Summary
We have been using Onyx, a non-adhesive liquid polymer, to treat cerebral AVMs endovascularly since 1999. During this time we have treated 45 consecutive, unselected patients.
From the outset this product brought about a change in our approach to treating this type of lesion because of the different injection behaviour observed for this material compared with the adhesive Histoacryl that had been employed until then.
The object of this article is to assess the results achieved by our team using this new embolic agent, following angiographic and clinical follow-up of cases for a minimum of six months and a maximum of five years (mean: two years).
We propose new categories of cerebral AVM based on the expected behaviour of Onyx within the nidus.
Our appraisal indicates that we have improved our angiographic results, achieving complete occlusion of the malformation in 22% of cases and over 80-% closure in 69% of cases. The morbimortality rate for the procedure was 18%.
Key words: AVMs, embolization, therapeutic neuroradiology, Onyx
Introduction
Taki et Al1 were the first to describe Onyx for cerebral AVMs, and subsequently Murayama et Al2 published clinical findings at UCLA. Jahan et Al3 described the injection protocol in a series of 23 patients with a total of 129 pedicles treated. This article emphasizes the critical need for slow, controlled injection of the solvent, DMSO, which has been demonstrated to be toxic to the arterial wall4,5.
A review of the literature reveals that there have been no assessments of the results achieved using this product, which still has not been approved by the FDA in the U.S.
Onyx appears to offer certain theoretical and experimental advantages over cyanoacrylates. First, it is not an adhesive, which allows easier, more controlled injection, with the option of halting and resuming a few minutes later, even after control angiography.
Another advantage is that the stream of Onyx does not break up during injection, thereby avoiding the formation of small bubbles that may travel out of control, as frequently happens with Histoacryl. Its behaviour is thus more predictable.
Furthermore, during injections small amounts of embolization material can be refluxed and used to block blood flow in the feeder vessel with the microcatheter threaded inside, thus producing the conditions interventional neuroradiologists consider optimum for infusing embolic agents.
There are also certain disadvantages, such as the need to be aware of the risk of serious consequences ensuing from rapid injection of DMSO into the AVM, hence the 0.25 cc of DMSO filling the dead space in the catheter should be injected over no less than 60 s.
Materials and Methods
From October 1999 to October 2004 we treated a total of 45 patients ranging in age from 5 to 66 years (mean: 35 years). The series consisted of 23 females and 22 males.
The clinical symptoms on presenting at the hospital were cerebral haemorrhage in 23 cases, epileptic seizures in 11 cases, and headache in 9 cases. Two cases were incidental findings on performing brain MRI scans for other causes, pituitary adenoma in one case and Chiari malformation in the other.
Follow-up ranged from a minimum of six months (two patients) to a maximum of five years (two patients), the mean being two years.
Cerebral angiography was performed at the final examination in all cases.
Low Onyx concentrations (6%, 6.5%, and 8%) were used for the first three years with the compatible FlowRider microcatheter. Starting in 2003 the new Onyx (18%, 20%, and 34%) and the UltraFlow catheter were used.
All procedures were performed in a high performance digital angiography room (Philips Integris Allura monoplane) under general anaesthesia. Systemic heparin was not administered in any of the cases.
Heparin was added to the coaxial perfusion and rinsing fluids.
The same preparatory and injection protocols were used in all cases, namely, the Onyx was shaken in the vial for 20 min before use, 0.8 cc DMSO was loaded into the yellow syringe when the microcatheter had been inserted to the desired position and rinsed out with 10 cc of saline solution without any added heparin. An amount of 0.25 cc of DMSO was injected into the UltraFlow catheter, exactly filling the dead space.
An amount of 0.25 cc of Onyx was loaded into the white syringe, topped up to exactly 1 cc, and injected. Injection was slow and nonstop, taking at least 60 s, pushing the DMSO out into the AVM.
It is important to connect the Onyx syringe to the UltraFlow hub quickly, without allowing any air to enter. The syringe should be held vertically with the tip pointing downwards on connection, after which it should immediately be turned over 180º so that its tip is pointing upwards and the injection started directly. This will ensure a sharp interface distinctly dividing the DMSO from the Onyx inside the catheter.
Thanks to the greater opacity of the 20-% Onyx solution, this enables clear visualization of the progress of the Onyx as it moves along the catheter.
We likewise consider catheter placement to be crucial and always select an arterial pedicle that allows access to the interior of the nidus. Where this is not possible, the catheter is inserted through the feeder vessel as close to the nidus as possible without touching the arterial wall. Possible normal branches originating from the vessel are studied exhaustively for at least 3 cm before it enters the nidus.
The choice of optimum projection for the injection is also critical. We attach greater importance to visualizing the origin of the vein or veins draining the nidal compartment being embolized. Wherever possible we try to distance the catheter from the nidus and the veins to control reflux in the feeder vessel.
An image of an earlier injection of contrast material through the microcatheter visualizing the artery, the nidus, and the drainage vein is displayed on the reference screen.
Having a radiographic film viewer in the procedure room has in some cases proven to be invaluable, allowing the image of a global angiography scan taken through the guide catheter to be viewed and thus allowing identification of the rest of the feeder arteries that might undergo backflow filling.
The Onyx is injected slowly yet continuously and monitored fluoroscopically by roadmapping. Using the new higher concentration Onyx affording greater radioopacity, the polymer can be seen to advance in the form of a stream that very seldom breaks up and slowly fills all the interstices of the malformation.
Injection is not halted if the Onyx is flowing properly into the interior of the nidus, but it is stopped when the embolic agent reaches the origin of the vein or if we suspect that backflow is taking place and we are not sure of the extent of filling or which feeder arteries are involved. Reflux into the catheterized vessel is allowed, with filling of the entire lumen of the vessel being permitted over a length of about 1.5 cm.
At this point, with injection halted, we wait 2 min and during that time perform control angiography through the guide catheter to check the morphology of the Onyx injected, using a projection orthogonal to the projection used for injection.
Next, after new roadmapping without contrast material, Onyx injection is resumed, and in many cases the embolization material can be seen to re-enter the malformation and fill other parts of the nidus.
This sequence is repeated several times until blood flow is blocked by occlusion of the feeder vessel's lumen by a plug measuring at most 1.5 cm.
Injection should always be halted when the Onyx has reached the origin of the vein. We have observed that in a large majority of cases, repeat injection of Onyx changes direction and fills new compartments.
Injection is complete when there is no further distal filling, when flow repeatedly proceeds in the direction of the vein, or when backflow fills other feeder arteries that may be normal branches.
Wherever possible the catheter should be removed by a sharp tug after first stretching the microcatheter to straighten out any kinks and reduce any points of contact with the wall of the artery used for access.
We have never injected more than three pedicles in a single session.
On completion of the procedure the patient is moved to the intensive care unit, intubated, and hypotension is monitored for at least 24 h.
After the patient regains consciousness and has been extubated, a normal neurological examination is performed, and the patient is then moved to the ward for neurological and vital signs monitoring for four days.
Another embolization session can be held after approximately two months. Radiosurgery or surgery can be carried out after about that same time, though angiography must be repeated immediately before any of these procedures.
Results
The following angiographic results have been achieved after an average of 2.5 embolization sessions per patient:
- 10 patients had 100 % occlusion on control angiography immediately after the final embolization session, for a complete occlusion rate of 22 %. In two patients aged 5 and 9 years initially treated after suffering various cerebral haemorrhages, the immediate outcome was complete occlusion. However, on follow-up angiographic controls two and four years later, respectively, the malformations were seen to have recurred, with angiogenesis and new nidal structures that required fresh embolization. The final result was 80-% closure and referral for radiosurgery.
- 15 patients (34%) had over 90-% closure with persistent occlusion on examination prior to radiosurgery or surgery.
- 16 patients (35%) had over 80-% closure.
- 4 patients (9%) had less than 60-% closure.
At the time of this review assessment, our results are 32 patients whose cerebral AVMs have been completely cured, 8 by embolization alone, 12 by embolization and surgery, and 12 by embolization and radiosurgery. The remaining 13 patients are in follow-up subsequent to radiosurgery.
Technical complications during the procedure involved four arterial perforations by the microguidewire in the latter stages of distal catheterization, all resolved by immediate injection of Onyx without clinical symptoms. Two of these involved perforating arteries. Two cases of entrapment of the catheter, both in 1999 after injection procedures lasting 45 and 60 min, also without clinical symptoms. One case of catheter rupture due to Onyx injection pressure, with the embolic agent leaking into the internal carotid artery and adhering to the artery wall without migrating or occluding blood flow, again without clinical symptoms. The catheter involved was a first generation FlowRider.
Morbidity resulting in permanent deficit occurred in seven cases (15.5%): four cases of hemiparesis, one case of hemiparesis with aphasia, one case of hemianopsia, and one case of unilateral deafness.
Of these seven cases, two were ischemic complications. One was produced by embolization of a branch of the right AICA feeding a cerebellar AVM. The patient had two right AICAs and it was impossible to tell which was functional. The second complication occurred during closure of a temporal-occipital AVM.
Of the remaining five cases, three were haemorrhages that occurred between 24 h to one week after the last embolization session resulting in over 80-% closure of the nidus.
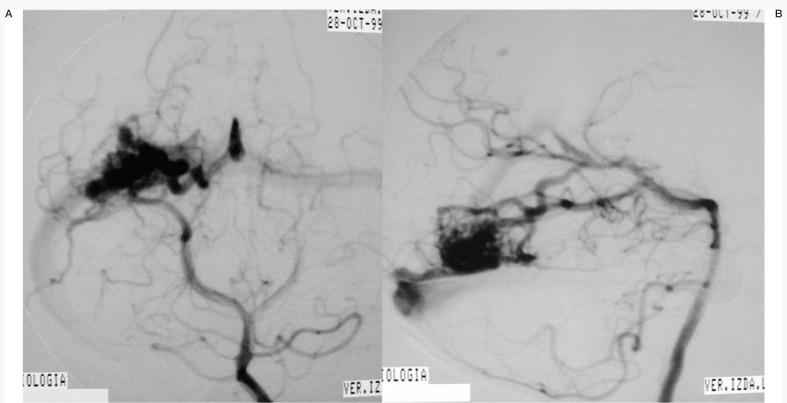
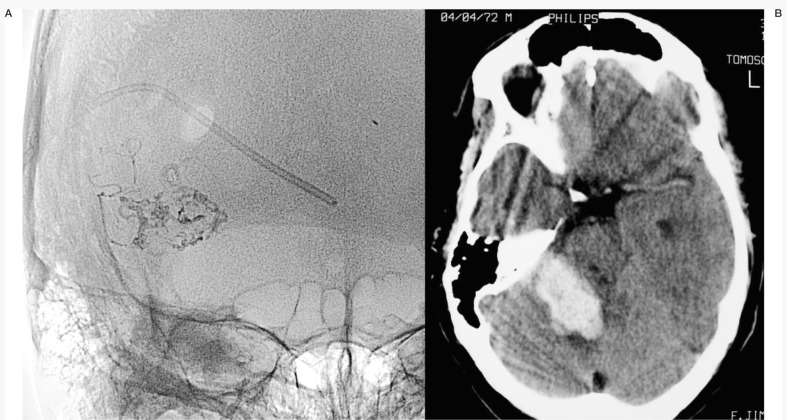
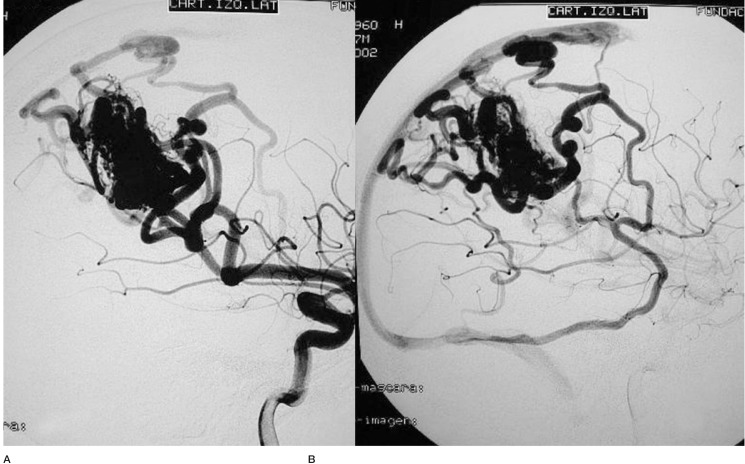
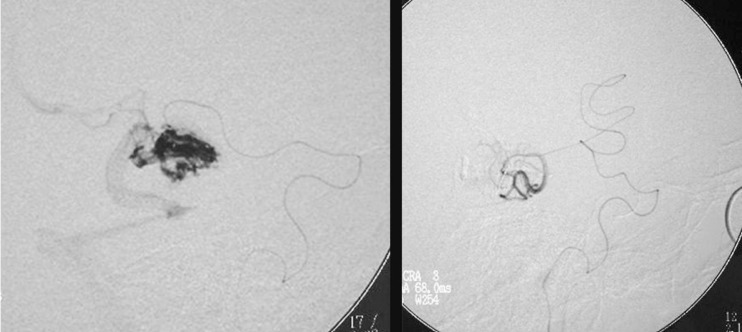
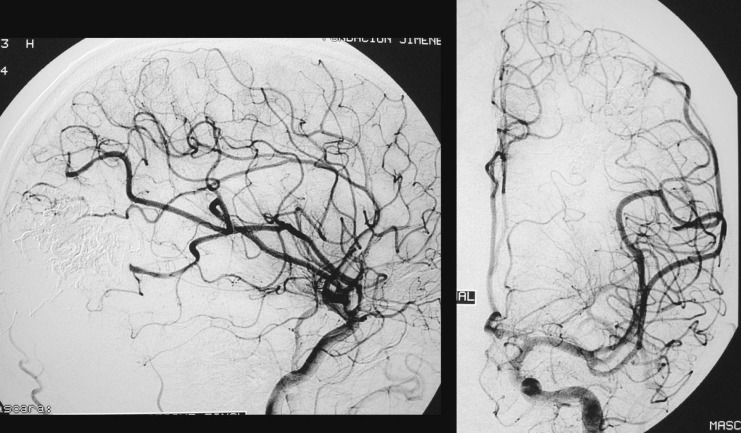
One of the patients (Case 1: figures 1, 2 and 3) had an AVM at the base of the right temporal lobe and presented with temporal lobe seizures that were not responsive to medication. Three pedicular injections of Onyx occluded the malformation angiographically. Eight hours later the patient suffered diminished awareness, and a brain CT scan revealed a right cerebellar haematoma compressing the brainstem. The haematoma was evacuated without touching the AVM. The patient was left with right hemiparesis.
Figure 1.
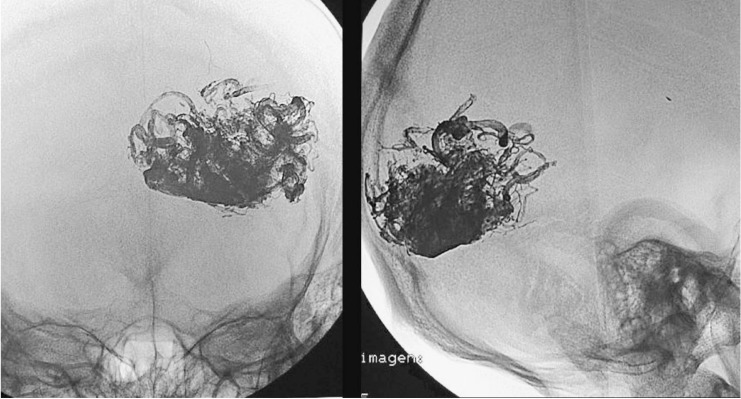
Case 1: Vertebral arteriography. Right medial temporal basal AVM. Supratentorial. Feeder branches from the posterior cerebral (medial temporal) artery. Venous drainage into the superior petrosal sinus and deep drainage into the basilar vein.
Figure 2.
Case 1: Vertebral arteriography performed immediately post-embolization. AVM and feeder arteries not discernible angiographically. Pedicular injection.
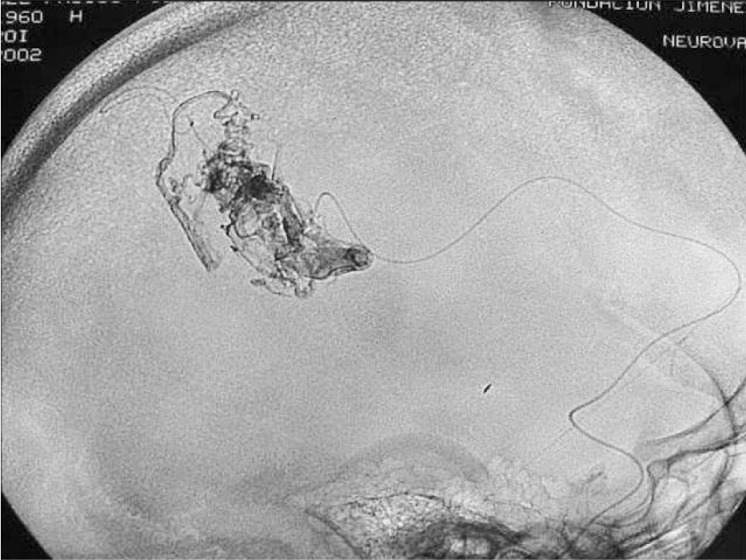
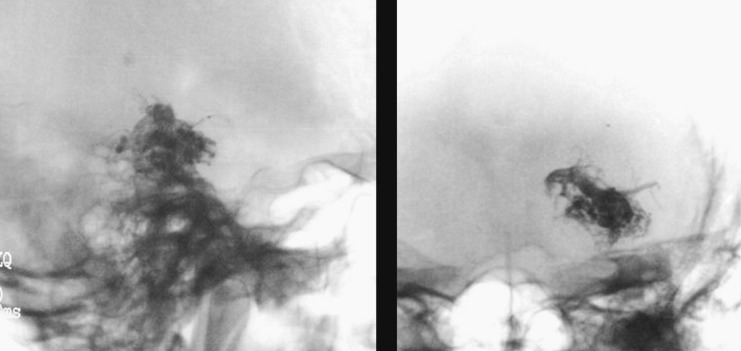
Figure 3.
Case 1: Onyx cast and simple axial CT scan. Pedicular injection. Right cerebellar haematoma slightly compressing the brainstem. Infratentorial location not directly related to the AVM.
The haemorrhage was probably caused by progressive thrombosis of the superior petrous sinus draining the superior right hemicerebellum.
The second patient had a left middle parietal cortical-subcortical AVM with intraventricular haemorrhage. Cerebral angiography was performed a few hours after the start of bleeding. A single injection of 9 cc of Onyx over 50 min. At the end of injection the Onyx was observed to pass into the ventricular cavity. 95-% closure. A few hours later massive intraventricular haemorrhage and smaller intracerebral haemorrhage. Immediate evacuation with slight right hemiparesis on discharge. A new angiographic examination performed prior to discharge showed complete resection of the AVM.
The third hemorrhagic complication was in a patient with a left frontal cortical-subcortical AVM 95-% closed after three injections of Onyx, in one session 7 cc being injected over 45 min. 0.5 cc of DMSO was inadvertently injected at high speed into a feeder artery pedicle. Massive haemorrhage occurred 3 h after the procedure, requiring immediate evacuation with extirpation of the haematoma and the AVM. On discharge AVM not discernible angiographically, right hemiparesis and aphasia.
In two cases of frontal AVM haemorrhage occurred one month after the last session while the patients were awaiting radiosurgery. Angiography had shown 80-% closure, and the Onyx had not entered the vein in either case. Following surgical evacuation an additional final embolization procedure was performed, and the patients were referred for radiosurgery. One patient was left with right hemiparesis.
There was one death (2 %) due to a massive cerebral haematoma five days after the third procedure to embolize a giant AVM in a 62 year-old patient with a history of six previous haemorrhages with a course of over 20 years. The final injection during the third embolization session was 6 cc over 35 min, with passage of the Onyx into the principal drainage vein. The patient spent 48 h in the ICU with monitoring for hypotension.
Discussion
Cerebral AVMs have posed a therapeutic challenge for us since 1980. A broad gamut of embolization materials has been tried, not always with satisfactory results.
In 1999 we decided to employ a new embolic agent designed specifically for the endovascular treatment of cerebral AVMs. "ONYXTM Liquid embolic System consists of ethylene vinyl alcohol co-polymer ( EVOH) disolved in Dimethyl sulfoxide (DMSO), and micronized tantalum for radiopacity.
DMSO acts as a solvent allowing the material to be injected through a microcatheter. Upon contact with the bloodstream DMSO precipitates, polymerization begins and the targeted area is embolized.
Onyx LES is presented in 3 different viscosities Onyx 18 , 20 and 34.
At the outset we envisaged a curative treatment for many lesions of this type and realized that we had to change the philosophy underpinning the therapeutic approach to these malformations, until then regarded as being amenable only to presurgical or preradiotherapeutic procedures.
Now, based on the experience gained over these past years and critical appraisal of our results, today we take a more global view of treatment for our patients, and our final objective is to cure AVMs completely using a combination of three methods, endovascular intervention, surgery, and radiotherapy.
The reason for treating cerebral AVMs is, first and foremost, to eliminate the risk of haemorrhage and in the second place to physically remove the lesion for good.
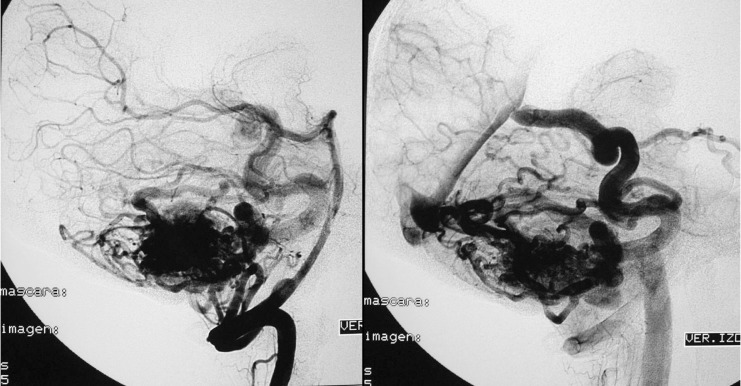
When the decision to undertake endovascular treatment as a first step is made, the therapeutic approach has to be chosen, i.e., the purpose of intervention will be either curative or preliminary, in preparation for a subsequent treatment option. This choice will depend on the size, location, and angioarchitecture of the AVM (Case 2: figures 4, 5, 6 and 7). Another factor that influences decision-making is the way the lesion presents clinically, since in certain cases conservative approaches are feasible, for instance, when detection is an accidental finding or patients are more than 80 years old, or it may simply be possible to perform diagnostic angiography only in certain cases in which haematomas need to be evacuated. We have to wait for the haematoma to be resolved and for the patient's clinical condition to be stabilized before embolization of the AVM can be undertaken.
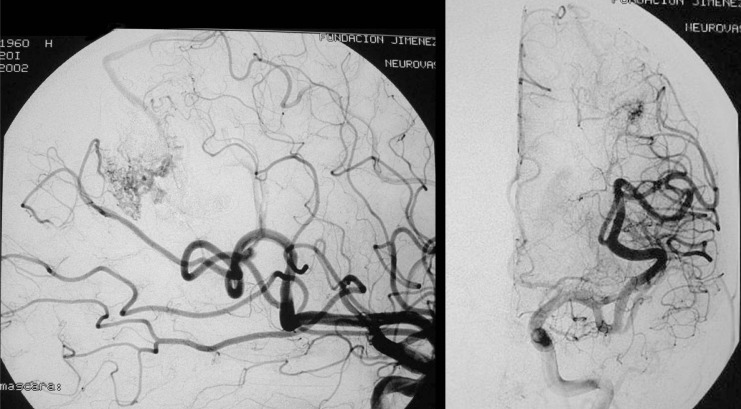
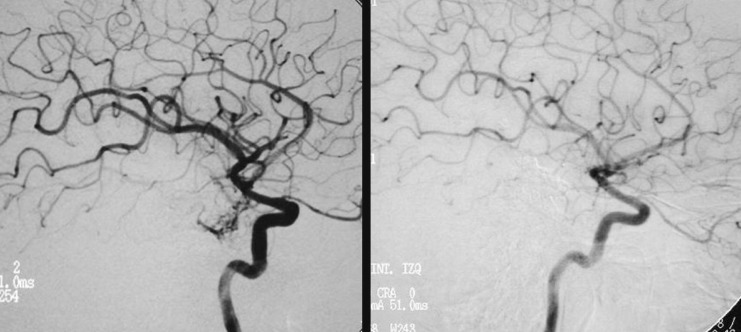
Figure 4.
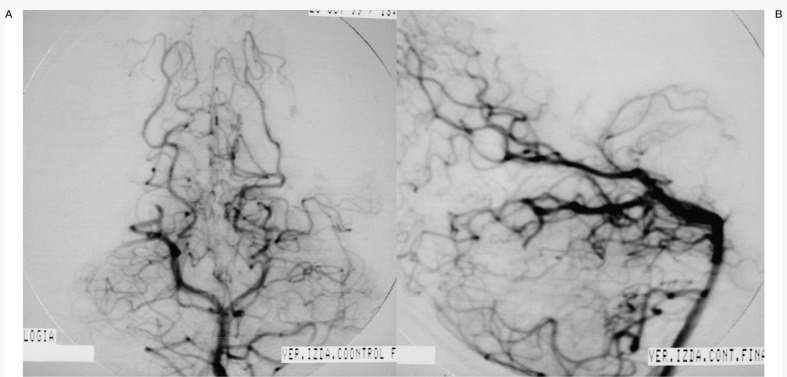
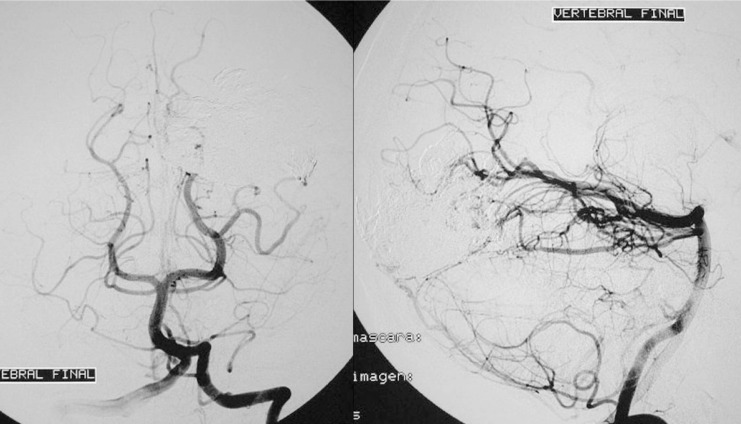
Case 2: Left carotid arteriography, AP projection, arterial and venous phases. Posterior parietal AVM with a compact nidus and multiple drainage veins. Some deep venous drainage.
Figure 5.
Case 2: Left carotid arteriography, lateral projection. Arterial and venous phases. High-risk AVM because of its location in a very eloquent area.
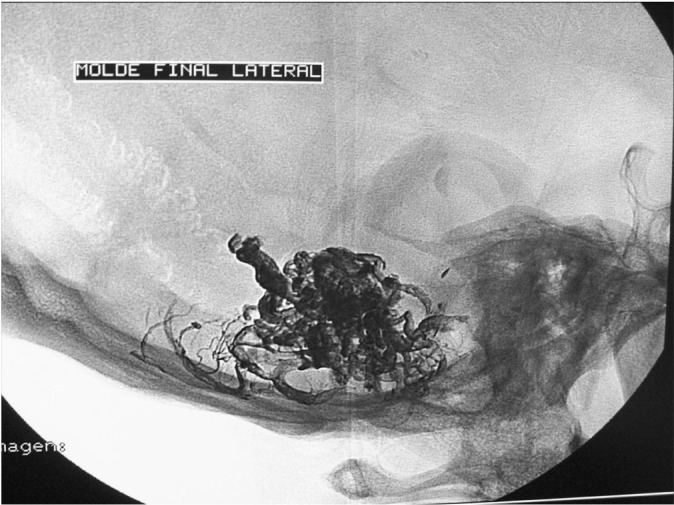
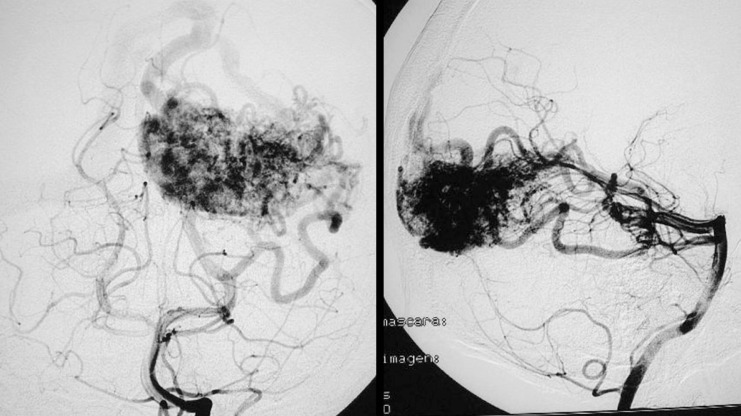
Figure 6.
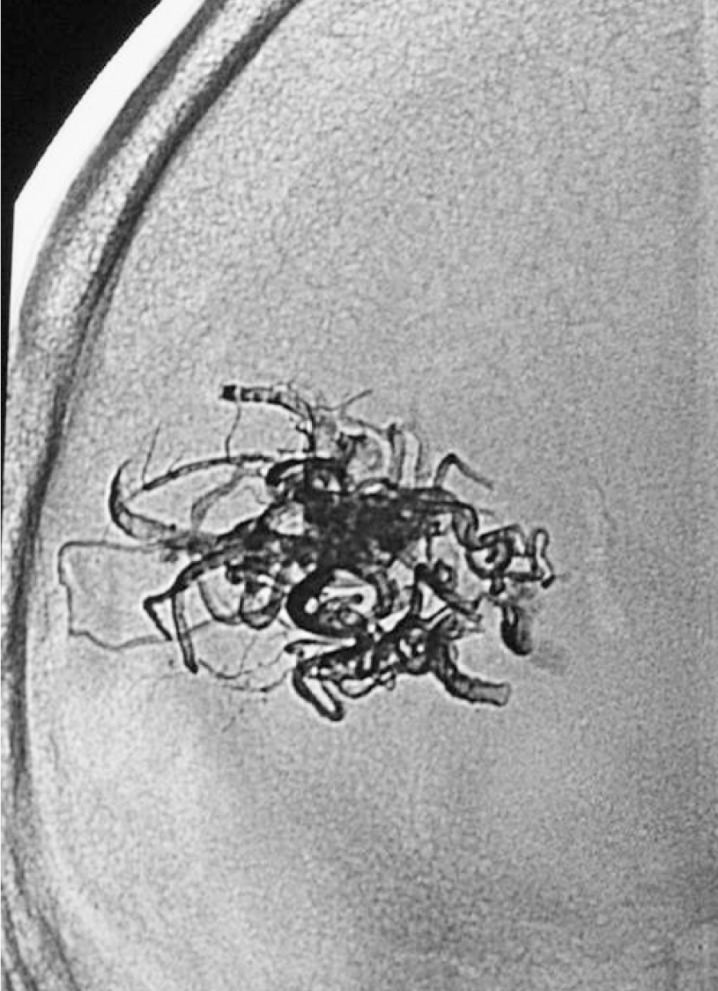
Case 2: Onyx cast after a single embolization session with two injections of Onyx 18, one pedicular injection and one intranidal injection, using the reflux technique.
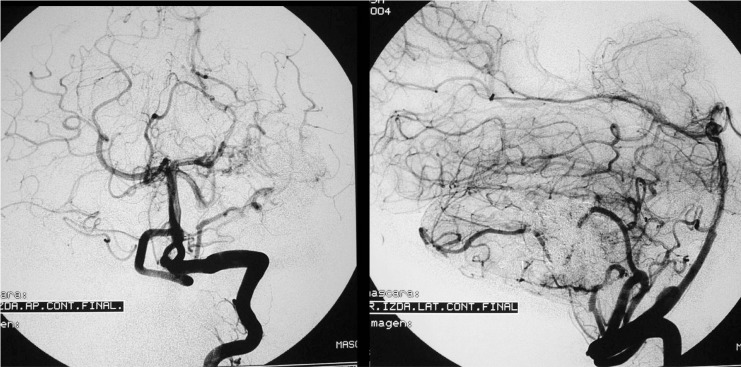
Figure 7.
Case 2: Control arteriography immediately on conclusion of the procedure. AVM remnant referred for radiosurgery.
Today, MRI scans and MR angiography are able to pinpoint the size and location of the AVM. Cerebral diffusion and perfusion sequences have been used to assess the condition of the brain in the region around the malformation.
A detailed study of all the features of an AVM requires selective angiography with multiple projections of the internal, external, and vertebral carotid arteries to try to identify all the feeders, the malformed nidus, and the drainage veins.
However, on many occasions high-quality angiographic imaging still does not produce sufficient input for a therapeutic decision6, because it fails to demonstrate the small feeders, intranidal aneurysms, direct AV fistulas, and separate compartments within the nidus.
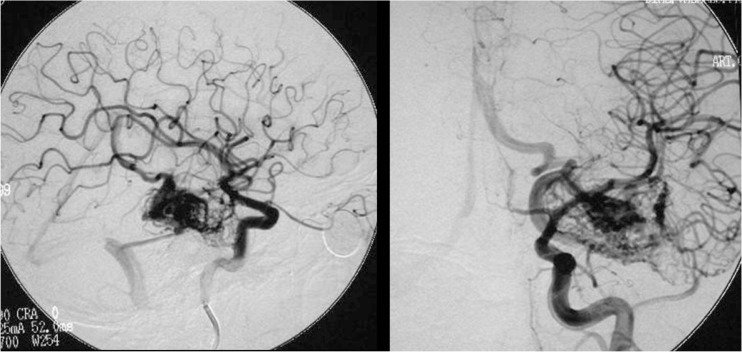
All these factors make necessary superselective imaging of all feeders, though in most cases this type of examination is performed only after the decision to treat the lesion endovascularly has already been taken (Case 3: figures 8, 9,10 and 11).
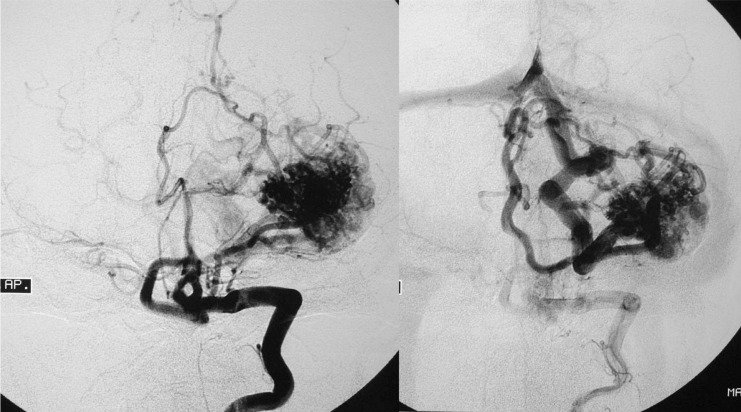
Figure 8.
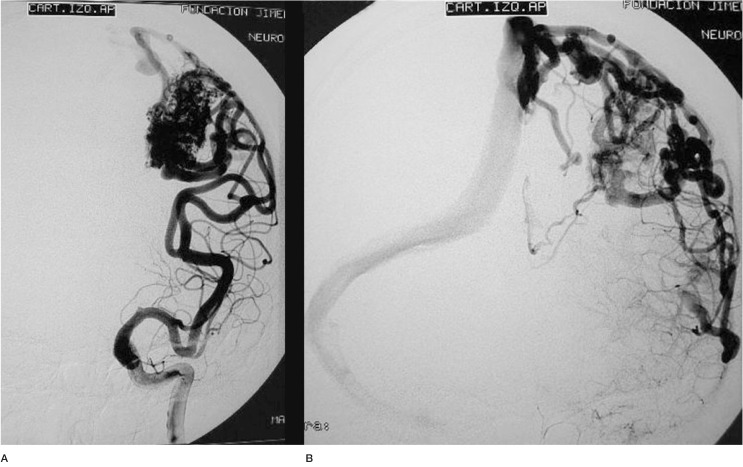
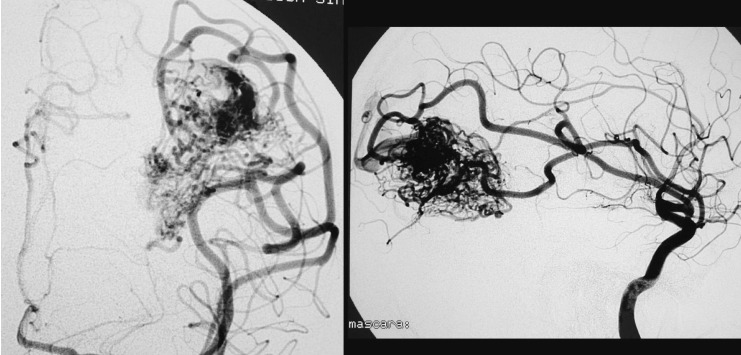
Case 3: Left vertebral arteriography. AP projection. Arterial and venous phases. Left cerebellar AVM
Figure 9.
Case 3: Vertebral arteriography, lateral projection. Arterial and venous phases. Principal feeder from the AICA. Profuse, dilated, ectasic drainage.
Figure 10.
Case 3: Onyx cast after two embolization sessions and one injection of 6 cc of Onyx 18 over 45 min using the reflux technique.
Figure 11.
Case 3: Vertebral arteriography after the second session. AVM not discernible angiographically.
Reliable comparisons of the results of cerebral AVM embolization is extremely difficult because of the wide range of different embolization materials and embolization methods employed by different authors making the reports. In various series published in the 1990s, complete occlusion of the AVM was achieved in 10-20% of cases 7,8,9. Wikholm 10 reported a cure rate of 13% with a 1.3-% mortality rate and a 6.7-% permanent morbidity rate.
In our series 10 cases (22%) were completely cured. These were unselected patients who had been referred to our department by neurologists and neurosurgeons at our own hospital or at other hospitals. The same method was employed in all cases by the same team of neuroradiologists, always using Onyx. The treatment strategy pursued a definitive cure in all cases except for three AVMs in the basal ganglion. The two cases in which reopening of the nidus occurred 2 and 4 years after treatment involved a 5-year-old and a 9-year-old child, respectively, who had undergone treatment during the acute phase due to repeated haemorrhaging and neurological deterioration. In both cases pial angiogenesis was observed to have taken place, probably secondary to compressive ischemia caused by the hematomas.
More than 90-% closure was achieved in 15 patients and more than 80-% closure in 16 patients, i.e., angiographic closure of more than 80 % of initial AVM volume was achieved in 91 % of our cases (Case 4: figures 12,13,14 and 15).
Figure 12.
Case 4: Young patient with seizures not responsive to medication. Right carotid arteriography. Medial temporal AVM with superficial and deep drainage.
Figure 13.
Case 4: Catheterization of the nidus via the anterior choroidal artery. Selective catheterization of a temporal branch in front of the nidus.
Figure 14.
Case 4: Onyx cast via the choroidal artery over 60 min using the reflux technique.
Figure 15.
Case 4. A) Immediate control angiography. Small nidal remnant. B) Control angiography after radiosurgery two years later. AVM not discernible angiographically.
A long injection lasting over 30 min was achieved after refluxing in all our patients during at least one embolization session, delivering a mean amount of 2 cc of Onyx. These injections have demonstrated the interconnections between the different, seemingly independent, compartments of the nidus and in many cases are able to occlude small, non-catheterized feeder arteries, including dural branches, which are filled by backflow.
The main technical complication encountered using this type of material is small perforations of the terminal arteries feeding the AVM produced by attempting highly distal insertion of the catheter over the guidewire. This occurred in four of our cases, all of which were resolved without any clinical symptoms by quickly injecting Onyx.
Another potential technical complication is the inability to remove the catheter after an extremely long injection with reflux, allowing filling of more than 2 cm. In such cases it is best not to persist in attempting to remove the microcatheter but to leave it in place with the end underneath the skin at the site of the femoral puncture. This complication occurred in two of our cases in the early years of the series, in both cases using the FlowRider microcatheter. In one of the cases papaverine was administered for 24 h, after which time the catheter still could not be removed.
Withdrawal of the catheter is another source of complications, because the drag on the AVM may tear small vessels, causing haemorrhage. The catheter should be drawn out with a sharp tug after pulling the microcatheter taut to reduce contact with the walls of the blood vessel used for access. This manoeuvre should always be performed while the patient is not under the effect of heparin.
The most frequent clinical complication was post-embolization cerebral haemorrhage. There are many possible causes of this complication, and it has been reviewed in a number of studies12,11,14,13,15,16,17.
It always results from increased perfusion pressure in a part of the malformation unused to receiving that amount of blood' brought about by redirection of blood flow either inside or immediately outside the AVM.
Significant blockage or stricture of AVM drainage leads to increased pressure at the outlet of a compartment or neighbouring compartments. Deruty18 described four haemorrhages in 40 patients (10 %) in the week after embolization and demonstrated occlusion of the principal drainage vein.
Normal perfusion pressure breakthrough in the brain was first described by Spetzler et Al19, who observed that the normal brain parenchyma surrounding an AVM is subject to arterial steal, with chronic hypoperfusion and impaired autoregulation. Acute reduction of AVM flow restores normal flow to the surrounding arterioles without proper autoregulation, giving rise to oedema of the capillary bed and haemorrhage. There is experimental evidence that vasomotor regulation may be altered following a prolonged period of arteriolar inactivity20.
Folkow 21 demonstrated that arterioles subject to chronic hypotension underwent structural changes resulting in loss of the middle layer and increased lumen size.
Gradual thrombosis of a vein that was draining both the AVM and normal brain tissue is another cause of post-embolization haemorrhage, with obstruction leading to venous haemorrhagic infarction. The site tends to be located outside the malformation, and its clinical behaviour is less aggressive than massive rupture of the AVM arteries22.
Ischemic complications caused by occlusion of normal vessels in the vicinity of the AVM are less common, because most injections are carried out intranidally or very close to the nidus.
The most recent publication dealing with the frequency of this type of haemorrhage placed the frequency at 3%23.
Certain angiographic findings can help predict an increased likelihood of rupture:
- Occluded or retarded flow in one of the principal veins.
- Stagnation of contrast material inside the nidus.
- Subtotal occlusion of a small AVM leaving part of the nidus unembolized.
- Occlusion of a direct fistula within the nidus, producing redirection of flow.
Where these findings are present antihypertensive medication should be administered to the patient in the ICU for several days24.
Assessment of our results has revealed a final morbidity rate of 20 %. In at least two cases (1 and 5) the cause was a technical deficiency attributable more to the radiologist than to the product, and in a third case (4) the cause was inappropriate indications for treatment, that is, embolization should not have been attempted during the hyperacute stage of haemorrhage.
In all the remaining cases, technical conditions were identical.
Detailed analysis of the angioarchitecture of the AVMs in those cases in which complications occurred has not revealed any risk factors except one' namely' all the cases involved malformations having a volume larger than 15 cm3 and an ectasic' high-volume' or distinctly aneurysmal principal drainage vein.
Yet in other malformations having these same features total or subtotal closure was achieved.
Results have been very good for malformations of the cerebellum and the temporal pole and not as good for deep malformations and malformations of the corpus callosum.
In the cases in which the malformation was extirpated following embolization using Onyx' the neurosurgeons observed that the embolized vessels were flexible and there was hardly any perilesional inflammation.
We have observed two distinct intranidal morphologies that are helpful in predicting AVM behaviour during embolization using Onyx.
In the glomerular type of AVM' nidus appearance resembles a cluster of small microlobules with a well-defined margin and one or two dilated arterial branches reaching the centre' and the drainage veins issue from the margins (Case 5: figures 16, 17, and 18)
Figure 16.
Case 5: Vertebral arteriography. AP and lateral projections. Large left occipital AVM in a young patient suffering from headache but without any neurological deficit. Glomerular type AVM.
Figure 17.
Case 5: Onyx cast after three embolization sessions including an intranidal injection of 8 cc of Onyx over 40 min using the reflux technique.
Figure 18.
Case 5: Final arteriography, AVM not discernible angiographically
For this type of AVM' injection of the Onyx is always slow and gradual' with centrifugal filling of all or a large part of the nidus. Refluxing to block flow during injection yields more complete filling.
The microtubular type of AVM has winding microtubules extending into the centre of the AVM from arteries that are not particularly dilated and often traverse the nidus. The margin of the nidus is less well-defined' and ordinarily there are more drainage veins than in glomerular AVMs. Injection of the Onyx tends to be less regular' even when performed from within the nidus itself' and very seldom can large amounts of Onyx be injected' even where flow can be blocked by refluxing. This type of malformation often presents internal haemorrhage and exhibits pial angiogenesis (Case 6: figures 19, 20 and 21).
Figure 19.
Case 6: Left carotid arteriography. Left temporo-parietal AVM with a microtubular type nidus.
Figure 20.
Case 6: Onyx cast after three embolization sessions using intranidal and pedicular injections.
Figure 21.
Case 6: Immediate final arteriography. AVM not discernible angiographically.
Conclusions
For us' the development of Onyx as an embolization agent has meant a complete change in our approach to all types of cerebral AVMs> even those deemed untreatable by other specialists.
We have learned that the method of treatment is fully reproducible but that the procedure must be performed by individuals with considerable experience in this method of treatment and that treatment protocols must be strictly adhered to.
Further research on the protocols should be continued to decrease the complication rate and increase the percentage of complete AVM closure.
References
- 1.Taki W, Kikuchi H, et al. Embolization of arteriovenous malformations using EVAL mixture (a new liquid embolization material. Neuroradiology. 1990;33:195–196. [Google Scholar]
- 2.Murayama Y, Viñuela F, et al. Non-adhesive liquid embolic agent for the treatment of cerebral AVM: clinical results at UCLA. Interventional Neuroradiology. 1999;5:78–82. [Google Scholar]
- 3.Jahan R, Murayama Y, et al. Embolization of arteriovenous malformatios with Onix: cinicopathological experience in 23 patients. Neurosurgery. 2001;48:984–997. doi: 10.1097/00006123-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chaloupka JC, Viñuela F, et al. Technical feasibility and histopathologic studies of ethylen vinyl coplolymer EVAL using a swin endovascular embolization model. Am J Neuroradiol. 1994;15:1107–1115. [PMC free article] [PubMed] [Google Scholar]
- 5.Sampey D, Ditmore QM, et al. Histological changes in brain tissue and vasculature after intracarotid infusion of organic solvents in rats. Neuroradiology. 1996;38:291–294. doi: 10.1007/BF00596551. [DOI] [PubMed] [Google Scholar]
- 6.Naktad PH, Nornes H. Superselective angiograhy, embolization an surgery in treatment of arteriovenous malformations of the brain. Neuroradiology. 1994;36:410–413. doi: 10.1007/BF00612131. [DOI] [PubMed] [Google Scholar]
- 7.Berenstein A, Choi IS. Surgical neuroangiography of intracranial lesions. Radiol Clin North Am. 1988;26:1143–1151. [PubMed] [Google Scholar]
- 8.Grziska U, Westphal M, et al. A joint protocol for the neurosurgical and neuroradiologic treatment of arteriovenous malformations: indications, technique and results in 76 cases. Surg Neurol. 1993;40:476–484. doi: 10.1016/0090-3019(93)90050-b. [DOI] [PubMed] [Google Scholar]
- 9.Guo WY, Wikholm K, et al. Combined embolization and ganma knife radiosurgery for cerebral arteriovenous malformations. Acta Radiol. 1993;34:600–606. [PubMed] [Google Scholar]
- 10.Wikholm K, Lindquist C, et al. Embolization of cerebral arteriovenous malformations. Part I. Technique morpholgy and complications. Neurosurgery. 1996;39:448–459. doi: 10.1097/00006123-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kader A, Joung WL, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34:801–808. doi: 10.1227/00006123-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Marks MP, Lane B, et al. Hemorrhage in intracerebral arteriovenous malformations: angiographics determinants. Radiology. 1990;176:807–813. doi: 10.1148/radiology.176.3.2389040. [DOI] [PubMed] [Google Scholar]
- 13.Nataf F, Meder JF, et al. Angioarchitecture associated with hemorrhage in cerebral arteriovenous malformations: a pronostic statistical model. Neuroradiology. 1997;39:52–58. doi: 10.1007/s002340050367. [DOI] [PubMed] [Google Scholar]
- 14.Turjman F, Massoud TF, et al. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery. 1995;37:856–860. doi: 10.1227/00006123-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Thompson RC, Steinberg GK, et al. The management of patients with arteriovenous malformations and associated intracranial aneurysms. Neurosurgery. 1998;43:202–212. doi: 10.1097/00006123-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Meisel HJ, Mansmann U, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000;46:793–800. doi: 10.1097/00006123-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Piotin M, Ross IB, et al. Intracranial arterial aneurysms associated with arteriovenous malformations: endovascular treatment. Radiology. 2001;220:506–513. doi: 10.1148/radiology.220.2.r01au09506. [DOI] [PubMed] [Google Scholar]
- 18.Deruty R, Pelissou-Guyotat I, et al. Therapeutic risk in multidisciplinary approach of cerebral arteriovenous malformations. Neurochirurgie. 1996;42:35–43. [PubMed] [Google Scholar]
- 19.Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 20.Nornes H, Grip A. hemodynamic aspects of cerebral arteriovenous malformations. J Neurosug. 1980;53:456–464. doi: 10.3171/jns.1980.53.4.0456. [DOI] [PubMed] [Google Scholar]
- 21.Folkow B, Gurevich M, et al. The hemodynamic consecuences of regional hypotension in spontaneously hypertensive and normotensive rats. Acta Physiol Scand. 1971;83:532–541. doi: 10.1111/j.1748-1716.1971.tb05111.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Rodhan NRF, Sundt TM, jr, et al. A theory for the hemodynamic complications following resection of intracerebral arteriovenous malformations. J Neurosurg. 1993;78:167–175. doi: 10.3171/jns.1993.78.2.0167. [DOI] [PubMed] [Google Scholar]
- 23.Piccard L, Costa EDA, et al. Acute spontaneous hemorrhage after embolization of cerebral arteriovenous malformations With N-butil cyanoacrilate. J Neuroradiol. 2001;28:147–165. [PubMed] [Google Scholar]
- 24.Massoud TF, Hademenos GJ, et al. Can induction of systemic hypotension help prevent nidus rupture complicating cerebral arteriovenous malformations embolization? Analysis underlying mechanisms achieved using a theoretical model. Am J Neuroradiol. 2000;21:1255–1267. [PMC free article] [PubMed] [Google Scholar]
- 25.Akin ED, Perkins E, et al. Surgical handling characteristics of an ethylene vinil alcohol copolymer compared with N-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J Neurosurgery. 2003;98:366–370. doi: 10.3171/jns.2003.98.2.0366. [DOI] [PubMed] [Google Scholar]