Abstract
Suppression of ipsilateral distortion product otoacoustic emissions (DPOAEs) by contralateral noise is used in humans and animals to assay the strength of sound-evoked negative feedback from the medial olivocochlear (MOC) efferent pathway. However, depending on species and anesthesia, contributions of other feedback systems to the middle or inner ear can cloud the interpretation. Here, contributions of MOC and middle-ear muscle reflexes, as well as autonomic feedback, to contra-noise suppression in anesthetized mice are dissected by selectively eliminating each pathway by surgical transection, pharmacological blockade, or targeted gene deletion. When ipsilateral DPOAEs were evoked by low-level primaries, contra-noise suppression was typically ∼1 dB with contra-noise levels around 95 dB SPL, and it always disappeared upon contralateral cochlear destruction. Lack of middle-ear muscle contribution was suggested by persistence of contra-noise suppression after paralysis with curare, tensor tympani cauterization, or section of the facial nerve. Contribution of cochlear sympathetics was ruled out by studying mutant mice lacking adrenergic signaling (dopamine β-hydroxylase knockouts). Surprisingly, contra-noise effects on low-level DPOAEs were also not diminished by eliminating the MOC system pharmacologically (strychnine), surgically, or by deletion of relevant cholinergic receptors (α9/α10). In contrast, when ipsilateral DPOAEs were evoked by high-level primaries, the contra-noise suppression, although comparable in magnitude, was largely eliminated by MOC blockade or section. Possible alternate pathways are discussed for the source of contra-noise-evoked effects at low ipsilateral levels.
Keywords: efferent, inner ear, middle-ear muscles, sympathetic nervous system
the mammalian cochlea receives neuronal feedback from the olivocochlear (OC) system, a group of cholinergic neurons originating in the superior olivary complex and projecting to the hair cells and/or neurons in the organ of Corti. The OC system has two components: 1) a medial (M)OC system, with cell bodies located primarily in the ventral nucleus of the trapezoid body, that projects via myelinated fibers to outer hair cells (OHCs) and 2) a lateral (L)OC system originating near the lateral superior olive and projecting via unmyelinated axons primarily to the dendrites of cochlear afferent neurons in the inner hair cell area (for review see Guinan 2006).
Electrically activating LOC neurons leads to slow (over tens of seconds) changes in cochlear nerve excitability (Groff and Liberman 2003), which are important in balancing the outputs of the two ears to maintain accuracy in binaural hearing (Darrow et al. 2006). In contrast, electrically activating MOC fibers leads to rapid (over tens of milliseconds) decreases in cochlear sensitivity, which are useful in minimizing the masking effects of continuous background noise (Winslow and Sachs 1987) and in protecting the ear from acoustic overstimulation (Rajan 1995). A more extensive discussion of the functional significance of these feedback systems can be found in a recent review (see Guinan 2006).
MOC neurons can also be activated by sound: they represent the effector arm of a sound-evoked, negative-feedback reflex (Liberman and Brown 1986). This MOC reflex can be assayed noninvasively via its effects on otoacoustic emissions (OAEs) (Collet et al. 1990; Puel and Rebillard 1990). Distortion product (DP)OAEs are commonly used, because they are relatively large in experimental mammals (see, e.g., Puria et al. 1996; Shera and Guinan 1999). They are evoked when two primary tones are presented at the appropriate frequency and level ratios. Nonlinearities in hair cell mechano-electric transduction produce distortions in the receptor potential, including a prominent component at the frequency equal to 2f1-f2 (where f2 and f1 are the higher and lower frequencies of the two stimulus tones). The distorted receptor potential, in turn, drives OHC electromotility, which moves the sensory epithelium and creates pressure waves that are transmitted back through the middle ear to the ear canal, where they can be measured with a low-noise microphone.
One common assay of MOC reflex strength, in humans as well as animals, is the suppression of ipsilateral OAEs by contralateral noise (Collet et al. 1990; Puria et al. 1996). This assay exploits the fact that roughly a third of MOC fibers to each ear respond best to sound in the opposite ear (Liberman 1988). When activated, these MOC fibers suppress the OHC's contributions to cochlear amplification, thereby decreasing the ipsilaterally evoked OAEs. Interpreting the contra-noise suppression assay is complicated, because contralateral sound can also activate the middle-ear muscle reflex (Cacace et al. 1991), especially the stapedius muscle, which can reduce sound transmission into the ipsilateral cochlea and attenuate cochlear responses (Goodman and Keefe 2006). In humans, the relative contributions can be teased apart by careful analysis of the OAE response phase (Guinan et al. 2003) or the ear's input impedance, which is increased by middle-ear muscle contraction (Goodman and Keefe 2006). A further challenge in animal work is that reflex strength for middle-ear muscles and MOC efferents can be differentially modulated by the depth and/or type of anesthesia (Boyev et al. 2002). The relative contributions of each reflex to contralateral-sound suppression can be identified, in animals, by eliminating one or the other feedback system surgically, pharmacologically, or genetically. Such analyses have suggested that in barbiturate-anesthetized cats contra-noise effects are dominated by the MOC system (Liberman et al. 1996; Puria et al. 1996), whereas in ketamine-anesthetized rats effects are dominated by the middle-ear muscles (Relkin et al. 2005).
Despite numerous prior studies in anesthetized mice using contra-noise suppression as an assay for MOC activity (Frisina et al. 2007; Jacobson et al. 2003; Zettel et al. 2007; Zhu et al. 2007), a clear demonstration of the OC system's role in this sound-evoked effect is lacking. Here, we use a combination of pharmacological blockers, brain stem lesions, and transgenic mice to show that, although contralateral-noise suppression is usually measurable in the anesthetized mouse, the contribution of the MOC system to the effect depends strongly on stimulus parameters: when the ipsilateral, OAE-evoking stimuli are at relatively high levels (70–80 dB SPL), the contra-noise suppression is mediated largely by the MOC system; however, with low-level (25–35 dB SPL) ipsilateral stimuli, a robust contra-noise suppression can be observed that is not mediated by the MOC or the middle-ear muscle systems or by the autonomic innervation of the inner ear vasculature (Spoendlin and Lichtensteiger 1966).
MATERIALS AND METHODS
Animals and General Procedures
Unless otherwise stated, all experiments were performed on CBA/CaJ mice at 6–8 wk of age in a soundproof chamber maintained at ∼32°C. The three transgenic mouse lines used were hybrids between 129 substrains and C57BL/6, as described in prior reports: α9 acetylcholine (ACh) receptor knockouts (Vetter et al. 1999), α10 ACh receptor knockouts (Vetter et al. 2007), and dopamine β-hydroxylase knockouts (Thomas and Palmiter 1997). For physiological testing, all mice were anesthetized with urethane (1.2 g/kg ip) and xylazine (20 mg/kg im) or with ketamine (100 mg/kg im) and xylazine (20 mg/kg im), except for dopamine β-hydroxylase mice, in which pentobarbital (71.2 mg/kg ip) was used because these mutants cannot tolerate the other anesthetics. Mice were only included in the present study if cochlear function was normal, as assessed by measurement of DPOAEs, as described below. All experimental procedures were approved by the Animal Care Committee of the Massachusetts Eye and Ear Infirmary.
DPOAEs and Contra-Noise Assays
For measurement of DPOAEs, a small slit was made in the ear canal to allow careful microscopic evaluation of the tympanic membrane. Acoustic stimuli were delivered with a custom acoustic assembly consisting of two electrostatic drivers (EC-1, Tucker Davis Technologies) to generate primary tones and a Knowles miniature microphone (EK-3103) to record ear canal sound pressure via an indwelling probe tube, concentric and coterminal with the sound port of the acoustic system. Before the experiments, the probe microphone was calibrated in a small cavity terminated by a ¼-in. condenser microphone (Bruel and Kjaer).
Stimuli were generated digitally (National Instruments, digital input-output board 6052E), and ear canal sound pressure was amplified and digitally sampled at 4 μs. Primary tones were set so that the frequency ratio (f2/f1) was 1.2 and the f2 level was 10 dB below the f1 level (unless otherwise specified). Both waveform and spectral averaging were used to increase the signal-to-noise ratio of the recorded ear canal sound pressure: a spectrum was computed from the average of 20 consecutive waveform buffers (each 33 ms in duration); this process was repeated four times, and the four spectra were averaged. The amplitude of the DPOAE at 2f1-f2 was extracted from the averaged spectra, along with the noise floor at nearby points in the spectrum. No distortion at 2f1-f2 was measurable in a passive coupler, with f2 = 16 kHz at the highest sound pressures presented (85 dB SPL).
To measure modulation of ipsilateral DPOAEs by contralateral noise, DPOAEs at 2f1-f2 (with f2 = 16 kHz) were repeatedly measured before, during, and after presentation of a continuous broadband contralateral noise via a closed acoustic system. Except for a notch between 25 and 30 kHz, the noise spectrum at the output of the acoustic system was flat (±10 dB) from 1 kHz to 40 kHz. The threshold for acoustic cross talk was determined by monitoring the output of the probe tube microphone in the ipsilateral acoustic system.
For the contralateral-noise assay, ipsilateral DPOAEs were evoked by either low-level or high-level primaries. For the low-level assay, the primary tones were always set to evoke a DPOAE of approximately −5 dB SPL, i.e., 10 dB above the noise floor. For the high-level assay, f2 was fixed at a level near the nonmonotonicity in the amplitude-vs.-level function (usually 75 dB SPL), and f1 was varied in 1-dB steps from 10 dB below f2 up to the f2 level. We set the level near the nonmonotonicity because sound-evoked ipsilateral MOC effects are maximal in this range. We varied the f1 level because the size and sign of the MOC effects are sensitive to primary level ratio for primaries near this nonmonotonicity but not for primary levels near threshold (Kujawa and Liberman 2001; Maison and Liberman 2000).
Shock-Evoked MOC Activation
For electrical stimulation of the OC bundle, a posterior craniotomy and partial cerebellar aspiration were performed to expose the floor of the IVth ventricle. Shocks (monophasic pulses, 150-μs duration, 200/s) were applied through fine silver wires (0.4-mm spacing) placed along the midline, spanning the OC decussation. Shock threshold for facial twitches was determined, and then muscle paralysis was induced with α-d-tubocurarine (1.25 mg/kg ip). The animal was connected to a respirator via a tracheal cannula, and shock levels were raised to 6 dB above twitch threshold. Once the stimulation electrode was positioned and the shock levels set the primaries were turned on, and repeated measures of DPOAE amplitude were obtained before, during, and after a 180-s continuous shock train to the OC bundle.
Brain Stem Lesions and Histological Verification
Brain stem cuts in acute experiments were made with a microknife positioned on the basis of surface landmarks visible on the floor of the IVth ventricle after cerebellar aspiration. For chronic lesions of the superior olivary complex, as described previously (Darrow et al. 2007), the mouse was held in a stereotaxic apparatus by a snout clamp and ear bars. The skin overlaying the skull was slit and retracted to reveal the bregma and lambdoidal sutures. Rongeurs were used to make an opening in the skull over the right lambdoidal suture. A micropipette filled with a 10 mM solution of the neurotoxin melittin was lowered into the brain at a position 0.49 mm caudal and 0.12 mm lateral to the bregma. At a depth of 0.69 mm, 0.2 μl of melittin was injected with a 10-μl syringe (Hamilton). After injection the scalp was sutured, and the animal was placed in a padded cage with heat lights. To assess the success of the cuts and lesions, brain stems were fixed in 10% formalin, cryoprotected (30% sucrose), and cut on a freezing microtome at 40 μm in the transverse plane. Sections were treated histochemically to reveal acetylcholinesterase activity (Osen and Roth 1969), mounted on glass slides, air-dried, and coverslipped.
RESULTS
Contra-Noise Effects on DPOAEs Evoked by Low-Level Primaries
Activating the MOC system suppresses cochlear responses by decreasing the normal contribution of OHCs to the amplification of sound-driven cochlear vibrations (see, e.g., Guinan 2006). Because the OHC amplifier normally has its greatest effect at low sound pressure levels (Ruggero and Rich 1991), the most dramatic suppressive effects of MOC activation, when expressed as a change in response amplitude, are typically seen on responses to low-level acoustic stimuli (Maison et al. 2007). Thus our first approach was to use stimuli evoking a DPOAE of 0 dB SPL, i.e., ∼10 dB above the measurement noise floor, which typically requires an f2 level of 25–35 dB SPL (with f1 level 10 dB higher). We used primary tones with f2 = 16 kHz for a number of reasons: 1) this frequency region has the lowest DPOAE thresholds in a wide variety of mouse models (e.g., Maison et al. 2006, 2010); 2) this cochlear region shows the maximum density of MOC terminals on OHCs in mouse (Maison et al. 2003); and 3) shock-evoked OC activation in mouse has its maximum effects on DPOAEs evoked with f2 at 16 kHz, and shock-evoked OC effects are highly reproducible at 16 kHz from animal to animal (Maison et al. 2006, 2010).
To assess MOC reflex effects, DPOAE amplitudes were measured before, during, and after a 200- to 450-s period during which continuous broadband noise was presented to the contralateral ear (e.g., Fig. 1A). Relatively long noise stimuli were used, because sound-evoked MOC effects can increase over tens of seconds because of a slow buildup of sound-evoked discharge in MOC fibers (Larsen and Liberman 2009).
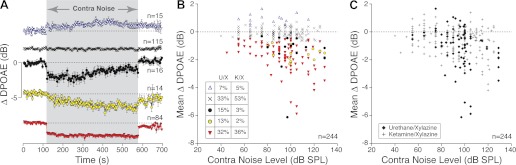
Fig. 1.
The time course and sign of contra-noise effects are variable. Contra-noise effects were measured 244 times in 52 mice, with ipsilateral f2 at 16 kHz and primary levels set to produce a distortion product otoacoustic emissions (DPOAE) of 0 dB SPL. A: each of the 244 runs was assessed by one-way ANOVA: in 115 cases (black Xs), responses during the contra noise were statistically indistinguishable from those before noise onset (P > 0.01). Runs with statistically significant effects were classified into 1 of 4 response types, and mean (±SE) ΔDPOAE is plotted for each: 1) enhancement (purple triangles), 2) rapid-onset suppression with slow adaptation (black circles), 3) slow-onset, buildup suppression (yellow circles), or 4) nonadapting suppression (red triangles). Time resolution is ∼4.5 s/point. ΔDPOAE is normalized re the mean prenoise DPOAE amplitude. B: mean ΔDPOAE (averaged over the contra-noise epoch) for each of the 244 runs is plotted as a function of contra-noise level, color-coded according to response “type” shown in A. Inset: relative frequency of occurrence of each response type for each anesthesia regimen. U/X, urethane-xylazine; K/X, ketamine-xylazine. C: same data as in B, but coded according to anesthetic regimen.
Under these stimulus conditions, contra-noise effects were often, but not always, detectable whether the mouse was anesthetized with ketamine-xylazine or urethane-xylazine (Fig. 1C). By one-way ANOVA, the contra noise caused a statistically significant change in ipsilateral DPOAE amplitudes roughly half the time (115/244 runs; Fig. 1A). The size of the effect generally increased with increasing contralateral noise levels (Fig. 1, B and C). However, the time course, and even the sign, of the effect on ipsilateral responses were different in different animals. We sometimes saw a nonadapting suppression clearly synchronized with the onset and offset of the contra noise (Fig. 1A); sometimes there was a slowly increasing or decreasing suppression, and occasionally there was a small but significant enhancement of ipsilateral response (Fig. 1A). Repeated runs in the same animal typically generated one response type, and all response types were seen in both ketamine-xylazine- and urethane-xylazine-anesthetized animals (see Fig. 1B, inset).
Contribution of acoustic cross talk.
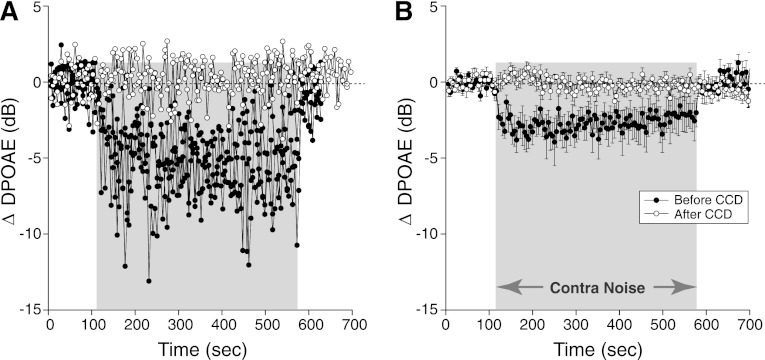
Acoustic cross talk, i.e., direct stimulation of the ipsilateral ear by the contralateral noise, is a possible source of artifact, especially with the relatively high noise levels used. Indeed, when the contralateral noise exceeded 95 dB SPL, the response of the ipsilateral microphone increased. To rule out this type of acoustic cross talk, we measured contra-noise effects in nine animals with robust suppressive effects, before and after destruction of the contralateral cochlea. As shown in Fig. 2, the destruction completely eliminated the suppression. Thus the effect does not arise simply by transmission of the contralateral stimulus to the ipsilateral ear.
Fig. 2.
Contra-noise suppression of DPOAEs disappears after contralateral cochlear destruction (CCD). A: results from 1 animal immediately before and immediately after CCD: multiple runs are superimposed (3 before and 2 after CCD). B: mean (±SE) data from 9 animals: 27 runs before and 18 runs after CCD. Mean contra-noise level was 101 dB SPL, ipsilateral f2 was at 16 kHz, and primary levels were set to produce a DPOAE of 0 dB SPL. Key in B applies to both panels.
Contribution of olivocochlear efferents.
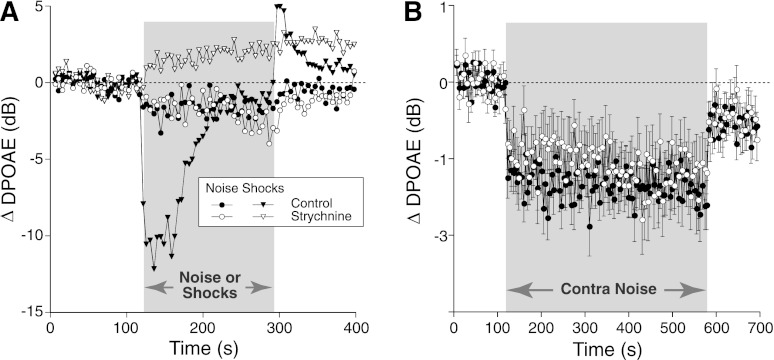
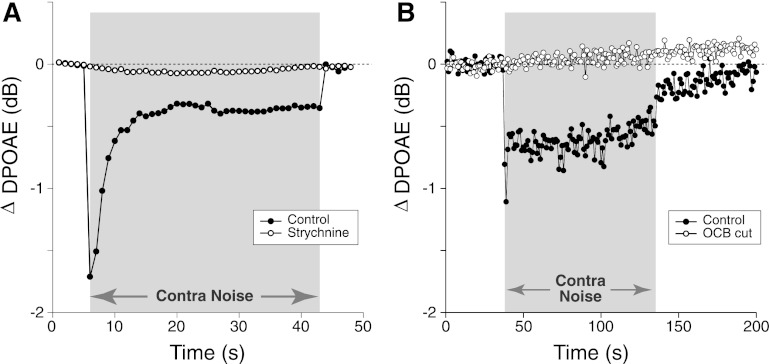
Strychnine is a potent blocker of the α9/α10 ACh receptors (Rothlin et al. 1999), which mediate suppressive MOC effects on OHCs in vivo (Sridhar et al. 1995). When given systemically at 10 mg/kg in mouse, strychnine completely blocks the DPOAE suppression evoked by shocking the OC bundle, leaving only a slow DPOAE enhancement of unknown origin (Maison et al. 2007). In one animal in the present study (Fig. 3A), electrical shocks were interleaved with contralateral noise, while ipsilateral DPOAEs were measured before and 30 min after strychnine injection. Whereas the robust shock-evoked MOC suppression disappeared, the contra-noise suppression persisted (Fig. 3A). The strychnine resistance of contra-noise suppression was confirmed in a larger group of mice (Fig. 3B) without interleaved electric stimulation of the OC bundle.
Fig. 3.
Contra-noise suppression persists after a strychnine dose that blocks shock-evoked medial olivocochlear (MOC) suppression. A: in 1 animal, shock-evoked and contra-noise-evoked effects were both measured before and after systemic strychnine (10 mg/kg). Strychnine blocked shock-evoked suppression (filled triangles, mean of 3 runs), leaving only a slow enhancement (open triangles, mean of 2 runs) as described in a prior report (Maison et al. 2007), while producing little effect on contra-noise suppression (filled vs. open circles, mean of 3 runs each). B: mean (±SE) effects of strychnine at 10 mg/kg, as measured 30 min after injection (mean of 59 runs from 39 animals). Mean contra-noise level was 102 dB SPL, ipsilateral f2 was at 16 kHz, and primary levels were set to produce a DPOAE of 0 dB SPL. Key in A applies to both panels.
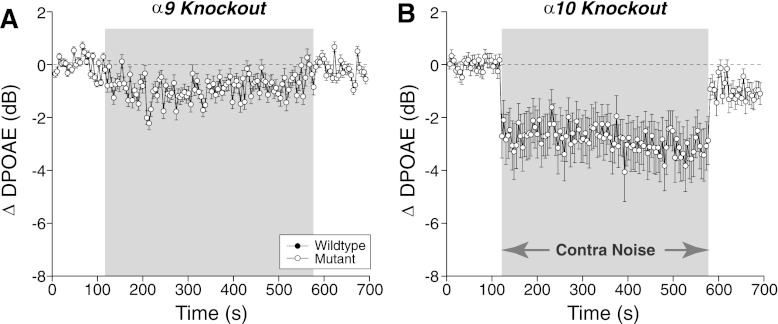
To further assess the role of the MOC system, we studied mutant mice lacking either the α9 (Fig. 4A) or α10 (Fig. 4B) ACh receptors. The cholinergic receptors on OHCs are heteromers of these two types of subunits, and deletion of either one renders animals functionally de-efferented, i.e., shock-evoked MOC suppression of DPOAEs is completely abolished in both α9 and α10 knockouts (Vetter et al. 1999, 2007). Thus the persistence of contra-noise suppression in both these mutant lines is further evidence against a role for the MOC system (Fig. 4).
Fig. 4.
Contra-noise suppression is normal in mice with mutations in the ACh receptors designed to block or enhance MOC effects. A: contra-noise effects in mice lacking the α9 ACh subunit: mean (±SE) ΔDPOAE from 8 runs in 3 mice; mean contra-noise level was 102 dB SPL. B: contra-noise effects mice lacking the α10 ACh subunit: mean (±SE) ΔDPOAE from 18 runs in 4 mice; mean contra-noise level was 99 dB SPL. For both panels, the ipsilateral f2 was at 16 kHz and primary levels were set to produce a DPOAE of 0 dB SPL.
As a final test of OC involvement, and to rule out possible contributions of the LOC system, for which the peripheral receptors are less well understood, contra-noise suppression was measured before and after acute surgical transection of the OC bundle. Success of the lesion was confirmed via brain stem sections through the olivary complex stained for acetylcholinesterase, the transmitter-degrading enzyme, which labels the cell bodies and axons of cholinergic neurons (Fig. 5A). As seen in Fig. 5B, the contra-noise suppression was unaffected by the cut, which clearly transected the OC bundle to the ipsilateral ear. To rule out participation by OC cells whose axons might course separately from the main OC bundle, the entire superior olivary complex was destroyed on one side (Fig. 6A) in several animals by stereotaxic injection of a neurotoxin (Le Prell et al. 2003). These lesions appeared to eliminate all MOC and LOC cells on one side. As shown in Fig. 6B, a robust contra-noise suppression could still be measured in these chronically de-efferented ears.
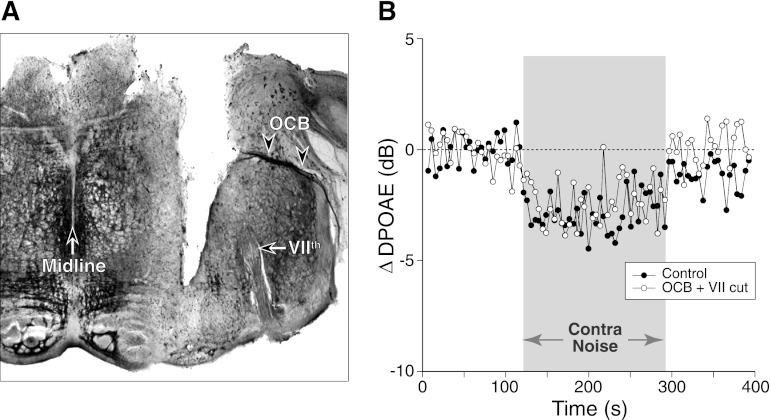
Fig. 5.
Contra-noise suppression persists after acute section of the olivocochlear bundle (OCB) and VIIth (facial) nerve. A: acetylcholinesterase-stained brain section shows a knife cut transecting the entire OCB (arrowheads) to the right ear (see Fig. 10, “side cut”) and the VIIth cranial nerve, the source of innervation for the stapedius muscle. B: mean ΔDPOAE from 2 runs before and 2 runs after the OCB transection shown in A. Contra-noise level was 98 dB SPL, ipsilateral f2 was at 16 kHz, and primary levels were set to produce a DPOAE of 0 dB SPL.
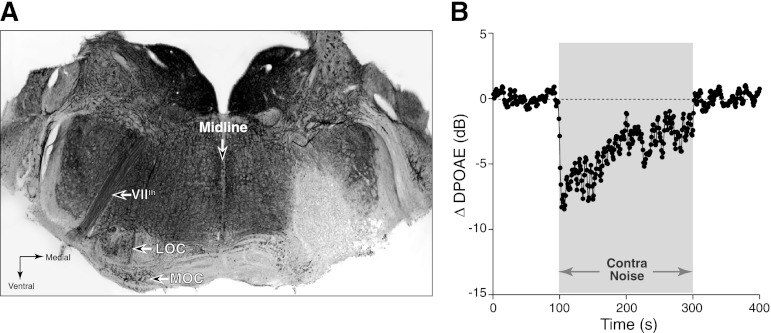
Fig. 6.
Contra-noise suppression persists after chronic lesion of MOC and lateral olivocochlear (LOC) neurons in the brain stem. A: acetylcholinesterase-stained brain stem section shows complete loss of LOC cells and near-complete loss of MOC cells on the right side, 1 wk after injection of neurotoxin. On the intact (left) side, LOC cell bodies are visible within the S-shaped lateral superior olive; MOC cell bodies are visible in the ventral nucleus of the trapezoid body, ventro-medial to the LOC group. B: contra-noise effects persist on the lesion side: mean ΔDPOAE from 6 runs is shown. Contra-noise level was 102 dB SPL, ipsilateral f2 was at 16 kHz, and primary levels were set to produce a DPOAE of 0 dB SPL. Time resolution is ∼1 s/point.
Contribution of middle-ear muscles.
To assess the middle-ear reflex contribution to contra-noise effects, we compared results in a large number of animals before and after muscle paralysis with α-d-tubocurarine, at a dose that abolishes facial twitches evoked by electrically shocking the facial nerve. As shown in Fig. 7A, the contra-noise effect was clearly not blocked by the paralysis. Indeed, on average, the mean effect was larger after curarization. In the same assays, we looked for impedance changes caused by middle-ear muscle contractions, by measuring ear canal sound pressure to short, ipsilateral 500-Hz tone bursts interleaved between presentations of the DPOAE-evoking tones (Goodman and Keefe 2006). A contra-noise-evoked shift in ipsilateral 500-Hz sound pressure would have suggested middle-ear muscle contraction, but no such shift was seen in the unparalyzed state (Fig. 7A). Furthermore, the efficacy of the paralytic is suggested by the elimination of small sound pressure level variations at 500 Hz that were seen before curarization (Fig. 7A). The slow drift in mean 500-Hz SPL may arise from instability in the speakers: note the high gain of the vertical scale for this measurement (right-hand axis in Fig. 7A).
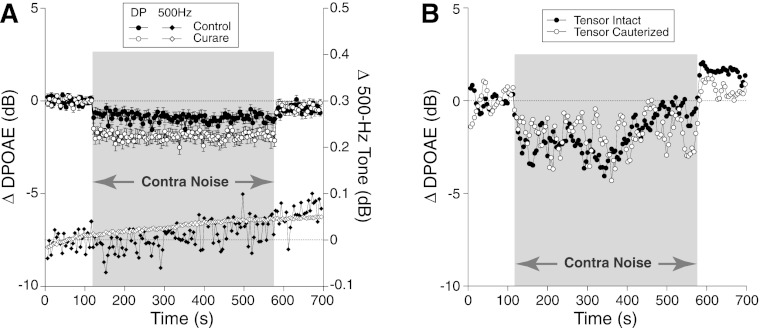
Fig. 7.
Contra-noise suppression persists after paralysis or lesion of middle-ear muscles. A: mean contra-noise suppression (filled and open circles; left y-axis) and mean ear canal SPL to an ipsilateral 500-Hz tone (filled and open diamonds; right y-axis) before and after paralysis with curare. Changes in 500-Hz SPL indicate impedance changes caused by middle-ear muscle contractions (Goodman and Keefe 2006). Data are means (±SE) from 18 animals: 38 runs before and 40 runs after curare. Mean contra-noise level was 106 dB SPL, ipsilateral f2 was at 16 kHz, and primary levels were set to produce a DPOAE of 0 dB SPL. B: contra-noise suppression persists after cauterization of the tensor tympani: data from a single experiment.
The lack of involvement of the stapedius reflex is further supported by the persistence of contra-noise suppression after transection of the facial (VIIth) nerve (Fig. 6). To further rule out a role for the tensor tympani, which is innervated by the Vth rather than the VIIth cranial nerve, we showed that contra-noise suppression was unaffected by cauterizing the tensor's tendon at its insertion into the malleus (Fig. 7B).
Contribution of the adrenergic system.
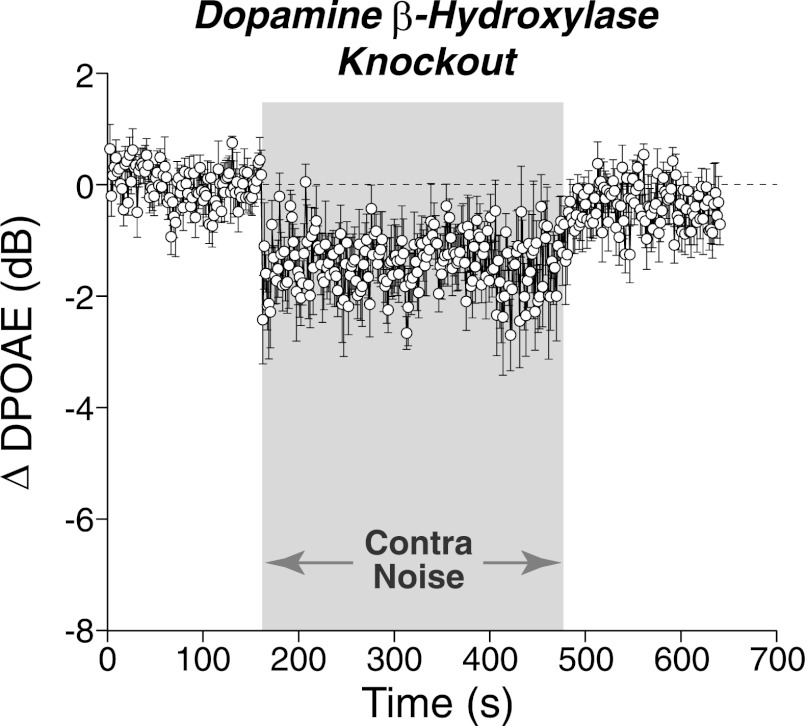
The vasculature and neurons of the inner ear receive adrenergic innervation from the cervical sympathetic chain (Hozawa et al. 1989; Spoendlin and Lichtensteiger 1966), and adrenergic receptors may also be expressed by cells of the organ of Corti and stria vascularis, despite a lack of direct sympathetic innervation (Fauser et al. 2004). Although it is not known whether activity in these adrenergic neurons is modulated by sound on the timescale studied here, changes in cochlear blood flow induced by the cochlear sympathetics could enhance or suppress DPOAEs. To probe this idea, contra-sound effects were measured in mice that lack the gene for dopamine β-hydroxylase. Such mice are effectively sympathectomized (Thomas and Palmiter 1997), as this enzyme is required for the conversion of dopamine to norepinephrine and epinephrine. As shown in Fig. 8, despite the absence of adrenergic signaling, contralateral-noise suppression was still observed.
Fig. 8.
Contra-noise suppression persists in mice lacking adrenergic signaling. Data are means (±SE) of 8 runs from 4 mutant animals lacking the gene for dopamine β-hydroxylase. Mean contra-noise level was 94 dB SPL, ipsilateral f2 was at 16 kHz, and primary levels were set to produce a DPOAE of 0 dB SPL. Time resolution is ∼1.6 s/point.
Contra-Noise Effects on DPOAEs Evoked by High-Level Primaries
In a final series of experiments, primary tones were set at higher sound pressure levels (f2 = 65–85 dB SPL). Although MOC feedback will cause smaller proportional reductions when cochlear responses are evoked at higher SPLs, the amount of MOC feedback will increase with increasing level of the acoustic stimuli, and binaural interactions in the MOC reflex (Liberman 1988) could enhance MOC-mediated contra-noise effects (see MOC Reflex Strength in Mice and the Importance of Assay Parameters).
As summarized in Fig. 9, contra-noise suppression of high-level ipsilateral DPOAEs, although small in magnitude, is clear-cut and reproducible. In contrast to effects seen with low-level DPOAEs, the phenomenon is dominated by contributions of the MOC pathway. These suppressive effects are no larger than those seen with low-level DPOAEs; however, the contra-noise effect is almost completely blocked by strychnine (Fig. 9A) or by cutting the entire OC bundle (Fig. 9B). The effect is also greatly reduced by cutting the OC bundle at the midline (data not shown), which should interrupt only the ipsilaterally responsive MOC efferents (Fig. 10). This is consistent with the idea that the effect is primarily mediated by contra-noise facilitation of the ipsilaterally responsive MOC pathway (see MOC Reflex Strength in Mice and the Importance of Assay Parameters).
Fig. 9.
Contra-noise suppression of DPOAEs evoked by high-level primaries is blocked by strychnine or cutting the OCB. A: contra-noise suppression in 1 animal before and 30 min after strychnine injection at 10 mg/kg (same dosage as for Fig. 3). B: contra-noise suppression in 1 animal before and immediately after an OCB lesion similar to that in Fig. 5A. For both panels, data are means of 11 runs: f1 was at 75 dB SPL, and f2 was raised in 1-dB steps from 65 to 75 dB SPL (see Maison and Liberman 2000). The f2 frequency was 16 kHz, and mean contra-noise level was 98 dB SPL. Time resolution is ∼1 s/point.
Fig. 10.
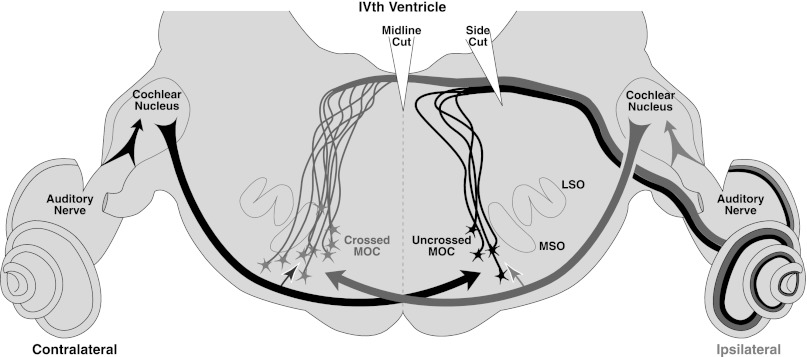
Schematic of the crossed and uncrossed reflex pathways for sound-evoked MOC feedback to 1 ear. Cochlear nucleus neurons on each side project to MOC cells on the opposite side of the brain (bold arrows), which drives their responses to monaural sound in the dominant ear: 2/3 of the MOC neurons projecting to each ear are driven by ipsilateral sound (black), while only 1/3 are driven by contralateral sound (gray). The binaural facilitation of MOC neurons by sound from the nondominant ear is schematized by the smaller, paler arrows to each MOC cell cluster. Cuts to the dorsal surface illustrate why a midline cut interrupts only the ipsilaterally responsive MOC neurons whereas a side cut can interrupt both ipsilateral and contralateral reflexes. LSO, lateral superior olive; MSO, medial superior olive.
DISCUSSION
MOC vs. Middle-Ear Muscle Feedback and Effects of Anesthesia
The auditory periphery in mammals is equipped with two binaural, sound-evoked, negative-feedback pathways: 1) the MOC system projecting bilaterally from the ventral nucleus of the trapezoid body to the bases of the OHCs, where it suppresses cochlear responses by turning down the gain of the cochlear amplifier, and 2) the stapedius muscle, innervated by a branch of the VIIth nerve, which, when activated to contract, stiffens the ossicular chain and reduces sound transmission through the middle ear (Liberman and Guinan 1998). Although there is a second middle-ear muscle, the tensor tympani (innervated by the Vth nerve), its activation is not typically evoked by acoustic stimulation (Mukerji et al. 2010).
Because contra-sound activation of either the MOC or middle-ear muscle reflexes can suppress ipsilateral cochlear responses, and because these reflexes can be differentially affected by anesthesia (both anesthesia depth and anesthetic pharmacology), contra-sound tests need to be carefully validated, for every new species-anesthesia combination, to separate MOC effects from the effects of middle-ear muscle. As illustrated here, the roles of each feedback system can be isolated by surgical, pharmacological, genetic, or acoustic means. Although the peripheral effects of both systems are mediated by ACh, the receptors on skeletal muscles, such as the stapedius, and those on the OHCs differ in their pharmacology: muscle ACh receptors are blocked by the paralytic curare (Fig. 7), whereas ACh receptors on OHCs, α9 and α10 subunits of the nicotinic type, are most effectively blocked by strychnine (Fig. 3) (Elgoyhen et al. 2001; Rothlin et al. 1999). Because these OHC receptors are not widely expressed in the body, they can be targeted for genetic deletion without embryonic lethality: prior studies have shown that MOC effects are eliminated by deletion of either the α9 or α10 subunit gene (Vetter et al. 1999, 2007). Middle-ear muscle activation can also be inferred from changes in the ear's input impedance, which, when present, change ipsilateral ear canal sound pressure synchronously with the onset (and offset) of the contra-noise stimuli. Muscle activation will also produce characteristic phase changes in the OAEs that have been used to parse MOC and middle-ear effects in humans (Guinan et al. 2003).
Using these as well as other reflex-specific manipulations, prior studies have shown that contra-noise suppression in the barbiturate-anesthetized cat is dominated by MOC effects (Liberman et al. 1996; Puria et al. 1996), whereas contra-noise suppression in the ketamine-anesthetized rat is dominated by middle-ear effects (Relkin et al. 2005). In fentanyl-droperidol-anesthetized guinea pigs, both feedback pathways are inactivated when a surgical plane of anesthesia is attained (Boyev et al. 2002), thereby eliminating the robust contra-noise effect seen in awake animals that is MOC mediated. In contrast, in the rabbit there is no measurable contra-noise suppression from the MOCs, even when the animals are fully awake (Whitehead et al. 1991). In awake humans, MOC effects dominate the contra-noise suppression when the contra noise is below ∼65 dB SPL, but the middle-ear muscles dominate the effects when contra-noise levels are above that level (Guinan et al. 2003).
Despite extensive use of contra-noise suppression as a test for MOC reflexes in the anesthetized mouse (Frisina et al. 2007; Jacobson et al. 2003; Zettel et al. 2007; Zhu et al. 2007), no validation of the MOC contributions to these effects had been carried out prior to the present study. As shown here, the extent of MOC contributions to the contra-noise suppression observed in either the ketamine-xylazine- or urethane-xylazine-anesthetized mouse depends on the level of the ipsilateral stimuli: with low-level ipsilateral stimuli (25–35 dB SPL), effects are dominated by an unknown mechanism that is neither middle ear nor MOC in origin (see Origins of Non-MOC Contra-Noise Effects in Mice), whereas with high-level ipsilateral stimuli (70–80 dB SPL), the effects are dominated by the MOC system (Fig. 9).
Stimulus parameters in other studies of contra-noise suppression of OAEs in mice (Zhu et al. 2007) included an intermediate level of OAE-evoking stimuli (50–65 dB SPL) and a lower level of contralateral noise (55 dB SPL). We were unable to produce any repeatable suppression with these parameters in our anesthetized mice (data not shown), despite the fact that both studies use CBA/CaJ mice and ketamine-xylazine anesthesia. One major paradigm difference is that prior studies of contra-noise suppression in mice swept the primary frequencies from 5.6 to 44.8 kHz in 1/8th-octave steps (Zhu et al. 2007). This may be an important different because of complex interactions between the two intracochlear sources of DPOAE generation that can produce frequency-specific variations in the measured size (and sign) of contra-noise effects (Henin et al. 2011). Although maximum effects in prior studies (∼2.5 dB) were seen for f2 ∼8 kHz (Zhu et al. 2007), suppression at 16 kHz was not much smaller (∼1.5 dB), which makes it comparable to the effects seen here, although with higher contra-noise levels. Despite the differences in stimulus parameters, the present results raise significant questions as to whether the contra-noise effects in prior mouse studies were indeed mediated by the OC system.
MOC Reflex Strength in Mice and the Importance of Assay Parameters
To understand why MOC involvement in contra-noise suppression depends on the level of the ipsilateral stimuli, it helps to review the neural circuitry and binaural response properties of single MOC neurons. Roughly two-thirds of MOC cells projecting to each ear are responsive to ipsilateral sound, while only a third are responsive to contralateral sound (Liberman and Brown 1986). This general projection pattern has been observed in cats (Guinan et al. 1984), guinea pigs (Robertson et al. 1987), and mice (Brown and Levine 2008). The brain stem circuitry of the MOC system is schematized in Fig. 10: given the crossed nature of the ascending projections from cochlear nucleus to MOC cells of origin, the ipsilaterally responsive MOC cells are found on the contralateral side of the brain stem, and vice versa (Liberman and Brown 1986). With monaural acoustic stimulation, most MOC cells respond only to sound in one ear; however, the sound-evoked responses of monaural MOC cells are greatly enhanced when sound is added to the other ear (Liberman 1988). This phenomenon, known as “binaural facilitation,” suggests an additional projection to each cell group from the nondominant ear, as schematized by the smaller arrows projecting to the MOC cell bodies in Fig. 10.
Assays of MOC reflex strength typically use high-level contralateral noise, to maximize sound-evoked activation of contralaterally responsive MOC neurons, coupled with low-level ipsilateral stimuli, to keep the cochlear responses in a range where the cochlear amplifier has maximum effect and thus in a range where any sound-evoked MOC feedback can produce a robust suppression (Puria et al. 1996). Indeed, in unanesthetized CBA/CaJ mice, there is a robust contra-noise suppression of low-level DPOAEs that largely disappears in the α9 knockout mouse (Chambers et al. 2011). The magnitude of the OC-mediated suppression in unanesthetized wild-type mice (5–6 dB) is significantly greater than the mean effects in the present study (Fig. 1). Together, the mouse data suggest that sound-evoked OC activity is so attenuated by ketamine-xylazine, or urethane-xylazine, anesthesia that suppression of low-level OAEs is undetectable, even with the relatively high contra-noise sound levels used here.
As the sound pressure of the ipsilateral (OAE eliciting) stimuli is raised, the magnitude of MOC-mediated OAE suppression steadily decreases, but only if MOC activity stays constant, as when MOC activity is evoked by shocking the OC bundle in a deeply anesthetized animal (see Fig. 1 from Maison et al. 2007). However, when MOC activity is sound evoked, as in the present study, raising the level of the ipsilateral stimuli also increases the amount of MOC feedback. This can happen for two reasons: either 1) the ipsilateral stimuli become intense enough to activate the large population of ipsilaterally responsive MOC cells (70% of the OC terminals on OHCs) and the addition of contralateral sound enhances their activity by binaural facilitation and thereby suppresses the OAE and/or 2) the ipsilateral stimuli become intense enough to enhance the responsiveness of the contralaterally responsive MOC cells (the remaining 30%), again by binaural facilitation (Liberman 1988). Support for hypothesis 1 is provided by the observation that the contra-noise effect is largely abolished by cutting the OC bundle at the midline (data not shown), which interrupts only the ipsilaterally responsive MOC cells. In contrast, when OC-mediated contra-sound suppression is observable with low-level ipsilateral stimuli, as in the barbiturate-anesthetized cat, the effect is not diminished by cutting the OC bundle at the midline; it is only eliminated when the contralaterally responsive fibers are interrupted by a more laterally positioned cut (see Fig. 6 in Liberman et al. 1996 and the schematic in Fig. 10).
Origins of Non-MOC Contra-Noise Effects in Mice
In the present study, we document a small, but highly significant, contra-noise suppression of the DPOAEs, which is not mediated by the OC system, either MOC or LOC, or the middle-ear muscles, either the stapedius or the tensor tympani. By using a mutant mouse lacking the synthetic enzyme (dopamine β-hydroxylase) required to convert dopamine into epinephrine or norepinephrine (Thomas and Palmiter 1997), we have also demonstrated that this contra-noise effect is not mediated by the sympathetic innervation of the inner ear, which projects from the cervical sympathetic chain both to the smooth muscles of the cochlear vasculature as well as directly to the axons of cochlear nerve fibers in the osseous spiral lamina (Spoendlin and Lichtensteiger 1966). The observation that the phenomenon disappears after contralateral cochlear destruction proves that it is not simply related to acoustic cross talk from the contralateral to the ipsilateral ear. The similar magnitude of this contra-noise effect in genetically de-efferented awake mice (lacking the α9 ACh receptor) and in anesthetized mice that are either genetically, surgically, or pharmacologically de-efferented suggests that the phenomenon is not anesthesia sensitive. It is a variable phenomenon, with variable onset and offset time courses, and it is not always clearly demonstrable in every mouse. Indeed, it may typically represent a mixture of mechanisms, which could sometimes include some small MOC contributions (depending on depth of anesthesia), but clearly is dominated by a mechanism that is not mediated by any of the “classic” neuronal feedback pathways that could link the two ears.
On the basis of the current understanding of cochlear structure and function, there are only two other plausible hypotheses we can construct for the origin of this effect. The first is inspired by the recent discovery (Graham and Vetter 2011) of cochlear receptors for corticotropin-releasing factor, a molecule best known for its upstream role in the hypothalamic-pituitary-adrenal axis and its control of the systemic stress response. Receptors for corticotropin-releasing factor are present on OHCs, and it is conceivable that this ligand is released into the bloodstream in response to high-level acoustic stimulation. It seems unlikely that such a mechanism could underlie a change in OHC function that has onset and offset time constants as fast as a few seconds, as is sometimes observed for the contra-noise effect (Fig. 1A). However, it is conceivable that such a mechanism could contribute to the contra-noise effects that have a much slower and more gradual onset (e.g., Fig. 1A). Unfortunately, the receptor knockout mouse has greatly elevated DPOAE thresholds, likely due to a role for the pathway in cochlear development (Graham and Vetter 2011). Thus a test requiring a response to low-level ipsilateral stimuli cannot be studied.
The second hypothesis is inspired by the report that type II cochlear nerve fibers, classically described as the “afferent” innervation of the OHCs, are actually both afferent and efferent in nature, with most peripheral terminals of type II neurons forming reciprocal synapses with the OHCs (Thiers et al. 2008). The transmitter(s) and receptor(s) mediating this putative bidirectional signaling are currently unknown. If the central projections of these type IIs also engage in reciprocal signaling with their cochlear nucleus targets, an additional putative pathway for interaural communication would exist, since there are well-studied central pathways that provide each cochlear nucleus with neural inputs from the other side (Bledsoe et al. 2009).
GRANTS
This work was supported by National Institutes of Health Grants RO1 DC-0188 (M. C. Liberman), RO1 DC-006258 (D. E. Vetter), RO1 MH-063352 (S. A. Thomas), P30 DC-5029 (M. C. Liberman), and P30 NS-047243 (D. E. Vetter).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.F.M. and M.C.L. conception and design of research; S.F.M. and H.U. performed experiments; S.F.M. and M.C.L. analyzed data; S.F.M. and M.C.L. interpreted results of experiments; S.F.M. and M.C.L. prepared figures; S.F.M. and M.C.L. drafted manuscript; S.F.M. and M.C.L. edited and revised manuscript; S.F.M., H.U., D.E.V., A.B.E., S.A.T., and M.C.L. approved final version of manuscript.
REFERENCES
- Bledsoe SC, Jr, Koehler S, Tucci DL, Zhou J, Le Prell C, Shore SE. Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways. J Neurophysiol 102: 886–900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyev KP, Liberman MC, Brown MC. Effects of anesthesia on efferent-mediated adaptation of the DPOAE. J Assoc Res Otolaryngol 3: 362–373, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Levine JL. Dendrites of medial olivocochlear neurons in mouse. Neuroscience 154: 147–159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacace AT, Margolis RH, Relkin EM. Threshold and suprathreshold temporal integration effects in the crossed and uncrossed human acoustic stapedius reflex. J Acoust Soc Am 89: 1255–1261, 1991 [DOI] [PubMed] [Google Scholar]
- Chambers AR, Hancock KE, Maison SF, Liberman MC, Polley DB. Sound-evoked olivocochlear activation in unanesthetized mice. J Assoc Res Otolaryngol 22: 222–223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A. Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear Res 43: 251–262, 1990 [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Cochlear efferent feedback balances interaural sensitivity. Nat Neurosci 9: 1474–1476, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J Neurophysiol 97: 1775–1785, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. Alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA 98: 3501–3506, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser C, Schimanski S, Wangemann P. Localization of beta1-adrenergic receptors in the cochlea and the vestibular labyrinth. J Membr Biol 201: 25–32, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD, Newman SR, Zhu X. Auditory efferent activation in CBA mice exceeds that of C57s for varying levels of noise. J Acoust Soc Am 121: EL29–EL34, 2007 [DOI] [PubMed] [Google Scholar]
- Goodman SS, Keefe DH. Simultaneous measurement of noise-activated middle-ear muscle reflex and stimulus frequency otoacoustic emissions. J Assoc Res Otolaryngol 7: 125–139, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CE, Vetter DE. The mouse cochlea expresses a local hypothalamic-pituitary-adrenal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity. J Neurosci 31: 1267–1278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff JA, Liberman MC. Modulation of cochlear afferent response by the lateral olivocochlear system: activation via electrical stimulation of the inferior colliculus. J Neurophysiol 90: 3178–3200, 2003 [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27: 589–607, 2006 [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol 4: 521–540, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Warr WB, Norris BE. Topographic organization of the olivocochlear projections from the lateral and medial zones of the superior olivary complex. J Comp Neurol 226: 21–27, 1984 [DOI] [PubMed] [Google Scholar]
- Henin S, Thompson S, Abdelrazeq S, Long GR. Changes in amplitude and phase of distortion-product otoacoustic emission fine-structure and separated components during efferent activation. J Acoust Soc Am 129: 2068–2079, 2011 [DOI] [PubMed] [Google Scholar]
- Hozawa J, Kimura RS, Takahashi S. Sympathetic nervous system in the guinea pig cochlea. Ear Res Jpn 20: 111–112, 1989 [Google Scholar]
- Jacobson M, Kim S, Romney J, Zhu X, Frisina RD. Contralateral suppression of distortion-product otoacoustic emissions declines with age: a comparison of findings in CBA mice with human listeners. Laryngoscope 113: 1707–1713, 2003 [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Effects of olivocochlear feedback on distortion product otoacoustic emissions in guinea pig. J Assoc Res Otolaryngol 2: 268–278, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen E, Liberman MC. Slow build-up of cochlear suppression during sustained contralateral noise: central modulation of olivocochlear efferents? Hear Res 256: 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Shore SE, Hughes LF, Bledsoe SC., Jr Disruption of lateral efferent pathways: functional changes in auditory evoked responses. J Assoc Res Otolaryngol 4: 276–290, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Response properties of cochlear efferent neurons: monaural vs. binaural stimulation and the effects of noise. J Neurophysiol 60: 1779–1798, 1988 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res 24: 17–36, 1986 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Guinan JJ., Jr Feedback control of the auditory periphery: anti-masking effects of middle ear muscles vs. olivocochlear efferents. J Commun Disord 31: 471–483, 1998 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Puria S, Guinan JJ., Jr The ipsilaterally evoked olivocochlear reflex causes rapid adaptation of the 2f1-f2 distortion product otoacoustic emission. J Acoust Soc Am 99: 3572–3584, 1996 [DOI] [PubMed] [Google Scholar]
- Maison SF, Adams JC, Liberman MC. Olivocochlear innervation in mouse: immunocytochemical maps, crossed vs. uncrossed contributions and colocalization of ACh, GABA, and CGRP. J Comp Neurol 455: 406–416, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a non-invasive assay of olivocochlear reflex strength. J Neurosci 20: 4701–4707, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Liu XP, Vetter DE, Eatock RA, Nathanson NM, Wess J, Liberman MC. Muscarinic signaling in the cochlea: presynaptic and postsynaptic effects on efferent feedback and afferent excitability. J Neurosci 30: 6751–6762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Rosahl TW, Homanics GE, Liberman MC. Functional role of GABAergic innervation of the cochlea: phenotypic analysis of mice lacking GABAA receptor subunits alpha1, alpha2, alpha5, alpha6, beta2, beta3, or delta. J Neurosci 26: 10315–10326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Vetter DE, Liberman MC. A novel effect of cochlear efferents: in vivo response enhancement does not require alpha9 cholinergic receptors. J Neurophysiol 97: 3269–3278, 2007 [DOI] [PubMed] [Google Scholar]
- Mukerji S, Windsor AM, Lee DJ. Auditory brainstem circuits that mediate the middle ear muscle reflex. Trends Amplif 14: 170–191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen KK, Roth K. Histochemical localization of cholinesterases in the cochlear nuclei of the cat, with notes on the origin of acetylcholinesterase-positive afferents and the superior olive. Brain Res 16: 165–185, 1969 [DOI] [PubMed] [Google Scholar]
- Puel JL, Rebillard G. Effect of contralateral sound stimulation on the distortion product 2F1-F2: evidence that the medial efferent system is involved. J Acoust Soc Am 87: 1630–1635, 1990 [DOI] [PubMed] [Google Scholar]
- Puria S, Guinan JJ, Jr, Liberman MC. Olivocochlear reflex assays: effects of contralateral sound on compound action potentials versus ear-canal distortion products. J Acoust Soc Am 99: 500–507, 1996 [DOI] [PubMed] [Google Scholar]
- Rajan R. Involvement of cochlear efferent pathways in protective effects elicited with binaural loud sound exposure in cats. J Neurophysiol 74: 582–597, 1995 [DOI] [PubMed] [Google Scholar]
- Relkin EM, Sterns A, Azeredo W, Prieve BA, Woods CI. Physiological mechanisms of onset adaptation and contralateral suppression of DPOAEs in the rat. J Assoc Res Otolaryngol 6: 119–135, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Cole KS, Harvey AR. Brainstem organization of efferent projections to the guinea pig cochlea studied using the fluorescent tracers fast blue and diamidino yellow. Exp Brain Res 66: 449–457, 1987 [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Katz E, Verbitsky M, Elgoyhen AB. The alpha9 nicotinic acetylcholine receptor shares pharmacological properties with type A gamma-aminobutyric acid, glycine, and type 3 serotonin receptors. Mol Pharmacol 55: 248–254, 1999 [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC. Furosemide alters organ of Corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci 11: 1057–1067, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ., Jr Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am 105: 782–798, 1999 [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Lichtensteiger W. The adrenergic innervation of the labyrinth. Acta Otolaryngol (Stockh) 61: 423–434, 1966 [PubMed] [Google Scholar]
- Sridhar TS, Liberman MC, Brown MC, Sewell WF. A novel cholinergic “slow effect” of efferent stimulation on cochlear potentials in the guinea pig. J Neurosci 15: 3667–3678, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers FA, Nadol JB, Jr, Liberman MC. Reciprocal synapses between outer hair cells and their afferent terminals: evidence for a local neural network in the mammalian cochlea. J Assoc Res Otolaryngol 9: 477–489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell 91: 583–592, 1997 [DOI] [PubMed] [Google Scholar]
- Vetter DE, Katz E, Maison SF, Taranda J, Turcan S, Ballestero J, Liberman MC, Elgoyhen AB, Boulter J. The alpha10 nicotinic acetylcholine receptor subunit is required for normal synaptic function and integrity of the olivocochlear system. Proc Natl Acad Sci USA 104: 20594–20599, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23: 93–103, 1999 [DOI] [PubMed] [Google Scholar]
- Whitehead ML, Martin GK, Lonsbury-Martin BL. Effects of the crossed acoustic reflex on distortion-product otoacoustic emissions in awake rabbits. Hear Res 51: 55–72, 1991 [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol 57: 1002–1021, 1987 [DOI] [PubMed] [Google Scholar]
- Zettel ML, Zhu X, O'Neill WE, Frisina RD. Age-related decline in Kv3.1b expression in the mouse auditory brainstem correlates with functional deficits in the medial olivocochlear efferent system. J Assoc Res Otolaryngol 8: 280–293, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Vasilyeva ON, Kim S, Jacobson M, Romney J, Waterman MS, Tuttle D, Frisina RD. Auditory efferent feedback system deficits precede age-related hearing loss: contralateral suppression of otoacoustic emissions in mice. J Comp Neurol 503: 593–604, 2007 [DOI] [PubMed] [Google Scholar]