Abstract
Drosophila melanogaster is a valuable model system for the neural basis of complex behavior, but an inability to routinely interrogate physiologic connections within central neural networks of the fly brain remains a fundamental barrier to progress in the field. To address this problem, we have introduced a simple method of measuring functional connectivity based on the independent expression of the mammalian P2X2 purinoreceptor and genetically encoded Ca2+ and cAMP sensors within separate genetically defined subsets of neurons in the adult brain. We show that such independent expression is capable of specifically rendering defined sets of neurons excitable by pulses of bath-applied ATP in a manner compatible with high-resolution Ca2+ and cAMP imaging in putative follower neurons. Furthermore, we establish that this approach is sufficiently sensitive for the detection of excitatory and modulatory connections deep within larval and adult brains. This technically facile approach can now be used in wild-type and mutant genetic backgrounds to address functional connectivity within neuronal networks governing a wide range of complex behaviors in the fly. Furthermore, the effectiveness of this approach in the fly brain suggests that similar methods using appropriate heterologous receptors might be adopted for other widely used model systems.
Keywords: live imaging, circadian, circuitry, GCaMP, Epac1-camps
despite its relative simplicity the nervous system of Drosophila melanogaster is capable of producing a remarkable repertoire of complex behaviors (Weiner 1999). Work on Drosophila has identified discrete networks of neurons that govern circadian timekeeping (Nitabach and Taghert 2008), courtship (Villella et al. 2008), memory (McGuire et al. 2005), sleep (Crocker and Sehgal 2010), feeding (Melcher et al. 2007), and decision-making (e.g., Dickson 2008; Peabody et al. 2009). The study of these and other neural networks in the fly continues to enrich and inform our understanding of the neural control of animal behavior. For many of these central brain networks the pattern and physiologic basis of their constituent connections have been proposed; however, due to the electrophysiologic inaccessibility of much of the fly CNS, many aspects of these network models remain unchallenged experimentally. The development of technically feasible methods to test for the presence and physiologic nature of connections between defined neuronal classes of the fly CNS will therefore be critical for progress in the field.
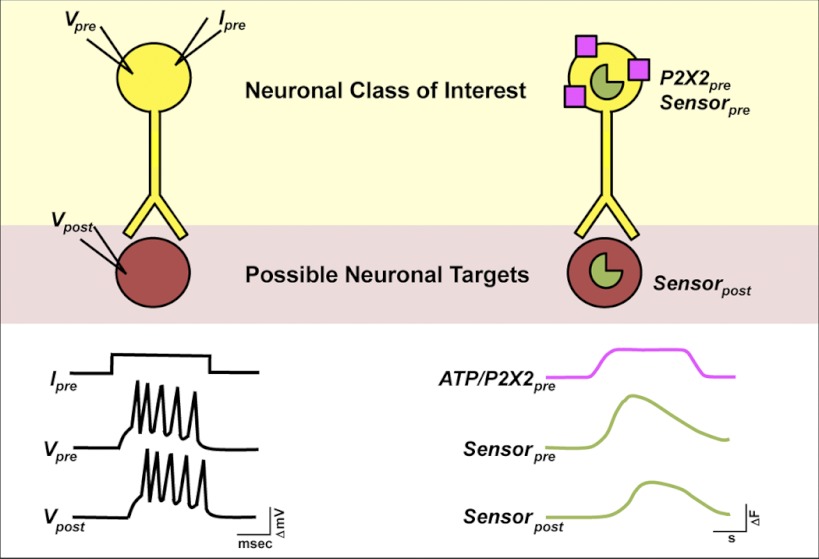
The ability to address the nature of connections between pairs of identified neurons has been one of the great strengths of large invertebrate model systems (Kandel 1976). The stereotyped and large neurons of these organisms are accessible to multiple recording and stimulating electrodes, making it possible to stimulate activity in a neuron of interest while measuring electrophysiologic responses in putative follower neurons (e.g., Kandel et al. 1967; Willows and Hoyle 1969; Fig. 1). Unfortunately, such multielectrode experiments are not feasible for most central neural networks of the Drosophila brain. The electrophysiologic inaccessibility of many central fly neurons has been surmounted somewhat by the use of genetically encoded sensors for neuronal excitation and second-messenger signaling (e.g., Lissandron 2007, #201; Ruta 2010, #268; Shafer 2008, #47; Tian 2009, #80; Tomchik 2009, #102; Wang 2003, #189; Yu 2003, #188) and the physiologic responses of single deeply situated neurons can now be routinely observed in the fly brain using live imaging techniques. Combining these techniques with an ability to acutely activate subsets of neurons would allow for existing models of neural connectivity to be tested and the downstream targets of neurons of interest to be identified physiologically.
Fig. 1.
Schematic of dual binary, ATP/P2X2 excitation approach to network interrogation. Left: an electrophysiologic approach to connectivity in invertebrate nervous systems. The investigator stimulates a neuron of interest with depolarizing current while simultaneously recording membrane voltage in putative follower neurons (e.g., Kandel et al. 1967). Right: a physiogenetic approach to connectivity in the Drosophila nervous system. Depolarizing current is induced in neuronal classes of interest through ATP gating of transgenic P2X2 receptors (shown in purple), whereas Ca2+ or cAMP levels are simultaneously monitored in putative follower neurons using genetically encoded sensors (shown in green). Note the differing time scales between methods.
Several genetically encoded triggers of neural excitation have been successfully used in Drosophila in conjunction with various chemical or physical triggering methods (reviewed in Venken et al. 2011). The first instance of such triggering in the fly used the photochemical excitation of neurons expressing transgenic P2X2 receptor, a mammalian ATP receptor that is not encoded by the Drosophila genome (Lima and Miesenböck 2005; Littleton and Ganetzky 2000). The mammalian thermosensitive TRPV1 channel has been used to excite fly sensory neurons using its ligand capsaicin (Marella et al. 2006) and ectopic expression of the Drosophila thermosensitive TRPA1 channel has also been used to activate multiple neuron types with pulses of high temperature (e.g., Parisky et al. 2008). Furthermore, the mammalian cold-sensitive TRPM8 channel has been used with both low-temperature pulses and menthol vapor as exogenous excitation triggers in the fly (Peabody et al. 2009). Finally, several groups have used the bacterial opsin Channelrhodopsin-2 (ChR2) to trigger neuronal excitation in Drosophila with blue light (e.g., Pulver et al. 2009; Schroll et al. 2006; Zimmermann et al. 2009). The fact that ChR2 is maximally activated by blue wavelengths makes it problematic for use in live imaging experiments, since GFP-based sensors must be excited with the same wavelengths that activate opsin conductance (Guo et al. 2009). The recent development of red-shifted optogenetic controls (Yizhar et al. 2011) and Ca2+ sensors (Zhao et al. 2011) may ultimately circumvent this problem, but these newly developed tools have not yet been successfully introduced to Drosophila. The use of temperature pulses to trigger the opening of TRPA1 or TRPM8 channels during live imaging experiments is also problematic, because acute shifts in temperature can cause significant movement of imaging targets within the explanted brain during high-resolution imaging, which makes the analysis of single-neuron somata difficult (Q. Zhang and O. Shafer, unpublished observations). For these reasons we have opted for ligand-gated triggering of transgenic receptors as a means for acute neuronal excitation. The feasibility of combining ATP excitation of P2X2-expressing fly neurons to attain biologically relevant neural excitation during behavioral and physiologic experiments has already been established for both larval and adult nervous systems (e.g., Hu et al. 2010; Lima and Miesenböck 2005). We have therefore chosen ATP/P2X2 excitation for use in our live imaging experiments.
In Drosophila the Gal4/UAS system is a powerful and versatile method of transgene expression that has been the tool of choice for directing sensor expression in specific neuronal classes within the fly brain (Brand and Perrimon 1993; Venken et al. 2011). The recent development of alternative binary expression systems, the LexA and Q systems (Lai and Lee 2006; Potter et al. 2010), now makes it possible to independently direct P2X2 and sensor expression within different neuronal classes. Here we have used the simultaneous use of the Gal4 and LexA systems for the independent dual binary expression of P2X2 and genetically encoded sensors of Ca2+ or cAMP, thereby allowing for the acute excitation of defined neuronal populations during the simultaneous live imaging of Ca2+ and cAMP dynamics within putative neuronal targets (Fig. 1).
Here we establish the feasibility of the simultaneous use of the GAL4 and LexA systems to render defined groups of neurons excitable by pulses of bath-applied ATP while simultaneously and independently expressing the Ca2+ sensor GCaMP3.0 or the cAMP sensor Epac1-camps in putative follower neurons. We present proof of principle experiments that establish the efficacy of this method for detecting established and/or predicted excitatory and modulatory connections within larval and adult brains, concentrating on the well-characterized circadian clock neuron network of the fly (Nitabach and Taghert 2008), the constituent physiologic connections of which have remained largely unexamined. The LexAop-P2X2, LexAop-GCaMP3.0, and LexAop-Epac1-camps lines we have used for these studies, along with large and growing number of existing GAL4, UAS, and LexA lines, constitute a useful and technically facile toolkit for the interrogation of central neuronal networks in the Drosophila brain.
METHODS
Fly stocks and rearing.
Flies were reared on cornmeal-yeast-sucrose media at 25°C under a 12:12 light:dark cycle or under the diurnal conditions of the lab. All Gal4 and UAS lines used in this study have been previously described: Pdf(M)-Gal4;; and ;Pdf(bmrj)-Gal4; (Renn et al. 1999), ;UAS-GCaMP3.0; (Tian et al. 2009), ;;UAS-P2X2 (Lima and Miesenböck 2005), ;;Clock(4.1M)-Gal4 (Zhang et al. 2010a,b), ;Clock(-856[8.2/2])-Gal4; (Gummadova et al. 2009), ;c929-Gal4; (Hewes et al. 2000), ;Rh6-Gal4; (Pichaud and Desplan 2001), ;UAS-Epac1-camps(50A); (Shafer and Taghert 2009), and ;Cha(7.4)-Gal4/CyO; (Salvaterra and Kitamoto 2001). The ;Pdf-LexA; line has also been described previously (Shang et al. 2008). The creation of the LexAop-P2X2, LexAop-GCaMP3.0, and LexAop-Epac1-camps lines is described in the following text. Stable lines carrying combinations of these elements were created using standard Drosophila genetic techniques.
Creation of LexAop P2X2 and sensor lines.
We used the LexA-response element containing pLOT vector (Lai and Lee 2006) for the creation of LexAop-GCaM3.0, LexAop-Epac1-camps, and LexAop-P2X2 plasmids. GCaMP3.0 (Tian et al. 2009) was obtained in a pEGFP-N1 vector from Addgene (Cambridge, MA; plasmid #22692) and digested with EagI. The resulting GCaMP3.0-containing fragment was gel purified, digested with BglII, and subsequently PCR purified using a QIAquick PCR Purification Kit (Qiagen, Valencia, CA). In parallel, pLOT vector was digested with EagI and BglII, and treated with CIP alkaline phosphatase (New England Biolabs, Ipswich, MA) following manufacturer's instructions. The GCaMP3.0 fragment was ligated with the linearized pLOT vector with a Quick Ligation Kit from New England Biolabs. Epac1-camps (Nikolaev et al. 2004) was sequentially digested from the pUAST-Epac1-camps plasmid (Shafer et al. 2008) using XhoI and BglII, and PCR purified. This Epac1-camps fragment was cloned into pLOT as above using sequential XhoI and BglII restriction digests of pLOT. The P2X2 trimer (Lima and Miesenböck 2005) was obtained as the Gateway entry clone pENTRA1_P2X2 from G. Miesenböck (Oxford University). We created a pLOT Gateway vector by cutting pLOT with KPN1, generating blunt ends using T4 DNA Polymerase (Invitrogen), and inserting the chloramphenicol/ccdB-resistant Gateway cassette A using T4 DNA Ligase following manufacturer's instructions (Invitrogen). We transformed OmniMAX 2T1R cells (Invitrogen) with the resulting pLOT-Gateway vector, selected ampicillin- and chloramphenicol-resistant clones for vector propagation, and purified the pLOT-Gateway vector using a Qiagen Mini Prep kit (Qiagen). The transfer of the P2X2 trimer from pENTRA1_P2X2 to the pLOT-Gateway vector was accomplished via LR recombination reaction according to manufacturer's instructions (Invitrogen) using LR II clonase (Invitrogen).
All three LexAop plasmids were extracted and purified using a Qiagen Mini Prep kit. Purified plasmids were sent to Genetic Services, Inc. (Cambridge, MA), where they were injected into w1118 embryos. We isolated and mapped several independent transgenic lines for each LexAop element using standard fly genetic techniques. The specific lines used here were: w;LexAop-GCaMP3.0(4B);, w;LexAop-Epac1-camps(1A);, w;LexAop-P2X2(7);, and w;;LexAop-P2X2(1).
Dissections, solutions, and test compound delivery.
Flies were anesthetized on CO2 and brains were dissected into room temperature hemolymph-like saline (HL3) consisting of (in mM): 70 NaCl, 5 KCl, 1.5 CaCl2, 20 MgCl2, 10 NaHCO3, 5 trehalose, 115 sucrose, 5 HEPES; pH 7.1 (Stewart et al. 1994). For larval brain dissections, third instar (nonwandering) larvae were removed from the food and brains were dissected directly into HL3, keeping the eye disks and ventral nerve cord intact. Mouth hooks continued to move after dissections and were therefore removed to prevent brain movement during imaging experiments. All brains were allowed to adhere to the bottom of 35-mm FALCON culture dishes (Becton Dickenson Labware, Franklin Lakes, NJ) under a drop of HL3 contained within a petri dish insert (Bioscience Tools, San Diego, CA) for directing perfusion flow. Brains were imaged 5 to 10 min after dissection to allow for optimum baseline stabilization and settling of the brain to the dish. Perfusion flow was established over the brain with a gravity-fed PS-8H perfusion system (Bioscience Tools). Test compounds were delivered to mounted brains by switching perfusion flow from the main HL3 line to another channel containing diluted compound for desired durations followed by a return to HL3 flow. All test compounds were dissolved in HL3. To control for the effects of switching channels, we perfused HL3 for 30 s from a second vehicle channel as a vehicle control. Adenosine 5[prime]-triphosphate disodium salt hydrate (ATP), guanosine 5[prime]-triphosphate disodium salt hydrate (GTP), and carbamoylcholine chloride (carbachol) were purchased from Sigma-Aldrich (St. Louis, MO).
Live imaging and analysis.
Live imaging was performed using an Olympus FV1000 laser-scanning microscope (Olympus, Center Valley, PA) under a ×20 (0.50 N/A W, UMPlan FL N) or ×60 (1.10 N/A W, FUMFL N) objective (Olympus, Center Valley, PA). Regions of interest (ROIs) were selected over single neuronal somata or, in the case of Bolwig's nerve, over the length of a nerve. For GCaMP3.0 imaging experiments, frames were scanned with a 488-nm laser at 1—10 Hz for 5 min and GCaMP emission was directed to a photomultiplier tube by means of a DM405/488 dichroic mirror. Scanning frequencies for GCaMP3.0 imaging were kept constant within experiments, but varied between experiments. Experiments involving multiple neuronal classes demanded larger scanning areas and therefore lower scan rates. Epac1-camps FRET imaging was performed by scanning frames with a 440-nm laser at a frequency of 1 Hz for 5 min. CFP and YFP emission was separated by means of a SDM510 dichroic mirror.
For each neuron within an optical section, ROIs were drawn over somata using Fluoview software (Olympus). Raw intensity values for GCaMP3.0 emission or Epac1-camps CFP and YFP emission were recorded as mean pixel intensities (value range: 0—4,095) for each ROI at each time point and exported from Fluoview. Data transformations (see details in the following text) were conducted using custom software developed in Matlab (The MathWorks, Natick MA). For GCaMP3.0 experiments, raw intensity traces were filtered with a 10-point moving average to remove high-frequency noise and then normalized to percentage fluorescence changes (ΔF/F0) using the following equation
where Fn is a raw intensity value recorded at each point in time and F0 is the baseline fluorescence value, calculated from the average of the raw intensity values in the first 10 s of recording from each trace. Maximum GCaMP3.0 fluorescence change values (max ΔF/F0) were determined as the maximum percentage change observed for each trace over the entire duration of each imaging experiment. Maximum values for each treatment and genotype were averaged to calculate the mean maximum change from baseline. To remove the direct excitatory effects of 488-nm light on Bolwig's Nerve (BN) (Yuan et al. 2011) from our analysis, which we observed during the start of a subset of our 488-nm scans, the F0 for all larval BN experiments was calculated from the average fluorescent intensities observed during the 15 s preceding the stimulus onset, by which time the baseline GCaMP3.0 fluorescence had stabilized following the light-induced excitation of the nerve.
For Epac1-camps data processing, we corrected YPF intensity values for spillover from the CFP channel by the following equation
where YFPsoc is the spillover—corrected YFP intensity, YFP and CFP are the raw intensity values, and 0.444 is the proportion of CFP emission that spills over into the YFP channel on our imaging system. The inverse FRET ratio, which is proportional to increases in cAMP, was calculated by taking the ratio of CFP/YFPSOC at all time points for each ROI. Each ratio trace was filtered with a 10-point moving average. All spillover-corrected and filtered Epac1-camps inverse FRET traces were normalized to the first time point to an initial value of “1.0.” Filtered, corrected, and normalized inverse FRET traces were expressed as percentage inverse FRET changes and averaged for each treatment and neuron type to create mean inverse FRET traces. The maximum percentage inverse FRET change was determined for every neuron based on the entire duration of the experiment. Such maximum inverse FRET changes were averaged for each treatment and neuron type to determine the mean maximum inverse FRET change. For most Epac1-camps inverse FRET traces, a spontaneous and gradual increase in inverse FRET was observed due to a slow photobleaching of YFP, as has been described previously for this sensor (Börner et al. 2011; Shafer et al. 2008). To correct for these spontaneous changes, we determined the mean inverse FRET increase for 10—20 untreated or vehicle treated neurons of a particular genotype, depending on the nature of the experiment. This mean trace was then subtracted from each individual experimental trace to generate corrected inverse FRET traces.
To statistically compare maximum changes in GCaMP3.0 fluorescence or Epac1-camps inverse FRET ratio between the vehicle and test compounds, we used a Kruskal—Wallis one-way ANOVA with a Dunn's multiple comparison test. Pairwise comparisons of maximum changes in GCaMP3.0 fluorescence or inverse Epac1-camps FRET in response to test compound or vehicle perfusion were made using the Mann—Whitney U test. All plots and statistical tests were generated and performed using Prism 5 (GraphPad, San Diego CA). Figures were constructed in Adobe Illustrator and Photoshop (Adobe Systems, San Jose, CA). To obtain intensity-mapped images representing select time points before, during, and after ATP/P2X2 stimulation, single frames were captured from intensity-mapped still images using Fluoview. These images were imported to Photoshop (Adobe Systems, San Diego CA), and trimmed to size.
RESULTS
Controlled excitation of P2X2-expressing deep brain neurons with perfused ATP is compatible with high-resolution live imaging.
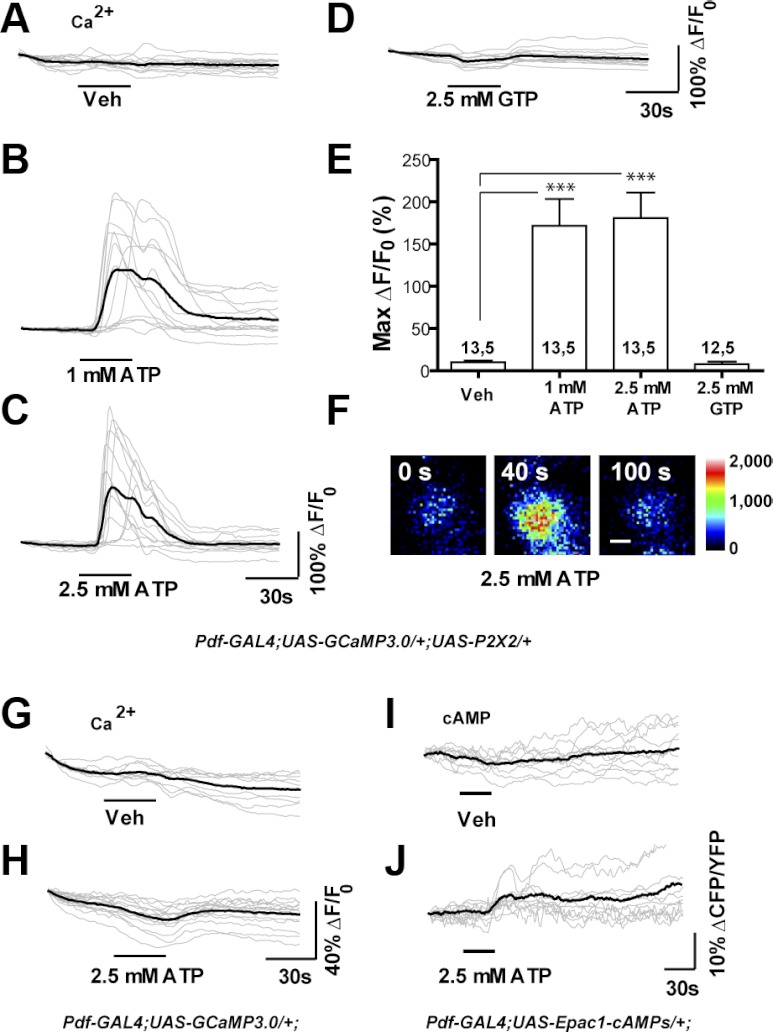
Previous work has established that neurons expressing transgenic P2X2 receptor in Drosophila can be excited at biologically relevant levels through the global uncaging of ATP in freely moving flies (Lima and Miesenböck 2005) or through the puffing of ATP on explanted brains during electrophysiologic recordings of superficial brain neurons (Hu et al. 2010). We wondered if the simple perfusion of ATP across the explanted brain could provide a reliable and technically facile means of exciting deeply situated adult neurons in a manner compatible with high-resolution live imaging. We therefore used a Pdf-Gal4 driver to coexpress UAS-GCaMP3.0 (Tian et al. 2009) and UAS-P2X2 (Lima and Miesenböck 2005) in the small ventral lateral neurons (s-LNvs). These cells are critical circadian pacemaker neurons whose small somata and deep position within the central brain make them difficult neurons to investigate electrophysiologically (Cao and Nitabach 2008). Compared with vehicle controls (Fig. 2A), 30-s perfusions of 1 or 2.5 mM ATP resulted in significant GCaMP3.0 fluorescence increases, thereby revealing acute excitation of the s-LNvs (Fig. 2, B, C, E, F). In contrast, 30-s perfusions of 2.5 mM GTP did not result in significant increases in on GCaMP3.0 fluorescence, instead causing very small decreases in fluorescence during perfusion (Fig. 2, D and E). The latencies of the s-LNv responses to 1 mM ATP were less consistent compared with the responses to 2.5 mM, although a few s-LNvs did display relatively late responses to the higher dose (Fig. 2, B and C). Many of the GCaMP3.0 fluorescence increases displayed by the s-LNvs following 1 mM ATP perfusion were markedly bimodal, unlike the majority of responses to 2.5 mM (Fig. 2, B and C). This was reminiscent of s-LNv GCaMP3.0 responses to nicotinic acetylcholine receptor activation. Carbachol (CCh) excitation of s-LNv nicotinic acetylcholine receptors (nAChRs), which like P2X2 are expected to gate both Na+ and Ca2+ upon ligand binding, results in bimodal GCaMP3.0 responses at low CCh concentrations but in single fluorescence peaks at high concentrations (Lelito and Shafer 2012). It is possible that, in the case of bimodal responses, the first peak reflects the direct gating of Ca2+ through P2X2, whereas the second peak represents Ca2+ entry through voltage-gated Ca2+ channels or the release of intracellular Ca2+.
Fig. 2.
Bath application of ATP results in the excitation of P2X2-expressing deep brain neurons during live imaging experiments. A–D: individual (gray) and mean (black) traces of Pdf(M)-Gal4;UAS-GCaMP3.0/+;UAS-P2X2/+ s-LNv responses to 30-s perfusion of (A) vehicle (N = 13 neurons from 5 brains [13,5]), (B) 1 mM ATP (N = 13,5) (C) 2.5 mM ATP (N = 13,5), and (D) 2.5 mM GTP (N = 12,5). Test compounds were perfused after a 35-s baseline interval and responses were recorded for a total of 150 s. E: histogram summarizing the mean maximum percentage increase in GCaMP3.0 fluorescence displayed by the neurons plotted in A–D. Perfusion of 1 and 2.5 mM ATP caused fluorescence increases that were significantly greater than vehicle control (P < 0.0001, by Mann—Whitney U test). The perfusion of 2.5 mM GTP did not cause significant fluorescence increases relative to the vehicle control (P = 0.6302 by Mann—Whitney U test). The two numbers displayed within or above each bar of the histogram indicate the number of neurons and the number of brains examined, respectively. F: representative intensity mapped micrographs of a single Pdf(M)-Gal4;UAS-GCaMP3.0/+;UAS-P2X2/+ s-LNv before (0 s), during (40 s), and after (100 s) its response to bath-applied 2.5 mM ATP. The scale bar in F = 2.5 μm. G and H: characterization of ATP's P2X2-independent effects on GCaMP3.0 fluorescence: unlike vehicle perfusion (G), 30-s 2.5 mM ATP perfusion (H) caused a slight but consistent decrease in GCaMP3.0 fluorescence. I and J: characterization of ATP's P2X2-independent effects on Epac1-camps inverse FRET levels. Unlike vehicle perfusion (I), 30-s 2.5 mM ATP perfusion caused a slight increase in inverse FRET (J), due to a decrease in YFP emission.
The Drosophila genome does not encode a P2X2 receptor homolog and previous studies suggest that there are no acute behavioral or physiologic effects of ATP in the absence of transgenic P2X2 (Lima and Miesenböck 2005; Littleton and Ganetzky 2000). Nevertheless, it is still possible that bath-applied ATP might have previously uncharacterized effects on the physiology of fly neurons, possibly through effects on the conserved ATP sensitive K+ channel (Kim and Rulifson 2004), or might have effects on properties of the genetically encoded sensors themselves (Willemse et al. 2007). We therefore treated brains expressing UAS-GCaMP3.0 or UAS-Epac1-camps in the absence of transgenic P2X2 expression with 30-s perfusions of 2.5 mM ATP to determine if ATP had measurable effects on GCaMP3.0 fluorescence or the inverse Epac1-camps FRET ratio (CFP/YFP), which are directly proportional to Ca2+ and cAMP levels, respectively. The 30-s perfusions of 2.5 mM ATP resulted in very small but consistent transient decreases in GCaMP3.0 fluorescence relative to vehicle controls (Fig. 2, G and H). Bath-applied ATP also caused a consistent increase in Epac1-camps inverse FRET values relative to vehicle controls (Fig. 2, I and J). However, the evaluation of raw CFP and YFP traces revealed that this change was not due to bona fide FRET changes, but rather to decreases in YFP fluorescence, reminiscent of GCaMP3.0 fluorescence loss (Fig. 2J and data not shown). We therefore conclude that bath-applied ATP has only small and easily accounted for effects on GCaMP3.0 fluorescence and Epac1-camps inverse FRET.
Taken together, these results indicate that deeply situated P2X2-expressing neurons can be excited by the controlled perfusion of ATP across the explanted brain in a manner compatible with high-resolution GCaMP3.0 and Epac1-camps live imaging within single neuronal somata. Furthermore, the absence of ATP excitation in neurons lacking transgenic P2X2 expression confirms that, as expected from previous work (Lima and Miesenböck 2005), ATP did not excite the s-LNvs in the absence of specifically directed P2X2 expression and had only minor effects on genetically encoded sensors.
LexA operator-driven sensors and P2X2 for dual binary expression experiments.
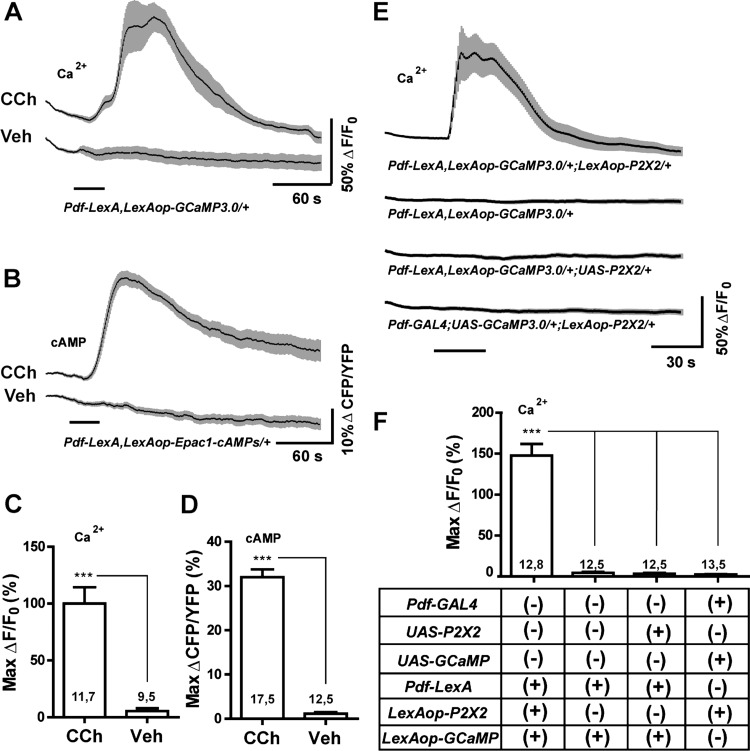
Our proposed method of circuit interrogation requires the independent expression of the P2X2 receptor and genetically encoded sensors in neurons of interest and their putative follower neurons (Fig. 1). To complement existing UAS-Sensor and UAS-P2X2 lines and the large number of existing GAL4 and LexA drivers, we have created a series of transgenic flies containing GCaMP3.0, Epac1-camps, and P2X2 elements under the control of the LexA operator (LexAop) (Lai and Lee 2006). We tested the functionality of our LexAop-GCaMP3.0 and LexAop-Epac1-camps elements within s-LNvs using the previously described Pdf-LexA element (Shang et al. 2008). The adult s-LNvs respond to the general cholinergic agonist carbachol (CCh) with rapid Ca2+ and cAMP increases (Lelito and Shafer 2012). LexAop-driven GCaMP3.0 and Epac1-camps were indeed capable of detecting significant increases in Ca2+ and cAMP in response to 30-s perfusions of 10−4 M CCh (Fig. 3, A—D). Along with evidence presented below, these results indicate that our LexAop-GCaMP3.0 and LexAop-Epac1-camps elements are suitable for the observation of Ca2+ and cAMP dynamics within single somata of deeply situated neurons of the adult brain.
Fig. 3.
LexA operator-driven P2X2 and genetically encoded sensors for excitation and live imaging. A: mean GCaMP3.0 traces for Pdf-LexA(7M),LexAop-GCaMP3.0(4A)/CyO s-LNvs to 30-s perfusions of 10−4 M carbachol (CCh) or vehicle (Veh). B: mean Epac1-camps inverse FRET traces for Pdf-LexA(7M),LexAop-Epac1-camps(1A)/CyO s-LNvs to 30-s perfusions of 10−4 M CCh or Veh. C and D: maximum changes in GCaMP3.0 fluorescence (C) or Epac1-camps inverse FRET increases (D) of s-LNv corresponding to the data in A and B, respectively. Numbers on the histograms indicate the number of neurons and brains sampled. Both LexAop-driven sensors displayed significant responses to CCh relative to Veh controls (P = 0.0004 for GCaMP3.0 fluorescence and P < 0.0001 for Epac1-camps inverse FRET by Mann—Whitney U test). E: mean GCaMP3.0 traces for the s-LNvs of the genotypes indicated below the plots to 30-s perfusions of 10−3 M ATP. Pdf-LexA—driven expression of LexAop-P2X2 rendered s-LNvs sensitive to bath-applied ATP. UAS-P2X2 and LexAop-P2X2 elements did not render neurons sensitive to ATP in the absence of their appropriate Gal4 or LexA drivers. F: summary of maximum Ca2+ responses of s-LNv in E. *** indicates P < 0.001 by Kruskal—Wallis one-way ANOVA and a Dunn's multiple comparison test. Numbers on the histogram is in C, D, and F indicate the number of neurons and brains sampled. For A, B, and E, the time of perfusion is indicated by the bars under the plots and the gray-shaded regions surrounding the mean plots indicate SE, as do the error bars in C, D, and F.
We tested the functionality of our LexAop-P2X2 element by coexpressing it with LexAop-GCaMP3.0 in the s-LNvs using Pdf-LexA. The s-LNvs of Pdf-LexA,LexAop-GCaMP3.0/+;LexAop-P2X2/+ brains displayed clear increases in GCaMP3.0 fluorescence in response to 30-s perfusions of 1 mM ATP, indicating that the LexAop-driven P2X2 element had rendered the s-LNvs sensitive to bath-applied ATP (Fig. 3, E and F). Importantly, the LexAop-P2X2 element rendered the s-LNvs sensitive to ATP only when driven by the Pdf-LexA driver: when UAS-GCaMP3.0 was driven in the s-LNvs with Pdf-GAL4 in flies carrying the LexAop-P2X2 element, ATP had no significant effects on GCaMP3.0 fluorescence (Fig. 3, E and F). This observation, along with work presented in the following text, indicates that the presence of the LexAop-P2X2 element does not cause significant P2X2 expression in the absence of LexA drivers. The same was true for the previously described UAS-P2X2 element (Fig. 3, E and F; Lima and Miesenböck 2005). We conclude that, like its UAS counterpart, our LexAop-P2X2 element is capable of specifically rendering deeply situated adult neurons excitable by bath-applied ATP.
Bath-applied ATP reliably and repeatedly activates P2X2-expressing neurons of the adult brain.
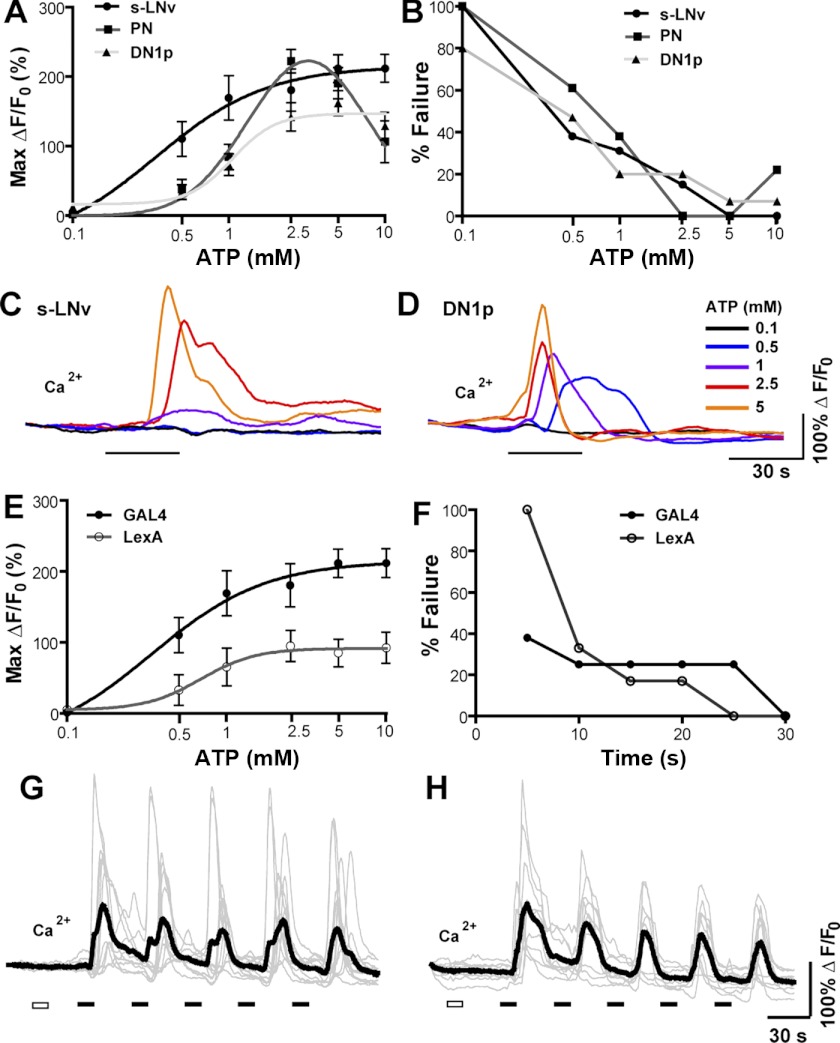
Having acquired the genetic elements necessary for dual binary control of P2X2 and sensor expression, we sought to determine the most reliable means of exciting deep brain neurons expressing UAS-P2X2 and LexAop-P2X2 elements using bath-applied ATP. We first imaged the somata of three different classes of neuron coexpressing P2X2 and GCaMP3.0: the s-LNvs and DN1p clock neurons [using Pdf(bmrj)-GAL4 and Clock(4.1M)-Gal4, respectively] and the olfactory projection neurons [PNs; using Cha(7.4)-Gal4] and compared the effects of 30-s perfusions of a range of ATP concentrations on GCaMP3.0 fluorescence (Fig. 4A). For all three neuron types, 30-s perfusions of 0.1 mM ATP had no significant effects on GCaMP3.0 fluorescence. Higher concentrations resulted in dose-dependent increases in Ca2+ responses, with the s-LNvs and DN1ps displaying sigmoidal response curves and the PNs (the most superficial of the neurons tested) displaying a biphasic response curve with diminished response magnitudes at doses >5 mM (Fig. 4A). We also compared the effects of these ATP concentrations between s-LNvs expressing GCaMP3.0 and P2X2 using either the GAL4 or LexA expression system. The LexA-expressing s-LNvs displayed significant GCaMP3.0 responses over the same range of ATP concentrations as their GAL4-expressing counterparts, but did so with lower response amplitudes, most likely due to lower levels of transgene expression (Fig. 4E). Nevertheless, the LexA-expressing s-LNvs displayed maximum fluorescence changes (ΔF/F0) approaching 100%, amplitudes on par with the GCaMP3.0 responses displayed by fly sensory neurons subjected to acute sensory excitation (Tian et al. 2009). As shown in Fig. 4, C and D, the excitatory responses of single P2X2-expressing neurons to a series of increasing ATP doses were proportional to the concentration of perfused ATP. Thus, the excitatory responses of single neurons can be controlled through the manipulation of ATP dose, thereby making it possible to excite neurons at a range of intensities.
Fig. 4.
Bath-applied ATP reliably and repeatedly activates deeply situated P2X2-expressing neurons in the explanted adult brain. A: dose—response curves for the excitation of P2X2-expressing s-LNvs (N = 13,5), DN1ps (N = 15,5), and olfactory projection neurons (PN, N = 18,5)) by 30-s perfusions of ATP. The genotypes used for each neuronal class where Pdf-Gal4;UAS-GCaMP3.0/+;UAS-P2X2/+ for s-LNvs, ;UAS-GCaMP3.0/+; Clock(4.1M)-Gal4/UAS-P2X2 for DN1ps, and ;Cha(7.4)-Gal4/UAS-GCaMP3.0;UAS-P2X2/+ for PNs. Values represent the mean maximum increase in GCaMP3.0 fluorescence (ΔF/F0) detected during the 150 s following ATP perfusion. B: failure rate curves for 30-s ATP perfusions over a range of concentrations for s-LNvs, DN1ps, and PNs based on the data shown in A. A maximum GCaMP3.0 fluorescence increase of <25% was considered a failure to excite. C and D: GCaMP3.0 responses of a single s-LNv (C) and DN1p (D) cell body to increasing ATP concentrations (0.1–5 mM), each delivered for 30 s. Single neurons displayed graded responses to increasing ATP doses. E: dose—response curves for s-LNv excitation in response to 30-s ATP perfusions comparing s-LNvs from Pdf-Gal4;UAS-GCaMP3.0/+;UAS-P2X2/+ (N = 13,5) and ;Pdf-LexA,LexAop-GCaMP3.0/LexAop-P2X2; (N = 10,5) brains. F: failure rates of s-LNv excitation by various durations of 2.5 mM ATP perfusions comparing s-LNvs excited using the GAL4 (N = 8,5) and LexA (N = 10,5) systems. Genotypes were identical to those used in E. G: individual (gray) and mean (black) GCaMP3.0 traces for repeatedly activated s-LNvs from Pdf-Gal4;UAS-GCaMP3.0/+;UAS-P2X2 brains (N=11,5). H: individual (gray) and mean (black) GCaMP3.0 traces for repeatedly activated s-LNvs from Pdf-LexA,LexAop-GCaMP3.0/LexAopP2X2 brains (N = 10,5). For G and H the white rectangles indicate 30 s of vehicle perfusion and black rectangles indicate 30 s of 2.5 mM ATP perfusion, with 90-s intervals between ATP perfusions.
Our results suggest that 30-s perfusions of 1—5 mM ATP result in significant neuronal excitation for all three neuron types we tested. To gauge the reliability of such ATP/P2X2 excitation we analyzed how often each of these 30-s ATP treatments failed to excite the P2X2-expressing s-LNvs, DN1ps, and PNs. We defined a failure conservatively as any ATP-treated neuron displaying less than a 25% maximal increase in GCaMP3.0 fluorescence. For all three neuron types, failure rates were <50% for 1 mM ATP perfusions and approached zero at higher concentrations (Fig. 4B). Our choice of 30-s perfusions was based on previous experiments involving the bath application of neurotransmitters and receptor agonists (Lelito and Shafer 2012). We wondered if shorter applications of ATP might still yield sufficient excitation of the s-LNvs, the most deeply situated of the neurons tested, using both the LexA and Gal4 expression systems. We therefore determined the failure rates for various durations of 2.5 mM ATP for s-LNvs coexpressing GCaMP3.0 and P2X2 with either LexA or Gal4 drivers. For perfusion durations of 10 to 20 s, failure rates for both genotypes were all near 30%. Failure rates reached zero at perfusion durations of 25 s for LexA s-LNvs and at 30 s for GAL4 s-LNvs (Fig. 4F).
The ability to excite the same set of P2X2-expressing neurons repeatedly would allow for multiple sets of putative follower neurons residing in different focal planes to be investigated in the same brain preparation. We therefore asked if P2X2-mediated excitation by bath-applied ATP could be used to repeatedly stimulate deep brain neurons. Indeed, repeated 30-s perfusions of 2.5 mM ATP resulted in reliable repeated excitation of s-LNvs expressing either GAL4- or LexA-driven P2X2 (Fig. 4, G and H). Although the baseline fluorescence of these neurons displayed a slow and steady drop in intensity, there was no significant difference in the mean maximum GCaMP3.0 fluorescence increases displayed in response to the first and last (fifth) 30-s perfusion of ATP, when compared with the baseline fluorescence preceding each ATP pulse. For repeated excitation using the GAL4 system to coexpress GCaMP3.0 and P2X2 expression (Fig. 4G), the first ATP perfusion caused a mean maximum GCaMP3.0 increase of 126.6 ± 32.9% and the fifth and final pulse caused a mean increase of 114.5 ± 21.9% (P = 0.8438 by Mann—Whitney U test). For repeated excitation using the LexA system (Fig. 4H) the first ATP perfusion caused a mean maximum GCaMP3.0 increase of 145.3 ± 19.1% and final pulse caused a mean increase of 94.1 ± 18.8% (P = 0.0524 by Mann—Whitney U test). Thus, P2X2-expressing neurons can be repeatedly activated in the same dissected brain without a significant rundown in excitation.
Based on these results, we conclude that 30-s perfusions of 1—5 mM ATP result in robust, reliable, and repeatable excitation of deep brain P2X2-expressing neurons, using either the GAL4 or LexA expression system to drive the expression of P2X2. However, we note that different neuronal types display differing profiles of excitation, indicating that specific excitation parameters should be determined empirically for every neuron class and genotype to be excited.
Dual binary expression of P2X2 and genetically encoded sensors allow for the specific excitation of neuronal subsets during live imaging experiments.
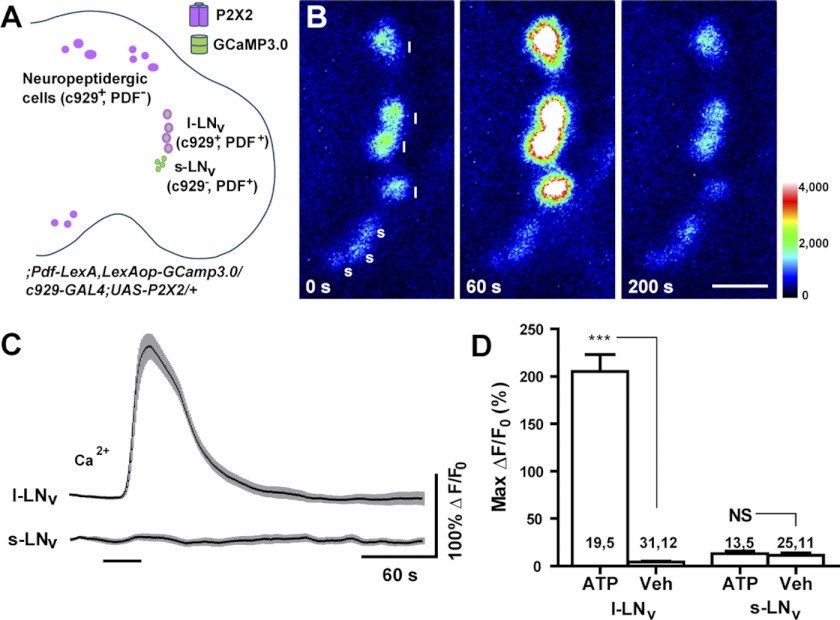
Having confirmed the efficacy of our LexAop-driven sensor and P2X2 elements, we next sought to confirm that the simultaneous use of the GAL4 and LexA systems could render specific neuron classes excitable by ATP during high-resolution imaging experiments. We therefore used the Pdf-LexA element to drive LexAop-GCaMP3.0 expression in both the s-LNvs and the large ventrolateral neurons (l-LNvs) of the circadian clock network, while simultaneously using the c929-GAL4 element, which is expressed by the l-LNvs but not the s-LNvs, to drive P2X2 in the l-LNvs and in the many other peptidergic neurons expressing this GAL4 driver (Fig 5A; Hewes et al. 2000). Thus, the l-LNvs of ;Pdf-LexA,LexAop-GCaMP3.0/c929-GAL4;UAS-P2X2/+ brains will express P2X2, whereas the s-LNvs will not. If the specific dual binary expression of P2X2 and GCaMP3.0 were successful, the l-LNvs would be expected to display acute GCaMP3.0 responses to bath-applied ATP, whereas the s-LNvs would not. As predicted, 30-s perfusions of 1 mM ATP resulted in the excitation of the l-LNvs, but did not excite the s-LNvs imaged within the same focal planes (Fig. 5, B–D). This result, along with the experiments presented in the following text, indicate that the simultaneous use of the GAL4 and LexA systems for the independent expression of P2X2 and genetically encoded sensors, makes possible the specific excitation of neuronal subsets in a manner compatible with high-resolution live imaging experiments. This result also suggests that the excitation of the l-LNvs, neurons important for the control of arousal, sleep, and the integration of circadian light input (Parisky et al. 2008; Shang et al. 2008; Sheeba et al. 2008), does not result in large acute Ca2+ increases in the critical s-LNv pacemaker neurons.
Fig. 5.
Independent expression of P2X2 and genetically encoded sensor in the fly brain by dual binary systems supports the excitation of specific neuronal subsets. A: schematic diagram showing the expression patterns of P2X2 (magenta) and GCaMP3.0 sensor (green) in the experimental fly brain, whose full genotype is shown. B: intensity-mapped stills of l-LNv and s-LNv GCaMP3.0 fluorescence before (T = 0 s), during (T = 60 s), and after (T = 200 s) perfusion of 1 mM ATP. The l-LNvs but not the s-LNvs responded to ATP. The colors of the legend indicate pixel intensity values. Each “l” indicates a l-LNv and each “s” indicates a s-LNv. C: mean GCaMP3.0 fluorescence traces of l-LNvs and s-LNvs to 30-s perfusions of 1 mM ATP (indicated by the bar under the plots). Sample sizes for these plots are shown in D. The gray-shaded regions surrounding the mean plots indicate SE. D: summary of maximum GCaMP3.0 fluorescence increases displayed by the l-LNv and s-LNv to bath-applied ATP and vehicle (Veh). *** indicates a significant difference between ATP and Veh (P < 0.001) and NS indicates nonsignificance by Mann—Whitney U test. The two numbers displayed within or above each bar of the histogram indicate the number of neurons and the number of brains examined, respectively.
Gal4-based excitation and LexA-based live imaging for an established excitatory connection in the larval brain.
We next sought to determine if our proposed method of addressing functional connectivity was sufficiently sensitive to detect an established neuronal connection in Drosophila. We were motivated to propose the present approach to circuit analysis because there are few well-established synaptic connections in our circuitry of interest, the circadian clock neuron network. One of the only fully confirmed synaptic connections in the Drosophila clock network is the excitatory connection between Bolwig's organ (BO), the maggot eye, and the LNv clock neurons, which persist through metamorphosis to become the adult s-LNvs (Fig. 6A; Helfrich-Förster et al. 2007). BO projects directly to the larval optic neuropil via Bolwig's nerve (BN), where its terminals reside in close apposition to LNv arbors (Helfrich-Förster et al. 2002; Malpel et al. 2002). BN expresses ChAT, an enzyme required for acetylcholine (ACh) synthesis (Yasuyama and Salvaterra 1999) and ChAT is required in BN for photic resetting of larval clock neurons (Keene et al. 2011). Dissociated and cultured larval LNvs are directly excited by bath-applied ACh and nicotine (Wegener et al. 2004). Finally, Yuan and colleagues (2011) have recently shown that blue-light stimulation of BO causes acute Ca2+ increases in the larval LNvs clock neurons. Thus, the BN to LNv connection in the larval brain offers a well-established excitatory connection in the clock network on which to test our method for addressing connectivity.
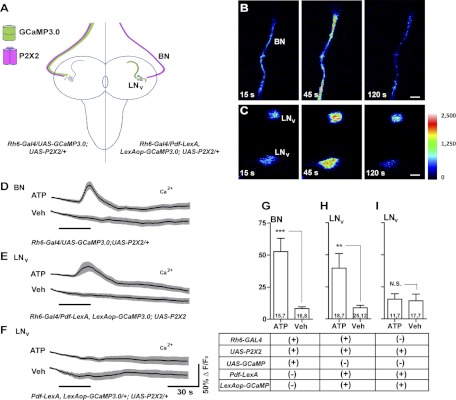
Fig. 6.
Gal4-based excitation and LexA-based live imaging for an established excitatory connection in the larval brain. A: schematic diagram of Bolwig's Nerve (BN) and larval LNv anatomy. The expression of GCaMP3.0 (green) and P2X2 (magenta) are indicated for two experimental genotypes. B: ATP/P2X2 excitation of BN. Single-plane intensity mapped confocal images of GCaMP3.0 fluorescence in the BN of an Rh6-Gal4/UAS-GCaMP3.0;UAS-P2X2/+ larva before (15 s), during (45 s), and after (120 s) the start of 30-s 5 mM ATP perfusion. C: single-plane intensity mapped confocal images of GCaMP3.0 fluorescence in two larval LNvs of a Pdf-LexA,LexAop-GCaMP3.0/Rh6-Gal4;UAS-P2X2/+ larva before (15 s), during (45 s), and after (120 s) their response to BN excitation. The look-up table represents pixel intensity values for both B and C. D: mean GCaMP3.0 fluorescence traces for BNs of ;Rh6-gal4/UAS-GCaMP3.0;UAS-P2X2/+ larval brains treated with 30-s perfusions (black bar) of 5 mM ATP or vehicle (Veh). E: mean GCaMP3.0 fluorescence traces recorded from the LNvs of Pdf-LexA,LexAop-GCaMP3.0/Rh6-Gal4;UAS-P2X2/+ larval brains in response to 30-s perfusions of 5 mM ATP or Veh. F: mean GCaMP3.0 fluorescence traces recorded from the LNvs of Pdf-LexA,LexAop-GCaMP3.0/+;UAS-P2X2/+ larval brains in response to 30-s perfusions of 5 mM ATP or Veh. For D–F, error bars indicate SE. G–I: summary histograms of the maximum GCaMP3.0 fluorescence increases displayed by the BNs (G) and s-LNvs (H and I) of the genotypes shown in D–F. The two numbers displayed within each bar of the histogram indicate the number of neurons and the number of brains examined, respectively. Asterisks indicate a significant difference in maximum fluorescence increase between ATP and Veh treatments and “N.S.” indicates no significant difference by Mann—Whitney U test (***P < 0.001 and **P < 0.01). The error bars in G represent the SE.
Under our experimental conditions, we found it necessary to remove the larval mouth hooks to prevent brain movement during imaging. Mouth hook removal was associated with the loss of BO, leaving only the afferent BNs associated with the eye disks and central brain (Fig. 6, A and B). We therefore first confirmed that excitation of BN was possible in the absence of BO by coexpressing P2X2 and GCaMP3.0 in BN using the Rh6-Gal4 driver, which is expressed in a subset of BN axons (Fig. 6A; Keene et al. 2011). We found that 30-s perfusions of 5 mM ATP caused reliable Ca2+ responses in BNs of ;Rh6-GAL4/UAS-GCaMP3.0;UAS-P2X2/+ brains, indicating the successful excitation of BNs (Fig. 6, B, D, and G).
Having confirmed successful ATP/P2X2 excitation of BN in our preparation, we asked if the predicted excitatory responses could be detected in larval LNvs in response to BN excitation. We therefore created ;Rh6-Gal4/Pdf-lexA, LexAop-GCaMP3.0; UAS-P2X2/+ larvae to independently express P2X2 in BN and GCaMP3.0 in the LNvs (Fig. 6A). Consistent with previous reports, we observed no Rh6-GAL4 driver expression in the LNvs or in any other central neurons of the larval brain (e.g., Keene et al. 2011 and data not shown). All 30-s perfusions of 5 mM ATP caused significant GCaMP3.0 fluorescence increases in the LNvs of Rh6-Gal4/Pdf-lexA, LexAop-GCaMP3.0; UAS-P2X2/+ brains (Fig. 6, C, E, and H). To confirm that the LNv responses to ATP perfusion were due to the specific excitation of the BN and not to the leaky expression of UAS-P2X2 in non-BN cell types or native responses of larval LNvs to ATP, we repeated the experiment on brains dissected from ;Pdf-lexA, LexAop-GCaMP3.0/+; UAS-P2X2/+ larvae, which lacked the R6-GAL4 element and therefore would not have driven P2X2 expression specifically in BN. The LNvs of these flies did not display significant changes in GCaMP3.0 fluorescence following 30-s perfusions of 5 mM ATP (Fig. 6, F and I), indicating that nonspecific UAS-P2X2 expression or native ATP responses had not caused the Ca2+ responses displayed by the LNvs following the ATP/P2X2 excitation of BN. We conclude that our method of addressing connectivity was sufficiently sensitive to detect an established excitatory connection deep within the larval brain.
LexA-based excitation and GAL4-based live imaging to test a predicted peptidergic connection in the adult central brain.
The circadian clock neuron network of the adult fly consists of approximately 150 neurons that express conserved molecular clockwork (Nitabach and Taghert 2008). Understanding the connective properties of this network was our motivation for developing a means for interrogating the physiologic connections between neuronal classes deep within the fly brain. The s-LNvs are critical neuronal pacemakers required for the maintenance of robust rhythms in sleep and activity in the fly under constant darkness and temperature (Grima et al. 2004; Renn et al. 1999; Shafer and Taghert 2009; Stoleru et al. 2004). A large and growing body of anatomic, genetic, and physiologic evidence suggests that the clock neuron network is coordinated through modulatory connections between the s-LNvs and the various classes of dorsal clock neurons. The s-LNvs project to the dorsal brain, where their terminals comingle with terminals from the dorsal clock neuron classes (Helfrich-Förster et al. 2007; Kaneko and Hall 2000). The s-LNvs express the neuropeptide pigment-dispersing factor (PDF), the genetic loss of which causes a weakening or loss of free-running behavioral rhythms (Helfrich-Förster 1995; Renn et al. 1999; Shafer and Taghert 2009) and a loss of synchronization among various clock neuron classes (Lin et al. 2004). PDF signals through PDFR, a G-protein—coupled receptor (GPCR) that signals through cAMP increases (Hyun et al. 2005; Lear et al. 2005; Mertens et al. 2005) and is expressed by dorsal clock neurons (Im and Taghert 2010). Finally, the dorsal neuron classes respond to bath-applied PDF peptide with cAMP increases (Shafer et al. 2008). Taken together, these findings provide strong evidence for a neuromodulatory connection between the s-LNvs and dorsal clock neurons in the adult fly brain. Thus, the current prevailing model predicts that the excitation of the s-LNvs will result in acute cAMP increases within dorsal clock neurons.
Nevertheless, the physiologic nature of this proposed connection has not been confirmed experimentally. Indeed, recent work has shown that the s-LNvs also express short neuropeptide F (sNPF) (Johard et al. 2009), which encodes four peptides whose GPCR would likely antagonize PDFR signaling (Garczynski et al. 2007; Mertens et al. 2002; Reale et al. 2004). The coexpression of potentially antagonistic peptides in the s-LNvs suggests that the excitation of these neurons might in fact cause cAMP decreases in dorsal clock neuron classes. Determining the functional nature of this proposed connection therefore requires the ability to experimentally interrogate its physiology. We therefore set out to determine the nature of the predicted connection between the s-LNv pacemakers and the LNds, which are among the predicted neuronal targets of the s-LNvs (Im and Taghert 2010; Shafer et al. 2008) and are thought to play a critical role in the control of the fly's evening bout of daily activity (Grima et al. 2004; Stoleru et al. 2004).
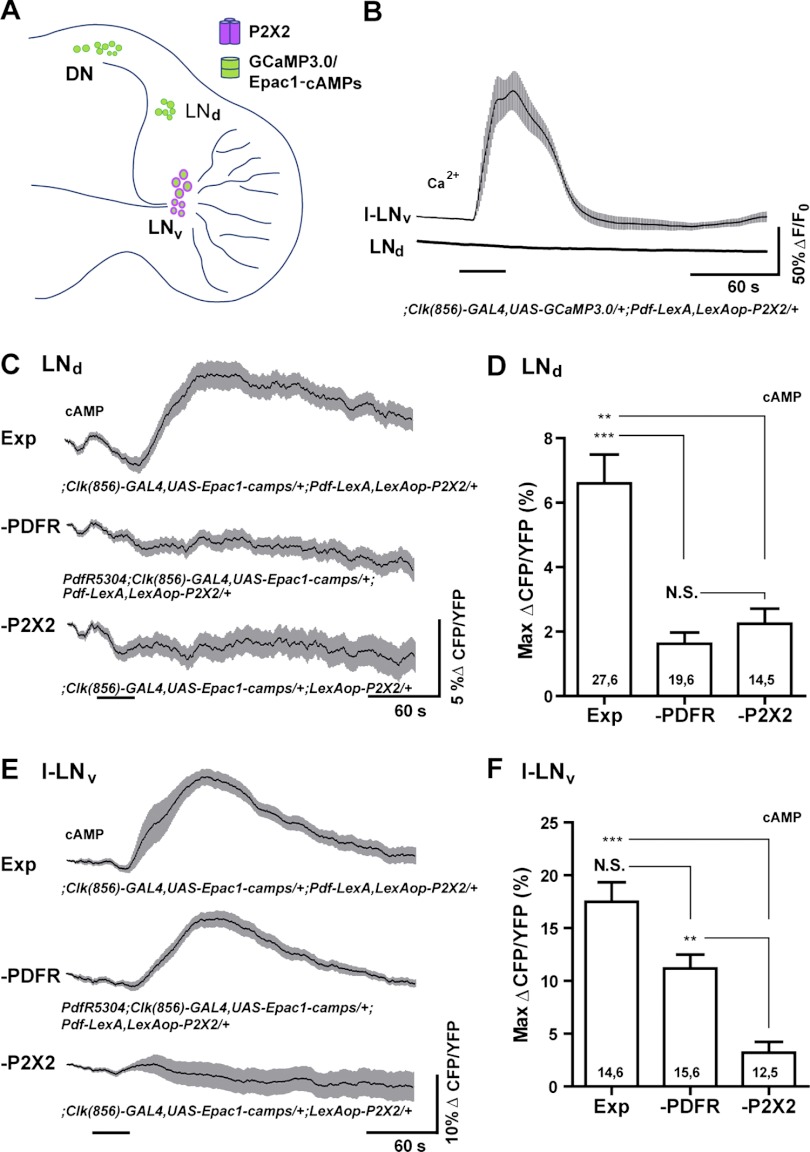
To investigate the proposed connection between the s-LNvs and the LNd clock neurons, we drove P2X2 expression specifically in the l-LNvs and s-LNvs using Pdf-LexA, while driving GCaMP3.0 or Epac1-camps expression with Clock(856)-GAL4, which is expressed throughout most of clock neuron network (Fig. 7A; Gummadova et al. 2009). Note that although Pdf-LexA drives LexAop-P2X2 in both the l-LNvs and s-LNvs, only the s-LNvs send projections to the dorsal brain, whereas the l-LNvs project to both optic lobes (Fig. 7A; Helfrich-Förster et al. 2007). For brains dissected from ;Clock(856)-GAL4,UAS-GCaMP3.0/+;Pdf-LexA,LexAop-P2X2/+ flies, excitation of the l-LNvs and s-LNvs with 30-s perfusions of 1 mM ATP caused clear increases in GCaMP3.0 fluorescence in the LNvs, but had no measurable effects on the LNds residing in the same optical sections, suggesting that LNv excitation does not cause large acute Ca2+ increases or acute excitation in the LNds (Fig. 7B). In contrast, 30-s perfusions of 1 mM ATP across ;Clock(856)-GAL4,UAS-Epac1-camps/+;Pdf-LexA,LexAop-P2X2/+ brains resulted in significant increases in Epac1-camps inverse FRET within the LNds, consistent with cAMP increases in response to LNv excitation (Fig. 7, C and D). Direct ATP/P2X2 excitation of the l-LNvs and s-LNvs caused significant increases in Epac1-camps inverse FRET (Fig. 7, E and F, and data not shown), indicating a strong coupling of neuronal excitation and cAMP production in these neurons. The large increase in LNd inverse Epac1-camps FRET was preceded by a small and transient decrease in inverse FRET (Fig. 7C). However, this decrease was not caused by LNv excitation, because we observed a similar initial decrease in mean inverse FRET in control brains lacking the Pdf-LexA element for driving LexAop-P2X2 expression in the LNvs (Fig. 7C).
Fig. 7.
LexA-based excitation and GAL4-based live imaging to test a predicted peptidergic connection deep within the adult brain. A: schematic diagram showing the expression of P2X2 and genetically encoded sensors in the experimental brain for testing the predicted physiologic connection between the LNv and the LNd clock neurons. B: mean GCaMP3.0 fluorescence traces of the l-LNvs and LNds during their responses to a 30-s bath application of 1 mM ATP (indicated by the bar under the plots). l-LNv and LNd plots were recorded simultaneously from the same optical sections of a ;Clock(856)-GAL4,UAS-GCaMP3.0/+;Pdf-LexA,LexAop-P2X2/+ brain. Shaded regions surrounding the mean plots indicate SE. Excitation of the LNvs had no measurable effects on GCaMP3.0 fluorescence in the LNds. For l-LNvs, N = 14 neurons from 6 brains (14,6). For s-LNvs, N = 17,6. C: mean Epac1-camps inverse FRET traces of the LNds during excitation of the LNvs in ;Clock(856)-GAL4,UAS-Epac1-camps/+;Pdf-LexA,LexAop-P2X2/+ brains (“Exp”). LNv excitation resulted in increases in cAMP in the LNds. This response was absent in PdfR5304;Clock(856)-GAL4,UAS-Epac1-camps/+;Pdf-LexA,LexAop-P2X2/+ brains (“—PDFR”), which lacked PDF receptor function, and in ;Clock(856)-GAL4,UAS-Epac1-camps/+; LexAop-P2X2/+ brains, which lacked a LexA driver for the P2X2 element (“—P2X2”). D: summary histogram of the mean maximum increases in Epac1-camps inverse FRET for the LNd data shown in C. E: mean Epac1-camps inverse FRET traces for l-LNvs imaged simultaneously with the LNds shown in C. Plots displayed as for C. ATP/P2X2 excitation of the LNvs resulted in cAMP increases in both wild type (“Exp”) and PdfR5304 (“—PDF”) backgrounds. The l-LNvs showed no cAMP increases in response to ATP in the absence of a LexA driver for the P2X2 element (“—P2X2”). F: summary histogram of maximum increases in Epac1-camps inverse FRET for the l-LNv data shown in E. For D and F, the two numbers within or above each bar of the histogram indicate the number of neurons and the number of brains examined respectively. ***P < 0.001; **P < 0.01; NS, nonsignificance by Kruskal—Wallis one-way ANOVA and Dunn's multiple comparisons test. The mean plots in C and E were corrected for spontaneous FRET drift by subtracting the mean inverse FRET traces of l-LNv and LNd neurons from vehicle treated ;Clock(856)-GAL4,UAS-Epac1-camps/+;Pdf-LexA,LexAop-P2X2/+ (“Exp”) brains (see methods for details).
The LNd cAMP response to bath-applied ATP required P2X2 expression in the LNvs, because brains carrying the LexAop-P2X2 element but lacking the Pdf-LexA driver failed to show cAMP increases in either the LNds or LNvs (Fig. 7, C–F; “—P2X2”). Furthermore, the LNd cAMP response to LNv excitation required functional PDF receptor, because ATP perfusion over brains from PdfR5304;Clock(856-GAL4,UAS-Epac1-camps/+;Pdf-LexA,LexAop-P2X2/+ flies failed to produce significant changes in LNd Epac1-camps inverse FRET levels (Fig. 7, C and D; “—PDFR”), despite clear excitation of LNvs within the same optical sections (Fig. 7, E and F; “—PDFR”).
Our results indicate that the excitation of the l-LNvs and s-LNvs results in acute cAMP increases in the LNds and that this response requires functional PDF receptor signaling (Fig. 7, C and D). Thus, our method of connectivity analysis was sufficiently sensitive to experimentally confirm a predicted modulatory connection deep within the adult brain. Given the thorough vetting of GCaMP3.0 and Epac1-camps sensors in the fly CNS by previous studies (e.g., Shafer et al. 2008; Tian et al. 2009), the lack of GCaMP3.0 responses in the face of clear Epac1-camps responses in the LNds following LNv excitation suggests that the LNds are not strongly excited by the LNvs and that the LNv-to-LNd connection acts predominantly as a modulator of LNd cAMP signaling. Thus, the connection between these neuronal classes could not have been detected with Ca2+ imaging alone, which argues for the use of diverse sensor types in the investigation of functional connectivity. The efficacy of this approach to circuit interrogation now makes possible a wider analysis of the patterns of clock network connections, and an investigation of how these connections might change over the course of the circadian cycle or in response to changing environmental conditions such as photoperiod and temperature. Furthermore, these experiments establish the feasibility of conducting such experiments in mutant backgrounds of choice.
DISCUSSION
Animal behavior is an emergent property of neural networks and is shaped by the pattern and nature of the connections between their constituent neurons. Connectivity is therefore an abiding problem in neuroscience, and understanding how it governs complex behavior is a fundamental goal of the field (Lichtman and Sanes 2008). Here we have introduced a method for addressing the physiologic connections between discrete neuronal classes in Drosophila. We have shown that the independent dual binary expression of the vertebrate purinergic P2X2 receptor and genetically encoded sensors makes possible the specific excitation of neuronal classes of interest while simultaneously imaging Ca2+ and cAMP dynamics within putative follower neurons. Our proof of principle experiments establish this “physiogenetic” approach as a technically facile method of investigating physiologic connections between electrophysiologically inaccessible neuronal classes of the Drosophila CNS.
Although the method we introduce here makes possible the detection of neural connections in regions of the brain where multielectrode electrophysiologic experiments are not possible, it is important to note its limitations relative to electrophysiologic techniques. For example, the use of bath-applied ATP to excite P2X2-expressing neurons does not offer the fine temporal control associated with the depolarization of neurons by brief current injections (Fig. 1). Likewise, genetically encoded sensors of neural signaling have not yet attained the sensitivity and temporal resolution of electrodes for detecting small changes in membrane voltage or modest excitatory/inhibitory responses. Thus, connections producing subthreshold excitatory input or only very weak excitation in follower neurons might be missed using the approach we have described. Furthermore, some inhibitory connections may not be detectable using existing genetically encoded sensors (e.g., Lelito and Shafer 2012). Thus, for any pair of neuronal classes, the absence of both cAMP and Ca2+ responses in a putative follower neuron is not in itself compelling evidence for a complete lack of connection. Despite these limitations, the work presented here establishes that our method of addressing functional connectivity is sufficiently sensitive to detect both excitatory and modulatory connections between electrophysiologically inaccessible neuronal classes within the adult fly brain, thereby allowing for the analysis of functional connectivity in regions of the brain where electrodes cannot be used. We therefore believe that this method will be immediately useful for the investigation of connectivity within a variety of electrophysiologically inaccessible networks in the fly brain.
The ultimate cellular resolution afforded by this approach is currently limited by the number of available highly specific LexA and Gal4 drivers for directed P2X2 expression. This is no less true for the widespread use of these same drivers for the experimental manipulation of neuronal function and behavior, a limitation that has not prevented the field from learning a great deal about the neuronal classes underlying a wide range of behaviors (Simpson and Stephen 2009). Nevertheless, the current supply of specific drivers allows for many hypothesized connections between neuronal classes to be experimentally tested using the approach we have described, and the production of highly specific genetic drivers continues apace (e.g., Bohm et al. 2010; Luan and White 2007; Pfeiffer et al. 2008, 2010). Furthermore, in instances when sufficiently specific drivers prove unattainable, increased specificity of ATP/P2X2 excitation can be realized through localized puffing of ATP (Hu et al. 2010; Huang et al. 2010) or through the focal liberation of caged ATP using focused laser light (Z. Yao and O.T. Shafer, unpublished observations).
Although the methods described here allow for connections between discrete neuronal classes to be detected and characterized, they do not currently allow for a differentiation between monosynaptic (direct) and polysynaptic (indirect) connections. This limitation does not preclude the usefulness of the technique, which can nevertheless reveal the presence and physiologic nature of connections between defined neuronal classes, whether monosynaptic or polysynaptic. Furthermore, it may be possible in the future to adapt established pharmacologic methods for determining if a given downstream response to ATP/P2X2 excitation is monosynaptic or polysynaptic. For example, the use of bathing saline containing high concentrations of divalent cations (e.g., Kandel et al. 1967) or tetrodotoxin (e.g., Mizunami 1990) to block the synaptic release from or the firing of interposed neurons could be compatible with this technique if P2X2, a nonselective cation channel, can drive sufficiently high Ca2+ in the presynaptic terminals of P2X2-expressing neurons in the presence of these manipulations. We are currently investigating these possibilities in multiple neuronal types.
Although other methods to detect physiologic connectivity have recently been used in the fly brain (e.g., Hu et al. 2010; Ruta et al. 2010), we feel that the approach outlined here has the virtue of a relative technical simplicity, requiring only standard confocal or epifluorescent microscopy and a means of delivering controlled perfusions of ATP solutions. Thus, the LexAop-driven P2X2, GCaMP3.0, and Epac1-camps elements we describe here, in combination with the large number of available Gal4, UAS, and LexA elements, constitute a flexible and technically facile toolkit for the interrogation of central neuronal networks in the fly. These tools can now be used to address functional connectivity within neuronal networks governing a wide range of behaviors in Drosophila. Furthermore, Drosophila photoreceptors and ligand-gated receptors have been successfully introduced into mammalian neurons (Morita et al. 2006; Zemelman et al. 2002), suggesting that an approach similar to the one described here using appropriate heterologous receptors could be used to investigate the physiologic connections between neuronal ensembles within other model systems.
GRANTS
This work was supported by an National Institutes of Health Pathway to Independence Award (National Institute of Neurological Disorders and Stroke Grant R00-NS-62953) to O. T. Shafer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Z.Y., A.M.M., K.R.L., T.M., and O.T.S. conception and design of research; Z.Y., A.M.M., K.R.L., and T.M. performed experiments; Z.Y., A.M.M., K.R.L., and T.M. analyzed data; Z.Y., A.M.M., K.R.L., T.M., and O.T.S. interpreted results of experiments; Z.Y., A.M.M., and K.R.L. prepared figures; Z.Y., A.M.M., K.R.L., and O.T.S. drafted manuscript; O.T.S. edited and revised manuscript; O.T.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Gero Miesenböck, Paul Taghert, Vivek Jayaraman, Michael Rosbash, Nick Glossop, Paul Hardin, Patrick Emery, and the Bloomington Drosophila Stock Center for providing fly stocks used in this study; Bing Yi and Jerry Rubin for sharing LexA and LexAop plasmids; and Robert Denver and John Kuwada for helpful comments on the manuscript.
REFERENCES
- Bohm RA, Welch WP, Goodnight LK, Cox LW, Henry LG, Gunter TC, Bao H, Zhang B. A genetic mosaic approach for neural circuit mapping in Drosophila. Proc Natl Acad Sci USA 107: 16378–16383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börner S, Schwede F, Schlipp A, Berisha F, Calebiro D, Lohse MJ, Nikolaev VO. FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells. Nat Protocols 6: 427–438, 2011 [DOI] [PubMed] [Google Scholar]
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci 28: 6493–6501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Sehgal A. Genetic analysis of sleep. Genes Dev 24: 1220–1235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science 322: 904–909, 2008 [DOI] [PubMed] [Google Scholar]
- Garczynski SF, Crim JW, Brown MR. Characterization and expression of the short neuropeptide F receptor in the African malaria mosquito, Anopheles gambiae. Peptides 28: 109–118, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431: 869–873, 2004 [DOI] [PubMed] [Google Scholar]
- Gummadova JO, Coutts GA, Glossop NRJ. Analysis of the Drosophila clock promoter reveals heterogeneity in expression between subgroups of central oscillator cells and identifies a novel enhancer region. J Biol Rhythms 24: 353–367, 2009 [DOI] [PubMed] [Google Scholar]
- Guo ZV, Hart AC, Ramanathan S. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat Methods 6: 891–896, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci USA 92: 612–616, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, Meinertzhagen IA, Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci 22: 9255–9266, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C, Shafer OT, Wulbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol 500: 47–70, 2007 [DOI] [PubMed] [Google Scholar]
- Hewes RS, Schaefer AM, Taghert PH. The cryptocephal gene (ATF4) encodes multiple basic-leucine zipper proteins controlling molting and metamorphosis in Drosophila. Genetics 155: 1711–1723, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu A, Zhang W, Wang Z. Functional feedback from mushroom bodies to antennal lobes in the Drosophila olfactory pathway. Proc Natl Acad Sci USA 107: 10262–10267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang W, Qiao W, Hu A, Wang Z. Functional connectivity and selective odor responses of excitatory local interneurons in Drosophila antennal lobe. Neuron 67: 1021–1033, 2010 [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, Bae E, Kim J. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron 48: 267–278, 2005 [DOI] [PubMed] [Google Scholar]
- Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J Comp Neurol 518: 1925–1945, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johard HAD, Yoishii T, Dircksen H, Cusumano P, Rouyer F, Helfrich-Förster C, Nässel DR. Peptidergic clock neurons in Drosophila: ion transport peptide and short neuropeptide F in subsets of dorsal and ventral lateral neurons. J Comp Neurol 516: 59–73, 2009 [DOI] [PubMed] [Google Scholar]
- Kandel ER. Cellular Basis of Behavior: An Introduction to Behavioral Neurobiology. San Francisco: Freeman, 1976, p. 727 [Google Scholar]
- Kandel ER, Frazier WT, Waziri R, Coggeshall RE. Direct and common connections among identified neurons in Aplysia. J Neurophysiol 30: 1352–1376, 1967 [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 422: 66–94, 2000 [DOI] [PubMed] [Google Scholar]
- Keene AC, Mazzoni EO, Zhen J, Younger MA, Yamaguchi S, Blau J, Desplan C, Sprecher SG. Distinct visual pathways mediate Drosophila larval light avoidance and circadian clock entrainment. J Neurosci 31: 6527–6534, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431: 316–320, 2004 [DOI] [PubMed] [Google Scholar]
- Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci 9: 703–709, 2006 [DOI] [PubMed] [Google Scholar]
- Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron 48: 221–227, 2005 [DOI] [PubMed] [Google Scholar]
- Lelito KR, Shafer OT., 3rd Reciprocal cholinergic and GABAergic modulation of the small ventrolateral pacemaker neurons of Drosophila's circadian clock neuron network. J Neurophysiol 107: 2096–2108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JW, Sanes JR. Ome sweet ome: what can the genome tell us about the connectome? Curr Opin Neurobiol 18: 346–353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121: 141–152, 2005 [DOI] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci 24: 7951–7957, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron 26: 35–43, 2000 [DOI] [PubMed] [Google Scholar]
- Luan H, White BH. Combinatorial methods for refined neuronal gene targeting. Curr Opin Neurobiol 17: 572–580, 2007 [DOI] [PubMed] [Google Scholar]
- Malpel S, Klarsfeld A, Rouyer F. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development 129: 1443–1453, 2002 [DOI] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49: 285–295, 2006 [DOI] [PubMed] [Google Scholar]
- McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol 76: 328–347, 2005 [DOI] [PubMed] [Google Scholar]
- Melcher C, Bader R, Pankratz MJ. Amino acids, taste circuits, and feeding behavior in Drosophila: towards understanding the psychology of feeding in flies and man. J Endocrinol 192: 467–472, 2007 [DOI] [PubMed] [Google Scholar]
- Mertens I, Meeusen T, Huybrechts R, De Loof A, Schoofs L. Characterization of the short neuropeptide F receptor from Drosophila melanogaster. Biochem Biophys Res Commun 297: 1140–1148, 2002 [DOI] [PubMed] [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 48: 213–219, 2005 [DOI] [PubMed] [Google Scholar]
- Mizunami M. Nonlinear signal transmission between second- and third-order neurons of cockroach ocelli. J Gen Physiol 95: 297–317, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Susuki J, Amino H, Yoshiki F, Moizumi S, Kudo Y. Use of the exogenous Drosophila octopamine receptor gene to study Gq-coupled receptor-mediated responses in mammalian neurons. Neuroscience 137: 545–553, 2006 [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279: 37215–37218, 2004 [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol 18: R84—37215–R93, 2008 [DOI] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJL, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60: 672–682, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody NC, Pohl JB, Diao F, Vreede AP, Sandstrom DJ, Wang H, Zelensky PK, White BH. Characterization of the decision network for wing expansion in Drosophila using targeted expression of the TRPM8 channel. J Neurosci 29: 3343–3353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TTB, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, Mungall C, Svirskas R, Kadonaga JT, Doe CQ, Eisen MB, Celniker SE, Rubin GM. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci USA 105: 9715–9720, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TTB, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics 186: 735–755, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichaud F, Desplan C. A new visualization approach for identifying mutations that affect differentiation and organization of the Drosophila ommatidia. Development 128: 815–826, 2001 [DOI] [PubMed] [Google Scholar]
- Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell 141: 536–548, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol 101: 3075–3088, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale V, Chatwin HM, Evans PD. The activation of G-protein gated inwardly rectifying K+ channels by a cloned Drosophila melanogaster neuropeptide F-like receptor. Eur J Neurosci 19: 570–576, 2004 [DOI] [PubMed] [Google Scholar]
- Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A PDF neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99: 791–802, 1999 [DOI] [PubMed] [Google Scholar]
- Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 468: 686–690, 2010 [DOI] [PubMed] [Google Scholar]
- Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Gene Expr Patterns 1: 73–82, 2001 [DOI] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Vl̂ler T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 16: 1741–1747, 2006 [DOI] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 58: 223–237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Taghert PH. RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: the anatomical basis of a neuropeptide's circadian functions. PLoS ONE 4: e8298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Griffith L, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci USA 105: 19587–19594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol 18: 1537–1545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JH, Stephen FG. Mapping and manipulating neural circuits in the fly brain. In: Advances in Genetics. Waltham, MA: Elsevier/Academic Press, 2009, p. 79–143 [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431: 862–868, 2004 [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 6: 875–881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJT, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron 72: 202–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A, Hall JC, Jeffrey CH. Neurogenetics of courtship and mating in Drosophila. In: Advances in Genetics. Waltham, MA: Elsevier/Academic Press, 2008, p. 67–184 [DOI] [PubMed] [Google Scholar]
- Wegener C, Hamasaka Y, Nässel DR. Acetylcholine increases intracellular Ca2+ via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila. J Neurophysiol 91: 912–923, 2004 [DOI] [PubMed] [Google Scholar]
- Weiner J. Time, Love, Memory. New York: Vintage Books, 1999 [Google Scholar]
- Willemse M, Janssen E, Lange FD, Wieringa B, Fransen J. ATP and FRET: a cautionary note. Nat Biotech 25: 170–172, 2007 [DOI] [PubMed] [Google Scholar]
- Willows AOD, Hoyle G. Neuronal network triggering a fixed action pattern. Science 166: 1549–1551, 1969 [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Salvaterra PM. Localization of choline acetyltransferase-expressing neurons in Drosophila nervous system. Microsc Res Tech 45: 65–79, 1999 [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron 71: 9–34, 2011 [DOI] [PubMed] [Google Scholar]
- Yuan Q, Xiang Y, Yan Z, Han C, Jan LY, Jan YN. Light-induced structural and functional plasticity in Drosophila larval visual system. Science 333: 1458–1462, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Ng M, Miesenböck G. Selective photostimulation of genetically charged neurons. Neuron 33: 15–22, 2002 [DOI] [PubMed] [Google Scholar]
- Zhang L, Chung BY, Lear BC, Kilman VL, Liu Y, Mahesh G, Meissner RA, Hardin PE, Allada R. DN1p circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr Biol 20: 591–599, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr Biol 20: 600–605, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, Campbell RE. An expanded palette of genetically encoded Ca2+ indicators. Science 333: 1888–1891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G, Wang Lp Vaughan AG, Manoli DS, Zhang F, Deisseroth K, Baker BS, Scott MP. Manipulation of an innate escape response in Drosophila: photoexcitation of acj6 neurons induces the escape response. PLoS ONE 4: e5100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]