Abstract
Virtually nothing is known about the activity of morphologically identified neurons in freely moving mammals. Here we describe stabilization and positioning techniques that allow juxtacellular recordings from labeled single neurons in awake, freely moving animals. This method involves the use of a friction-based device that allows stabilization of the recording pipette by friction forces. Friction is generated by a clamplike mechanism that tightens a sliding pipette holder to a preimplanted pipette guide. The interacting surfaces are smoothed to optical quality (<5-nm roughness) to enable micrometer stepping precision of the device during operation. Our method allows recordings from identified neurons in freely moving animals, and thus opens new perspectives for analyzing the role of identified neurons in the control of behavior.
Keywords: animal behavior, friction stabilization, neuron morphology
from the pioneering work of Hubel and Wiesel (1977) it became clear that single-neuron activity provides crucial insights into brain function. Thus, to explore the relationship between neural activity and behavior, extracellular recordings have been performed for decades. Extracellular recordings make it possible to record simultaneously from a large number of cells, and the recording stability allows monitoring of cellular activity over extended periods of time (Buzsáki 2004; Csiscvari et al. 2003; McNaughton et al. 1983; Nicolelis et al. 1997; Recce and O'Keefe 1989; Thompson and Best 1990; Wilson and McNaughton 1993). These favorable features make it the method of choice when population activity from neuronal ensembles is used as a functional correlate of behavior. One major limitation is that these methods do not allow the identification of the recorded neurons, thereby leaving the identity of putative cellular elements unresolved (Chorev et al. 2009). As a consequence, despite a large body of evidence pointing to the existence of strong structure-function relationships in the nervous system (Buhl et al. 1996; Chagnac-Amitai et al. 1990; Klausberger and Somogyi 2008; de Kock et al. 2007; de Kock and Sakmann 2009; Oberlaender et al. 2011), anatomical correlates of brain activity during animal behaviors have remained largely unexplored.
Enormous advances have been made in recent years regarding the generation of new tools allowing the visualization of cellular morphologies and single-cell microcircuits (Marshel et al. 2010; Osakada et al. 2011; Scanziani and Häusser 2009; Wickersham et al. 2007). Historically, the juxtacellular labeling technique was the first method that provided the means of identifying extracellularly recorded units (Pinault 1994, 1996; Pinault and Deschênes 1998). The close contact between the recorded cell and the pipette tip (hence termed “juxtacellular” configuration) allows electrical transfer of small dyes (e.g., biocytin) and more recently also DNA (Judkewitz et al. 2009; Kitamura et al. 2008; Marshel et al. 2010; Rae and Levis 2002; Rathenberg et al. 2003) into the recorded cell by alternating current injections. This particularly easy, powerful, and reliable tool has been successfully applied to explore structure-function relationships of neuronal activity in a number of different organisms during anesthetized (Herfst and Brecht 2008; Joshi and Hawken 2006; Pilowsky and Makeham 2001; Pinault 2003; Simpson et al. 2005; Sugiyama et al. 2011; Toney and Daws 2006) and awake (Houweling et al. 2010; Houweling and Brecht 2008; de Kock and Sakmann 2009) states. Despite providing a major step forward in the visualization of neuronal morphologies, application of this labeling technique is still limited to mechanically stable preparations (i.e., anesthetized or awake head-fixed animals), where animal movement and/or brain pulsations are minimized to an extent that is compatible with conventional single-cell recording techniques.
We developed a new method, which allowed us to record single-cell activity during unrestrained animal behavior and to recover cell morphologies at high rates, making it possible to explore the relationship between neural activity, morphology, and behavior. Our key development consisted of a new friction-based pipette-positioning device that provided mechanical stability of the recording during animal movement. Here we provide a description of this device and its mechanics and an overview of its performance. Our data indicate that friction stabilization is an effective means for recording identified neurons in freely moving animals.
METHODS
In vivo juxtacellular recordings.
Juxtacellular recording and labeling of single neurons was performed in freely moving Wistar rats (∼P30–50) according to previously published procedures (Houweling et al. 2010). A total of 165 rats were used in the present study. Thick-walled borosilicate glass pipettes with filament (1.5-mm outer diameter, 0.87-mm inner diameter; 3–6 MΩ in brain tissue) (Hilgenberg, Malsfeld, Germany) were filled with Ringer solution containing (in mM) 135 NaCl, 5.4 KCl, 5 HEPES, 1.8 CaCl2, and 1 MgCl2 (pH 7.2) as well as biocytin (2–3%). Standard surgical preparation, pipette anchoring, and anesthesia/wake-up procedures were performed as described previously (Burgalossi et al. 2011; Lee et al. 2006, 2009). Animals were either preimplanted under ketamine-xylazine anesthesia (intraperitoneal doses of 100 and 5 mg/kg, respectively) or directly anesthetized with an intraperitoneal dose of a mixture of medetomidine (225 μg/kg), midazolam (6 mg/kg), and fentanyl (7.5 μg/kg). This anesthetic mix was antagonized with a subcutaneous dose of a mixture of atipamezole (1 mg/kg), flumazenil (600 μg/kg), and naloxone (180 μg/kg) (Lee et al. 2006). Except for one recording targeted to the barrel cortex (3 mm posterior to bregma and 5 mm lateral from the midline) and 12 recordings targeted to the dorsal hippocampus (“unterminated recordings,” data set in Fig. 4B), all recordings were performed at variable depths (∼1,000–5,000 μm) at the coordinates for targeting the medial entorhinal cortex (0.2–0.8 mm anterior to the transverse sinus and 4.5–5 mm lateral to the midline, left hemisphere). A fraction of recordings targeted to the medial entorhinal cortex (n = 46) have been published elsewhere (Burgalossi et al. 2011). The juxtacellular signal was amplified by an ELC Ultra miniature headstage (npi electronic, Tamm, Germany) and an ELC-03XS amplifier (npi electronic) and sampled at 20–50 kHz by a LIH 1600 data-acquisition interface (HEKA Electronic, Lambrecht/Pfalz, Germany) under the control of PatchMaster 2.20 software (HEKA Electronic). The location of the animal was tracked at 25 Hz with the Digital Lynx videotracking system (Neuralynx, Bozeman, MT) by visualizing the two LEDs (red and blue) separated by 4.5 cm on the rat's head. All experimental procedures were performed according to German guidelines on animal welfare and were reviewed and approved by local ethics committees.
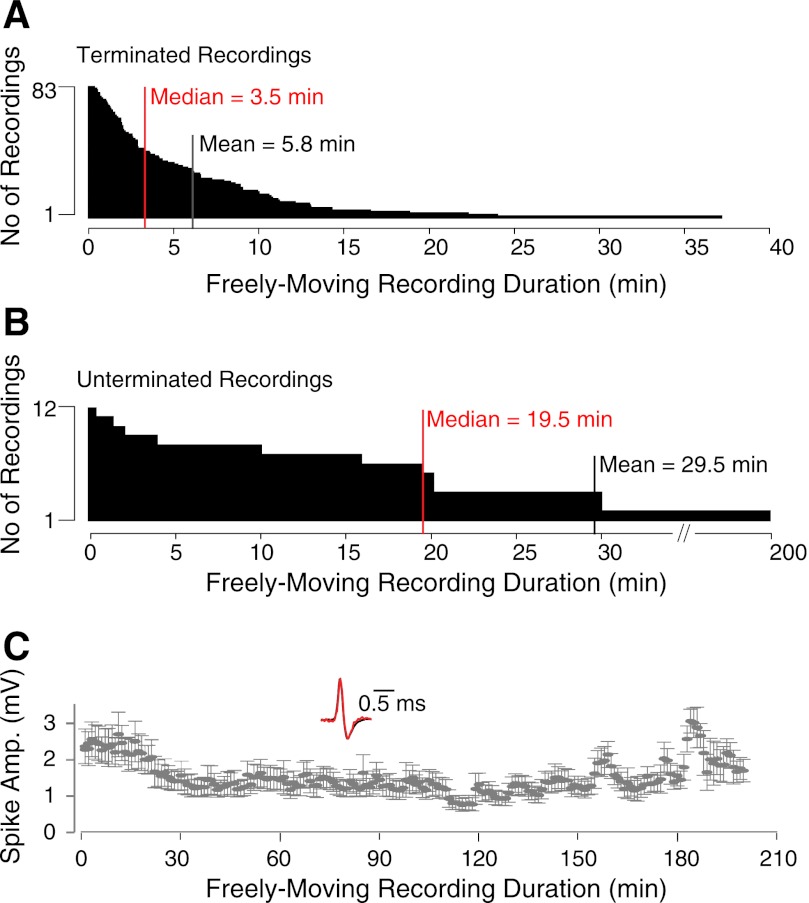
Fig. 4.
Juxtacellular recording durations during freely moving behavior. A: distribution of recording durations for all deliberately terminated freely moving juxtacellular recordings (n = 83). B: distribution of recording durations for a subset of unterminated freely moving juxtacellular recordings (n = 12) targeted to the dorsal hippocampus. C: average ± SD spike amplitudes (bin size = 1 min) for the longest freely moving unterminated recording shown in B (total duration = 3.3 h). Inset: superimposed normalized spike waveforms at the beginning (red) and end (black) of the recording session.
Histological analysis.
At the end of recording, the animal was injected with an overdose of ketamine or urethane and quickly perfused transcardially with 0.1 M phosphate-buffered saline followed by a 4% paraformaldehyde solution. To reveal the morphology of juxtacellularly labeled cells, 100- to 150-μm-thick brain slices were processed with the avidin-biotin-peroxidase method as previously described (Epsztein et al. 2010; Lee et al. 2006, 2009). In Fig. 5A, cytochrome oxidase staining was performed as previously described (Burgalossi et al. 2011), and the neuron was manually reconstructed with Neurolucida software (MBF Bioscience, Williston, VT) and displayed as a two-dimensional projection. The micrograph of the biocytin-filled neuron (Fig. 5A) was generated by two-dimensional projection of a z-stack, in order to achieve an extended-focus view.
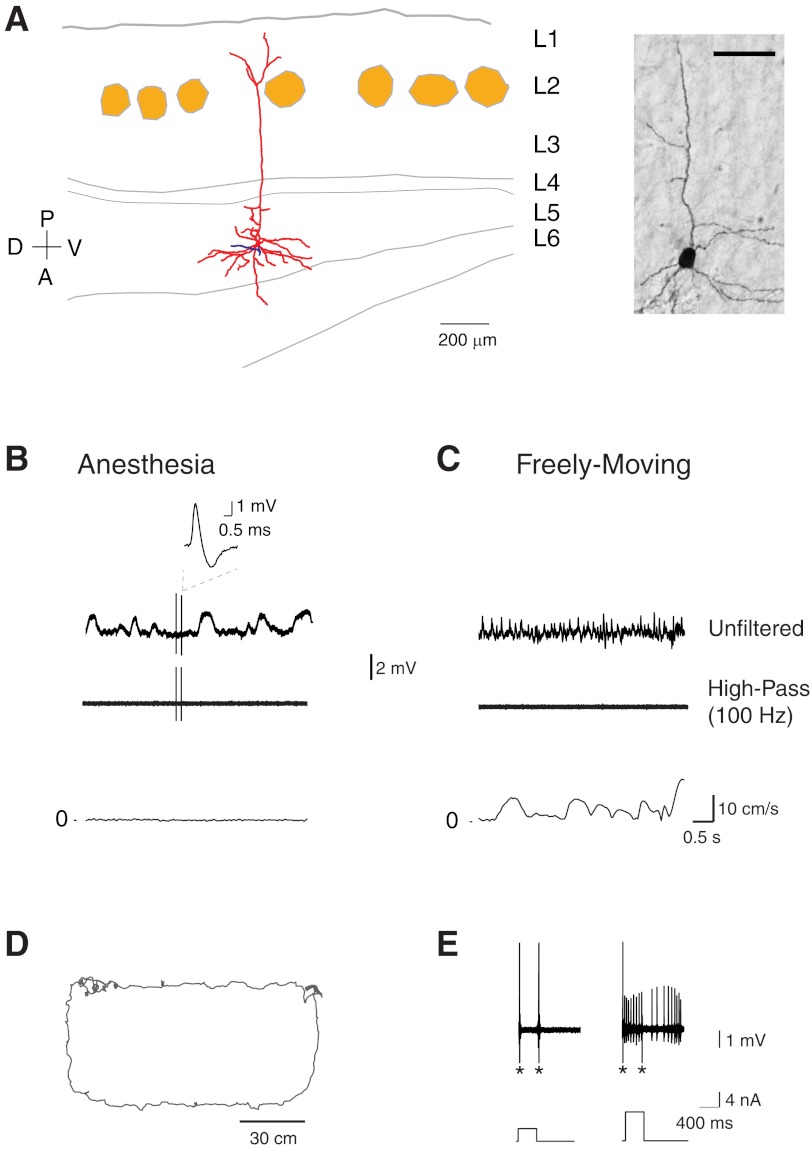
Fig. 5.
Identified silent cells in freely moving animals. A, left: reconstruction of the somatodendritic (red) and axonal (blue) morphology of a layer 5 pyramidal neuron in medial entorhinal cortex with a superimposed outline of small layer 2 patches (light brown) identified by cytochrome oxidase staining. D, dorsal; V, ventral; A, anterior; P, posterior. Right: micrograph of the biocytin-labeled neuron reconstructed on left. Scale bar, 50 μm. B, top: representative unfiltered and high-pass-filtered spike traces recorded from the cell shown in A before the animal woke up and explored the arena. A magnification of the spike is shown. Note the slow wave oscillations (∼1 Hz) during anesthesia in the unfiltered trace. Bottom: speed plot corresponding to the traces shown above. C, top: representative unfiltered and high-pass-filtered spike traces recorded during freely moving behavior from the same cell shown in A. Note the faster LFP oscillations compared with the anesthetized period and the absence of spikes. Bottom: speed plot corresponding to the traces shown above. D: trajectory of the rat (gray) during running in a 120 × 60-cm “O”-shaped maze. E: action potential firing (top) induced by squared current pulses of increasing amplitude (bottom) for the cell shown in A. These pulses were delivered at the end of the freely moving recording session to confirm the presence of the silent cell. Note that this cell kept discharging after the current pulse. Asterisks indicate stimulation artifacts (truncated for display purposes).
Data analysis.
For spike analysis, juxtacellular traces were high-pass filtered at 100 Hz, and a three-dimensional analysis using time and the first two principal components of the waveform was performed to visualize and assess the stability of spike amplitude over time and to isolate spikes from recording artifacts. Juxtacellularly recorded spikes were differentiated from “extracellularly” recorded spikes by the presence of a large positive polarity (leading and dominant positive peak > 1 mV) and by the ability to modulate cell firing with nanocurrents. All included recordings satisfied these criteria. The duration of the freely moving recordings was defined as the time at which the rat moved after waking up from the anesthesia to the time of the last included spike. A fraction of recordings (21 of 83; 25%) were deliberately terminated to improve the rate and quality of cell recovery (Burgalossi et al. 2011). Silent cells were fired after the animals had sampled the behavioral arena at least once. Spike stability over time during freely moving behavior was assessed by normalizing the duration of each recording (bin size = 10%) and averaging the number of spikes within each bin. The position of the rat was defined as the midpoint between the two head-mounted LEDs. Running speed analysis was performed with a cutoff of 0.2 cm/s applied to the speed averaged across a 600-ms rectangular sliding window. Data in the text are expressed as means ± SD.
Manufacturing of friction device.
Friction devices were made out of magnesium. The pipette holder was cut with a wire electrical discharge machining (EDM), while the pipette guide was generated by wire EDM and milling. The total mass of the friction device (without the glass pipette) was 2.12 g (pipette holder = 0.82 g; pipette guide = 1.30 g). The surface of the pipette guide and the internal surface of the pipette holder were processed with a micromilling machine (MMC1100, LT Ultra-Precision Technology). Titanium nitride (TiN) coating was generated by a sputtering system (Bestec). Recoating of the pipette guide is possible and can be performed in case of deterioration of the TiN film, which will typically become apparent by the disappearance of the golden color of the pipette guide surface.
Finite element method simulations and stepping tests.
To obtain the pipette tip displacement during an acceleration of 5 g (∼50 m/s2) of the rat, a finite element method (FEM) simulation was performed; 50 m/s2 corresponds to approximately three times the maximal head acceleration of rats during exploratory behavior (Ledberg and Robbe 2011; Sunderam et al. 2007; Venkatraman et al. 2007, 2010). For the simulation, the assembly was meshed with curve-based tetrahedral volume elements for best model mapping. The bottom side of the base plate was restrained in order to match the experimental condition of cementing the base on the rat's skull. A force of 0.106 N was applied on the pipette holder (arrows in Fig. 1C) representing the acceleration along the z-axis (= parallel to the recording pipette axis). Given that acrylic anchoring (Fig. 2A) factually prevents movements of the recording pipette in the x-y plane, the results of the FEM simulation under force application along the z-axis are displayed in Fig. 1C. Force application along the x- and y-axes also resulted in maximal tip displacements < 1 μm (0.18 and 0.03 μm, respectively). The distal-most sites on the pipette holder were always chosen as application points (as in Fig. 1C), as these result in maximal tip displacements given the largest moments of force. The equations for the simulation were computed with an iterative equation solver to identify displacement and stress of the assembly. The displacement value of 0.159 μm of the pipette tip is the absolute translation of the tip considering the elastic deformation of the components. The resulting maximum stress is <2% of the yield point. The preload force, generated by the friction clamp and acting upon the pipette guide, depends on both geometry and preload displacement. According to FEM simulation, the preload force displays a value of ∼13.9 N. The pipette clamping force was simulated at 22.2 N in combination with a 1.5-mm-diameter pipette. The maximum force upon the pipette during the insertion process is ∼44.4 N, which does not damage an intact pipette. The maximum stress caused by the pipette insertion is ∼100.5 MPa, which is below the stress yield (155 MPa) and does not lead to plastic deformation of the magnesium frame. Static friction values were estimated by measuring the force needed to move the pipette holder along the z-axis of the pipette guide (typically in the 0.7–1 N range). For testing the stepping behavior of the friction device in the micrometer range, step sizes of 6, 4, 2, and 1 μm were imposed by an external motor (z-axis of the MMC1100) and stepping of the pipette was measured online with a chromatic sensor (Fries Research & Technology; resolution = 6 nm).
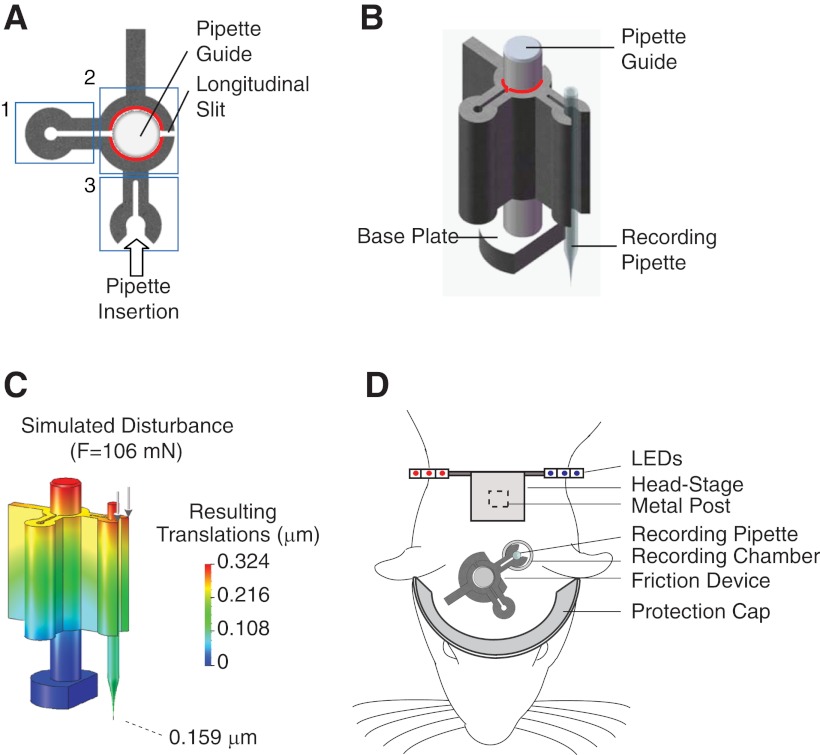
Fig. 1.
Mechanics of the friction-based device. A: top view of the assembled device. Boxes highlight the friction clamp (1), the central core (2) with friction interface indicated in red, and the pipette-holding arm (3). Modified from Burgalossi et al. (2011), with permission from Elsevier. B: 3-dimensional view of the assembled device with the recording pipette in position. The friction interface is highlighted in red. C: color-coded maximal translations of the device according to finite element method (FEM) simulation under the application of 106 mN (arrows), corresponding to the assumed 5 g maximal acceleration of the rat (see methods). The base of the pipette guide is the fixed point. Maximal translation of the pipette tip is shown. D: schematic representation showing the position of the individual implanted components relative to a rat's head.
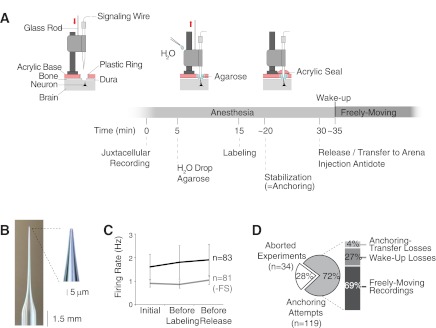
Fig. 2.
Steps and performance of the juxtacellular recording technique in freely moving animals. A: schematic representation of the steps to perform juxtacellular recordings in freely moving animals. Time 0 refers to the initial establishment of the juxtacellular configuration. Note that the “agarose” step can also be performed before, and not necessarily after, establishing the juxtacellular configuration. Components modified from Burgalossi et al. (2011), with permission from Elsevier. B: recording electrode with long thin taper used for juxtacellular recordings. Inset: higher magnification of the tip. C: average firing rates computed during the 1st minute of juxtacellular recording (“initial”), “before labeling,” and “before release” of the animal from the head-fixation frame. The averages are calculated separately for all neurons (n = 83, black) and without the 2 fast-spiking cells (−FS) in our data set (n = 81, gray). Error bars represent SD. D: overview of the performance of the friction device for obtaining juxtacellular recordings in freely moving animals: 28% of the experiments were interrupted because of inability to obtain stable juxtacellular recordings (“aborted experiments”); the percentages for “anchoring-transfer losses,” “wake-up losses,” and “freely moving recordings” refer to the subset of experiments (72%) in which 1 stable juxtacellular recording in the target region was obtained and stabilization was attempted by application of acrylic (“anchoring attempts”).
RESULTS
Design and mechanics of friction-based device.
A fundamental problem in single-neuron recordings from behaving animals is the mechanical stability of the recording. As shown by Lee et al. (2006, 2009), mechanical stability can be greatly improved by minimizing relative movement of brain and pipette by “head-anchoring” the pipette, i.e., by cementing the recording pipette in place and to the skull. In our present approach we sought to further improve stability by using an extremely rigid friction-based pipette positioning system. Whereas previous head-anchored recordings were combined with whole cell recordings, we combined our friction-based pipette positioning with juxtacellular recordings, which do not provide recording access to the cell interior but are easier to obtain. The mechanical design of our new device had to satisfy the following criteria: 1) high “stiffness” (i.e., mechanical stability) of the recording pipette, 2) stepping precision and reproducibility in the micrometer range (2–6 μm), 3) small size to fit the space constraints of a rat's skull, and 4) ease of operation.
Since the production process involved the employment of diamond tools for ultraprecise micromilling (see below), the choice of the construction materials was restricted to nonferrous metals. Magnesium was chosen as the construction material because it showed the most favorable combination of low density together with a good modulus of elasticity and good machining properties with diamond tools. Low density ensures lightness of the device, while elastic properties of the material are an essential prerequisite for the generation of high spring forces (see below) and prevent undesired deformation of the material.
The pipette stabilization device consists of two individual components: a sliding pipette holder and a pipette guide (Fig. 1, A and B). The pipette guide (29-mm total length) has a larger base, which allows it to be cemented on the rat's skull. The pipette holder is designed to hold the pipette, which is laterally inserted through a snap mechanism between two springs (Fig. 1, A and B). Given the relatively large force generated by the two springs upon pipette insertion (22 N according to FEM simulation; see methods), our lateral insertion and stabilization design confers better stiffness compared with conventional axial insertion devices. The pipette holder is designed to slide along the pipette guide on the z-axis. The device is operated in conjunction with a conventional external stepping motor, which is used to step down the pipette holder relative to the pipette guide in a controlled fashion.
Friction force is the functional basic principle of the device. To ensure both a high static friction force (the major determinant for mechanical stability of the pipette) and stepping microprecision of the device during operation, we designed a “friction clamp” mechanism (Fig. 1A) and we “smoothed” the interacting surfaces to optical quality (average roughness < 5 nm) by a micromilling process. The friction clamp generates a preload force (∼14 N according to FEM simulation; see methods) by spring mechanisms, which clamps the pipette holder tightly onto the pipette guide (Fig. 1B). Surface smoothing was necessary to minimize “stick-slip” behavior upon operation of the device. “Stick-slip” refers to the sudden jump in velocity of an object that is observed when an applied force overcomes static friction. “Smoothing” the interacting surfaces reduces stick-slip by minimizing the difference between the static and dynamic friction. As a result, under these conditions, with minimal surface roughness in order to achieve reliable micrometer stepping performance of the device, the resulting friction forces are primarily determined by the design of the friction clamp. This confers the additional advantage that friction forces can be reliably predicted based just on the design geometry of the clamp.
Given the geometry and curvatures of the interacting surfaces of our device, conventional surface polishing (Oberg et al. 2000) and milling processes (Groover and Mikell 2007; Oberg et al. 2000) could not be employed. We therefore generated optical surfaces with an ultraprecise micromilling machine (LT-Ultra MMC1100). The high rotation speed of the diamond milling tool, together with high-positioning resolution of the processed material during milling (<100 nm), guaranteed uniform machining with minimal error variability on the processed surfaces. This process ensured regular and reliable stepping of the device upon application of external forces (e.g., via conventional stepping motors) down to 1-μm step size (average step size = 1.04 ± 0.07 μm, n = 20 test steps; see methods). The final production step involved the coating of the pipette guide surface with TiN, an extremely hard ceramic material that is used to prevent surface abrasion by frictional forces (Pierson 1996). This protective coating therefore considerably lengthens the lifetime of the friction interface and allows long-term and repetitive use of the same friction devices (to date, we have not observed any detectable rundown in performance over the course of >3 yr). The TiN coating was generated by a conventional “sputtering” process, a procedure used for thin-film deposition (∼150 nm) that consists of ejecting material from a “source” (solid Ti, in the presence of N2) that then deposits onto a “target” (in the form of TiN) (Behrisch 1981).
Measurements revealed a necessary force of ∼0.9 N to overcome the static friction in axial direction. Assuming a maximum acceleration of the rat of 5 g (Ledberg and Robbe 2011; Sunderam et al. 2007; Venkatraman et al. 2007, 2010) the corresponding maximal force acting on the pipette holder will be ∼106 mN. FEM simulation showed that under these conditions the resulting translations of the pipette tip were <1 μm (Fig. 1C; see methods). The large security factor of ∼8 between static friction and the maximal theoretical force generated by the rat upon movement is responsible for the high stability of the pipette tip during the assumed maximal acceleration of the rat.
Figure 1D shows the spatial configuration of the implanted components. The full implant can be assembled just prior to the recording on naive animals, a procedure that typically takes 1 h. Alternatively, it can be assembled in a two-step procedure, with surgery, craniotomy, and metal post implantation performed a few days before the experiment. In fact, while deterioration of the brain exposure has dramatic effects on the success rate of whole cell recordings, it has only minimal effects on juxtacellular recordings, which on the other hand can be successfully established up to several weeks after the initial opening of the cranial window (Houweling et al. 2010). The two-step procedure confers the advantage that the animal is fully recovered from the surgery at the moment the experiment is performed.
Craniotomy and implantation of a metal post for head fixation (Fig. 1D) are performed according to standard procedures (Houweling and Brecht 2008; Voigt et al. 2008). The craniotomy is then sealed with silicone, and animals are returned to their home cage (2-step procedure). Two to three days later animals are anesthetized with antagonizable anesthesia (Lee et al. 2006, 2009), and the remaining components of the implant (pipette positioning device and protective aluminum ring) are cemented on the acrylic base just prior to the experiment, a procedure that typically requires not more than 30 min. In conjunction with a conventional stereotaxic apparatus, the assembled device can be positioned so that the pipette tip is centered on the craniotomy. The base of the guide is then cemented in place with dental acrylic. The implant is finalized by cementing an aluminum-covered plastic ring (Fig. 1D) that protects the recording pipette during the freely moving animal behavior. Once the implant is fully assembled, the miniaturized preamplifier with LEDs for tracking can be attached on the metal post (Fig. 1D) with the use of double-sided tape or mild glues.
Juxtacellular recordings in freely moving animals with friction-based device.
Figure 2A shows the steps for performing juxtacellular recordings in freely moving animals. “Blind” juxtacellular recording and labeling were performed according to previously established procedures and validation criteria (Pinault 1996). Instead of the sharp electrodes used by others (i.e., Pinault 1996) we used patchlike, low-resistance recording pipettes, which were pushed through the intact dura (as in Houweling and Brecht 2008; Houweling et al. 2010; Voigt et al. 2008; see Fig. 2A), as this is expected to add mechanical stability to the preparation by minimizing brain tissue motion. Typically, large peak-to-peak spike signals (>2 mV) and biphasic action potential shapes (see Fig. 3, C and D) are mostly indicative for a somatic/perisomatic location of the recording pipette tip (Henze et al. 2000; Kamondi et al. 1998), and this condition is most favorable for optimal cell labeling (see also Fig. S1D in Burgalossi et al. 2011 for additional representative spike waveforms). Once a juxtacellular recording that satisfies these criteria is obtained, firing rate and spike shapes are closely monitored for ∼5 min. Mechanical damage of the recorded cell is in fact known to occur if the recording pipette is pushed too close to the recorded cell (Pinault 1996). In this context, firing rate and spike shapes are the most suitable indicators for cellular damage by the recording pipette. If firing frequency increases over time and/or spikes become broader or display an up-going “intracellular-like” shape, the cell should be discarded and the electrode further advanced into the target area or retracted for a subsequent penetration. Once a stable recording configuration is achieved, with unaltered firing rate and spike shapes during the first ∼5 min, then a water drop is carefully applied to the friction interface (with the help of a long, flexible pipette tip) to further increase the static friction force (Fig. 2A). Ringer solution in the recording chamber is removed and exchanged with a 3% agarose solution cooled at 30°C (Fig. 2A). The activity of the cell is monitored for an additional 10 min, during which the water is allowed to fully spread by capillary action across the interface. After that, squared current pulses are used to label the cell (Pinault 1994, 1996). If signs of cellular damage are observed during and/or after the labeling procedure, in particular increases in firing rates that do not return to prelabeling baseline levels over the course of a few minutes, the cell should be discarded. In our data set, firing rates before and after labeling did not differ significantly (before labeling = 1.8 ± 0.7 Hz, after labeling = 1.9 ± 0.6 Hz; Fig. 2C). In our experience, the risk of damage can be minimized by reducing labeling times (<2 min), which can be sufficient for optimal labeling if a relatively high concentration of biocytin is used (2–3%; see methods). After successful labeling, an acrylic seal is applied very carefully on top of the agarose layer (with the help of a syringe with a 20-gauge needle) to anchor the recording pipette relative to the skull. The acrylic is left to harden for 10 min (Fig. 2A), after which the animal is carefully released from head fixation, moved to the behavioral arena, and injected with the antidote. Wake-up typically occurs in 2–3 min, after which the animal spontaneously engages in exploratory behavior and the functional properties of the recorded cells can be assessed (Burgalossi et al. 2011).
Fig. 3.
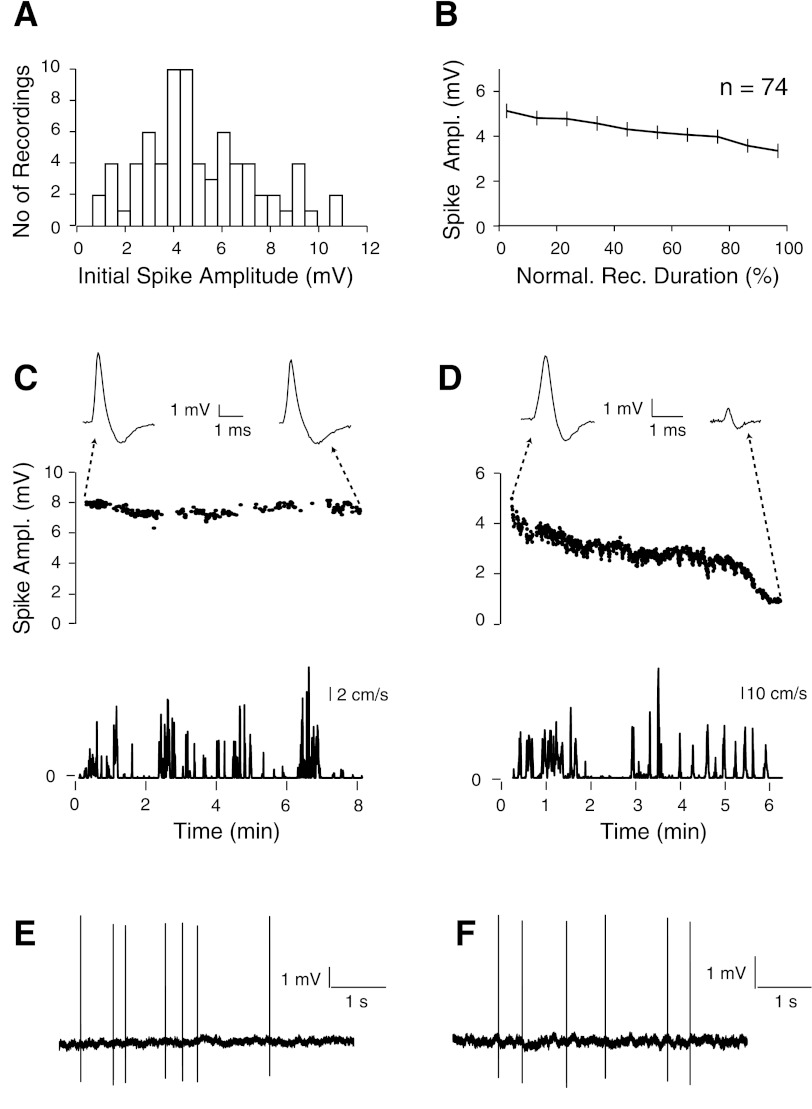
Stability of spike signals during freely moving animal behavior. A: distribution of the initial peak-to-peak spike amplitudes, calculated as the average during the first 10% of each freely moving recording session. B: distribution of spike amplitudes over time for all freely moving recordings. Recording duration was normalized; bin size = 10%. Error bars represent SE. C and D, top: representative recordings where spike amplitudes were either stable (C) or slowly decreased during the freely moving recording session (D). On top, representative spike waveforms from the beginning and the end of each recording session are shown. Bottom: speed plots for the corresponding plots shown above (same timescale). E and F: representative raw spike traces for the recordings shown in C and D, respectively.
In our experience, electrodes with a long thin taper together with a relatively large tip opening (∼2- to 3-μm outer diameter) were most effective in obtaining stable juxtacellular recordings (Fig. 2B). The thin taper also minimizes the damage to the surrounding brain tissue when recordings from deep target areas are performed (such as most recordings in the present study). Despite the large tip size, the electrode resistance in the brain tissue was typically in the 3–6 MΩ range, probably because of the contribution of the long thin taper. It is, however, important to note that given the large tip size of our juxtacellular electrodes, one would expect at least an undersampling of small local circuit neurons (i.e., interneurons), based on the theoretically lower chances of establishing a juxtacellular configuration with smaller cell somata. Higher-impedance electrodes might therefore be needed for reliable sampling and labeling of interneurons, as shown by others (Klausberger et al. 2003).
In a subset of experiments (n = 34 penetrations, 75 recordings), we quantified the performance of our electrodes for obtaining juxtacellular recordings. On average, we obtained 2.8 ± 2.4 recordings per penetration, as indicated by the ∼2–3 times increase in tip resistance and the presence of either spontaneous or evoked spikes, and 42 of the 75 recordings satisfied our stability criteria (see Fig. 2C). Obtaining stable juxtacellular recording required on average 4.7 ± 3.1 penetrations per experiment in the target region, and a fraction of experiments (28%; Fig. 2D) were aborted prior to wake-up because of inability to obtain stable configurations. The relatively large tip size was probably responsible for the lower number of juxtacellular configurations that could be established per penetration compared with electrodes with standard tip sizes (∼1 μm) (data not shown). In addition, the inability of the present design of the friction drive to retract the pipette (Fig. 1B and Fig. 2A) could possibly account for the low rate of stable juxtacellular recordings. Consistent with previous work, cortical neurons showed low levels of activity under anesthesia, and their firing rates were stable over the course of the anesthetized phase of the experiment (that is, from the initial establishment of the juxtacellular recording to the transfer of the animal into the arena) (Fig. 2C).
Altogether, in 119 experiments we attempted to stabilize a juxtacellular recording with application of acrylic (Fig. 2D). Of these, only a minor subset were lost during head anchoring or animal transfer to the behavioral arena (5 of 119; 4% of the total), while 27% were lost during wake-up, similar to previous studies (Lee et al. 2006, 2009). These latter losses are probably due to the change in blood pressure and concomitant brain tissue movements occurring upon reversal of the anesthesia and wake-up. We obtained 83 juxtacellular recordings from freely moving animals out of the 119 experiments where we attempted to stabilize a recording by application of acrylic. This yielded a recording success rate of 69% over all anchoring attempts, which corresponds to an overall rate of 50% over all experiments (Fig. 2D). These recordings were obtained at variable depths from the brain surface (∼1,000–5,000 μm) and in different brain areas (entorhinal cortex, n = 107; retrosplenial cortex, n = 11; barrel cortex, n = 1). Recording durations were variable, with 42% in our data set lasting >5 min and up to 37 min when left uninterrupted (Fig. 4A). Given that a fraction of recordings (25%; 21 of 83) were deliberately terminated to improve the rate of cell recovery, the average recording duration (5.8 ± 6.1 min) provides an underestimate of the total recording length that can be obtained with our method. To estimate the maximal recording lengths that can be obtained with our method, we performed a subset of recordings (n = 12) that, in contrast to the previous data set (Fig. 4A), were not deliberately terminated. As shown in Fig. 4B, these recordings lasted on average 29.5 ± 55.0 min (median 19.5 min) and up to 3.3 h. Remarkably, spike amplitude and shape remained relatively stable during this long period of time, indicating that long-lasting recordings (several hours) in freely moving animals can indeed be obtained with our method when recordings are not deliberately terminated.
The initial peak-to-peak spike amplitude during freely moving behavior was on average 5.1 ± 2.3 mV (Fig. 3A), corresponding to 46.4 ± 25.9 times above the root mean square amplitude of the noise (see Fig. 3, E and F, for example traces). This large signal-to-noise level allows unequivocal spike identification. This is particularly relevant for detection of spikes within bursts, where spike signals can undergo dramatic attenuation (Buzsáki et al. 1996; Fee et al. 1996; Harris et al. 2000), and thereby most likely fall below the detection limit of classical extracellular techniques. Recorded spike signals were typically stable during the freely moving recording session (Fig. 3B) and were lost either by sudden disappearance of the signal or by a slow decrease of the signal amplitude over time (Fig. 3, C and D). Recording losses were not always correlated with mechanical events (i.e., head shakes, head bumps), and were probably caused by internal brain tissue displacements relative to the skull.
For 60 of the 83 freely moving recordings, we were able to identify the recorded cell. These cases included cells with intact dendritic arbor, where morphology could be classified (n = 39), and cells with incomplete morphology due to partial filling and/or partial damage (n = 21). In the remaining 23 cases either no staining was observed or multiple cells located in close proximity were labeled, rendering identification of the recorded cell impossible. Multiple cell labeling can be reduced by both lowering the concentration of biocytin and shortening the incubation time of the brain slices in the substrate-developing solution (Burgalossi et al. 2011; see methods).
Our rates of cell recovery from freely moving animals (72%, 60 of 83) are comparable to those reported from anesthetized preparations (Pinault 1996). The quality of cell recovery, however, appears to be lower in freely moving animals, with incomplete morphologies in 35% of cases (21 of 60), where dendrites were only partially visible and somata partially/fully destroyed. These cases could likely arise from end-point cellular damage, which is usually accompanied by a sudden loss of the recording and a typical “dying-out” spike burst. Ending the experiment before recording loss occurs, together with quick perfusion of the animal, improves the quality of cell recovery. In all cases, however, recording sites can be clearly identified by biocytin spillover at the ejection site. Since only one cell per animal is recorded, our method always provides unequivocal identification of the recording site, even when cell recovery has failed.
Visualizing silent cells during awake, freely moving behaviors.
Given the high rate of cell recovery, our technique provides the means to systematically explore the structure-function relationship of single neurons recorded during freely moving animal behaviors (Burgalossi et al. 2011). This approach is particularly suitable to the study of spatial cognition, where spatial exploration by the animal is required for assessing the functional properties of the recorded cells (Derdikman and Moser 2010).
An additional advantage of the juxtacellular recording technique over conventional extracellular methods is that neurons can be identified irrespective of their spiking activity. Unlike extracellular recordings, the juxtacellular configuration requires the establishment of close contact with the recorded cell, which can be monitored by the increase in resistance at the electrode tip. The presence of a silent (i.e., nonspiking) cell can be confirmed by inducing action potential firing with positive current injections (Zhang and Deschênes 1997). Figure 5 shows a representative recording from a layer 5 pyramidal cell in medial entorhinal cortex (Fig. 5A) that did not spike during exploration of a novel environment (Fig. 5, B–D). The activity of the cell was monitored while the rat sampled the arena (Fig. 5D), and at the end of the trial the cell was fired by current injection (Fig. 5E).
In our data set, we recorded nine silent cells. Since damage or mechanical stress on the recorded cell caused by the measuring electrode often leads to artifactual increases in spiking activity, the fact that we could record silent cells, which did not spike for up to 4.3 min (in our present data set) during exploration, indicates that our method does not lead to major alterations of the physiology of the recorded cells.
DISCUSSION
Previous methods for pipette stabilization during freely moving behavior involved either active stabilization of microdrives (Fee 2000; Long et al. 2010) or head-anchoring strategies (Lee et al. 2006, 2009). Here we introduce static friction force as a novel principle for pipette positioning and stabilization in awake, freely moving animals. Our new device is easy to operate and does not contain active (e.g., electric and/or electronic) components but is operated in conjunction with an external standard manipulator. These features contribute to the lightness of the device and to the remarkably low levels of electric noise interference on the recorded signal (Fig. 3, C and D). The design of the friction clamp allows precise control of the friction forces, which are generated by mechanical springs and can therefore be adjusted according to the required stepping precision. In particular, by smoothing the interacting surfaces to optical quality, we were able to achieve an excellent compromise between micrometer stepping precision of the device and high static friction forces (∼0.9 N), which are key for recording stability.
Our device appeared to provide much better mechanical stiffness of the recording pipette compared with previous stabilization methods (Lee et al. 2009), particularly during the head anchoring process, during which the pipette is firmly anchored to the skull by the application of dental cement. Approximately 30% of intracellular recordings were lost at this step with previous stabilization procedures because of pipette drifting during hardening of the acrylic (Lee et al. 2006, 2009). The very low rates of recording losses during head anchoring and upon release of the rat from the head fixation frame (5 of 119) indicate that the rigidity by which the pipette is held by the friction device is certainly a key factor for stability. Note, however, that this is a comparison between intracellular recordings on the one hand and juxtacellular recordings on the other hand. Whether the enhanced mechanical stability also yields higher recording rates in the patch-clamp configuration remains to be determined.
The juxtacellular recording and labeling technique has been extensively described in the past (Pinault 1994, 1996) and widely used to study suprathreshold activity from identified neurons in vivo. In our present method, the close proximity of the recording pipette tip to the recorded cell, in combination with the mechanical disturbances arising from freely moving behaviors, could potentially alter the physiological firing properties of the recorded cells. However, the firing rates of the medial entorhinal cortex neurons we recorded juxtacellularly in freely moving animals (Burgalossi et al. 2011) appeared to be within the range reported by other studies done with standard extracellular techniques (Hafting et al. 2005; Sargolini et al. 2006), indicating that our method might induce only minimal disturbances of the physiology of the cell recorded during animal movement. Compared with whole cell recordings, juxtacellular recordings provide information about the spiking output of the cell without the subthreshold activity that reflects the synaptic inputs that the cell receives. Unlike whole cell recordings, however, the intracellular composition of the recorded cell is not altered by the juxtacellular configuration, which makes it possible to probe the molecular identity of the recorded cell with post hoc immunohistochemical staining (Simpson et al. 2005; Somogyi and Klausberger 2005).
The combined use of juxtacellular labeling with pipette friction-stabilization allowed the recovery of neuronal morphologies at high rates from freely moving animals, indicating that our present method is a useful tool for studying the cellular basis of neural activity during freely moving behaviors. Recorded spike signals were typically stable during animal movement, and the higher signal-to-noise ratio compared with classical extracellular techniques allowed for unequivocal identification of the spike signals.
Classical extracellular recording techniques allow the same units to be recorded over long periods of time during freely moving behaviors (McNaughton et al. 1983; Nicolelis et al. 1997; Recce and O'Keefe 1989; Thompson and Best 1990; Wilson and McNaughton 1993), possibly because small variations in electrode position relative to the recorded units, which are likely to occur as a result of mechanical disturbances, have minor effects on the recorded signals. On the other hand, juxtacellular recordings are established by positioning the recording pipette tip in close proximity to the recorded cell (Houweling et al. 2010; Pinault 1996), and mechanical stability in the micrometer range is needed to maintain a stable configuration over time. The sensitivity of this method to small variations in the spatial configuration is most probably responsible for the limited duration of our recordings. In our present data set, 41% of all recordings lasted >5 min (max duration = 37 min), and a fraction of recordings were deliberately terminated when sufficient sampling of the environment was obtained (Burgalossi et al. 2011). Deliberately unterminated recordings, on the other hand, lasted on average ∼5 times longer, with 7 of 12 recordings lasting >15 min (max duration = 3.3 h; see Fig. 4). This preliminary data set indicates that long-lasting, stable juxtacellular recordings in freely moving animals can indeed be obtained with our method.
Our recording stabilization procedures were based on the “head anchoring” principle, by which rigidity of the recording pipette relative to the skull is the key determinant of recording success and stability (Lee et al. 2006, 2009). In this configuration, displacements of brain tissue relative to the pipette tip cannot be prevented or counteracted, in contrast to “active stabilization” procedures developed by others (Fee 2000). Despite the fact that in our preparation internal brain movements are minimized by 1) the intact dura, 2) the agarose application on the brain surface, and 3) the acrylic seal (see Fig. 2A), these movements represent the most likely source for recording instability and loss. In this context, it is worth mentioning that recording stability could differ depending on the target brain region, with more posterior cortical areas (as in the data set in Fig. 4A) being theoretically more susceptible to brain tissue displacements than internal areas (i.e., hippocampus, thalamus; see also Fig. 4, B and C), especially to posteriorly coupled motion of the spinal cord occurring during extension and contractions of the torso (Fee 2000).
One limitation of the present method is the use of antagonizable anesthesia. Physiological and behavioral data are collected after pharmacological antagonism of anesthesia and rapid transition of the animal to the awake state. Although several studies suggest that our wake-up protocol might have only limited effects on the physiology of spatial representations (Burgalossi et al. 2011; Epsztein et al. 2010, 2011; Lee et al. 2006, 2009), its potential effects on animal behavior and physiology cannot be excluded and should therefore be carefully considered. From the point of view of mechanical stability, anesthetized preparations provide great advantages over awake, head-fixed preparations. Under these conditions, in fact, given the absence of animal movement and the highly reduced brain pulsations, stable juxtacellular recordings can be easily established and kept for long periods of time (>30 min; Fig. 2A), which are required for staining and stabilization procedures before the animal is transferred to the behavioral arena and woken up by injection of the antagonist. In principle, the present method can be applied also to awake, unanesthetized animals pretrained to head fixation. This preparation would provide several key advantages, which include the absence of anesthesia and the possibility of pretraining the animal to perform more complex behavioral tasks. As expected, however, because of the higher mechanical instability of this experimental configuration, overall success rates appear to be lower compared with the use of initially anesthetized preparations (A. Burgalossi and M. Brecht, work in progress).
In its present state our method comes with inevitable technical and ethical costs, since it is limited to one recording attempt per animal and requires a larger number of experimental animals compared with, for example, classical in vivo recording techniques. Here, two directions for future technical improvements are worth emphasizing (A. Burgalossi and M. Brecht, work in progress), which could theoretically result in longer recording lengths and higher success rate, along with a reduction of the associated technical and ethical costs: 1) the use of motorized friction-based drives, by which the position of the pipette relative to the cell can be carefully adjusted over time, and 2) the implementation of a removable stabilization seal (instead of dental acrylic), by which more than one recording attempt per animal can be performed. The latter, along with the use of different fluorescent dyes for neuronal labeling, would also allow multiple recordings to be performed in the same animal.
Computational models and quantifications suggested sparse coding in the cortex, with a large fraction of cells being silent during active behaviors (Huber et al. 2008; Thompson and Best 1989; Wolfe et al. 2010). In CA1, for example, a large fraction of cells (>50%) are silent in a given environment (Epsztein et al. 2011; Thompson and Best 1989; Wilson and McNaughton 1993). Recent work suggested that silent and nonsilent cells might receive distinct synaptic inputs and therefore be embedded in distinct subnetworks (Epsztein et al. 2011). The reason for the existence of silent cells is still unclear, and their role in brain coding and behavior has been poorly addressed given the current lack of robust methods to study them. Silent cells can be recorded via patch clamp (Epsztein et al. 2011) and two-photon imaging techniques (Dombeck et al. 2010; Helmchen et al. 2001), but these approaches are either technically challenging or limited to optically accessible cortical regions. Our approach allows recording and labeling of silent cells at any brain depth, since cells can be identified independently from their spiking activity as an increase in resistance at the electrode tip. We therefore envision that our method would be most suitable for exploring anatomical correlates of neurons that do not actively participate in the encoding of given behaviors (Shoham et al. 2006; Wolfe et al. 2010). We conclude that friction-stabilization allows recordings from single, morphologically identified neurons in freely behaving animals, and thus provides a novel tool for correlative studies of single-cell morphology, activity, and behavior.
GRANTS
This work was supported by Neurocure, the Bernstein Center for Computational Neuroscience (BMBF) and Humboldt University, the EU Biotact-grant, and the Neuro-behavior ERC grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.H., A.B., M.S., and M.B. conception and design of research; L.H., A.B., K.H., and J.J.T. performed experiments; L.H., A.B., K.H., J.J.T., M.S., and M.B. approved final version of manuscript; A.B. and J.J.T. analyzed data; A.B., M.S., and M.B. interpreted results of experiments; A.B. and J.J.T. prepared figures; A.B. drafted manuscript; M.B. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Brigitte Geue, Maik Kunert, Undine Schneeweiß, and Arnold Stern for outstanding technical assistance and Moritz von Heimendahl for MATLAB programming.
Present address of L. Herfst: Luzac College Eindhoven, Jonckbloetlaan 13, 5615 EM Eindhoven, The Netherlands.
REFERENCES
- Behrisch R. Sputtering by Particle Bombardment. Berlin: Springer, 1981 [Google Scholar]
- Brinksmeier E, Gläbe R. Advances in precision machining of steel. Ann CIRP 50: 385–388, 2001 [Google Scholar]
- Brinksmeier E, Lucca DA, Walter A. Chemical aspects of machining processes. Ann CIRP 53: 685–699, 2004 [Google Scholar]
- Buhl EH, Szilágyi T, Halasy K, Somogyi P. Physiological properties of anatomically identified basket and bistratified cells in the CA1 area of the rat hippocampus in vitro. Hippocampus 6: 294–305, 1996 [DOI] [PubMed] [Google Scholar]
- Burgalossi A, Herfst L, von Heimendahl M, Förste H, Haskic K, Schmidt M, Brecht M. Microcircuits of functionally identified neurons in the rat medial entorhinal cortex. Neuron 70: 773–786, 2011 [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci 7: 446–451, 2004 [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Penttonen M, Nádasdy Z, Bragin A. Pattern and inhibition-dependent invasion of pyramidal cell dendrites by fast spikes in the hippocampus in vivo. Proc Natl Acad Sci USA 93: 9921–9925, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Luhmann HJ, Prince DA. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol 296: 598–613, 1990 [DOI] [PubMed] [Google Scholar]
- Chorev E, Epsztein J, Houweling AR, Lee AK, Brecht M. Electrophysiological recordings from behaving animals—going beyond spikes. Curr Opin Neurobiol 19: 513–519, 2009 [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Henze DA, Jamieson B, Harris KD, Sirota A, Barthó P, Wise KD, Buzsáki G. Massively parallel recording of unit and local field potentials with silicon-based electrodes. J Neurophysiol 90: 1314–1323, 2003 [DOI] [PubMed] [Google Scholar]
- Derdikman D, Moser EI. A manifold of spatial maps in the brain. Trends Cogn Sci 14: 561–569, 2010 [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci 13: 1433–1440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epsztein J, Brecht M, Lee AK. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron 70: 109–120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epsztein J, Lee AK, Chorev E, Brecht M. Impact of spikelets on hippocampal CA1 pyramidal cell activity during spatial exploration. Science 327: 474–477, 2010 [DOI] [PubMed] [Google Scholar]
- Fee MS. Active stabilization of electrodes for intracellular recording in awake behaving animals. Neuron 27: 461–468, 2000 [DOI] [PubMed] [Google Scholar]
- Fee MS, Mitra PP, Kleinfeld D. Automatic sorting of multiple unit neuronal signals in the presence of anisotropic and non-Gaussian variability. J Neurosci Methods 69: 175–188, 1996 [DOI] [PubMed] [Google Scholar]
- Groover D, Mikell P. Theory of Metal Machining. Fundamentals of Modern Manufacturing (3rd ed.). Hoboken: Wiley, 2007 [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature 436: 801–806, 2005 [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsáki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84: 401–414, 2000 [DOI] [PubMed] [Google Scholar]
- Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope. High-resolution brain imaging in freely moving animals. Neuron 31: 903–912, 2001 [DOI] [PubMed] [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsáki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol 84: 390–400, 2000 [DOI] [PubMed] [Google Scholar]
- Herfst LJ, Brecht M. Whisker movements evoked by stimulation of single motor neurons in the facial nucleus of the rat. J Neurophysiol 99: 2821–2832, 2008 [DOI] [PubMed] [Google Scholar]
- Houweling AR, Brecht M. Behavioural report of single neuron stimulation in somatosensory cortex. Nature 451: 65–68, 2008 [DOI] [PubMed] [Google Scholar]
- Houweling AR, Doron G, Voigt BC, Herfst LJ, Brecht M. Nanostimulation: manipulation of single neuron activity by juxtacellular current injection. J Neurophysiol 103: 1696–1704, 2010 [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci 198: 1–59, 1977 [DOI] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromádka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451: 61–64, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Hawken MJ. Loose-patch-juxtacellular recording in vivo—a method for functional characterization and labeling of neurons in macaque V1. J Neurosci Methods 156: 37–49, 2006 [DOI] [PubMed] [Google Scholar]
- Judkewitz B, Rizzi M, Kitamura K, Häusser M. Targeted single-cell electroporation of mammalian neurons in vivo. Nat Protoc 4: 862–869, 2009 [DOI] [PubMed] [Google Scholar]
- Kamondi A, Acsády L, Buzsáki G. Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. J Neurosci 18: 3919–3928, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Judkewitz B, Kano M, Denk W, Häusser M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nat Methods 5: 61–67, 2008 [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421: 844–848, 2003 [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321: 53–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CPJ, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol 581: 139–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CPJ, Sakmann B. Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. Proc Natl Acad Sci USA 106: 16446–16450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledberg A, Robbe D. Locomotion-related oscillatory body movements at 6–12 Hz modulate the hippocampal theta rhythm. PLoS One 6: e27575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Epsztein J, Brecht M. Head-anchored whole-cell recordings in freely moving rats. Nat Protoc 4: 385–392, 2009 [DOI] [PubMed] [Google Scholar]
- Lee AK, Manns ID, Sakmann B, Brecht M. Whole-cell recordings in freely moving rats. Neuron 51: 399–407, 2006 [DOI] [PubMed] [Google Scholar]
- Long MA, Jin DZ, Fee MS. Support for a synaptic chain model of neuronal sequence generation. Nature 468: 394–399, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Mori T, Nielsen KJ, Callaway EM. Targeting single neuronal networks for gene expression and cell labeling in vivo. Neuron 67: 562–574, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, O'Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods 8: 391–397, 1983 [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Ghazanfar AA, Faggin BM, Votaw S, Oliveira LM. Reconstructing the engram: simultaneous, multisite, many single neuron recordings. Neuron 18: 529–537, 1997 [DOI] [PubMed] [Google Scholar]
- Oberg E, Jones FD, Horton HL, Ryffel HH. Machinery's Handbook (26th ed.). New York: Industrial Press, 2000 [Google Scholar]
- Oberlaender M, Boudewijns ZSRM, Kleele T, Mansvelder HD, Sakmann B, de Kock CPJ. Three-dimensional axon morphologies of individual layer 5 neurons indicate cell type-specific intracortical pathways for whisker motion and touch. Proc Natl Acad Sci USA 108: 4188–4193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, Callaway EM. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron 71: 617–631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson HO. Handbook Of Refractory Carbides and Nitrides: Properties, Characteristics, Processing, and Applications. New York: William Andrew, 1996 [Google Scholar]
- Pilowsky PM, Makeham J. Juxtacellular labeling of identified neurons: kiss the cells and make them dye. J Comp Neurol 433: 1–3, 2001 [DOI] [PubMed] [Google Scholar]
- Pinault D. Golgi-like labeling of a single neuron recorded extracellularly. Neurosci Lett 170: 255–260, 1994 [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods 65: 113–136, 1996 [DOI] [PubMed] [Google Scholar]
- Pinault D. Cellular interactions in the rat somatosensory thalamocortical system during normal and epileptic 5–9 Hz oscillations. J Physiol 552: 881–905, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D, Deschênes M. Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: a three-dimensional, graphic, and morphometric analysis. J Comp Neurol 391: 180–203, 1998 [DOI] [PubMed] [Google Scholar]
- Rae JL, Levis RA. Single-cell electroporation. Pflügers Arch 443: 664–670, 2002 [DOI] [PubMed] [Google Scholar]
- Rathenberg J, Nevian T, Witzemann V. High-efficiency transfection of individual neurons using modified electrophysiology techniques. J Neurosci Methods 126: 91–98, 2003 [DOI] [PubMed] [Google Scholar]
- Recce M, O'Keefe J. The tetrode: a new technique for multi-unit extracellular recording. Soc Neurosci Abstr, 1989 [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312: 758–762, 2006 [DOI] [PubMed] [Google Scholar]
- Scanziani M, Häusser M. Electrophysiology in the age of light. Nature 461: 930–939, 2009 [DOI] [PubMed] [Google Scholar]
- Shoham S, O'Connor DH, Segev R. How silent is the brain: is there a “dark matter” problem in neuroscience? J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192: 777–784, 2006 [DOI] [PubMed] [Google Scholar]
- Simpson JI, Hulscher HC, Sabel-Goedknegt E, Ruigrok TJH. Between in and out: linking morphology and physiology of cerebellar cortical interneurons. Prog Brain Res 148: 329–340, 2005 [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562: 9–26, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderam S, Chernyy N, Peixoto N, Mason JP, Weinstein SL, Schiff SJ, Gluckman BJ. Improved sleep-wake and behavior discrimination using MEMS accelerometers. J Neurosci Methods 163: 373–383, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Shiba K, Nakazawa K, Suzuki T, Umezaki T, Ezure K, Abo N, Yoshihara T, Hisa Y. Axonal projections of medullary swallowing neurons in guinea pigs. J Comp Neurol 519: 2193–2211, 2011 [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Place cells and silent cells in the hippocampus of freely-behaving rats. J Neurosci 9: 2382–2390, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney GM, Daws LC. Juxtacellular labeling and chemical phenotyping of extracellularly recorded neurons in vivo. Methods Mol Biol 337: 127–137, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt BC, Brecht M, Houweling AR. Behavioral detectability of single-cell stimulation in the ventral posterior medial nucleus of the thalamus. J Neurosci 28: 12362–12367, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman S, Long JD, Pister KS, Carmena JM. Wireless inertial sensors for monitoring animal behavior. Conf Proc IEEE Eng Med Biol Soc 2007: 378–381, 2007 [DOI] [PubMed] [Google Scholar]
- Venkatraman S, Jin X, Costa RM, Carmena JM. Investigating neural correlates of behavior in freely behaving rodents using inertial sensors. J Neurophysiol 104: 569–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods 4: 47–49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science 261: 1055–1058, 1993 [DOI] [PubMed] [Google Scholar]
- Wolfe J, Houweling AR, Brecht M. Sparse and powerful cortical spikes. Curr Opin Neurobiol 20: 306–312, 2010 [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Deschênes M. Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. J Neurosci 17: 6365–79, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]