Abstract
The activity of supragranular pyramidal neurons in the dorsolateral prefrontal cortex (DLPFC) neurons is hypothesized to be a key contributor to the cellular basis of working memory in primates. Therefore, the intrinsic membrane properties, a crucial determinant of a neuron's functional properties, are important for the role of DLPFC pyramidal neurons in working memory. The present study aimed to investigate the biophysical properties of pyramidal cells in layer 2/3 of monkey DLPFC to create an unbiased electrophysiological classification of these cells. Whole cell voltage recordings in the slice preparation were performed in 77 pyramidal cells, and 24 electrophysiological measures of their passive and active intrinsic membrane properties were analyzed. Based on the results of cluster analysis of 16 independent electrophysiological variables, 4 distinct electrophysiological classes of monkey pyramidal cells were determined. Two classes contain regular-spiking neurons with low and high excitability and constitute 52% of the pyramidal cells sampled. These subclasses of regular-spiking neurons mostly differ in their input resistance, minimum current that evoked firing, and current-to-frequency transduction properties. A third class of pyramidal cells includes low-threshold spiking cells (17%), which fire a burst of three-five spikes followed by regular firing at all suprathreshold current intensities. The last class consists of cells with an intermediate firing pattern (31%). These cells have two modes of firing response, regular spiking and bursting discharge, depending on the strength of stimulation and resting membrane potential. Our results show that diversity in the functional properties of DLPFC pyramidal cells may contribute to heterogeneous modes of information processing during working memory and other cognitive operations that engage the activity of cortical circuits in the superficial layers of the DLPFC.
Keywords: firing pattern, intrinsic membrane properties, low-threshold spiking, regular spiking
the prefrontal cortex (PFC), one of the largest and most differentiated neocortical areas of the primate brain, mediates complex behaviors that are characteristic of primates in general and of humans in particular (Wood and Grafman 2003). Working memory, the ability to hold information in mind for a short period of time, is a critical cognitive process underlying complex behaviors in primates (Baddeley 2003; Fuster 1997; Goldman-Rakic 1995). In vivo, cells in the dorsolateral PFC (DLPFC) frequently remain active during the delay period of a delayed-response trial, and thus DLPFC neuron activity is considered the cellular correlate of mnemonic events in the working memory process (Goldman-Rakic 1995; Miller et al. 1996). Although the delay period activity has been reported in many other cortical and subcortical areas (Curtis and Lee 2010; Hikosaka et al. 2000; Watanabe and Funahashi 2004), DLPFC cell firing during the delay period is more resistant to distractors than in other regions (Artchakov et al. 2009; Miller et al. 1996).
The exact mechanisms underlying sustained delay-related activity of DLPFC neurons are still largely unclear. The specific organization of neuronal circuitry in this brain area, especially in layer 2/3 (Gonzalez-Burgos et al. 2000; Kritzer and Goldman-Rakic 1995; Melchitzky et al. 1998, 2001; Pucak et al. 1996; Selemon and Goldman-Rakic 1988), and distinctive morphological and electrophysiological properties of these neurons may contribute to the unique features of delay-related firing in PFC. For example, anatomical observations of pyramidal cells in human and macaque monkey PFC revealed a higher number of dendritic branches and higher spine density than their counterparts in other cortical regions (Elston 2003; Elston et al. 2001; Jacobs et al. 2001). Such increased dendritic complexity and spine density of PFC neurons may reflect the integration of a large number of incoming inputs of diverse origin. Furthermore, it may also indicate a specialization of DLPFC neurons in recurrent excitation, a proposed mechanism for generation of persistent delay-related activity (Wang 2001).
The intrinsic firing properties of pyramidal neurons may be a crucial determinant of in vivo firing and behavioral performance. For instance, age-related changes of intrinsic membrane properties of monkey DLPFC neurons correlate with age-related changes in working memory performance (Chang et al. 2005; Luebke et al. 2010; Luebke and Amatrudo 2010) and thus could explain the age-related changes in working memory task-related firing of monkey DLPFC neurons in vivo (Wang et al. 2011). Despite the importance of intrinsic membrane properties of primate DLPFC pyramidal neurons, only a few studies have reported detailed data on the electrophysiological properties of primate DLPFC pyramidal cells, focusing mostly on layer 5 pyramidal neurons (Chang and Luebke 2007). We have performed multiple studies of neurons, synapses, and small circuits in layer 3 of monkey DLPFC, addressing mostly properties of GABA neurons (Gonzalez-Burgos et al. 2004, 2005a, 2009; Krimer et al. 2005; Povysheva et al. 2007, 2008; Zaitsev et al. 2005, 2009). Moreover, our studies of layer 3 pyramidal neurons from monkey DLPFC were focused on synaptic properties (Gonzalez-Burgos et al. 2004, 2008; Povysheva et al. 2006) and neuromodulation (Gonzalez-Burgos et al. 2005b; Henze et al. 2000; Urban et al. 2002), but no detailed description of the electrophysiological subclasses of layer 3 pyramidal cells was obtained.
Current classifications of pyramidal cells are based on the cells' firing patterns as well as the shape of their action potentials (Connors and Gutnick 1990; de la Pena and Geijo-Barrientos 1996; Degenetais et al. 2002; Foehring et al. 1991; Larkman and Mason 1990; Maravall et al. 2004; Yang et al. 1996), whereas the subthreshold membrane properties are often overlooked (but see Kawaguchi 1993; Schwindt et al. 1997). Recently, we have shown that multivariate statistical analysis of a large neuronal data set is a powerful classification tool that allowed us to reliably define multiple subclasses of GABA neurons in layer 3 of monkey PFC (Krimer et al. 2005; Zaitsev et al. 2005, 2009). In the present study we employed cluster analysis for unbiased delineation of the physiological groups of pyramidal cells in layer 2/3 of monkey DLPFC. Sixteen electrophysiological measures of subthreshold, single spike, and firing pattern properties have been chosen for this multivariate statistical analysis. We describe four distinct electrophysiological classes of pyramidal cells in DLPFC, including two classes of regular spiking (RS) cells with different input resistance, low-threshold spiking (LTS) cells, and a class of cells with intermediate properties.
MATERIALS AND METHODS
Slice Preparation
Twelve experimentally naive young adult (3.5–6 kg; 3.5–4-years old), male, long-tailed macaque monkeys (Macaca fascicularis) were used in this study. All studies were conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. The procedure used to obtain tissue from the DLPFC has been previously described in detail (Gonzalez-Burgos et al. 2004). Briefly, animals were treated with ketamine hydrochloride (25 mg/kg im), dexamethasone phosphate (0.5 mg/kg im), and atropine sulfate (0.05 mg/kg sc); an endotracheal tube was inserted, and anesthesia was maintained with 1% halothane in a 28% O2-air mixture. Monkeys were placed in a stereotaxic apparatus, and a craniotomy was performed over the DLPFC in one hemisphere. The dura was removed in a location determined by stereotaxic coordinates and by the position of relevant sulcal landmarks, and a small block of tissue was excised containing both the medial and lateral banks of the principal sulcus (area 46) as well as a small adjacent portion of dorsal area 9. After the surgery, the animals were treated with an antibiotic (chloramphenicol, 15 mg/kg im) and an analgesic (hydromorphone, 0.02 mg/kg im) three times a day for 3 days. All animals recovered quickly with no impairments in eating or drinking and no overt behavioral deficits. In most cases, the animals underwent the same procedure 2–4 wk later to obtain tissue from a nonhomotopic portion of the contralateral areas 46 and 9. During the second procedure, after the craniotomy, the animal was given an overdose of pentobarbital sodium (30 mg/kg) and perfused through the heart with ice-cold modified artificial cerebrospinal fluid before the tissue block was quickly excised. Subsequent treatment of the tissue was the same for both procedures. Previous studies have demonstrated that the initial biopsy did not affect the properties of neurons obtained in the contralateral hemisphere (Gonzalez-Burgos et al. 2000, 2004; Henze et al. 2000).
The tissue blocks were placed in ice-cold Ringer solution containing (in mM) 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgSO4, 26 NaHCO3, and 10 dextrose, pH 7.4, perfused with a 95%O2-5%CO2 gas mixture. Coronal 350-μm-thick slices were cut from each block using a Vibratome (VT 1000S Leica, Wetzlar, Germany) and incubated for 1 h at 36°C and at room temperature thereafter, or at room temperature from the beginning. For recordings, slices were submerged in a chamber mounted on the microscope and perfused with Ringer solution at 32°C. Other brain slices from these animals were used and reported in studies of interneurons (Povysheva et al. 2006, 2008; Zaitsev et al. 2005, 2009).
Electrophysiological Recordings
Pyramidal cells in layer 2/3 were visualized using infrared differential interference contrast (DIC) videomicroscopy and identified on the basis of their triangular soma and the presence of an apical dendrite. Patch electrodes with open-tip resistances of 5–10 MΩ were filled with a solution containing (in mM) 114 potassium gluconate, 6 KCl, 4 MgATP, 0.3 GTP, 10 HEPES, 0.5% biocytin, and pH 7.25 adjusted with KOH. Whole cell current-clamp recordings were performed after seal resistance of at least 1.5–2 GΩ was reached. Voltages were amplified using Intracellular Electrometers IE-210 (Warner Instrument, Hamden, CT) or MultiClamp 700A (Axon Instruments, Union City, CA) operating in a bridge-balance mode, filtered online at 4–5 kHz, and acquired on a personal computer at a sampling rate of 20 kHz using Power 1401 interface and Signal 2 or Signal 3 software (CED, Cambridge, UK). Access resistance typically was 15–30 MΩ and remained relatively stable during experiments (≤30% increase) for the cells included in the analysis. Membrane potential was not corrected for the liquid junction potential. To characterize the intrinsic membrane properties of neurons, hyper- and depolarizing current steps of 500-ms duration were applied in 5- to 10-pA increments at 0.2 Hz with two repetitions. Recordings of intrinsic membrane properties were done immediately after membrane rupture and lasted less than 5 min. All included cells had resting potentials more negative than −50 mV and overshooting spikes.
Electrophysiological Analysis
A number of electrophysiological parameters were measured. Resting membrane potential (RMP; in mV) was determined as the stable membrane potential (no holding current applied) reached a few minutes after breaking the membrane.
Subthreshold membrane properties.
Input resistance (Rin; in MΩ), is the slope of the regression line fitted to the voltage-current (V-I) curve (usually between −50 and −10 pA) as measured at the end of the 500-ms voltage responses. The membrane time constant τ (in ms) was determined from the monoexponential curve best fitting the average voltage response to the small hyperpolarizing current steps. Rheobase (I0; in pA) is the minimum current that evoked firing. Sag (in %) was measured during a 500-ms hyperpolarizing current step (50 pA) as the percent change between the most negative membrane potential (Vhmax) and steady-state membrane potential at the end of the step (Vhss): (Vhmax − Vhss)/Vhss × 100%. Rebound depolarization (RD; in %) was measured after a 500-ms hyperpolarizing current step (50 pA) as a maximal positive voltage deflection above RMP (Vrdmax) and normalized to the amplitude of steady-state voltage deflection during that hyperpolarizing current step: Vrdmax/Vhss × 100%. Hump (in %) was estimated at the depolarizing current step that preceded spiking as the percent change between the maximal (Vdmax) and steady-state membrane potential: (Vdmax − Vdss)/Vdss × 100%.
Action potential properties.
Action potential (AP) properties were analyzed at I0. Time to first spike (TFS; in ms) is the time from the beginning of stimulation to the first spike. Action potential threshold (APT; in mV) is the membrane potential at the point at which the interpolated rate of voltage rise (dV/dt) reached >10 mV/ms. Action potential amplitude (APA; in mV) was measured from the threshold to the peak. Action potential duration (APD; in ms) is the spike width at its half-amplitude. Amplitude of the afterhyperpolarization (AHP; in mV) was measured from the APT to the most negative membrane potential after the spike. Amplitude of the fast component of the AHP (fAHP; in mV) was measured from the APT to the hyperpolarization peak or, if present, to the onset of the medium component of the AHP (mAHP). The latter was determined as the crossing point of a marked slowing in the voltage drop to <5 mV/ms. The depolarizing afterpotential (ADP; in mV) was measured as the positive voltage deflection between fast and medium AHPs. Amplitude of the mAHP (in mV) was measured from the peak ADP to the peak AHP. If ADP was absent, mAHP was measured from the end of the fAHP. The time interval tAHP (in ms) was measured as the interval between onset of AHP and the hyperpolarization peak.
Firing pattern properties.
To determine firing pattern properties, two times the I0 was applied, except for the interspike interval between the first and second spikes at minimal suprathreshold current (ISI1–2; in ms). Steady-state frequency (fss; in Hz), the reciprocal of the average of the four to nine interspike intervals (ISIs), was measured within the last 250 ms of the response to depolarizing current pulses, where firing frequency remained relatively stable. Late frequency adaptation (FFAL; in %), is the percent decrease in the frequency from onset (reciprocal to the 1st ISI) to steady-state frequency. Initial frequency adaptation (FFAI; in %) is the percent decrease in frequency, measured as the reciprocal of the first and second ISIs.
Frequency-current relationship properties.y
Frequency-current relationship (f-I) properties were calculated for the first and second instantaneous firing frequency (1/ISI). fn_k (in Hz/pA) is the slope of linear trend for the instantaneous firing frequency n (1/ISIn) vs. current (for n = 1, 2). fn_threshold (in Hz) is the intercept of the extrapolated f-I linear fit with the frequency axis (for n = 1, 2).
Histological Processing and Morphological Analysis
After recordings were made, slices were immersed in 4% paraformaldehyde in 0.1 M phosphate buffer for 24–72 h at 4°C and then cryoprotected (33% glycerol and 33% ethylene glycol in 0.1 M phosphate buffer) and stored at −80°C. To visualize biocytin, about one-half of the slices were incubated with streptavidin-Alexa Fluor 633 conjugate (Invitrogen; dilution 1:500) for 24–48 h at 4°C in phosphate buffer containing 0.4% Triton X-100. Pyramidal cells were imaged using an Olympus Fluoview 500 confocal laser scanning microscope equipped with a ×20/0.80 N.A. oil-immersion objective. The remaining slices were serially resectioned at 40–50 μm, and then the sections were treated with 1% H2O2 for 2–3 h at room temperature, rinsed, and incubated with the avidin-biotin-peroxidase complex (1:100; Vector Laboratories, Burlingame, CA) in phosphate buffer for 4 h at room temperature. Sections were rinsed, stained with 3,3′-diaminobenzidine, mounted on gelatin-coated glass slides, dehydrated, and coverslipped. Some of these pyramidal neurons were reconstructed using the Neurolucida tracing system (MicroBrightField, Williston, VT).
Statistical Analysis
All statistical tests were performed using Statistica 6.1 software (StatSoft, Tulsa, OK). Unless otherwise stated, all data are means and standard error of measurement. To divide pyramidal cells into groups based on electrophysiological properties, the cluster analysis was employed, following Ward's hierarchical clustering algorithm with Euclidean distance, which reduced cluster size by consecutively merging data points based on the least possible increase in the within-group sum of squared deviation (Johnson and Wichern 1998; Ward 1963). Before the cluster analysis was performed, all variables were normalized to their z scores. The statistical significance between group means was tested using ANOVA followed by Fisher's least significant difference (LDS) post hoc tests (multiple comparison tests).
RESULTS
Classification Based on Subthreshold Responses, AP, and Firing Pattern Properties
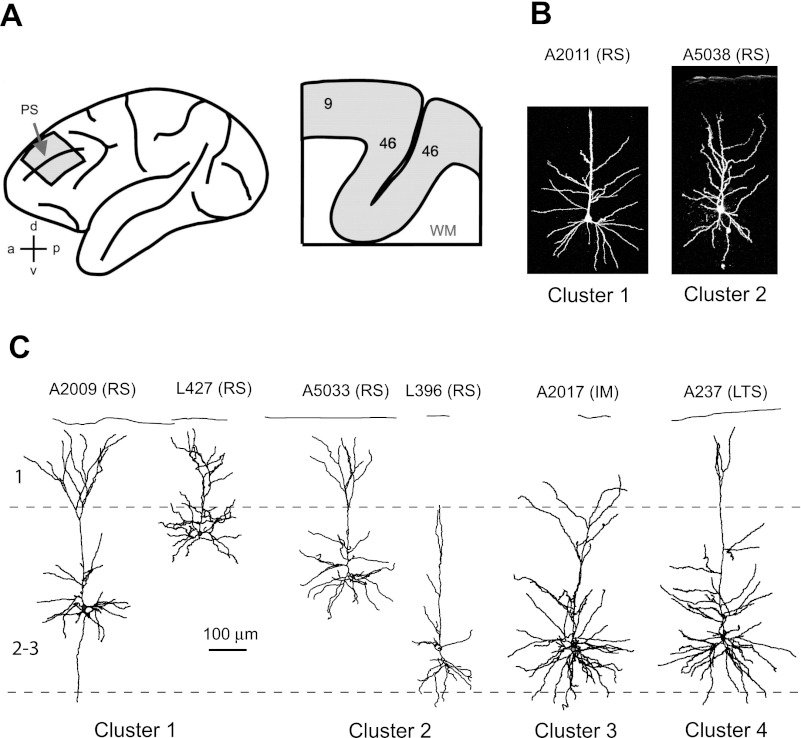
Seventy-seven pyramidal cells from 12 monkeys were included in this study (4–12 neurons per animal). Neurons were identified as pyramidal cells based on biocytin labeling following electrophysiological recording; in each case, these cells had clear apical and basal dendrites that were densely covered with spines. The somata of cells were located across the depth of layer 2/3 (between 300 and 800 μm from the pial surface). Representative examples of reconstructed layer 2/3 pyramidal cells are shown in Fig. 1.
Fig. 1.
Morphological properties of monkey dorsolateral prefrontal cortex (DLPFC) pyramidal cells. A: lateral view (left) of the macaque monkey cortex showing the approximate location of the DLPFC tissue blocks removed during surgery (see materials and methods) and schematic view (right) of a typical coronal slice containing portions of DLPFC areas 9 and 46, including the medial and lateral banks of the principal sulcus (PS). Representative examples are confocal (B) and Neurolucida-reconstructed layer 2/3 pyramidal cells (C). Labels above cells indicate the experimental identification number of cells and, in parentheses, their electrophysiological type based on subjective classification: RS, regular spiking; IM, intermediate; LTS, low-threshold spiking. Cluster numbers are based on the cluster solutions obtained with Ward's clustering method (see text for details). Note that although the majority of layer 2/3 pyramidal cell apical tufts reach the pial surface and begin to branch exuberantly at the layer 2/1 junction, some pyramidal cells of layer 2/3 resemble slender pyramidal cells from layer 5. Scale is the same for B and C.
According to the current qualitative electrophysiological classifications, pyramidal cells can be grouped into cells that fire bursts of multiple spikes [intrinsic bursting neurons, also termed LTS cells] and RS cells that fire trains of single spikes during current pulse injection; RS cells are a predominant electrophysiological type of pyramidal neurons in layer 2/3 (Chang and Luebke 2007; de la Pena and Geijo-Barrientos 1996; Degenetais et al. 2002; Foehring et al. 1991; Hattox and Nelson 2007; Tasker et al. 1996; Yang et al. 1996). Different subclasses of RS pyramidal cells are described based on somewhat arbitrary criteria such as the degree of changes in AP threshold and spike frequency adaptation across a sustained train of APs (Agmon and Connors 1992; Chang and Luebke 2007; Degenetais et al. 2002; Nunez et al. 1993); however, the tentative correspondence between groups identified in previous studies is not always obvious (Degenetais et al. 2002).
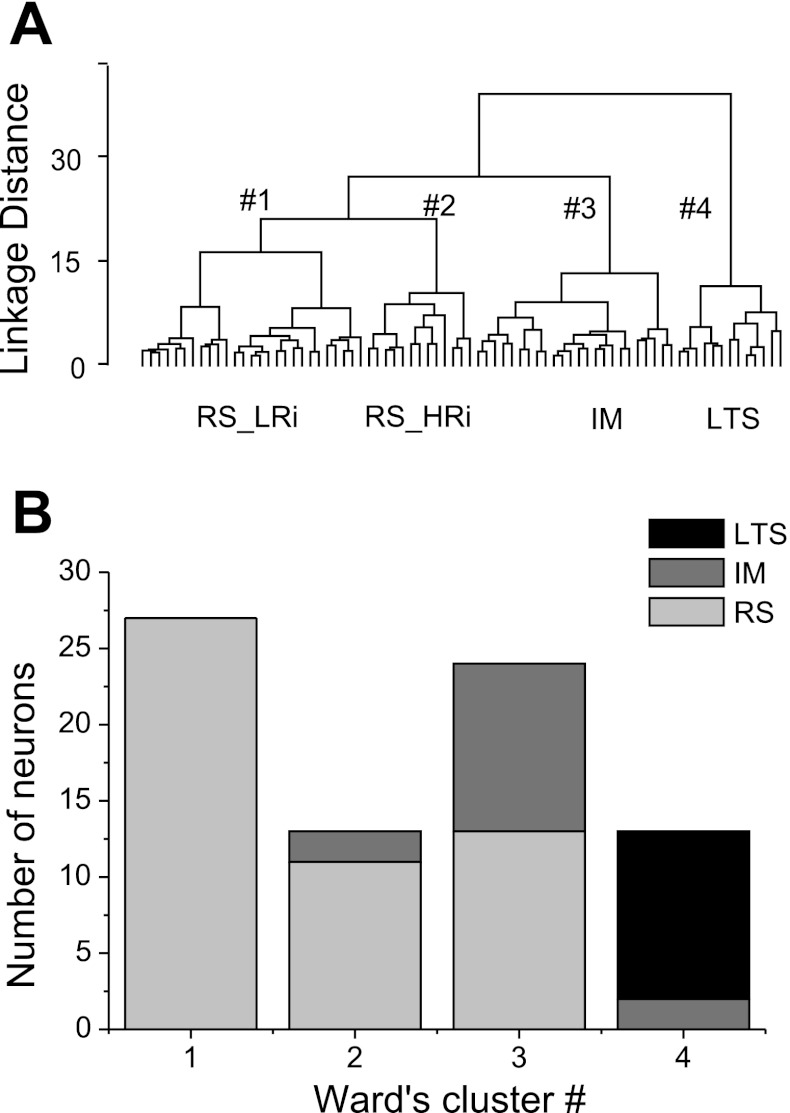
In our view, a reliable electrophysiological classification of pyramidal cells should be based on the most functionally relevant aspects of a neuron's electrophysiological properties. Recently, we showed that a multivariate statistical approach to the classification of cortical interneurons is superior to “subjective” analyses, because it permits multiple parameters to be considered in concert, which is very difficult to accomplish with any type of subjective analysis (Krimer et al. 2005; Zaitsev et al. 2005). Thus, to determine which electrophysiological classes of pyramidal neurons could be segregated using a multivariate statistical approach, we performed a cluster analysis. First, we determined which variables should be included. Because a neuron's functions depend on its 1) subthreshold responses, 2) AP, and 3) firing pattern properties, we chose 24 electrophysiological variables in an effort to comprehensively describe these properties (see materials and methods for details). It is important that selected variables are independent and describe different parameters of neuronal behavior. Therefore, we calculated correlation coefficients to determine whether any of the measures were correlated. Weak-to-moderate correlations (r = 0.20–0.40) were found between many variables; however, stronger correlations (r > 0.50) were observed only between a few variables. For example, strong correlations were found between Rin and I0 (r = −0.73), between sag, hump, and RD (r = 0.66–0.86), between f1_k, f2_k, and fss (r = 0.56–0.73), and between f1_threshold and f2_threshold (r = 0.72). Of pairs of strongly correlated parameters, only one was included into cluster analysis. Instead of sag, hump, and RD, we used the derivative parameter equal to the sum of values of sag, hump, and RD. In total, 16 variables were chosen, namely, Rin, τ, APT, APA, APD, AHP, mAHP, ADP, tAHP, fss, FFAL, FFAI, ISI1–2, f1_k, f1_threshold, (sag + hump + RD); the mean of absolute correlation coefficients between the 16 variables was 0.21 ± 0.01 (P > 0.05). All variables were normalized, and then Ward's hierarchical clustering algorithm with Euclidean distance was used for classification. The obtained hierarchical tree suggested four main electrophysiological classes of pyramidal cells (Fig. 2A).
Fig. 2.
Cluster analysis-based physiological classification of pyramidal neurons in monkey DLPFC. A: the dendrogram obtained through Ward's hierarchical clustering algorithm revealed 4 clusters, demonstrating the presence of distinct physiological groups of pyramidal neurons. RS_LRi, RS cells with low input resistance; RS_HRi, RS cells with high input resistance. B: the number of neurons of each subjectively characterized electrophysiological type in the 4 clusters obtained with Ward's clustering method. Clusters 1 and 2 included mostly RS cells, cluster 3 included IM and RS cells, and cluster 4 consisted of LTS cells.
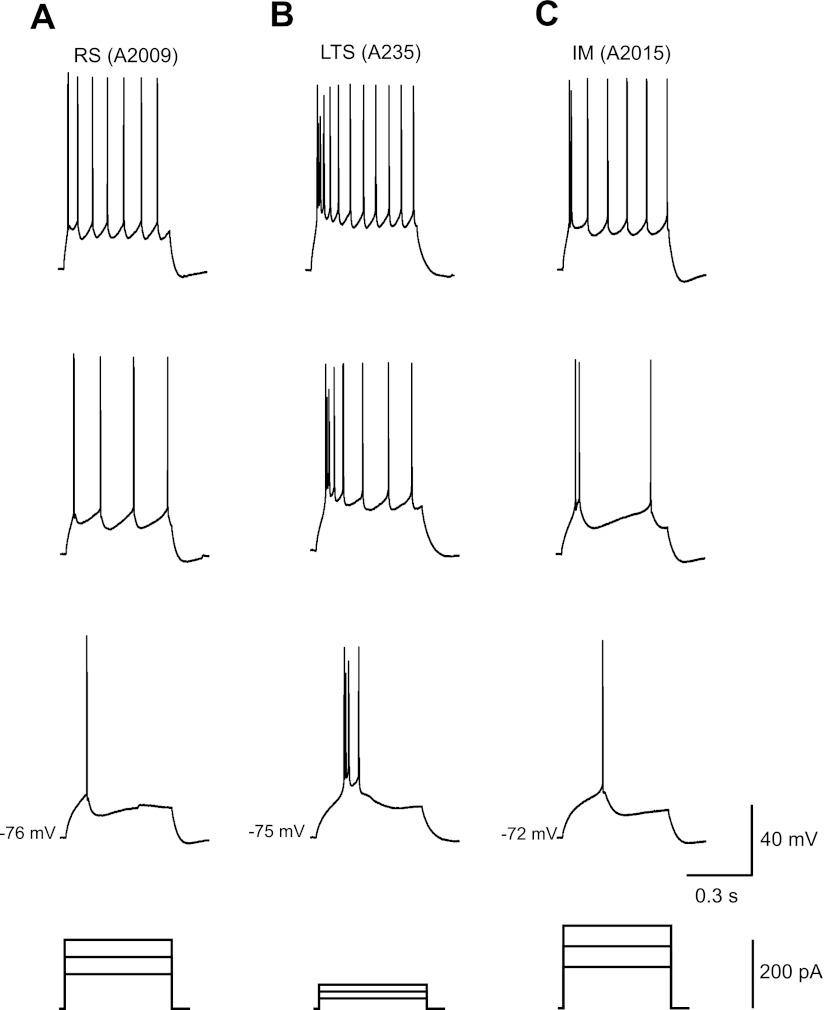
Next, we checked whether the cells in each cluster had firing properties that matched some of the properties proposed in previous studies (Fig. 2B). We found that clusters 1 (n = 27) and 2 (n = 13) included mostly RS cells. These cells generated trains of single spikes with relatively low frequencies and prominent frequency adaptation (Fig. 3A). Both the firing frequency and the frequency adaptation almost invariably increased with an increase in intensity of the stimulating current. Cluster 4 (n = 13) consisted mostly of LTS cells, which discharged with a burst of two to five fast spikes in response to just suprathreshold depolarizing current steps (Fig. 3B). During the initial burst, AP threshold slightly increased and the amplitude slightly decreased. After the initial spike burst, LTS neurons fired single spikes with progressive spike frequency adaptation and stable spike threshold and spike amplitude. Cluster 3 (n = 24) included RS cells (n = 9) and pyramidal cells (n = 15), which exhibited firing properties intermediate (IM) between RS and LTS types (Fig. 3C). The same cell might exhibit a RS pattern at just above firing threshold stimulation but show a pattern more similar to that of LTS cells at an increased level of depolarization current.
Fig. 3.
Representative examples of layer 2/3 pyramidal cell firing pattern from monkey PFC. A–C: subsequent sweeps with spikes beginning with the response to the first suprathreshold current step recorded from the typical RS (cluster 1) (A), LTS (cluster 4) (B), and IM (cluster 3)pyramidal cells (C).
The result of the cluster analysis indicates the degree of similarity among neurons but does not assign statistical significance to the grouping. To determine whether the clusters contained cells with statistically distinct properties, we applied the ANOVA test to all the measured variables. For any pair of clusters, the Fisher's LSD post hoc test was thereafter applied to compare the means (Table 1). ANOVA revealed significant differences between clusters for 21 of 24 electrophysiological variables, confirming the statistical significance of the obtained classification.
Table 1.
Electrophysiological characteristics of pyramidal cells from different clusters
| Characteristics | RS_LRi | RS_HRi | IM | LTS | F Ratio | P Value | Fisher's LSD |
|---|---|---|---|---|---|---|---|
| RMP, mV | −73.2 ± 1.1 | −71.3 ± 1.9 | −71.8 ± 1.0 | −71.7 ± 1.1 | 0.5 | 0.70 | (1,2,3,4) |
| Rin, MΩ | 174 ± 16 | 332 ± 28 | 118 ± 7 | 261 ± 42 | 18.1 | <0.001 | (3); (1); (2,4) |
| τ, ms | 19.6 ± 0.9 | 20.8 ± 2.3 | 17.2 ± 0.7 | 25.3 ± 3.0 | 4.5 | <0.01 | (1,2,3); (2,4) |
| I0, pA | 106 ± 8 | 52 ± 9 | 120 ± 7 | 60 ± 12 | 12.8 | <0.001 | (2,4); (1,3) |
| Sag, % | 9.7 ± 1.2 | 6.6 ± 0.9 | 20.2 ± 2.6 | 13.2 ± 2.0 | 9.1 | <0.001 | (1,2,4); (3) |
| RD, % | 8.9 ± 1.4 | 7.3 ± 0.9 | 19.0 ± 2.0 | 14.8 ± 2.4 | 8.8 | <0.001 | (1,2); (3; 4) |
| Hump, %) | 11.8 ± 1.9 | 8.6 ± 2.3 | 31.2 ± 3.7 | 32.3 ± 4.6 | 14.2 | <0.001 | (1,2); (3; 4) |
| TFS, ms | 136 ± 10 | 150 ± 20 | 117 ± 8 | 126 ± 17 | 1.2 | 0.31 | (1,2,3,4) |
| APT, mV | −40.4 ± 0.9 | −40.0 ± 0.9 | −41.4 ± 0.7 | −44 ± 0.8 | 3.1 | <0.05 | (1,2,3); (3,4) |
| APA, mV | 82 ± 2 | 64 ± 4 | 82 ± 2 | 78 ± 2 | 10.9 | <0.001 | (2); (1,3,4) |
| APD, ms | 0.93 ± 0.02 | 0.88 ± 0.04 | 0.90 ± 0.03 | 1.12 ± 0.11 | 3.3 | <0.05 | (1,2,3); (4) |
| AHP, mV | 11.9 ± 0.8 | 9.6 ± 1.0 | 10.4 ± 0.6 | 6.6 ± 1.0 | 5.9 | <0.01 | (4); (1,2,3) |
| fAHP, mV | 7.2 ± 1.5 | 5.5 ± 1.7 | 5.5 ± 1.1 | 6.0 ± 1.0 | 0.40 | 0.75 | (1,2,3,4) |
| ADP, mV | 0.84 ± 0.21 | 0.52 ± 0.28 | 2.4 ± 0.35 | n/a | 6.7 | <0.001 | (1,2); (3) |
| mAHP, mV | 5.5 ± 0.7 | 4.6 ± 0.9 | 7.3 ± 0.6 | n/a | 10.1 | <0.001 | (1,2); (3) |
| tAHP, ms | 37 ± 4 | 32 ± 6 | 61 ± 6 | 1.3 ± 0.2 | 19.8 | <0.001 | (4); (1,2); (3) |
| f1_k, Hz/pA | 0.42 ± 0.04 | 1.22 ± 0.15 | 0.70 ± 0.06 | 0.49 ± 0.07 | 19.4 | <0.001 | (1,4); (3,4); (2) |
| f1_threshold, Hz | 1.1 ± 1.2 | 1.0 ± 5.4 | 16.8 ± 7.7 | 102 ± 15 | 34.1 | <0.001 | (1,2,3); (4) |
| f2_k, Hz/pA | 0.25 ± 0.03 | 0.92 ± 0.17 | 0.33 ± 0.05 | 0.55 ± 0.07 | 14.6 | <0.001 | (1,3); (3,4); (2) |
| f2_threshold, Hz | 1.67 ± 0.65 | 0.52 ± 2.17 | −1.1 ± 1.1 | 39 ± 10 | 23.5 | <0.001 | (1,2,3); (4) |
| ISI1-2, ms | 215 ± 13 | 165 ± 28 | 186 ± 24 | 27 ± 15 | 13.2 | <0.001 | (4); (1,2,3) |
| FFAI, % | 37 ± 2 | 37 ± 5 | 66 ± 3 | 42 ± 3 | 21.7 | <0.001 | (1,2,4); (3) |
| FFAL, % | 50 ± 2 | 58 ± 5 | 76 ± 2 | 86 ± 2 | 34.4 | <0.001 | (1,2); (3); (4) |
| fss, Hz | 16.3 ± 0.7 | 26.6 ± 2.4 | 17.1 ± 0.9 | 16.5 ± 1.0 | 13.9 | <0.001 | (1,3,4); (2) |
Values are means ± SE of electrophysiological measurements in regular-spiking cells with low (RS_LRi; cluster 1; n = 27) and high input resistance (RS_HRi; cluster 2; n = 13), intermediate-spiking cells (IM; cluster 3; n = 24), and low-threshold-spiking cells (LTS; cluster 4; n = 13). See text for definition of electrophysiological characteristics. Following cluster analysis, Fisher's post hoc least significant difference (LSD) test was applied to compare the means for any pair of clusters 1–4 with statistically distinct properties; cluster numbers grouped within parentheses for a measurement indicate no significant difference between the indicated clusters, whereas different sets of parentheses for each measurement indicate significant differences between cells in each respective group of clusters.
Analyzing the results of the Fisher's LSD post hoc test, we found that cells from cluster 4 had the most distinctive properties because they were different from cells in the other clusters in the maximal number of electrophysiological properties (9 of 24), including almost all single-spike properties and f-I relationship properties. Cluster 3 was different from all other clusters in its subthreshold properties (Rin, sag), different kinetics of afterpotentials, and firing frequency adaptation. Clusters 1 and 2, both consisting of RS cells, differed from each other in Rin, f-I relationship properties, and some other properties (Table 1). On the basis of electrophysiological properties of cells included in each cluster, we named the clusters accordingly: 1) RS cells with low input resistance (RS_LRi), 2) RS cells with high input resistance (RS_HRi), 3) IM cells, and 4) LTS cells. Below we provide a comprehensive comparison of pyramidal cells from each cluster.
Subthreshold Membrane Properties
RMP was the only parameter that did not differ between electrophysiological classes of pyramidal cells (ANOVA, F = 0.5, P = 0.70). The resting potential had a mean value of 72.2 ± 0.6 mV for the total pyramidal cell population following a normal distribution (Shapiro-Wilk's W-test, W = 0.98, P = 0.22; Kolmogorov-Smirnov test, d = 0.08, P > 0.20).
Rin showed remarkable differences between the electrophysiological classes. A Fisher's LSD test revealed that pyramidal cell classes could be subdivided into three distinct groups (Table 1). The lowest Rin was exhibited by IM cells (118 ± 7 MΩ), whereas LTS cells showed relatively large Rin (261 ± 42 MΩ). RS_LRi cells had almost two times smaller Rin than RS_HRi cells (174 ± 16 vs. 332 ± 28 MΩ; Fig. 4). As a consequence, RS_HRi and LTS cells had lower I0 than RS_LRi and IM neurons (Table 1). Membrane time constant τ was maximal in LTS cells, and thus this cell type may be more efficient at summation of excitatory synaptic potentials.
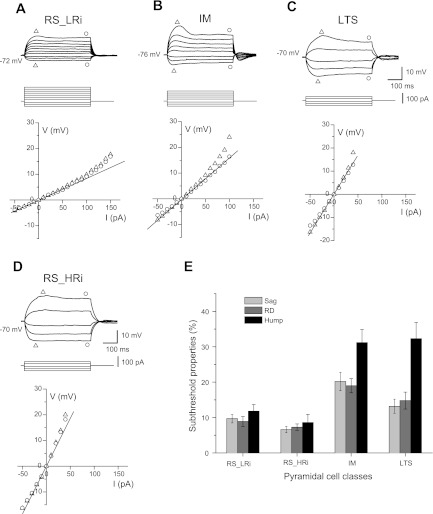
Fig. 4.
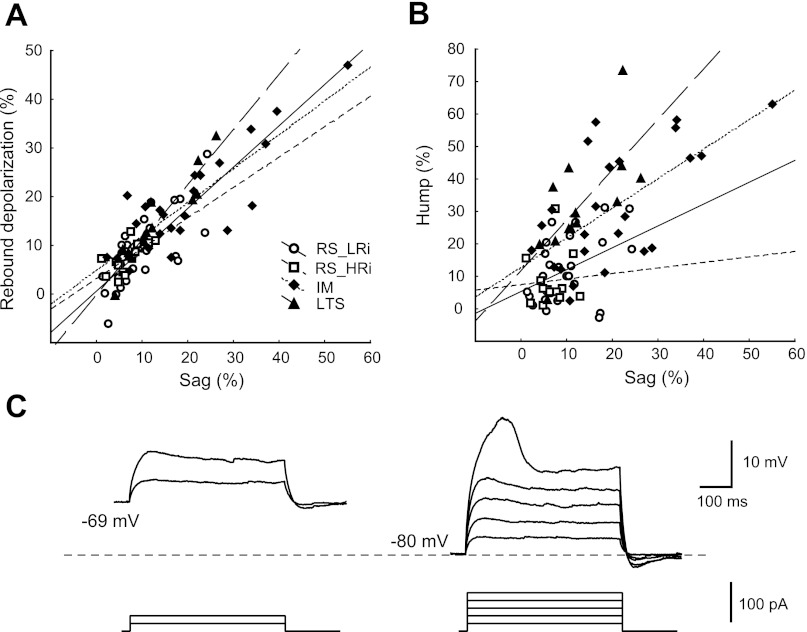
Subthreshold membrane properties of the typical RS_HRi, RS_LRi, IM, and LTS cells in monkey DLPFC. A–D: voltage deflections to injection of a series of hyperpolarizing and subthreshold depolarizing current steps (500 ms, 10 pA per step) of a typical neuron from each cluster. Graphs show voltage-current (V-I) plots, where triangles indicate the maximal voltage deflection from the resting membrane potential (RMP) and circles indicate the voltage deflection at the end of the step. Note that a time-dependent rectification in response to hyperpolarizing current steps and rebound depolarization (RD) was larger in IM and LTS cells than in RS cells. In response to subthreshold depolarizing current steps, LTS and IM cells exhibited humplike upward voltage deflection; this was not typical for either class of RS cells. E: bar graph summarizing the mean values of sag, hump, and RD in all 4 classes. Note the different ratio between hump and sag in IM and LTS cells.
In response to hyperpolarizing current steps, IM pyramidal cells (cluster 3) exhibited a large time-dependent rectification that produced a delayed depolarizing sag, shifting the membrane potential toward the RMP. The sag was more pronounced when the cells were hyperpolarized more than 10–20 mV relative to the RMP, suggesting it was likely produced by the current through hyperpolarization-activated and cyclic nucleotide-gated channels (Ih; Pape 1996; Robinson 2003). Whereas pyramidal cells from cluster 3 exhibited the largest sag (20.2 ± 2.6%), cells in the other clusters typically showed no detectable sag or a small sag (7–13%) (Figs. 4 and 5).
Fig. 5.
Properties of sag, hump and RD in monkey DLPFC pyramidal neurons. A: sag vs. RD plot indicates a strong correlation (r = 0.86, P < 0.001) between these parameters. B: sag vs. hump plot. Note that the slope of the regression line for sag vs. hump differs between pyramidal cell classes: LTS cells has the steepest slope. Symbols are as indicated in A. C: voltage dependence of humplike depolarization in LTS pyramidal cell. The amplitude of hump increases as the depolarizing current step is applied from more hyperpolarized membrane potentials.
The majority of pyramidal cells that exhibited profound depolarizing sag during hyperpolarizing current steps showed a transient RD after the end of the hyperpolarizing current. The strong correlation between sag and RD (r = 0.86, P < 0.001; Fig. 5A) indicates that the ionic mechanisms of both phenomena are similar and that Ih can contribute to the RD, as well. In contrast to previous reports (de la Pena and Geijo-Barrientos 1996; Degenetais et al. 2002; Yang et al. 1996), the RD in our conditions never caused rebound spikes in IM cells, which had the largest sag amplitude, or in other clusters.
In response to subthreshold depolarizing current steps, almost all LTS cells and IM cells exhibited a humplike depolarizing voltage deflection, which was not observed in either class of RS cells (Figs. 4 and 5). The initial bursts of APs in LTS neurons suggest the involvement of low-threshold calcium channels underlying the bursting mechanism. In agreement with this, the amplitude of the humplike depolarization was voltage dependent in that it increased as the depolarizing current step was applied from more hyperpolarized membrane potentials (Fig. 5C). In the total population of cells, the hump amplitude was significantly correlated with the amplitude of the sag (r = 0.66, P < 0.01; Fig. 5B) and of the rebound depolarization (r = 0.67, P < 0.001; Fig. 5A), indicating that Ih may contribute to the hump, as well. It is worth noting that the slope of the regression line for sag vs. hump differed between pyramidal cell classes (Fig. 5B): LTS cells had the steepest slope, and although LTS cells had smaller sag than IM cells (LTS sag: 13.2 ± 2.0%; IM sag: 20.2 ± 2.6%), the hump amplitude was similar (LTS hump: 32.3 ± 4.6%; IM hump: 31.2 ± 3.7%).
Single-Spike Properties
The properties of individual APs were evaluated from the first spikes evoked by minimal suprathreshold current. The latency to the first spike did not differ between cell groups, with an average value of 131 ± 6 ms. APT was considerably lower in LTS (−44.0 ± 0.8 mV) than in RS pyramidal cells (−40.4 ± 0.9 mV in RS_LRi and −40.0 ± 0.9 mV in RS_HRi), indicating a greater excitability of the LTS cell class. LTS cells had the longest spike duration at half-maximal amplitude (1.12 ± 0.11 ms) compared with other pyramidal cell classes (0.88–0.93 ms). The amplitude of the first spike was significantly smaller in RS_LRi pyramidal cells (64 ± 4 mV) than in all other cell classes.
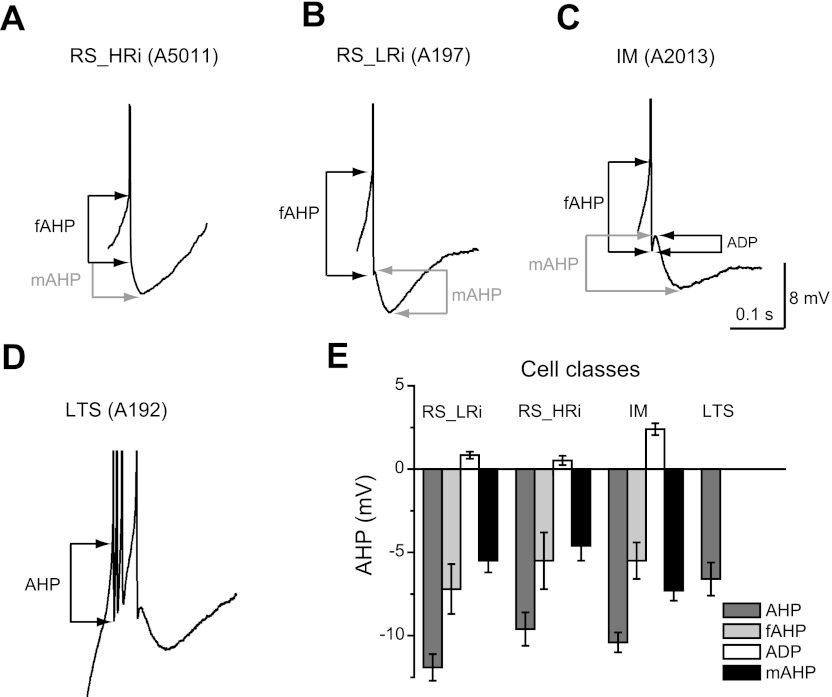
In the majority of neurons, the first AP in a train was followed by the same sequence of afterpotentials; a fast AHP was followed by a depolarizing afterpotential and then by a medium AHP. However, the depolarizing afterpotential amplitude varied significantly between cells and was undetectable in some neurons (Fig. 6). The depolarizing afterpotential was generally more prominent in IM cells (2.4 ± 0.4 mV) than in RS cells (0.8 ± 0.2 mV in RS_LRi and 0.5 ± 0.3 mV in RS_HRi). The medium AHP was larger in amplitude and almost twice as long in duration in RS_LRi than in RS_HRi cells. In LTS cells a prominent depolarizing afterpotential led to the next spike, which completely masked a medium-component AHP. After the initial burst of spikes, LTS cells fired single spikes with the same sequence of afterpotentials as RS pyramidal cells.
Fig. 6.
Sequences of afterpotentials in different electrophysiological classes of pyramidal cells in monkey DLPFC. A–D: in the majority of pyramidal neurons, the first action potential in a train was followed by the same sequence of afterpotentials: a fast afterhyperpolarization (fAHP) was followed by a depolarizing afterpotential (ADP) and then by a medium afterhyperpolarization (mAHP). The ADP was generally more prominent in IM cells (C) than in RS cells (B); moreover, the ADP was undetectable in some RS neurons (A). In LTS cells, a prominent ADP led to the next spike, which completely masked a medium-component AHP (D). After the initial burst of spikes, LTS cells fired single spikes with the same sequence of afterpotentials as RS pyramidal cells. E: bar graph summarizing the measurements of amplitude of different components of AHP.
Firing Pattern Properties
Onset response.
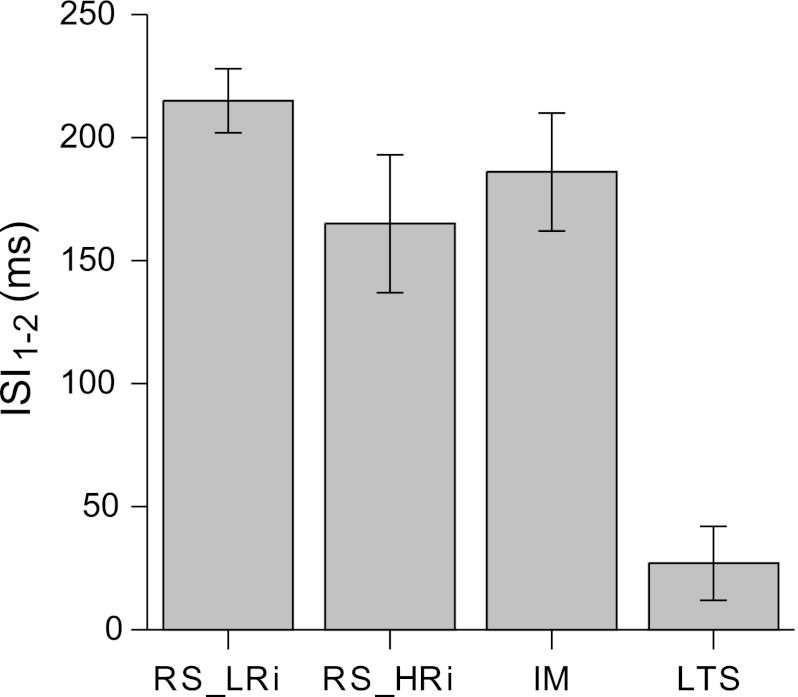
The most remarkable electrophysiological feature of LTS cells compared with other cell groups is their ability to fire bursts of APs at the beginning of the response to just suprathreshold depolarizing current pulses. A typical layer 2/3 LTS pyramidal cell fired just one burst consisting of three to four spikes followed by a train of single spikes. In contrast, both classes of RS and IM cells typically generated trains of single spikes with low frequencies in response to just suprathreshold depolarizing current pulses. To quantify this aspect of firing pattern, the ISI1–2 in the train was selected. ISI1–2 was measured at just suprathreshold depolarizing current pulses. We found that LTS cells exhibited the shortest ISI1–2 (27 ± 15 ms), whereas the mean value of ISI1–2 in other pyramidal cell groups was several times larger (Fig. 7, Table 1).
Fig. 7.
First interspike intervals (ISI1–2) in different electrophysiological classes of pyramidal cells in monkey DLPFC. LTS pyramidal cells exhibit burst firing to just suprathreshold depolarizing current pulses, and thus they have the shortest ISI1–2 (30.5 ± 19.2 ms). In other pyramidal cell groups, the mean value of ISI1–2 is several times larger.
Firing frequency adaptation.
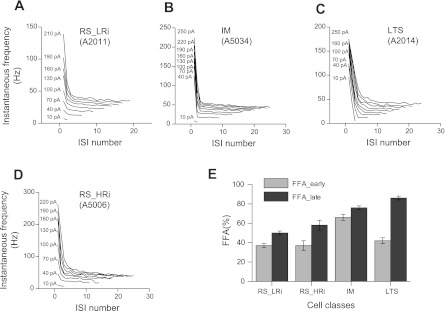
To characterize the firing frequency adaptation, the instantaneous spike frequency was plotted against the ISI number. With near-threshold depolarizing current steps, both classes of RS cells displayed a nonadapting or weakly adapting firing pattern, whereas the LTS cells exhibited strong firing frequency adaptation (Fig. 8). An increase of current enhanced firing frequency adaptation in RS and IM cells. In RS pyramidal cells, firing frequency adaptation developed within the first three to six spikes (the first 100–200 ms after the onset of the current pulses); later, the firing frequency remained relatively stable until the end of the current pulse. In response to an increase of current magnitude, most IM cells fired a pair of spikes at the beginning of the train. The initial spike doublet was followed by an almost nonadapting firing pattern. To compare the degree of adaptation between cell classes, the firing frequency adaptation was estimated at a current intensity two times the I0. Late and initial firing frequency adaptations were calculated as the percentage of decrease in the frequency from the onset instantaneous frequency (reciprocal of the 1st ISI) to steady-state frequency (late adaptation) and to the 2nd instantaneous frequency (initial adaptation). The late adaptation was stronger in LTS (86 ± 2%) and IM cells (76 ± 2%) than in RS cells (50 ± 2% in RS_LRi and 58 ± 5% in RS_HRi). The largest initial firing frequency adaptation was found for IM cells (66 ± 3%); in all other pyramidal cell classes, it was only about 40% (Fig. 8E, Table 1).
Fig. 8.
Firing frequency adaptation in different electrophysiological classes of pyramidal cells in monkey DLPFC. A–D: plots of instantaneous spike frequencies in the sequential sweeps for representative examples of pyramidal cells of 4 different electrophysiological classes. Note that with near-threshold depolarizing current steps, both classes of RS cells (A and B) display nonadapting or weakly adapting firing pattern, whereas the LTS cells (C) exhibit strong firing frequency adaptation; with larger depolarizing current steps, plots for RS and LTS cells look alike. In contrast, in IM cells (D), the initial spike doublet is followed by an almost nonadapting firing with the full range of depolarizing current steps, forming the characteristic plot. E: bar graph summarizing the mean values of early and late firing frequency adaptation (FFA) in the 4 classes. Note the different ratio between FFA_early and FFA_late in IM and LTS cells.
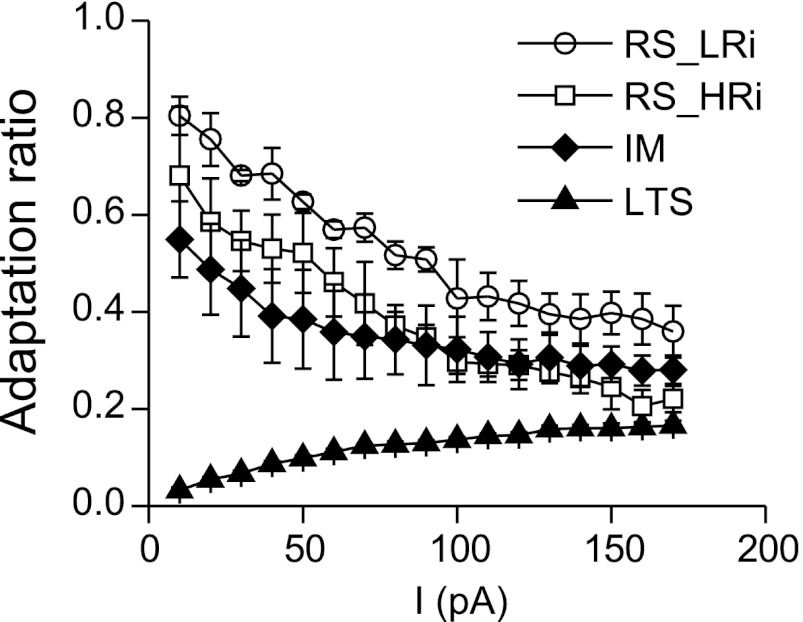
Interestingly, the changes in firing frequency adaptation with increasing current were cell type specific. To estimate this property, an adaptation ratio was calculated as the ratio of the first to the last ISI so that stronger firing frequency adaptation is represented by a smaller adaptation ratio. In RS cells, adaptation ratio strongly decreased with an increase of the stimulation current, whereas in LTS cells, adaptation ratio increased. In the majority of the IM cells, a fast initial decrease of adaptation ratio was followed by a stable adaptation ratio (Fig. 9). When depolarizing current steps reached 150–200 pA above firing threshold, a similar adaptation ratio of 0.2–0.4 was observed almost in all pyramidal cells. Thus the differences in firing pattern between pyramidal cell classes reduced with increasing levels of depolarization.
Fig. 9.
Changes in adaptation ratio in different electrophysiological classes of pyramidal cells in monkey DLPFC. The changes in FFA with increasing current are cell type specific. In RS cells, adaptation ratio strongly decreased with an increase of the stimulation current, whereas in LTS cells, adaptation ratio increased. When depolarizing current steps reached 150–200 pA above firing threshold, the similar adaptation ratio of 0.2–0.4 was observed in almost all pyramidal cells.
Current-to-Frequency Transduction in Different Electrophysiological Classes of Pyramidal Cells in Monkey DLPFC
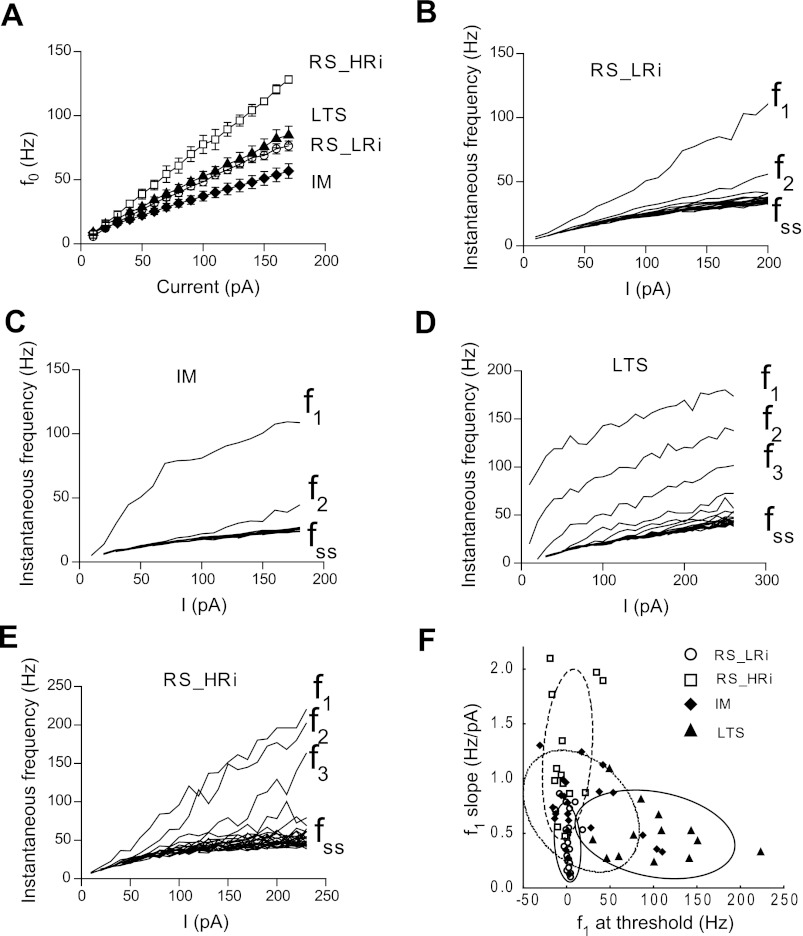
An essential characteristic of neuronal function is the transduction of synaptic inputs into discharge. Thus we compared how the different electrophysiological classes of pyramidal cells transform a steady depolarizing input into AP discharge. First, we investigated how rapidly monkey pyramidal cells start firing in response to different intensity depolarizing current steps. The instantaneous frequency of the first spike (f0) (calculated as 1/latency to the 1st spike) rose linearly with the increase of stimulation current. The slope of current vs. instantaneous frequency was significantly different between pyramidal cell classes, being maximal in RS_HRi pyramidal cells (0.75 ± 0.01 Hz/pA), minimal in IM cells (0.30 ± 0.01 Hz/pA), and intermediate in LTS (0.48 ± 0.01 Hz/pA) and RS_LRi cells (0.46 ± 0.01 Hz/pA) (Fig. 10A).
Fig. 10.
Current-to-frequency transduction (f/I) in different electrophysiological classes of pyramidal cells in monkey DLPFC. A: the averaged instantaneous frequency of the first spike (f0; calculated as 1/latency to the 1st spike) plotted against current strength for 4 electrophysiological classes of pyramidal cells. B–E: representative examples of f/I plots for pyramidal cells from different electrophysiological classes. The frequencies corresponding to the first (f1), second (f2), etc., spikes increase monotonically with the current strength in RS cells of both classes (B and E) and in the IM cell (C). In contrast, the LTS cell (D) shows an abrupt onset of repetitive firing with increasing current for the f1 and f2 frequencies. F: a plot of f1_threshold vs. f1_slope demonstrates the separation of RS and LTS pyramidal cells. fss, steady-state frequency.
Next, we examined how the discharge frequencies of different electrophysiological classes of pyramidal cells changed with the increase of current steps. Because pyramidal cells exhibit firing frequency adaptation, we studied changes of initial and steady-state frequencies by plotting them against the amount of current injected (f/I plot). The instantaneous frequency corresponding to the first ISI (f1) rose more steeply with an increase of injected depolarizing current than other instantaneous frequencies in all electrophysiological classes. At the same time, the relationship between f1 and injected current was cell class specific. In the majority of RS (30/40 cells) and IM cells (16/24 cells), f1 increased linearly with current intensity. In some RS cells (10/40 cells) from both RS_LRi and RS_HRi classes, the f1/I plot had two distinct regions, with an initial shallow slope being followed by a slightly steeper slope. In contrast, one-third of IM cells (8/24) and all LTS cells exhibited a flattening of the slope (Fig. 10, C and D). The presence of two ranges on the f1/I plot is reflected in the linear fit parameters. The intercept of linear fit (f1_threshold) was negative if an initial shallow slope was followed by a steeper slope and positive in cases of flattening of the f1/I curve. We found that f1_threshold in RS cells was close to 0 Hz (1.1 ± 1.2 in RS_LRi and 1.0 ± 5.4 in RS_HRi, whereas f1_threshold was 102 ± 15 Hz in the LTS cell cluster and 17 ± 8 Hz in the IM cell cluster). The slopes of the f1/I curve were significantly different between pyramidal cell classes, being maximal in RS_HRi pyramidal cells (1.22 ± 0.15 Hz/pA) and about two times smaller in other cell groups (Table 1). Thus RS and LTS pyramidal cells were clearly separated in the f1_threshold vs. f1_slope plot (Fig. 10F). The f2/I plots had similar structure; however, a smaller number of cells exhibited two distinct regions. Steady-state frequency increased linearly with current in all cell types, and their dynamics were similar.
DISCUSSION
In this study, we examined passive and active intrinsic membrane properties of layer 2/3 pyramidal cells of monkey DLPFC and found that these neurons are electrophysiologically diverse. Our results are in contrast with data obtained from rodents, where almost all supragranular pyramidal cells have the characteristic RS firing pattern and pyramidal neurons with dissimilar firing patterns can be found only in infragranular layers (Agmon and Connors 1989; Connors and Gutnick 1990; de la Pena and Geijo-Barrientos 1996; Degenetais et al. 2002; Hattox and Nelson 2007; Mason and Larkman 1990; Otsuka and Kawaguchi 2011). However, electrophysiologically diverse pyramidal neurons were found in layer 2/3 in cat and human neocortex (Chen et al. 1996; Foehring et al. 1991; Nowak et al. 2003; Nunez et al. 1993; Tasker et al. 1996). Thus the monkey neocortex, like human and cat neocortex, appears to differ from the rodent neocortex in having a greater heterogeneity in the electrophysiological properties of layer 2/3 pyramidal cells.
Factors Influencing Electrophysiological Properties In Vitro
However, the direct comparison of our results with other in vivo and in vitro studies should be done with some caution, because many experimental factors may influence the active and passive membrane properties of pyramidal neurons in vitro including composition of the intracellular solution and ionic medium, the temperature of the preparation during the electrophysiological recordings, and the degree of dendritic tree damage caused by slice preparation. For example, dendrotomy significantly increases Rin and AP and AHP amplitudes and hyperpolarizes the AP threshold (Bekkers and Häusser 2007). Because some dendritic branches of pyramidal cells are trimmed during the slice preparation, it is possible that the electrophysiological differences between at least some cell classes are due to differences in the degree of dendritic damage. However, several lines of evidence combined indicate that this factor did not confound the present classification. First, if we assume that classes of RS_LRi and RS_HRi pyramidal cells represent the same population of RS cells in which RS_HRi cells are more damaged by slicing, then RS_HRi pyramidal cells should also have larger AP and AHP amplitudes and a more hyperpolarized APT than RS_LRi cells, as indicated by a previous experimental study (Bekkers and Häusser 2007). In striking contrast, RS_HRi cells have smaller AP amplitude and similar AP threshold and amplitudes of AHP compared with RS_LRi pyramidal cells (Table 1). Second, all pyramidal cells included in this study had clear apical and basal dendrites, and thus it is unlikely that differences in dendritic tree trimming during slice preparation significantly affected their electrophysiological properties.
The ionic medium composition may determine the electrophysiological type of cells. Neuronal types like chattering cells or neurons with high-frequency spike bursts have been described in the cat's visual, motor, and association cortical areas in vivo (Gray and McCormick 1996; Nowak et al. 2003; Steriade et al. 1998). However, to be detected in slices they require specific ionic conditions, such as a 1.2 mM calcium concentration (Brumberg et al. 2000) that appears to be closer to that of in vivo-like ionic media at 1.2–1.4 mM (Hansen 1985). In the present study, we used Ringer solution containing 2 mM CaCl2, the most typical composition for in vitro studies, and thus the presence of LTS and IM cells in layer 2/3 of monkey PFC is species specific, at least for these in vitro conditions.
Multiple reports have demonstrated effects of the main internal anion on the shape and the amplitude of AHP, especially on its medium and slow component (Kaczorowski et al. 2007; Zhang et al. 1994). For example, the AHP is, on average, smaller in potassium gluconate immediately after the whole cell configuration is obtained and remains constant for the duration of the recording, whereas in potassium methylsulfate it is comparable to the AHP measured in the perforated-patch configuration. Thus the direct comparison of results obtained with sharp electrodes and patch electrodes with different compositions of the intracellular solution should be done with some caution. In our experiments, potassium gluconate-based intracellular solution was used, and although all registrations of active and passive membrane properties were done during the first 5 min after whole cell configuration was reached, we could not exclude an underestimation of the medium component of AHP. Because medium and slow AHP regulate firing frequency and its adaptation (Faber and Sah 2007; Sah and Faber 2002), these parameters also might be affected.
Membrane properties of neurons are highly sensitive to temperature (Ali et al. 2007; Lee et al. 2005; Thompson et al. 1985; Volgushev et al. 2000) and are altered by reductions of only 5–10°C from normal body temperature (Thompson et al. 1985). We performed our experiments at 32°C, the most typical temperature for such types of experiments. Thus it should be noted that these cooling of neurons may result in an increase in Rin, slowing of AP kinetics, and an increase in the amplitude and duration of medium and slow AHP compared with the normal body temperature.
Electrophysiological Classes of Layer 2/3 Pyramidal Cells in Monkey DLPFC
We classified monkey DLPFC pyramidal cells into four electrophysiological classes based on the results of cluster analysis. Two classes contain cells with RS firing pattern (RS_HRi cells and RS_LRi) and constitute 52% of the total pyramidal cell population, another class includes LTS cells (17%), and the last class consists of cells with an intermediate firing pattern (31%). The two classes of RS pyramidal cells differ in Rin, membrane time constant τ, I0, AP amplitude, and fss of firing (see Table 1 for a complete list of differences). Because of the differences in intrinsic properties, RS_HRi cells are more excitable than RS_LRi pyramidal cells, discharging with a higher frequency than RS_LRi pyramidal at the same level of stimulation. Thus the two classes of RS cells may play a different functional role in DLPFC circuitry. In previous classifications, RS cells were not separated on the basis of Rin, with only a few exceptions to our knowledge (Kawaguchi 1993; Schwindt et al. 1997). Usually, RS pyramidal cells were divided into two or three subgroups based primarily on the degree of changes in AP threshold and spike frequency adaptation across a sustained train of APs (Agmon and Connors 1992; Chang and Luebke 2007; Degenetais et al. 2002). In contrast to these reports, we usually did not observe the changes in AP threshold or amplitude across the train of APs in monkey DLPFC RS cells in a range of applied depolarization current. Also, in our sample we did not detect cells with properties known as RS fast-adapting pyramidal cells, which fire a brief train of 2–10 APs followed by a depolarizing plateau (Chang and Luebke 2007; Degenetais et al. 2002; Nunez et al. 1993; Otsuka and Kawaguchi 2008). The monkey DLPFC RS cells described in this study are most similar to neurons previously reported in rodents as RS1 (Agmon and Connors 1992), RS (Kawaguchi 1993; Mason and Larkman 1990; Yang et al. 1996), or slow-adapting RS1 cells (Degenetais et al. 2002); in cats as RS slow-adapting (Nunez et al. 1993) or RS cells (Chen et al. 1996; Nowak et al. 2003); in monkey as non-LTS RS1 cells (Chang and Luebke 2007); and in humans as RS (Foehring et al. 1991) and non-LTS cells (Tasker et al. 1996). Similar to RS cells from other species, RS cells in monkey DLPFC had little or no anomalous inward rectification detected in either the hyperpolarized or the depolarized membrane voltage range.
Here we report that at least one-third of pyramidal cells in layer 2/3 of monkey DLPFC exhibit LTS and IM firing patterns. In the LTS cells, the burst of APs was evoked at I0, whereas a larger current is required to evoke bursts in IM pyramidal cells. The bursts were typically two to five spikes long; two-spike bursts were most common in IM cells, whereas LTS cells commonly fired bursts with three to five spikes. As mentioned above, cells with burst firing are usually found only in deep layers in the rodent neocortex (de la Pena and Geijo-Barrientos 1996; Larkum et al. 2007; Mason and Larkman 1990; Schwindt et al. 1997). For example, in rodent medial PFC, LTS cells constitute up to 64–68% in layers 5 and 6 and <5% in layer 2/3 (de la Pena and Geijo-Barrientos 1996; Otsuka and Kawaguchi 2011; Yang et al. 1996). However, it was reported that two initial high-frequency spikes (“doublets”) can be evoked in cells in all cortical layers (Agmon and Connors 1992; Connors 1984; McCormick et al. 1985). It is also worth noting that LTS cells in human neocortex are more similar to monkey IM cells than to monkey LTS cells, because at depolarized potentials (more positive than −69 mV), LTS neurons in human cortex respond with RS firing, whereas at more hyperpolarized potentials they respond with two- or, rarely, three-spike bursts that ride on a hump and are followed by rhythmic spiking (Foehring et al. 1991; Tasker et al. 1996).
The LTS firing pattern is believed to be generated by low-threshold calcium channels (LTCC) (de la Pena and Geijo-Barrientos 1996; Yang et al. 1996). In the present study, we did not directly investigate the ionic basis of the LTS firing pattern in monkey PFC neurons; however, some observations suggest the presence of LTCCs in monkey LTS and IM pyramidal cells. For example, we observed that the humplike depolarization in LTS and IM neurons was much more intense if the depolarizing current was applied at a more hyperpolarized membrane potential. In contrast, RS pyramidal cells typically did not exhibit hump at any membrane potential. Another sign of LTCC contribution is the presence of postspike ADP (Yang et al. 1996). Although the ionic basis for the postspike ADPs differs widely in various central neurons (Higashi et al. 1993), in rat PFC neurons, Ca2+ entry plays a critical role in electrogenesis of ADPs (Yang et al. 1996). IM cells possessed a large postspike ADP, in LTS cells the bursting complex also was derived from a ADP that followed the first AP, whereas ADP was less prominent or completely absent in RS pyramidal cells.
Functional Significance of the Findings
The DLPFC plays a key role in complex cognitive functions, for instance, working memory. In fact, it is generally assumed that the sustained firing of DLPFC neurons during the delay period of working memory tasks represents the active holding of relevant information required for correct task performance (Goldman-Rakic 1995). The ability of DLPFC pyramidal neurons to sustain delay period firing during working memory tasks may be influenced by the intrinsic membrane properties of these cells. For example, strong firing frequency adaptation during sustained excitatory input opposes the production of persistent delay-related firing via recurrent excitation (Carter and Wang 2007; Pinto and Ermentrout 2001). Consequently, strong spike frequency adaptation may decrease the efficacy of storage of information in working memory, as suggested by computational modeling studies (Carter and Wang 2007). Interestingly, in the present study we did not observe fast-adapting RS cells (Chang and Luebke 2007; Degenetais et al. 2002; Nunez et al. 1993; Otsuka and Kawaguchi 2008). Moreover, no firing frequency adaptation was observed in RS cells if low-intensity depolarizing current was applied (Fig. 9). Under larger depolarizing current steps, the firing frequency remained relatively stable after the short period of rate adaptation: LTS and RS_HRi cells adapted within the first three to six spikes (the first 100–200 ms after the onset of the current pulse), and IM and RS_LRi cells did not change their firing rates after the first ISI (Fig. 8, A, B, and E). These data suggest that among the different pyramidal cell classes described in this study, RS cells appear to have the most appropriate electrophysiological properties for maintaining persistent delay-related firing.
During working memory tasks, some primate DLPFC neurons display brief cue- or response-related activity instead of sustained delay period-related firing (Goldman-Rakic 1996). An important question is, therefore, whether differences in biophysical membrane properties, which strongly modulate the response to brief or sustained excitatory input, contribute to the different patterns of task-related firing of primate DLPFC neurons. However, many, if not most, individual DLPFC neurons respond in more than one phase of the trial, i.e., during the cue, delay, and/or response periods (Goldman-Rakic 1996). Thus the heterogeneity of task-related activity of most primate DLPFC neurons cannot be determined by biophysical properties alone and probably requires the combined effects of intrinsic membrane properties, specific morphological properties, and functional connectivity. For example, the composite firing profile of DLPFC cells with activity in more than one phase of the task may be due to inputs from neurons whose activation is simpler and related to only one phase (Goldman-Rakic 1996).
Neuronal activity in primate DLPFC is crucial for cognitive control, a cognitive mechanism allowing coordination of lower level sensory and motor operations and memory to achieve a specific goal (Miller 2000; Miller and Cohen 2001). Cognitive control must implicate complex forms of information processing in DLPFC circuits, which may require specific forms of information transmission via DLPFC neuron activity. Interestingly, intrinsic bursting neurons may play unique roles in information transmission combined with specific forms of short-term synaptic plasticity. For instance, bursts may enhance transmission of information to downstream neurons (Lisman 1997), enable coding of changes in network firing rate (Abbott et al. 1997), or enhance recruitment of specific interneuron subtypes (Berger et al. 2010). Importantly, whereas LTS cells had bursting properties irrespective of the depolarizing stimulus strength, IM cells displayed two modes of response, regular spiking and bursting firing, depending on the strength of stimulation and membrane potential value. Thus the proportion of monkey DLPFC neurons with bursting activity may vary according to the overall level of network activity.
Differential excitability of pyramidal cell classes and the diversity of current-to-frequency transduction properties may play an important role in cortical network functioning. For example, different cell types would be involved in firing with different strengths of synaptic stimulation: LTS and RS_HRi pyramidal cells need smaller depolarizing current to fire, and moreover, because they have a longer membrane time constant, they may be more efficient at summation of excitatory synaptic potentials. In contrast, IM and RS_LRi will be activated with stronger stimuli. RS cells are well suited to perform a linear transduction of synaptic inputs to spike output over a wide range of currents with different gain; the RS_LRi cells have a significantly smaller gain (f/I curve slope) than the RS_HRi cells and would thus fire fewer spikes in response to the same synaptic stimulation. It is worth noting that the cell excitability and the transduction gain are regulated by many neuromodulators, which have multiple effects on intrinsic membrane properties and synaptic neurotransmission (Saar and Barkai 2009; Seamans and Yang 2004). For example, dopamine neuromodulation strongly affects the excitability of primate DLPFC pyramidal neurons (Henze et al. 2000) and their firing in vivo during working memory tasks (Sawaguchi 2001; Wang et al. 2004; Williams and Goldman-Rakic 1995). Moreover, extracellular dopamine levels in monkey DLPFC rise at the beginning of a working memory task and stay elevated across many trials (Watanabe et al. 1997). A number of studies in both rat and primate PFC have confirmed that, at least under certain conditions, dopamine via D1 receptors increases the excitability of PFC neurons in vitro and in vivo (Seamans and Yang 2004) and thus changes their output gain. Our present results suggest that heterogeneity in the intrinsic firing properties of pyramidal cells may contribute to information processing in superficial layers of the DLPFC during cognitive function. Further studies are necessary to fully understand the complex interactions between biophysical properties of individual DLPFC neurons and other multiple factors that ultimately determine their patterns of activity in vivo during cognitive tasks.
GRANTS
This work was supported by National Institute of Mental Health Grants MH043784 and MH084053. A. Zaitsev was also supported by Russian Foundation for Basic Research Grant 11-04-00912а and by the Program for Basic Research of the Presidium of the Russian Academy of Sciences (Molecular and Cell Biology).
DISCLOSURES
D. A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd., and Pfizer and in 2010–2012 served as a consultant in the areas of target identification and validation and new compound development to Bristol-Myers Squibb.
AUTHOR CONTRIBUTIONS
Author contributions: A.V.Z., N.V.P., G.G.-B., and D.A.L. conception and design of research; A.V.Z., N.V.P., G.G.-B., and D.A.L. performed experiments; A.V.Z. analyzed data; A.V.Z., N.V.P., G.G.-B., and D.A.L. interpreted results of experiments; A.V.Z. prepared figures; A.V.Z. and G.G.-B. drafted manuscript; A.V.Z., N.V.P., G.G.-B., and D.A.L. edited and revised manuscript; A.V.Z., N.V.P., G.G.-B., and D.A.L. approved final version of manuscript.
REFERENCES
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science 275: 220–224, 1997 [DOI] [PubMed] [Google Scholar]
- Agmon A, Connors BW. Correlation between intrinsic firing patterns and thalamocortical synaptic responses of neurons in mouse barrel cortex. J Neurosci 12: 319–329, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A, Connors BW. Repetitive burst-firing neurons in the deep layers of mouse somatosensory cortex. Neurosci Lett 99: 137–141, 1989 [DOI] [PubMed] [Google Scholar]
- Ali AB, Bannister AP, Thomson AM. Robust correlations between action potential duration and the properties of synaptic connections in layer 4 interneurones in neocortical slices from juvenile rats and adult rat and cat. J Physiol 580: 149–169, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artchakov D, Tikhonravov D, Ma Y, Neuvonen T, Linnankoski I, Carlson S. Distracters impair and create working memory-related neuronal activity in the prefrontal cortex. Cereb Cortex 19: 2680–2689, 2009 [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci 4: 829–839, 2003 [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Häusser M. Targeted dendrotomy reveals active and passive contributions of the dendritic tree to synaptic integration and neuronal output. Proc Natl Acad Sci USA 104: 11447–11452, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger TK, Silberberg G, Perin R, Markram H. Brief bursts self-inhibit and correlate the pyramidal network. PLoS Biol 8: pii: e1000473, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg JC, Nowak LG, McCormick DA. Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J Neurosci 20: 4829–4843, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter E, Wang XJ. Cannabinoid-mediated disinhibition and working memory: dynamical interplay of multiple feedback mechanisms in a continuous attractor model of prefrontal cortex. Cereb Cortex 17, Suppl 1: i16–i26, 2007 [DOI] [PubMed] [Google Scholar]
- Chang YM, Luebke JI. Electrophysiological diversity of layer 5 pyramidal cells in the prefrontal cortex of the rhesus monkey: in vitro slice studies. J Neurophysiol 98: 2622–2632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex 15: 409–418, 2005 [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang JJ, Hu GY, Wu CP. Electrophysiological and morphological properties of pyramidal and nonpyramidal neurons in the cat motor cortex in vitro. Neuroscience 73: 39–55, 1996 [DOI] [PubMed] [Google Scholar]
- Connors BW. Initiation of synchronized neuronal bursting in neocortex. Nature 310: 685–687, 1984 [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13: 99–104, 1990 [DOI] [PubMed] [Google Scholar]
- Curtis CE, Lee D. Beyond working memory: the role of persistent activity in decision making. Trends Cogn Sci 14: 216–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pena E, Geijo-Barrientos E. Laminar localization, morphology, and physiological properties of pyramidal neurons that have the low-threshold calcium current in the guinea-pig medial frontal cortex. J Neurosci 16: 5301–5311, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Electrophysiological properties of pyramidal neurons in the rat prefrontal cortex: an in vivo intracellular recording study. Cereb Cortex 12: 1–16, 2002 [DOI] [PubMed] [Google Scholar]
- Elston GN. Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex 13: 1124–1138, 2003 [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J. The pyramidal cell in cognition: a comparative study in human and monkey. J Neurosci 21: RC163, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol 34: 1077–1083, 2007 [DOI] [PubMed] [Google Scholar]
- Foehring RC, Lorenzon NM, Herron P, Wilson CJ. Correlation of physiologically and morphologically identified neuronal types in human association cortex in vitro. J Neurophysiol 66: 1825–1837, 1991 [DOI] [PubMed] [Google Scholar]
- Fuster JM. Network memory. Trends Neurosci 20: 451–459, 1997 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron 14: 477–485, 1995 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA 93: 13473–13480, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex 10: 82–92, 2000 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol 93: 942–953, 2005a [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Krimer LS, Urban NN, Barrionuevo G, Lewis DA. Synaptic efficacy during repetitive activation of excitatory inputs in primate dorsolateral prefrontal cortex. Cereb Cortex 14: 530–542, 2004 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Seamans JK, Lewis DA, Barrionuevo G. Dopaminergic modulation of short-term synaptic plasticity in fast-spiking interneurons of primate dorsolateral prefrontal cortex. J Neurophysiol 94: 4168–4177, 2005b [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex 18: 626–637, 2008 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Rotaru DC, Zaitsev AV, Povysheva NV, Lewis DA. GABA transporter GAT1 prevents spillover at proximal and distal GABA synapses onto primate prefrontal cortex neurons. J Neurophysiol 101: 533–547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science 274: 109–113, 1996 [DOI] [PubMed] [Google Scholar]
- Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev 65: 101–148, 1985 [DOI] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol 98: 3330–3340, 2007 [DOI] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol 84: 2799–2809, 2000 [DOI] [PubMed] [Google Scholar]
- Higashi H, Tanaka E, Inokuchi H, Nishi S. Ionic mechanisms underlying the depolarizing and hyperpolarizing afterpotentials of single spike in guinea-pig cingulate cortical neurons. Neuroscience 55: 129–138, 1993 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000 [DOI] [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Treml M. Regional dendritic and spine variation in human cerebral cortex: a quantitative Golgi study. Cereb Cortex 11: 558–571, 2001 [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. Upper Saddle River, NJ: Prentice Hall, 1998 [Google Scholar]
- Kaczorowski CC, Disterhoft J, Spruston N. Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. J Physiol 578: 799–818, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol 69: 416–431, 1993 [DOI] [PubMed] [Google Scholar]
- Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzalez-Burgos G, Povysheva NV, Iyengar S, Barrionuevo G, Lewis DA. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2–3 of monkey dorsolateral prefrontal cortex. J Neurophysiol 94: 3009–3022, 2005 [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol 359: 131–143, 1995 [DOI] [PubMed] [Google Scholar]
- Larkman A, Mason A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci 10: 1407–1414, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Waters J, Sakmann B, Helmchen F. Dendritic spikes in apical dendrites of neocortical layer 2/3 pyramidal neurons. J Neurosci 27: 8999–9008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Callaway JC, Foehring RC. Effects of temperature on calcium transients and Ca2+-dependent afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol 93: 2012–2020, 2005 [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 20: 38–43, 1997 [DOI] [PubMed] [Google Scholar]
- Luebke J, Barbas H, Peters A. Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Res Rev 62: 212–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Amatrudo JM. Age-related increase of sIAHP in prefrontal pyramidal cells of monkeys: relationship to cognition. Neurobiol Aging 33: 1085–1095, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravall M, Stern EA, Svoboda K. Development of intrinsic properties and excitability of layer 2/3 pyramidal neurons during a critical period for sensory maps in rat barrel cortex. J Neurophysiol 92: 144–156, 2004 [DOI] [PubMed] [Google Scholar]
- Mason A, Larkman A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. J Neurosci 10: 1415–1428, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985 [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Synaptic targets of the intrinsic axon collaterals of supragranular pyramidal neurons in monkey prefrontal cortex. J Comp Neurol 430: 209–221, 2001 [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol 390: 211–224, 1998 [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci 1: 59–65, 2000 [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001 [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16: 5154–5167, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol 89: 1541–1566, 2003 [DOI] [PubMed] [Google Scholar]
- Nunez A, Amzica F, Steriade M. Electrophysiology of cat association cortical cells in vivo: intrinsic properties and synaptic responses. J Neurophysiol 70: 418–430, 1993 [DOI] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Cell diversity and connection specificity between callosal projection neurons in the frontal cortex. J Neurosci 31: 3862–3870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Firing-pattern-dependent specificity of cortical excitatory feed-forward subnetworks. J Neurosci 28: 11186–11195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58: 299–327, 1996 [DOI] [PubMed] [Google Scholar]
- Pinto D, Ermentrout G. Spatially structured activity in synaptically coupled neuronal networks: I. Traveling fronts and pulses. SIAM J Appl Math 62: 206, 2001 [Google Scholar]
- Povysheva NV, Gonzalez-Burgos G, Zaitsev AV, Kroner S, Barrionuevo G, Lewis DA, Krimer LS. Properties of excitatory synaptic responses in fast-spiking interneurons and pyramidal cells from monkey and rat prefrontal cortex. Cereb Cortex 16: 541–552, 2006 [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Kroner S, Krimer OA, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Electrophysiological differences between neurogliaform cells from monkey and rat prefrontal cortex. J Neurophysiol 97: 1030–1039, 2007 [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J Neurophysiol 100: 2348–2360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucak ML, Levitt JB, Lund JS, Lewis DA. Patterns of intrinsic and associational circuitry in monkey prefrontal cortex. J Comp Neurol 376: 614–630, 1996 [DOI] [PubMed] [Google Scholar]
- Robinson RB. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480, 2003 [DOI] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-lasting maintenance of learning-induced enhanced neuronal excitability: mechanisms and functional significance. Mol Neurobiol 39: 171–177, 2009 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–353, 2002 [DOI] [PubMed] [Google Scholar]
- Sawaguchi T. The effects of dopamine and its antagonists on directional delay-period activity of prefrontal neurons in monkeys during an oculomotor delayed-response task. Neurosci Res 41: 115–128, 2001 [DOI] [PubMed] [Google Scholar]
- Schwindt P, O'Brien JA, Crill W. Quantitative analysis of firing properties of pyramidal neurons from layer 5 of rat sensorimotor cortex. J Neurophysiol 77: 2484–2498, 1997 [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74: 1–58, 2004 [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8: 4049–4068, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Durmuller N, Grenier F. Dynamic properties of corticothalamic neurons and local cortical interneurons generating fast rhythmic (30–40 Hz) spike bursts. J Neurophysiol 79: 483–490, 1998 [DOI] [PubMed] [Google Scholar]
- Tasker JG, Hoffman NW, Kim YI, Fisher RS, Peacock WJ, Dudek FE. Electrical properties of neocortical neurons in slices from children with intractable epilepsy. J Neurophysiol 75: 931–939, 1996 [DOI] [PubMed] [Google Scholar]
- Thompson SM, Masukawa LM, Prince DA. Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci 5: 817–824, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NN, Gonzalez-Burgos G, Henze DA, Lewis DA, Barrionuevo G. Selective reduction by dopamine of excitatory synaptic inputs to pyramidal neurons in primate prefrontal cortex. J Physiol 539: 707–712, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol 522: 59–76, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature 476: 210–213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science 303: 853–856, 2004 [DOI] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci 24: 455–463, 2001 [DOI] [PubMed] [Google Scholar]
- Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58: 236–244, 1963 [Google Scholar]
- Watanabe M, Kodama T, Hikosaka K. Increase of extracellular dopamine in primate prefrontal cortex during a working memory task. J Neurophysiol 78: 2795–2798, 1997 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Funahashi S. Neuronal activity throughout the primate mediodorsal nucleus of the thalamus during oculomotor delayed-responses. I. Cue-, delay-, and response-period activity. J Neurophysiol 92: 1738–1755, 2004 [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376: 572–575, 1995 [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci 4: 139–147, 2003 [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properties of layers V-VI principal pyramidal cells in rat prefrontal cortex in vitro. J Neurosci 16: 1904–1921, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex 15: 1178–1186, 2005 [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, Lewis DA. Interneuron diversity in layers 2–3 of monkey prefrontal cortex. Cereb Cortex 19: 1597–1615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Weiner JL, Valiante TA, Velumian AA, Watson PL, Jahromi SS, Schertzer S, Pennefather P, Carlen PL. Whole-cell recording of the Ca2+-dependent slow afterhyperpolarization in hippocampal neurones: effects of internally applied anions. Pflügers Arch 426: 247–253, 1994 [DOI] [PubMed] [Google Scholar]