Abstract
The public pays large sums of money to watch skilled motor performance. Notably, however, in recent decades motor skill learning (performance improvement beyond baseline levels) has received less experimental attention than motor adaptation (return to baseline performance in the setting of an external perturbation). Motor skill can be assessed at the levels of task success and movement quality, but the link between these levels remains poorly understood. We devised a motor skill task that required visually guided curved movements of the wrist without a perturbation, and we defined skill learning at the task level as a change in the speed–accuracy trade-off function (SAF). Practice in restricted speed ranges led to a global shift of the SAF. We asked how the SAF shift maps onto changes in trajectory kinematics, to establish a link between task-level performance and fine motor control. Although there were small changes in mean trajectory, improved performance largely consisted of reduction in trial-to-trial variability and increase in movement smoothness. We found evidence for improved feedback control, which could explain the reduction in variability but does not preclude other explanations such as an increased signal-to-noise ratio in cortical representations. Interestingly, submovement structure remained learning invariant. The global generalization of the SAF across a wide range of difficulty suggests that skill for this task is represented in a temporally scalable network. We propose that motor skill acquisition can be characterized as a slow reduction in movement variability, which is distinct from faster model-based learning that reduces systematic error in adaptation paradigms.
Keywords: reaching, pointing, wrist, movement, speed–accuracy trade-off, motor control
society arguably rewards motor skill above everything else, at least judging by the salaries of professional athletes. Conversely, loss of motor function caused by neurological injury and disease carries immense cost to individuals and society. Both attainment of high levels of skill and the rehabilitation of lost skills depend on motor learning, which makes the study of motor skill learning of great scientific and practical interest. The challenge is to agree upon a working definition of motor skill and design laboratory-based tasks that capture real-world manifestations of motor skill learning.
The majority of recent research on the neuroscience of motor learning has focused on motor adaptation (Bedford 1989; Bock 1992; Caithness et al. 2004; Cunningham 1989; Krakauer et al. 1999, 2000; Miall et al. 2004; Rabe et al. 2009; Sainburg and Wang 2002; Shadmehr and Mussa-Ivaldi 1994; Shadmehr et al. 2010; Simani et al. 2007; Smith et al. 2006; Thoroughman and Shadmehr 2000; Welch 1978; Wolpert et al. 1995), which consists of a change in motor performance driven by a perturbation, such as a change in the environment. The goal of adaptation is reduction of systematic error induced by the perturbation, and this occurs through adjustment of an internal model that maps motor commands onto predicted sensory outcomes (forward model; Shadmehr et al. 2010). In adaptation, a prediction error (i.e., the discrepancy between predicted and observed movement) drives learning in an obligatory manner: the forward model changes to reduce this error regardless of the subject's wishes (Mazzoni and Krakauer 2006). Reward can substitute for error information (Izawa and Shadmehr 2011) but is not necessary for adaptation to occur. Adaptation occurs relatively rapidly, typically over tens of minutes, and has a task-defined endpoint: elimination of the systematic error caused by the perturbation. Real-life behaviors that require adaptation include recalibration of sensorimotor mappings (looking and reaching while wearing glasses, switching to driving on the opposite side of the road in another country) and coping with changes in effectors (moving with fatigued muscles).
Adaptation tasks do not, for the most part, require an improvement of motor execution itself. In adaptation to visuomotor rotation, for example, subjects need to learn to map a particular movement direction in hand space onto a new cursor direction in visual space. Thus rotation adaptation leads to a new visuomotor mapping that allows the selection of the correct arm movement direction to be accurate in visual space. Subjects do not, however, need to learn anything new about how to execute the required movements in hand space. Indeed, the movements required by the new visuomotor mappings are movements that subjects were able to execute at baseline, which were simply elicited by different targets. Adaptation, at least early on, is not associated with changes in the quality of execution of the new movement, which can be captured by variable error around movement endpoint (Krakauer et al. 2000). Quality of execution is not emphasized in these paradigms because the learned movement is bounded by the quality of execution of the unperturbed baseline movement.
Here we are interested in the kind of motor learning that occurs in the absence of perturbation and in which the main performance goal is reduction of variable error (Deutsch and Newell 2004; Guo and Raymond 2010; Hung et al. 2008; Liu et al. 2006; Logan 1988; Muller and Sternad 2004; Ranganathan and Newell 2010). Performance is limited by task difficulty, often in the form of a trade-off between speed and accuracy. Learning consists of breaking through this limit (i.e., improving the speed–accuracy trade-off) (Reis et al. 2009; Sanes et al. 1990). Real-life examples include many sports (Yarrow et al. 2009). Learning tasks of this type do not generally have a built-in limit of performance: there is no systematic error to reduce to zero, and final performance is different from baseline. The limit of maximum performance cannot usually be predicted in advance, and improvement can continue for years. Reward and motivation play a prominent role. Although a universally accepted definition of motor skill learning does not exist, the features just listed capture the concepts of motor skill learning proposed by Guthrie (1952), Welford (1968), Willingham (1998), and Schmidt and Lee (2005), all of which share an emphasis on speed, accuracy (precision), and efficiency. This kind of learning, which we would operationally define as a core component of skill learning, has not been subjected to the same kind of detailed kinematic analysis as adaptation.
In this study we sought to investigate motor skill learning defined as an improved trade-off between speed and accuracy using a task that did not involve a perturbation and was amenable to a detailed kinematic analysis. We acknowledge that skill has a more colloquial usage that goes beyond improvement in the speed–accuracy trade-off. Here we use skill to mean “motor acuity,” in analogy with perceptual acuity but exemplified in the motor case by the ability to move faster and more accurately. We take this approach because we feel we are justified in our conceptual distinction between learning to return to baseline performance and improvement beyond baseline performance. This distinction does not preclude performance improvement at asymptote in adaptation paradigms.
Although skill is manifested as improved performance, there must be underlying changes in movement that are the basis for improved performance. In many motor-learning tasks, kinematics and performance are one and the same (e.g., movement direction corresponds to success or failure in a visuomotor rotation task). It is desirable to study learning in a task where these two measures are more loosely coupled, because this is the situation in many real-world tasks in which the relationship between movement details and outcome score is complex. For example, Sternad and colleagues have studied a virtual skittles task where subjects swing a ball, hanging on a string, through a curved trajectory to knock down a skittle (Muller and Sternad 2004). Accuracy depended on particular pairings of the angle and speed of release of the ball from the hand. With practice, subjects learned the optimal speed–angle pairings that allow the skittle to be hit by the ball. Here movement kinematics had redundancy, in that multiple speed–angle pairs can lead to success. However, the kinematics of motor execution was compressed to a single time point (the moment of release). It would be of interest to study the entire movement that led to that final time point. Improvement of motor execution was only one component of motor skill learning in this task. Although this improvement was reflected in a better performance (reduced variable error), larger changes were also shown in other components: learning the best task-specific strategy and identifying the optimal region of the speed–angle subspace were at least as important as reducing trial-to-trial movement variability. We take this as evidence that the rate-limiting step in such a complex task is learning the performance subspace, and not overcoming any inherent execution-related difficulty in releasing the ball at the requisite position and speed. Similarly, Newell and colleagues studied a gyroscope-based task and found that a critical point in learning was the identification of a particular movement sequence that resonated with the gyroscope's rotational inertial properties (Liu et al. 2006). Again, we would argue that here the rate-limiting step is learning the rule for how to move the gyroscope and not the actual execution of the rule.
In contrast to the tasks described so far, motor tasks that emphasize repetitive or sequential finger tapping (Karni et al. 1995; Muellbacher et al. 2000; Rosenkranz et al. 2007; Walker et al. 2002; Wu et al. 2004) do emphasize skill, in that they require subjects to accurately execute a short repeating sequence of finger taps at higher and higher speeds. Although this task speaks to an important feature of skilled behavior, which is an improved relationship between task difficulty and performance, movement execution is usually measured using two separate variables, one indicating movement difficulty, such as movement time (MT), and the other the quality of performance, such as accuracy. This is problematic because skill acquisition can be inferred only if both variables change in the expected direction (shorter MT, increased accuracy) (Sanes et al. 1990). Ascertaining whether skill has been acquired is not possible when these variables change in opposite directions. An increase in speed, accompanied by a decrease in accuracy, could reflect either a change in skill or simply a shift to another range of performance along an unchanged trade-off between speed and accuracy.
Deriving a speed–accuracy trade-off function (SAF) has been proposed as a preferred metric for execution assessment (Wickelgren 1977). We recently argued that to detect and quantify skill acquisition as a changed relationship between speed and accuracy, it is first necessary to empirically derive the SAF for that task at baseline (Reis et al. 2009). In this framework, a perturbation causes performance to worsen relative to baseline, and adaptation would represent a return of performance back to the baseline SAF. A fundamental feature of skilled performance, which has received limited attention in previous studies, is the coexistence of two kinds of measure: an explicit, usually binary, measure of success at the task level, such as scoring a goal or getting a tennis serve in, a measure that can be captured by a SAF (Plamondon and Alimi 1997; Reis et al. 2009), and continuous kinematic measures of movement execution or quality (Ghilardi et al. 2009). A crucial concept regarding skilled performance is that successful goal attainment and the trajectory kinematics associated with this attainment are distinct, because only the former is explicitly required by the task and there is likely redundancy between the former and the latter.
With these considerations in mind we devised a motor skill task in which skilled performance required accurate execution of an entire trajectory without a perturbation component and examined the effect of learning at the task level through examination of changes in shape of the SAF and at the movement level through analysis of trajectory kinematics. First, we examined how the SAF changes with practice at a single difficulty level, to determine whether skill for a task is controlled locally or globally. Second, we studied the kinematic changes that accompany improved performance, to establish whether the quality of movements changes with increased skill. Third, we compared how the SAF changes when practice is conducted at different speeds, to establish how skill learning is affected by training at different difficulty levels.
METHODS
Fifty right-handed subjects (28 females, 18–38 years of age), naïve to the task, participated in the study. All subjects gave written informed consent and received a small compensation to participate in the study, which was approved by the Columbia University Institutional Review Board. Subjects were randomly assigned to one of four groups.
General Approach
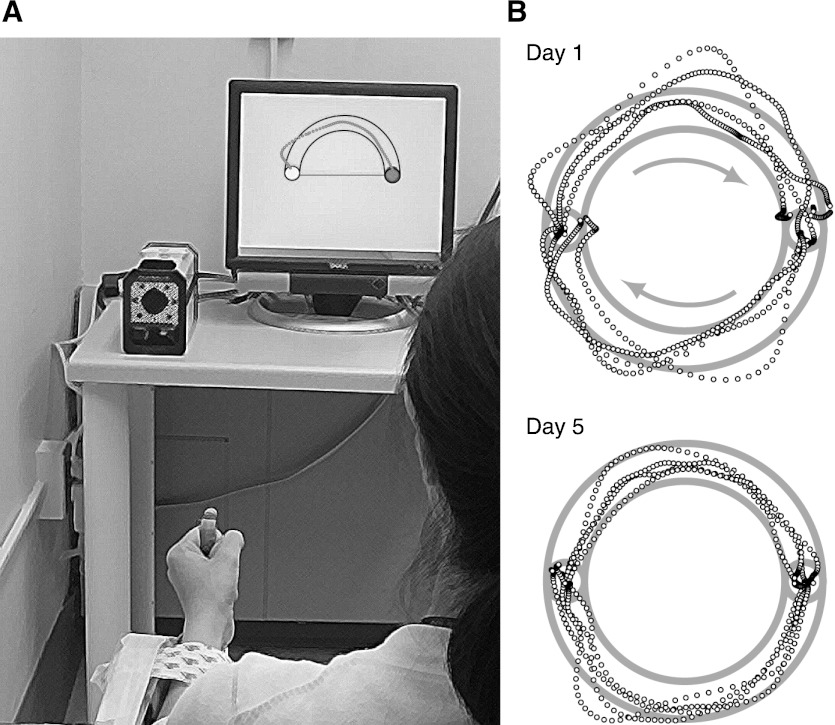
All subjects performed a pointing task by moving a computer screen cursor with their left wrist (Fig. 1A). The left wrist, rather than the right, was chosen to maximize the dynamic range of learning, based on the assumption that subjects would have worse initial performance with their nondominant hand. The task goal was to guide the cursor through a semicircular channel from one end to the other without hitting the edges.
Fig. 1.
The arc-pointing task. A: experimental apparatus. Subjects guided a screen cursor by making a pointing movements with their fist through wrist flexion–extension and pronation–supination. An infrared camera recorded the position of a retroreflective marker attached to the knuckle. B: sample hand paths before and after training. The task was to move the cursor in a clockwise direction from one circle to the other through a circular channel. Representative trajectories from a fast test speed condition (goal movement time was 500 ms) before training (day 1, top panel) and after training (day 5, bottom panel). After training, trajectories were more likely to be within the channel than before training.
Apparatus
Subjects sat facing a computer monitor with their left forearm splinted on a table and controlled a screen cursor by rotating their hand (held closed into a fist with surgical adhesive tape) around the wrist (Fig. 1A). The splint prevented forearm supination, so that screen x and y positions were mapped, respectively, to wrist flexion–extension and radial–ulnar deviation. A desktop computer (Apple, Cupertino, CA) was used to control the visual display and to collect cursor position data through custom software. A Qualisys (Gothenburg, Sweden) Proreflex infrared camera recorded pointing direction as the position of a spherical reflective marker on the index finger's proximal interphalangeal joint (knuckle), at a sampling rate of 100 Hz.
The screen was calibrated so that a 1-cm deviation of the index knuckle caused a 4-cm deviation of the screen cursor. Given that the distance from radial head to the marker was 12 cm on average across subjects, this calibration resulted in a mapping of 0.84 cm of screen cursor movement per degree of wrist rotation. The screen was placed at a distance of 140 cm from the subject and had dimensions of 32.2 × 28.8 cm. The target set consisted of two horizontally separated circles (diameter of 0.7 cm). The distance between the targets was normalized according to the subject's fist size (the distance between the proximal interphalangeal joint and the radial styloid process in the wrist, with the hand closed into a fist), and was typically 4.4 cm on the screen. The targets were connected by two semicircular channels (upper and lower). The width of the channel was the same as the targets' diameter (0.7 cm). The targets and the channels were always visible. The cursor's diameter was 0.1 cm.
The placement of the targets required subjects to make movements (arcs of 4.4-cm diameter on the screen, i.e., 1.1 cm of knuckle deviation) that spanned the middle half of their full range of wrist excursion, which was ∼4 cm of knuckle deviation. At the time of calibration, the experimenter passively moved each subject's wrist horizontally and vertically while observing the screen cursor, and carefully adjusted the forearm's angle so that all cursor positions on the screen could be achieved while keeping the wrist well away from the limits of its range of motion. In this manner, we minimized the possibility that biomechanical limits on range of motion would influence subjects' movements.
Task
The task goal was to move the cursor from one circle to the other through the arc channel in the clockwise direction (upper channel for movements starting in left circle; lower channel for movements starting in right circle), without touching or crossing the channel's edges (Fig. 1B). Thus, only one channel was presented in each trial, and channels alternated between successive trials. At the beginning of each trial, one of the circles became white (start circle) and the other red (target), and subjects placed the cursor in the start circle. After a variable delay (400 to 1,600 ms), the target changed from red to green and a tone was played (“ok-to-go” signal). The instruction was to start the movement at any time after the ok-to-go signal, and to move the cursor from start circle to target (according to the speed requirements of the block). Note that this was not a reaction time protocol: subjects were clearly instructed to start moving only when ready. The trial ended after the cursor entered and stayed in the target for 200 ms.
The cursor was visible throughout the movement. After each trial, the entire trajectory of the cursor appeared as a series of circles on the screen (“knowledge of performance” [KP]). The cursor path shown as KP feedback was colored according to the position of the cursor with respect to the channel; the portions of the path inside the channel were white, and the portions outside the channel red. A reward was given if the entire movement was inside the channel (and if the MT requirements were met). During test sessions, KP was not shown if the movement was outside the required MT range. Instead, an instruction (“go faster” or “go slower”) appeared on the screen, directing subjects to adjust their speed to the current MT requirements. Cursor path and reward were shown for 1.5 s, after which the target of the previous trial became the start circle, and another trial started, with the same parameters except that the channel was the one not used in the previous trial.
During testing and training sessions, subjects were required to make the movements in a predefined MT range. In the beginning of these sessions' blocks, subjects observed a computer-generated demonstration of the cursor moving through the channel in the required MT. The cursor moved along the center of the channel at uniform speed such that the MT was the middle MT value for the required range. The MT of the cursor in the demonstration trial was chosen as the middle value of this range simply to illustrate the approximate MT that subjects should aim for. Reward was given only if the movement was in the required MT range. If not, subjects were instructed to adjust their speed on the next trial. The task's reward structure was as follows. Valid movements (inside the channel and within MT range in constrained blocks) were followed by a pleasant sound and were rewarded with symbolic coins in proportion to their MT. Invalid movements (movements with any point outside the channel or with MT outside the required range in constrained blocks) were followed by a neutral sound (a click). The coins were shown on the screen after each trial, and the cumulative number of coins, across all trials for a given session, was continuously displayed at the top of the screen. The goal was to accumulate as many coins as possible during each session. The total number of coins at the end of each day was explicitly reported to the subjects. Thus, control over MT was achieved by a combination of reward (only movements within the required range could be rewarded), feedback (movements outside range were followed by an instruction to adjust speed and KP was not given), and selection (movements outside the MT range were discarded from further analysis).
Study Design
Subjects participated in the study for 5 consecutive days (Monday to Friday). Performance-estimation sessions (testing sessions) and the training sessions were carried out on separate days; testing sessions were conducted before and after training, on days 1 and 5, and training sessions on days 2, 3, and 4. These days were consecutive for all subjects: day 1 (testing session before training) was always a Monday; training days (2, 3, 4) were always Tuesday, Wednesday, and Thursday; and day 5 (testing session after training) was always Friday. Whereas an increase in accuracy with a concomitant decrease in MT implies a shift of the SAF, we considered it important to sample the SAF directly in separate testing sessions with constrained MTs. These testing sessions afforded several benefits. First, they were a means to assess skill across a range of speeds, and thus a range of difficulty levels, rather than assessing performance changes at only one particular level of difficulty. In this manner, it was possible to test for generalization, because changes in skill were measured not only at the trained speed, but also at untrained ones. Second, the testing sessions reduced the potential confound of exploratory behavior during training. It is plausible that, during training, subjects might explore their performance limits by varying their trajectories from trial to trial in a search for ways to achieve higher performance. Indeed, the fact that the goal of learning is to reach a new level of performance, not available before learning, makes some amount of exploration of movement parameters during training sessions quite likely. Testing sessions were designed to minimize such exploration because subjects were asked to perform at their best level and because the required speed for a given movement changed relatively frequently (see the following text).
Testing sessions.
Speed–accuracy functions (SAF) were probed (identically for all groups) on day 1 and day 5 by collecting movements at five predefined time ranges (in ms: 240–420, 400–600, 640–960, 800–1,200, 1,200–1,800). For all speeds but the fastest, MT ranges were chosen to be proportional to the required MT. For the fastest speed the range was chosen to be slightly wider. Typically, 20 movements within each time range were collected in each test session, in two separate blocks. Movements outside the required MT ranges were discarded. Thus, a testing session was composed of 10 blocks, each composed of 10 movements within a single MT range. The order of the blocks was interleaved to counterbalance possible sequential effects from one block to the next. KP was shown only if the movement was in the correct speed range. At the beginning of each block, a demonstration trial was shown (see earlier text), which indicated the target MT for that block. Between blocks, subjects had a 10-s rest. Before the testing session, subjects completed a warm-up block of 40 movements without any speed constraints and with reward proportional to their speed.
Training sessions.
Training sessions were carried out on days 2, 3, and 4. Each day's training was composed of 3 blocks of 120 movements each. Between blocks, each subject's hand was released from the splint, and subjects rested for 5 min. Within blocks, subjects had a 10-s rest every 30 movements. Four experimental groups had the same testing sessions, and differed only in the training protocol: 1) Medium group (n = 18), trained at target MT of 620 ms (required range, 520–780 ms); successful movements were rewarded with 4 coins. 2) Slow group (n = 10), trained at target MT of 1,200 ms (960–1,440 ms); successful movements were rewarded with 3 coins. 3) Fast group (n = 17), trained at target MT of 450 ms (360–540 ms); successful movements were rewarded with 5 coins. 4) Control group (n = 5), no training sessions (performed only testing sessions on days 1 and 5). The MT training range for the Medium group was chosen as slightly faster than the average MT found in a pilot study in which subjects were free to choose their movement speed during training. During training, subjects were rewarded only if they executed the movement in the channel and within the specified time range. KP was given for all movements. A demonstration of the required MT was shown every 30 movements.
Data Analysis
Trajectories were analyzed both online and offline. Online analysis was necessary to calculate MT to determine whether subjects had moved at the required speed. For online analysis, cursor position was decomposed into radial and angular components (r, θ), with the origin at the center of the circle defined by the two channels, after each trial. Movement onset and end times were defined as the times when angular position increased (in the clockwise direction) by 10° and 170°, respectively, relative to the angle of the start circle. A trial was considered a success (“in channel”) if the cursor's radial position never exceeded the channel's boundaries, and if the cursor entered and stayed inside the target for at least 200 ms. These calculations were performed by the custom software that controlled the experiment.
For offline analysis, we used custom routines written within the Igor software package (WaveMetrics, Lake Oswego, OR). Cursor position data were low-pass filtered (zero-lag, third-order Butterworth filter, cutoff frequency 14 Hz). For submovement analysis, data were filtered after each additional differentiation (to obtain velocity, acceleration, and jerk). Movement onset, end, MT, and trial success were calculated in the same manner as for online analysis. All the kinematic variables were calculated after discarding the first and last 10°, which correspond to the segments of cursor movement within the start circle and within the target.
Performance Analysis
Since the performance measure p (proportion of movements in channel) is bounded between 0 and 1, changes in p at a given MT are not additively comparable across MTs. One could therefore observe larger performance changes for initial values of p around 0.5 compared with values near 1, not because subject's skill has improved more at 0.5 but because performance cannot improve beyond 1. To allow comparison of the improvement across MTs, we transformed the data to z values using the logit transformation, logit(p), z = ln[p/(1 − p)], a common procedure for transforming binary outcomes into unbounded variables (Agresti 1989; Bonnet et al. 2008; Davison and Tustin 1978). The resulting variable z can be considered a more accurate reflection of skill, because it is not bounded at either end of the SAF. We thus refer to changes in z as skill learning in our task. The logit function maps (0, 1) to (−∞, +∞), and is the solution for z in the logistic function p = (1 + e−z)−1. In the context of an SAF, z can be a linear function of speed, or rather MT, such that z = b0 + b1* MT. Because the logit transformation (z = ln[p/(1 − p)]) is not defined for p = 1 and p = 0, we adjusted the accuracy measure p before applying the logit transformation according to the following rule. For p < 1, padj = p + 1/(2n). For p = 1, padj = p − 1/(2n), where n denotes the number of trials in condition (at least 18).
The same approach was taken in the investigation of feedforward and feedback control changes. The proportion of movements in the “critical zone” and proportion of successful feedback corrections were transformed to z values using logit transformation.
Analysis of Trajectory Mean and Variability
To compare the effect of practice on trajectory, the trial-by-trial mean and variance were computed (over all trials in a given speed range) from time-normalized radial positions. The result was five mean trajectories and their variance on both day 1 and day 5 for each subject, which allowed comparison of mean trajectory and trajectory variability between the two testing sessions (i.e., before and after the three practice sessions). Trajectories were normalized by interpolating the sampled radial position between 10° and 170° to 200 points evenly spaced in time. Variance and average radial position were computed for each time-normalized point in every subject, day, and test speed. Thus, for every analysis, every subject contributed two matrices (one for each testing session, i.e., day 1 and day 5) of 200 (time-normalized points) × 5 (speeds). Time normalization of trajectories was performed only for the analysis of trajectory mean and variability.
Submovement Analysis
To examine the kinematic structure of movements, we measured movement segmentation by identifying peaks in the time course of jerk. Such segmentation has generally been described in the velocity profile, where a submovement can appear as a peak (Novak et al. 2002; Rohrer and Hogan 2003). However, submovements can blend into each other if one starts before the previous one ended. This can hide velocity peaks because they become separated not by a velocity minimum but by an inflection point. Analysis of higher time derivatives, such as acceleration and jerk, allows the identification of more submovements that might otherwise be hidden in the velocity profile. A triplet of jerk peaks, positive then negative then positive, generally corresponds to a single peak in the velocity profile. Such a triplet of peaks is identifiable even when the velocity profile has an inflection point.
Based on these considerations, we defined a submovement as the segment between two positive jerk peaks in a positive—negative–positive triplet of jerk peaks (minimum peak jerk value, 200 cm/s3). Submovement duration was the time from one positive jerk peak to the next one. Submovement amplitude was the difference between the first positive peak and the subsequent minimum. This method is only one of several possible methods for identifying submovements (Meyer et al. 1988; Milner 1992; Novak et al. 2002; Rohrer and Hogan 2003). It makes fewer assumptions than other methods but may also identify fewer submovements. No time normalization of the trajectories was performed for the calculation of submovements.
To avoid the possibility that submovement analysis might be affected by artifacts of the specific filtering method used, we also performed this analysis after filtering the raw position data with a smoothing spline (Reinsch 1967), with a smoothing factor of 0.003, instead of the third-order Butterworth filter. This approach yielded the same pattern of results for submovements as the analysis based on Butterworth-filtered data, and will not be reported further.
Statistical Tests
Statistical analysis was performed in JMP (SAS Institute, Cary, NC) and through custom routines written in Matlab (The MathWorks, Natick, MA). The effects of conditions were compared through repeated-measures ANOVA with subject as within factors.
Random field Gaussian distribution correction for temporal correlation in the data.
To detect differences in variance and mean radial position, a two-way ANOVA was run repeatedly for every normalized time point (n = 200). Thus, for every time point, an ANOVA was run on within-subject variance or mean radial position measures from every speed and testing day. Such an approach raises the need to correct for the multiple comparisons (because each ANOVA was run 200 times, once for each time point). When correcting for the probability of false positives due to the multiple comparisons, we took into consideration the temporal correlations in the data that resulted from temporal smoothing. Thus, corrected thresholds were computed based on estimating the number of truly independent samples present within the sampled vector using random field theory (Worsley et al. 1992). Assuming a full-width-at-half-maximum (i.e., the effective full-width-at-half-maximum of a Gaussian kernel used to smooth white noise errors) of 40 time points, and a one-dimensional size of 200, t and F random field thresholds were computed (Worsley et al. 2004) to provide a false-positive rate of 0.05 per test, corrected for multiple comparisons across the multiple time points.
RESULTS
We first describe changes in task performance and kinematics through detailed analysis of data from subjects trained at medium speed (Medium group). We then compare learning across all three training groups (Slow, Medium, Fast).
Practice Led to a Change in the Speed–Accuracy Trade-Off Function
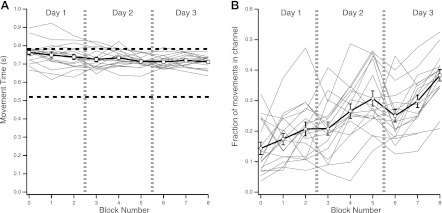
The Medium group trained in a range of MTs between 520 and 780 ms on days 2, 3, and 4. Average accuracy for the first and last training blocks showed a significant improvement [t(17)= 27.68, P < 0.0001; Fig. 2A]. The increase in accuracy was accompanied by a significant decrease in MT [t(17)= 2.92, P = 0.0095; Fig. 2B].
Fig. 2.
Performance during training blocks, shown as individual subject data (thin gray traces) and as a group average ± SE (thick black trace). A: movement time (MT) as a function of training block (120 trials, 3 blocks per daily session). Training sessions took place on 3 consecutive days for all subjects. With training, average MT and its intersubject variability decreased. Also shown is the imposed MT range (horizontal dashed lines; i.e., MTs for which a movement within the channel was rewarded). B: fraction of movements within the channel as a function of training block. With training, the proportion of successful movements increased.
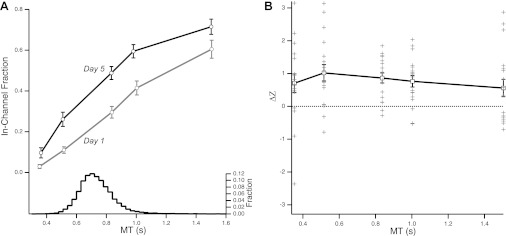
The SAF for baseline performance (day 1 performance) showed a monotonic relationship between speed and accuracy (Fig. 3A). After training in the range of movement times shown by the histogram, subjects showed improvement at all test speeds, which indicates generalization across difficulty levels of the improvement achieved during training (Fig. 3A).We calculated performance change using Δz, defined as z5 − z1 (i.e., the difference between day 5 and day 1; see methods). Positive values of Δz indicate improved movement accuracy for a given MT range (Fig. 3B). One-way ANOVA for the effect of MT on Δz resulted in a significant intercept term [t(17) = 6.85, P < 0.0001], indicating overall improvement in the task, and a nonsignificant effect of MT [F(4,68) = 0.356, P = 0.839], indicating that the improvement did not differ between trained and untrained speeds. Importantly, there was no significant change in z for the Control group, who were tested on days 1 and 5 but did not get any training on days 2, 3, or 4. This indicates that the test sessions themselves did not lead to appreciable skill learning [t(4) = 1.1, P = 0.284].
Fig. 3.
Improvement in performance after training. A: proportion of within-channel movements on day 1 (gray) and day 5 (black), plotted as a function of test movement time (MT). The testing session days (days 1 and 5) occurred a day before and a day after the 3 training days, respectively, for all subjects. Subjects were required to move in five different MT ranges. The x-axis shows the average MT value achieved by subjects for each imposed MT range. The histogram indicates the average distribution of movement times during training (collapsed across the 3 days of training). The traces represent speed–accuracy trade-off functions (SAF). There is a change in the SAF before and after training, manifested as improved performance (greater rate of successful movements) at all MTs. Note that improvement is observed at speeds that were not experienced during training (generalization). Error bars denote SE. B: logit-transformed change in performance (Δz) as a function of MT. Solid line indicates average Δz; gray markers indicate performance of single subjects. All average values of Δz are >0, indicating global improvement in accuracy.
Improvement in Accuracy Was Accompanied by Specific Changes in Trajectory Kinematics
We asked whether increases in the probability of success in the arc-pointing task were accompanied by specific changes in trajectory kinematics. Importantly, we sought to dissociate changes in kinematics related to learning from changes that were a function of movement speed (i.e., related to control). This was accomplished by testing performance at fixed MTs before and after training, which allowed us to separate effects of day (skill) from the effects of speed (task difficulty).
Skill acquisition was associated with a large reduction in trajectory variability.
A feature that has been proposed to characterize skilled motor performance is lower trial-to-trial variability in task-relevant dimensions compared with novice performance (Barden et al. 2005; Madeleine and Madsen 2009; Muller and Sternad 2009; Scholz and Schoner 1999). We therefore computed trial-to-trial variability (in a point-by-point comparison) for the cursor's radial position, which was the task-relevant dimension for the task because the cursor had to remain in the channel. Mean variability plots for each test MT are shown for days 1 and 5 in Fig. 4, A and B. On day 1 there was an increase in variability as movement speed increased and as trajectories unfolded in the channel (Fig. 4A). On day 5, there was a marked reduction in overall variability and a flattening of speed- and position-related variability dependencies (Fig. 4B). Variability changes were measured using two-way ANOVA with effects of day (day 1, before training; day 5, after training), speed (5 test speeds), and day × speed interaction. An ANOVA was performed at each time-normalized point (i.e., 200 times). Correction for multiple comparisons was done using random Gaussian field theory (Worsley et al. 2004), an approach that takes into consideration the temporal correlations in the data (see methods). All three effects (day, speed, and day × speed interaction) reached significance levels (Fig. 4, C–E). Note that day and speed effects were much more pronounced than the interaction between speed and day.
Fig. 4.
Changes in trial-to-trial path variability between day 1 and day 5. This variability is the variation in the cursor trajectory's radial position across trials, for a given speed, across all trials in a testing session (day 1 or day 5). Movement duration was first normalized to 200 points. Variability was computed for every subject, time point, and test speed before and after training (see methods). A: average variance as a function of normalized time and test MT on day 1. Variance was greater for later portions of the trajectory and for higher speeds. B: average variance as a function of normalized time and test MT on day 5. Although the same trends seen in day 1 persist, there is a noticeable reduction in overall variance levels. C: day effect on variance (F values) as a function of normalized time. Larger F values indicate higher probability that variability changed with training. Dotted horizontal line represents the threshold (corrected for multiple comparisons) above which F values are statistically significant. A significant change in variability after training can be seen throughout most of the trajectory. D: speed effect (F values) as a function of normalized time. The changes in the variability measures between test speeds are most marked in the late portion of the trajectory. E: day × speed interaction effect (F values) as a function of normalized time. Compared with the main effects, the interaction is smaller and reaches significant levels only for brief portions of the trajectory.
To confirm that variability decreased after training, we performed a post hoc “leave-one-out” analysis. The input for this analysis was a single variance measure from each subject from day 1 and day 5, from a single normalized time point. The point was chosen based on the maximal day effect from a two-way ANOVA that was run on the remaining subjects in the experiment (n = 17). Thus the data for the post hoc test were selected independently of the activation of the individual subjects, and therefore the two analyses (the first-step ANOVA and the post hoc analysis on day effect) are not dependent. A paired t-test on the variance measures from day 1 and day 5 revealed a significant decrease in variance after training [t(17) = 5.27, P < 0.0001].
Skill was associated with a change in average trajectory.
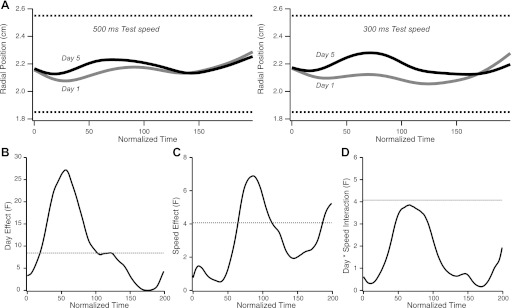
A given task and a given physical plant may be associated with a unique solution for best performance. This solution may become “known” early during familiarization with the task and then attempted with increasing success with practice. Alternatively, the unique solution itself may evolve through training. Thus in the arc-skill task subjects could either show the same mean trajectory or show qualitatively different mean trajectories at the beginning and end of training. The average movement path was measured for the radial position of the cursor for each of the five test speeds. Even though trajectory failures occurred more frequently for the fast speeds, the average trajectory, even before training, was inside the channel for all test speeds (Fig. 5A). A two-way ANOVA on mean trajectory showed an effect of day, speed, and a nearly significant day × speed interaction (Fig. 5, B–D). This result indicates that the average trajectory for each speed changed after training and that the average trajectory for each speed was different.
Fig. 5.
Changes in average movement path. A: time course of average radial position of movements in the upper arc, for the two fastest MT ranges, centered at 500 ms (left) and 300 ms (right). Radial cursor position is plotted against normalized time. Average path was computed by averaging time-normalized trajectories across trials and subjects. The average trajectories are well inside the channel (depicted by the horizontal dotted lines). B: day effect (F values) as a function of normalized time. Dotted horizontal line represents the threshold (corrected for multiple comparisons) above which F values are statistically significant. Significant changes in average radial position after training can be seen along the first half of the trajectory. C: speed effect (F values) as a function of normalized time. The changes in average radial position reach significance around the center of the trajectory. D: day × speed interaction effect (F values) as a function of normalized time. Compared with the main effects, the interaction is much smaller, approaching significant levels in the first half of the trajectory.
Training led to an improvement in feedforward and feedback control.
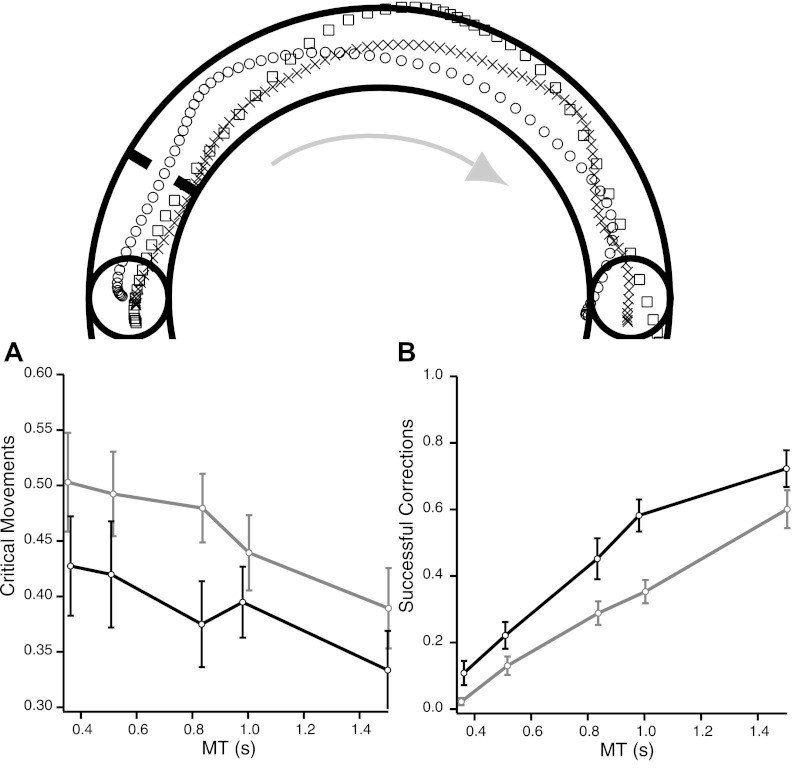
Training could lead to better feedforward and/or feedback control. Although a feedforward improvement would lead to decreased trajectory variability around the average path from the beginning of the movement, feedback improvements should become apparent when trajectories make large deviations from the mean trajectory later in the movement. We defined feedforward planning error to have occurred when the cursor veered close to the channel edge early in the trajectory (30° from the center of the starting circle), because we predicted that this would increase the risk of failing to remain in channel. A movement was classified as being in a “critical zone” if it was >1SD (computed based on the distribution of movements from all subjects before and after training) from the channel center at 30° but nevertheless still in channel. The feedforward error measure was the proportion of movements that reached the critical zone. We also used this critical-zone classification for our definition of online feedback correction (i.e., the proportion of trials that were successful despite the cursor having entered the critical zone at the beginning of the movement). We found that both the feedforward and feedback measures improved with training. To compare the changes in proportions of critical-zone movements and successful corrections, data were transformed to z values using the logit transformation (see methods).
A two-way ANOVA (with effects of MT and day) showed a clear reduction of the proportion of movements in the critical zone after training [F(1,161) = 7.928, P = 0.0055, Fig. 6A]. In addition, the proportion of movements that entered the critical zone and yet remained inside the channel increased after learning increased [F(1,156) = 9.991, P < 0.0019, Fig. 6B]. Thus, with training, subjects were able to reduce the number of trials that entered the critical zone early in the trajectory and increased their ability to steer themselves out of trouble in the event that they did enter the critical zone.
Fig. 6.
Improvement in feedforward and feedback control. “Critical-zone” movements were defined based on the distribution of the radial positions at the beginning of the movement (at 30°, thick black lines). Top: representative trajectories for a movement outside the critical zone (circles), a movement in the critical zone that stayed in the channel (×'s), and a movement in the critical zone that exited the channel (squares). A: proportion of movements in the critical zone before (gray) and after (black) training, as a function of test MT. After training, fewer movements entered the critical zone, indicating improved movement planning. B: proportion of movements in the critical zone that stayed inside the channel as a function of test MT before (gray) and after (black) training. After training, the proportion of successful corrections increased for all speeds.
Although this analysis suggests possible changes in feedforward and feedback control, it is limited because we did not manipulate the relevant variables to establish cause–effect relationships. Nevertheless, we consider this approach worthwhile because there are no straightforward manipulations to conclusively distinguish between improvements in feedforward and feedback control. For example, whereas sudden visual perturbations of the cursor could be used to study feedback corrections, this would not address online correction of self-generated errors, which may involve other mechanisms. Similarly, removing visual feedback and observing changes in endpoint variability would not isolate feedforward control because corrections could still occur based on proprioception and efference copy signals.
Skill was associated with decreased movement effort, without changes in submovement number or duration.
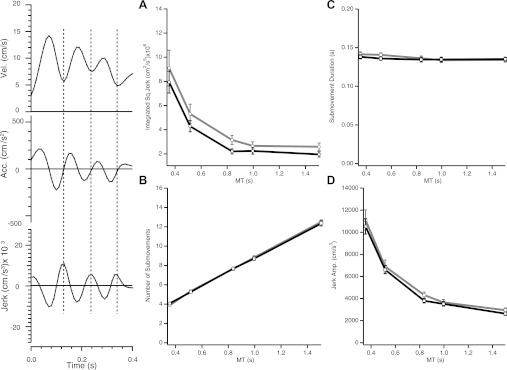
Skilled movements are typically characterized by their graceful trajectories. This feature can be quantified by movement smoothness, which has been suggested as an optimization criterion for planning of reaching movements (Flash and Hogan 1985). Thus, skill acquisition could be a process of trajectory optimization that would result in smoother trajectories after training. Such a change could correspond to the “improved efficiency” that is included in some definitions of motor skill learning (e.g., Guthrie 1952; Pear 1948; Proctor and Dutta 1995). We computed trajectory smoothness by integrating the squared jerk (rate of change of acceleration) profile along the entire trajectory (smaller values of integrated square jerk indicate greater smoothness). We did not normalize this measure of smoothness across speeds, because we were interested in smoothness changes that accompanied training, not changes in speed. For higher movement speeds, the integrated square jerk could be smaller (due to shorter movement duration) or larger (due to higher jerk values associated with higher speeds). As it happened, integrated square jerk increased with movement speed [F(4,153) = 50.87, P < 0.0001]. Importantly, there was a significant decrease of integrated square jerk following training, as seen by a significant day effect [F(1,153) = 6.33, P = 0.0129] and a nonsignificant day × speed interaction [F(4,153) = 0.1647, P = 0.96]. These results indicate that movement smoothness increased with training across all test speeds (Fig. 7A).
Fig. 7.
Submovement analysis. Movements were divided into segments according to the peaks in the time course of jerk (second time derivative of tangential velocity). Plots on left represent, for a sample movement, the time course of tangential velocity (top), acceleration (middle), and jerk, for the same trajectory (bottom). A submovement was defined as the portion of trajectory between successive positive jerk peaks (vertical dotted lines). A: integrated square jerk across the whole trajectory as a function of MT for day 1 (gray) and day 5 (black). As movement time increases, trajectory smoothness increases, seen as a reduction in integrated square jerk. Trajectory smoothness also increases with training. Error bars denote SE. B: number of submovements as a function of test MT for day 1 (gray) and day 5 (black). Although there is a clear increase in the number of submovements with MT, there is no change with training. C: mean submovement duration (peak-to-peak time differences) as a function of MT for day 1 (gray) and day 5 (black). As movement time increases the mean submovement duration slightly decreases. There is no change with training. D: mean submovement amplitude (peak-to-peak jerk amplitude) as a function of MT for day 1 (gray) and day 5 (black). As movement time increases the mean jerk pulse decreases. However, there is no change with training.
Wrist trajectories were composed of a series of peaks in the velocity profile. Such irregularities may reflect submovements, that is, segmentation of the overall movement into individually controlled components (Milner 1992; Morasso and Mussa Ivaldi 1982; Vallbo and Wessberg 1993; Viviani and Terzuolo 1982). Given that submovements can reflect online corrections of an ongoing trajectory or an optimal discrete controlled unit for the task (Milner 1992), a question of considerable interest is whether an increase in trajectory smoothness is the result of a decrease in the number of submovements. We thus hypothesized that the segmentation observed on day 1 indeed represented submovements, and asked how submovements are related to movement speed (i.e., how does submovement structure change across the execution range?) and testing day (how does submovement structure change with skill learning?).
We defined a submovement as a peak in the time course of tangential acceleration, detected as a triplet of peaks in the time course of jerk (a negative peak surrounded by two positive peaks; see Fig. 7 and methods). We calculated the mean number of submovements per trial, their mean duration (time between two positive jerk peaks), and their mean amplitude (peak-to-peak amplitude of the jerk peak triplet) of submovements. We tested for a relationship of these measures with execution and learning by performing individual two-way (MT, testing day) repeated-measures ANOVAs on each measure. Submovement number showed a clear increase with MT and their amplitude decreased with MT (Fig. 7, B and D). Both submovement number and amplitude varied more than threefold across the range of speeds tested [submovement number: F(4,153) = 1,639, P < 0.0001; amplitude F(4,153) = 160, P < 0.0001]. Duration, on the other hand, varied negligibly with MT: although the effect was significant [F(4,153) = 4.176, P = 0.003], the difference between duration at the fastest and slowest speeds was only 6 ms (from 140 to 134 ms; Fig. 7C), which is at the limit of our apparatus' measurement sensitivity (sampling rate 100 Hz). There was no significant effect of testing day on mean submovement number [F(1,153) = 0.1293, P = 0.72], duration [F(1,153) = 2.16, P = 0.14], or amplitude of submovements [F(1,153) = 2.56, P = 0.11]. We obtained similar results (i.e., significant speed effect on duration, amplitude, and number, and no significant day effects) when we filtered the raw position data using a different filtering method (smoothing splines), which suggests that the duration invariance is not an artifact induced by filtering. Figure 8 depicts representative velocity and jerk profiles before and after training for fast and slow movements, and demonstrates the training-induced reduction in integrated jerk with no changes in the submovement's structure.
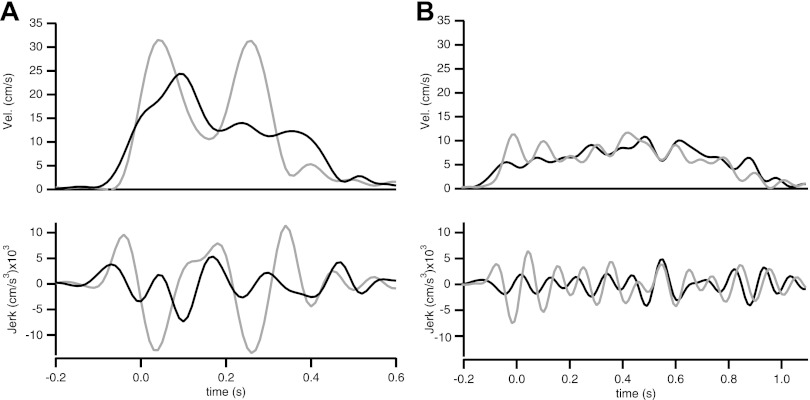
Fig. 8.
Representative velocity and jerk profiles before and after training, demonstrating the lack of change in number of submovements and the reduction in overall jerk amplitude. A: velocity (top) and jerk (bottom) profiles of a fast movement before (gray) and after (black) training. B: velocity (top) and jerk (bottom) profiles of a slow movement before (gray) and after (black) training.
The invariance of submovement structure could either indicate a hard constraint, perhaps related to a limitation on how curved wrist movements can be controlled, or an effect of visual feedback-specific corrections. To address these two alternatives we tested the effect of removing online visual feedback. We performed an additional experiment in which five subjects from our group of participants (and thus previously trained on the arc-skill task) were asked to perform the arc-skill task again. After an initial warm-up block, subjects performed a block of 100 movements without online visual feedback, and a short block of 20 movements with visual feedback. Comparison of the duration of the submovements revealed no significant difference in the average duration of submovements with or without visual feedback [F(1,4) = 0.037, P = 0.85]. This result supports the idea that submovements of fixed average duration are a kinematic feature of curved movements, irrespective of whether visual feedback is present. It is of course also possible that movement segmentation is an idiosyncratic feature of the biomechanics of the wrist joint. However, a type of movement segmentation was also found for elbow movement, with or without visual feedback (Doeringer and Hogan 1998). This generality argues against (but does not exclude) a biomechanical origin for movement segmentation. Another possible concern is that movement segmentations might represent physiologic tremor. Excluding this possibility on a quantitative basis is difficult (Vallbo and Wessberg 1993). However, visual inspection of the trajectories did not show stereotyped cycles of back-and-forth oscillations, which makes tremor unlikely. It should be noted that, although the channel had a constant curvature, our task did not require paths of constant curvature but only paths that stayed within two radial boundaries, which could have varying or constant curvature. Our finding was that paths had varying curvature.
Training in Different Speed Ranges Led to Similar Changes in the Speed–Accuracy Trade-Off Function
We started our investigation by assessing how subjects changed their speed–accuracy trade-off with training. The first finding was that subjects showed increased accuracy at speeds that extended beyond their training range, which raised the question, would generalization look different if training was constrained to a different speed range? We therefore trained two additional groups of subjects, one at a slower constrained speed and one at a faster constrained speed, respectively. The speeds experienced during training by these two groups, as well as those experienced by the group described earlier, are indicated by the histograms in Fig. 9A. As for the initial (medium-speed) group, we probed accuracy at five test MTs before and after training (Fig. 9A). Interestingly we saw a similar change in the SAF for the three training speeds; that is, the shape of the SAF after training was similar regardless of training range. This result was supported by comparison, between groups, of changes in the performance measure, Δz: although there was an overall improvement in performance [Δz was significantly different from 0, t(41) = 9.79, P < 0.0001], there was no difference between groups in the amount of improvement across all speeds [F(2,41) = 0.078, P = 0.925 for group effect; F(4,164) = 2.46, P = 0.0474 for speed effect; and F(8,164) = 1.298, P = 0.248 for day × speed interaction; Fig. 9B]. The lack of interaction indicates that similar improvements occurred within each speed range, regardless of the speed practiced during training.
Fig. 9.
Comparison of performance between groups that trained at three speed ranges. A: proportion of within-channel movements on day 1 (dotted lines) and day 5 (solid lines), plotted as a function of test movement time (MT), for three groups that trained at different speeds. Intensity of traces increases with training speed (light gray, Slow training group; dark gray, Medium training group; black, Fast training group). The histogram indicates the average distributions of movement times during training (collapsed across the 3 days of training). The traces represent speed–accuracy trade-off functions (SAF). All groups show improvement across the whole test range, indicating broad generalization across MT values ranging from 300 to 1,800 ms, and thus across a wide range of difficulty. Error bars denote SE. B: logit-transformed change in performance (Δz) as a function of MT for the three groups. All average values of Δz are >0, indicating global improvement in accuracy.
DISCUSSION
We sought to characterize the structure of motor skill learning at the levels of task performance and of underlying kinematics by studying changes in the speed–accuracy relationship for a wrist precision guidance task. We emphasized the need to distinguish learning-related changes in kinematics from those associated with greater control demands when movements are executed at higher speeds. Training within a restricted speed range led to improvements that generalized to outside the trained range, as indicated by a change in the overall SAF for the task. Skill acquisition, which we defined as a training-related change in the SAF, was accompanied by a reduction in trajectory variability and small but significant changes in trajectory shape and smoothness. These kinematic changes occurred without changes in submovement number or duration. Finally, constraining training to specific speed ranges led to a similar shift of the SAF across test speeds.
Before discussing specific aspects of our results, it is worthwhile clarifying several novel aspects of our study. A large body of research has addressed motor skill learning in the past century (seminal reviews include Adams 1987; Guthrie 1952; Pear 1948; Proctor and Dutta 1995; Schmidt and Lee 2005; Welford 1968). A liberal use of the term “motor skill learning,” however, coupled with the more recent recognition that motor learning appears to be comprised of distinct processes that can be distinguished at the computational and implementation (neural substrate) levels (Bastian 2006; Izawa and Shadmehr 2011; Krakauer and Mazzoni 2011; Shmuelof and Krakauer 2011; Wolpert et al. 2011), makes it potentially difficult to recognize what has been established about motor skill learning.
First, we based our investigation on an operational definition of a core component of motor skill learning, suggested by Reis et al. (2009). With few exceptions (Guthrie 1952; Schmidt and Lee 2005; Welford 1968; Willingham 1998), prior studies have usually relied instead on a common intuition about motor skill learning. Our approach offers an inroad into studying motor learning that is more resistant to arguments about definitions themselves: our results establish properties of how a speed–accuracy trade-off changes with practice, whatever term one may wish to use for this type of learning.
Second, we assessed motor skill learning as a change in an overall ability. We sampled subjects' motor ability, defined as performance on a speed–accuracy task, across multiple levels of difficulty before and after training, which elicited performance that ranged from very successful to poor. By sampling across the entire speed–accuracy function, we thus measured how subjects' overall motor ability changed with training. This approach is in contrast to the more common method of focusing on performance at a single level of difficulty: performance of a task in one condition is poor before learning, and it improves, with unchanged difficulty, after practice. Although this approach allows a detailed analysis of the time course of learning (e.g., Newell et al. 2001), the change in the overall ability to perform a task remains unknown. Our focus on difficulty, imposed by a speed–accuracy trade-off, also made our analysis of generalization and transfer directly relevant to the improvement of motor ability: we tested generalization along the same dimension (speed) that inherently imposed a limit on performance. In analogy with studies of perceptual skill learning, such as discriminating between sound pairs of varying pitch separation (Amitay et al. 2006), we used generalization to probe the expansion of an ability (in this case a motor ability) along its difficulty axis.
Third, we analyzed the entire kinematics of the movement that leads to an outcome at the performance level (i.e., to success or failure). Most studies of motor skill learning have recorded outcome-level measures (e.g., points scored, time on target), single-point kinematics (the angle and speed of the hand at the time of release of a dart or a ball on a string; e.g., Muller and Sternad 2004), or measures that collapse an entire trajectory to a single number (such as root mean square error; e.g., Wulf and Lee 1993; but see also Cohen and Sternad 2012; Georgopoulos et al. 1981). In our task, the whole curved trajectory of the wrist was part of the skilled task, because leaving the channel at any point along the trajectory would lead to loss of points. This task feature effectively imposed a speed–accuracy trade-off on the entire trajectory, unlike traditional speed–accuracy tasks in which hitting a target is all that matters.
Motor Skill Learning as a Reduction in Trajectory Variability
Although ample evidence exists that improvement in skill is associated with a reduction in variability in task performance (Deutsch and Newell 2004; Guo and Raymond 2010; Hung et al. 2008; Liu et al. 2006; Logan 1988; Muller and Sternad 2004; Ranganathan and Newell 2010) it is curious that, aside from two elegant studies of the structure of performance variability in skill tasks (Cohen and Sternad 2009; Muller and Sternad 2004), the emphasis of recent motor learning studies and computational theories has been on reduction of constant error using adaptation paradigms (Bedford 1989; Bock 1992; Caithness et al. 2004; Cunningham 1989; Krakauer et al. 1999, 2000; Miall et al. 2004; Rabe et al. 2009; Sainburg and Wang 2002; Shadmehr and Mussa-Ivaldi 1994; Shadmehr et al. 2010; Simani et al. 2007; Smith et al. 2006; Thoroughman and Shadmehr 2000; Welch 1978; Wolpert et al. 1995). In such adaptation studies, variable error often does not decrease (Krakauer et al. 2000) or is not measured at all because it is the return to mean baseline performance that is of interest. Variability could have many sources, ranging from a deliberate increase in exploration to find a solution for a complex task (Izawa and Shadmehr 2011; Newell and McDonald 1992; Olveczky et al. 2005) to variability induced by constraints of the system, that is, execution noise (Apker et al. 2010; Sanger 2006; van Beers et al. 2004), sensory noise (Munuera et al. 2009; Osborne et al. 2005; van Beers 2007), and planning noise (Churchland et al. 2006; van Beers 2007). We chose to focus on variability due to noise because it defines the bound on baseline performance (Fitts 1954). We minimized the contribution of exploration to variability by having a dedicated test session outside the training context that enforced movement speed, and by devising a task with a solution that was clear from the first trial. Our underlying assumption was that when maximal skill is required in task performance, exploration is minimized. In songbird learning, higher variability appears to be related to exploration and learning. Interestingly, this variability is reduced when male birds perform a song for a female bird, compared with when they practice the song in isolation (Doupe et al. 2005). Variability measures obtained from day 1 showed an increase in variability with test speed, consistent with signal dependent noise (Fig. 4). After training, we observed an overall reduction in variability across all speeds, which we define as an increase in motor acuity.
Our results are consistent with the reduction in variability demonstrated in monkeys practicing fast accurate reaching (Georgopoulos et al. 1981) and in human subjects practicing the virtual skittles task (Cohen and Sternad 2009; Muller and Sternad 2004), where execution variability was shown to decrease over days. Although the approach in the virtual skittles task was to mathematically divide task variability into three different components (covariation, tolerance, and noise), we isolated execution variability through the constraints of the task design. We constrained covariation by choosing a single joint movement with no redundancy, which subjects are able to perform even at baseline. We minimized the need for exploration by choosing a task with a “simple” spatial solution (the channel provides clear visual indication of which trajectories are valid) and by separating performance estimation from training. The approaches used by Sternad and colleagues and in our study both aim to study execution variability by examining variability in task performance. Covariation and the choice of coordinate frames, however, can have powerful effects on the observed variability (Sternad et al. 2010) and potentially mask the true extent of noise that is actually deleterious to performance. Our task offers a natural choice of coordinate frame (polar coordinates) in which to measure performance, because the task is defined by the need to control radial position throughout the movement, and there is no redundancy or covariation to influence observed variability. Thus our task provides a direct measure of task-relevant variability in a task-relevant coordinate frame.
Unlike variability, the learning-related change in mean trajectory was small (Fig. 5A). This is quite different from adaptation paradigms, in which there are large changes in mean trajectory over the course of learning. In adaptation experiments, movement trajectories are either directly perturbed and have to be changed back to baseline-like trajectories, as in force-field adaptation, or the visual trajectory is perturbed and large changes in arm trajectory are required, as in visuomotor rotation. Large changes in mean trajectory to reduce constant error are thought to occur through the updating of a forward model via sensory prediction errors (Mazzoni and Krakauer 2006; Shadmehr et al. 2010; Synofzik et al. 2008; Tseng et al. 2007; Wagner and Smith 2008; Wolpert and Miall 1996). The forward model then guides changes in the controller.
How should one fit this study's results into existing ways of thinking about motor learning? Here we provide some speculation on this question. A fundamental concern is whether variability reduction can be formulated in the same way as constant error reduction (i.e., in terms of forward model-based learning; Shadmehr et al. 2010). The output of an improved forward model could be combined with sensory information to improve state estimation. From this perspective, a scenario that could explain our results is that trajectory reoptimization occurred between days 1 and 5 from learning a better forward model of the task, and that variability reduction was the result of improved feedback gains from better state estimation with the new forward model. Support for this idea comes from the fact that we saw both a small but significant change in mean trajectory and improved feedback corrections with training. Thus one could interpret variability reduction within the current framework of optimal feedback control (Diedrichsen et al. 2010; Todorov and Jordan 2002), based on the idea that an optimal control policy can be computed once system identification (adaptation) is complete (Nagengast et al. 2009).
A model-based framework, however, does not readily explain why it takes longer to reduce variability than to reduce systematic error. Admittedly, we tested subjects on only two days and so cannot separate out different time courses for changes in the mean and variability of the trajectory, but there is ample evidence that mean errors are reduced faster than variable errors (Cohen and Sternad 2009; Ghilardi et al. 2000; Muller and Sternad 2004; Newell et al. 2001). To posit state estimation as the bottleneck for motor skill learning only pushes the explanation for this difference in time scales from the motor execution side to the state estimation side.
Alternatively, reduction in variability may occur through a model-free mechanism rather than a model-based one. Whereas in model-based learning a forward model in the cerebellum is used to derive a controller (Imamizu et al. 2000; Synofzik et al. 2008; Tseng et al. 2007), in model-free learning (Kaelbling et al. 1996; Sutton and Barto 1998), the controller is learned directly through trial and error (Criscimagna-Hemminger et al. 2010; Huang et al. 2011; Kaelbling et al. 1996; Sutton and Barto 1998). Support for the idea that there are cerebellum-independent forms of motor learning comes from a study that showed that patients with cerebellar degeneration can reduce small systematic errors without a forward model, but do so through trial and error instead (Criscimagna-Hemminger et al. 2010). Several recent studies of motor learning that did not require adaptation to a perturbation, but required trial-and-error learning, support the idea that practice through trial and error leads to changes in motor cortical representations. Rats trained in a reach-to-grasp task showed an improved signal/noise ratio for neural spiking in motor cortex, and this was correlated with reduced muscle recruitment variability (Kargo and Nitz 2004). Practice of novel motor tasks promotes dendritic spine formation in the motor cortex of the mouse (Xu et al. 2009), and blocking dopamine receptors in motor cortex completely abolishes skill learning in rats (Hosp et al. 2011).
Several studies have suggested that variability of eye movements is largely the result of noisy sensory estimation rather than motor noise (Munuera et al. 2009; Osborne et al. 2005; van Beers 2007). The possibility that improvement in performance variability for arm movements may similarly result from improved sensory processing is supported by the finding that proprioceptive acuity improves with motor learning (Wong et al. 2011). Thus fast model-based learning in the cerebellum and slower model-free learning in sensory and motor cortex could provide an explanation for the difference in time scales for reduction in systematic and variable errors.
Submovements as Constraints on How Skill Is Acquired
Our task design separated training from testing sessions, which comprised a range of imposed speeds, and thereby enabled us to identify trajectory features associated with required speed and not changeable with learning. The velocity profiles of trajectories in our study were segmented into multiple peaks. Whereas velocity profiles became smoother after training, their segmentation did not change with training or with removal of visual feedback, and was characterized by an average segment duration that was invariant across a range of speeds and across training sessions.
Velocity profile segmentation is a common feature of straight and curved movements, and has been interpreted to indicate the presence of submovements. These have been hypothesized to represent elementary units of motor execution (Houk et al. 2007; Milner 1992; Morasso and Mussa Ivaldi 1982; Polyakov et al. 2009; Vallbo and Wessberg 1993; Viviani and Terzuolo 1982). The stability, across training, in the structure of movement segmentation in the present study may indicate that submovements are elementary structures, perhaps reflecting motor commands that descend in a pulsatile fashion (Vallbo and Wessberg 1993). Alternatively, the segmentation pattern could reflect biomechanical constraints of the wrist joint, which are known to result in higher variability and curvature compared with arm movements (Charles and Hogan 2010). In either case, the invariance in mean submovement duration and number with training observed in our study suggests that curved wrist movements have a basic structure that cannot be changed by learning. The changes in mean trajectory and trajectory variability that mediate performance improvement with learning must respect this invariant execution structure.
We also found that skill is associated with more efficient movement execution, as indicated by the reduction in trajectory irregularity that accompanies learning. This change is consistent with minimization of movement effort predicted by optimal feedback control (Todorov and Jordan 2002). Taken together these results suggest that, if indeed movement segmentation indicates a submovement organization of aimed movements, then skill is achieved through a more efficient concatenation of movement segments, rather than by changes in the movement segments themselves.
Generalization of Motor Skill
Generalization in skill learning can suggest features of how skill is controlled and neurally represented, just as generalization in adaptation can provide insight into functional and neural bases of sensorimotor mappings (Shadmehr 2004; Tanaka et al. 2009). Crucially, we tested for generalization across levels of difficulty (speed), which, for a task characterized by an SAF, serves as a window into the functional organization of motor skill. We found that training caused the SAF to shift as a whole, and that training with different speed regimens yielded similar SAF shifts.
The functional implication is that motor skill is achieved through global control across a wide range of difficulty, suggesting a common process responsible for performance across speeds. In other words, the SAF does not reflect local trade-off functions specific to narrow ranges of difficulty but rather an overall control system that is modified as a whole with practice, regardless of whether practice occurs with easy or difficult regimens. This surprising finding contradicts the “challenge point” hypothesis, which posits that practice at a specific difficulty level yields optimal information for learning (Guadagnoli and Lee 2004). Instead, our results echo what has been observed in perceptual skill learning: practicing auditory discrimination at any of three difficulty levels leads to similar performance improvement at all three levels (Amitay et al. 2006). Note that such a global effect does not preclude the possibility of additional local effects of training.
Generalization to other speeds, and the similarity of benefit obtained from practicing at different speeds, suggest that curved wrist movements are represented in a network that controls wrist movement across a range of speeds, rather than in speed-specific cortical modules. These findings are consistent with the broad generalization seen across movement speeds for motor adaptation (Goodbody and Wolpert 1998; Joiner et al. 2011; Krakauer et al. 2000; but also see Kitazawa et al. 1997) and with a neural representation of movement speed as a modulator of neural activity across large populations of premotor and motor cortical neurons (Moran and Schwartz 1999). From a computational standpoint, our finding that the SAF shifts across all speeds is consistent with the idea of “dynamic movement primitives” (Pastor et al. 2009) in which movements can be represented in kinematic coordinates as differential equations that are spatially and temporally invariant (i.e., movements are self-similar for a change in speed).
Our demonstration that local speed training can lead to a global shift of the SAF suggests an important mechanistic similarity between motor skill learning and perceptual learning. Like skill learning, perceptual learning leads to new levels of performance, reduction in variability, and shifts in psychometric functions (Sagi 2011). Both processes are associated with expansion of cortical representations (Kleim et al. 2004; Recanzone et al. 1992) and increased synaptic density (Xu et al. 2009; Yang et al. 2009). Unlike perception, however, which effectively results in a decision, motor control results in kinematics, which have a complex relationship to task success. Thus, unlike studies of perceptual skill learning, motor skill learning may offer a window into the intermediate behavioral variables that are directly controlled by the brain to achieve better task-level performance.
Two previous studies have addressed aspects of motor skill learning specifically relevant to our results. A previous study (Corcos et al. 1993) reported a reduction of endpoint variability in a task that required fast elbow flexions over different angular distances. This reduction generalized from practice at one movement distance to other distances. Interpreting this change as improvement of motor skill, however, is complicated by two aspects of the study. First, accuracy was not emphasized in the task. Second, the relationship between distance and accuracy was nonmonotonic before practice, and became flat after practice. The task was not, therefore, a speed–accuracy trade-off task. These previous results are consistent with ours in demonstrating transfer of variability reduction to other movements but do not inform us on the effect of difficulty on generalization.
Fitts's law was shown to change with practice in a study by Abrams and Pratt (1993). The major finding was that, after practice of fast visually guided tapping movements of a stylus, the primary submovement comprised a larger fraction of total movement time, whereas the fraction of time devoted to corrective submovements was reduced. These results suggested an improvement in feedback control, but there was also a possible improvement in movement planning, because the time before movement initiation increased with practice. Both of these interpretations are consistent with our findings. In the study by Abrams and Pratt, submovement number and duration were not calculated for submovements beyond the primary one. Comparison with our submovement findings is also precluded by the fact that submovements in a curved movement, which are usually present throughout the movement, cannot be assumed to be equivalent to corrective submovements in a straight movement, which are mainly present at the end of the movement.
Conclusion
We studied a nonperturbation task where difficulty is imposed by a speed–accuracy trade-off that applies to an entire trajectory. Although motor skills can include abilities beyond speed and accuracy, moving fast and accurately can be considered a central goal of motor skill learning. Our results suggest that task-level improvement in this type of learning is captured globally by changes in the SAF and at the kinematic level by changes in mean trajectory, reduced trajectory variability, and improved feedback gains. These kinematic changes are accomplished with invariant isochronous submovements. We conclude that improved performance (skill) is driven mainly by reductions in variability, and we conjecture that this improvement is brought about by changes in motor and sensory cortical representations that increase the neural signal-to-noise ratio. This increase in signal over noise could be accomplished by recruiting more neurons or by tuning individual neurons more finely. We also suggest that these skill mechanisms are distinct from the model-based mechanisms in cerebellum that can quickly reduce systematic errors.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant R01-NS-052804 (to J.W.K., P.M.), Machiah Foundation/Jewish Community Federation (to L.S.), and the Parkinson's Disease Foundation (to P.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.S., J.W.K., and P.M. conception and design of research; L.S. and P.M. performed experiments; L.S. analyzed data; L.S., J.W.K., and P.M. interpreted results of experiments; L.S. prepared figures; L.S., J.W.K., and P.M. drafted manuscript; L.S., J.W.K., and P.M. edited and revised manuscript; L.S., J.W.K., and P.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank E. Zarahn for assistance with statistical analysis and insightful discussion; T. Pearson, A. Wilson, J. Camilo Cortes, and R. J. Delnicki for technical assistance; R. S. Sainburg for sharing experiment-control software; and A. Haith for useful comments on the manuscript.
Present address of L. Shmuelof: Department of Computer Science and Applied Mathematics, Weizmann Institute of Science, Rehovot, Israel 76100.
REFERENCES
- Abrams RA, Pratt J. Rapid aimed limb movements: differential effects of practice on component submovements. J Mot Behav 25: 288–298, 1993 [DOI] [PubMed] [Google Scholar]
- Adams JA. Historical review and appraisal of research on the learning, retention, and transfer of human motor-skills. Psychol Bull 101: 41–74, 1987 [Google Scholar]
- Agresti A. Tutorial on modeling ordered categorical response data. Psychol Bull 105: 290–301, 1989 [DOI] [PubMed] [Google Scholar]
- Amitay S, Irwin A, Moore DR. Discrimination learning induced by training with identical stimuli. Nat Neurosci 9: 1446–1448, 2006 [DOI] [PubMed] [Google Scholar]
- Apker GA, Darling TK, Buneo CA. Interacting noise sources shape patterns of arm movement variability in three-dimensional space. J Neurophysiol 104: 2654–2666, 2010 [DOI] [PubMed] [Google Scholar]
- Barden JM, Balyk R, James Raso V, Moreau M, Bagnall K. Repetitive pointing to remembered proprioceptive targets improves 3D hand positioning accuracy. Hum Mov Sci 24: 184–205, 2005 [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol 16: 645–649, 2006 [DOI] [PubMed] [Google Scholar]
- Bedford FL. Constraints on learning new mappings between perceptual dimensions. J Exp Psychol Hum Percept Perform 15: 232–248, 1989 [Google Scholar]
- Bock O. Adaptation of aimed arm movements to sensorimotor discordance: evidence for direction-independent gain control. Behav Brain Res 51: 41–50, 1992 [DOI] [PubMed] [Google Scholar]
- Bonnet C, Fauquet Ars J, Estaun Ferrer S. Reaction times as a measure of uncertainty. Psicothema 20: 43–48, 2008 [PubMed] [Google Scholar]
- Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci 24: 8662–8671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles SK, Hogan N. The curvature and variability of wrist and arm movements. Exp Brain Res 203: 63–73, 2010 [DOI] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV. A central source of movement variability. Neuron 52: 1085–1096, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RG, Sternad D. Variability in motor learning: relocating, channeling and reducing noise. Exp Brain Res 193: 69–83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RG, Sternad D. State space analysis of timing: exploiting task redundancy to reduce sensitivity to timing. J Neurophysiol 107: 618–627, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcos DM, Jaric S, Agarwal GC, Gottlieb GL. Principles for learning single-joint movements. I. Enhanced performance by practice. Exp Brain Res 94: 499–513, 1993 [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol 103: 2275–2284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham HA. Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol Hum Percept Perform 15: 493–506, 1989 [DOI] [PubMed] [Google Scholar]
- Davison MC, Tustin RD. The relation between the generalized matching law and signal-detection theory. J Exp Anal Behav 29: 331–336, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch KM, Newell KM. Changes in the structure of children's isometric force variability with practice. J Exp Child Psychol 88: 319–333, 2004 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R, Ivry RB. The coordination of movement: optimal feedback control and beyond. Trends Cogn Sci 14: 31–39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeringer JA, Hogan N. Intermittency in preplanned elbow movements persists in the absence of visual feedback. J Neurophysiol 80: 1787–1799, 1998 [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends Neurosci 28: 353–363, 2005 [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47: 381–391, 1954 [PubMed] [Google Scholar]
- Flash T, Hogan N. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci 5: 1688–1703, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Massey JT. Spatial trajectories and reaction times of aimed movements: effects of practice, uncertainty, and change in target location. J Neurophysiol 46: 725–743, 1981 [DOI] [PubMed] [Google Scholar]
- Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res 871: 127–145, 2000 [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Moisello C, Silvestri G, Ghez C, Krakauer JW. Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently. J Neurophysiol 101: 2218–2229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbody SJ, Wolpert DM. Temporal and amplitude generalization in motor learning. J Neurophysiol 79: 1825–1838, 1998 [DOI] [PubMed] [Google Scholar]
- Guadagnoli MA, Lee TD. Challenge point: a framework for conceptualizing the effects of various practice conditions in motor learning. J Mot Behav 36: 212–224, 2004 [DOI] [PubMed] [Google Scholar]
- Guo CC, Raymond JL. Motor learning reduces eye movement variability through reweighting of sensory inputs. J Neurosci 30: 16241–16248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie ER. The Psychology of Learning (rev. ed.) New York: Harper & Brothers, 1952 [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci 31: 2481–2487, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC, Bastianen C, Fansler D, Fishbach A, Fraser D, Reber PJ, Roy SA, Simo LS. Action selection and refinement in subcortical loops through basal ganglia and cerebellum. Philos Trans R Soc Lond B Biol Sci 362: 1573–1583, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70: 787–801, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YC, Kaminski TR, Fineman J, Monroe J, Gentile AM. Learning a multi-joint throwing task: a morphometric analysis of skill development. Exp Brain Res 191: 197–208, 2008 [DOI] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403: 192–195, 2000 [DOI] [PubMed] [Google Scholar]
- Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput Biol 7: e1002012, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Ajayi O, Sing GC, Smith MA. Linear hypergeneralization of learned dynamics across movement speeds reveals anisotropic, gain-encoding primitives for motor adaptation. J Neurophysiol 105: 45–59, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelbling LP, Littman ML, Moore AW. Reinforcement learning: a survey. J Artif Intell Res 4: 237–285, 1996 [Google Scholar]
- Kargo WJ, Nitz DA. Improvements in the signal-to-noise ratio of motor cortex cells distinguish early versus late phases of motor skill learning. J Neurosci 24: 5560–5569, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158, 1995 [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Kimura T, Uka T. Prism adaptation of reaching movements: specificity for the velocity of reaching. J Neurosci 17: 1481–1492, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci 24: 628–633, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Ghez C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 2: 1026–1031, 1999 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol 21: 636–644, 2011 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YT, Mayer-Kress G, Newell KM. Qualitative and quantitative change in the dynamics of motor learning. J Exp Psychol Hum Percept Perform 32: 380–393, 2006 [DOI] [PubMed] [Google Scholar]
- Logan GD. Toward an instance theory of automatization. Psychol Rev 95: 492–527, 1988 [Google Scholar]
- Madeleine P, Madsen TM. Changes in the amount and structure of motor variability during a deboning process are associated with work experience and neck-shoulder discomfort. Appl Ergon 40: 887–894, 2009 [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Abrams RA, Kornblum S, Wright CE, Smith JE. Optimality in human motor performance: ideal control of rapid aimed movements. Psychol Rev 95: 340–370, 1988 [DOI] [PubMed] [Google Scholar]
- Miall RC, Jenkinson N, Kulkarni K. Adaptation to rotated visual feedback: a re-examination of motor interference. Exp Brain Res 154: 201–210, 2004 [DOI] [PubMed] [Google Scholar]
- Milner TE. A model for the generation of movements requiring endpoint precision. Neuroscience 49: 487–496, 1992 [DOI] [PubMed] [Google Scholar]
- Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J Neurophysiol 82: 2676–2692, 1999 [DOI] [PubMed] [Google Scholar]
- Morasso P, Mussa Ivaldi F. A trajectory formation and handwriting: a computational model. Biol Cybern 45: 131–142, 1982 [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 111: 344–349, 2000 [DOI] [PubMed] [Google Scholar]
- Muller H, Sternad D. Decomposition of variability in the execution of goal-oriented tasks: three components of skill improvement. J Exp Psychol Hum Percept Perform 30: 212–233, 2004 [DOI] [PubMed] [Google Scholar]
- Muller H, Sternad D. Motor learning: changes in the structure of variability in a redundant task. Adv Exp Med Biol 629: 439–456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munuera J, Morel P, Duhamel JR, Deneve S. Optimal sensorimotor control in eye movement sequences. J Neurosci 29: 3026–3035, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagengast AJ, Braun DA, Wolpert DM. Optimal control predicts human performance on objects with internal degrees of freedom. PLoS Comput Biol 5: e1000419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KM, Liu YT, Mayer-Kress G. Time scales in motor learning and development. Psychol Rev 108: 57–82, 2001 [DOI] [PubMed] [Google Scholar]
- Newell KM, McDonald PV. Searching for solutions to the coordination function: learning as exploratory behavior. In: Tutorials in Motor Behavior II, edited by Stelmach GE, Requin J. Amsterdam: Elsevier, 1992, p. 517–532 [Google Scholar]
- Novak KE, Miller LE, Houk JC. The use of overlapping submovements in the control of rapid hand movements. Exp Brain Res 144: 351–364, 2002 [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol 3: e153, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LC, Lisberger SG, Bialek W. A sensory source for motor variation. Nature 437: 412–416, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor P, Hoffmann H, Asfour T, Schaal S. Learning and generalization of motor skills by learning from demonstration. In: Proceedings of the 2009 International Conference on Robotics and Automation, Kobe, Japan Cambridge, MA: MIT Press, 2009 [Google Scholar]
- Pear TH. Professor Bartlett on skill. Occup Psychol 22: 92, 1948 [PubMed] [Google Scholar]
- Plamondon R, Alimi AM. Speed/accuracy trade-offs in target-directed movements. Behav Brain Sci 20: 279–303, 1997 [DOI] [PubMed] [Google Scholar]
- Polyakov F, Drori R, Ben-Shaul Y, Abeles M, Flash T. A compact representation of drawing movements with sequences of parabolic primitives. PLoS Comput Biol 5: e1000427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor RW, Dutta A. Skill Acquisition and Human Performance. Thousand Oaks, CA: Sage, 1995 [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 101: 1961–1971, 2009 [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Newell KM. Influence of motor learning on utilizing path redundancy. Neurosci Lett 469: 416–420, 2010 [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Schreiner CE. Changes in the distributed temporal response properties of SI cortical neurons reflect improvements in performance on a temporally based tactile discrimination task. J Neurophysiol 67: 1071–1091, 1992 [DOI] [PubMed] [Google Scholar]
- Reinsch CH. Smoothing by spline functions. Numer Math 10: 177–183, 1967 [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106: 1590–1595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Hogan N. Avoiding spurious submovement decompositions: a globally optimal algorithm. Biol Cybern 89: 190–199, 2003 [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Kacar A, Rothwell JC. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci 27: 12058–12066, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi D. Perceptual learning in vision research. Vision Res 51: 1552–1566, 2011 [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145: 437–447, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Dimitrov B, Hallett M. Motor learning in patients with cerebellar dysfunction. Brain 113: 103–120, 1990 [DOI] [PubMed] [Google Scholar]
- Sanger TD. Arm trajectories in dyskinetic cerebral palsy have increased random variability. J Child Neurol 21: 551–557, 2006 [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. Urbana-Champaign, IL: Human Kinetics, 2005 [Google Scholar]
- Scholz JP, Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126: 289–306, 1999 [DOI] [PubMed] [Google Scholar]
- Shadmehr R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum Mov Sci 23: 543–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010 [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron 72: 469–476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simani MC, McGuire LM, Sabes PN. Visual-shift adaptation is composed of separable sensory and task-dependent effects. J Neurophysiol 98: 2827–2841, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4: e179, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternad D, Park SW, Muller H, Hogan N. Coordinate dependence of variability analysis. PLoS Comput Biol 6: e1000751, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press, 1998 [Google Scholar]
- Synofzik M, Lindner A, Thier P. The cerebellum updates predictions about the visual consequences of one's behavior. Curr Biol 18: 814–818, 2008 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Sejnowski TJ, Krakauer JW. Adaptation to visuomotor rotation through interaction between posterior parietal and motor cortical areas. J Neurophysiol 102: 2921–2932, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature 407: 742–747, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci 5: 1226–1235, 2002 [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Wessberg J. Organization of motor output in slow finger movements in man. J Physiol 469: 673–691, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers RJ. The sources of variability in saccadic eye movements. J Neurosci 27: 8757–8770, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers RJ, Haggard P, Wolpert DM. The role of execution noise in movement variability. J Neurophysiol 91: 1050–1063, 2004 [DOI] [PubMed] [Google Scholar]
- Viviani P, Terzuolo C. Trajectory determines movement dynamics. Neuroscience 7: 431–437, 1982 [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Smith MA. Shared internal models for feedforward and feedback control. J Neurosci 28: 10663–10673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35: 205–211, 2002 [DOI] [PubMed] [Google Scholar]
- Welch RB. Perceptual Modification: Adapting to Altered Sensory Environments. New York: Academic Press, 1978 [Google Scholar]
- Welford AT. Fundamentals of Skill. London: Methuen, 1968 [Google Scholar]
- Wickelgren WA. Speed–accuracy tradeoff and information-processing dynamics. Acta Psychol 41: 67–85, 1977 [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychol Rev 105: 558–584, 1998 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci 12: 739–751, 2011 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. Are arm trajectories planned in kinematic or dynamic coordinates? An adaptation study. Exp Brain Res 103: 460–470, 1995 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw 9: 1265–1279, 1996 [DOI] [PubMed] [Google Scholar]
- Wong JD, Wilson ET, Gribble PL. Spatially selective enhancement of proprioceptive acuity following motor learning. J Neurophysiol 105: 2512–2521, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. Neuroimage Suppl 23 1: S189–S195, 2004 [DOI] [PubMed] [Google Scholar]
- Wu T, Kansaku K, Hallett M. How self-initiated memorized movements become automatic: a functional MRI study. J Neurophysiol 91: 1690–1698, 2004 [DOI] [PubMed] [Google Scholar]
- Wulf G, Lee TD. Contextual interference in movements of the same class: differential effects on program and parameter learning. J Mot Behav 25: 254–263, 1993 [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462: 915–919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature 462: 920–924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarrow K, Brown P, Krakauer JW. Inside the brain of an elite athlete: the neural processes that support high achievement in sports. Nat Rev Neurosci 10: 585–596, 2009 [DOI] [PubMed] [Google Scholar]