Introduction
Onyx is a new liquid embolic system (Micro Therapeutics, Inc., Irvine, CA) consisting of ethylene-vinyl alcohol copolymer (EVOH), formed of 48 mol/L ethylene and 52 mol/L vinyl alcohol, dissolved in dimethyl-sulfoxide (DMSO) and mixed with micronized tantalum powder (35% weight per volume) for radiopaque visualization. In its low density form it is predominantly used worldwide in the treatment of cerebral 1-6 or spinal7,8 arteriovenous malformations (AVMs), other more rare indications are hypervascularized tumors9, peripheral AVMs or arteriovenous fistulas (AVFs)10, and there is also an extensive use in experimental work 11-12. Onyx LD means low density of EVOH in the range of 6 to 8 % (Onyx 18 to 34) compared to high density Onyx 500+ (containing 20 % of EVOH) used for aneurysm occlusions. The basics of Onyx LD liquid system and the main principles of its application in endovascular AVM therapy are described and discussed in this chapter.
Preparation of Onyx liquid embolic system
The ready-to-use vials of Onyx LD (1,5 ml) are placed on a mixer and have to be shaken for at least 20 minutes to obtain a homogenous solution consisting of the embolic component and the tantalum powder. The microcatheter is flushed with saline and subsequently after the dead space volume is filled with 0.23 to 0.26 ml of pure DMSO depending on witch catheter is deployed (Ultraflow™ or Marathon™, Micro Therapeutics, Inc., Irvine, CA). It is important to follow these steps very carefully in order to avoid unintentional precipitation of Onyx, which immediately begins when there is contact to water, saline solution or blood. Therefore, the catheter hub requires to be flushed with DMSO also. After the air-bubble-free aspiration of the Onyx in a dedicated syringe, the syringe is connected to the microcatheter producing a clear interface between Onyx and DMSO by positioning the syringe vertically immediately after the connection or, more simply, by using a special Interface-Adapter.
Application of Onyx LD in central AVMs
The most important factor for successful embolization of cerebral or spinal AVMs is the intranidal positioning of the microcatheter (figure 1). Beside clinical experiences experimental data using a hydraulic pump model or the rete mirabile14 of the swine also clearly demonstrated that even positioning the tip of the catheter just shortly below the level of the "nidus" in the feeding artery may lead to incomplete filling of the nidus, and that the volume and the extension of Onyx reflux is much higher than it is for intranidal positioning (figure 2).
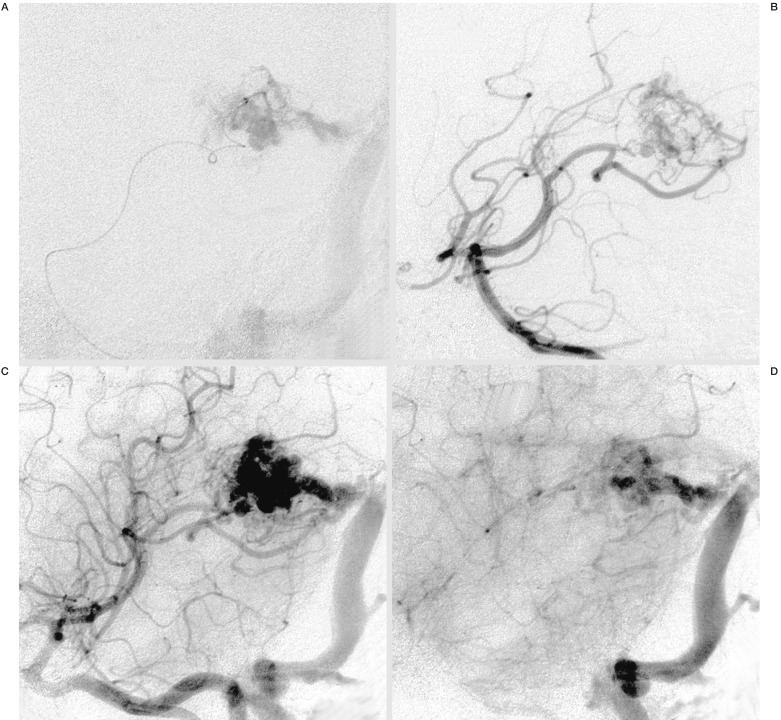
Figure 1.
A) Intranidal positioning of the Microcatheter, Marathon™, in a small AVM of the left trigonum in an oblique projection and superselective injection of contrast media. B-D) Angiogram of the right vertebral artery in an early and later arterial (B,C) and venous phase (D) in the same projection, demonstrating the small arterial feeders and nidus with moderate flow and the main venous draining vein into the left sinus transversus.
Figure 2.
Results of Onyx 20 injection in a hydraulic pump model with a simulated arterial flow of 60 ml/min. The "nidus" is in a tube consisting of compressed foam and extends within the length marked by the needles (40 mm). A) "Intranidal" positioning of the Microcatheter, Ultraflow, in the proximal part of the foam with little reflux and homogenous filling of the entire "nidus". B) Postioning of the same microcatheter just below the level of the "nidus" with huge reflux and filling up only half of the foam.
The angioarchitecture of the malformation is a determining factor for EVOH concentration to be used. The viscosity of Onyx nearly doubles from Onyx LD 18 to 34, the numbers in Onyx 18,20 and 34 are related to the unit of centipoise, the hundreth of a poise, the unit of viscosity (1 poise=dyne sec/cm2 or 1.0g/cm/sec). The main criteria for the choice of Onyx 18, 20 or 34 (concentration of EVOH of 6; 6,5 and 8%) are the distance from tip of the microcatheter to the venous part of the malformation, flow rate and fistulous versus plexiform appearance of the nidus. Nevertheless beside the clearly defined differences in the viscosity from Onyx 18 to 34, there is lack of experimental data for a rational selection of one or more of the substances. Our own ongoing studies using the rete mirabile of the swine under different flow conditions are promising to fill this gap. Actually, there is a widespread use of different Onyx densities, dependent on the investigator, and, at the moment, no precise or binding recommendations for the best choice are possible.
The first step in starting Onyx injection is normally to produce a plug around the tip of the catheter to establish and increase a pressure gradient to the distal part within the nidus, which is supported by pushing Onyx carefully into the nidal part of the malformation.
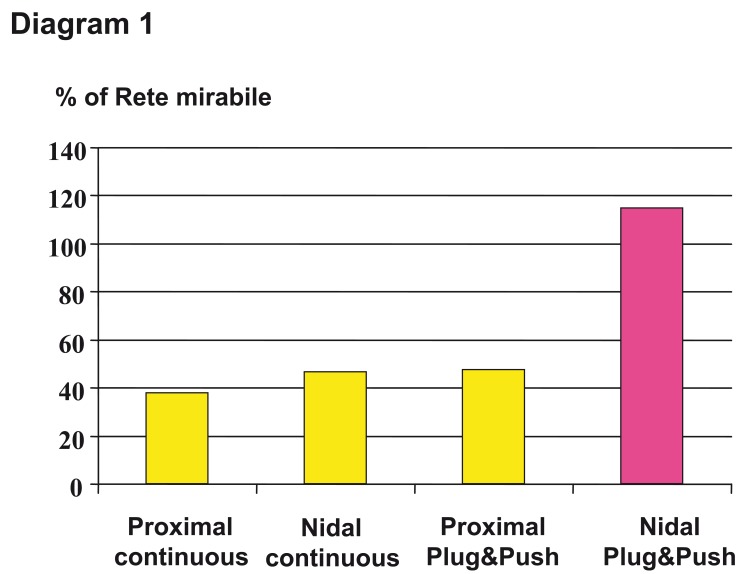
This so-called "plug and push" technique allows a complete occlusion of over 100 percent in the rete mirabile of the swine in experimental work, even if there is no arteriovenous shunt over the rete mirabile. The differences in the filling results using "plug and push" and other injection techniques are demonstrated in diagram 1.
Diagram 1.
Results of different injection techniques (continuous versus plug&push technique) and microcatheter positions below (proximal) and within the "nidus" (nidal). Satisfactory result only after intranidal postioning and using the plug and push technique. The over one hundred percent filling in this experimental work using the rete mirabile of the swine as a biological AVM model is explained by ongoing further embolization of the contralateral part of the rete.
The injection of Onyx is slow and ranges mostly between 0.1 and 0.2 ml per minute and should not exceed over 0.3 ml to maintain the microcatheter injection pressure at a safe level and to avoid vasospasm and angionecrosis. Care should be taken when injecting the initial drop of Onyx since the catheter is filled with pure DMSO.
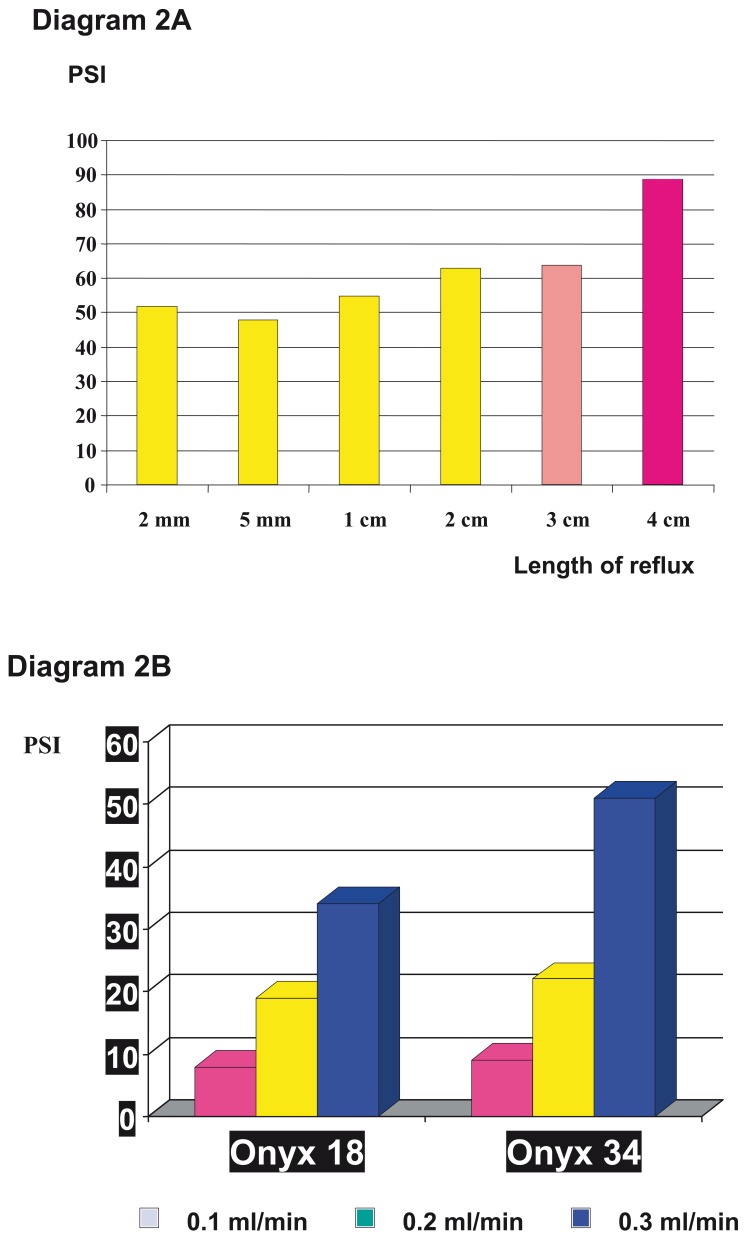
The experimental data from Murayama et al, published in 198815, clearly demonstrates that an excessively fast injection of DMSO during Onyx delivery increases the risk and safety of the application. When 0.5 ml of pure DMSO is injected in 30 to 120 seconds neither vasospasm nor any other complications are observed. Injection of the same volume within 5 to 15 seconds may lead to vasospasm and angionecrosis. These data were confirmed by Chaloupka et Al 16 and a long term follow-up study of 29 patients, who where observed over a period of up to 5 years after embolization, and showed neither cardiomyopathy nor neoplasms after the treatment using DMSO as an organic solvent17. Besides these safety aspects, fast injection speed only produces more reflux and with it increasing pressure within the catheter (diagram 2 A,B).
Diagram 2.
Demonstration of the increase of pressure inside the microcatheter A) after a length of reflux over 3 cm, B) and dependance on the injection speed and viscosity of Onyx.
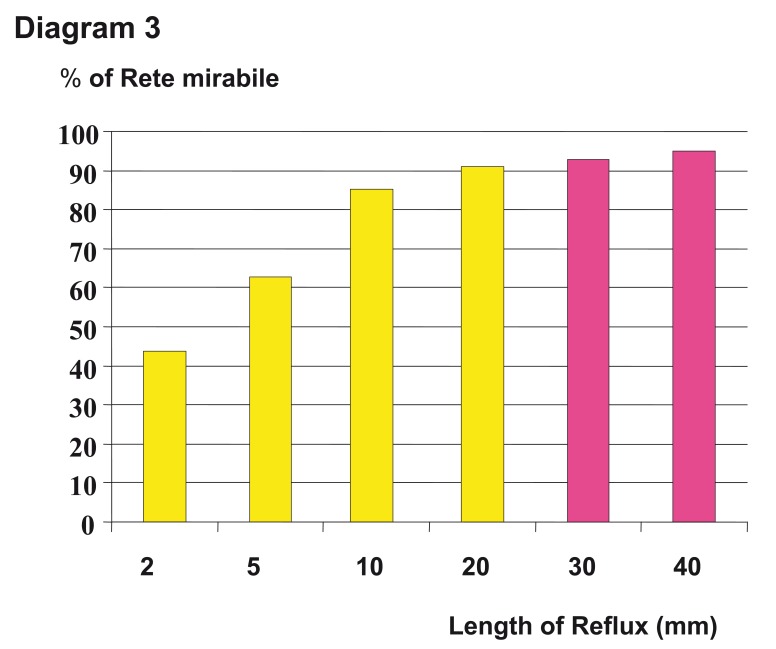
The extent of reflux is normally accepted from 1 to 2 cm, beyond this, according to animal experiments, an improvement of the embolization result can not be expected (diagram 3).
Diagram 3.
Different results of the filling of the rete mirabile in swine regarding the length of reflux. Beyond a length of 2 cm there is no significant increase of filling.
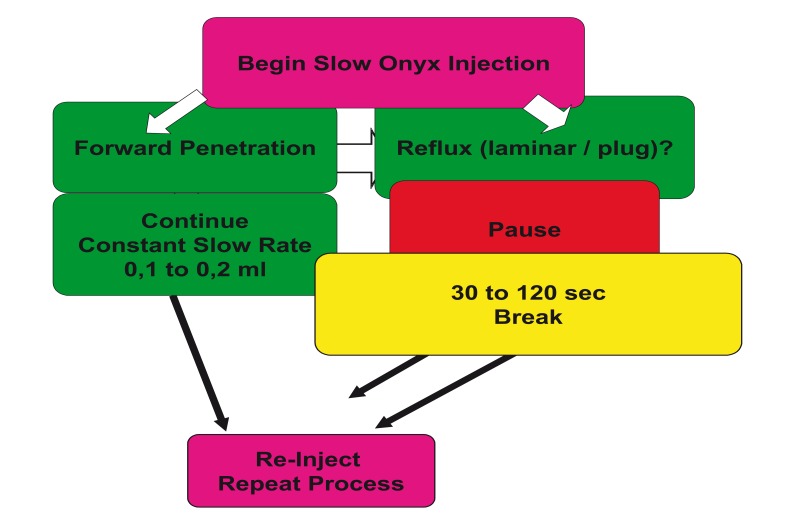
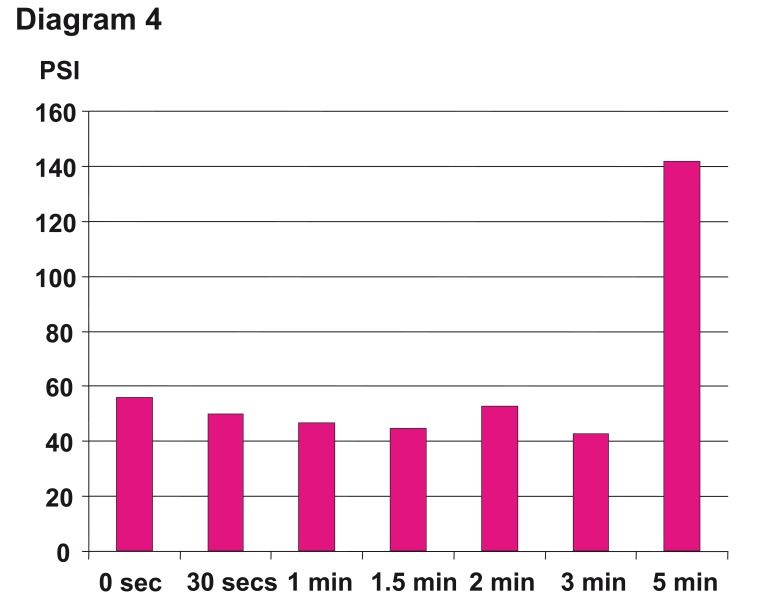
The Onyx application continues at intervals of injection and break phases, where the breaks normally last between 30 seconds and two minutes, depending on the volume and the time of the injection phase. The break should not exceed 2 to 3 minutes and can be dangerous after this period due to increasing pressure within the microcatheter with high risk of unintentional catheter tip occlusion (diagram 4). With the slow injection of Onyx, the breaks and the intranidal positioning of the microcatheter, a homogenous spongy polymeric cast is created (figure 3 A,B ). The polymer solidifies from out-to-inside. In figure 4 the injection pattern during the interventional session is illustrated.
Figure 3.
Two demonstrations of a homogeneous spongic cast after embolization of AVMS, both in ap and lateral projections. A) Same patient as in Figure 1. Cast after injection 0.9 ml of Onyx 20 after single catherization and 90% angiographic occlusion of the AVM. B) Another patient with a giant temporal AVM on the left side. Cast after injection of 26 ml of Onyx in five sessions using Onyx of different viscosities and 90% occlusion of the AVM. After embolization and surgical resection the patient was clinically asymptomatic.
Figure 4.
Summary and illustration of the decision process during the Onyx injection from the beginning of the application in repeat and reinject cycles.
Diagram 4.
Different times of breaks (waiting time) after the Onyx injection period from zero to 5 minutes and the influence on the pressure inside the microcatheter clearly demonstrates that beyond 3 min, there is an immense increase of the pressure and with it risk of unintentional microcatheter occlusion. Two minutes maximum is recommended in the Instructions For Use.
Summarizing the reasons for the necessity of the slow injection and the injection/break intervals of Onyx application, there is an increase of the angiotocixity with enhancing injection speed, an increase of the pressure within the microcatheter and the length of the produced reflux, as well as the disadvantage of obtaining inhomogenities of the spongic cast.
Surgical Resection and Histopathological Examinations
Inflammatory reaction can be observed in the histopathological examination after resection of the AVM and preoperative endovascular embolization with ethylene vinyl alcohol copolymer. However, the imflammatory response to the nidal tissue is usually mild, especially compared to n-butyl 2 cyanoacrylate (NBCA). The common findings in the histopathological examination are a homogenous distribution of EVOH in the small nidal vessels. Tantalum is predominantly located at the wall of the vessels and red thrombus material is inconstantly found in the lumen. Although both, recanalization and ongoing thrombosis in angiographic controls after embolizations, as observed in clinical reports18, the vessel occlusion after embolization had to be defined as a permanent occlusion. Observation of angionecrosis in the specimens after surgical resection of the AVM is rare. For surgeon the resection seems to be easier to handle19 because the Onyx cast is soft, formable and elastic20.
Withdrawal of the Microcatheter
Although the Onyx liquid embolic system is a nonadhesive agent, problems with the withdrawal of the microcatheter after the embolization can occur. This depends on the depth of the postioning of the microcatheter within the nidus, presence of loops in the feeding arteries and tortuosity of the intracranial vessels, the volume of Onyx and the injection time during the treatment session, and may be due to the occurrence of vasospasm. Our own experiences, personal communications with other interventional groups and company informations confirm that the problem of withdrawal of the microcatheter occur in less than 5 percent of all interventional sessions worldwide. This is a serious problem.
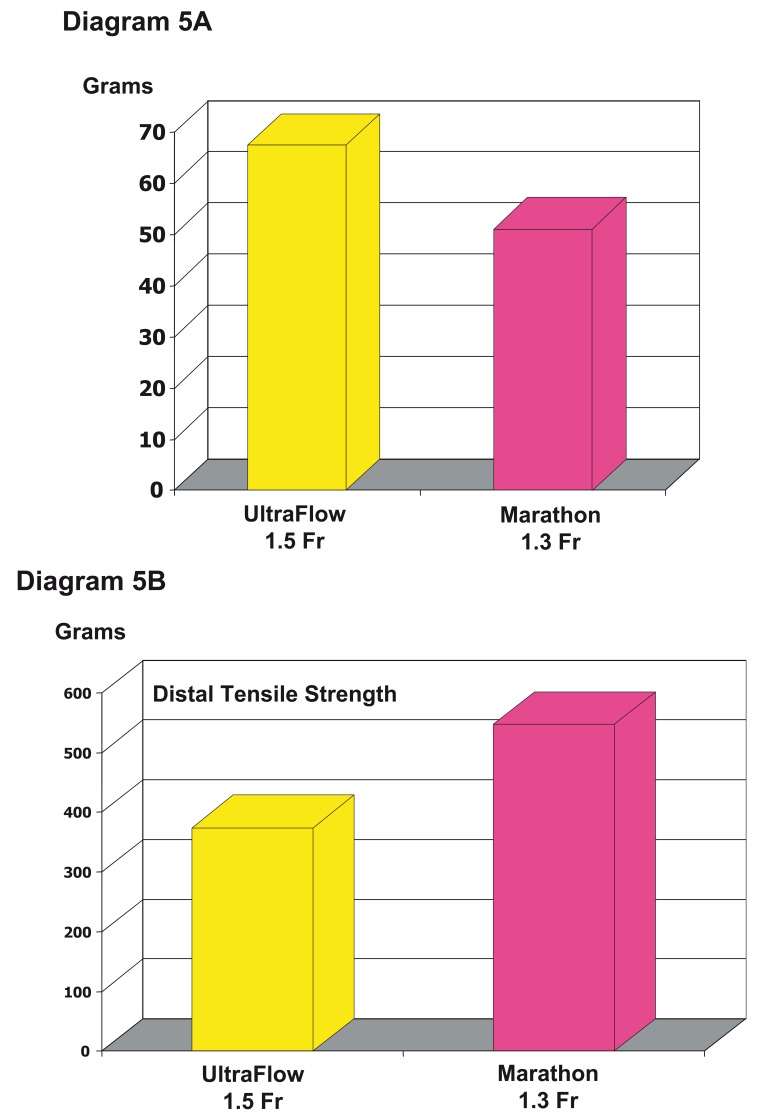
The influence of the length of the reflux produced during the embolization is one but not the most important factor of this problem. Special strategies have been developed to overcome this problem and growing experiences with Onyx reduce the risk. Now, there is a new microcatheter available, which is smaller (1.3 vs 1.5 Fr), also flow-directed and braided in the distal part with nitinol. Our own first and limited experiences using this new microcatheter show that there are no problems of withdrawing the microcatheter and the procedure seems much easier. We hope that with this new microcatheter the "withdrawal" - problem is one of the past. Experimental data support this hope. There is less detachment force necessary to retrieve the new microcatheter and an increase of the tensile strength of the distal catheter tip in comparison of both flow directed microcatheters (Diagram 5 A,B).
Diagram 5.
Differences between Ultraflow™ and Marathon™ regarding the soft and nitinol braided distal tip due to the detachment force (A) and the tensile strength (B). A) Comparison of the forces necessary to retrieve the microcatheters out of a cast with a reflux of 3 cm demonstrating that the detachment force in the braided one is 25% less. B) The distal tensile strength is one third greater in the braided one and demonstrates the increase stretch resistance of the distal, soft part of the microcatheter. This allows more tension to be brought to the tip and with it to pull out the catheter with more ease out of the cast.
Conclusions and Summary
Onyx LD liquid embolic system is a promising nonadhesive permanent occlusive agent for embolizing cerebral and spinal arteriovenous malformations. Compared to NBCA, the preparation, application and the behavior of the two substances is completely different. Only the learning curve to control the embolization of AVMs safely and effectively seems similar in both. Reducing this learning curve is the target of the described preparations and applications of Onyx LD.
References
- 1.Boulos AS, Levy EI, et al. Evolution of neuroendovascular intervention: A review of advancement in device technology. Neurosurgery. 2004;54:438–453. doi: 10.1227/01.neu.0000103672.96785.42. [DOI] [PubMed] [Google Scholar]
- 2.Florio F, Lauriola W, et al. Endovascular treatment of intracranial arterio-venous malformations with Onyx embolization: preliminary experience. Radiol Med. 2003;106:512–520. [PubMed] [Google Scholar]
- 3.Howington JU, Kerber CW, et al. Liquid embolic agents in the treatment of intracranial arteriovenous malformations. Neurosurg Clin N Am. 2005;16:355–363. doi: 10.1016/j.nec.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Jahan R, Murayama Y, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48:984–985. doi: 10.1097/00006123-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Song D, Leng B, et al. Onyx in the treatment of large and giant aneurysms and arteriovenous malformations. Chines Medical Jounal. 2004;117:1869–1872. [PubMed] [Google Scholar]
- 6.Taylor CL, Dutton K, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810–812. doi: 10.3171/jns.2004.100.5.0810. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux AJ, Chir B, et al. Embolization of spinal cord arteriovenous malformations with an ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide (Onyx liquid embolic system) J Neurosurg. 2000;93:304–308. doi: 10.3171/spi.2000.93.2.0304. [DOI] [PubMed] [Google Scholar]
- 8.Warakaulle DR, Aviv RI, et al. Embolization of spinal dural arteriovenous fistulae with Onyx. Neuroradiology. 2003;45:110–112. doi: 10.1007/s00234-002-0936-2. [DOI] [PubMed] [Google Scholar]
- 9.Gobin YP, Murayama Y, et al. Head and neck hypervascular lesions: Embolization with ethylene vinyl alcohol copolymer - Laboratory evaluation in swine and clinical evaluation in humans. Radiology. 2001;221:309–317. doi: 10.1148/radiol.2212001140. [DOI] [PubMed] [Google Scholar]
- 10.Numan F, Omeroglu A, et al. Embolization of peripheral vascular malformations with ethylene vinyl alcohol copolymer (Onyx) JVIR. 2004;15:939–946. doi: 10.1097/01.RVI.0000130862.23109.52. [DOI] [PubMed] [Google Scholar]
- 11.Abdala N, Levitin A, et al. Use of ethylene vinyl alcohol copolymer for tubal sterilization by selective catheterization in rabbits. JVIR. 2001;12:979–984. doi: 10.1016/s1051-0443(07)61579-6. [DOI] [PubMed] [Google Scholar]
- 12.Hamada J, Yutaka K, et al. A nonadhesive liquid embolic agent composed of ethylene vinyl alcohol copolymer and ethanol mixture for the treatment of cerebral arteriovenous malformations: experimental study. J Neurosurg. 2002;97:889–895. doi: 10.3171/jns.2002.97.4.0889. [DOI] [PubMed] [Google Scholar]
- 13.Wright KC, Greff RJ, et al. Experimental evaluation of cellulose acetate NF and ethylene-vinyl alcohol copolymer for selective arterial embolization. J Vasc Interv Radiol. 1999;10:1207–1018. doi: 10.1016/s1051-0443(99)70221-6. [DOI] [PubMed] [Google Scholar]
- 14.Massoud TF, Ji C, et al. An experimental arteriovenous malformation model in swine: Anatomic basis and construction technique. Am J Neuroradiol. 1994;15:1537–1545. [PMC free article] [PubMed] [Google Scholar]
- 15.Murayama Y, Viñuela F, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous Malformations: Preliminary histopatholigical studies in swine rete mirabile. Neurosurgery. 1998;43:1164–1175. doi: 10.1097/00006123-199811000-00081. [DOI] [PubMed] [Google Scholar]
- 16.Chaloupka J, Huddle D, et al. A reexamination of the angiotoxicity of superselective injection of DMSO in the swine rete embolization model. Am J Neuroradiol. 1999;20:401–410. [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiu K, Nishida A, et al. The long-term follow-up patients treated with liquid embolic agent dissolved in dimethyl sulfoxid (DMSO); Oral Presentation at the WITNC; September; Seoul. 2001. [Google Scholar]
- 18.Larages A, Lobato RD, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients (comment) Neurosurgery. 2002;51:1525–1526. [PubMed] [Google Scholar]
- 19.Akin ED, Perkins E, et al. Surgical handling characteristics of an ethylene vinyl alcohol copolymer compared with n-butyl cyanoacrylate used for embolization of vessels in an arteriovenous resection model in swine. J Neurosurg. 2003;98:366–370. doi: 10.3171/jns.2003.98.2.0366. [DOI] [PubMed] [Google Scholar]
- 20.Duffner F, Ritz R, et al. Combined therapy of cerebral arteriovenous malformations: histological differences between a non-adhesive liquid embolic agent and n-butyl 2-cyanoacrylate (NBCA) Clin Neuropathol. 2002;21:13–17. [PubMed] [Google Scholar]