuronidation, UPLC, position, regiospecific
INTRODUCTION
Flavonoids are a class of phytochemicals widely distributed in the plant world and are believed to possess a myriad of beneficial effects, but their poor bioavailabilities have severely limited their potentials as agents that can be developed as drugs (1–8). This is because low bioavailabilities mean large variability in individual exposure, which would require a larger population for clinical trials to demonstrate effectiveness (7–13). The extensive metabolism of flavonoids by glucuronidation is the main reason for their poor in vivo bioavailabilities, with sulfation designated as the secondary contributing factor (10, 11, 14). O-methylation has also been reported as a contributing metabolic pathway for various flavonoids (15). Phase II metabolism is considered as the major detoxification pathway in the human body. Usually, the addition of glucuronic acid and sulfate moiety to the structure of flavonoids through glucuronidation and sulfation reactions makes the flavonoids more hydrophilic, which can then be eliminated through bile and kidney (16, 17).
In order to overcome the problem of their low bioavailabilities, it is becoming increasingly urgent to understand the structure-metabolism relationship (SMR) between flavonoids and various UDP-glucuronosyltransferase (or UGT) isoforms. However, development of this understanding had been impeded by the lack of UGT crystal structure and very limited qualitative and quantitative information about the regiospecific glucuronidation of large numbers of structurally diverse flavonoids.
Approximately 150 articles on the topic of flavonoid glucuronidation have been published since 2000, which is 20% of total number of glucuronidation paper published during that period, according to a PubMed search of UGT and metabolism on Oct 16, 2009. However, it was interesting to note that only 10–15% of the published literature on flavonoid glucuronidation had information about the position of flavonoid glucuronides, and more than half of which were about glucuronides of a single flavonol, quercetin. We hypothesized that this was because most laboratories did not have access to a convenient method that could identify the position of glucuronidation.
NMR would be one of the best if not the best methods to determine the position of glucuronidation in a flavonoid. In an earlier paper from this lab, we identified the position of prunetin glucuronidation via the use of NMR, which is quite time-consuming (18). It was affordable to us because only one glucuronide was formed when using a particular UGT (e.g., UGT1A10) isoform. It would be exceeding time consuming and difficult if we had to separate two or more glucuronides using liquid chromatography.
To improve upon this time consuming method, Dr. Brodbelt and co-workers had used the positive and negative electrospray ionization mass spectrometry (EI-MS) coupled with collision-induced dissociation (CID) to identify the position of flavonoid glucuronidation (19, 20). This group used EI-MS followed by post-column complexation of flavonoid with transition metal such as magnesium(II), cobalt(II), nickel(II) and auxillary ligand such as 4,7-dimethyl-1,10-phenanthroline and 4,7-diphenyl-1,10-phenanthroline. Upon CID, this complex yielded product ions that were used to identify the position of glucuronidation (19, 20). Whereas this method significantly advanced our ability to identify the position of glucuronidation, it remains complex and requires the use of complex instrumentation and reagents. On the other hand, this work demonstrated that it is possible to modernize a classical method that used flavonoid-metal complex to identify the position of glycosylation or glucuronidation using purified flavonoids (19–21). Other groups have also reported the use of ESI-MS coupled with HPLC-UV for detection of the position of glycosylation or glucuronidation in flavonoids (21–25).
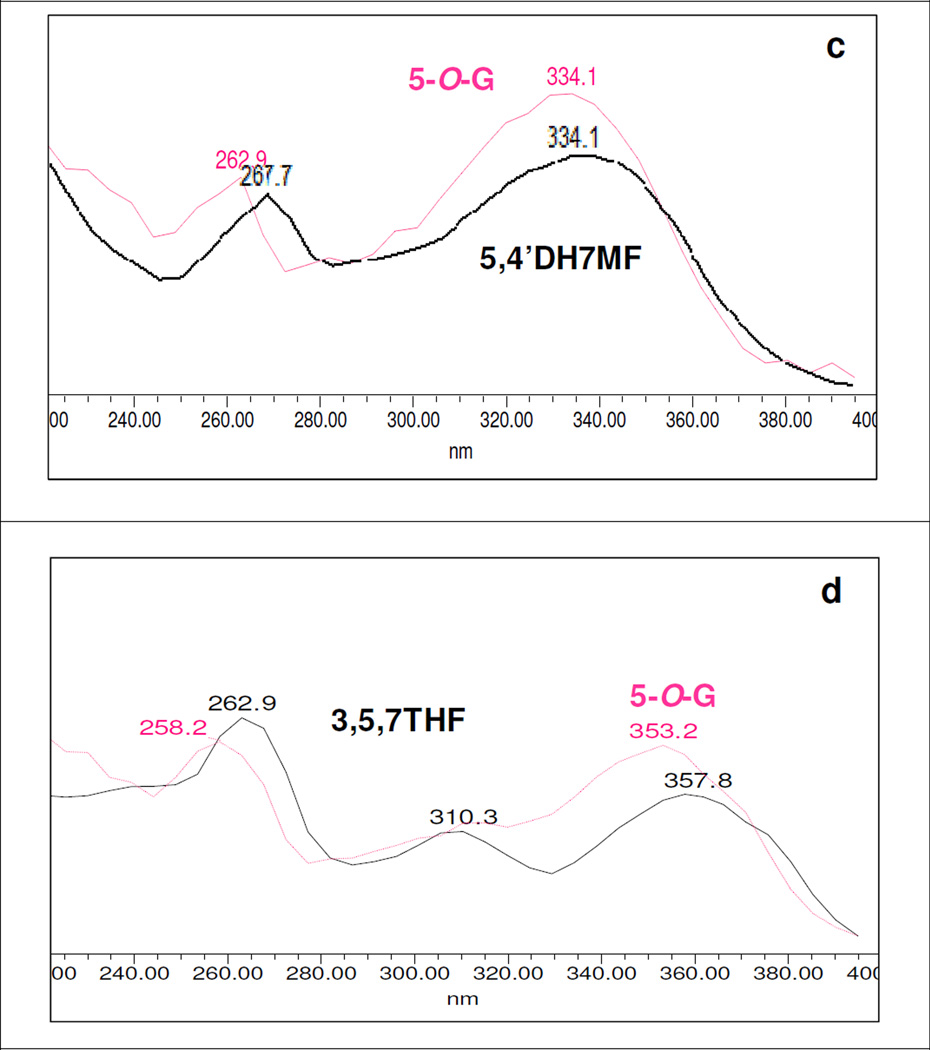
Recently, we became aware of 1970s literatures that showed position of glycosylation of flavonoids can significantly change the UV spectra of their respective aglycones (26). Although the observations were mainly based on work with purified flavonoids with glycosylation (with a variety of sugar moieties at different positions (26), the potentials exist to adapt it to modern analytical techniques such as UPLC with online UV spectra without having to first purified the compounds. Because flavones and flavonols are two of the major subclasses of flavonoids, each numbered in hundreds and commercially available, they were selected as the model compounds for the present study (Fig.1), and we used the term “flavonoids” in this paper to represent these two subclasses of flavonoids. They were also selected because they consistently showed (with rare exception) two major absorption peaks (or λmax) in the region of 240–280nm (commonly referred to as Band II) and 300–380 nm (commonly referred to as Band I). Spectra of flavones and flavonols (with 3-hydroxyl group) could be differentiated by the Band I λmax. Band I λmax of flavones occurred at 304–350 nm while that of flavonols occurred at longer wavelengths of 352–385 nm (26). Lastly, these flavonoids were selected because multiple hydroxyl positions (e.g., 3, 5, 7, and/or 4’ position) may be glucuronidated, which allowed us to determine the preferred position for glucuronidation in a flavonoid with multiple hydroxyl groups. The latter is important since most of the flavonoids in nature have multiple hydroxyl groups.
Fig.1.
Backbone of selected flavonoids used for the present study with one or more hydroxyl and/or methoxyl group substitutions. In this paper, term “flavonoid” has been used to represent the compounds from sub-classes flavone and flavonol only. Band I was considered to be associated with absorption due to B-ring cinnamoyl system (mark in Fig.1), and Band II with absorption due to A-ring benzoyl system (mark in Fig.1).
Therefore, the purpose of this paper is to establish a fast, economical and easily accessible method to determine position of mono-O-glucuronidation of flavonols and flavones. We hypothesized that this new method would cost much less with respect to time and efforts, and demand less chemistry and mass spectrometry expertise. Such a method should be very helpful in furthering the research of SMR of UGTs and role of UGT metabolism in drug disposition. It would also raise the quality of the future research endeavors in the field of flavonoid metabolism as it would be much easier for other researchers to use the same approach to identify the position of flavonoid glucuronidation.
EXPERIMENTAL SECTION
Expressed human UGT isoforms (Supersomes™) 1A1, 1A7, 1A8, 1A9 and 1A10 were purchased from BD Biosciences (Woburn, MA). β-D-Glucuronidase without sulfatase (product No. G7396), uridine diphosphoglucuronic acid (UDPGA), alamethicin, D-saccharic-1,4-lactone monohydrate, and magnesium chloride were purchased from Sigma-Aldrich (St Louis, MO). Ammonium acetate (HPLC grade) was purchased from J.T. Baker (Phillipsburg, NT). The UGT isoforms for generating the glucuronides were selected based on the preliminary and published studies (27) from our lab, which showed that 1A1, 1A7, 1A8, 1A9 and 1A10 were the major UGT isoforms to glucuronidate flavonoids. Also, certain UGT isoforms could produce sufficient amount of regiospecific glucuronides of a flavonoid with multiple hydroxyl groups so that its online UV spectra will be of high quality (higher signal to noise ratio).”
Thirty-six (36) flavonoids belonging to the flavones or flavonols subclass with one or more hydroxyl and/or methoxyl substitutions at different positions were purchased from Indofine Chemicals (Somerville, NJ). The selected compounds consisted of 18 flavonoids with one hydroxyl group (i.e., 3-hydroxylflavone (or 3HF), 4’HF, 5HF, 6HF, 7HF, 3-hydroxyl-4’-methoxyflavone (or 3H4’MF), 3H5MF, 3H6MF, 3H7MF, 4’H6MF, 4’H7MF, 5H7MF, 6H4’MF, 6H7MF, 7H4’MF, 5,7-dimethoxy-3-hydroxylflavone (or 5,7DM3HF), 6,4’DM3HF, 7,4’DM3HF); 13 flavonoids with two hydroxyl groups (i.e., 3,4’-dihydroxylflavone (or 3,4’DHF), 3,5DHF, 3,6DHF, 3,7DHF, 5,4’DHF, 5,6DHF, 5,7DHF, 6,4’DHF, 6,7DHF, 7,4’DHF, 5,4’-dihydroxyl-7-methoxyflavone (or 5,4’DH7MF), 5,6DH7MF, 5,7DH8MF); 4 flavonoids with three hydroxyl groups (i.e., 3,6,4’-trihydroxylflavone (or 3,6,4’THF), galangin (or 3,5,7THF), resokaempferol (or 3,7,4’THF), and apigenin (or 5,7,4’THF)); and one flavonoid with four hydroxyl groups (i.e., kaempferol (or 3,5,7,4’ tetrahydroxylflavone)). All other materials (typically analytical grade or better) were used as received.
Generation of Flavonoids Glucuronide(s) by UGTs
UGT1A9 was used to generate most of the flavonoid glucuronides unless otherwise specified, and UGT1A1 was used to generate glucuronides of 3,4’DHF, 3,6DHF, 3,7DHF, 5,6DHF, 6,4’DHF, 6,7DHF, 7,4’DHF, 3,6,4’THF, 5,6DH7MF, galangin and resokaempferol. UGT 1A7, 1A8, and 1A10 were additionally used for generating certain regiospecific glucuronides of kaempferol. Different UGTs were used because of two reasons. First, these isoforms were able to generate enough metabolite of mono-hydroxyl flavonoid to produce good quality UV spectra. Second, for certain multi-hydroxyl flavonoids, different UGT isoforms preferentially generate a regiospecific glucuronide in large quantities, allowing us to derive good UV spectra needed for position idenntification.
The incubation procedure producing glucuronides(s) was essentially the same as published previously (18, 28). Briefly, incubation procedures using recombinant UGT isoforms (SupersomesTM) were as follows: (1) Supersomes™ (final concentration in range of 0.0125–0.05 mg of protein per mL as optimum for the reaction, magnesium chloride (0.88 mM), saccharolactone (4.4 mM), alamethicin (0.022 mg/mL), 10 or 25 µM concentration of flavonoid in a 50 mM potassium phosphate buffer (pH 7.4), and UDPGA (3.5 mM, add last) were mixed; (2) the mixture (final volume 200 µL) was incubated at 37°C for overnight so as to generate sufficient amounts of flavonoid glucuronides; and (3) the reaction was stopped by the addition of 50µL of 94% acetonitrile/6% glacial acetic acid containing 50 µM internal standard. Testosterone was used as an internal standard for most compounds except for 3,7DHF where 5-hydroxylflavone was used as internal standard; and for 3HF, 3,5DHF, resokaempferol and galangin where formononetin was used as an internal standard.
Generation of Online UV Spectra of Flavonoids and Their Glucuronides Using UPLC
The Waters Acquity UPLC (Ultra performance liquid chromatography) system equipped with a photodiode array detector (PDA), sample manager, binary solvent manager, Empower software, and BEH C18 column (1.7 µm, 2.1 × 50 mm) was used to separate each of the flavonoid and its glucuronides(s) in the sample and obtain the corresponding UV spectra.
A general LC method was used for all the analysis unless otherwise specified. The parameters used were: mobile phase A, 100% aqueous buffer (2.5mM ammonium acetate, pH 4.5); mobile phase B, 100% acetonitrile; flow rate 0.45 mL/min; gradient: 0 min, 10% B, 0 to 2 min, 10–20%B, 2 to 3 min, 20–70%B, 3 to 3.5 min, 70%B, 3.5 to 4 min, 70–10%B; and injection volume, 10 µL. For kaempferol, different mobile phase A and gradient method were adopted: mobile phase A, 0.2% v/v formic acid and gradient: 0 min, 10% B, 0 to 2 min, 10–20%B, 2 to 3 min, 20–40%B, 3 to 3.5 min 40–50%B, 3.5 to 4 min, 50–70%B, 4 to 4.5 min, 70%B, and 4.5 to 5 min, 70–10%B. Kaempferol needed different condition for better separation of the three glucuronide(s) formed.
The absorbance values (y-axis scales) of UV spectral plots of each flavonoid and its metabolite(s) were normalized to the same dimensions with respect to each other using the normalization tool of the Empower software. The spectral plots of flavonoids and the corresponding metabolite(s) were displayed along the y-axis such that the axis range was 100% of the absorbance values range.
Confirmation of Glucuronides Using Hydrolysis with β-D-Glucuronidase Enzyme
The glucuronidation reaction was run with UGT isoform(s) to almost complete substrate exhaustion at a substrate concentration of 25 µM. Glucuronides produced by UGTs incubation were further purified using solid phase extraction. After a PolarPlus® Octadecyl C18 Speedisk® 10µm solid phase extraction column (J.T. Baker, NJ; column volume, 3 ml) was washed with 2 mL methanol and 1 mL water, samples were loaded on to the column. 1mL water was then used to clear up the salts and saccharolactone, and successive 2 mL methanol elution fractions containing glucuronides were then collected. This was followed by 2 hours of air dying, and the residue was reconstituted with potassium phosphate butter (pH 7.4). A 500 µL portion of the metabolite solution was taken and 415 unit/mL of β-D-glucuronidase enzyme was added to hydrolyze the metabolites (into aglycone) at 37°C for 5 mins. The control reaction was run using equivalent volume of purified water in the place of enzyme solution.
Confirmation of Degree of Substitution of Flavonoids Using UPLC/MS/MS
Glucuronide(s) of flavonoids with more than one hydroxyl group in their structure were analyzed to confirm for their degree(s) of glucuronic acid substitution (i.e. mono- or di-glucuronides) using UPLC/MS/MS method. Similar UPLC conditions as explained above were used to separate the flavonoids and their respective glucuronides(s). The effluent from Waters Acquity UPLC system was introduced into an API 3200 Qtrap triple-quadrupole mass spectrometer (Applied Biosystem/MDS SCIEX, Foster City, CA) mounted with a TurboIonSpray™ source. The working parameters for the mass spectrometers and compound-specific MS conditions were optimized. The mass of each individual peak of separated glucuronides was measured using QSMS mode. In MS2 scan, precursor ion mode was used to confirm the identity of each peak as glucuronide, in which Q3 was held to measure the occurrence of the aglycone fragment ion and Q1 is scanned for the glucuronide(s) ions that result in the corresponding aglycone ion (29, 30).
RESULTS AND DISCUSSION
Generation and Confirmation of Flavonoids Glucuronide(s) by UGT Isoforms
36 selected flavones and flavonols with one or more hydroxyl groups at different positions (3, 5, 6, 7 or 4’) (Fig.1) in their structure were glucuronidated using either UGT 1A1, 1A7, 1A8, 1A9 or 1A10 isoforms. All flavonoids with one hydroxyl group formed single mono-O-glucuronides, regardless of the presence of the methoxyl group. Among flavonoids with two or more hydroxyl groups, 3,5DHF, 5,7DHF, 5,6DH7MF, 5,7DH8MF, apigenin formed only one major detectable glucuronide; while 3,4’DHF, 3,6DHF, 3,7DHF, 5,4’DHF, 6,4’DHF, 7,4’DHF, and 5,4’DH7MF formed 2 major glucuronides; and 3,6,4’THF, galangin, resokaempferol, kaempferol formed 3 major glucuronides (Fig. S2.33-S2.36 in supporting information). “Kaempferol formed kaempferol-3-O-glucuronide (or 3-O-G of kaempferol) and kaempferol-7-O-G (or 7-O-G of kaempferol) as the major metabolites, while kaempferol-4’-O-G (or 4’-O-G of kaempferol) as the minor metabolite with the selected UGT isoforms.”
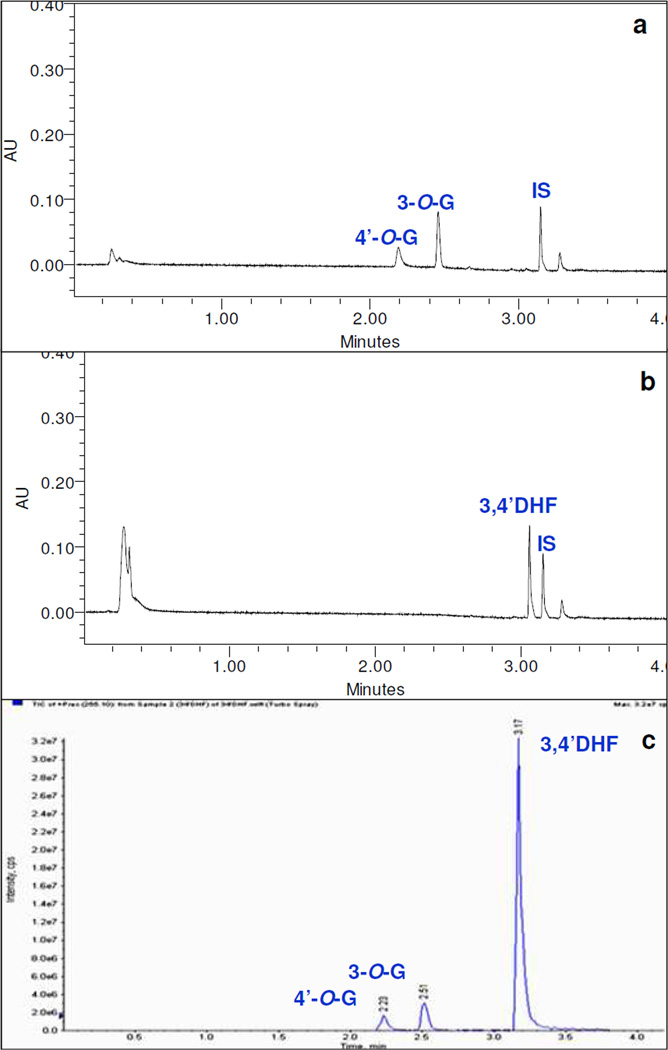
The metabolite peaks were first confirmed as glucuronides by hydrolyzing them with β-D-glucuronidase as described in the method. All mono- or di- glucuronides peaks disappeared to give respective aglycone peaks. Fig. 2 panel A showed the UPLC chromatograms of glucuronides of 3,4’DHF hydrolyzed with β-D-glucuronidase and their respective control samples. Similar results were obtained for other flavonoids and shown in Figure S1 panel A of the supporting information (SI). Galangin and kaempferol also showed one more mono-O-glucuronide most probably substituted at 5-hydroxyl group but the concentration was too low for proper UV spectrum analysis. Also, galangin and kaempferol formed a minor di-glucuronide (confirmed by UPLC/MS/MS) but UV spectrum shift method (i.e., change in λmax) was not able to identify the positions of glucuronidation for galangin and kaempferol di-glucuronides (Figure S1 panel A, SI).
Fig.2.
Panel A UPLC chromatograms at 254nm of (top: control sample and bottom: sample after hydrolysis with β-D-Glucuronidase) and Panel B LC/MS/MS scans of 3,4’DHF and its glucuronides generated by either UGT1A1 at an incubation concentration of 25 µM.
Confirmation of Degree of Substitution of Flavonoids by UPLC/MS/MS
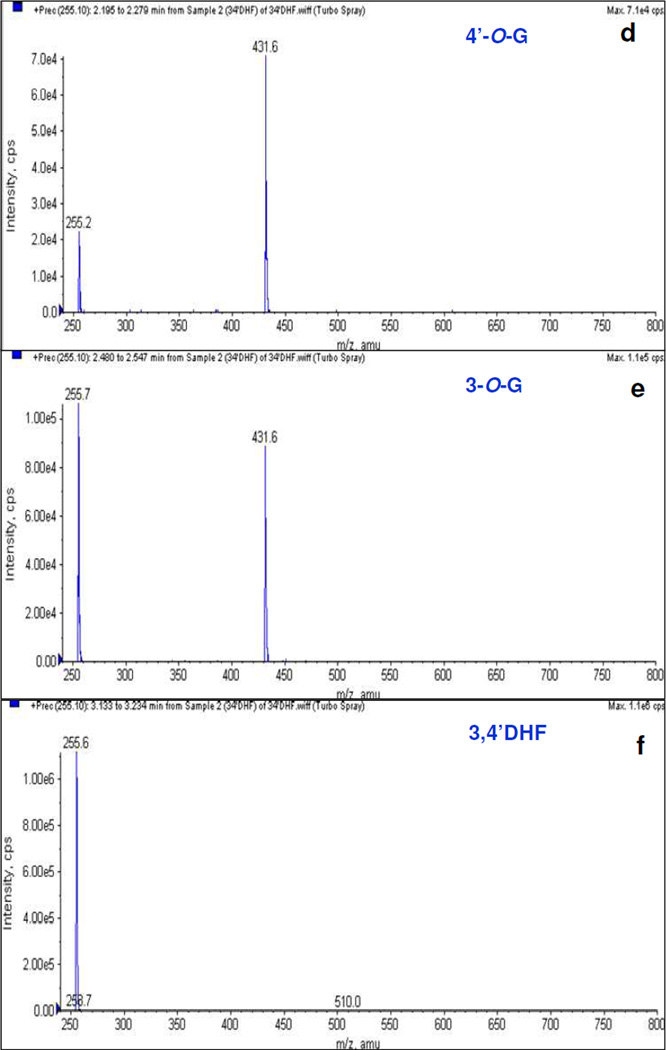
Although treatment with glucuronidase were able to show that the metabolites were indeed glucuronides, LC/MS/MS method was needed to show if the glucuronide was mono- or di- or tri-glucuronides. The results indicated that glucuronidation of all selected flavonoids, even those with multiple hydroxyl groups, generated only one or more detectable mono-O-glucuronide(s) except for galangin and kaempferol, whose glucuronidation also generated one di-glucuronide each. Galangin di-glucuronide was formed by UGT1A7 and 1A9 at 10 and 25µM substrate concentrations, whereas kaempferol di-glucuronide was formed by UGT1A10 only at the highest concentration (25µM).
Flavonoids mono- or di- glucuronides were identified by MS and MS2 (precursor ion) full scan modes. The main working parameters for a few compounds on which mass spectrometer were set had been shown in Table S1 of supporting information. The conjugates produced strong mono-O-glucuronide molecular ions under the conditions used. Fig. 2 panel B showed MS scans of glucuronides of 3,4’DHF (m/z 431 [M+1]+) as examples. These diagnostic ions were also shown to form the respective aglycone by losing a glucuronic acid moiety (Fig. S2 panel B). Additionally, MS scans of glucuronides of 3HF (m/z 415 [M+1]+); 3,5DHF (m/z 431 [M+1]+); 3,7DHF (m/z 429 [M-1]-); galangin (m/z 445 [M-1]-); resokaempferol (m/z 445 [M-1]-); and kaempferol (m/z 461 [M-1]-) could be found in the supporting information (Figure S1 panel B of SI).
Confirmation of Position of Glucuronidation
The sites of mono-O-glucuronidation of flavonoids were determined based on the λmax shifts in their characteristic UV absorption spectra (26, 31). Fig. 3–6 showed the UV spectra of 16 selected flavonoids and their corresponding single mono-O-glucuronide at 3, 7, 4’ or 5 positions as examples, and others were more or less similar (see Figure S2 of SI). A systematic analysis of UV spectrum change as the result of glucuronidation of 18 different flavonoids with one hydroxyl groups at 3, 4’, 5, 6 or 7 showed how λmax shifted due to glucuronidation (Figures S2.1-S2.18, SI), which were to be detailed below.
Fig.3.
UV spectra of (a) 3HF; (b) 3H5M; (c) 3,5DHF; and (d) resokaempferol (3,7,4’THF) (solid black line) and their 3-O-glucuronides (red dotted line) generated by either UGT1A1, 1A7, 1A8, 1A9, or 1A10 at an incubation concentration of 10 or 25 µM.
Fig.6.
UV spectra of (a) 5HF; (b) 5H7M; (c) 5,4’DH7MF; and (d) galangin (3,5,7THF) (solid black line) and their 5-O-glucuronides (magenta dotted line) generated by either UGT1A1, 1A7, 1A8, 1A9, or 1A10 at an incubation concentration of 10 or 25 µM.
The analysis demonstrated how positions of glucuronidation of 18 flavonoids (with more than one hydroxyl groups) were solved based on the changes in λmax value or spectrum shift obtained from glucuronidation of 18 flavonoids with only one hydroxyl group. To simplify the data presentation, results are organized according to the position of glucuronidation of the hydroxyl group in the flavonoid structure.
In case where there were more than one mono-O-glucuronides, we were able to identify each of mono-O-glucuronide by identifying either the maximal number of mono-O-glucuronides possible (i.e., three mono-O-glucuronide for a tri-hydroxyflavone) or by eliminating 5-OH group as a group for glucuronidation in flavonoid with multiple hydroxyl groups since 5-O-glucuronidaton was very slow and normally not detectable under current reaction conditions.
Effects of Glucuronidation at 3-Hydroxyl Group on λmax Shift in UV Spectra
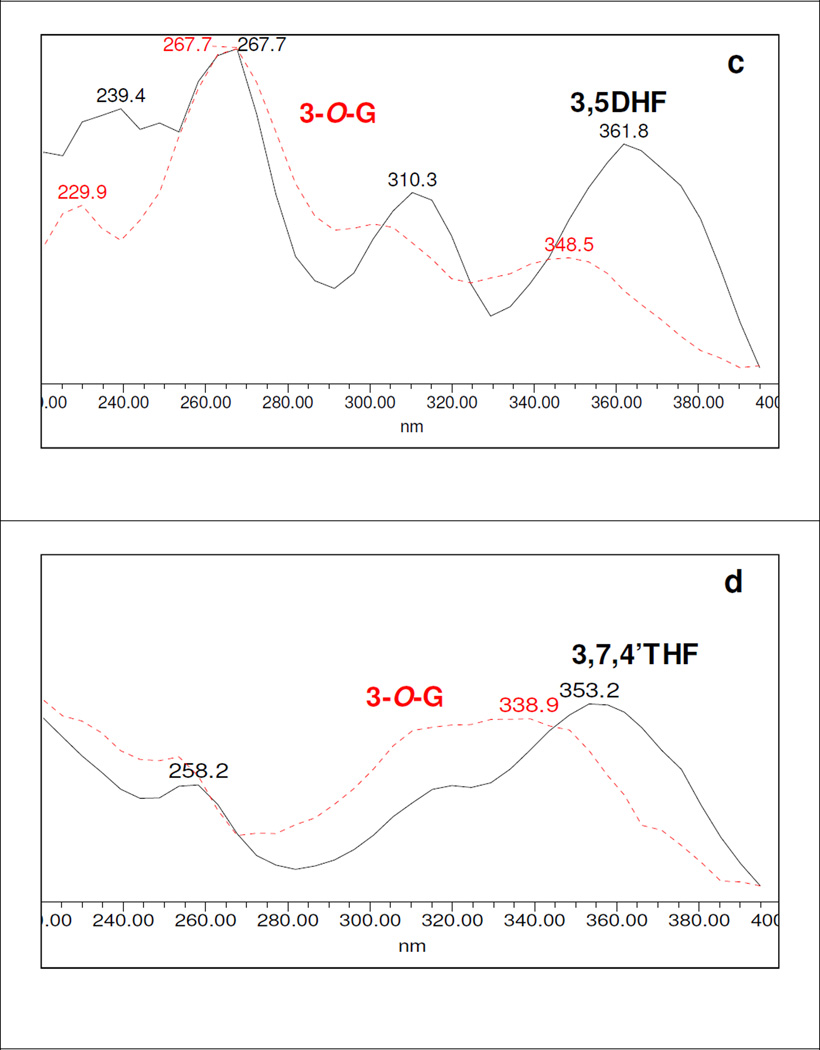
The resulted UV spectra showed that the glucuronidation of hydroxyl position at C3 resulted in either diminishment of UV absorption and/or an approximately 14–29 nm hypsochromic shift in Band I λmax, but no consistent shift in Band II λmax (Figures S2.1-S2.8, SI). The results were tabulated in Table 1. As examples, shifts in Band I and Band II λmax of 3-O-glucuronide of 3HF, 3H5MF, 3,5DHF and resokaempferol (3,7,4’THF) had been shown in Fig. 3a-3d.
Table 1.
Diagnostic shift in Band I of the 3-O-glucuronides in relation to corresponding aglycone
| Compound | Aglycone or Glucuronide (O-G) |
λmax, nm | Diagnostic shifts in Band I in relation to aglycone |
Figure number in SI |
|
|---|---|---|---|---|---|
| Band II | Band I | ||||
| 3HF | Aglycone | 239.4 | 343.7 | 2.1 | |
| 3-O-G | 248.8 | / | Band I disappeared | 2.1 | |
| 3H4’MF | Aglycone | 234.7 | 353.2 | 2.2 | |
| 3-O-G | ND* | 329.4 | −23.8 nm | 2.2 | |
| 3H5MF | Aglycone | 262.9 | 357.8 | 2.3 | |
| 3-O-G | 262.9 | 338.9 | −18.9 nm | 2.3 | |
| 3H6MF | Aglycone | 253.5 | 329.4 | 2.4 | |
| 3-O-G | 262.9 | 310.2 | −19.1 nm | 2.4 | |
| 3H7MF | Aglycone | 253.5 | 338.9 | 2.5 | |
| 3-O-G | 248.8 | 310.3 | −28.6 nm | 2.5 | |
| 5,7DM3H | Aglycone | 253.5 | 348.5 | 2.6 | |
| 3-O-G | ND* | 329.4 | −19.1 nm | 2.6 | |
| 6,4'DM3HF | Aglycone | ND* | 348.5 | 2.7 | |
| 3-O-G | ND* | 334.1 | −14.4 nm | 2.7 | |
| 7,4'DM3HF | Aglycone | 262.9 | 348.5 | 2.8 | |
| 3-O-G | 262.9 | 329.4 | −19.1 nm | 2.8 | |
| 3,4’DHF | Aglycone | 234.7 | 357.8 | 2.19 | |
| 3-O-G | 234.7 | 329.4 | −28.4 nm | 2.19 | |
| 3,5DHF | Aglycone | 267.7 | 361.8 | 2.20 | |
| 3-O-G | 267.7 | 348.5 | −13.3 nm | 2.20 | |
| 3,6DHF | Aglycone | 258.2 | 329.4 | 2.21 | |
| 3-O-G | 253.5 | 315.1 | −14.3 nm | 2.21 | |
| 3,7DHF | Aglycone | 253.5 | 338.9 | 2.22 | |
| 3-O-G | 248.8 | 315.1 | −23.8 nm | 2.22 | |
| Galangin | Aglycone | 262.9 | 357.8 | 2.33 | |
| 3-O-G | 262.9 | / | Band I disappeared | 2.33 | |
| 3,6,4’THF | Aglycone | ND* | 357.8 | 2.34 | |
| 3-O-G | 267.7 | 338.9 | −18.9 nm | 2.34 | |
| Resokaempferol | Aglycone | 253.5 | 353.2 | 2.35 | |
| 3-O-G | ND* | 338.9 | −14.3 nm | 2.35 | |
| Kaempferol | Aglycone | 262.9 | 366.1 | 2.36 | |
| 3-O-G | 262.9 | 348.5 | −17.6 nm | 2.36 | |
ND means not detected by the Empower software in the spectra
All flavonoids with 3-hydroxyl group alone or with one or more hydroxyl group(s) at other position(s) were shown to form at least one 3-O-glucuronide with a hypsochromic shift in Band I λmax in the above-mentioned range. For example, glucuronidation of 3-hydroxyl group of 3HF cause the disappearance of Band II in the UV spectra of 3-O-glucuronide of 3HF (Fig.3a). 3-O-Glucuronidation of 3H5M, 3,5DHF and respokaempferol caused hyposchromic Band I λmax shift of 18.9, 13.3 and 14.3 nm, respectively Fig.3 (b, c, d). Table 1 showed diagnostic hypsochromic shift in Band I λmax of the UV spectra of 3-O-glucuronides of flavonols.
Detailed spectra data (corresponding to values in Table 1) were shown in Fig.S3 (a-d) and Figures S2.19-S2.22 and Figures S2.33-S2.36 of the SI. The λmax shift of unknown flavonoid (those with more than one hydroxyl group) glucuronides was predicted correctly based on the shifts in λmax of the known flavonoid with only one hydroxyl group to form 3-O-glucuronides.
The prediction was made easy because all 3-O-glucuronides showed Band I λmax hyposchromic shifts within the range of ~14–29 nm. The smallest Band I λmax shift (13.3 nm) was displayed by 3-O-glucuronide of 3,5DHF. This strongly suggested that a Band I λmax hypsochromic shift of an unknown flavonol glucuronide in the range of ~13–30 nm without a corresponding shift in Band II λmax value could be used as a spectrum tool to determine the position of glucuronidation of flavonols (or flavones with a 3-hydroxyl group).
Effects of Glucuronidation at 7-Hydroxyl Position on λmax Shift in UV Spectra
The glucuronidation of 7-hydroxyl group had no or minimal effect on the Band I or Band II λmax values in the UV spectrum of flavonoid glucuronides (Fig. 4a-4b). UV spectra of 7HF, 7H4’M, 5,7DHF and apigenin (5,7,4’THF) and their respective 7-O-glucuronides were shown in Fig.4a-4d, as examples. All selected flavonoids with a free hydroxyl group at C7 formed a 7-O-glucuronide. None of the formed 7-O-glucuronides showed any change in their spectra or λmax values except for 7-O-glucuronide of 3,7DHF, which showed a hyposchromic shift of −4.7nm in Band II λmax (Table 2).
Fig.4.
UV spectra of (a) 7HF; (b) 7H4’M; (c) 5,7DHF; and (d) apigenin (5,7,4’THF) (solid black line) and their 7-O-glucuronides (blue dotted line) generated by either UGT1A1, 1A7, 1A8, 1A9, or 1A10 at an incubation concentration of 10 or 25 µM.
Table 2.
Diagnostic shift in Band I and II of the 7-O-glucuronides in relation to corresponding aglycone
| Compound | Aglycone or Glucuronide (O-G) |
λmax, nm | Diagnostic shifts in Band I and II in relation to aglycones |
Figure number in SI |
|
|---|---|---|---|---|---|
| Band II | Band I | ||||
| 7HF | Aglycone | 253.5 | 310.3 | 2.17 | |
| 7-O-G | 253.5 | 310.3 | No change | 2.17 | |
| 7H4’MF | Aglycone | ND* | 329.4 | 2.18 | |
| 7-O-G | ND* | 329.4 | No change | 2.18 | |
| 3,7DHF | Aglycone | 253.5 | 338.9 | 2.22 | |
| 7-O-G | 248.8 | 338.9 | No change (Band I) −4.7 nm (Band II) |
2.22 | |
| 5,7DHF | Aglycone | 267.7 | 310.3 | 2.25 | |
| 7-O-G | 267.7 | 310.3 | No change | 2.25 | |
| 6,7DHF | Aglycone | 267.7 | 319.9 | 2.27 | |
| 7-O-G | 267.7 | 319.9 | No change | 2.27 | |
| 7,4’DHF | Aglycone | ND* | 334.1 | 2.28 | |
| 7-O-G | 253.5 | 334.1 | No change | 2.28 | |
| 5,7DH8MF | Aglycone | 272.4 | 343.7 | 2.31 | |
| 7-O-G | 272.4 | 343.7 | No change | 2.31 | |
| Apigenin | Aglycone | 267.7 | 338.9 | 2.32 | |
| 7-O-G | 267.7 | 338.9 | No change | 2.32 | |
| Galangin | Aglycone | 262.9 | 357.8 | 2.33 | |
| 7-O-G | 262.9 | 357.8 | No change | 2.33 | |
| Resokaempferol | Aglycone | 253.5 | 353.2 | 2.35 | |
| 7-O-G | 253.5 | 353.2 | No change | 2.35 | |
| Kaempferol | Aglycone | 262.9 | 366.1 | 2.36 | |
| 7-O-G | 262.9 | 366.1 | No change | 2.36 | |
ND means not detected by the Empower software in the spectra
In case of 3,7DHF, one glucuronide showed a hypsochromic shift of ~23.8 nm in Band I λmax with no shift in Band II λmax while the other showed a hyposchromic shift of -4.7nm in Band II λmax but no change in Band I (Figure S2.22, SI). These results indicated that the one glucuronide with a shift of ~23.8 nm in Band I λmax was glucuronidated at the 3-hydroxyl group while the other one was glucuronidated at the 7-hydroxyl group. Similarly, 5,7DHF formed only one glucuronide (Fig. 4c) which did not show any change in UV scan, confirming that 7-hydroxyl group was glucuronidated in this case.
In case of both 6,7DHF (Figure S2.27, SI) and 7,4’DHF (Figure S2.28, SI), one glucuronides showed no shift at all while the other showed the hypsochromic shift of ~10 nm in Band I λmax, suggesting that the one with no shift in λmax was glucuronidated at 7-hydroxyl group while the other was glucuronidated at 4’-hydroxyl group or 6-hydroxyl group, respectively. How glucuronidation at 4’- and 6’-hydroxyl groups affected UV spectra were to be discussed below in their respective sections.
Glucuronidation of apigenin (Fig. 4d), galangin (Figure S2.33, SI), resokaempferol (Figure S2.35, SI), and kaempferol (Figure S2.36, SI) at 7-O position also did not change their respective UV spectra. Based on this, we deduced that in most cases (perhaps with rare exceptions not found here), no change in UV spectra of an unknown flavone/flavonol glucuronide was diagnostic of the fact that the position of glucuronidation was 7-hydroxyl group.
Effects of Glucuronidation at 4’-Hydroxyl Group on λmax Shift in UV Spectra
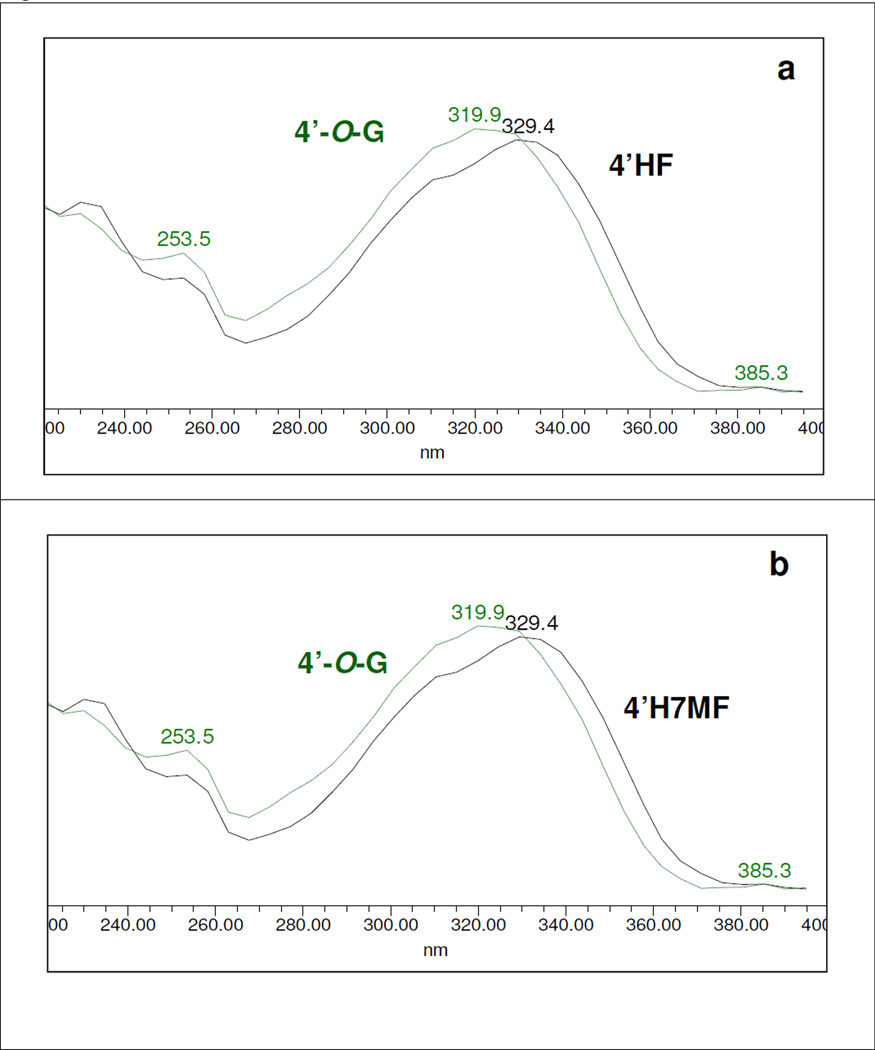
The glucuronidation of 4’-hydroxyl group resulted in approximately 4–10 nm hypsochromic shift in Band I λmax but no consistent shift in Band II λmax (Figures S2.9-S2.11, SI). The results were tabulated in Table 3. UV spectra of 4’HF, 4’H7M, 5,4’DHF and 3,6,4’THF and their respective 4’-O-glucuronides were shown in Fig.5a-5d, as examples.
Table 3.
Diagnostic shift in Band I of the 4’-O-glucuronides in relation to corresponding aglycone
| Compound | Aglycone or Glucuronide (O-G) |
λmax, nm | Diagnostic shifts in Band I in relation to aglycones |
Figure number in SI |
|
|---|---|---|---|---|---|
| Band II | Band I | ||||
| 4’HF | Aglycone | 253.5 | 324.6 | 2.9 | |
| 4’-O-G | 248.8 | 315.1 | −9.5 nm | 2.9 | |
| 4’H6MF | Aglycone | 272.4 | 329.4 | 2.10 | |
| 4’-O-G | 272.4 | 324.6 | −4.8 nm | 2.10 | |
| 4’H7MF | Aglycone | ND* | 329.4 | 2.11 | |
| 4’-O-G | 253.5 | 319.9 | −9.5 nm | 2.11 | |
| 3,4’DHF | Aglycone | 234.7 | 357.8 | 2.19 | |
| 4’-O-G | ND* | 353.2 | −4.6 nm | 2.19 | |
| 5,4’DHF | Aglycone | 267.7 | 329.4 | 2.23 | |
| 4’-O-G | 272.4 | 315.1 | −9.5 nm | 2.23 | |
| 6,4’DHF | Aglycone | 272.4 | 329.4 | 2.26 | |
| 4’-O-G | 272.4 | 319.9 | −9.5 nm | 2.26 | |
| 7,4’DHF | Aglycone | 253.5 | 334.1 | 2.28 | |
| 4’-O-G | 253.5 | 324.6 | −9.5 nm | 2.28 | |
| 5,4’DH7MF | Aglycone | 267.7 | 338.9 | 2.29 | |
| 4’-O-G | 267.7 | 324.6 | −9.5 nm | 2.29 | |
| 3,6,4’THF | Aglycone | ND* | 357.8 | 2.34 | |
| 4’-O-G | 258.2 | 348.5 | −9.3 nm | 2.34 | |
| Resokaempferol | Aglycone | 253.5 | 353.2 | 2.35 | |
| 4’-O-G | 253.5 | 348.5 | −4.7 nm | 2.35 | |
| Kaempferol | Aglycone | 262.9 | 366.1 | 2.36 | |
| 4’-O-G | 262.9 | 357.8 | −8.3 nm | 2.36 | |
ND means not detected by the Empower software in the spectra
Fig.5.
UV spectra of (a) 4’HF; (b) 4’H7M; (c) 5,4’DHF; and (d) 3,6,4’THF (solid black line) and their 4’-O-glucuronides (green dotted line) generated by either UGT1A1, 1A7, 1A8, 1A9, or 1A10 at an incubation concentration of 10 or 25 µM.
In case of 3,4’DHF, one glucuronide showed a hypsochromic shift of ~28.4 nm in Band I λmax while the other showed a shift of only ~5 nm in Band I λmax with no shift in Band II λmax (Figure S2.19, SI). These results indicated that the glucuronide with ~28.4 nm shift in Band I λmax was glucuronidated at 3-hydroxyl group while the other was glucuronidated at 4’-hydroxyl group. This was consistent with the information obtained from the glucuronidation of known 3- and 4’-hydroxyl groups (Table 3).
Similarly, 7-O-glucuronide of 7,4’DHF showed no spectrum shift at all while 4’-O-glucuronide of 7,4’DHF showed a hypsochromic shift of ~9.5 nm in Band I λmax (Figure S2.28, SI), consistent with the information obtained from spectra shift of known 4’-O-glucuronides. Also, 4’-O-glucuronide of resokaempferol (Figure S2.35, SI) and kaempferol (Figure S2.36, SI) showed a hypsochromic shift of ~4.7 and 8.3 nm in Band I λmax, respectively (Table 3). Since 3-O-glucuronide and 7-O-glucuronide of these two compounds were easily recognized as shown earlier, it was possible to identify 4’-O-glucuronides of these two compounds by rule of elimination also.
Based on above observations, we determined the 4’-O-glucuronides of 5,4’DHF (Fig. 5c), 6,4’DHF (Figure S2.26, SI), 3,6,4’THF (Fig. 5d) and 5,4’DH7MF (Figure S2.29, SI), all of which showed a hypsochromic shift of about ~9.5 nm in Band I λmax (Table 3). Therefore, we deduced that glucuronidation at 4’-hydroxyl group would show a consistent and diagnostic hypsochromic shift in Band I λmax in the range of 4–10 nm.
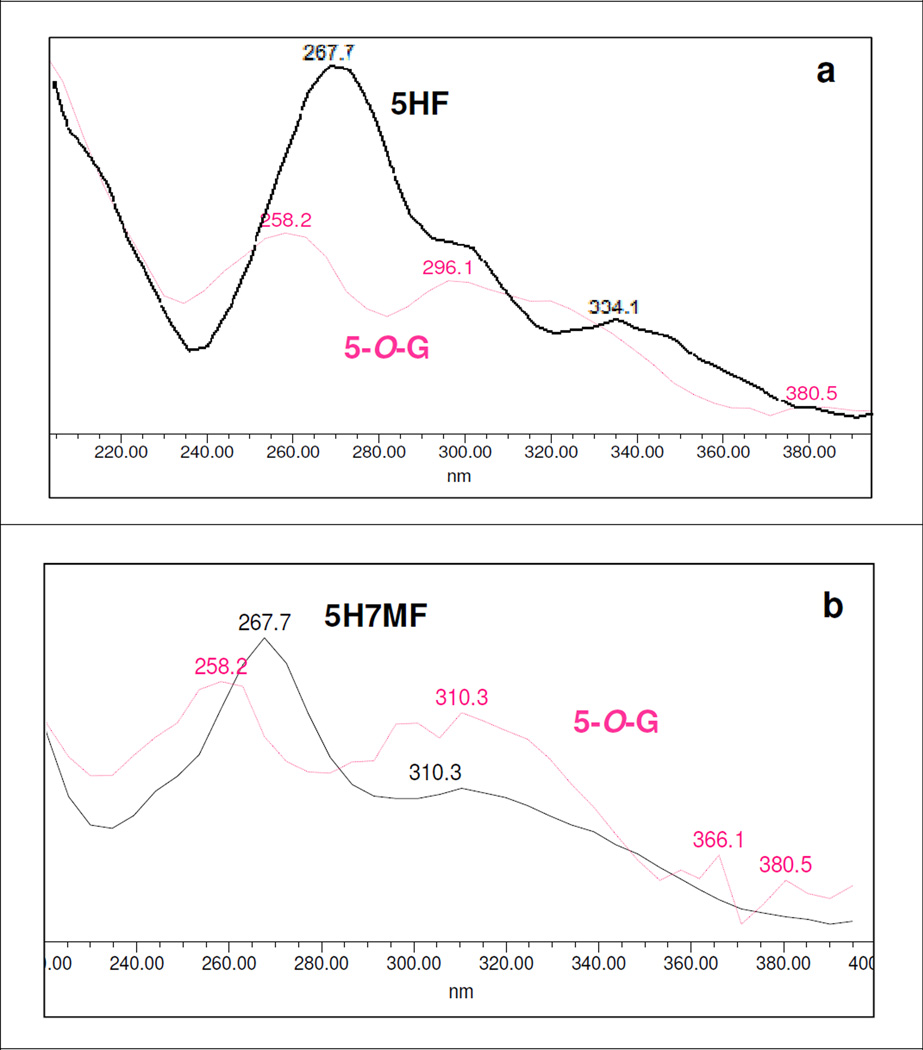
Effects of Glucuronidation at 5-Hydroxyl Group on λmax Shift in UV Spectra
The glucuronidation of 5-hydroxyl group could cause hypsochromic shift of ~10 nm in Band II λmax (Fig. 6a-6b), while no consistent shifts in λmax of Band I could be observed (Figures S2.12-S2.13, SI), as shown in Table 4. Among the selected multi-hydroxyl flavonoids, there were 9 flavonoids with a free 5-hydroxyl group but only four flavonoids (or about 44%) formed 5-O-glucuronide in detectable amounts, strongly suggesting that in presence of additional hydroxyl group(s), 5-hydroxyl group was not the favored position of O-glucuronidation.
Table 4.
Diagnostic shift in Band II of the 5-O-glucuronides in relation to corresponding aglycone
| Compound | Aglycone or Glucuronide (O-G) |
λmax, nm | Diagnostic shifts in Band II in relation to Aglycones |
Figure number in SI |
|
|---|---|---|---|---|---|
| Band II | Band I | ||||
| 5HF | Aglycone | 267.7 | 334.1 | 2.12 | |
| 5-O-G | 258.2 | 324.1 | −9.5 nm | 2.12 | |
| 5H7MF | Aglycone | 267.7 | 310.3 | 2.13 | |
| 5-O-G | 258.2 | 310.3 | −9.5 nm | 2.13 | |
| 5,4’DHF | Aglycone | 267.7 | 329.4 | 2.23 | |
| 5-O-G | 262.9 | 334.1 | −4.8 nm | 2.23 | |
| 5,6DHF | Aglycone | 281.9 | ND* | 2.24 | |
| 5-O-G | 272.4 | 310.3 | −9.5 nm | 2.24 | |
| 5,4’DH7MF | Aglycone | 267.7 | 338.9 | 2.29 | |
| 5-O-G | 258.2 | 334.1 | −4.8 nm | 2.29 | |
| Galangin | Aglycone | 262.9 | 357.8 | 2.33 | |
| 5-O-G | 258.2 | 353.2 | −4.7 nm | 2.33 | |
ND means not detected by the Empower software in the spectra
In case of 5,4’DHF (Fig. 6c), 5,4’DH7MF (Figure S2.29, SI) and galangin (3,5,7THF) (Fig. 6d), the UV spectra of 5-O-glucuronides showed hypsochromic shift of only ~5 nm in Band II λmax. However, in both cases, 5-O-glucuronides could also be deduced based on rule of elimination, suggesting that glucuronidation of 5-hydroxy group could also cause a hypsochromic shift in range of ~5 nm. Whereas the UV spectra of 5-O-glucuronide of 5,6DHF (Figure S2.24, SI) showed hypsochromic shift of ~9.5 nm in Band II λmax, which conformed to the UV spectra of glucuronides of 5HF and 5H7MF. Based on this, we deduced that glucuronidation at 5-hydroxyl group would show a consistent and diagnostic hypsochromic shift in Band II λmax in the range of 4–10 nm.
Effect of Glucuronidation at 6-Hydroxyl Group on λmax Shift in UV Spectra
The glucuronidation of 6-hydroxyl group can cause either no change or random change in Band I and/or II λmax values (Figures S2.14-S2.16, SI). So, in most cases glucuronidation at 6-hydroxyl group was determined by rule of elimination. The results showed that as compared to diagnostic spectral shift for other glucuronides, 6-O-glucuronides did not have any consistent shift in either Band I or Band II λmax. Results obtained by the rule of elimination were shown in Table 5.
Table 5.
Diagnostic shift in Band I and II of the 6-O-glucuronides in relation to the corresponding aglycone
| Compound | Aglycone or Glucuronide (O-G) |
λmax, nm | Diagnostic shifts in Band I and II in relation to aglycones |
Figure number in SI |
|
|---|---|---|---|---|---|
| Band II | Band I | ||||
| 6HF | Aglycone | 267.7 | 305.6 | 2.14 | |
| 6-O-G | 262.9 | 305.6 | No change (Band I) −4.8 nm (Band II) |
2.14 | |
| 6H4’MF | Aglycone | 277.1 | 310.3 | 2.15 | |
| 6-O-G | 277.1 | 310.3 | No change (Band I) No change (Band II) |
2.15 | |
| 6H7MF | Aglycone | 262.9 | 305.6 | 2.16 | |
| 6-O-G | 262.9 | 310.3 | +4.7 nm (Band I) No change (Band II) |
2.16 | |
| 3,6DHF | Aglycone | 258.2 | 329.4 | 2.21 | |
| 6-O-G | 253.5 | 329.4 | No change (Band I) −4.7 nm (Band II) |
2.21 | |
| 5,6DHF | Aglycone | 281.9 | ND* | 2.24 | |
| 6-O-G | 277.1 | ND* | −4.8 nm (Band II) | 2.24 | |
| 6,4’DHF | Aglycone | 272.4 | 329.4 | 2.26 | |
| 6-O-G | 267.7 | 329.4 | No change (Band I) −4.7 nm (Band II) |
2.26 | |
| 6,7DHF | Aglycone | 267.7 | 319.9 | 2.27 | |
| 6-O-G | 267.7 | 310.3 | −9.6 nm (Band I) No change (Band II) |
2.27 | |
| 5,6DH7MF | Aglycone | 277.1 | 319.9 | 2.30 | |
| 6-O-G | 272.4 | 319.9 | No change (Band I) −4.7 nm (Band II) |
2.30 | |
| 3,6,4’THF | Aglycone | ND* | 357.8 | 2.34 | |
| 6-O-G | ND* | 353.2 | −4.6 nm (Band I) | 2.34 | |
ND means not detected by the Empower software in the spectra
The glucuronidation at 6-OH position did not produce any consistent λmax shift. Moreover, 5-O-glucuronidation did not show consistent shift in the Band I, whereas 4’-O-glucuronidation did not show consistent shift in the Band II. This might pose a difficulty in the identification of the position of glucuronidation in the flavonoids where these groups occur together. To solve this, the amount of different mono-O-glucuronides formed in the mixture could also be used as additional information. In general, the rank of preference for position of glucuronidation was found to be 6-O-G > 4’-O-G > 5-O-G, such that in any of the tested compound, 5-O-G was the least formed glucuronide. Therefore, for mono-O-glucuronides where diagnostic shifts in λmax do not provide clear and sufficient information, relative rates of mono-O-glucuronides formation can be helpful in the identification of position of glucuronidation. Since the relative rates of formation would depend on the organism and enzyme isoforms used, only the relative rates of formation different mono-O-glucuronides of a flavonol/flavone by a standardized enzymatic system such as commercially available human recombinant UGT isoforms could be used as diagnostics for comparison.
Challenges of Using λmax Shift in UV Spectra to Determine the Regiospecific Glucuronide
For certain flavonoids which had hydroxyl group(s) only in ring A but not in the ring(s) B and/or C in their structures (e.g., 5,7-DHF), if one diagnostic band did not show a very high peak in the UV spectra in relation to the other diagnostic band (at least ~20%), the smaller band was not always reliable for determining the diagnostic shifts in λmax. In this case, relative formation rates of different regiospecific glucuronides (e.g., 6-O-G> 4’-O-G > 5-O-G) would be helpful.
The λmax values of Band I and Band II in the UV spectra of the flavones and flavonols and their respective mono-O-glucuronides at different positions were somewhat dependent (within a few nm) on the chromatographic conditions used such as pH of mobile phases, solvent used, and gradient speed. However, none of the selected flavonoid showed any deviation from the conclusion made in this paper in that the diagnostic λmax shift values stayed relative constant (within the range).
The elution order of the regiospecific mono-O-glucuronides could have been used to identify the specific metabolite. However, the elution order was shown to change randomly with the chromatographic conditions used as well as with the position of free hydroxyl groups in the structure of flavonoids, and there was no specific order pattern that can be used to identify the position of glucuronidation (as shown in Figure S1, Panel A, SI).
Use of Spectrum λmax Shift Method to Identify Mono-O-glucuronides of Quercetin
We used human recombinant UGT1A9 and UGT1A10 to generate the four mono-O-glucuronides of quercetin. UV λmax shift of Band I and II of the four mono-O-glucuronides with retention times are shown in Table 7. UGT1A9 formed glucuronides I, II and IV whereas UGT1A10 formed glucuronides I, II and III. Based on the diagnostic shift in λmax of Band I and II of the four mono-O-glucuronides of quercetin (Fig.7), we were able to identify the glucuronide-II and glucuronide-III as 3-O-G and 4’-O-G, respectively (Table 7). However, it was difficult to identify the position of glucuronidation of glucuronides I and IV which generated almost similar λmax shift patterns. Based on the published literature on the elution order (20, 25, 32, 33) of quercetin glucuronides, we could identify the glucuronide-I and glucuronide-IV as 7-O-G and 3’-O-G, respectively.
Table 7.
Diagnostic shift in Band I and II of the mono-O-glucuronides of quercetin in relation to the quercetin
| Compound/Glucuronide (Retention time) (Color of line in Fig. 7) |
λmax, nm | Diagnostic shifts in Band I and II |
Position of O- glucuronidation |
|
|---|---|---|---|---|
| Band II | Band I | |||
|
Quercetin (2.87 min) (Black solid) |
255.7 | 373.6 | ||
|
Glucuronide-I (1.04 min) (Red dotted) |
255.7 | 371.2 | −2.4 nm (Band I) No change (Band II) |
7-O-G |
|
Glucuronide-II (1.25 min) (Blue dashed) |
258.2 | 356.2 | −17.4 nm (Band I) +2.5 nm (Band II) |
3-O-G |
|
Glucuronide-III (1.47 min) (Green dotted dashed) |
253.3 | 366.2 | −7.4 nm (Band I) − 2.4 nm (Band II) |
4’-O-G |
|
Glucuronide-IV (1.80 min) (Brown double dotted dashed) |
253.3 | 371.2 | − 2.4 nm (Band I) − 2.4 nm (Band II) |
3’-O-G |
Fig.7.
UV spectra of quercetin (3,5,7,3’,4’-pentahydroxyflavone) (solid black line) and its four mono-O-glucuronides generated by UGT1A8 and UGT1A9 at an incubation concentration of 10µM.
Additional supporting evidence for the identification of glucuronide-I and glucuronide-IV as 7-O-G and 3’-O-G, respectively, was provided by the rates of formation of these quercetin glucuronides by UGTs. In our study, UGT1A9 formed 7-O-G, 3-O-G and 3’-O-G at 1.29±0.21, 1.43±0.23, 0.7±0.09 nmol/min/mg, respectively. UGT1A10 formed 7-O-G, 3-O-G and 4’-O-G at 0.8±0.06, 0.57±0.09, 0.33±0.12 nmol/min/mg, respectively. This was consistent with the published reports on the formation of quercetin glucuronides by human liver, intestine and UGT isoforms (32, 34). This suggested that the available information in literature on the rates of formation of glucuronides and elution order of the mono-O-glucuronides of particular flavonoid could also be useful in identifying the position of glucuronidation. However, if such information is unavailable, it would be very difficult to differentiation between glucuronidation at position 3’-O vs. 7-O.
Possible Application of the Spectrum λmax Shift Method
The newly established method of identifying the position of glucuronidation will enable multiple different areas of research: glucuronidation reaction including isoform-specific glucuronidation and structure-metabolism relationship, glucuronide transport, and/or UGT polymorphism. This method could also be used to give more precise structural information about flavonoid glucuronides, which is a significant improvement as most of the papers published regarding flavonoid glucuronidation were not able to pinpoint the position of the glucuronide group.
Isoform-specific glucuronidation is closely related to the regiospecific glucuronidation of flavonoids by various UGT isoforms. Therefore, identification of the glucuronide position could facilitate the determination of whether a specific UGT isoform prefer a particular hydroxyl position. For example, in the case of 3,4’DHF, UGT1A9 only glucuronidated 3-hydroxyl group while UGT1A1 glucuronidated both 3- and 4’-hydroxyl groups. On the other hand, UGT1A9 did not glucuronidate 4’-hydroxyl group in resokaempferol (3,7,4’THF), but UGT1A1 was able to metabolize all the three hydroxyl groups. This suggested that 4’ position was not a favored position of glucuronidation by UGT1A9, although the same group was not unfavored by UGT1A1. Assuming that glucuronidation of a hydroxyl group in a particular flavonoid is exclusively favored by one particular UGT isoform, the specific glucuronide level might be used as a diagnostic tool to determine the expression levels of a specific isoform in vivo.
Second, the identification of position of glucuronidation of a compound could also help to understand the relationship between the structure of glucuronides and their transport by efflux transporter. Jeong et al. (2004) showed that total (apical and basolateral) excretion rates of the raloxifene-4’-O-glucuronide from the Caco-2 cells monolayer and the rates of its formation in the cell lysate were ~7.6 and ~2.2 pmol/min/monolayer, respectively. In other words, that the excretion rates of the raloxifene-4’-O-glucuronide from the Caco-2 cells monolayer were 3.4 times higher than the rate of its formation in the cell lysate (35). It suggested that the efflux transporter(s) involved was/were favoring the excretion of raloxifene-4’-O-glucuronide from the cell such that the glucuronidation reaction was favored in the forward direction.
Recent data from our lab also showed that the rates of formation of the two mono-O-glucuronides of 3,7DHF, 3-O-glucuronide and 7-O-glucuronide in Caco-2 cell lysate were 19.27±1.9 and 4.75±1.7 nmol/hr/mg of protein (4-fold difference), respectively, where as the rates of total excretion of 3-O-glucuronide and 7-O-glucuronide of 3,7DHF in Caco-2 cell monolayer were 0.75±0.03 and 0.66±0.03 nmol/hr/mg of protein (1.1 fold difference), respectively. Both examples discussed above suggested that the excretion of flavonoids glucuronides in an organ or cell line was not only decided by the activities of UGT isoforms, but also influenced by the activities of responsible efflux transporter(s).
Third, the knowledge about preference of position to be glucuronidated in the flavonoids structure and excretion of glucuronides could also be used to generate the in silico quantitative structure-activity relationship models for UGTs and various efflux transporters such as MRP2 and BCRP. Smith et al. (2003) used mapping of glucuronidation site as one of the feature to generate common-feature pharmacophores of UGT1A4 (36). Also, Williamson et al. (2007) used interaction of quercetin glucuronides, 3-O-β-D-glucuronide, 7-O-β-D-glucuronide, 3’-O-β-D-glucuronide and 4’-O-β-D-glucuronide with MRP2, to assess the predictive power of an in silico generated 3-dimensional homology model of MRP2 (37).
This technique could be successfully utilized for the identification of position of glucuronidation in the samples generated in different experimental matrix including in vitro samples such as microsomal samples and in vivo samples such as plasma. It is however important that samples should be reasonably cleaned using solid or liquid phase extraction such that experimental matrix does not interfere with UV spectra. Also, sufficient quantity of analyte should be present in the sample so that it can be processed to obtain good quality UV spectra.
On the other hand, the shifts in λmax values were not consistent across different conjugation. The methylation at a particular hydroxyl position in the structure of flavones or flavonols did not cause the similar shifts in λmax as the substitution by glucuronic acid at the same position (see Figure S2, SI). Sulfates of 4’HF, 5HF and 7HF did not always lead to similar shifts in λmax as glucuronides (data not shown here). Therefore, a systematic study on mono-sulfates of flavonoids is required to define the diagnostic shifts in λmax based on position of sulfation.
In conclusion, we developed a λmax shift method to identify the position of glucuronidation for mono-O-glucuronides of flavones and flavonols, and this method is simpler and faster than NMR or EI-MS/CID coupled with metal complexation. This method does not require the purification of glucuronides or the use of shift regents. The only requirements are that we need sufficient amount (~1–5 µM) of metabolites and aglycones to generate good quality spectra, and that the position of hydroxyl group is at 3, 4’, 5 and/or 7, single or in combo. Information about the position of glucuronidation is expected to serve as a very important tool in furthering the study of metabolism by UGT isoforms and role of efflux transporters in excretion of glucuronides.
Supplementary Material
Table 6.
Effect of regiospecific glucuronidation on the λmax of Band I and Band II in the UV spectrum of flavones and flavonols
| Position of Glucuronidation |
Effect on λmax | |
|---|---|---|
| Band I | Band II | |
| 3-O | ~13–30 nm hypsochromic shift in λmax | No consistent shift in λmax |
| 7-O | No change in λmax | No change in λmax |
| 4’-O | ~4–10 nm hypsochromic shift in λmax | No consistent shift in λmax |
| 5-O | No consistent shift in λmax | ~4–10 nm hypsochromic shift in λmax |
| 6-O | No consistent shift in λmax | No consistent shift in λmax |
ACKNOWLEDGEMENT
This work was supported by NIH Grant No. GM070737 and a training fellowship from the Pharmacoinformatics Training Program of the Keck Center of the Gulf Coast Consortia (NIH Grant No. 5 R90 DK071505-03).
ABBREVIATIONS USED
- UGT
UDP-glucuronosyltransferases
- UDPGA
uridine diphosphoglucuronic acid
- UPLC
ultraperformance liquid chromatography
- MS
mass spectroscopy
- NMR
nuclear magnetic resonance
- UV
ultraviolet
- PDA
photodiode array detector
- SMR
structure metabolism relationship
- HF
Hydroxyflavone
- DHF
dihydroxyflavone
- THF
trihydroxyflavone
- MHF
methoxy- hydroxyflavone
- DMHF
dimethoxy- hydroxyflavones
- DHMF
dihydroxy- methoxyflavone
- MRP2
multi-resistance protein 2
- BCRP
breast cancer resistance protein
- SI
supporting information; EI-MS/CID
Footnotes
SUPPORTING INFORMATION AVAILABLE
UPLC Chromatograms and LC/MS/MS scans of 3HF, 3,5DHF, 3,7DHF, 3,5,7THF 3,7,4’THF (resokaempferol) and 3,5,7,4’-tetrahydroxyflavone (kaempferol) (and their glucuronides; UV spectra of 36 selected flavonoids and their corresponding glucuronides; and UPLC/MS/MS optimized ion source and compound parameters for precursor ion scan for 3HF, 3,4’DHF, 3,5DHF, 3,7DHF, 3,5,7THF 3,7,4’THF (resokaempferol) and 3,5,7,4’-tetrahydroxyflavone (kaempferol).
References
- 1.Kelly GE, Joannou GE, Reeder AY, Nelson C, Waring MA. The variable metabolic response to dietary isoflavones in humans. Proc Soc Exp Biol Med. 1995;208(1):40–43. doi: 10.3181/00379727-208-43829. [DOI] [PubMed] [Google Scholar]
- 2.Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, Heizer WD, Thomas BF, Hill JM, Crowell JA, Zeisel SH. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75(1):126–136. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 3.Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med. 2004;7:36. 829–837. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Feng WY. Metabolism of green tea catechins: an overview. Curr Drug Metab. 2006;7(7):755–809. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- 5.Erlund I, Kosonen T, Alfthan G, Maenpaa J, Perttunen K, Kenraali J, Parantainen J, Aro A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur J Clin Pharmacol. 2000;56(8):545–553. doi: 10.1007/s002280000197. [DOI] [PubMed] [Google Scholar]
- 6.Sesink AL, O'Leary KA, Hollman PC. Quercetin glucuronides but not glucosides are present in human plasma after consumption of quercetin-3-glucoside or quercetin-4'-glucoside. J Nutr. 2001;131(7):1938–1941. doi: 10.1093/jn/131.7.1938. [DOI] [PubMed] [Google Scholar]
- 7.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81(1 Suppl):243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 8.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 9.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. Plasma phytoestrogens are not altered by probiotic consumption in postmenopausal women with and without a history of breast cancer. J Nutr. 2004;134(8):1998–2003. doi: 10.1093/jn/134.8.1998. [DOI] [PubMed] [Google Scholar]
- 10.Hu M, Chen J, Lin H. Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther. 2003;307(1):314–321. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther. 2003;304(3):1228–1235. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 12.Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143–146. doi: 10.1111/j.1365-2125.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perabo FG, Von Low EC, Ellinger J, von Rucker A, Muller SC, Bastian PJ. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2008;11(1):6–12. doi: 10.1038/sj.pcan.4501000. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30(4):370–377. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 15.Hollman PC, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother. 1997;51(8):305–310. doi: 10.1016/s0753-3322(97)88045-6. [DOI] [PubMed] [Google Scholar]
- 16.Miners JO, Smith PA, Sorich MJ, McKinnon RA, Mackenzie PI. Predicting human drug glucuronidation parameters: application of in vitro and in silico modeling approaches. Annu Rev Pharmacol Toxicol. 2004;44:1–25. doi: 10.1146/annurev.pharmtox.44.101802.121546. [DOI] [PubMed] [Google Scholar]
- 17.Burchell B. Transformation Reactions: Glucuronidation. In: Woolf TF, editor. Handbook of drug metabolism. New York: Dekker; 1999. pp. xi–596. [Google Scholar]
- 18.Joseph TB, Wang SW, Liu X, Kulkarni KH, Wang J, Xu H, Hu M. Disposition of flavonoids via enteric recycling: enzyme stability affects characterization of prunetin glucuronidation across species, organs, and UGT isoforms. Mol Pharm. 2007;4(6):883–894. doi: 10.1021/mp700135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis BD, Needs PW, Kroon PA, Brodbelt JS. Identification of isomeric flavonoid glucuronides in urine and plasma by metal complexation and LC-ESI-MS/MS. J Mass Spectrom. 2006;41(7):911–920. doi: 10.1002/jms.1050. [DOI] [PubMed] [Google Scholar]
- 20.Davis BD, Brodbelt JS. Regioselectivity of human UDP-glucuronosyl-transferase 1A1 in the synthesis of flavonoid glucuronides determined by metal complexation and tandem mass spectrometry. J Am Soc Mass Spectrom. 2008;19(2):246–256. doi: 10.1016/j.jasms.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuyckens F, Claeys M. Determination of the glycosylation site in flavonoid mono-O-glycosides by collision-induced dissociation of electrospray-generated deprotonated and sodiated molecules. J Mass Spectrom. 2005;40(3):364–372. doi: 10.1002/jms.794. [DOI] [PubMed] [Google Scholar]
- 22.Dueñas M, Mingo-Chornet H, Pérez-Alonso JJ, Paola-Naranjo RD, González-Paramás AM, Santos-Buelga C. Preparation of quercetin glucuronides and characterization by HPLC–DAD–ESI/MS. European Food Research and Technology. 2008;227(4):1069–1076. [Google Scholar]
- 23.Wittig J, Herderich M, Graefe EU, Veit M. Identification of quercetin glucuronides in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;753(2):237–243. doi: 10.1016/s0378-4347(00)00549-1. [DOI] [PubMed] [Google Scholar]
- 24.Mullen W, Boitier A, Stewart AJ, Crozier A. Flavonoid metabolites in human plasma and urine after the consumption of red onions: analysis by liquid chromatography with photodiode array and full scan tandem mass spectrometric detection. J Chromatogr A. 2004;1058(1–2):163–168. [PubMed] [Google Scholar]
- 25.Day AJ, Bao Y, Morgan MR, Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic Biol Med. 2000;29(12):1234–1243. doi: 10.1016/s0891-5849(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 26.Mabry TJ, Markham KR, Thomas MB. The systematic identification of flavonoids. New York: Springer-Verlag; 1970. pp. xi–354. [Google Scholar]
- 27.Tang L, Singh R, Liu Z, Hu M. Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones. Mol Pharm. 2009;6(5):1466–1482. doi: 10.1021/mp8002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Tam VH, Hu M. Disposition of flavonoids via enteric recycling: determination of the UDP-glucuronosyltransferase isoforms responsible for the metabolism of flavonoids in intact Caco-2 TC7 cells using siRNA. Mol Pharm. 2007;4(6):873–882. doi: 10.1021/mp0601190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King R, Fernandez-Metzler C. The use of Qtrap technology in drug metabolism. Curr Drug Metab. 2006;7(5):541–545. doi: 10.2174/138920006777697936. [DOI] [PubMed] [Google Scholar]
- 30.Clarke NJ, Rindgen D, Korfmacher WA, Cox KA. Systematic LC/MS metabolite identification in drug discovery. Anal Chem. 2001;73(15):430A–439A. doi: 10.1021/ac012480y. [DOI] [PubMed] [Google Scholar]
- 31.Markham KR. Techniques of flavonoid identification. London; New York: Academic Press; 1982. pp. xi–113. [Google Scholar]
- 32.Boersma MG, van der Woude H, Bogaards J, Boeren S, Vervoort J, Cnubben NH, van Iersel ML, van Bladeren PJ, Rietjens IM. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem Res Toxicol. 2002;15(5):662–670. doi: 10.1021/tx0101705. [DOI] [PubMed] [Google Scholar]
- 33.Day AJ, Mellon F, Barron D, Sarrazin G, Morgan MR, Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res. 2001;35(6):941–952. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- 34.van der Woude H, Boersma MG, Vervoort J, Rietjens IM. Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem Res Toxicol. 2004;17(11):1520–1530. doi: 10.1021/tx049826v. [DOI] [PubMed] [Google Scholar]
- 35.Jeong EJ, Lin H, Hu M. Disposition mechanisms of raloxifene in the human intestinal Caco-2 model. J Pharmacol Exp Ther. 2004;310(1):376–385. doi: 10.1124/jpet.103.063925. [DOI] [PubMed] [Google Scholar]
- 36.Smith PA, Sorich MJ, McKinnon RA, Miners JO. Pharmacophore and quantitative structure-activity relationship modeling: complementary approaches for the rationalization and prediction of UDP-glucuronosyltransferase 1A4 substrate selectivity. J Med Chem. 2003;46(9):1617–1626. doi: 10.1021/jm020397c. [DOI] [PubMed] [Google Scholar]
- 37.Williamson G, Aeberli I, Miguet L, Zhang Z, Sanchez MB, Crespy V, Barron D, Needs P, Kroon PA, Glavinas H, Krajcsi P, Grigorov M. Interaction of positional isomers of quercetin glucuronides with the transporter ABCC2 (cMOAT, MRP2) Drug Metab Dispos. 2007;35(8):1262–1268. doi: 10.1124/dmd.106.014241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.