Abstract
Clinical evidence has suggested that subtle changes in deep brain stimulation (DBS) settings can have differential effects on bradykinesia and rigidity in patients with Parkinson's disease. In this study, we first investigated the degree of improvement in bradykinesia and rigidity during targeted globus pallidus DBS in three 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated rhesus macaques. Behavioral outcomes of DBS were then coupled with detailed, subject-specific computational models of neurons in the globus pallidus internus (GPi), globus pallidus externus (GPe), and internal capsule (IC) to determine which neuronal pathways when modulated with high-frequency electrical stimulation best correlate with improvement in motor symptoms. The modeling results support the hypothesis that multiple neuronal pathways can underlie the therapeutic effect of DBS on parkinsonian bradykinesia and rigidity. Across all three subjects, improvements in rigidity correlated most strongly with spread of neuronal activation into IC, driving a small percentage of fibers within this tract (<10% on average). The most robust effect on bradykinesia resulted from stimulating a combination of sensorimotor axonal projections within the GP, specifically at the site of the medial medullary lamina. Thus the beneficial effects of pallidal DBS for parkinsonian symptoms may occur from multiple targets within and near the target nucleus.
Keywords: Parkinson's disease, globus pallidus
clinical evidence from parkinson's disease (PD) patients with deep brain stimulation (DBS) implants has shown that making subtle adjustments to a patient's stimulator settings can have differential effects on bradykinesia and rigidity scores, regardless of whether the DBS target is the subthalamic nucleus (STN) or the globus pallidus (GP) (Bejjani et al. 1998; Butson et al. 2011; Krack et al. 1998; Yelnik et al. 2000). These studies, along with a recent case study of STN-DBS in an 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated nonhuman primate (Xu et al. 2011), support the mechanistic hypothesis that treating both bradykinesia and rigidity with DBS may require direct stimulation of more than one neuronal pathway within the brain. It remains unclear, however, which neuronal pathways to target for relieving parkinsonian bradykinesia and rigidity and to what extent those pathways are involved in the therapeutic mechanisms of DBS.
Computational models provide a framework to address such hypotheses by coupling model predictions of the volume of tissue activated in the brain to symptomatological outcome measures. An earlier study of ours investigated the neuronal pathways targeted during therapeutic STN-DBS in parkinsonian nonhuman primates (Miocinovic et al. 2006). We have now investigated the therapeutic mechanisms of GP-DBS in terms of how activating pallidofugal [GP externus (GPe) and GP internus (GPi)] and internal capsular (IC) output with high-frequency stimulation affects the severity of bradykinesia and limb rigidity in parkinsonian nonhuman primates. Our computational models of GP-DBS have previously shown that stimulation activates not only GPi efferents, but also axonal projections extending from GPe that synapse en passant through GPi en route to STN (Johnson and McIntyre 2008). At the same time, because of the close proximity of the GP to the corticospinal and corticobulbar tracts of IC (Schmahmann and Pandya 2006), GP-DBS may also influence activity patterns within these fiber tracts, in turn generating a therapeutic effect (Ashby and Rothwell 2000; Johnson et al. 2009). Antidromic activation of motor cortex has also been reported during STN-DBS (Dejean et al. 2009; Gradinaru et al. 2009; Li et al. 2007). Indeed, transcranial stimulation, at levels thought to produce weak activation of the corticospinal tract pathway, has been reported to improve several parkinsonian motor symptoms, especially rigidity (Fregni et al. 2006; Lefaucheur et al. 2004). However, at higher levels of stimulation through electrodes near IC, strong muscle contractions can also appear (Ashby et al. 1998; Butson et al. 2007; Tommasi et al. 2008) and thus worsen bradykinesia and rigidity outcome measures (Xu et al. 2011).
The GP provides an opportune target structure to investigate the mechanisms of DBS on bradykinesia and rigidity, because unlike the STN, which receives direct projections from motor cortical areas (Afsharpour 1985), electrical stimulation with an activation volume confined to the GP is unlikely to generate antidromic responses in motor cortex (Parent and Hazrati 1995). Moreover, the GP, which is significantly larger in volume than the STN (Yelnik 2002), is more conducive to spatial targeting with macroscale electrodes. In this study, three MPTP-treated nonhuman primates with moderate parkinsonian motor symptoms were implanted unilaterally with a scaled-down version of the human DBS lead and were evaluated behaviorally across a range of stimulation voltages and electrode configurations. Computational neuron models of DBS (Johnson and McIntyre 2008) were tailored to each subject's brain anatomy and were used to investigate how modulation of the pallidofugal and IC pathways influences behavioral outcome measures of parkinsonian limb rigidity and bradykinesia.
MATERIALS AND METHODS
Animals.
We studied three female rhesus macaques (Macaca mulatta). All surgical procedures and behavioral protocols were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic and complied with United States Public Health Service policy on the humane care and use of laboratory animals.
Surgical procedures.
Using aseptic surgical procedures under isoflurane anesthesia, mild to moderate hemiparkinsonian states were induced through unilateral intracarotid infusions of MPTP (0.4–0.6 mg/kg over a 10-min period). In two of the three nonhuman primates (NE and RA), additional intramuscular injections of MPTP were administered to augment parkinsonian motor symptoms (0.2–0.4 mg/kg daily treatment over a 4- to 5-day period). All three animals reached a moderately parkinsonian state (Fig. 1A) such that akinetic symptoms did not prevent them from performing a reach-and-retrieval task for food reward.
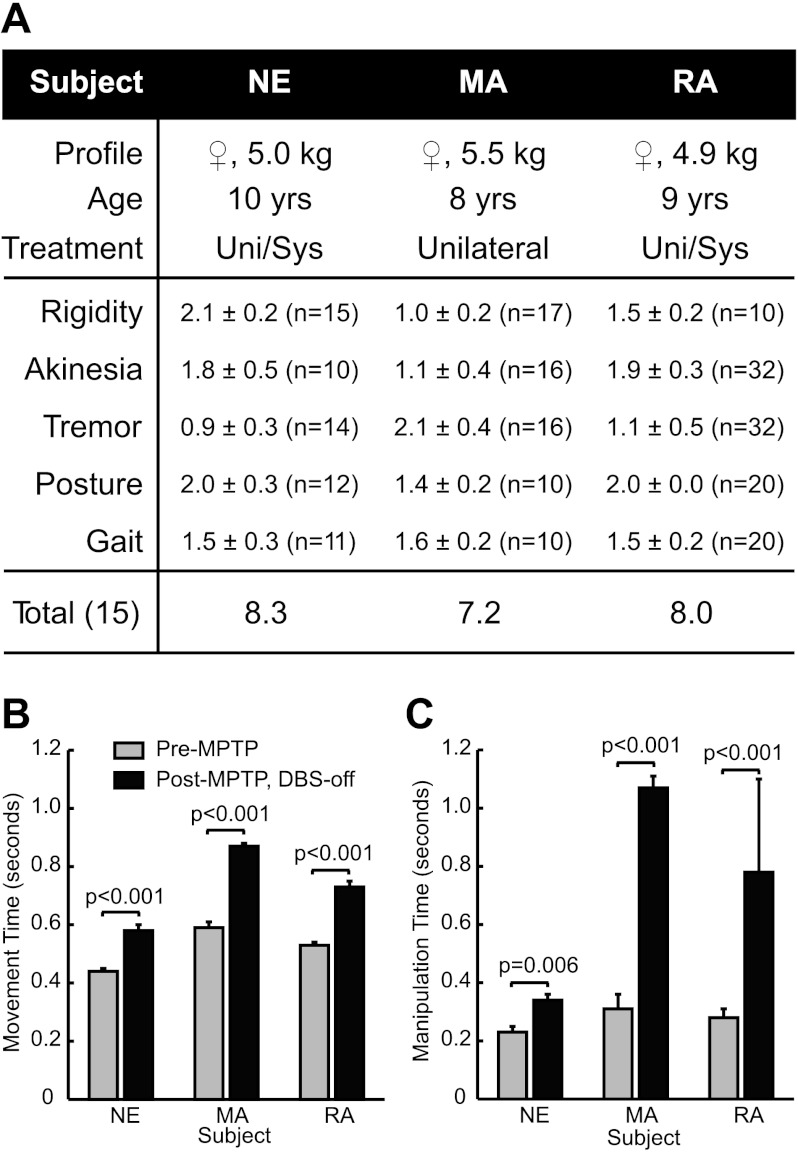
Fig. 1.
Characterization of parkinsonian motor signs in the deep brain stimulation-implanted (DBS-OFF) state. A: the average severities of parkinsonian motor signs were evaluated before 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment and again following MPTP treatment and DBS lead implantation (3: severe; 0: no symptoms). The number of testing sessions is given by n in each case. Scores are reported as ±SE following MPTP treatment in the DBS-OFF state. MPTP treatment elicited a moderate parkinsonian state in each subject. Bradykinesia (B) and finger dexterity (C) were quantified in terms of performance on a reach-and-retrieval task for food reward. Following administration of MPTP, movements became significantly slower, and the ability to manipulate small objects became impaired. Uni, unilateral; Sys, systemic.
Neurosurgical planning incorporated computed tomography (CT) scans and T1/T2-weighted magnetic resonance images acquired from each animal under propofol anesthesia (Miocinovic et al. 2007). Surgical procedures used to implant the cranial chambers and implantable pulse generators (IPG) are described in detail elsewhere (Hashimoto et al. 2003; Johnson et al. 2009). The smaller 17-mm DBS chamber held a 30–35° angle from vertical in the coronal plane. An IPG (Medtronic Itrel II) was placed subcutaneously below the scapula, and an extension cable was routed subcutaneously between the IPG and the DBS chamber. Analgesics were provided before and after these procedures.
Following a recovery period, microelectrode recordings were performed through the DBS chamber to verify coordinates and boundaries of each pallidal segment before DBS lead implantation. Microdrive-guided insertions were made with a single tungsten microelectrode (FHC) or a linear array of 12 microelectrodes on a tungsten carrier with each axial site separated by 300 μm (NeuroNexus). Spike recordings were sampled at 25 kHz and band-pass filtered between 250 and 5,000 Hz (AlphaOmega). The GPe and GPi were identified by rates and burst patterns characteristic of a parkinsonian state, and regions within these nuclei were differentiated by their responses to sensorimotor manipulation (Filion et al. 1988; Filion and Tremblay 1991). Microstimulation (10–100 μA) was used to identify the corticospinal tract of IC, and recordings of evoked potentials in response to a strobe light were used to establish the coordinates of the optic tract. The microelectrode track that yielded well-defined sensorimotor representations in both pallidal segments and high thresholds for contralateral muscle contraction side effects was chosen for implantation of the DBS lead.
The DBS lead used in this study was a scaled-down version of the human DBS lead and consisted of four cylindrical electrode contacts (750-μm diameter, 500-μm height) each separated by 500 μm (Advanced Bionics and NuMED). Following insertion to the target depth, the DBS lead was secured with glue to an interlocking ring that fit within a circular groove in the metal chamber (Elder et al. 2005). Contact wires from each of the DBS electrodes were either linked directly to the IPG or connected to a switch that enabled stimulation through either the IPG or an external device. Within 1 wk of the implantation, postoperative CT scans were performed to verify the orientation and position of the DBS lead within the GP.
Behavior.
The animals were housed on a 12:12-h light-dark cycle. Behavioral tests to assess parkinsonian motor symptoms were conducted at approximately the same time each day for a given animal. Before and after implantation of the DBS lead, baseline parkinsonian motor scores were collected using a modified version of the Unified Parkinson's Disease Rating Scale (UPDRS) to ensure that a substantial therapeutic effect did not occur from the implantation process (Fig. 1A). An examiner, who was blinded to the condition of the animal, rated the severity of the animals' rigidity, akinesia, resting tremor, posture, and gait (0, no symptoms, to 3, severe symptoms). Scores were averaged over multiple behavioral testing sessions, each consisting of observing the animal sitting in a chair and ambulating in a plastic enclosure. Akinesia was assessed by the amount of spontaneous movement over a 30-min interval. Although each of these parkinsonian symptoms was present at varying degrees in the three animals, we only evaluated rigidity and bradykinesia (slowness of movement) in the context of DBS.
Rigidity and bradykinesia were evaluated in baseline sessions and during DBS sessions. With the nonhuman primate awake and resting comfortably in its chair, an examiner blinded to the applied DBS setting assessed muscle rigidity according to the degree of resistance to passive movements of the elbow, shoulder, hip, and knee joints contralateral to the implanted DBS lead (0, no symptoms, to 3, severe symptoms). Rigidity scores were averaged across joints for each session. Bradykinesia was assessed through a self-paced, reach-and-retrieval task for food reward. Video of each trial was recorded at 30 frames per second, and frame-by-frame analysis was used to calculate and differentiate the movement time of each trial, defined as the summation of reach and retrieval times, from manipulation time (Johnson et al. 2009). Comparisons between a pre-MPTP state and a post-MPTP/DBS-implanted (DBS-OFF) state showed significant increases in movement time and time to grasp the food reward (Fig. 1, B and C), which is consistent with bradykinesia and impaired fine manipulation in human PD (Berardelli et al. 2001). These two measures (rigidity and bradykinesia) were used for all DBS-OFF/ON trials.
DBS-ON sessions were conducted at least 1 mo after lead implantation to reduce susceptibility of the voltage fields to known changes in electrode-tissue impedances (Lempka et al. 2009). Stimulation settings consisted of a sustained pulse train at 135 Hz with charge-balanced pulses (90-μs long cathodic phase, 3.5-ms long anodic phase). Voltages ranged from 0 to 3 V for monopolar and 0 to 6 V for bipolar configurations. Multiple trials were conducted over multiple days for each DBS setting. Parkinsonian motor signs were examined after 1–5 min of stimulation. In the case when multiple DBS settings were examined on a single testing session, trials were separated by at least 10 min of no stimulation. Several factors constrained the number of DBS settings that could be examined during the programming process, including the necessity for washout periods following DBS and the induction of contralateral muscle contractions during DBS at higher voltages. A summed total of 87 different simulation parameter sets were investigated.
Neuron and electrical field models.
A nonhuman primate brain atlas (Martin and Bowden 2000) was edge-warped to preoperative MR images (Edgewarp). Surface reconstructions of the external and internal GP were generated in a three-dimensional nonuniform rational basis spline modeling environment (Rhinoceros). The MRI-based reconstructions were adjusted with minor scale and translation corrections to match the borders of the nuclei identified during microelectrode mapping sessions.
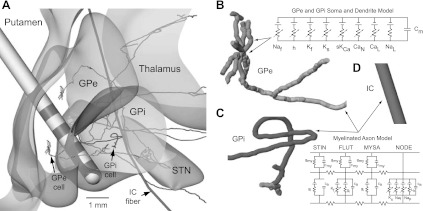
The computational neuron models, described in detail elsewhere (Johnson and McIntyre 2008), consisted of a population of GPe and GPi neurons that were reconstructed from nonhuman primate biotinylated dextran amine-labeled pallidal neurons (Parent et al. 2001; Sato et al. 2000; Fig. 2). These morphologies were converted into a series of compartments with lengths and thicknesses defined individually for the soma, dendrites, and axons of the pallidal neurons. The GPe neuron morphologies were distributed randomly within the reconstruction of each subject's GPe such that the axonal processes passed either through GPi (n = 370) or directly into the IC (n = 630) en route to STN (Parent and Parent 2002). GPi neurons (n = 1,000) were randomly distributed within the nucleus such that their axonal processes coursed medially along the lenticular fasciculus or ansa lenticularis (Baron et al. 2001; Parent and Parent 2004). The sensorimotor territories of each subject's GPe and GPi were estimated within the posterolateral portion of each pallidal segment according to 1) each subject's sensorimotor responses observed during microelectrode mapping sessions and 2) sensorimotor regions identified through previous histological studies (Francois et al. 1994; Hoover and Strick 1993). Computational neuron models of GPe and GPi were distributed within their respective pallidal segments and labeled according to whether their soma was located within a sensorimotor region. Analysis of the computational model parameter space was constrained to these sensorimotor regions of GP. Model parameters and properties for the pallidal neurons were consistent with those reported previously (Johnson and McIntyre 2008). In addition to the pallidal neuron models, axonal models of motor-related IC fibers (mIC; n = 100) were developed in the context of each subject's MRI-based reconstruction of IC and were distributed uniformly and posterior to the capsular genu (Schmahmann and Pandya 2006). The IC myelinated axon models were represented with nodes of Ranvier (NODE), myelin attachment segments (MYSA), paranode main segments (FLUT), and internode segments (STIN). As previously described (McIntyre et al. 2002), the nodes were instantiated with a membrane capacitance as well as nonlinear fast Na+, persistent Na+, slow K+, and linear leakage conductances. Both MYSA and STIN segments were modeled with a parallel circuit containing a membrane capacitance and linear conductance. The FLUT segments consisted of similar passive membrane properties with the addition of a fast K+ conductance.
Fig. 2.
A computational model of globus pallidus (GP)-DBS (Johnson and McIntyre 2008). The model provided a quantitative prediction of the local cellular effects of DBS in the GP and internal capsule (IC). A: coronal view of the overall model system, which consisted of populations of GP externus (GPe), GP internus (GPi), and motor-related IC (mIC) neurons. Shown are single reconstructions of each neuron type. GPe neurons projected either to subthalamic nucleus (STN) directly through IC or through GPi before projecting through IC. B–D: the neuron models consisted of multiple compartments instantiated with passive and active membrane properties in the soma, dendrites, and axon segments. See Johnson and McIntyre (2008) and McIntyre et al. (2002) for more details on the neuron models. Note that the fiber diameters are not drawn to scale. NAf, fast Na+; h, hyperpolarization-activated cyclic nucleotide-gated (HCN) channel; CaN, N-type Ca2+; CaL, L-type Ca2+; Nap, persistent Na+; Lk, linear leakage; Cm, membrane capacitance; gmy, myelin sheath conductance; cmy, myelin sheath capacitance; ca, internodal axolemma capacitance; gi, internodal axolemma conductance; Kf, fast K+; Ks, slow K+; sKCa, small-conductance Ca2+-activated K+ channel; STIN, internode segments; FLUT, paranode main segments; MYSA, myelin attachment segments; NODE, nodes of Ranvier.
A model of the implanted DBS lead was positioned within the pallidal reconstruction according to a coregistration of preoperative MRI and postoperative CT imaging. Neurons with model compartments found to overlap spatially with the DBS lead were removed from subsequent analysis. An axisymmetric finite-element model simulating the voltage distribution of DBS in neural tissue was applied in COMSOL Multiphysics (Johnson and McIntyre 2008; Miocinovic et al. 2006). The model consisted of a 57% voltage drop at the electrode-tissue interface and a 250-μm thick encapsulation layer (0.18 S/m) between the electrode and bulk tissue (0.20 S/m; Miocinovic et al. 2009). In the NEURON v6.1 programming environment (Hines and Carnevale 1997), multicompartment cable models of sensorimotor GPe and GPi neurons and mIC fibers were simulated with the amplitude of the filtered extracellular stimulation waveform scaled by the finite-element model voltage distributions (Johnson and McIntyre 2008).
Statistical analysis.
Statistical analyses (ANOVA, P < 0.05) were performed for comparisons between clinically rated parkinsonian motor symptom scores in the normal and post-MPTP/DBS-OFF states. These tests were also used to investigate differences in each subject's motor performance on a reach-and-retrieval task. In the three subjects investigated in this study, on average, increasing the voltage of DBS resulted in a decrease in the severity of parkinsonian motor signs up until the voltage-induced contralateral muscle contractions, which confounded the therapeutic effect. The relationships between model predictions and behavioral outcome measures were thus estimated to be second-order in nature. These relationships were examined with polynomial regression analysis and with F-test statistics to investigate whether the second-order regression explained a significant proportion of the variance (P < 0.05).
Histology.
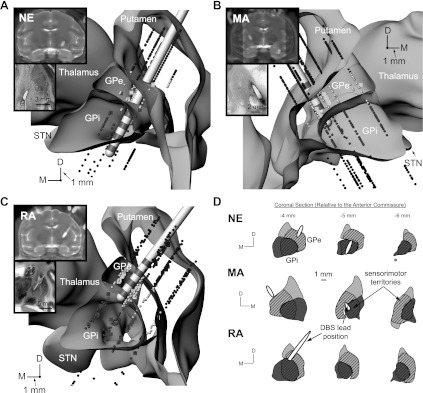
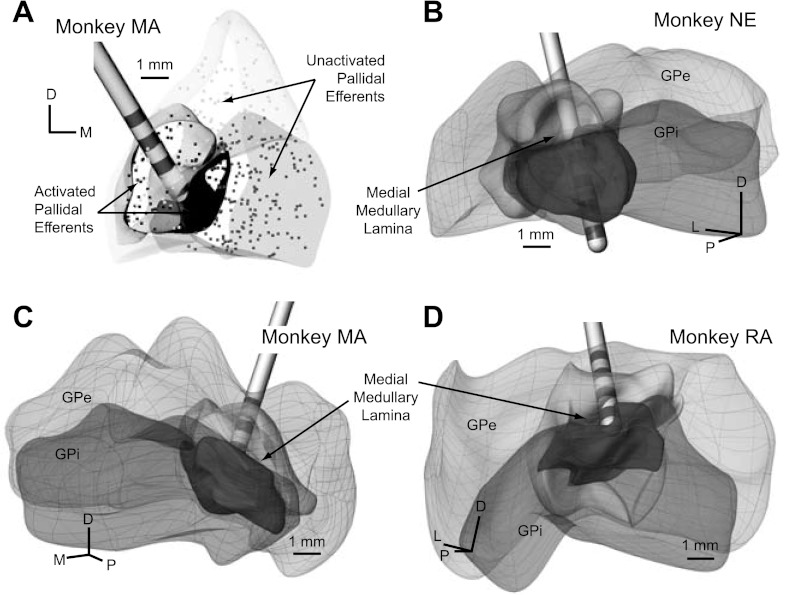
At the conclusion of the experiments, the animals were deeply anesthetized, given an overdose of pentobarbital (100 mg/kg), and perfused with a 10% paraformaldehyde solution. The brains were removed and processed with histological techniques (50-μm sections in either coronal or sagittal planes and Nissl-stained). For each animal, the DBS lead location was identified within the histological slices, which in all three cases validated the imaging-based results of the DBS lead within the GP (Fig. 3).
Fig. 3.
DBS lead localization in the GP. A–C: volumetric reconstructions of the leads within the GP were generated by coregistration and segmentation of preoperative MRI and postoperative computed tomography scans (inset). Histology (inset) was also performed for each lead implant. Stereotactic implantation coordinates for the DBS leads were established by means of microelectrode recordings in which regions of putamen, GPe, GPi, and optic tract were each identified. Cube markers represent cells identified as responsive to sensorimotor manipulation. D: sensorimotor regions (cross-hatched regions) of GPe and GPi were estimated from these recordings as well as from previous histological studies (Francois et al. 1994; Hoover and Strick 1993). D, dorsal; M, medial.
RESULTS
Estimation of the DBS parameter space.
The anatomic coordinates and orientations of the three DBS leads were implanted along different trajectories through the GP, which enabled stimulating a diverse population of GPe and GPi neurons across the three subjects (Fig. 3, A–C). The DBS lead in subject NE had its three proximal electrode contacts in GPi and its distal electrode contact near the border of optic tract. The DBS lead in subjects MA and RA had its two middle electrode contacts in GPe primarily. For both of these implants, the distal electrode contact was located slightly medial to the medial medullary lamina, with the proximal contact at the border between GPe and putamen. To target a larger proportion of GPe efferents projecting directly into IC en route to STN, the DBS lead in subject RA was positioned near the medial border of GPe and IC. In contrast, the DBS lead in subject MA was positioned more ventral and posterior to sample a larger proportion of GPe efferents projecting through GPi.
Segmentation of the GPe and GPi was further demarcated into sensorimotor and nonsensorimotor territories as described in materials and methods (cross-hatched and non-cross-hatched portions, respectively, in Fig. 3D). The volumetric reconstruction of the DBS lead was positioned within this framework according to each subject's histology and postoperative CT scan. All three DBS leads were found to overlap spatially with a small percentage of the total number of GPe and GPi model neurons (GPe, GPi; NE: 6.4, 7.1%; MA: 5.9, 2.1%; RA: 5.0, 1.1%) and a slightly larger percentage of GPe and GPi model neurons within the sensorimotor territory in each pallidal segment (NE: 17.0, 12.0%; MA: 11.1, 4.6%; RA: 9.3, 3.7%).
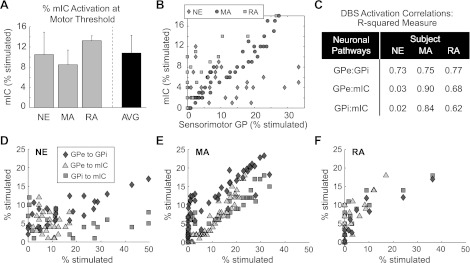
Computational neuron models of mIC fibers were also simulated for all DBS settings evaluated in the nonhuman primates, including those settings that were at or above threshold for inducing contralateral muscle contractions in the upper and lower limbs. The models predicted that 10.8 ± 3.5% of mIC fibers were activated on observing contralateral muscle contractions, a percentage that was consistent across animals and across electrode configurations (Fig. 4A). To assess the degree to which DBS in each monkey was able to activate one pathway over another, the modeling results underwent correlation analysis. Overall, the models predicted that a broad combination of pallidal neuron to IC fiber activation could be evaluated behaviorally in the nonhuman primates (Fig. 4B). Specifically, across the three subjects, there was a positive correlation between activation of GPe output and activation of GPi output for all DBS settings due in large part to activation of GPe neurons projecting through GPi (Fig. 4, C–F). Subjects MA and RA also showed positive correlations between activation of GPe and mIC as well as activation between GPi and mIC pathways. However, in the case of subject NE, there was no clear relationship between activation of mIC fibers and either GPe or GPi pathways suggesting that stimulation settings could dissociate activation of those pathways.
Fig. 4.
Correlations between model predictions of GPi, GPe, and IC activation with DBS. A: the threshold to elicit contralateral muscle contractions in the face, upper extremities, and/or lower extremities with DBS was found to parallel activating at least 11% of mIC fibers on average across the 3 nonhuman primates (error bars = SD). B: DBS programming involved assessing rigidity and bradykinesia for 87 different stimulation settings, which according to the models resulted in a broad range of GP-to-IC fiber activation ratios. C–F: correlation analysis between model predictions of the sensorimotor pathways affected (GPe, GPi, and mIC) for all 3 subjects. The axes for each plot are shown by a notation of the form GPe:GPi, for example, which signifies percent activation of GPe on the x-axis and percent activation of GPi on the y-axis across all evaluated DBS settings in each subject.
Identification of therapeutic pallidal DBS settings.
The severity of parkinsonian rigidity and bradykinesia was quantified for a range of DBS settings, which consisted of variations in both electrode configuration and stimulation amplitude. Pulse width and frequency were unchanged. A total of 87 different stimulation settings were examined behaviorally across the 3 subjects (42 for rigidity and 55 for bradykinesia with 10 settings common between the 2 behavioral examinations). Thresholds for evoking muscle contractions with stimulation were observed between −2 and −3 V for cathodic-monopolar DBS and −4 and −7 V for bipolar DBS. Muscle contractions first appeared as either an orofacial (RA) or upper extremity (NE and MA) contraction, likely reflecting slightly different anterior-to-posterior implant trajectories (Landy et al. 2000).
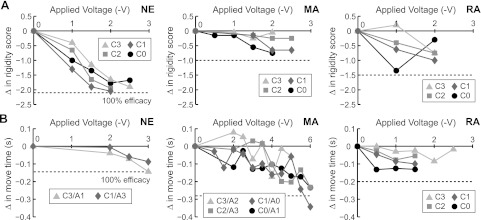
Although improvement in rigidity and bradykinesia with pallidal DBS was observed in all three subjects, the degree of improvement depended on the precise electrode configuration and applied stimulation voltage (Fig. 5). Increasing the DBS voltage resulted in a progressive improvement in rigidity for all electrode configurations even at small voltages in subject NE and RA. For all other cases (rigidity in subject MA and bradykinesia in all 3 subjects), symptoms remained unchanged at low stimulation voltages and improved at higher voltages. We observed that further increase in stimulus amplitude often resulted in tonic muscle contractions. In subject RA, for instance, monopolar DBS using the distal electrode as the cathode (−2 V) induced tonic muscle contractions in the face and upper limb and supplanted benefit on upper limb rigidity during DBS. In the same animal, monopolar DBS using the proximal electrode as the cathode (−3 V) induced arm dyskinesias, which prolonged movement times on the reach-and-retrieval task.
Fig. 5.
Characterization of rigidity and bradykinesia in the DBS-ON state. The degree of improvement (Δ) in parkinsonian rigidity (A) and parkinsonian bradykinesia (B) with pallidal DBS depended on electrode configuration and stimulation voltage in each of the 3 subjects. Electrode configurations are represented by a letter (C, cathode; A, anode) and by a number (0 is the distal electrode, and 3 is the proximal electrode on the DBS lead). Instances of cathodic monopolar DBS used a distant ground [e.g., implantable pulse generator (IPG) can] as the anode electrode.
Neural correlates for improving rigidity and bradykinesia.
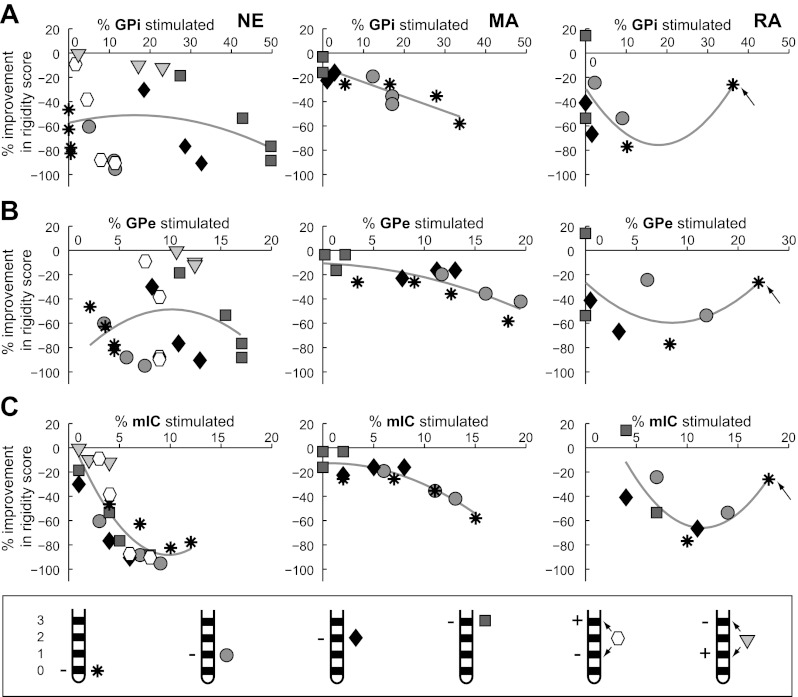
Pallidal DBS improved rigidity using electrode contacts in GPi or GPe or even in regions outside of the GP. Comparisons between 1) model predictions of the percentage of sensorimotor GPi and GPe efferents driven at or above the stimulation frequency of 135 Hz and 2) the percent change in averaged rigidity score across all tested DBS settings showed trends with greater pallidal efferent activation paralleling less muscle rigidity in subject MA (GPe/GPi, r2 = 0.78/0.66) and to a lesser extent subject RA (GPe/GPi, r2 = 0.32/0.27; Fig. 6, A and B). In subject NE, there also was a clear relationship between increasing the cathodic voltage at one of the three electrode contacts located within GPi and decreased rigidity. However, for the electrode located ventral to GPi, rigidity improved by 83% despite only driving 0.5% of GPi and 4.5% of GPe neurons. In this animal, there was no consistent relationship for overall sensorimotor GPe or GPi efferent activation and rigidity (GPe: r2 = 0.08; GPi: r2 = 0.06). In contrast, there was a consistent relationship in subject NE between activation of fibers in IC and reduction in rigidity (r2 = 0.71; Fig. 6C). This relationship was also strong for subjects MA (r2 = 0.81) and RA (r2 = 0.61). Indeed, the most therapeutic stimulation setting for each subject was found using the electrode closest to IC (NE: contact 1; MA: contact 0; RA: contact 0).
Fig. 6.
Relationships between rigidity and the neuronal pathways modulated by GP-DBS. The computational model was used to calculate the activation of GPe (A), GPi (B), and mIC (C) output activity at each experimentally tested DBS setting. Markers signify different monopolar (asterisk: cathode 0/distal contact; circle: cathode 1; diamond: cathode 2; square: cathode 3/proximal contact) and bipolar (hexagon: cathode 1/anode 3; downward-pointing triangle: cathode 3/anode 1) electrode configurations. Arrows signify a DBS setting that yielded contralateral muscle contractions. Gray lines show the polynomial regression of all data points.
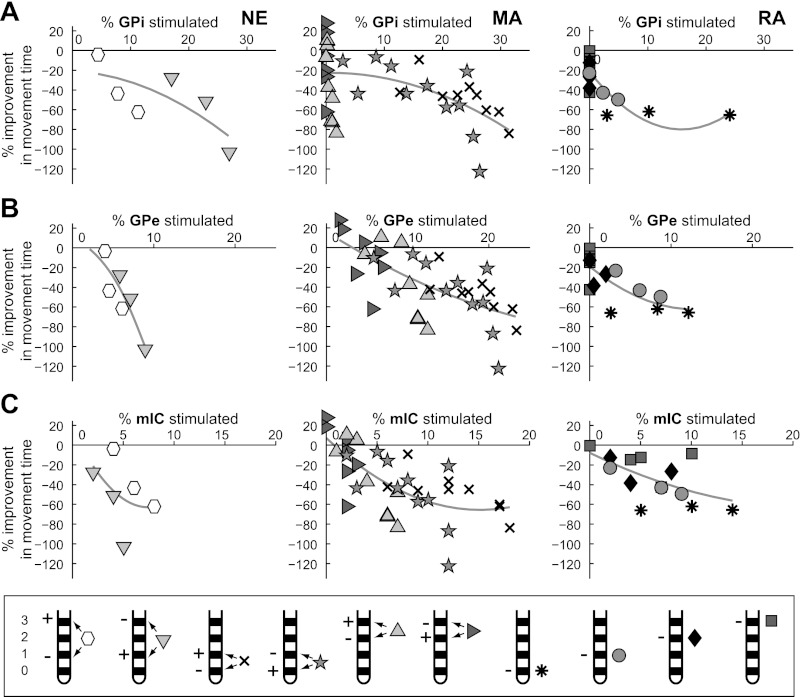
For most electrode configurations, increased activation of any one of the neuronal pathways (GPe, GPi, and mIC) paralleled a decrease in bradykinesia with DBS (Fig. 7). In subject NE, the most consistent relationship for decreasing bradykinesia was with activation of GPe efferent projections. Interestingly, in subjects MA and RA in which the active electrodes were located within GPe, the model results indicated that bradykinesia was relieved without directly activating GPi efferent activity. Together, these results suggest that GPe or some other fiber passing through GPe may be a therapeutic target for controlling bradykinesia. The data also showed trends between improvement in bradykinesia and activation of fibers in IC for each electrode configuration. However, the relationship was not consistent when grouping all electrode configurations together for a given subject (NE: r2 = 0.20; MA: r2 = 0.47; RA: r2 = 0.20; Fig. 7C). Although the simulation results for rigidity indicated that there was a relative lack of spatial stimulation precision necessary to have a therapeutic effect, relieving bradykinesia with stimulation required more spatially selective targeting. Across the three subjects, the most therapeutic electrode configurations for bradykinesia were those that activated GPe and GPi efferents near the medial medullary lamina between GPe and GPi. The computational models predicted that in these instances at least 10% of pallidal neurons in both sensorimotor GPe and GPi were directly activated by the stimulation (Fig. 8). Stimulation settings that activated a greater percentage of pallidal projections near the ventral border of GPi (subject NE) or near the dorsolateral border of GPe (subjects MA and RA) resulted in less improvement in bradykinesia for stimulation amplitudes equivalent to those used with electrodes at the border between GPe and GPi.
Fig. 7.
Relationships between bradykinesia and the neuronal pathways modulated by GP-DBS. The computational model was used to calculate the activation of GPe (A), GPi (B), and mIC (C) output activity at each experimentally tested DBS setting. Markers are identical to those in Fig. 6 with the addition of 4 bipolar electrode configurations (cross: cathode 0/anode 1; pentagram: cathode 1/anode 0; upward-pointing triangle: cathode 2/anode 3; right-pointing triangle: cathode 3/anode 2).
Fig. 8.
Pallidal regions modulated with the most effective DBS setting for bradykinesia in each of the 3 subjects. A: volumes of activated tissue were defined according to regions in which the efferent output was entrained to the high-frequency stimulation (>80%) as shown in the coronal view from subject MA. Black boxes represent the original soma locations of activated GPe and GPi efferents. B–D: oblique perspectives showing the spatial distribution of activated regions in each subject and their localization to the medial medullary lamina. L, lateral; P, posterior.
DISCUSSION
High-frequency electrical stimulation targeted to the GP is known to relieve motor symptoms in medication-refractory patients with PD (Volkmann et al. 2004). Although the most commonly accepted therapeutic target in the GP has been the posteroventral sensorimotor GPi, the precise pathways involved in treating each parkinsonian motor symptom are not well-characterized. We investigated how behavioral outcome measures of bradykinesia and rigidity vary according to DBS electrode configuration and voltage settings and further how these DBS settings relate to computational model predictions of the activated pathways within GPe, GPi, and IC. The results suggest that the therapeutic effect on rigidity correlated most closely with activating a small percentage of IC fibers, whereas eliciting an optimal therapeutic effect on bradykinesia required activating a large percentage of sensorimotor GPe and GPi neurons near the medial medullary lamina.
Mechanisms of pallidal DBS for managing parkinsonian bradykinesia.
The possibility of dissociating improvement in bradykinesia and rigidity with DBS was first suggested in a series of clinical studies with GPi-DBS patients (Bejjani et al. 1998; Krack et al. 1998; Yelnik et al. 2000). In these studies, high-frequency electrical stimulation targeted to the dorsal GPi was more effective at treating bradykinesia, whereas stimulation targeted to the ventral GPi at times worsened bradykinesia, potentially due to confounding capsular effects (Xu et al. 2011). Given the size of the DBS leads used in these studies, it is very likely that the dorsal site of stimulation was in GPe rather than dorsal GPi, which is consistent with observations in our study and previous results in humans undergoing acute stimulation in GPe (Vitek et al. 2004). The models in our study showed that improvement in bradykinesia was most significant when at least 10% of sensorimotor GPe efferent output (near the dorsomedial border with GPi) was entrained to the 135-Hz stimuli. The data also showed that bradykinesia could be relieved without direct activation of GPi efferents, suggesting that driving inhibitory axonal input into GPi is a therapeutic mechanism for improving bradykinesia with pallidal DBS (Kravitz et al. 2010; Vitek et al. 2004).
Pharmacological studies in nonhuman primates (Baron et al. 1992; Filion et al. 1991) and pallidotomy studies in humans (de Bie et al. 1999; Vitek et al. 2003) support the relationship between a reduction in GPi activity and improvement in parkinsonian bradykinesia. Electrical stimulation of GPe, using settings that improve bradykinesia, is known to have a prolonged inhibitory effect on STN and GPi activity (Vitek et al. 2012). Such modulation could be elicited through inhibition of STN-GPi neuronal activity (Kita et al. 2005), activation of GPe projections passing through GPi (Johnson and McIntyre 2008; Sato et al. 2000), and/or facilitation of the direct pathway by stimulation of striatofugal fibers synapsing on GPi neurons (Kravitz et al. 2010). Although the former and latter were not specifically modeled in our study, they can be inferred from the fact that the axonal fiber locations driven by the most therapeutic DBS settings for bradykinesia were located at a region that would drive inhibitory afferents to GPi without significant GPi efferent stimulation (Fig. 8).
It is important to note, however, the DBS in GPe also modulates the firing pattern of GPi neuronal activity (Vitek et al. 2012). Other studies have shown that modulating the firing patterns of neurons in GPi with STN-DBS parallels improvement in bradykinesia (Hashimoto et al. 2003). Regularizing the firing pattern of activity in GP is thought to limit the pathological information content being transmitted through the pallidofugal pathway to motor thalamus and brain-stem regions (Dorval et al. 2008, 2010; Grill et al. 2004), thereby freeing downstream networks to participate in motor control more effectively. Taken together, the present study in the context of these previous studies suggests that there are likely multiple physiological mechanisms by which DBS can improve bradykinesia.
Although our model-behavior data showed trends between improvement in bradykinesia and mIC fiber activation, the correlation was less robust than that for driving efferents from GPe and at higher levels, GPi. Nevertheless, the spread of stimulation into IC may have some aggregate effect on bradykinesia improvement (Ashby et al. 1998). Repeated transcranial magnetic stimulation over motor cortex (5 Hz, 10% below threshold for evoking contralateral muscle contractions) in patients with PD, for example, is known to reduce movement time on a peg-board test (Pascual-Leone et al. 1994a,b); whether such effects are due to direct activation of IC or induced through changes in intracortical network activity remains unclear.
Mechanisms of pallidal DBS for managing parkinsonian rigidity.
The concept of different optimal target volumes for managing bradykinesia and rigidity with DBS was recently described in a computational modeling study in a cohort of STN-DBS patients with PD (Butson et al. 2011). The model simulations predicted that the optimal target volumes for treating rigidity were lateral and closer to IC than the volumetric target for treating bradykinesia. Our model predictions are consistent with these results and further show that weak activation of IC (<10%) correlates with improvement in rigidity. It is important to note that this absolute percentage reflects the excitability of the axon models, which can vary depending on the passive and active membrane properties instantiated within the fiber models (McIntyre and Grill 2002; McIntyre et al. 2002). Stimulation of pallidal projections (both GPe and GPi) in subject MA showed strong correlations with a therapeutic effect on rigidity. However, there were strong correlations between the percent activation of GPe:GPi:mIC model fibers as shown in Fig. 4, suggesting that stimulation through this lead was not able to activate one pathway over another. The lack of spatial targeting necessary to manage rigidity with DBS is consistent with clinical findings in PD patients with GPi-DBS implants (Bejjani et al. 1998; Krack et al. 1998; Yelnik et al. 2000) and a recent case study in an MPTP-treated monkey implanted in the STN with a scaled-down version of the human DBS lead (Xu et al. 2011). These studies noted that rigidity could be improved using any of the four electrode contacts on the DBS lead regardless of implantation in GPe/GPi or STN so long as the stimulation was of sufficient amplitude.
DBS amplitudes below threshold for producing contralateral muscle contractions have been implicated in the modulation of IC fiber activity (Ashby et al. 1998; Kuhn et al. 2004). In PD patients, Ashby and colleagues (1998) reported short-latency facilitation and long-latency inhibition of voluntary muscle contraction after delivering a stimulus pulse in GPi using voltage settings that at higher stimulation frequencies produced a therapeutic effect on rigidity. They observed that the distal electrode contact always had the lowest threshold for short-latency facilitation, which based on their implant trajectories would be consistent with its proximity to IC. Continuous stimulation of this tract would likely drive motoneurons and inhibit the overall population of motor cortex through antidromic signaling (Gradinaru et al. 2009; Li et al. 2007), intracortical inhibition (Johnson et al. 2009), and/or decreased motor cortex excitability (Kuhn et al. 2003). However, since the IC fiber models used in our study were constructed as nonspecific projections, it was not possible to determine the origin of the fibers (e.g., corticospinal, corticobulbar, pallidofugal, or striatofugal).
There are several possible mechanisms by which IC stimulation could improve muscle rigidity. One explanation follows the logic of limiting the amount of supraspinal drive on α-motoneurons in the spinal cord, which are known to exhibit abnormally high activity in PD patients with significant parkinsonian rigidity (Landau et al. 1966). Indeed, IC lesions, which presumably decrease supraspinal tone on α-motoneurons, are known to have a temporary, beneficial effect on rigidity (Smith 1962). In our study, however, a small percentage of axonal fibers within IC were entrained at 135 Hz and not refractory to the high-frequency stimulation, which would seem at odds with the proposed explanation. It is possible, however, that excitation of a small percentage of fibers within IC reduces the excitability of spinal interneurons through recurrent inhibition from synchronous activation of α-motoneurons, thereby decreasing α-motoneuron activity during joint movements (Delwaide et al. 1993). An abnormal stretch reflex is also observed in PD patients (Pollock and Davis 1930), suggesting that altered Golgi tendon organ reflex activity (Burne and Lippold 1996) and/or static γ-motoneuron activity (Lee 1989) are present. Following administration of neuroleptics in animal models of parkinsonism (Ellenbroek et al. 1985; Steg 1964), γ-motoneuron activity is known to decrease. Thus it is also possible that parkinsonian rigidity could be relieved through DBS by altering the activity of static γ-motoneurons and turning down the sensitivity of the stretch reflex during joint movements (Grigg and Preston 1971). To resolve these questions, future studies should begin investigating the effects of GP-DBS or STN-DBS on brain-stem and spinal-cord activity.
The combination behavioral-modeling results from this study suggest that stimulating fibers of passage within or near GPi correlate with improving parkinsonian bradykinesia and rigidity during pallidal DBS. Improvements in parkinsonian rigidity correlated most consistently across the three subjects with spread of activation into IC, driving a small percentage of fibers within this tract (<10% on average). The most robust effect on bradykinesia resulted from stimulating a combination of sensorimotor axonal projections within the GP, specifically at the site of the medial medullary lamina. Future studies investigating motor cortex activity as well as brain-stem and spinal-cord activity may be useful for further defining differences in the mechanisms of action of DBS on parkinsonian rigidity and bradykinesia.
GRANTS
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health [Grants F32-NS-061541 (M. D. Johnson), R01-NS-047388, and R01-NS-037019].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.D.J., C.C.M., and J.L.V. conception and design of research; M.D.J., J.Z., and D.G. performed experiments; M.D.J., J.Z., and D.G. analyzed data; M.D.J., J.Z., D.G., C.C.M., and J.L.V. interpreted results of experiments; M.D.J. prepared figures; M.D.J. drafted manuscript; M.D.J., J.Z., D.G., C.C.M., and J.L.V. edited and revised manuscript; M.D.J., J.Z., D.G., C.C.M., and J.L.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Martin and André Parent for providing the camera lucida reconstructions of the nonhuman primate pallidal neurons, Weidong Xu, Svjetlana Miocinovic, Jennie Minnich, and Erin Bynum for technical assistance with the nonhuman primates, and Tom Foutz for helpful discussion.
REFERENCES
- Afsharpour S. Topographical projections of the cerebral cortex to the subthalamic nucleus. J Comp Neurol 236: 14–28, 1985 [DOI] [PubMed] [Google Scholar]
- Ashby P, Rothwell JC. Neurophysiologic aspects of deep brain stimulation. Neurology 55: S17–S20, 2000 [PubMed] [Google Scholar]
- Ashby P, Strafella A, Dostrovsky JO, Lozano A, Lang AE. Immediate motor effects of stimulation through electrodes implanted in the human globus pallidus. Stereotact Funct Neurosur 70: 1–18, 1998 [DOI] [PubMed] [Google Scholar]
- Baron MS, Sidibe M, DeLong MR, Smith Y. Course of motor and associative pallidothalamic projections in monkeys. J Comp Neurol 429: 490–501, 2001 [PubMed] [Google Scholar]
- Baron MS, Wichmann T, DeLong MR. Inactivation of the sensorimotor territory in the internal pallidum reverses parkinsonian signs in MPTP-treated monkeys (Abstract). Soc Neurosci Abstr 18: 69, 1992 [Google Scholar]
- Bejjani BP, Damier P, Arnulf I, Papadopoulos S, Bonnet AM, Vidailhet M, Agid Y, Pidoux B, Cornu P, Dormont D, Marsault C. Deep brain stimulation in Parkinson's disease: opposite effects of stimulation in the pallidum. Mov Disord 13: 969–970, 1998 [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain 124: 2131–2146, 2001 [DOI] [PubMed] [Google Scholar]
- Burne JA, Lippold OC. Loss of tendon organ inhibition in Parkinson's disease. Brain 119: 1115–1121, 1996 [DOI] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage 34: 661–670, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, Wolgamuth B, McIntyre CC. Probabilistic analysis of activation volumes generated during deep brain stimulation. Neuroimage 54: 2096–2104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bie RM, de Haan RJ, Nijssen PC, Rutgers AW, Beute GN, Bosch DA, Haaxma R, Schmand B, Schuurman PR, Staal MJ, Speelman JD. Unilateral pallidotomy in Parkinson's disease: a randomised, single-blind, multicentre trial. Lancet 354: 1665–1669, 1999 [DOI] [PubMed] [Google Scholar]
- Dejean C, Hyland B, Arbuthnott G. Cortical effects of subthalamic stimulation correlate with behavioral recovery from dopamine antagonist induced akinesia. Cereb Cortex 19: 1055–1063, 2009 [DOI] [PubMed] [Google Scholar]
- Delwaide PJ, Pepin JL, Maertens de Noordhout A. Contribution of reticular nuclei to the pathophysiology of parkinsonian rigidity. Adv Neurol 60: 381–385, 1993 [PubMed] [Google Scholar]
- Dorval AD, Kuncel AM, Birdno MJ, Turner DA, Grill WM. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol 104: 911–921, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Russo GS, Hashimoto T, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson's disease. J Neurophysiol 100: 2807–2818, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder CM, Hashimoto T, Zhang J, Vitek JL. Chronic implantation of deep brain stimulation leads in animal models of neurological disorders. J Neurosci Methods 142: 11–16, 2005 [DOI] [PubMed] [Google Scholar]
- Ellenbroek B, Schwarz M, Sontag KH, Jaspers R, Cools A. Muscular rigidity and delineation of a dopamine-specific neostriatal subregion: tonic EMG activity in rats. Brain Res 345: 132–140, 1985 [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 547: 142–151, 1991 [PubMed] [Google Scholar]
- Filion M, Tremblay L, Bedard PJ. Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res 444: 165–176, 1988 [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L, Bedard P. Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 547: 152–161, 1991 [PubMed] [Google Scholar]
- Francois C, Yelnik J, Percheron G, Fenelon G. Topographic distribution of the axonal endings from the sensorimotor and associative striatum in the macaque pallidum and substantia nigra. Exp Brain Res 102: 305–318, 1994 [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MT, Barbosa ER, Nitsche MA, Pascual-Leone A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson's disease. Mov Disord 21: 1693–1702, 2006 [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 324: 354–359, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg P, Preston JB. Baboon flexor and extensor fusimotor neurons and their modulation by motor cortex. J Neurophysiol 34: 428–436, 1971 [DOI] [PubMed] [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport 15: 1137–1140, 2004 [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23: 1916–1923, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput 9: 1179–1209, 1997 [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science 259: 819–821, 1993 [DOI] [PubMed] [Google Scholar]
- Johnson MD, McIntyre CC. Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J Neurophysiol 100: 2549–2563, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Vitek JL, McIntyre CC. Pallidal stimulation that improves parkinsonian motor symptoms also modulates neuronal firing patterns in primary motor cortex in the MPTP-treated monkey. Exp Neurol 219: 359–362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Tachibana Y, Nambu A, Chiken S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J Neurosci 25: 8611–8619, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krack P, Pollak P, Limousin P, Hoffmann D, Benazzouz A, Le Bas JF, Koudsie A, Benabid AL. Opposite motor effects of pallidal stimulation in Parkinson's disease. Ann Neurol 43: 180–192, 1998 [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Brandt SA, Kupsch A, Trottenberg T, Brocke J, Irlbacher K, Schneider GH, Meyer BU. Comparison of motor effects following subcortical electrical stimulation through electrodes in the globus pallidus internus and cortical transcranial magnetic stimulation. Exp Brain Res 155: 48–55, 2004 [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Meyer BU, Trottenberg T, Brandt SA, Schneider GH, Kupsch A. Modulation of motor cortex excitability by pallidal stimulation in patients with severe dystonia. Neurology 60: 768–774, 2003 [DOI] [PubMed] [Google Scholar]
- Landau WM, Struppler A, Mehls O. A comparative electromyographic study of the reactions to passive movement in parkinsonism and in normal subjects. Neurology 16: 34–48, 1966 [DOI] [PubMed] [Google Scholar]
- Landy HJ, Weiner WJ, Calancie B, Harris W, Shulman LM, Singer C, Abrams L, Bowen B. Electromyography during stereotactic pallidotomy for Parkinson's disease. Stereotact Funct Neurosur 74: 21–29, 2000 [DOI] [PubMed] [Google Scholar]
- Lee RG. Pathophysiology of rigidity and akinesia in Parkinson's disease. Eur Neurol 29, Suppl 1: 13–18, 1989 [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Von Raison F, Menard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson's disease. Clin Neurophysiol 115: 2530–2541, 2004 [DOI] [PubMed] [Google Scholar]
- Lempka SF, Miocinovic S, Johnson MD, Vitek JL, McIntyre CC. In vivo impedance spectroscopy of deep brain stimulation electrodes. J Neural Eng 6: 046001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol 98: 3525–3537, 2007 [DOI] [PubMed] [Google Scholar]
- Martin R, Bowden D. Primate Brain Maps: Structure of the Macaque Brain. Amsterdam: Elsevier Science, 2000 [Google Scholar]
- McIntyre CC, Grill WM. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J Neurophysiol 88: 1592–1604, 2002 [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Richardson AG, Grill WM. Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. J Neurophysiol 87: 995–1006, 2002 [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Lempka SF, Russo GS, Maks CB, Butson CR, Sakaie KE, Vitek JL, McIntyre CC. Experimental and theoretical characterization of the voltage distribution generated by deep brain stimulation. Exp Neurol 216: 166–176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, McIntyre CC. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol 96: 1569–1580, 2006 [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Zhang J, Xu W, Russo GS, Vitek JL, McIntyre CC. Stereotactic neurosurgical planning, recording and visualization for deep brain stimulation in non-human primates. J Neurosci Methods 162: 32–41, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 20: 91–127, 1995 [DOI] [PubMed] [Google Scholar]
- Parent M, Levesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol 439: 162–175, 2001 [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Axonal collateralization in primate basal ganglia and related thalamic nuclei. Thal Rel Sys 2: 71–86, 2002 [Google Scholar]
- Parent M, Parent A. The pallidofugal motor fiber system in primates. Parkinsonism Relat Disord 10: 203–211, 2004 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cammarota A, Grafman J, Hallett M. Akinesia in Parkinson's disease. II. Effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology 44: 892–898, 1994a [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cohen LG, Hallett M. Akinesia in Parkinson's disease. I. Shortening of simple reaction time with focal, single-pulse transcranial magnetic stimulation. Neurology 44: 884–891, 1994b [DOI] [PubMed] [Google Scholar]
- Pollock LJ, Davis L. Muscle tone in parkinsonian states. Arch Neurol Psychiatry 23: 303–319, 1930 [Google Scholar]
- Sato F, Lavallee P, Levesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J Comp Neurol 417: 17–31, 2000 [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford Univ. Press, 2006 [Google Scholar]
- Smith MC. Location of stereotactic lesions confirmed at necropsy. Br Med J 1: 900–906, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg G. Efferent muscle innervation and rigidity. Acta Physiol Scand Suppl, SUPPL 225: 1–53, 1964 [PubMed] [Google Scholar]
- Tommasi G, Krack P, Fraix V, Le Bas JF, Chabardes S, Benabid AL, Pollak P. Pyramidal tract side effects induced by deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry 79: 813–819, 2008 [DOI] [PubMed] [Google Scholar]
- Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, McDonald W, Haber M, Barnhart H, Wahlay N, Triche S, Mewes K, Chockkan V, Zhang JY, DeLong MR. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol 53: 558–569, 2003 [DOI] [PubMed] [Google Scholar]
- Vitek JL, Hashimoto T, Peoples J, DeLong MR, Bakay RA. Acute stimulation in the external segment of the globus pallidus improves parkinsonian motor signs. Mov Disord 19: 907–915, 2004 [DOI] [PubMed] [Google Scholar]
- Vitek JL, Zhang J, Hashimoto T, Russo GS, Baker KB. External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Exp Neurol 233: 581–586, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann J, Allert N, Voges J, Sturm V, Schnitzler A, Freund HJ. Long-term results of bilateral pallidal stimulation in Parkinson's disease. Ann Neurol 55: 871–875, 2004 [DOI] [PubMed] [Google Scholar]
- Xu W, Miocinovic S, Zhang J, Baker KB, McIntyre CC, Vitek JL. Dissociation of motor symptoms during deep brain stimulation of the subthalamic nucleus in the region of the internal capsule. Exp Neurol 228: 294–297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelnik J. Functional anatomy of the basal ganglia. Mov Disord 17, Suppl 3: S15–S21, 2002 [DOI] [PubMed] [Google Scholar]
- Yelnik J, Damier P, Bejjani B, Francois C, Gervais D, Dormont D, Arnulf I, Bonnet A, Cornu P, Pidoux B, Agid Y. Functional mapping of the human globus pallidus: contrasting effect of stimulation in the internal and external pallidum in Parkinson's disease. Neuroscience 101: 77–87, 2000 [DOI] [PubMed] [Google Scholar]