Summary
The endovascular approach to arteriovenous malformations (AVM) using different embolizing agents is a well-established treatment option.
This report assesses the results of our experience using a non "glueing" embolic material available for several years, commercially known as Onyx®.
We used Onyx to treat 34 consecutive patients in the last four years. All patients were treated in the same department by the same neuroradiological team, with a strictly repetitive technical strategy and procedural protocol.
All our patients presented AVMs with Spetzler Grade 3 or more, because in our Institution Grade 1 or 2 AVMs are directly treated by surgical approach. We adopt a multidisciplinary treatment approach (embolization, surgery, radiotherapy) by which embolization is construed as work in progress offering definitive treatment of AVMs without severe risks.
Embolization is mainly undertaken as the first step before surgery, to reduce flow and size of the AVM by a "targeted" technique. In addition to reducing lesion size, endovascular treatment aims to seal off AVM areas anatomically or haemodynamically complex for surgical treatment. Occasionally, the reduction in size allows a radiosurgical approach. Embolization seldom results in a definitive cure of AVMs.
At the end of multimodal approach, we obtained the complete and definitive cure of AVM in 21/34 patients (two complete obliteration with interventional technique, 19 in combination with surgery); to these were added 5/34 patients who received radiosurgical therapy.
No major complications arose during endovascular treatment. One patient had transitory (36 hour) impaired right arm pronation. The CT scan disclosed an asymptomatic mild SAH in the left sylvian fissure but no ischaemic areas.
One patient still in treatment died from fatal rebleeding (the clinical onset had been with haemorrhage two weeks before the session) 12 days after the embolization.
Excellent or good clinical outcome was obtained in 23/26 patients who completed the therapeutic protocol. Outcome was conditioned by focal symptoms present on admission in three patients due to haemorrhagic onset, but only one patient presented a severe disability on discharge.
In our view, the main problem of Onyx is that the apparently easier approach will probably lead to a wider diffusion of these procedures. AVMs are extremely difficult and dangerous to treat: this is not affected by the quality of the embolizing agents used and must be kept in mind at all times.
Key words: cerebral AVM, AVM embolization, Onyx
Introduction
The endovascular approach to arteriovenous malformations (AVM) using different embolizing agents is a well-established treatment option. The procedure allows complete closure of the malformation in around a quarter of all cases reported in different literature series with significant variability among different authors. The incidence of major complications also varies widely, probably because successful treatment is highly operator-dependent. In turn, operator-dependency is linked to use of a valid but difficult to manage embolizing agent like acrylic glue (Histoacryl). The choice of cyanoacrylate dilution and the speed and technique of glue injection in relation to microcatheter position are empirical skills difficult to transmit from one operator to another and often applied on the basis of personal experience. This means that although acrylic glue is a valid embolizing agent applied for many years, it remains difficult to use with significant risks for the patient. In addition, the potential risk of serious complications has prevented acrylic glue manufacturers giving the product the CE trademark for endovascular use, which is prohibited. This situation recently created difficult working conditions, especially from the legal standpoint, despite widespread application for many years (also acknowledged by the Italian Ministry of Health, which can authorise the use of glue on an individual patient basis).
This situation accounts for the ongoing search for safer alternative embolizing agents whose application can be standardised within limits feasible for any interventional procedure.
A new embolizing material, Onyx (Onyx System MicroTherapeutics, Inc., MTI), was marketed some years ago as a "foam" with the theoretical feature of progressive occlusion rather than immediate glueing.
We report the results obtained in 34 patients treated with Onyx in the last four years analysing the application technique and our impressions during treatment in relation to our past experience with cyanoacrylate glue.
Number of cases, age range and mean age
We treated 34 consecutive patients using Onyx in the last four years (table 1).
Table 1.
Case Record
| # | Sex | Age | Sessions | Pedicles |
|---|---|---|---|---|
| 1 | M | 60 | 2 | 3 |
| 2 | F | 39 | 3 | 6 |
| 3 | F | 31 | 1 | 2 |
| 4 | M | 21 | 2 | 3 |
| 5 | M | 69 | 1 | 1 |
| 6 | F | 38 | 2 | 3 |
| 7 | M | 43 | 4 | 6 |
| 8 | F | 21 | 1 | 2 |
| 9 | F | 35 | 5 | 7 |
| 10 | F | 19 | 2 | 2 |
| 11 | M | 18 | 6 | 8 |
| 12 | M | 35 | 1 | 1 |
| 13 | M | 22 | 2 | 4 |
| 14 | M | 23 | 2 | 3 |
| 15 | M | 71 | 1 | 2 |
| 16 | F | 33 | 1 | 2 |
| 17 | M | 18 | 4 | 6 |
| 18 | F | 30 | 5 | 7 |
| 19 | M | 37 | 5 | 6 |
| 20 | F | 32 | 2 | 2 |
| 21 | F | 33 | 1 | 2 |
| 22 | M | 38 | 1 | 1 |
| 23 | F | 55 | 2 | 3 |
| 24 | M | 51 | 3 | 6 |
| 25 | M | 22 | 2 | 3 |
| 26 | M | 31 | 1 | 1 |
| 27 | F | 47 | 1 | 1 |
| 28 | F | 43 | 4 | 6 |
| 29 | F | 26 | 5 | 6 |
| 30 | F | 51 | 4 | 4 |
| 31 | F | 44 | 2 | 2 |
| 32 | F | 52 | 1 | 1 |
| 33 | F | 16 | 1 | 2 |
| 34 | F | 38 | 3 | 4 |
All patients were treated in the same department by the same neuroradiological team with a strictly repetitive technical strategy and procedural protocol.
All our patients presented AVMs with Spetzler Grade 3 or more because in our Institution Grade 1 or 2 AVMs are directly treated by surgical approach.
We performed 83 treatment sessions (min. 1, max. 8, mean 2.4 sessions/patient), embolizing 118 pedicles (min. 1/session, max. 3/session, mean 1.4 pedicles/session).
The cohort comprised 17F and 17M, ranging in age from 6 to 71 years (mean age: 36.5 years): more than two thirds (23/34) of our patients were younger than 40 years.
Clinical presentation
Clinical presentation at onset and site of AVMs are reported in table 2.
Table 2.
Clinical Onset and AVM Site
| # | AVM Site | Clinical onset |
|---|---|---|
| 1 | LEFT, Temporo-occipital | Haemorrhage |
| 2 | LEFT, Temporo-occipital | Haemorrhage |
| 3 | LEFT, Pre-Rolandic | Epilepsy |
| 4 | Cerebellar vermis | Neurological deficit |
| 5 | RIGHT, Fronto-opercular | Haemorrhage |
| 6 | LEFT, Temporo-occipital | Haemorrhage |
| 7 | RIGHT, Fronto-temporal | Epilepsy |
| 8 | LEFT, Temporo-occipital | Haemorrhage |
| 9 | LEFT, Fronto-opercular | Epilepsy |
| 10 | RIGHT, Frontal | Headache |
| 11 | LEFT, Frontal | Epilepsy |
| 12 | Genus of Corpus callosum | Haemorrhage |
| 13 | Splenium of Corpus callosum | Haemorrhage |
| 14 | Splenium of Corpus callosum | Haemorrhage |
| 15 | RIGHT, cerebellar | Haemorrhage |
| 16 | LEFT, Frontal | Epilepsy |
| 17 | RIGHT, Pons and cerebellum | Haemorrhage |
| 18 | LEFT, Temporo-parietal | Epilepsy |
| 19 | LEFT, Temporal | Epilepsy |
| 20 | LEFT, Temporo-occipital | Epilepsy |
| 21 | LEFT, Frontal | Epilepsy |
| 22 | LEFT, Fronto-opercular | Haemorrhage |
| 23 | LEFT, Occipital | Neurological deficit |
| 24 | Splenium of Corpus callosum | Headache |
| 25 | LEFT, Temporal | Neurological deficit |
| 26 | LEFT, Temporal | Headache |
| 27 | LEFT, Temporal | Haemorrhage |
| 28 | RIGHT, Occipital | Neurological deficit |
| 29 | RIGHT, Fronto-opercular | Asymptomatic |
| 30 | RIGHT, Fronto-opercular | Headache |
| 31 | RIGHT, Frontal | Haemorrhage |
| 32 | LEFT, Fronto-temporal | Headache |
| 33 | RIGHT, Pons and cerebellum | Neurological deficit |
| 34 | RIGHT, Fronto-opercular | Headache |
Thirteen patients were admitted for intracranial haemorrhage. Seizures were present in nine patients, headache in six. AVM was an incidental finding in one patient undergoing MR scan for other reasons. The remaining patients had no focal neurological disorders (e.g., vertigo) or focal transitory neurological symptoms (e.g., visual impairment, TIAs).
Follow-up
After complete cure (embolization or embolization+surgery, see below), follow-up angiography was performed in all the patients at final discharge (figures 1-2). Patients who had undergone radiosurgery after embolization had an MRA examination every three months. None of our patients treated with radiosurgery after embolization has a follow-up > 14 months. We have scheduled follow-up angiography at 36 months after radiosurgery if MRA demonstrates a good, ongoing reduction of the nidus.
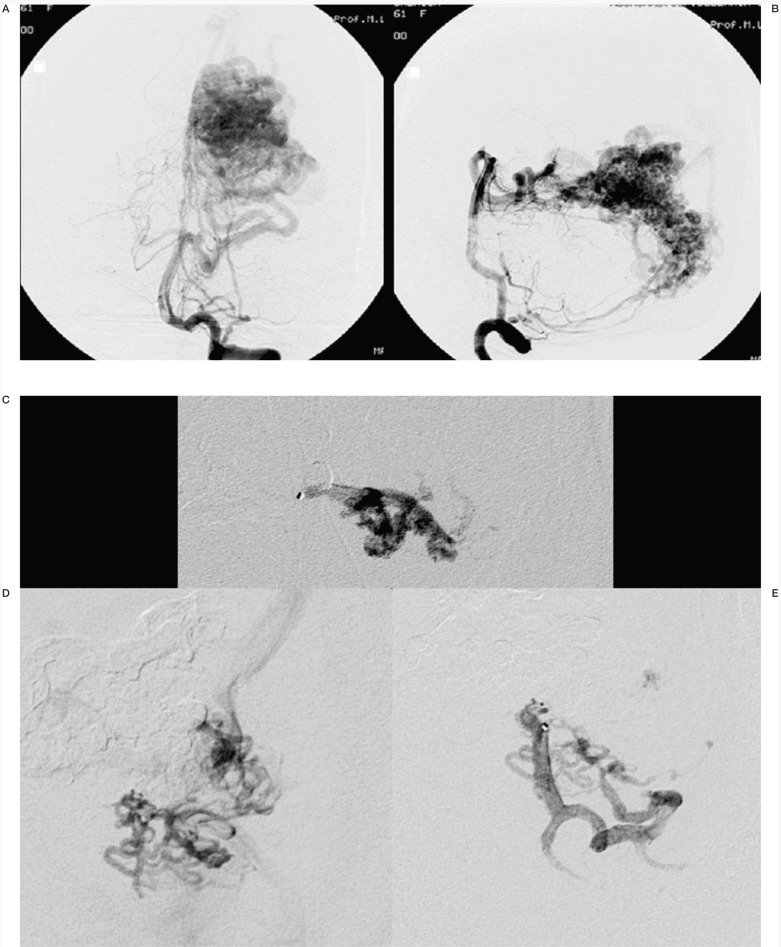
Figure 1.
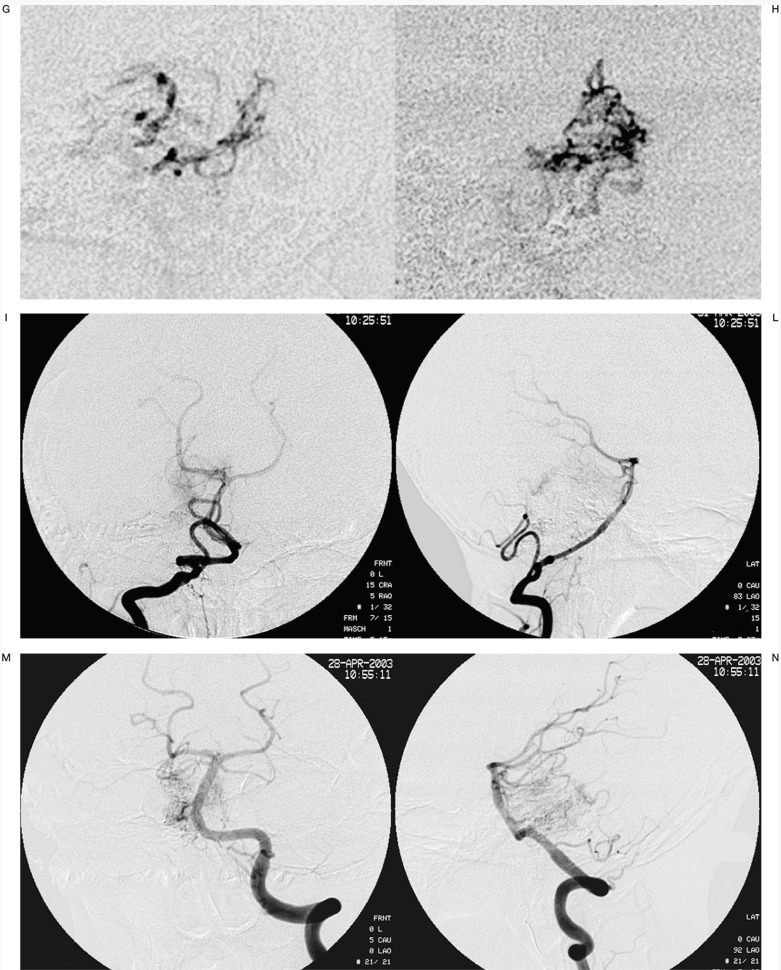
Case #2. DC, 39-year-old woman, clinical onset: haemorrhage. Left temporo-occipital AVM. A-B) Left vertebral angiography shows the large AVM. C-D-E) Three of the six pedicular Onyx injections. F-G) Onyx casts at the end of the second and third (last) sessions. H) Lateral angiogram at the end of the last session. I-L) Left vertebral angiography at the discharge, post surgery.
Embolizing material and procedure
Our angiographic suite is equipped with a 3D rotational DSA system (Bi plane GE Advantx).
All AVM procedures are performed under general anaesthesia with systemic heparinisation (5000 U in bolus when the guiding catheter is in place). A 5 or 6F guidecatheter (Cordis Envoy or Boston Guider Soft-tip) is placed in the parent vessel. The microcatheters we use are mainly Flowrider and Ultraflow 1.5 (MTI) on Silverspeed 10 wire (MTI). Baltacci (Balt) 1.5 and 1.2 are less commonly used. We perform AVM embolization with Onyx 18, or, rarely, Onyx 20.
Using Onyx, we strictly observe the directions for use recommended by the manufacturer. The Onyx must be shaken for at least 20 minutes before injection; failure to mix properly may result in inadequate suspension of tantalium, resulting in inadequate radiographic visualization. When the microcatheter is in place, we flush contrast from the microcatheter hub with 10 ml of saline and leave the syringe connected. To fill the catheter deadspace, we aspirate approximately 0.8 ml of sterile DMSO into its 1 ml syringe and inject it into the microcatheter (for Ultraflow, 0.25 ml), at a rate not greater than 0.3 ml/min. Then we remove the DMSO syringe and overfill and wash the luer hub with the remaining DMSO.
Immediately we connect the Onyx syringe to the microcatheter hub. The syringe should be held vertical with the tip downwards to connections, after which it should be turned over 180° to ensure an interface between DMSO and Onyx inside the microcatheter.
Inject at a steady rate not exceeding 0.3 ml/min to flush the deadspace volume (for Ultraflow, 0.25 ml) at a rate not greater than 0.3 ml/min.
Adequate fluoroscopic visualization must be maintained during Onyx delivery (better by slow subtracted angiographic series or non-injected roadmapping).
We do not allow more than 1-1.5 cm of Onyx to reflux back over the catheter tip to avoid difficulties in microcatheter removal.
When the Onyx has refluxed back over catheter tip, we stop the injection and wait for a few minutes (allowing time for angiographic control through the guiding catheter) before resuming the injection. Upon completion of Onyx injection, we wait a few seconds and then gently retract the catheter to separate the Onyx mass from the catheter.
In 3/118 pedicles in 3/34 patients with highflow fistulae we use NBCA glue (Glubran-2, GEM) which is as effective as Histoacryl and has CE-marking.
In one patient with a very large bleeding AVM, we started the treatment seven years ago. In the first 5/7 sessions, we used GDC coils (Boston scientific) to embolize a posterior cerebral artery flow related aneurysm, Liquid Coils (Boston Scientific) and Histoacryl. Presurgical embolization with Onyx was completed in the last two sessions, analyzed in this report.
Only the microcatheter is withdrawn following injection, the guide catheter remaining in place for the next microcatheter. No more than two injections are performed at one session in most cases, with further sessions scheduled at one to two week intervals. We have never injected more than three pedicles in a single session. Following the embolization procedure, the patient is extubated and returned to the neurosurgery ward and is normally discharged 72 hours later.
Therapeutic strategy
Embolization allows complete closure of the malformation in only a quarter of cases in literature series taken together, figures varying significantly from one institution to another. In most of the cases, treatment is continued for several months or years. Equally variable are the incidence rates of major complications.
For these reasons, our Institution adopts a multidisciplinary approach (embolization, surgery and radiotherapy) to treat patients with Grade 3 or more AVMs. The purpose of this therapeutic strategy is to cure AVMs completely in shortest possible time to eliminate the risk of bleeding and secondly to remove the lesion completely. These results must be achieved under the safest possible conditions for the patient.
Hence, we construe embolization as a work in progress. It mainly starts as the first step before surgery to reduce flow and size of AVM by a "targeted" technique (in addition to reducing lesion size, endovascular treatment aims to seal off AVM areas anatomically or haemodynamically complex for surgical treatment) (figure 2). Occasionally, the reduction of size allows a radiosurgical approach (figures 3-4-5). Embolization seldom results in a definitive cure of AVMs but at the end of multimodal approach, we obtained the complete and definitive cure of AVM.
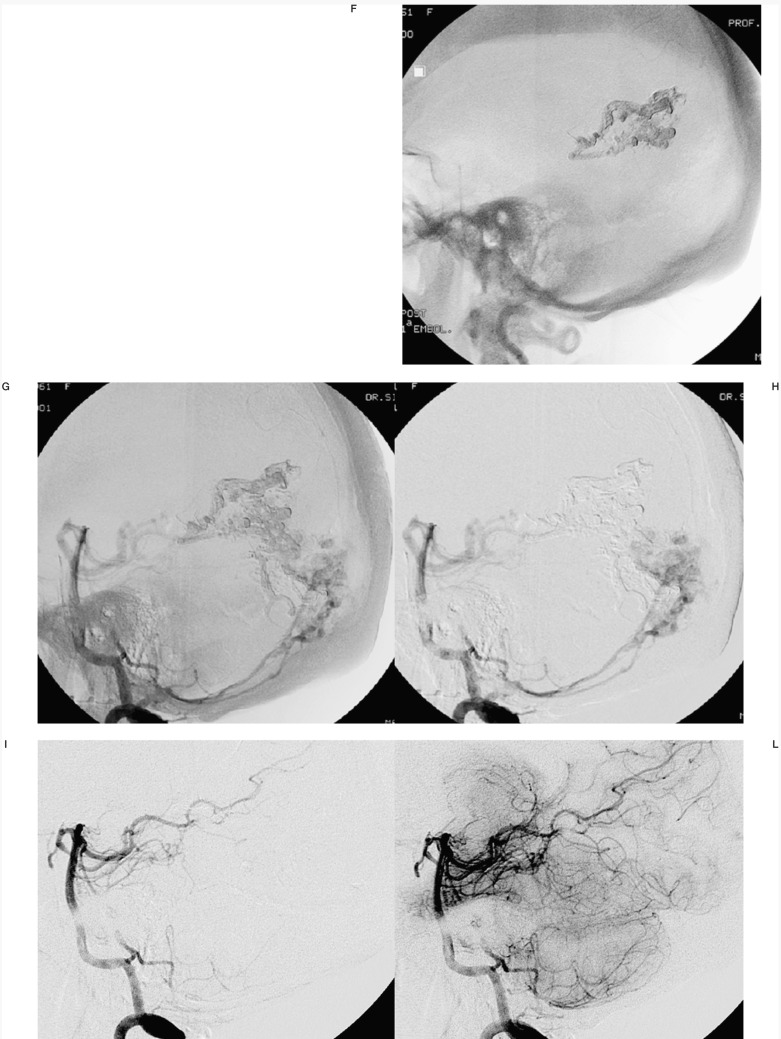
Figure 2.
Case #14. LTS, 23-year-old man, clinical onset: haemorrhage. Splenial and Parasplenial AVM. A-B) 3D angiography from right vertebral artery. C) Onyx casts of two of the three pedicular Onyx injections, with targeted embolization of the AVM deeper portion (D-E), anatomically complex for surgical treatment. F-G) 3D angiography from right vertebral artery at the end of the last session. H-I) Left vertebral and right carotid angiography at the discharge, post surgery.
Figure 3.
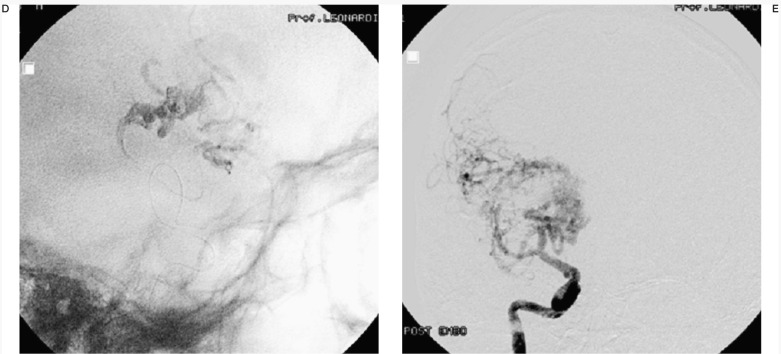
Case #7. SS, 43-year-old man, clinical onset: epilepsy, first seizure 16 months before. Right fronto-temporal AVM. A) Right carotid angiography shows the large AVM. B-C) Three of the six pedicular Onyx injections. D) Onyx cast at the end of the last session. E) After 4 embolization sessions nidal size is markedly reduced allowing a radiosurgical approach.
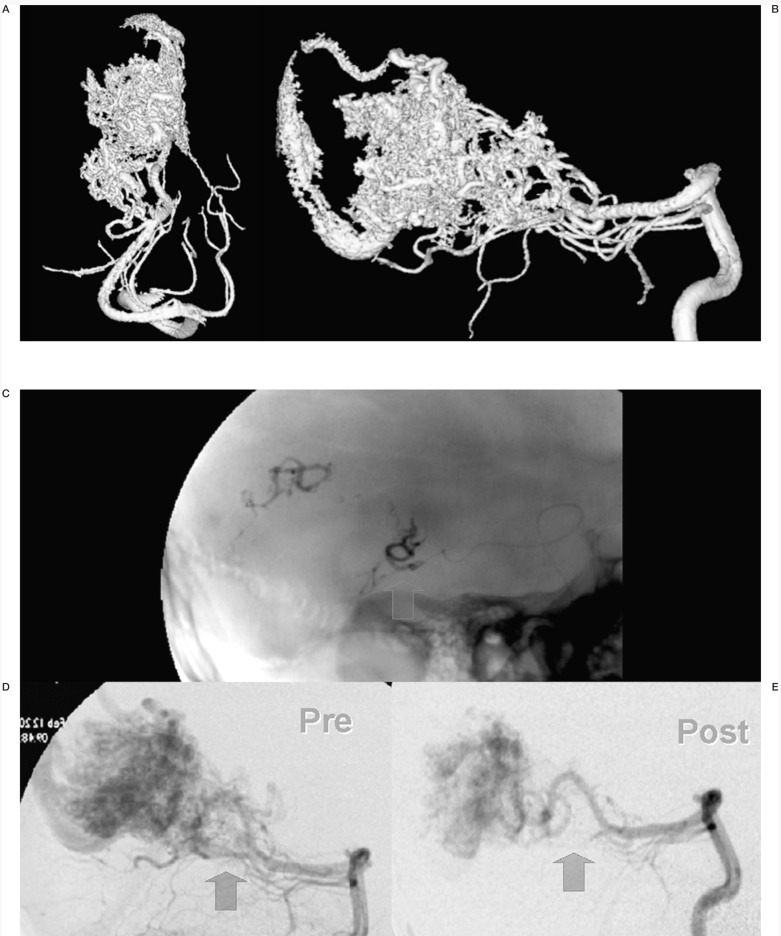
Figure 4.
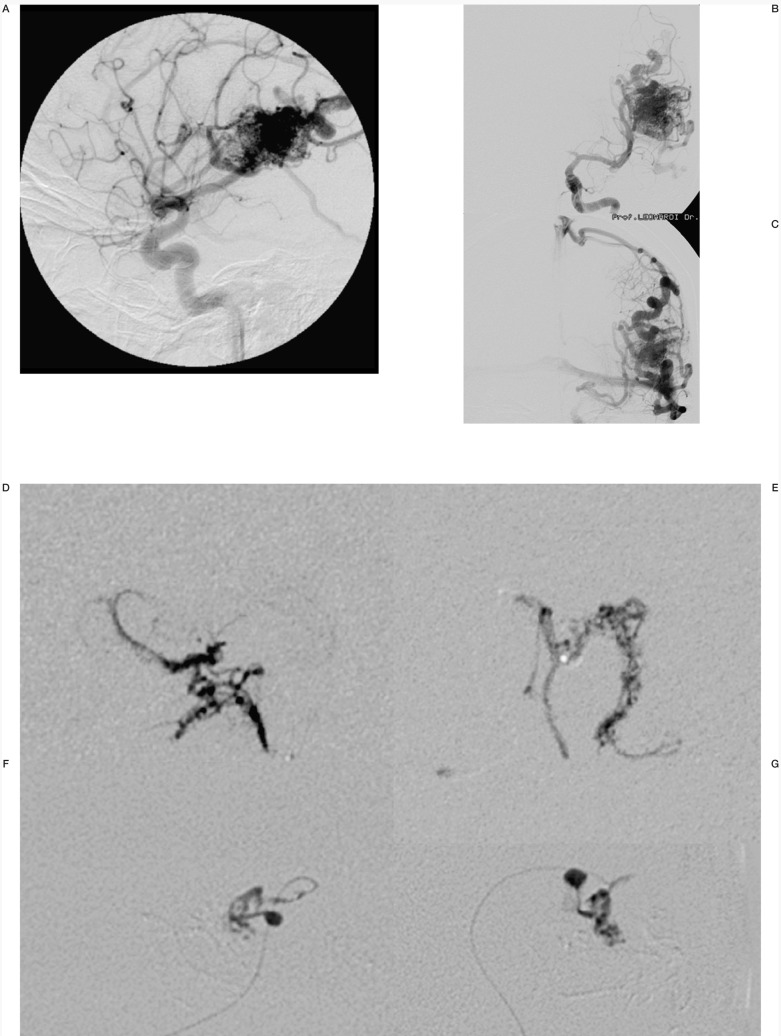
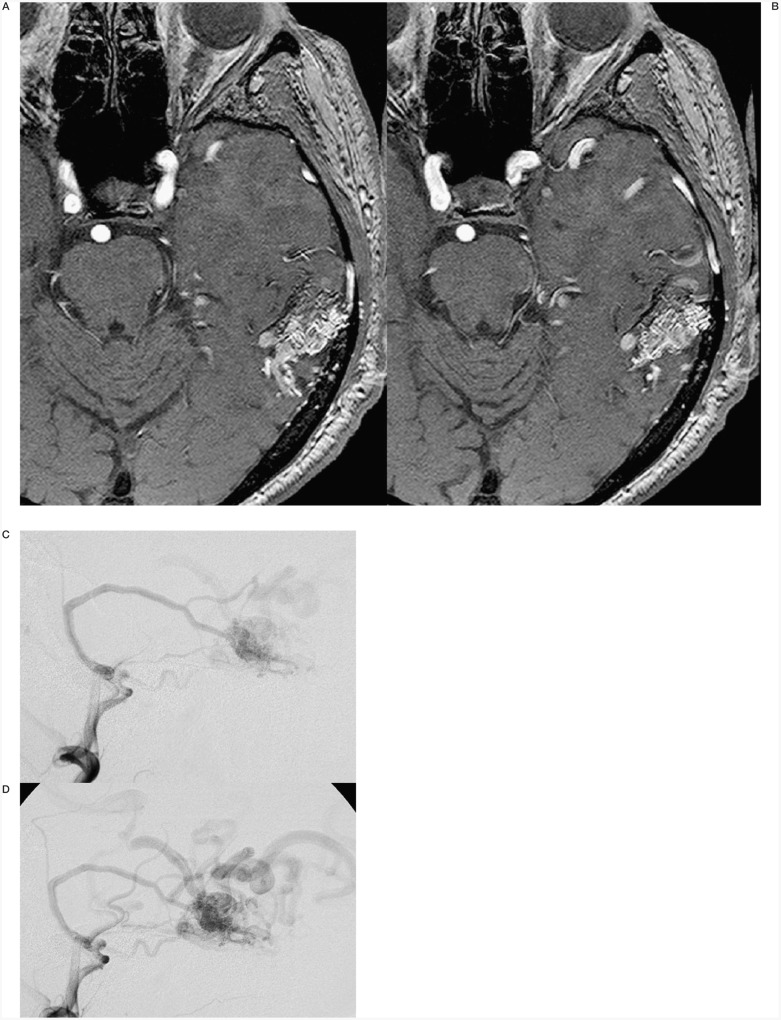
Case #9. NC, 35-year-old woman, clinical onset: epilepsy, first seizure 1 month before. Left fronto-opercular AVM. A-B-C) Left carotid angiography. D-E-F-G) Three of the seven pedicular Onyx injections (F-G) are two orthogonal projections of the same injection, with targeted occlusion of intranidal aneurysm).
H-I) Pre- and post-embolization 3D TOF MR scans at the same level; the patient underwent radiosurgery to complete the treatment.
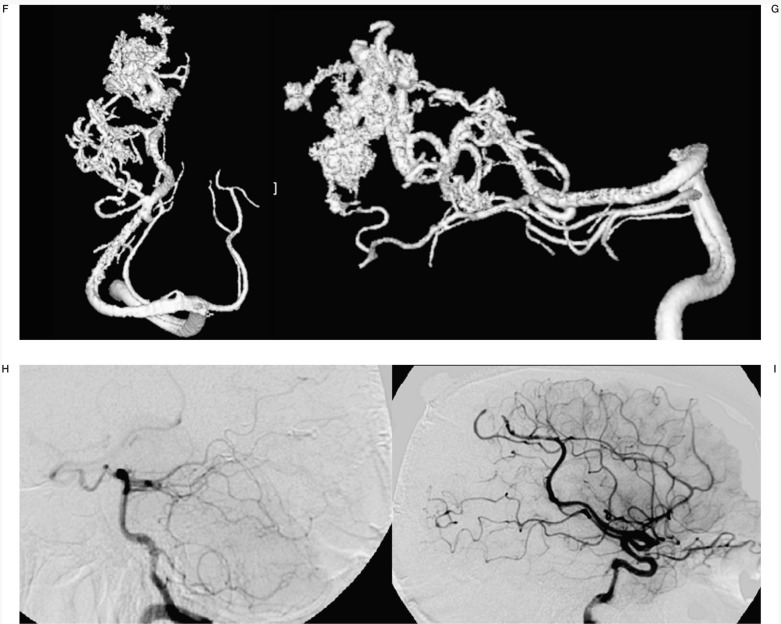
Figure 5.
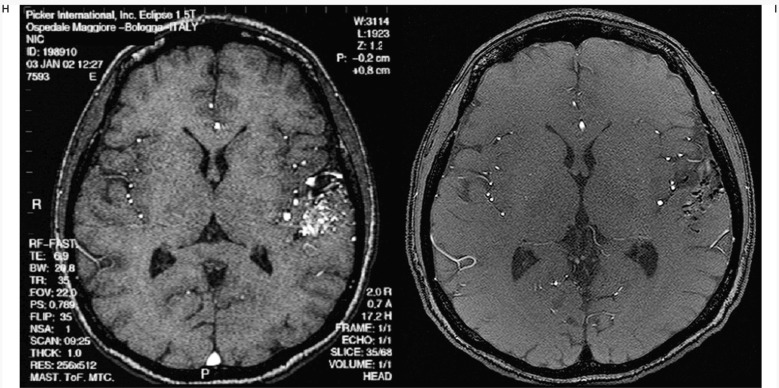
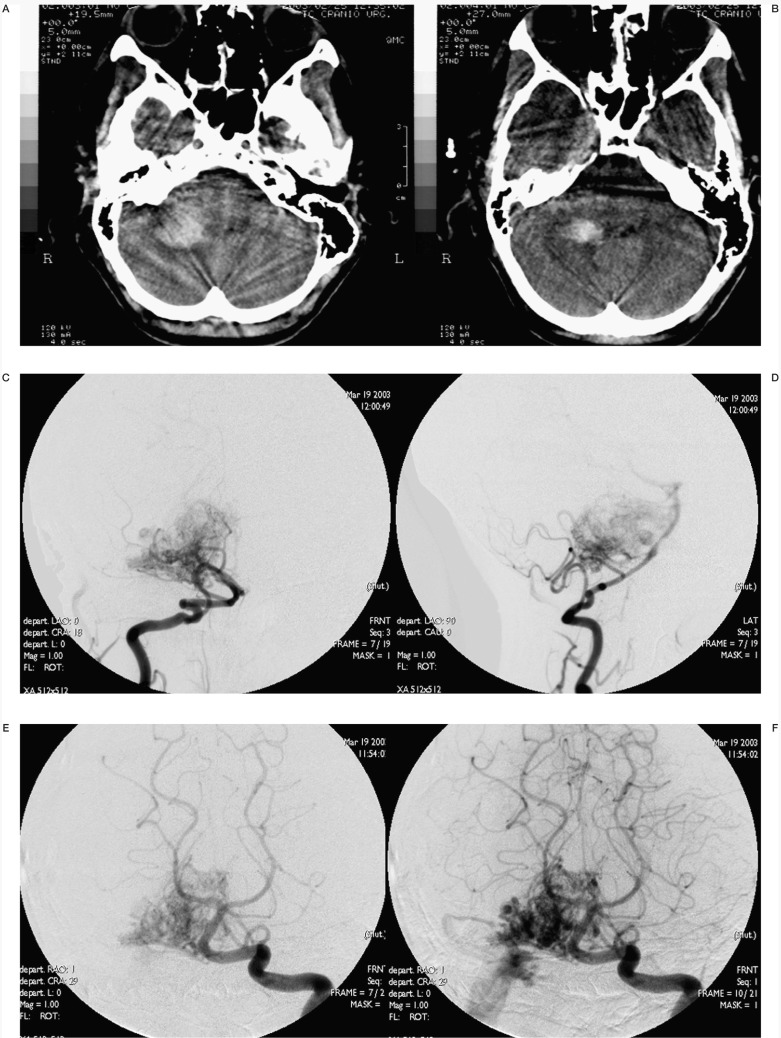
Case #17. LD, 18-year-old man, clinical onset: haemorrhage. Right ponto-cerebellar region AVM. A-B) Axial CT scans show the right ponto-cerebellar haematoma at clinical onset; C-D) right vertebral angiography. E-F) Left vertebral angiography. G-H) Two of the six pedicular Onyx injections. I-L-M-N) Right and left angiography at the end of the last session; the reduction of nidal size allowed a radiosurgical approach.
Results
Results are summarized in table 3. A stable post-embolization cure was achieved in two patients.
Table 3.
Therapeutic Strategy and AVM Site
| # | AVM Site | Therapeutic Strategy |
|---|---|---|
| 1 | LEFT, Temporo-occipital | Curative embolization |
| 2 | LEFT, Temporo-occipital | Embo+Surgery |
| 3 | LEFT, Pre-Rolandic | Still in treatment |
| 4 | Cerebellar vermis | Still in treatment |
| 5 | RIGHT, Fronto-opercular | Presurgical Embo. Died from rebleeding awaiting surgery |
| 6 | LEFT, Temporo-occipital | Embo+Surgery |
| 7 | RIGHT, Fronto-temporal | Embo+Radiosurgery |
| 8 | LEFT, Temporo-occipital | Embo+Surgery |
| 9 | LEFT, Fronto-opercular | Embo+Radiosurgery |
| 10 | RIGHT, Frontal | Embo+Surgery |
| 11 | LEFT, Frontal | Embo+Surgery |
| 12 | Genus of Corpus callosum | Embo+Surgery |
| 13 | Splenium of Corpus callosum | Embo+Radiosurgery |
| 14 | Splenium of Corpus callosum | Embo+Surgery |
| 15 | RIGHT, cerebellar | Curative embolization |
| 16 | LEFT, Frontal | Still in treatment |
| 17 | RIGHT, Pons and cerebellum | Embo+Radiosurgery |
| 18 | LEFT, Temporo-parietal | Embo+Radiosurgery |
| 19 | LEFT, Temporal | Still in treatment |
| 20 | LEFT, Temporo-occipital | Still in treatment |
| 21 | LEFT, Frontal | Embo+Surgery |
| 22 | LEFT, Fronto-opercular | Embo+Surgery |
| 23 | LEFT, Occipital | Embo+Surgery |
| 24 | Splenium of Corpus callosum | Embo+Surgery |
| 25 | LEFT, Temporal | Embo+Surgery |
| 26 | LEFT, Temporal | Embo+Radiosurgery |
| 27 | LEFT, Temporal | Embo+Surgery |
| 28 | RIGHT, Occipital | Embo+Radiosurgery |
| 29 | RIGHT, Fronto-opercular | Embo+Surgery |
| 30 | RIGHT, Fronto-opercular | Still in treatment |
| 31 | RIGHT, Frontal | Embo+Surgery |
| 32 | LEFT, Fronto-temporal | Embo+Surgery |
| 33 | RIGHT, Pons and cerebellum | Still in treatment |
| 34 | RIGHT, Fronto-opercular | Embo+Surgery |
In the others, embolization reduced AVM volume from 50 to 95%. Five patients underwent radiosurgery after the reduction in size of the AVM.
Eight patients are still in treatment, two (#3 and #4) are in doubt as whether to continue the therapeutic programme.
The remaining 19 completed the treatment with surgery. From the surgical standpoint, the main improvement in embolizing agent is that Onyx's properties (soft, foamy and non crystallizing) make it easier to manipulate than acrylic glues (figure 6).
Figure 6.
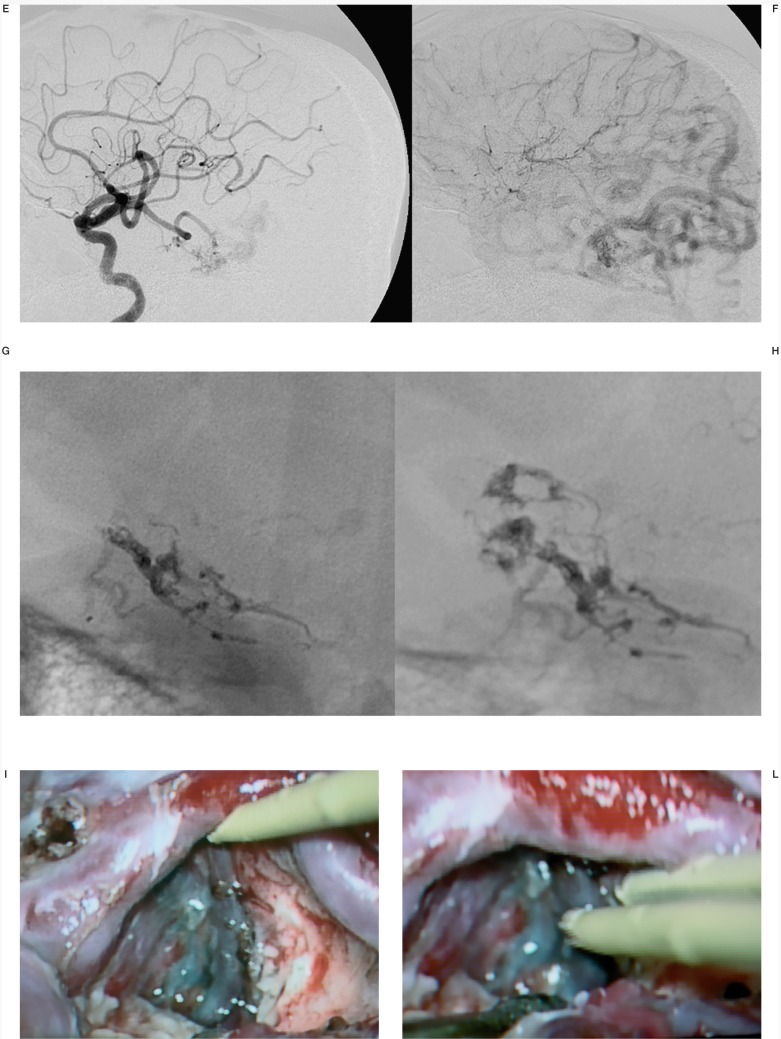
Case #25.TG, 22-year-old man, clinical onset: TIAs. Left temporal AVM. A-B) MRI shows the temporal AVM. C-D) External and E-F) internal left carotid angiography angiogram shows large occipito-temporal deep AVM and giant basilar aneurysm. G-H) Onyx cast at the end of the last session (two embolization sessions with three pedicular injections). I-L) Intraoperative pictures that show the embolized portions of the AVM in grey; the embolised vessels are soft and compressible between the noses of the bipolar coagulation.
Technical complications during embolization procedures
In one case we observed an Onyx intra-microcatheter polymerization (our mistake in DMSO injection?). We simply withdrew the microcatheter and went on with the session. Rupture of the microcatheter was reported in one case, with no problems for the patient.
In our experience (118 pedicles), we never encountered difficulties in microcatheter withdrawal at the end of the injection.
Morbidity and mortality
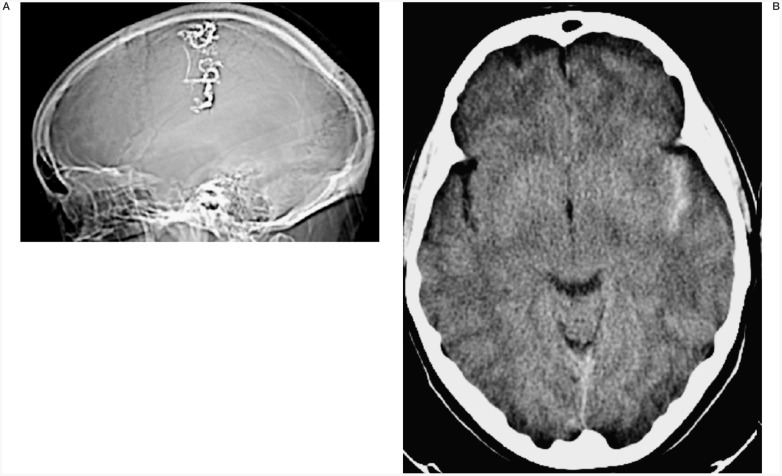
No major complications arose during endovascular treatment. One patient had transitory (36 hour) impaired right arm pronation. The CT scan showed an asymptomatic mild SAH in the left sylvian fissure but no ischaemic areas (figure 7).
Figure 7.
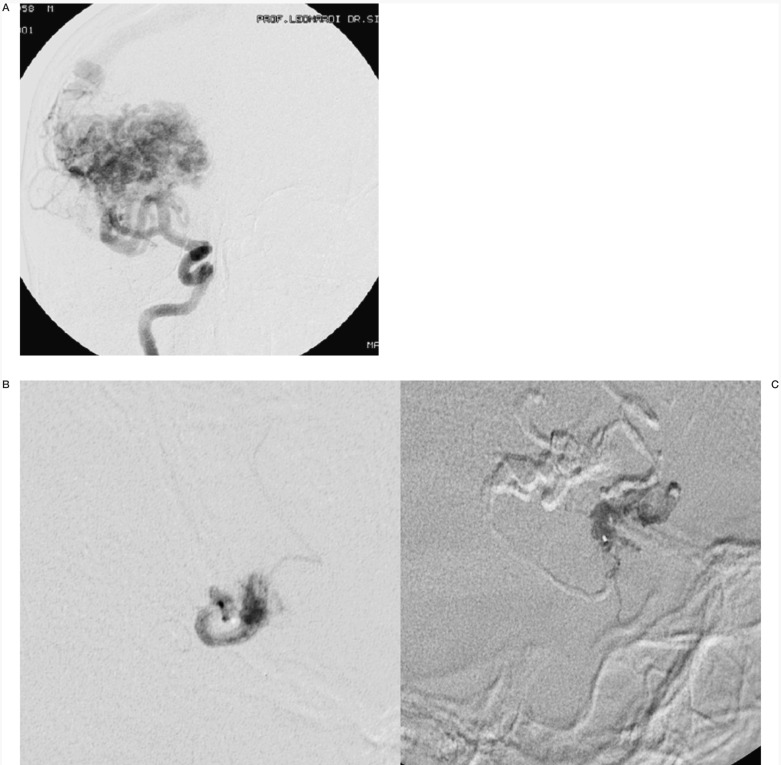
Case #3. SA, 31-year-old woman, clinical onset: epilepsy from adolescence. Left Rolandic AVM. CT scanogram shows the Onyx cast of the only session performed in this patient (two pedicular injections). CT scan with mild subarachnoid haemorrhage; the patient had transitory (36 hour) impaired right arm pronation and is in doubt as to whether to continue the therapeutic programme.
One patient (#5) still in treatment died from a fatal rebleeding (the clinical onset had been with haemorrhage two weeks before the session) 12 days after the embolization. During injection no venous passage of Onyx was observed and no stagnation was present after the treatment. The nidus was filled about 50%.
Clinical Outcome
Clinical outcome in relation to therapeutic strategy and clinical onset is reported in table 4. Among the 26/34 patients who completed the therapeutic programme, an excellent or good outcome was achieved in 23. Outcome was conditioned by focal symptoms present on admission in three patients due to haemorrhagic onset, but only one patient presented a severe disability on discharge.
Table 4.
Clinical Outcome in Relation Therapeutic Strategy and Clinical Onset
| # | Clinical Outcome | Therapeutic Strategy | Clinical Onset |
|---|---|---|---|
| 1 | Hemianopsia due to haemorrhagic onset | Curative embolization | Haemorrhage |
| 2 | Hemianopsia due to haemorrhagic onset | Embo+Surgery | Haemorrhage |
| 3 | – | Still in treatment | Epilepsy |
| 4 | – | Still in treatment | Neurological deficit |
| 5 | Died from rebleeding while in treatment | Presurgical Embo, | Haemorrhage |
| 6 | No deficit at discharge | Embo+Surgery | Haemorrhag |
| 7 | Excellent | Embo+Radiosurgery | Epilepsy |
| 8 | No deficit at discharge | Embo+Surgery | Haemorrhage |
| 9 | Excellent (still under antiepileptic therapy) | Embo+Radiosurgery | Epilepsy |
| 10 | Excellent | Embo+Surgery | Headache |
| 11 | Excellent | Embo+Surgery | Epilepsy |
| 12 | No deficit at discharge | Embo+Surgery | Haemorrhage |
| 13 | No deficit at discharge | Embo+Radiosurgery | Haemorrhage |
| 14 | No deficit at discharge | Embo+Surgery | Haemorrhage |
| 15 | Severe disability due to haemorrhagic onset | Curative embolization | Haemorrhage |
| 16 | – | Still in treatment | Epilepsy |
| 17 | Mild ataxia due to haemorrhagic onset | Embo+Radiosurgery | Haemorrhage |
| 18 | Excellent (still under antiepileptic terapy) | Embo+Radiosurgery | Epilepsy |
| 19 | – | Still in treatment | Epilepsy |
| 20 | – | Still in treatment | Epilepsy |
| 21 | Excellent (still under antiepileptic terapy) | Embo+Surgery | Epilepsy |
| 22 | No deficit at discharge | Embo+Surgery | Haemorrhage |
| 23 | Excellent | Embo+Surgery | Neurological deficit |
| 24 | Excellent | Embo+Surgery | Headache |
| 25 | Excellent | Embo+Surgery | Neurological deficit (TIA) |
| 26 | Excellent | Embo+Radiosurgery | Headache |
| 27 | No deficit at discharge | Embo+Surgery | Haemorrhage |
| 28 | Excellent | Embo+Radiosurgery | Neurological deficit |
| 29 | Excellent | Embo+Surgery | Asymptomatic |
| 30 | – | Still in treatment | Headache |
| 31 | No deficit at discharge | Embo+Surgery | Haemorrhage |
| 32 | Excellent | Embo+Surgery | Headache |
| 33 | – | Still in treatment | Neurological deficit |
| 34 | Excellent | Embo+Surgery | Headache |
Discussion and Conclusions
Onyx has several "a priori" advantages and disadvantages over acrylic glue as an embolizing agent. The main advantage is that the product's indications for use state that Onyx "…is intended for the embolization of brain arteriovenous malformations." and that Onyx bears the CE mark for this specific indication. The main disadvantage is that Onyx can only be used with DMSO compatible catheters of which few are available. Although these catheters are excellent, it means that the choice of microcatheter is limited in terms of type and size. We noted the impressions gained during clinical application of Onyx in our small series.
Favourable impressions
The most striking initial feature of Onyx is the inverted time frame of the procedure. The traditional "inject and run" approach adopted with acrylic glue is replaced by the need and possibility for slow progressive injection lasting several minutes. Even reflux around the tip of the microcatheter can be dealt with calmly by stopping the injection, waiting a few minutes and then resuming the injection exploiting occlusion of the afferent vessel to gain further progression of the embolizing agent into the nidus. During injection, it is not uncommon the see the Onyx run into nidal compartments not disclosed during diagnostic injection by the microcatheter before injection of the embolizing agent. We also found that he slow progressive technique of embolization with Onyx safeguarded against the danger ever present with cyanoacrylate of sudden diffusion of the glue into the venous compartment of the AVM with potentially catastrophic consequences. The main effect of this difference between acrylic glue and Onyx is that the focal point of the endovascular procedure shifts from the moment of injection of the embolizing agent to the search for the best position from which to start the injection. In addition, we found an increase in the parts of the nidus which could be embolized by a single injection.
Unfavourable impressions
Onyx appears less able to penetrate the nidus due to its high density. At the start of injection, there is often a marked difference between the angiographic image obtained by injection of contrast medium from the microcatheter and the cast formed after injection of Onyx. However, good nidal penetration is ensured by the flexibility and potential duration of the injection.
Initially, we were struck by the length and complexity of the preparatory phase prior to injection and were worried about maintaining the correct position of the microcatheter during treatment. This difficulty has now been overcome with experience by monitoring the stability of the catheter with periodic fluoroscopic control of its position also during flushing with saline solution and the injection of DMSO.
Technical and procedural features
Plainly, it is necessary to monitor the exact time taken to inject the Onyx. It is unadvisable to rely on direct fluoroscopic monitoring alone so we use a slow angiographic serie (one image a second for 60 seconds) or alternatively a subtracted scopy injection.
To enhance sensitivity with respect to reflux, it is advisable to choose a working position during injection offering the best longitudinal approach of the distal tip of the catheter associated with its dissociation from the nidus. This will avoid underestimating the degree of reflux which could hinder extraction of the catheter.
Conclusions
In our opinion, the main problem with Onyx is that the apparently easier approach will probably allow a wider diffusion of these procedures. A major problem may arise if the neuroradiologist's attention is focused on the technical aspects of the treatment rather the general problem of the pathophysiology of the AVM.
The availability of a supposedly "easier to use" embolizing agent may have positive effects if adopted with the extreme care and prudence that AVMs impose. On the contrary, it could have very negative effects if adopted with a more relaxed approach without in-depth assessment of the anatomical relationship, angioarchitecture and flow conditions of the AVM. AVMs are extremely difficult and dangerous: this is not affected by the quality of the embolizing agents used and must be kept in mind at all times.
References
Previously published data of the author or his group
- 1.Simonetti L, Cenni P, et al. Prime esperienze di embolizzazione di MAV cerebrali con Onyx. Analisi del trattamento di sei pazienti. Rivista di Neuroradiologia. 2001;14:251–256. [Google Scholar]
- 2.Leonardi M, Simonetti L, et al. Initial experience of cerebral AVM embolization with Onyx: analysis of treatment in seven patients. Neuroradiology. 2001;43:S65. [Google Scholar]
- 3.Leonardi M, Simonetti L. The use of Onyx in endovascular procedures. A personal experience. XVII Annual Meeting of the Japanese Society for Intravascular. Neurosurger. 2001;55 [Google Scholar]
- 4.Leonardi M, Simonetti L, et al. Cerebral AVM treated with Onyx: preliminary experience in eight patients. Journal of Neuroradiology. 2002;29:1S145C. [Google Scholar]
- 5.Leonardi M, Simonetti L, et al. Experience of cerebral AVM embolisation with Onyx: analysis of treatment in 17 patients. Interventional Neuroradiology. 2003;9:128. doi: 10.1177/15910199050110S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonardi M, Simonetti L, et al. Brain AVM embolization with Onyx: analysis of treatment of critical lesions. Neuroradiology. 2004;46:89–90. doi: 10.1177/15910199050110S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
Other selected references
- 1.Berenstein A, Lasjaunias P. Cerebral vascular malformations. Surgical Neuroangiography. Springer. 2004:609–734. [Google Scholar]
- 2.Jahan R, Murayama Y, et al. Embolisation of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48:984–95. doi: 10.1097/00006123-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Moret J, Picard L. Endovascular Treatment of Brain AVMs with Permanent Embolic Material. Rivista di Neuroradiologia. 1991;4:85–93. [Google Scholar]
- 4.Moret J, Hammani N, et al. Traitment des malforations arterioveneuse cerebral par l'Onyx: a propos d'une serie de 94 patients. J of Neuroradiology. 32:86. [Google Scholar]
- 5.Negoro M, Miyachi S, et al. The Selection and Results of AVM Treatment. Interventional Neuroradiology. 1999;5:167–68. doi: 10.1177/15910199990050S130. [DOI] [PubMed] [Google Scholar]
- 6.Valavanis A, Yasargyl MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Standard Neurosurg. 1998;24:131–214. doi: 10.1007/978-3-7091-6504-1_4. [DOI] [PubMed] [Google Scholar]