Abstract
During reviewing cases with AVM, the author noticed that stenotic and occlusive changes of the draining veins are commonly seen in high flow cerebral AVMs. However, little attention has been paid to these venous diseases until ectatic veins, generated in the upstream of the venous system, cause mass effect to the surrounding structures, or redistribution and shunting toward regional veins became insufficient after they are markedly overloaded or occluded. Cases with such venous abnormality are clinically important because of the possibility of dramatic improvement of neurological deficits after embolization of AVMs.
Following presenting treatment results of 177 AVM case, the author is going to present five cases with abnormality in the Galenic venous system and two cases with abnormality in cortical veins associating with high flow cerebral AVMs. Consideration will be made on symptomatology and pathophysiologic mechanism of venous abnormalities associating with high flow cerebral AVMs.
Key words: AVMs, embolization, therapeutic neuroradiology
Materials and Methodos
Patients
Among 576 cases with cerebral AVMs seen by the author during January 1990 - December 2004, 215 cases admitted to my department as candidates for embolization. There were 140 males and 75 females out of the 215 cases. Of whom 121 cases (%) presented with hemorrhage, 65 (%) with epilepsy, developed focal neurological deficits, showed cognitive/ mental impairment, suffered from headache, 3 had trigeminal neuralgia, non had combination of the above all and 11 were asymptomatic.
All cases underwent magnetic resonance imaging (MRI) and angiography in order to evaluate size of the nidus, eloquence of the adjacent brain, and pattern of venous drainage. Based upon these findings, they were classified into five groups according to Spetzler's grading system (table 1).
Table 1.
Classification of 215 Cases of AVM According to Spetzler's Grading System
| Grade | Number of cases |
|---|---|
| I | 7 |
| II | 40 |
| III | 72 |
| IV | 83 |
| V | 13 |
Number of patients who underwent various treatments and combination of treatments were summarized in the table 2. Informed consent was obtained in 177 of the 215 cases and embolization was performed.Among them, surgical resection of the nidus was performed on 102 cases and 51 underwent gamma knife therapy following embolization, and one case had combination of these three treatment modalities.
Table 2.
Number of Patients Who Underwent Various Treatments and Combination of Treatments
| Treatment Modality | Number of Cases |
|---|---|
| Embolization | 23 |
| Embolization + Surgery | 102 |
| Embolization + Gamma Knife Therapy |
51 |
| Embolization + Surgery + Gamma Knife Therapy |
1 |
| Gamma Knife Therapy | 15 |
| Surgery | 9 |
| Conservative Treatment | 14 |
Angiographically, 45 cases showed vessel wall irregularities and /or stenoses, and 3 cases presented with occlusion of Galenic system. There were 7 cases with abnormality in venous drainage system that played major pathophysiologic role in the formation of neurological manifestation; 6 with prominent ecstatic changes on draining veins and 1 case with occlusion of the deep venous system. All of these 7 cases will be presented as illustrative cases.
Microcatheters and Guidewires
According to the results of the evaluation of the physical properties of 16 currently available microcatheters and guidewires 1, the authors picked a combination of the most flexible and lubricated microcatheter (GT Catheter III; Terumo Corp, Tokyo) and the most lubricated and highly responsive guidewire (Guide Wire GT, Terumo Corp, Tokyo). As these materials were withdrawn by the manufacturer from the marked, the author was obliged to use the most commonly commercially available catheters and guide wires since August 1999.
Flow Control
After placing the tip of a microcatheter in the desired position, test injection of contrast material was repeated until desired flow status for depositing embolic material in the AVM nidus was generated by use of one or more of the following flow control techniques.
Systemic hypotension under general anesthesia
By lowering arterial pressure, better opacification of the nidus and delayed penetration of the contrast material into the drainage vein was attained. When the catheter tip was placed in the ideal position, mean arterial pressure was usually lowered to 50 mm Hg.
Carotid compression
If desired flow control was not obtained by systemic hypotension alone, manyual compression of the carotid artery was applied during injection of contrast material. By monitoring the behavior of contrast material on digital subtraction angiography, the degree of compression was adjusted.
Temporary balloon occlusion
More complicated but efficient flow control was performed, when necessary, by altering hemodynamics of cerebral AVMs by use of temporary balloon occlusion of feeding pedicles in two ways: 1) For extremely high flow AVMs, non-detachable balloon was placed in the proximal portion of the feeding pedicle in order to obtain better filling of the malformation bed and sloer penetration of the draining veins. 2) For AVMs with dual blood supply from the differenty arterial systems, a non-detachable balloon was inflated in one of the major feeding pedicles during injection of the embolic material into the other. This resulted in reversed flow within the nidus and enhanced penetration of liquid embolic material.
The tip of a microcatheter was placed into the nidus or at the entrance of the nidus by use of digital subtraction angiography (DSA) roadmap. Staged embolization was performed in 2 to 5 sessions (average: 2.8). Concerning embolic material, normal butyl cyanoacrylate (NBCA) was used in 127, non-adhesive liquid embolic material ethylene vinyl alcohol (EVAL) was used in 47 and both of these were used in 3 cases. In order to prevent liquid embolic material from migrating into the draining veins, flow restriction was performed using non-detachable balloon(s), systemic hypotension under general anesthesia, manual compression of a carotid artery and combination of 2 or 3 of these. In 14 out of 42 cases with prominent blood supply to the nidus through leptomeningeal anastomoses, embolization was performed using polyvinyl alcohol (PVA) particles after main feeding pedicles were embolized using a liquid embolic material.
Results
Clinical outcome of patients treated by the author was summarized in table 3. Range of follow up was 6 months to 18 years (mean 98 months). Spetzler grade I to III patients presented with no mortality and low treatment morbidity, and 83.8 % of grade IV patients were independent at the last follow up. One grade IV patient died after surgery, one grade V patient died after surgery and one grade V patient died of recurrent hemorrhage after surgery. There was no embolization related death.
Table 3.
Clinical Outcome of 102 Cases Treated by Embolization Plus Surgery
| Grade | Independent | Moderately Disabled |
Severely Disabled |
Dead |
|---|---|---|---|---|
| I | 4(100%) | 0 | 0 | 0 |
| II | 24(100%) | 0 | 0 | 0 |
| III | 29(96.7%) | 1(3.3%) | 0 | 0 |
| IV | 31(83.8%) | 3(8.1%) | 2(5.4%) | 1(2.9%) |
| V | 5(71.4%) | 0 | 0 | 2(28.6%) |
Early morphological results, right after completion of embolization, were summarized in table 4. Rate of complication of embolization was compared before and after the introduction of improved microcatheters and guide wires in table 5.
Table 4.
Results of Embolization in 177 cases
| Obliteration rate | Number | % |
|---|---|---|
| 100% | 10 | 5.6 |
| >90% | 69 | 39.2 |
| >75% | 33 | 18.6 |
| >50% | 56 | 31.6 |
| <50% | 9 | 5.0 |
Table 5.
Complications of Embolization in 177 cases
| Year | 1990-1993 | 1994-2004 |
| Number | 65 | 112 |
| Transient Deficit | 9 (13.8%) | 3 (2.7%) |
| Mild Deficit | 4 (6.2%) | 2 (1.8%) |
| Severe Deficit | 1 (1.5%) | 0 |
| Death | 2 (3.0%) | 0 |
Illustrative Cases
Case 1
A 25-year-old female had suffered right trigeminal neuralgia for the last two years. The pain increased in intensity and was not relieved by any kind of medication for the last three years. After having a CT at a nearby clinic, the patient was referred to our hospital under the diagnosis of cerebellopontine angle tumor. MRI performed at our department revealed a large AVM in the right cerebellar hemisphere (figures 1G,H ). On vertebral angiography, it was noted that the right anterior inferior cerebellar and the superior cerebellar arteries were the main feeding pedicles and the petrosal vein and its tributaries were the main draining veins. Because of a high flow AVM, transverse pontine vein, lateral mesencephalic vein and basal vein were markedly engorged (figures 1 A,B,C,D ). Embolization of the AVM was performed in three sessions using normal butyl cyanoacrylate (NBCA). The patient was relieved by trigeminal neuralgia right after completion of embolization that resulted in approximately 80 % occlusion of the nidus and a prominent shrinkage of the draining vein (figures 1 E,F,I,J ). Surgical resection of the nidus, performed 5 days after embolization, resulted in complete removal of the nidus, seventh and eighth cranial nerve palsy and moderate cerebellar ataxia on the right. After rehabilitation for half a year, the patient returned to normal life as a housewife leaving only a mild cerebellar ataxia on the right.
Figure 1.
(Case 1) - A, B, C, D) Vertebral angiography, Towne and lateral projection, revealed a large cerebellar hemispheric AVM with the petrosal vein and its tributaries as the main draining veins. Because of a high flow AVM and stenosis at Galenic-straight sinus junction, transverse pontine vein, lateral mesencephalic vein and basal vein were markedly engorged. E,F) Early and late arterial phase of vetebral angiography, Towne projection, taken after the completion of embolization, revealing that approximately 80 % of the nidus was occluded and a prominent shrinkage of the draining vein was attained. G,H) T1 weighted MRI showing a large AVM in the right cerebellar hemisphere. I,J) T1 weighted MRI after embolization disclosing extensive area with lack of flow void and thrombus formation in the AVM nidus.
Case 2
A 21-year-old male had a 5-year history of facial pain that became intractable for the last several months. The patient developed left hemiparesis, hemisensory disturbance, and homonymous hemianopsia 2 months after embolization, performed at an outside hospital using PVA particles (250-425 µm in diameter), for a large cerebellar hemispheric AVM (figures 2 A,B ). Vertebral angiography and CT revealed a rapidly enlarging varix, not seen before the first embolization, on engorged and tortuous draining vein (figures 2 C,D,E,F ). The patient was referred to the author, for the purpose of embolization, because of high possibility of rupture. Staged embolization using EVAL was performed at my department, and 85% comprehensive obliteration of the nidus was attained. During surgery performed 5 days after embolization, a dilated vein which was a tributary of the brachial vein was noted to compress the entry zone of the right trigeminal nerve. Post-surgical course was uneventful and the facial pain completely disappeared shortly after the surgery. Control angiography revealed a complete resection of the nidus. Control CT revealed thrombosis and prominent shrinkage of the varix at 1 and 6 months after surgery respectively (figures 2 G,H ). Left hemiparesis gradually improved and the only neurological deficit seen 2 years after the surgery was mild cerebellar ataxia.
Figure 2.
(Case 2) - A,B) Vertebral angiography, Towne and lateral projections, showing a massive right cerebellar hemispheric AVM and dilated Galenic system withprominent tortuosity. Note severe stenosis in Galen-straight sinus junction (arrow). C,D) Vertebral angiography, venous phase, revealed a rapidly enlarging varix on the basal vein of Rosenthal. E,F) CTs, taken before and 2 months after particulate embolization performed at an outside hospital. Note formation of a gigantic varix that is severely compressing the left posterior limb of the internal capsule, basal ganglia and thalamus. G,H) Enhanced CTs showing complete thrombosis at 1 month and prominent shrinkage of the varix at 3 months after embolization and resection of the AVM.
Case 3
A 39-year-old female had a very long history starting with presumably a rupture of the AVM in childhood and rapid deterioration of neurological status caused by the venous component of the AVM in middle age. 33 years ago the patient complained of sudden severe headache, lost consciousness and became hemiparetic. The patient had severe headache again and was diagnosed to have a subarachnoid hemorrhage (SAH) 15 years ago. After having an episode of SAH again a year ago the patient developed a progressive sensorimotor deterioration, emotional incontinence and dysphasia.
Right hemiparesis and hemihypesthesia became prominent 7 months prior to admission, emotional and urinary incontinence became prominent 6 months prior to admission, and the patient became aphasic 5 months prior to admission. After being divorced by her husband, she conducted a suicidal attempt in desperation (figure 3 G ). Disturbance of consciousness progressed further and she was comatose when the diagnosis of a massive cerebral AVM was made using CT two months later. In addition, the patient presented with conjugate deviation to the left, complete right hemiplegia and hemihypesthesia on admission. Cerebral angiography showed that the main feeding pedicles came from the insular segment of the middle cerebral arteries and the anterior choroidal artery. Interestingly, insular veins drained predominantly into the basal vein and caused marked enlargement and tortuosity of this vein (figures 3 A,B ). The posterior medial and lateral choroidal arteries, the anterior and middle temporal branches of the posterior cerebral arteries and the lateral striate arteries were also supplying blood to the AVM. CT showed that a huge varicose vein severely compressing the midbrain from laterally. There was no perinidal edema. As it was thought that the patient's cardinal neurological deficits were caused by mechanical compression to the upper brain stem by venous components and developed relatively recently, we expected that they may be reversible by embolization of the AVM. The main feeding pedicles were embolized in two sessions using EVAL. On completion of embolization, approximately 80% of the nidus was embolized. The embolization procedure brought about prominent decrease in degree of brainstem compression as a result of marked shrinkage of the nidus and the draining veins (figures 3 C,D,E,F). To our pleasant surprise, the patient showed spectacular recovery of disturbance in consciousness, right hemiplegia and aphasia shortly after the second session of embolization (figure 3 H).
Figure 3.
(Case 3) - A,B) Left carotid angiography, AP and lateral projection demonstrating a massive insular AVM. Note prominent stagnation of contrast material in the basal vein of Rosenthal that is markedly dilated due to abundant flow from the nidus and stenosis in the Galenic-straight sinus junction. C,D: Left carotid angiography, AP and lateral projection after embolization. Note prominent reduction in size of the nidus and marked shrinkage of the tortuous basal vein. E,F: CT taken before (left) and after (right) embolization. Note that a huge varicose vein severely compressing the midbrain from laterally before embolization, dramatically lost mass effect to the brain stem after embolization. G: The patient's right arm showing a scar formed by wrist cut with suicidal intent. H) The patient at 6 month follow up after surgery showing only a mild right hemiparesis.
When the author visited the patient at rehabilitation institution one month after embolization, she was alert and spoke fluently and only neurological deficits were moderate right hemiplegia and hemihypesthesia.
The referring neurosurgeon removed most of the nidus. And gamma knife therapy was performed for the small remaining portion around the posterior limb of the internal capsule. Angiographically complete cure was attained two years later. The patient has been conducting normal life as a housewife for the last 18 years having only a trace of right hemiparesis.
Case 4
54-year-old female
The patient had scintillating scotoma for 35 years prior to admission to our hospital. The patient recognized lack of the left half of her visual field for 10 years prior to admission. CT, performed at a regional university hospital, revealed a massive AVM spreading over the left posterior temporal to the occipital and parietal regions (figures 4 A,B). Numerous flecks of curvilinear calcification spreading over the left cerebral hemisphere was noted. The lesion was considered to be inoperable and treated conservatively there. The patient started having left hemiconvulsion 7 years prior to admission.
Figure 4.
(Case 4) - A,B) plain and enhanced CT showing a massive AVM extending over the left posterior temporal to the occipital and parietal regions. Note numerous flecks of curvilinear calcification spreading over the left cerebral hemisphere. C,D) arterial and venous phase of vertebral angiography, lateral projection, mainly opacifying the superior part of the nidus. Note lack of visualization of the Galenic system and engorgement of the cortical veins. E,F) arterial and venous phase of external carotid angiography, lateral projection, mainly opacifying the posterior part of the nidus. Note engorged cortical vein draining into the tentorial sinus. G,H) arterial and venous phase of internal carotid angiography, lateral projection, mainly opacifying the anterior part of the nidus draining into the superior draining cortical vein and superficial sylvian vein. No opacification of Galenic system. I,J) arterial and venous phase of the right carotid angiography taken after surgery. No residual nidus was identified. K,L) arterial and venous phase of the vertebral angiography taken after surgery. No residual nidus was identified.
The patient had increased incidence of focal seizures and developed progressive left hemiplegia one year prior to admission. On admission to our hospital, moderate disturbance in consciousness, severe right hemiparesis and hemihypesthesia were noted. Angiography showed a massive AVM with high flow fistulae within the nidus. Because of the occlusion of the Galenic system, there was an extensive dilatation of cortical veins around the nidus that drained into the superior sagittal, tentorial and paracavernous sinuses (figures 4 C-H). About 80 % of the nidus was occluded by embolization performed in 4 sessions.
The patient became alert and hemiparesis dramatically improved after the second session of embolization. The AVM nidus was completely resected spending 20 hours (figures 4 I-L). The patient has been conducting normal life as a housewife for the last 15 years with left homonymous hemianopsia and slight hemihypesthesia.
Case 5
62-year-old male complained of frequent severe headache for 16 years prior to admission. Cerebral angiography performed at nearby hospital at that time revealed a large, high flow AVM in the left parietal lobe. The AVM was judged to be inoperable at a regional university hospital and conservatively treated since then. The patient started having generalized seizure twice a year since 13 years prior to admission. Two months prior to admission, the patient became hemiparetic and aphasic transiently after seizure. The patient became disorientated since this episode. The only neurological abnormality on admission was moderate dementia. Angiography showed an AVM in the sensory cortex with markedly dilated tortuous cortical draining veins. Of note was very slow emptying of contrast material from the inferiorly draining vein into the basal vein of Rosenthal through the connecting vein (figures 5 A,B,C). This was caused by severe stenosis in the Galenic-straight sinus junction (figure 5D ). MRI demonstrated the relationship between the subcortically located nidus and the inferiorly draining vein that was connected to the basal vein (figure 5 E). By attaining 95% occlusion of the nidus by embolization, it was postulated that the remaining nidus would be easily resectable by surgery (figure 5 F). However, the patient suddenly obtunded 3 days after embolization.
Figure 5.
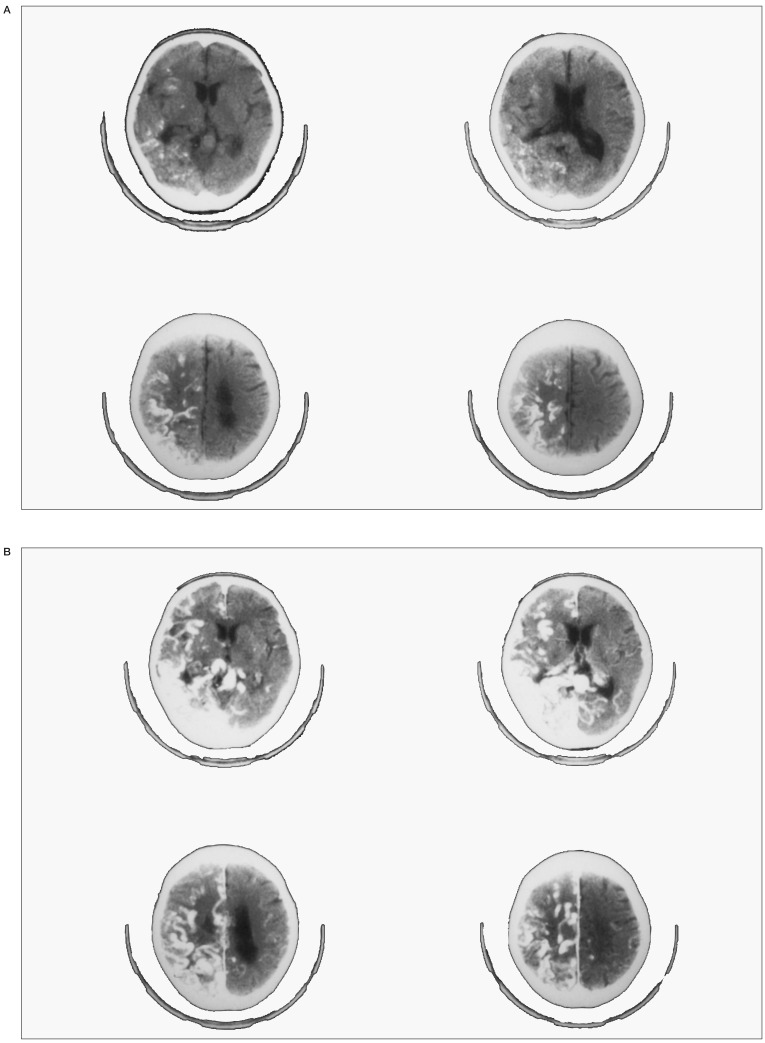
(Case 5) - A) Left carotid angiography, early arterial phase, showing all feeding pedicles coming from cortical branches of the middle cerebral artery. B) Left carotid angiography, late arterial phase, showing an AVM in the sensory cortex. C) Left carotid angiography, early venous phase, demonstrating markedly dilated tortuous cortical draining veins. Of note was very slow emptying of contrast material from the inferiorly draining vein. into the basal vein of Rosenthal through the connecting vein. D) Left carotid angiography, late venous phase revealed that stagnation in the inferiorly draining cortical vein , that was connected to the basal vein, was caused by severe stenosis in the Galenic-straight sinus junction. E) T1 weight MRI on admission showing the relationship between the subcortically located nidus and the inferiorly draining vein that was connected to the basal vein. F) T1 weight MRI immediately after embolization, showing disappearance of flow void in the nidus. G) Plain CT showing a large subcortical hematoma in the left parietal region. H) Intraoperative view showing an extensive thrombosis of the inferiorly draining cortical veins. The nidus was located in the subcortical area.
CT revealed a massive subcortical hematoma in the left parietal region (figure 3 G). Intraoperative view during the emergency hematoma evacuation showed an extensive thrombosis of the inferiorly draining cortical veins (figure 3 H). There was a quick recovery of disturbance in consciousness, but hemiplegia and aphasia persited.
Case 6
A 54-year-old male had a couple of episodes of severe headache about two years prior to admission. For 18 months prior to admission, he had focal seizures starting in his left hand. The seizure quickly spread to the other side of the body and increased in frequency.
And the patient had generalized convulsions once a week for the last three months. As cerebral angiography, performed at a local hospital, revealed an AVM with a huge varix in the right parietal lobe, the patient was referred to my department for the purpose of embolization (figures 6 A,B).
Figure 6.
(Case 6) - A,B) right carotid angiography, AP and lateral projection, revealed a huge varix embedded deep within the brain parenchyma. C,D) control angiography, following embolization, showing disappearance of the varix together with most of the nidus.
During embolization, liquid embolic material was injected, through a microcatheter with its tip placed within the nidus, until the column of the embolic material reach the varix. Approximately 95 % of the nidus was obliterated together with the varix (figures 6C,D ). Marked reduction in number of seizures was noted right after embolization.
Control angiography performed 2 years after gamma knife therapy showed complete disappearance of the nidus.
Case 7
A 54-year-old female noticed gradual onset of numbness of the left upper and lower extremities and gait disturbance approximately half a year prior to admission to our hospital. The patient also complained of chronic headache over the last 10 years. As MRI taken at a community hospital revealed multiple space-occupying lesions over the right cerebral hemisphere, she was referred to our hospital under the diagnosis of brain tumor (figures 7 A,B). On admission the patient showed slow mentation, slight left hemiparesis and hemihypesthesia, and papiledema on fundscopic examination. Cerebral angiography showed a posterior temporal AVM with relatively small nidus and large intranidal shunts. The main feeding pedicles came from the temporal branches of the middle cerebral arteries, but there was considerable contribution from the petrosquamosal branch of the middle meningeal artery and anterior and middle temporal branches of the posterior cerebral artery. The most outstanding finding, in this case, was that there was an enormous engorgement of the superiorly draining veins that finally emptied into the superior sagittal sinus. Inferiorly draining veins were not opacified, probably due to thrombosis of a huge varix on the superficial middle cerebral vein that was seen on MRI (figures 7 C,D,E,F). Most of the nidus was obliterated by embolization (figures 7 G,H) and the varicous vein was thrombosed (figures 7 I,J). The patient stopped complaining of headache, and hemiparesis and hemisensory disturbance showed quick recovery shortly after embolization. Control angiography performed 6 months after gamma knife therapy revealed complete obliteration of the nidus and the patient was neurologically intact.
Figure 7.
(Case 7) - A,B) T1 weighted coronal MRI showing a huge space occupying lesion spreading over the right temporal lobe. The lesion seems to have mixed intensity, but the area with flow void represents nidus and draining veins and high intensity globoid mass, located inferolaterlly, is a thrombosed varix of the superficial middle cerebral vein. C,D) right internal carotid injection, AP projection, arterial and venous phase. E,F) right internal carotid injection, lateral projection, arterial and venous phase. Cerebral angiography showed a posterior temporal AVM with relatively small nidus and large intranidal shunts. There was an enormous engorgement of the superiorly draining veins that finally emptied into the superior sagittal sinus. G,H) Control angiography following ambolization showed occlusion of most of the nidus and obliteration of the engorged draining vein. I,J) control T1 weighted coronal MRI, taken 1 week after embolization, showing an extensive thrombosis in the engorged draining vein.
Discussion
It seems obvious that aggressive embolization, aiming at cure by itself, generates higher rate of complete obliteration of the AVM nidus. However, as the other side of a coin, a higher rate of periprocedural hemorrhagic complication becomes unavoidable. As our motto was to generate higher complete cure rate with less complication, combination of some of the treatment modalitites is the rule, and heavily depending upon a single treatment modality is exception. This explains relatively low rate of complete obliteration by embolization alone in our series (table 4). Presurgical embolization was terminated when following strategically important factores were attained : 1) occlusion of the feeding pecdicles coming from deep, 2) obliteration of large arteriovenous shunts within the nidus, 3) obliteration of blood supply to the nidus via leptomeningeal anastmoses and 4) shrinkage of draining veins overlying the nidus. Embolization made surgical approach easy, and complete surgical resection of the nidus was attained in 96 out of 102 cases (94.1 %).The treatment results of 6 cases with a part of the nidus remaining after surgery were: 3 complete cure by gamma knife therapy, 1 died of delayed hemorrhage from the residual nidus and 2 patient refused an additional treatment.
Pre-gamma knife therapy embolization was terminated when 1) the nidus shrinked to fit the gamma knife focal spot, 2) intranidal high flow shunts were obliterated and 3) intranidal aneurysms and/or varices were obliterated. Such precautions made incidence of complication low, especially after 1994 when I switched to modern microcatheters and guidewires (table 5).
Several interesting points were noticed during reviewing author's cases. There is a considerable number of cases with steno-occlusive changes in the major draining vein among cases with massive nidus (42 per cent of the author's cases being classified into grade IV and V). Furthermore, neurological deficits quite often showed spectacular shrinkage in these cases immediately after effective embolization for the AVM. Inspired by these cases with abnormality in major draining veins, the author is going to make some consideration into pathophysiologic mechanism of neurological manifestation of massive AVMs. Galenic venous system is most frequently subject to steno-occlusive and/or ectatic change as vast amount of venous flow from deep seated AVMs converge to this venous system. The clinical manifestations related to the abnormalities of the venous system varied depending upon its anatomical location and degree of ectatic and stenotic changes. In case 1, the cerebellar AVM drained mostly into the superior petrosal vein and then to the lateral mesencephalic, basal and Galenic venous system. Compression by the prominently engorged transverse pontine vein, generated by reflux of blood flow, seemed relevant to trigeminal neuralgia. Intractable trigeminal neuralgia disappeared completely after the completion of embolization that resulted in collapse of draining veins. As the trigeminal nerve has close relationship to several venous components in the posterior fossa, trigeminal neuralgia could be caused by compression from normal veins2. However, trigeminal neuralgia is a rare manifestation of the posterior fossa AVM together with hemifacial spasm3,4.
In case 2, the cause of trigeminal neuralgia was also diagnosed to be compression by an ectatic draining vein of a high flow cerebellar AVM at a local university hospital. The dilated and tortuous appearance and the reflux of the venous flow were consistent with venous hypertension. This phenomenon was caused by a high-flow AVM and impaired venous outlets generated by stenosis of the vein of Galen. Following particulate embolization, the patient developed severe neurological deficits caused by compression to the upper brain stem by a newly formed gigantic varix on the basal vein. Particulate embolization probably occluded minor draining veins, in stead of the nidus, and enhanced the turbulent flow and induced intimal damage to the main draining vein. Shortly after liquid embolizasion at our institute, trigeminal neuralgia has completely disappeared. The residual nidus of the cerebellar AVM was completely resected by surgery. Only permanent deficit in this case was mild cerebellar ataxia. In case 3, rapid deterioration of neurological status before admission seemed caused by severe compression of the upper brain stem by the profusely ectatic basal vein of Rosenthal, and their dramatic recovery after embolization by rapid shrinkage of the ectatic vein. Though venous varices are considered important component that cause intracranial hemorrhage in cases with cerebral AVMs, cerebral and dural arteriovenous fistulae5,6,7 little attention has been paid to mass effect of gigantic varices generated on draining veins in the formation of neurological deficits and their dramatic reversibility after effective treatment for cerebral AVMs8,9.
Usually occlusion of Galenic system accompanying deep brain AVMs does not present with clinical evidence of dysfunction of deep cerebral structures thanks to very well developed collaterals 10. In case 4, numerous flecks of curvilinear calcification spreading over the left cerebral hemisphere suggest anoxia caused by longstanding venous hypertension. Also in this case, decompensation of collateral venous drainage after spontaneous occlusion of Galenic system seemed to have resulted in dysfunction of mesencephalon and upper brain stem. Dramatic reversal of clinical manifestation of this case seemed to be caused by disappearance of decompensation of venous return by the reduction of venous overload brought about by embolization. There were occasional deep AVM cases with infarction in the draining territories of deep venous structures as a result of rapid thrombosis of the Galenic system11. Of interest in case 5 was that stenotic change in the Galenic-straight sinus junction resulted in an extensive thrombosis in the cortical veins through a connecting vein. As stagnation in the draining system was already existed, extensive thrombosis in the major draining veins took place after a marked reduction of blood flow generated by a very efficient embolization. The author was sure that no embolic material escaped into the venous circulation during embolization in this case. Such complication might have been prevented if the patient were put on systemic heparinization after embolization.
Turjman et al statistically analyzed angioarchitectural characteristics and population demographics by means of multivariate analysis. And they found the following six parameters to be the most predictive of epilepsy: cortical location of the AVM, feeding by the middle cerebral artery, cortical location of the feeder, absence of aneurysm, presence of varix/varices in the venous drainage, and association of varix and absence of intranidal aneurysms. Their study helped us identify features that strongly correlate with epilepsy and identify goals of treatment for epileptogenic AVMs12. Our case 6 had all of these parameters, and focal and generalized seizures markedly decreased in frequency following embolization that obliterated 95% of the nidus and the varix. Pritz's report on 6 cases with ruptured AVM and an associated venous varix, recent hemorrhage was identified around the venous varix in 4 of the 5 patients who underwent MR.
A huge varix of our case may be a pseudoaneusym grew by repeating rupture of a varix generated on a main draining vein. In addition to eradication of fear of re-rupture, incidence of seizure decreased dramatically after embolization in this case.
Case 7 was characterized by an extensive engorgement of cortical draining veins that exerted prominent mass effect. After spontaneous thrombosis of the inferiorly draining vein, the superiorly draining vein enlarged further before admission. Visible nidus on angiography was small and this was almost completely embolized by one session. Slow mentation and neurological deficits gradually but completely recovered after embolization. Kurita et al reported on two cases with cerebral AVMs showing varicous dilatation of main cortical draining vein. And he described that brain edema can develop in patients with an unruptured AVM by venous congestion following spontaneous thrombosis of venous components 13.
Ectatic and occlusive diseases of the venous drainage system of cerebral AVMs are common, but pathomechanism is not well known. Based upon review of literature and observation of vessel wall irregularity with or without stenosis of the vein of Galen and its tributaries in 11 out of 53 cases with deep-seated cerebral AVMs, Vinuela et al. speculated that the angiographic changes are probably related to endothelial damage secondary to turbulent, unstable, and irregular flow. And concluded that if a vein draining an AVM is subjected to a high lateral pressure, it will become long, serpiginous, and slightly wider; and if it is subject to a highly turbulent flow it will markedly dilate 10.
In conclusion, formulating treatment strategy based upon careful analyses of angiographic findings is important. Do not give up curative treatment of the patients with massive AVMs, because the patients with venous diseases can make full recovery if curative treatments are instituted timely even though they are referred to you presenting coma, as was shown in illustrative cases.
References
- 1.Ogata N, Goto K, et al. An evaluation of the physical properties of current microcathters and guidewires - The development of the "Catheter-glide approach" in response to weaknesses of current materials. Interventional Neuroradiology. 1997;3:65–80. doi: 10.1177/159101999700300107. [DOI] [PubMed] [Google Scholar]
- 2.Matsushima T, Huynh-Le P, et al. Trigeminal neuralgia caused by venous compression. Neurosurgery. 2004;55:334–339. doi: 10.1227/01.neu.0000129552.87291.87. [DOI] [PubMed] [Google Scholar]
- 3.Silber MH, Sandok BA, et al. Vascular malformations of the posterior fossa - Clinical and radiologic features. Arch Neurol. 1987;44:965–969. doi: 10.1001/archneur.1987.00520210059020. [DOI] [PubMed] [Google Scholar]
- 4.Yang PJ, Higashida RT, et al. Intravascular embolization of a cerebellar arteriovenous malformation for treatment of hemifacial spasm. Am J Neuroradiol. 1989;10:403–405. [PMC free article] [PubMed] [Google Scholar]
- 5.Pritz MB. Ruptured supratentorial arteriovenous malformations associated with venous aneurysms. Acta Neurochir. 1994;128:150–162. doi: 10.1007/BF01400666. [DOI] [PubMed] [Google Scholar]
- 6.King WA, Martin NA. Intracerebral hemorrhage due to dural arteriovenous malformations and fistulae. Neurosurg Clin N Am. 1992;3:577–590. [PubMed] [Google Scholar]
- 7.Machida T, Hayashi N, et al. Posterior cranial fossa dural arteriovenous malformation with a varix mimicking a thrombosed aneurysm: Case report. Neuroradiology. 1993;35:210–211. doi: 10.1007/BF00588496. [DOI] [PubMed] [Google Scholar]
- 8.Nishino A, Sakurai Y, et al. superior petrosal sinus dural arteriovenous malformation with varix indenting brain stem-report of a case and review of the literature. No To shinkei. 1991;43:62–69. [PubMed] [Google Scholar]
- 9.Oda M, Takahashi JA, et al. Rendu-Osler-Weber disease with a giant intracerebral varix secondary to a high-flow pial AVF: Case report. Surg Neurol. 2004;61:353–356. doi: 10.1016/j.surneu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Viñuela F, Nombela L, et al. Stenotic and occlusive disease of the venous drainage system of deep brain AVM's. J Neurosurg. 1985;63:180–184. doi: 10.3171/jns.1985.63.2.0180. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JH, Williams JP, et al. Spontaneous thrombosis of deep cerebral veins: a complication of arteriovenous malformation. Stroke. 1975;6:164–171. doi: 10.1161/01.str.6.2.164. [DOI] [PubMed] [Google Scholar]
- 12.Turjman FT, Massoud TF, et al. Epilepsy associated with cerebral arteriovenous malformations: a multivariate analysis of angioarchitectural characteristics. AJNR Am J Neuroradiol. 1995;16:345–350. [PMC free article] [PubMed] [Google Scholar]
- 13.Kurita H, Shin M, et al. Congestive brain oedema associated with a pial arteriorvenous malformation with impaired venous drainage. Acta Neurochir. 2001;143:339–342. doi: 10.1007/s007010170087. [DOI] [PubMed] [Google Scholar]