Abstract
Rapidly stopping action engages a network in the brain including the right presupplementary motor area (preSMA), the right inferior frontal gyrus, and the basal ganglia. Yet the functional role of these different regions within the overall network still remains unclear. Here we focused on the role of the right preSMA in behavioral stopping. We hypothesized that the underlying neurocognitive function of this region is one or more of setting up a stopping rule in advance, modulating response tendencies (e.g., slowing down in anticipation of stopping), and implementing stopping when the stop signal occurs. We performed two experiments with magnetic resonance imaging (MRI)–guided, event-related, transcranial magnetic stimulation(TMS), during the performance of variants of the stop signal task. In experiment 1 we show that stimulation of the right preSMA versus vertex (control site) slowed the implementation of stopping (measured via stop signal reaction time) but had no influence on modulation of response tendencies. In experiment 2, we showed that stimulation of the right preSMA slowed implementation of stopping in a mechanistically selective form of stopping but had no influence on setting up stopping rules. The results go beyond the replication of prior findings by showing that TMS of the right preSMA impairs stopping behavior (including a behaviorally selective form of stopping) through a specific disruption of the implementation of stopping. Future studies are required to establish whether this was due to stimulation of the right preSMA itself or because of remote effects on the wider stopping network.
Keywords: dorsomedial frontal cortex, cognitive control, conditional stop signal task, selective stopping, stop signal reaction time
accumulating evidence shows that rapidly stopping action is implemented by a prefrontal-basal ganglia network including the right presupplementary motor area (preSMA), the right inferior frontal gyrus (IFG), and the basal ganglia (see reviews by Aron et al. 2007b; Chambers et al. 2009; Chikazoe 2010; Levy and Wagner 2011). Here, we focused on the functional role of the right preSMA using event-related transcranial magnetic stimulation (TMS).
Much research implicates the right preSMA in stopping. In functional magnetic resonance imaging (fMRI) studies, activation in the right preSMA (and usually also the anterior cingulate) is greater on trials for which participants successfully cancel a prepotent response compared with trials for which they do not (Aron and Poldrack 2006; Aron et al. 2007a; Boecker et al. 2010; Boehler et al. 2010; Cai and Leung 2009; Chevrier et al. 2007; Chikazoe et al. 2009b; Curtis et al. 2005; Hampshire et al. 2010; Sharp et al. 2010). Neurophysiologic studies have also shown activation in the preSMA prior to and during stopping (Chen et al. 2010; Swann et al. 2012). Several studies with macroelectrode stimulation in epilepsy patients show that preSMA stimulation leads to the arrest of ongoing vocal or manual movements (Luders et al. 1988; Mikuni et al. 2006; Swann et al. 2012). However, clear-cut evidence for the idea that the preSMA is critical for behavioral stopping is scant. In one study, stop-signal reaction time (SSRT) for a stop-signal task was longer in patients with a dorsomedial lesion versus controls; however, the damage was very broad, including both cingulate and superior frontal gyri (Floden and Stuss 2006). A single case report of a patient with a lesion of the preSMA extending to cingulate and superior frontal gyri also showed a behavioral stopping deficit for a stop-change task (Nachev et al. 2007). Thus, although these studies showed that dorsomedial damage impairs stopping, the lesions were not restricted to the preSMA. The same concern goes for a study of transcranial direct current stimulation over the dorsomedial frontal cortex; this also affected stopping, but the neural correlates are unclear (Hsu et al. 2011).
Here we used TMS to study how the right preSMA regulates stopping. In a previous TMS study by Chen et al. (2009) participants prepared to respond to a go signal and were sometimes required to cancel the response when a stop signal was subsequently presented. TMS was applied at the time of the go signal and also 100 ms after the go signal (but typically before the stop signal). Although stimulation over the left preSMA reliably prolonged SSRT compared with a vertex control site, the finding raises several questions. Why did stimulating before the stop signal affect stopping? Which underlying function was affected? It is possible that the elongated SSRT could have arisen due to disruption of the stopping network prior to the stop signal; due to disruption of stopping preparation; or due to disruption of the stopping process itself (perhaps via a lingering effect of TMS from the earlier time period).
Successfully stopping a response relies on multiple underlying functions including setting up a stopping rule (or configuration of the stopping network) in advance, modulating response tendencies (e.g., slowing down responses when stopping is likely) (Bogacz et al. 2010; Forstmann et al. 2008; Verbruggen and Logan 2009b), and implementing the stopping process when a signal occurs. The extant fMRI, lesion, and TMS studies do not clarify which of these functions are implemented by the preSMA. Indeed, the preSMA is likely to implement several of them, as other studies have pointed to its importance for other forms of action control including switching of responses and rules, processing conflict, and reconfiguring stimulus–response mappings (Brass and von Cramon 2002; Chen et al. 2010; Isoda and Hikosaka 2007; Matsuzaka and Tanji 1996; Matsuzaka et al. 1992; Nachev et al. 2008; Neubert et al. 2010; Ridderinkhof et al. 2004; Rushworth et al. 2002). Here we aimed to identify the functional role of the right preSMA in stopping by using event-related TMS during the performance of two variants of the stop-signal task. Importantly, we used a batwing-shaped TMS coil to target the right preSMA, and we used an MRI-based distance-adjusted threshold method to tailor the stimulation intensity to each specific subject (Cai et al. 2012). In so doing we aimed to complement and extend the TMS findings of Chen et al. (2009) by conducting two experiments to establish a more specific effect of preSMA stimulation, and to investigate which functions are implemented by the right preSMA that contribute to behavioral stopping.
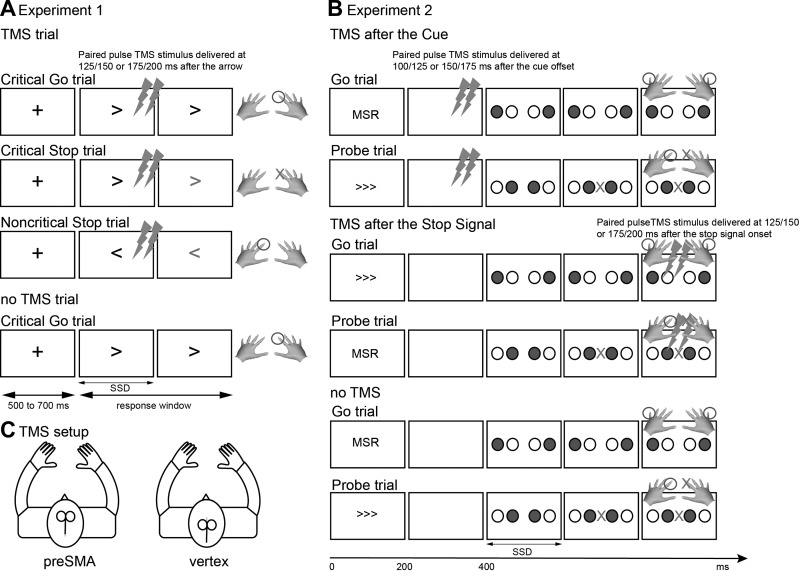
Experiment 1 was designed to test the role of the right preSMA in modulation of response tendencies versus implementation of the stopping process. For this, we used the conditional stop-signal task (Fig. 1A) (Aron et al. 2007a; De Jong et al. 1995; Jahfari et al. 2009). The conditional stop-signal task enables a richer assessment of response control than the standard stop-signal task used in previous studies (Chambers et al. 2006; Chen et al. 2009). On each trial an arrow (Go signal) is presented and participants are required to make a response. Occasionally, the arrow turns red (Stop signal) shortly after the appearance of the Go signal and participants need to cancel the response only if the arrow points in the critical direction. Thus, if the arrow points say to the right (the critical direction), then the participant needs to prepare to stop; if the arrow points say to the left (the noncritical direction), then the participant does not need to prepare to stop. On Go trials without a stop signal, RT is slower for critical than for noncritical trials. This indicates that participants slow down their responses and prepare to stop on critical trials. Using this task in combination with single-pulse TMS over the primary motor cortex, a previous study showed that the corticospinal excitability of the responding hand is reduced at 160 ms after the Go signal in critical versus noncritical trials (Jahfari et al. 2009). This suggests that participants proactively modulate response tendencies on critical trials. Thus, we can index the modulation of response tendencies via the behavioral slowing on critical versus noncritical trials, and we can index the implementation of stopping itself via SSRT. The process of setting up the stopping rule must contribute to both the modulation of response tendencies and the implementation of stopping; so effects of preSMA stimulation on either the response slowing or SSRT could indicate a disruption of setting up the stopping rule.
Fig. 1.
Tasks for experiments 1 and 2. A: experiment 1: conditional stop signal task. A leftward or rightward pointing arrow (go signal) was presented. Participants made quick button presses using either the left or right index finger. Occasionally (30%), the arrow turned to red (stop signal), here shown in gray. Participants tried to withhold the response only if the arrow pointed in the critical direction (e.g., right) and continue the response if the arrow pointed in the noncritical direction (e.g., left). Dual-pulse transcranial magnetic stimulation (TMS) was given at 125/150 or 175/200 ms after the onset of the go signal on half of the trials but not on the others. B: experiment 2, selective stopping task. Each trial began with a cue (“MSL,” “<<<,” “MSR,” or “>>>,” indicating maybe stop the left or right hand). The cue was presented for 200 ms, followed by a blank screen for 200 ms. Subsequently, two blue circles (go signal) and two white circles were presented. Participants made quick bimanual button press with either index or little fingers of both hands. Occasionally (30%), a red “X” (stop signal) was presented shortly after the go signal. Participants tried to withhold the response on the cued hand (e.g., the right hand for “MSR” or “>>>” cues) and to continue the response with the other. Dual-pulse TMS stimulation was given at 100/125 or 150/175 ms after the cue offset on one third of trials, or at 125/150 or 175/200 ms after the onset of the stop signal on another one third of trials. C: a batwing coil was placed over either the right preSMA or the vertex with the handle pointing posteriorly.
We used structural MRI to localize the right preSMA and a vertex control region in each participant. TMS was delivered after the go signal but before the stop signal at a range of times corresponding to previously observed changes in motor system excitability on critical versus noncritical trials (Jahfari et al. 2009). If preSMA (vs. vertex) stimulation disrupts the implementation of the stopping process, then this should lengthen SSRT; by contrast, if preSMA stimulation disrupts the modulation of response tendencies then this should reduce the ability of participants to slow down in anticipation of stopping. To anticipate the results, like Chen et al. (2009), we show that when TMS was delivered after the go signal, but before the stop signal, SSRT was lengthened for preSMA stimulation versus the vertex. However, we also show that the slowing down in anticipation of stopping (response modulation) was not changed. The elongation of SSRT could have two causes: a disruption in setting up the stopping rule or a disruption in implementing stopping. To dissociate these two accounts, we conducted experiment 2. This time we used a selective stop task, which involves a trial-by-trial changing stopping rule and allows us to dissociate setting up the stopping rule from implementing stopping (Aron and Verbruggen 2008; Cai et al. 2011; Claffey et al. 2010). Specifically, the selective stopping task has a foreknowledge period (e.g., Maybe Stop Right hand), a delay, a go stimulus (move both hands together) and, then, on some trials, a stop signal (requiring the subject to stop the cued hand and continue with the other). The stop signal is not informative of which hand to stop; therefore the participant has to encode the cue presented in the foreknowledge period. In a previous study (Cai et al. 2011), we could demonstrate that the stopping rule is set up even before the go signal. We demonstrated this by applying single-pulse TMS in the foreknowledge period to examine the corticomotor excitability of the right hand for “Maybe Stop Right” versus “Maybe Stop Left” and other conditions. We found that the corticomotor excitability of the right hand was reduced in the foreknowledge period for Maybe Stop Right versus the other conditions. In other words, even before subjects knew which response to make, they had already formed an action plan of which responses might need to be stopped. Because this process is clearly separated from the actual stopping process that occurs later (when a stop signal is presented), we could, in the current experiment 2, use TMS over the right preSMA to test the hypothesis that the right preSMA might be important for either setting up the stopping rule or implementing the stopping process. If preSMA (vs. vertex) stimulation disrupts setting up the stopping rule/network then this should lead to reduced selectivity of stopping (increased latency of continuing one response while stopping the other) when stimulation is given in the period of setting up the stopping process; by contrast, if preSMA stimulation disrupts the ability to implement the stopping process then SSRT should be lengthened when stimulation is given in the period of implementing the stopping process.
METHODS
Experiment 1
Participants.
Sixteen right-handed adults (six females, 18–23 years) provided consent according to an Institutional Review Board protocol at the University of California at San Diego and passed TMS safety screening.
Task.
We used the conditional stop-signal task (Fig. 1A). On each trial, a white cross (fixation) was presented in the center followed by a white leftward or rightward pointing arrow (go signal). The interval between the fixation and the go signal ranged from 500 to 700 ms. Participants executed speeded button-press responses using their right index or middle fingers. On 33% of trials, the arrow turned red (a stop signal) shortly after it was presented. Participants tried to cancel the initiated response only if the arrow pointed in the critical direction (e.g., right) but continued their response if the arrow pointed in the noncritical direction (e.g., left). The critical directions were counterbalanced across participants. On the critical trials, the delay between the go signal and the stop signal (stop-signal delay [SSD]) was dynamically adjusted in a staircase fashion to ensure that the probability of stopping converged on 50%. This allows a reliable estimation of the SSRT (Band et al. 2003; Verbruggen and Logan 2009a). The initial SSDs were 150 ms. If the participant failed to stop in a critical stop trial, then the SSD was reduced by 50 ms for the next stop trial. If the participant stopped in a critical stop trial, then the SSD was increased by 50 ms for the next stop trial. The SSDs in the noncritical stop trial were yoked to those in the critical stop trial. The limited response window was 1 s. The intertribal interval (ITI) varied between 2.5 and 3 s, which guarantees enough time to recharge the TMS machine on two consecutive pulse trials.
TMS method and apparatus.
We used event-related TMS to disrupt the neural activity in the right preSMA at specific time points while participants were doing the task. On each trial we delivered two pulses 25 ms apart. This dual-pulse method increases the chances of disrupting the underlying cognitive function while still preserving good temporal resolution. The dual pulses were generated with a MagStim 200–2 monophasic stimulator connected to a MagStim BiStim module (Magstim Co., Ltd, Spring Garden, Whitland, UK). The stimulation was delivered via a 70-mm batwing coil (type no. 15411). This coil is designed to stimulate deep cortical areas (e.g., the leg area of M1 or the preSMA). Motor thresholds were obtained using surface electromyography (EMG) from the first dorsal interosseous (FDI) of the right hand via 10-mm-diameter Ag-AgCl hydrogel electrodes (Medical Supplies, Inc., Newbury Park, CA). A ground electrode was placed over the styloid process (wrist) of the right ulna. The EMG signal was amplified via a Grass QP511 Quad AC Amplifier System Grass amplifier (Grass Technologies, West Warwick, RI), with a band-pass filter between 30 Hz and 1 kHz and a notch filter at 60 Hz. A CED Micro 1401 mk II acquisition system was used to sample data at 2 kHz. Data were displayed and recorded to disk using CED Signal v4 (Cambridge Electronic Design, Cambridge, UK).
Hot-spotting and resting motor threshold.
We identified the stimulation spot for the right FDI muscle in left primary motor cortex. The coil was initially placed 5 cm lateral and 2 cm anterior to the vertex and repositioned to where the largest MEP was observed consistently. The resting motor threshold (RMT) for the right FDI was defined as the minimum stimulator output required to induce MEPs of 0.1 mv peak-to-peak amplitude in 5 of 10 consecutive trials (Rossini et al. 1994).
MRI localization.
We acquired a high-resolution T1 structural MRI scan in each participant, at the Center for Functional MRI at University of California, San Diego (3D FSPGR: slice thickness, 1 mm; repetition time [TR], 7.8 s; echo time [TE], 3 s; matrix, 192 × 192; field of view [FOV], 256; 172 sagittal slices). For the TMS experiment, the structural MRI images were used to localize the stimulation spot for the right preSMA and to calculate the coil–cortex distance of the right preSMA and the left M1 for each participant (see the following text). MRI images were coregistered to participants' heads in space using a magnetic tracking device (miniBIRD 500; Ascension Technology Corp., Milton, VT) and MRI coregistration software (MRIReg and MRIcro). The coordinate for the right preSMA (x = 10, y = 6, z = 72) in Montreal Neurological Institute (MNI) space was determined from the group contrast of “critical Go-noncritical Go” from a previous fMRI study (Jahfari et al. 2009) and this MNI coordinate was registered to individual structural space (FLIRT, FMRIB, Oxford, UK) (Jenkinson and Smith 2001). Another reason to target the right preSMA rather than the left preSMA (as was done in Chen et al. 2009) is that many fMRI studies have reported the peak stopping-related activation of the preSMA in the right hemisphere (Aron and Poldrack 2006; Aron et al. 2007a; Boecker et al. 2010, 2011; Boehler et al. 2010; Cai and Leung 2009; Chao et al. 2009; Chikazoe et al. 2009b) and many studies point to a right-hemisphere white-matter network between the preSMA, the right IFG, and the basal ganglia (Aron et al. 2007a; Forstmann et al. 2012; King et al. 2011; Madsen et al. 2010; Swann et al. 2012). Therefore, we consider that the right preSMA is probably a more representative region for stopping than the left preSMA. The stimulation locus for the right preSMA was identified as the nearest voxel on the scalp surface to this preSMA coordinate. Vertex location is determined by the middle waypoint between nasion and inion. For both preSMA and vertex, the coil was oriented with the handle pointing in a posterior direction, inducing current along the posterior–anterior axis.
Distance adjusted thresholding.
This study aimed to stimulate the right preSMA and the vertex (control site). A key parameter is the choice of TMS stimulation intensity for these nonmotor regions. We used the distance-adjusted threshold method, which we recently validated for the Batwing Coil (Cai et al. 2012). In that study we derived a linear function relating coil–cortex distance and resting motor threshold (RMT): distance-adjusted RMTtarget site = RMTM1 + g × (Dtarget site − DM1), where RMTtarget site is the estimated RMT of a target site, RMTM1 is the RMT of the M1, Dtarget site is the coil-cortex distance of a target site, DM1 is the coil-cortex distance of the M1, and g is the gradient relating RMT to distance. Specifically, g reflects the extra amount of output (%) required to reach an equivalent level of stimulation at the motor cortex for every millimeter increment between the coil and cortex (in Cai et al. 2012, we estimate this parameter at 1.4%). The coil–cortex distance of the right preSMA is the distance between the stimulation locus of the right preSMA and the coordinate for the right preSMA in the individual space. The coil–cortex distance of M1 is the distance from the stimulation locus of the M1 during hot-spotting to its nearest voxel on the cortical surface. For each participant, we could thus calculate the distance-adjusted RMT of the right preSMA. The stimulation intensity for the right preSMA was initially determined as 110% of the distance-adjusted RMT at the depth of the right preSMA. For some subjects, the stimulation intensity was further adjusted to be comfortable. The average intensity was 55 ± 10% of the maximum stimulator output (∼98% of distance-adjusted RMT for the right preSMA: 57 ± 13%; RMT for the left M1: 39 ± 8%). The same stimulation intensity was used for the vertex.
Procedure.
Testing occurred on 4 different days. On day 1, the participant received a structural MRI scan. On day 2, we estimated the correct stimulation intensity for the right preSMA and the vertex using the distance threshold adjustment method. On days 3 and 4 participants performed the conditional stop signal task while TMS was administered over the right preSMA (experimental site) or the vertex (control site). The order of experimental (preSMA) and control sessions (vertex) was counterbalanced across participants. Participants practiced for 2 task blocks then completed 8 blocks of 48 trials proper. Each block had 16 no-signal and 8 signal trials for both critical and noncritical conditions. In half the trials (equally distributed for different types of trials), a dual pulse was delivered at 125/150 ms or at 175/200 ms after the go signal (TMS trials). No pulses were administered on the remaining half of trials (no TMS trials). Separate SSD staircases were applied for TMS and noTMS trials at experimental and control sessions. The stimulation times were determined from a study of the same task by Jahfari et al. (2009), in which corticomotor excitability for critical versus noncritical trials began to differentiate approximately 160 ms after the go signal. Two stimulation times were used to provide temporal jitter during the preparatory period (increasing the likelihood of observing a TMS effect) and they were collapsed for behavioral analysis. Initial analysis showed that stimulation time did not cause a significant behavioral difference.
Behavioral analysis.
SSRT was calculated by subtracting the average SSD for critical stop trials from the mean RT on critical go trials (De Jong et al. 1995; Jahfari et al. 2009; Logan et al. 1984; Verbruggen and Logan 2008). We indexed the process of modulating response tendencies by the response-slowing effect (i.e., mean Go critical RT − mean Go noncritical RT). To affirm that the TMS effect was not merely a result of distraction, we derived other behavioral indices, specifically: critical Go RT (the reaction time on critical Go trials), noncritical Go RT (the reaction time on noncritical Go trials), and Stop Accuracy (the accuracy of critical stop trials).
A 2 × 2 repeated-measures ANOVA was conducted to test the effect of locus (preSMA vs. vertex) and stimulation (TMS vs. noTMS) on key behavioral measures. This let us test two key predictions. First, if the right preSMA is functionally critical for stopping, we expected a significant interaction between locus and stimulation on SSRT. Second, if the right preSMA is functionally critical for modulating response tendencies, we expected a significant interaction between locus and stimulation on the response-slowing effect. Furthermore, if the TMS effect is driven by distraction or discomfort of stimulation, we expected a significant interaction between the locus and stimulation on the other behavioral measures (Critical Go RT, noncritical Go RT, and stop accuracy). Significant interactions were followed up by post hoc t-tests, corrected for multiple comparisons using the Holm–Bonferroni method (Levin 1996).
Experiment 2
Participants.
Seventeen participants were recruited for experiment 2 (6 female, 18–27 years old, 1 left-handed). Fourteen participants returned from experiment 1 and the other three were new. All subjects provided written consent and passed a TMS safety screen again. One participant was excluded because she could tolerate only 50% of distance-adjusted RMT.
Task.
In this experiment, participants did a selective stopping task. This consists of Go trials and Probe trials (Fig. 1B). Each trial began with a cue (“MSL,” “<<<,” or “MSR,” “>>>”: “MSL” and “<<<” indicate that participants may need to stop their left hand, whereas “MSR” and “>>>” indicate that participants may need to stop their right hand). The cue was presented for 200 ms, after which the screen turned black for 200 ms, followed by four circles in a horizontal row, each 2.3° in diameter. The two inner circles were separated from the outer ones by 1.2°. The four circles corresponded to left little and index and right index and little fingers. Either two inner or outer circles were filled blue (go signal) and the others were filled white. Participants made quick bimanual responses simultaneously to the two blue circles within a 1-s response window. A “decoupled response” message was presented for 2 s as a feedback warning if bimanual responses were separated by >70 ms. On 33% of trials, a red “X” (stop signal) was presented shortly after the go signal between the two inner circles (Probe trial). The SSD was dynamically adjusted to ensure that the probability of stopping approximated 50%. If the participant successfully stopped, the SSD increased by 50 ms on the next Probe trial, whereas if the participant failed to stop, the SSD decreased by 50 ms on the next Probe trial.
TMS method.
The TMS apparatus, stimulation sites, and procedure were identical to those in experiment 1. With three new participants, the average intensity after adjustment for comfort was 55 ± 10% of the maximum stimulator output (∼96% of distance-adjusted RMT for the right preSMA: 58 ± 11%; RMT for the left M1: 39 ± 8%). The same stimulation intensity was used for the vertex.
Procedure.
For the 3 new participants, testing occurred on 4 different days as in experiment 1. For the 14 participants who returned, testing occurred on 2 different days. Participants performed the selective stopping task while TMS was administered over the right preSMA (experimental site) or the vertex (control site). The order of experimental (preSMA) and control sessions (vertex) was counterbalanced across participants. Participants practiced for 2 task blocks then completed 10 blocks of 72 trials proper. Each block had 48 Go trials and 24 Probe trials. To interfere with the period corresponding to setting up the selective stopping rule, dual pulses were delivered at 100/125 ms or at 150/175 ms after the offset of the task cue on one third of trials (cueTMS). To interfere with the period corresponding to implementing the stopping process, dual pulses were delivered at 125/150 or 175/200 ms after the onset of the stop signal on Probe trials or after the average SSD on Go trials on another third of trials (ssdTMS). No pulse was given on the remainder of trials (noTMS). Four types of foreknowledge cue (“MSL,” “<<<,” “MSR,” and “>>>”), two types of go signal (two inner circles and two outer circles), and three types of stimulation (cueTMS, ssdTMS, and noTMS) were randomly distributed and balanced across Go and Probe trials. Separate SSD staircases were applied for cueTMS, ssdTMS, and noTMS trials at experimental and control sessions.
Behavioral analysis.
To examine the role of the right preSMA in setting up the selective stopping rule, we delivered TMS pulses after the foreknowledge cue (cueTMS). The behavioral indices for setting up the selective stopping rule in this experiment were the stopping interference effect and SSRT. The stopping interference effect is the degree of slowing that occurs for the continuing response when the other one is stopped (Aron and Verbruggen 2008; Cai et al. 2011). This is calculated by subtracting the RT on the alternative hand (noncued hand) in go trials from the RT on the alternative hand (noncued hand) in successful probe trials. If the selective stopping rule is not set properly then the degree of slowing should increase.
To examine the role of the right preSMA in implementing stopping, stimulation was given after the stop signal (ssdTMS). The behavioral index for implementing stopping was SSRT, which in this case was calculated by subtracting the average SSD on the probe trials from the mean RT on the cued hand on the Go trials (Aron and Verbruggen 2008; Cai et al. 2011; Claffey et al. 2010).
To make sure that any effect of stimulation on the stopping interference effect and SSRT did not simply result from the distraction or discomfort of TMS, we examined other behavioral measures, specifically: Go Cue RT (reaction time of the cued hand in go trials), Go Alt RT (reaction time of the alternative/noncued hand in go trials), Probe Accuracy (accuracy of probe trials), and Probe Alt RT (reaction time of the alternative/noncued hand in probe trials). See Aron and Verbruggen (2008) for more details.
A 2 × 3 ANOVA was conducted to test the effect of locus (preSMA vs. vertex) and stimulation (noTMS, cueTMS, and ssdTMS) on each behavioral measure. For the behavioral measures that showed a significant interaction, we further examined whether the interaction is driven by the contrast between noTMS and cueTMS or by the contrast between noTMS and ssdTMS. This was examined by a cue period 2 × 2 ANOVA of locus (preSMA vs. vertex) and stimulation (noTMS vs. cueTMS) and a stop signal delay period 2 × 2 ANOVA of locus (preSMA vs. vertex) and stimulation (noTMS vs. ssdTMS). Significant interactions were followed up by post hoc t-tests, corrected for multiple comparisons using the Holm–Bonferroni procedure as in experiment 1.
RESULTS
Experiment 1
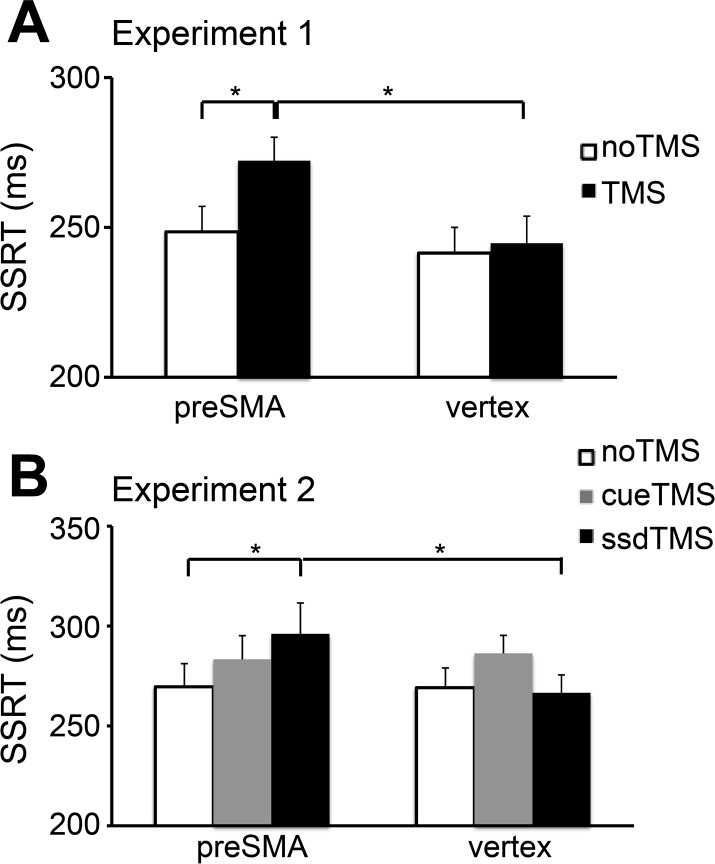
Table 1 and Fig. 2A show the behavioral data. There was a significant interaction between locus (preSMA vs. vertex) and stimulation (TMS vs. noTMS) on SSRT [F(1,15) = 5.30, P < 0.05], but no significant main effects of locus (P = 0.08) or stimulation (P = 0.051). Holm–Bonferroni corrected post hoc t-tests showed that SSRT on TMS trials in the preSMA session (272 ± 31 ms) was significantly longer than SSRT on noTMS trials [249 ± 34 ms; t(15) = 3.03, P < 0.01] and SSRT on TMS trials in the preSMA session was significantly longer than SSRT on TMS trials in the vertex session [245 ± 36 ms; t(15) = 3.31, P < 0.01].
Table 1.
Behavioral data for experiments 1 and 2
| preSMA |
Vertex |
|||
|---|---|---|---|---|
| Experiment 1 | noTMS | TMS | noTMS | TMS |
| Critical Go RT (ms) | 481 (85) | 478 (76) | 457 (55) | 454 (48) |
| Noncritical Go RT (ms) | 370 (37) | 361 (33) | 364 (29) | 359 (31) |
| Stop Accuracy (%) | 54 (4) | 53 (6) | 54 (3) | 54 (5) |
| SSRT (ms) | 249 (34) | 272 (31) | 241 (34) | 245 (36) |
| Response Slowing Effect (ms) | 111 (63) | 118 (61) | 92 (42) | 95 (37) |
| preSMA |
Vertex |
|||||
|---|---|---|---|---|---|---|
| Experiment 2 | noTMS | cueTMS | ssdTMS | noTMS | cueTMS | ssdTMS |
| Go Cue RT (ms) | 508 (65) | 498 (63) | 515 (67) | 505 (74) | 493 (74) | 509 (78) |
| Go Alt RT (ms) | 508 (66) | 497 (63) | 515 (68) | 504 (75) | 493 (73) | 510 (77) |
| Probe Accuracy (%) | 50 (9) | 48 (10) | 48 (10) | 51 (8) | 49 (8) | 50 (8) |
| Probe Correct Alt RT (ms) | 818 (159) | 805 (153) | 818 (176) | 780 (149) | 758 (164) | 779 (155) |
| Stopping Interference Effect (ms) | 310 (120) | 307 (112) | 303 (128) | 276 (112) | 264 (124) | 270 (113) |
| SSRT (ms) | 270 (46) | 283 (47) | 296 (62) | 269 (40) | 286 (36) | 267 (36) |
Fig. 2.
A: stop-signal reaction time (SSRT) for the TMS trials was significantly longer than that for the noTMS trials in the preSMA session (P < 0.01) and SSRT for the TMS trials in the preSMA session was significantly longer than that in the vertex session (P < 0.01) in experiment 1. B: SSRT for the ssdTMS trials was significantly longer than that for the noTMS trials in the preSMA session (P < 0.01) and SSRT for the ssdTMS trials in the preSMA session was significantly longer than that in the vertex session (P < 0.05) in experiment 2.
The effect of preSMA stimulation on SSRT was unlikely due to distraction or discomfort because there were no significant interactions between locus and stimulation for the response-slowing effect, critical Go RT, noncritical Go RT, and Stop accuracy (all values of P > 0.4). The fact that there was no significant interaction for the response-slowing effect suggests that preSMA stimulation did not modulate response tendencies.
Thus, although stimulation prior to the stop signal did not lead to a modulation of response tendencies, it did lengthen SSRT. This replicates the findings of Chen et al. (2009), although with greater potential spatial specificity since we used a distance-adjusted thresholding method and Batwing TMS coil, which collectively increase confidence that the stimulation specifically targeted the right preSMA rather than the wider/deeper dorsomedial frontal regions. It also suggests that the increased SSRT is not due to disruption of modulation of response tendencies. Nevertheless, the elongation in SSRT could relate to a disruption in setting up the stopping rule or it could relate to disruption in implementing stopping. Although stimulation did occur before the stop signal, the effects of TMS could persist for 150 ms or more (Izumi et al. 1997) (thus affecting the implementation of stopping stage). In addition, the conditional stop signal task does not require the participant to update the stopping rule trial by trial, because the critical direction remains the same throughout the whole experiment. To further dissociate the possible functional roles of the right preSMA, we conducted a second experiment with a selective stop paradigm (Fig. 1B) (Aron and Verbruggen 2008; Cai et al. 2011; Claffey et al. 2010).
Experiment 2
Table 1 and Fig. 2B show the behavioral data. There was a significant interaction between locus (preSMA vs. vertex) and stimulation (noTMS, cueTMS, and ssdTMS) on SSRT [F(2,30) = 6.19, P < 0.006] and a significant main effect of stimulation [F(2,30) = 5.18, P < 0.012], but no significant main effect of locus (P > 0.37). A follow-up 2 × 2 ANOVA for the cue period showed no interaction between locus (preSMA vs. vertex) and stimulation (noTMS vs. cueTMS) (P > 0.7). A follow-up 2 × 2 ANOVA for the stop signal period showed a significant interaction between locus (preSMA vs. vertex) and stimulation (noTMS vs. ssdTMS) [F(1,15) = 5.85, P = 0.029]. Paired t-tests showed that, for the preSMA session, SSRT on the ssdTMS trials (296 ± 62 ms) was significantly longer than SSRT on the noTMS trials (270 ± 46 ms) [t(15) = 3.15, P = 0.007] and SSRT on the ssdTMS trials for the preSMA session was significantly longer than SSRT on the ssdTMS trials for the vertex session (267 ± 36 ms) [t(15) = 2.16, P < 0.05].
These results were unlikely related to nonspecific effects of distraction/discomfort because there was no significant interaction between locus and stimulation for Go Cue RT, Go Alt RT, Probe Accuracy, and Probe Alt RT (all values of P > 0.3). The fact that there was no significant interaction between locus and stimulation for the stopping interference effect (P > 0.8) speaks against a role for the right preSMA in setting up the stopping rule. There was a significant main effect of locus for the stopping interference effect (P < 0.005); stopping interference effect was larger in the preSMA session than that in the vertex session. The generally increased interference effect in the preSMA session is probably attributable to the stopping difficulty induced by the ssdTMS trials.
The fact that we did not find an effect in the cue period does not rule out the possibility that TMS over the cue period does temporally disrupt the process of setting up the stopping rule; it's just that this process could take longer and finish before the stop signal on most trials; thus, disruption could be ineffective. Thus, the TMS effect on the stopping interference effect might not be observable because the selective stopping mechanism has already been established before the stop signal on the cueTMS trials. Nevertheless, we can still test the “setting up of the stopping rule” hypothesis using Go RT instead of the stopping interference effect, based on the psychological refractory period effect (Pashler 1994; Verbruggen et al. 2010). The psychological refractory period effect refers to the increase of response latency that is caused by a processing bottleneck when the delay between two decision processes is so short that the second decision has to be postponed until the first decision is complete. If TMS over the cue period increased the length of setting up a selective stopping mechanism, one would predict that Go RT would increase since response selection in the go trials would not start before setting up the stopping rule is finished. However, we did not see a significant interaction between locus and stimulation on either Go Cue RT or Go Alt RT (P values > 0.79). Again, the current result speaks against the “setting up of the stopping rule” hypothesis.
DISCUSSION
Many studies implicate the right preSMA in behavioral stopping, although its critical importance has not been strongly established and neither has its underlying functional role. In two experiments, using an MRI-based method for subject-specific calibration of TMS intensity, we show that stimulation over the right preSMA specifically affects the implementation of stopping. This was relative to a control region (the vertex) and unlikely due to distraction or other nonspecific effects, because it specifically affected SSRT but not other behavioral indices.
Although the effect of TMS over the preSMA (in the left hemisphere) on stopping has been examined in a previous study (Chen et al. 2009), the current study goes beyond their finding by showing that the TMS over the right preSMA specifically disrupted the implementation of stopping process but not others. Chen et al. (2009) applied TMS at the time of the go signal and also 100 ms after the go signal and found that SSRT was significantly longer when the TMS coil was placed over the left preSMA versus vertex. However, it is unclear which underlying processes were disrupted by the stimulation of the left preSMA. The elongated SSRT could have arisen from disrupting the setting up of the stopping network, the modulation of response tendencies, or the implementation of stopping itself (through lingering effects). Chen et al. (2009) could not test these different accounts because they used a simple stop-signal task. In the current study, we replicate the result from Chen et al. (2009), although we used the distance-adjusted thresholding method, which increases confidence that the effect relates to the right preSMA rather than other areas of the dorsomedial frontal cortex. Moreover, we show that, at least for that locus and stimulation time, the effect of preSMA stimulation was on SSRT and not on the modulation of response tendencies. We then provide further replication, in experiment 2, that preSMA stimulation elongates SSRT. This points to a disruption of the implementation of stopping because the stimulation after the stop signal was too late to affect setting up the stopping rule and no other behavioral measures were influenced. Taken together, experiments 1 and 2 suggest that the right preSMA (alone or via the wider stopping network) plays a critical role in implementing stopping and it can be differentiated from response tendency modulation or setting up the stopping rule.
TMS Affects Stopping via the Right preSMA Alone or the Wider Stopping Network
Although these findings are compatible with the hypothesis that the preSMA is itself critical for implementing the stopping process (Chen et al. 2009; Floden and Stuss 2006; Li et al. 2006; Mostofsky and Simmonds 2008; Nachev et al. 2007) there is an alternative possibility: that stimulating the preSMA had remote effects on other key nodes in the stopping network such as the right IFG or basal ganglia. Indeed, combined fMRI and TMS studies have revealed that TMS can induce changes in the blood oxygenation level–dependent (BOLD) signal in interconnected remote regions (Heinen et al. 2011; Ruff et al. 2006, 2008). For example, Ruff et al. (2006) showed that TMS over the frontal eye field can influence visual perception via modulating BOLD activity in the visual cortex. In relation to the current study, it is now quite well established that the preSMA along with the right IFG and subthalamic nucleus (STN) are activated in stopping and they are anatomically connected with each other (Aron et al. 2007a; Duann et al. 2009; Ford et al. 2010; Forstmann et al. 2010; Johansen-Berg et al. 2004; King et al. 2011). Indeed, macrostimulation of both the preSMA and the right IFG can induce motor arrest (Fried et al. 1991; Luders et al. 1988), and stimulation of the preSMA in a single patient with electrocorticography revealed evoked responses within 30 ms in the right IFG, corresponding well to a white-matter connection and also stopping-related task-evoked responses (Swann et al. 2012). It is thus possible that, by stimulating the right preSMA with TMS, we may have disturbed neuronal activity in the right IFG or basal ganglia, thus leading to impaired stopping behavior. A third alternative is that the entire network is needed to implement outright stopping, perhaps via long-range synchronization in the beta frequency band (Swann et al. 2011), and any perturbation to this network at any node could affect its function. Therefore, the conclusion we draw in the current study is that the right preSMA is either critical for implementing stopping, or it is part of a network that is critical, and stimulating it with TMS has an impact on the wider stopping network. Future studies are required to adjudicate between these possibilities, such as by using concurrent TMS-fMRI to examine the activity change in the right IFG and STN during TMS of the right preSMA while the participant stops action.
Stimulation of the preSMA Affects Both Standard (Global) and Selective Stopping
A distinction between standard (nonselective) and selective stopping has recently been made on the basis of behavioral and physiological studies (Aron and Verbruggen 2008; Greenhouse et al. 2012; Majid et al. 2011). The standard (nonselective) form of stopping is putatively implemented via the hyperdirect pathway to the subthalamic nucleus of the basal ganglia (Aron et al. 2007b; Forstmann et al. 2012; Jahfari et al. 2011; Ray et al. 2012), whereas the selective stopping could be implemented by the indirect pathway through the striatum. Although much evidence shows that the basic form of stopping recruits preSMA, right IFG, and the basal ganglia (Aron and Poldrack 2006; Boehler et al. 2010; Cai and Leung 2009, 2011; Chevrier et al. 2007; Chikazoe et al. 2009b; Curtis et al. 2005; Konishi et al. 1999; Leung and Cai 2007; Li et al. 2006; Rubia et al. 2001; Vink et al. 2005; Xue et al. 2008) and a white-matter network connecting these nodes (Aron et al. 2007a; Forstmann et al. 2012; King et al. 2011; Madsen et al. 2010), the neural substrates of selective stopping are not well established, with just one study to date showing activation of the preSMA and right IFG (see Coxon et al. 2009).
In our current experiment 2, we showed that stimulation of the preSMA affects mechanistically selective stopping and not just the standard, nonselective stopping that putatively occurs in experiment 1 and in Chen et al. (2009). Therefore, we suggest that the preSMA (or the network to which it is connected) is functionally critical for both global and selective stopping.
Role of the preSMA in Response Modulation and Setting Up the Stopping Rule
Although TMS over the right preSMA did affect the implementation of stopping, it did not affect the setting up of the stopping rule or the modulating of response tendencies. However, methodological considerations argue against a strong interpretation of these negative results. First, whereas event-related TMS has high temporal resolution, this comes with the downside that one could “miss” a cognitive process of interest. Although the stimulation times were carefully determined from previous studies (Cai et al. 2011; Claffey et al. 2010; Jahfari et al. 2009), it is possible that the TMS pulse missed the critical timing for setting up the stopping rule or modulating response tendencies. Second, although this form of TMS has reasonable local spatial resolution, this comes with the downside that one could “miss” a key functional area in a structure as large and deep as the preSMA. Although the stimulation loci were carefully chosen from the previous study and calibrated individually, it is likely that the preSMA has multiple functional subregions (Zhang et al. 2012). For example, the region that is critical for stopping might not overlap with the region that is critical for setting up the stopping rule and/or the one for modulating response tendencies. Indeed, fMRI studies do point to different loci in left/right anterior/posterior preSMA for different aspects of stopping, switching, and wider action control (Aron and Poldrack 2006; Boecker et al. 2010; Boehler et al. 2010; Brass and von Cramon 2002; Cai and Leung 2009; Chikazoe et al. 2009a,b; Cunnington et al. 2006; Dove et al. 2000; Fassbender et al. 2009; Leung and Cai 2007; Li et al. 2006; Luks et al. 2002, 2007; Rubia et al. 2001; Rushworth et al. 2002). Thus we might have affected the preSMA region for stopping (or for the stopping network) but missed the preSMA region critical for setting up the stopping rule and/or modulating response tendencies. Indeed, as we pointed out in the introduction, a role for the preSMA in setting up the stopping rule, or more widely, in task configuration for cognitive control is suggested by dozens of studies ranging from neurophysiology (Chen et al. 2010; Isoda and Hikosaka 2007; Matsuzaka and Tanji 1996; Matsuzaka et al. 1992) to fMRI (Brass and von Cramon 2002; Dove et al. 2000; Luks et al. 2002, 2007; Rushworth et al. 2002) to lesion and TMS (Chen et al. 2009; Floden and Stuss 2006; Nachev et al. 2007). Therefore, previous studies suggest that the preSMA is engaged in an early stage of action control, although the current study does not directly speak to this role. Future research is required to explore the effect of TMS at different times and at different preSMA subregions.
Conclusion
In two experiments we found that stimulation over the right preSMA affected the implementation of stopping. This was relative to a control region (the vertex) and unlikely due to distraction or other nonspecific effects, because it specifically affected SSRT and not other behavioral indices. Because we used an MRI-based method to tailor stimulation to individual subjects we can increase the confidence that this was due to a specific effect on the right preSMA rather than other areas within dorsomedial frontal cortex. Although these studies clarify that preSMA stimulation (at the specific locus we chose from fMRI studies) does affect the implementation of the stopping process, the lack of effects on setting up the stopping rule and modulating response tendencies are not easily interpreted. Further, because TMS at a node such as the right preSMA likely has effects on remote nodes in an overall, and well-established, white-matter network, we cannot distinguish between the possibilities that the right preSMA is itself critical for implementing stopping or that it participates in a network whose other nodes implement stopping or that the network as a whole performs this function. Nevertheless, the current study provides strong evidence for a critical role for the right preSMA in implementing stopping, either alone or within the network, buttressing a now substantial functional imaging literature on this network. The results encourage the future use of concurrent TMS-fMRI to further study the network dynamics of cognitive control, that is, the communication among the right preSMA, right IFG, the striatum, and the subthalamic nucleus while stopping a prepotent response.
GRANTS
This work was supported by a National Institute on Drug Abuse Grant DA-026452.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.C., F.V., C.D.C., and A.R.A. conception and design of research; W.C. and J.S.G. performed experiments; W.C. analyzed data; W.C., F.V., C.D.C., and A.R.A. interpreted results of experiments; W.C. prepared figures; W.C. and A.R.A. drafted manuscript; W.C., J.S.G., F.V., C.D.C., and A.R.A. edited and revised manuscript; W.C., J.S.G., F.V., C.D.C., and A.R.A. approved final version of manuscript.
REFERENCES
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27: 3743–3752, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci 27: 11860–11864, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 26: 2424–2433, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Verbruggen F. Stop the presses: dissociating a selective from a global mechanism for stopping. Psychol Sci 19: 1146–1153, 2008 [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 112: 105–142, 2003 [DOI] [PubMed] [Google Scholar]
- Boecker M, Drueke B, Vorhold V, Knops A, Philippen B, Gauggel S. When response inhibition is followed by response reengagement: an event-related fMRI study. Hum Brain Mapp 32: 94–106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain–conjunction analyses of the Stop-signal task. Neuroimage 52: 1621–1632, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacz R, Wagenmakers EJ, Forstmann BU, Nieuwenhuis S. The neural basis of the speed–accuracy tradeoff. Trends Neurosci 33: 10–16, 2010 [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex 12: 908–914, 2002 [DOI] [PubMed] [Google Scholar]
- Cai W, George J, Chambers CD, Stokes MG, Verbruggen F, Aron AR. Stimulating deep cortical structures with the Batwing Coil: how to determine the intensity for transcranial magnetic stimulation using coil-cortex distance. J Neurosci Methods 204: 238–241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Leung HC. Cortical activity during manual response inhibition guided by color and orientation cues. Brain Res 1261: 20–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Leung HC. Rule-guided executive control of response inhibition: functional topography of the inferior frontal cortex. PLoS One 6: e20840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Oldenkamp C, Aron AR. A proactive mechanism for selective suppression of response tendencies. J Neurosci 31: 5965–5969, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB. Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci 18: 444–455, 2006 [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 33: 631–646, 2009 [DOI] [PubMed] [Google Scholar]
- Chao HH, Luo X, Chang JL, Li CS. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time: an intra-subject analysis (Abstract). BMC Neurosci 10: 75, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. Neuroimage 44: 537–545, 2009 [DOI] [PubMed] [Google Scholar]
- Chen X, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci 30: 14657–14675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp 28: 1347–1358, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr Opin Psychiatry 23: 267–272, 2010 [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex 19: 146–152, 2009a [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci 29: 15870–15877, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claffey MP, Sheldon S, Stinear CM, Verbruggen F, Aron AR. Having a goal to stop action is associated with advance control of specific motor representations. Neuropsychologia 48: 541–548, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Stop and go: the neural basis of selective movement prevention. J Cogn Neurosci 21: 1193–1203, 2009 [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Robinson S, Moser E. The selection of intended actions and the observation of others' actions: a time-resolved fMRI study. Neuroimage 29: 1294–1302, 2006 [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D'Esposito M. Canceling planned action: an fMRI study of countermanding saccades. Cereb Cortex 15: 1281–1289, 2005 [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD. Strategies and mechanisms in nonselective and selective inhibitory motor control. J Exp Psychol Hum Percept Perform 21: 498–511, 1995 [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res 9: 103–109, 2000 [DOI] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 29: 10171–10179, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Hester R, Murphy K, Foxe JJ, Foxe DM, Garavan H. Prefrontal and midline interactions mediating behavioural control. Eur J Neurosci 29: 181–187, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci 18: 1843–1849, 2006 [DOI] [PubMed] [Google Scholar]
- Ford A, McGregor KM, Case K, Crosson B, White KD. Structural connectivity of Broca's area and medial frontal cortex. Neuroimage 52: 1230–1237, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Anwander A, Schafer A, Neumann J, Brown S, Wagenmakers EJ, Bogacz R, Turner R. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci USA 107: 15916–15920, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmakers EJ. Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci USA 105: 17538–17542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Keuken MC, Jahfari S, Bazin PL, Neumann J, Schäfer A, Anwander A, Turner R. Cortico-subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. Neuroimage 60: 370–375, 2012 [DOI] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci 11: 3656–3666, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Oldenkamp CL, Aron AR. Stopping a response has global or nonglobal effects on the motor system depending on preparation. J Neurophysiol 107: 384–392, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50: 1313–1319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen K, Ruff CC, Bjoertomt O, Schenkluhn B, Bestmann S, Blankenburg F, Driver J, Chambers CD. Concurrent TMS-fMRI reveals dynamic interhemispheric influences of the right parietal cortex during exogenously cued visuospatial attention. Eur J Neurosci 33: 991–1000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TY, Tseng LY, Yu JX, Kuo WJ, Hung DL, Tzeng OJ, Walsh V, Muggleton NG, Juan CH. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage 56: 2249–2257, 2011 [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci 10: 240–248, 2007 [DOI] [PubMed] [Google Scholar]
- Izumi S, Takase M, Arita M, Masakado Y, Kimura A, Chino N. Transcranial magnetic stimulation–induced changes in EEG and responses recorded from the scalp of healthy humans. Electroencephalogr Clin Neurophysiol 103: 319–322, 1997 [DOI] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with restraint: what are the neurocognitive mechanisms? J Cogn Neurosci 22: 1479–1492, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci 31: 6891–6899, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156, 2001 [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 101: 13335–13340, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AV, Linke J, Gass A, Hennerici MG, Tost H, Poupon C, Wessa M. Microstructure of a three-way anatomical network predicts individual differences in response inhibition: a tractography study. Neuroimage 59: 1949–1959, 2012 [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain 122: 981–991, 1999 [DOI] [PubMed] [Google Scholar]
- Leung HC, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci 27: 9893–9900, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B. On the Holm, Simes, and Hochberg multiple test procedures. Am J Public Health 86: 628–629, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann NY Acad Sci 1224: 40–62, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci 26: 186–192, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform 10: 276–291, 1984 [DOI] [PubMed] [Google Scholar]
- Luders H, Lesser RP, Dinner DS, Morris HH, Wyllie E, Godoy J. Localization of cortical function: new information from extraoperative monitoring of patients with epilepsy. Epilepsia 29(Suppl. 2): S56–S65, 1988 [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Dale CL, Hough MG. Preparatory allocation of attention and adjustments in conflict processing. Neuroimage 35: 949–958, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL. Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. Neuroimage 17: 792–802, 2002 [PubMed] [Google Scholar]
- Madsen KS, Baare WF, Vestergaard M, Skimminge A, Ejersbo LR, Ramsoy TZ, Gerlach C, Akeson P, Paulson OB, Jernigan TL. Response inhibition is associated with white matter microstructure in children. Neuropsychologia 48: 854–862, 2010 [DOI] [PubMed] [Google Scholar]
- Majid DS, Cai W, George JS, Verbruggen F, Aron AR. Transcranial magnetic stimulation reveals dissociable mechanisms for global versus selective corticomotor suppression underlying the stopping of action. Cereb Cortex 22: 363–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. J Neurophysiol 68: 653–662, 1992 [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Tanji J. Changing directions of forthcoming arm movements: neuronal activity in the presupplementary and supplementary motor area of monkey cerebral cortex. J Neurophysiol 76: 2327–2342, 1996 [DOI] [PubMed] [Google Scholar]
- Mikuni N, Ohara S, Ikeda A, Hayashi N, Nishida N, Taki J, Enatsu R, Matsumoto R, Shibasaki H, Hashimoto N. Evidence for a wide distribution of negative motor areas in the perirolandic cortex. Clin Neurophysiol 117: 33–40, 2006 [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci 20: 751–761, 2008 [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9: 856–869, 2008 [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage 36(Suppl. 2): T155–T163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci USA 107: 13240–13245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull 116: 220–244, 1994 [DOI] [PubMed] [Google Scholar]
- Ray NJ, Brittain J-S, Holland P, Joundi RA, Stein J, Aziz TZ, Jenkinson N. The role of the subthalamic nucleus in response inhibition: evidence from local field potential recordings in the human subthalamic nucleus. Neuroimage 60: 271–278, 2012 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science 306: 443–447, 2004 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994 [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage 13: 250–261, 2001 [DOI] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal vs. frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb Cortex 18: 817–827, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol 16: 1479–1488, 2006 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87: 2577–2592, 2002 [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA 107: 6106–6111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, Strunk J, George J, Aron AR. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson's disease. J Neurosci 31: 5721–5729, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Cai W, Pieters T, Claffey MP, George JS, Conners C, DiSano M, Aron AR, Tandon N. Roles for the presupplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage 59: 2860–2870, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci USA 107: 13966–13971, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci 12: 418–424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev 33: 647–661, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. J Exp Psychol Hum Percept Perform 35: 835–854, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp 25: 336–344, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex 18: 1923–1932, 2008 [DOI] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CS. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex 22: 99–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]