SUMMARY
Many physiological and behavioural responses to changes in environmental lighting conditions are mediated by extraocular photoreceptors. Here we investigate encephalic photoreception in Phreatichthys andruzzii, a typical cave-dwelling fish showing an extreme phenotype with complete anophthalmy and a reduction in size of associated brain structures. We firstly identified two P. andruzzii photopigments, orthologues of rod opsin and exo-rod opsin. In vitro, both opsins serve as light-absorbing photopigments with λmax around 500 nm when reconstituted with an A1 chromophore. When corrected for the summed absorption from the skin and skull, the spectral sensitivity profiles shifted to longer wavelengths (rod opsin: 521 nm; exo-rod opsin: 520 nm). We next explored the involvement of both opsins in the negative phototaxis reported for this species. A comparison of the spectral sensitivity of the photophobic response with the putative A2 absorbance spectra corrected for skin/skull absorbance indicates that the A2 versions of either or both of these pigments could explain the observed behavioural spectral sensitivity.
KEY WORDS: extraretinal photoreception, cavefish, rod opsin, exo-rod opsin, negative phototaxis
INTRODUCTION
In nonmammalian vertebrates, extraocular photoreceptors mediate many physiological and behavioural responses to changes in environmental lighting. These include the entrainment of circadian rhythms, sun compass orientation, changes in body pigmentation, as well as colouration and thermoregulation (Bertolucci and Foà, 2004). Extraocular photoreceptors are found in: (i) the pineal complex, involving intracranial (pineal and parapineal) and extracranial (frontal organ and parietal eye) elements, (2) the deep brain and (3) a variety of peripheral tissues (Shand and Foster, 1999; Peirson et al., 2009).
The existence of deep brain photoreceptors has been demonstrated in a wide range of nonmammalian vertebrate species (Underwood and Groos, 1982; Foster et al., 1994; Pasqualetti et al., 2003). Evidence for the existence of deep brain photoreceptors in fish was first described in the European minnow, Phoxinus phoxinus, by von Frisch and Scharrer (von Frisch, 1911; Scharrer, 1928). Minnows retain colour changes and dependent conditioned reflexes in response to light even after removal of both the eyes and the pineal gland.
The present study was initially aimed at identifying photopigments in the cavefish Phreatichthys andruzzii. Phreatichthys andruzzii is a member of the Cyprinidae family and inhabits subterranean cave systems in desert areas of central Somalia. It is a typical cave-dwelling vertebrate, showing an extreme troglomorphic phenotype with complete anophthalmy, together with the complete absence of optic nerves and chiasma, and an extreme reduction in size of the mesencephalic lobes (Ercolini and Berti, 1975; Berti et al., 2001). We have recently demonstrated that mutations in two widely expressed non-visual opsins, a melanopsin and a teleost multiple tissue (TMT)-opsin orthologue, are responsible for the blind clock phenotype in P. andruzzii (Cavallari et al., 2011). However, P. andruzzii, in common with several other cave-dwelling species (Langecker, 1989; Timmermann and Plath, 2009), displays strong negative phototaxis (Ercolini and Berti, 1975), indicating that it is not entirely insensitive to light.
Here, we report the identification of P. andruzzii orthologues of two teleost visual pigments, rod opsin, usually found in retinal rod photoreceptors, and exo-rod opsin, a closely related protein previously identified in the teleost pineal gland. We show that both form light-absorbing photopigments with λmax around 500 nm when reconstituted with an A1 (11-cis retinaldehyde) chromophore. To determine whether the presence of either or both of these pigments could be conferring negative phototaxis, we explored the spectral sensitivity of this behaviour. A comparison of these data indicates that either rod or exo-rod pigments employing an A2 chromophore could indeed represent the origin of the phototactic behaviour.
MATERIALS AND METHODS
Fish
Phreatichthys andruzzii Vinciguerra 1924 were originally collected near the village of Bud-Bud (04°11′19″N, 46°28′27″E, central Somalia) during the course of several expeditions (1968–1982), and then reproduced in the laboratory. Adult cavefish (N=10) were maintained and bred in darkness at a constant temperature (27±0.5°C) except during food administration and aquaria maintenance. They were fed three times per week with flake food and frozen Chironomidae larvae and Artemia sp. (Ocean Nutrition, Essen, Belgium).
PCR-based cDNA cloning
Total RNA was isolated from cavefish brains using Trizol reagent (Invitrogen, Milano, Italy) according to the manufacturer's instructions. To initially obtain partial cDNA sequences, single-stranded cDNA was synthesised using SuperScript III Reverse-Transcriptase (Invitrogen). Opsin gene sequences were amplified by PCR using Taq DNA Polymerase (Invitrogen) with primers designed by Primer3 software (Rozen and Skaletsky, 2000) against orthologous Danio rerio sequences (exo-rod opsin, GenBank NM131212; rod opsin, GenBank AF109368). The primers used for gene amplification were: forward (F), 5′-CTTCACCGTCACGCTCTACA-3′; reverse (R), 5′-GCAATCACCATGACAACCAC-3′ for exo-rod opsin, and F, 5′-TCACCATCGAGCACAAGAAG-3′; R, 5′-GGGATGAAGAAGTGCACGAT-3′ for rod opsin. Negative control reactions containing water or RNA instead of cDNA were included in the PCR reactions. Bands of the predicted sizes (510 bp for exo-rod opsin and 423 bp for rod opsin) were cloned into the pGEM®-T Easy Vector (Promega, Milano, Italy). The cavefish opsin cDNA fragments were sequenced and compared with the GenBank database using the BLAST algorithm (Altschul et al., 1990). Additional cDNA sequences were subsequently obtained using a 5′–3′ SMART RACE cDNA amplification kit (BD Bioscience-Clontech, Milano, Italy) and then coding sequences were deposited in GenBank (exo-rod opsin: GQ404491; rod opsin: JQ413240). Nucleotide coding sequences were converted into amino acid sequences using the Translate tool by ExPASy Proteomics (http://www.expasy.org/tools/dna.html) and then aligned using ClustalW2 (http://www.ebi.ac.uk/clustalw2).
In vitro opsin expression and absorbance spectra
Phreatichthys andruzzii opsins were expressed and reconstituted with the A1 chromophore 11-cis-retinal as described by Tarttelin et al. (Tarttelin et al., 2011). Briefly, full-length opsin open reading frames (ORFs) (primer pairs: exo-rod opsin F, 5′-AGGATCCGCCACCATGAATGGAACGGAAGGTCCAAACTTC-3′ and R, 5′-CGCTAGCAGCTGGAGCCACCTGACTGGACGAGACC-3′; rod opsin F, 5′-AGGATCCGCCACCATGAACGGTACAGAGGGACCGAATTTC-3′ and R, 5′-CTC TAGACGCCG GG GACACGGACGACACGGACGAC-3′) were appended with a sequence encoding the 1D4 epitope (TETSQVAPA), cloned into pcDNA5/FRT/TO (Invitrogen) and subsequently used to generate stably transfected tetracycline-inducible Flp-In™-293 cells (Invitrogen). Cells were cultured in HYPERflasks (Corning, Ewloe, UK) prior to opsin protein expression induction by the addition of 1 μg ml−1 tetracycline and 5 mmol l−1 sodium butyrate (Sigma-Aldrich, Poole, UK). Under dim red light, opsins were reconstituted with 50 μmol l−1 11-cis retinal and were then 1D4 column purified. Absorption spectra of the purified pigment samples were recorded on a dual beam spectrophotometer (UV-2450, Shimadzu, Kyoto, Japan). Photo-bleached samples were illuminated with 515 nm light for 5 min to enable the calculation of difference spectra. The λmax of each pigment was determined by fitting a standard A1 visual pigment template (Govardovskii et al., 2000) to the difference spectra using the Solver add-in in Microsoft Excel to generate best-fit curves. This template was kindly provided by D. M. Hunt (Institute of Ophthalmology, University College London, London, UK). The effect of using an A2 chromophore (11-cis 3-dehydroretinal) on λmax was estimated according to the formula A2λmax=exp[(A1λmax/400)+5.0] derived from A1λmax=400×[(lnA2λmax)−5.0] after Parry and Bowmaker (Parry and Bowmaker, 2000).
Spectral transmission
Both the skin overlying the roof of the skull and the roof of the skull were removed separately from five previously frozen fish and the spectral transmission (300–800 nm) of circular areas 3.5 mm in diameter was determined by mounting them in front of an integrating sphere within a spectrophotometer (UV-2101PC, Shimadzu). Due to uncertainties of the absolute transmission, all values were expressed relative to the transmission at 800 nm.
Negative phototaxis
Tests for negative phototactic behaviour were conducted in a windowless room. The test aquarium (75 l, 40×40×48 cm) was placed in an environmental chamber at 27±0.5°C. A black PVC partition was used to divide the aquarium into two halves in such a way that a small 5-cm-high gap was left above the bottom of the aquarium. This gap allowed the test fish to move from one compartment to the other. Both compartments of the aquarium contained a water filter system to keep the water clean and oxygenated. Filters were removed 30 min before the trial. One half of the aquarium was covered on all sides by black plastic foil and hence remained dark (the ‘dark zone’); the other half was not covered (the ‘light zone’). The light zone was illuminated using two halogen light sources (6423FO, 150 W, Philips, Milano, Italy) fed into the environmental chamber through fiber optics (GLI-156P, Fort, Bergamo, Italy). Using interference filters (Ø 45 mm; Optics Balzers AG, Balzers, Liechtenstein) we produced three types of monochromatic light at 644±5, 550±5 and 453±5 nm, respectively. The light source was installed 24 cm above the base of the aquarium in the middle of the water column. Irradiance was measured at the level of the black partition (31 cm from the interference filter) by a radiometer (DO9721, probe LP9021 RAD, spectral range 400–950 nm, sensibility 0.005 W m−2, DeltaOHM, Padova, Italy). The irradiance applied was normalised to obtain the same quanta flux for each monochromatic light source. The light intensity was adjusted using neutral density filters (Lee Filters, Andover, Hampshire, United Kingdom). For all lights the irradiance in the dark zone was not detectable (<0.005 W m−2). The maximum irradiance applied (0.1 W m−2) was comparable to the daily average twilight irradiance at the latitude (04°11′20″N) where the fish were collected (Kato and Loeb, 2003).
We performed tests with eight different irradiances (2.2×1017, 1.1×1017, 2.2×1016, 2.2×1015, 2.2×1014, 2.2×1013, 2.2×1012 and 2.2×1010 quanta m−2 s−1) for each monochromatic light source (a total amount of 240 individual tests). To initiate a test, a fish was removed from the breeding aquarium and introduced into the test aquarium 20 h prior to experimentation to allow acclimatisation to the new environment. The behaviour of each fish was tested under each monochromatic light source, irradiance and in darkness in a random order. The time spent in each compartment was determined during a 30 min period. The aquarium was equipped with infrared LEDs, which were not detected by the fish but allowed video recording (Lythgoe, 1988). Filming was carried out with an infrared video camera, and video data were later analysed on a PC.
Statistical analysis
All the results are expressed as means ± s.d. Student's t-test, repeated-measures one-way ANOVA and Dunnett's multiple comparison test were used to determine significant differences (P<0.05) using GraphPad Prism 4.0 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Identification of rod opsin and exo-rod opsin from P. andruzzii
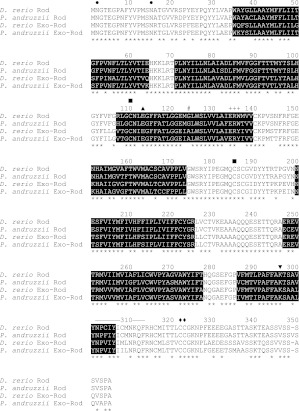
As a first step to explore the role of visual and non-visual opsins in the photophobic response, we exploited the close sequence similarity between zebrafish and P. andruzzii (Tarttelin et al., 2011) to clone rod opsin and exo-rod opsin gene orthologues from the cavefish. The P. andruzzii rod opsin full-length cDNA includes a 1068 bp ORF encoding a 355 amino acid protein, whereas the full-length exo-rod opsin cDNA contains a 1065 bp ORF encoding a 354 amino acid protein. Comparison of the predicted rod and exo-rod opsin protein sequences with their D. rerio counterparts (Fig. 1) indicates that they share 93 and 90% identity, respectively. As expected, only 74% identity exists between the rod and exo-rod opsins (Philp et al., 2000a).
Fig. 1.
Alignment of the rod opsin and exo-rod opsin amino acid sequences of Phreatichthys andruzzii with Danio rerio orthologues (GenBank: AB187811 and AB025312). Asterisks (*) indicate amino acid identity and alignment gaps are indicated by dashes (−). The seven transmembrane domains based upon orthology with the bovine rod opsin model (Palczewski et al., 2000) are indicated in black. Conserved residues and motifs are annotated: ●, N2 and N15; ▪, C110 and C187; ▴, E113; +, E134-R135-Y/W136 tripeptide; ▾, K296; −, 302-313 NPxxY(x)5,6F motif; ◆, C322 and C323. The proposed A124G spectral tuning site is also indicated (#). See Results for more details.
Inspection of the primary structure reveals that individual residues and motifs indicative of functional rod opsin photopigments are all present in both opsins. These include: (1) two amino-terminal glycosylation sites (N2 and N15) and two carboxyl-terminal palmitoylation sites (C322 and C323); (2) a pair of cysteines (C110 and C187) in the second and third extracellular loops, which form a disulphide bond essential to fold the protein into the correct tertiary structure; (3) a glutamic acid residue (E113) that serves as the Schiff's base counterion; (4) a conserved ERY[W] motif (134–136), necessary to bind the α-subunit of transducin; (5) a lysine (K296) in the seventh transmembrane domain acting as a Schiff's base linkage to the chromophore; and (6) a conserved NpxxY(x)5,6F motif (302–313), containing a NKQ motif (310–312) that assists in maintaining structural integrity upon photopigment activation. Thus, unlike the situation for Opn4m2 and TMT opsin, there is no evidence for disruptive mutations affecting these two cavefish opsins (Cavallari et al., 2011).
Absorption spectra
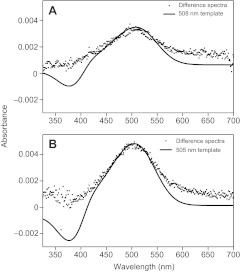
Both pigments were expressed in HEK293 cells, affinity purified and reconstituted with 11-cis retinaldehyde. UV-Vis spectroscopy of the reconstituted pigments revealed absorbance in the visible range for each pigment that was lost in favour of enhanced UV absorption following bright light exposure, indicating that both the rod and exo-rod pigments could be photo-bleached. The spectral sensitivity of these A1 photopigments was defined by fitting the dark-bleach difference spectra with the Govardovskii et al. (Govardovskii et al., 2000) opsin absorbance template (Fig. 2). The λmax predicted for P. andruzzii rod pigment is 508 nm (R2=0.97), whereas that of the exo-rod pigment is 505 nm (R2=0.99). The λmax values we report for both of the P. andruzzii pigments are slightly longer than their D. rerio counterparts: rod λmax is ~502 nm and exo-rod λmax is ~498 nm (Tarttelin et al., 2011). Comparison of the primary structure of the two P. andruzzii opsins reveals that a single A124G substitution is likely to account for the 3 nm difference observed between the two photopigments (Hunt et al., 2001).
Fig. 2.
Dark-bleach difference spectrum for P. andruzzii (A) rod and (B) exo-rod opsin photopigments fitted with A1 template (Govardovskii et al., 2000) giving a rod pigment λmax of 508 nm and an exo-rod pigment λmax of 505 nm.
Negative phototaxis
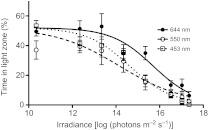
In our aquarium system designed for assaying negative phototactic behaviour, at all wavelengths tested, the fish (N=10) showed a photophobic behaviour that significantly decreased in response to reduction in light intensity (RM one-way ANOVA, 644 nm: F8,72=16.01, P<0.0001; 550 nm: F8,72=11.78, P<0.0001; 453 nm: F8,72=24.84, P<0.0001; Fig. 3). Dunnett's post hoc tests showed that a significant photophobic behaviour was present at 1017–1016 quanta m−2 s−1 under the 644 nm monochromatic light. The threshold of sensitivity was lower for shorter wavelengths (453–550 nm: 1014–1013 quanta m−2 s−1 versus 644 nm: 1017–1016 quanta m−2 s−1).
Fig. 3.
Mean (±s.d.) percentage of time spent in the light zone in cavefish. Each fish was tested at eight light irradiances for each monochromatic light. In constant darkness conditions, the fish spent the same percentage of time in both zones of the aquarium system (paired Student's t-test, t9=0.76, P>0.4).
Comparing spectral sensitivity of reconstituted pigments and phototaxis
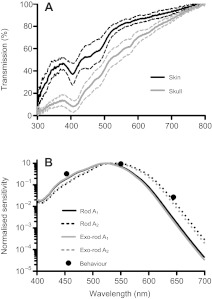
In an attempt to determine whether either rod and/or exo-rod opsin photopigments could subserve the phototactic behaviour of this species, we next undertook a quantitative comparison of spectral sensitivity data for these pigments with that of the behavioural response. As both sequences were cloned from brain cDNA, their spectral sensitivity in vivo is influenced by the transmission properties of the overlying skin and skull. We therefore measured the spectral transmission properties for both the skin and the skull over the range of 300–800 nm (Fig. 4A). This revealed a positive correlation between transmission and wavelength, with a marked dip around 400 nm, corresponding to the absorption of oxygenated haemoglobin.
Fig. 4.
(A) Spectral transmission properties of cavefish skin and skull over the range 300–800 nm. A positive correlation between transmission and wavelength was found, with a marked dip around 400 nm, corresponding to the absorption of oxygenated haemoglobin. (B) Absorbance spectra for rod and exo-rod opsins corrected for predicted pre-receptoral filtering by the skin and skull. The spectra for the A1 pigments are derived from the measured absorbance of these pigments in vitro when reconstituted with 11-cis retinal (Fig. 2). The A2 spectra are predictions based upon the method of Parry and Bowmaker (Parry and Bowmaker, 2000). The relative sensitivities of the phototactic response at the three wavelengths tested (644, 550 and 453 nm) are shown as filled circles. Those points depict the irradiance required to induce a half-maximal phototactic response (EC50) at the wavelength of peak sensitivity (550 nm) divided by the EC50 at the test wavelength.
When the spectral sensitivity profiles for both rod and exo-rod opsins, calculated by in vitro spectroscopy, were corrected for the summed absorption from the skin and the skull, their λmax shifted to longer wavelengths (521 and 520 nm, respectively; Fig. 4B). To determine whether these corrected spectra are consistent with the observed behavioural responses to 644, 550 and 453 nm, we calculated the effective irradiance of each of the stimuli presented in the behavioural experiment for each pigment. This was achieved by multiplying the applied irradiance by the relative sensitivity of the pigment to that wavelength. If the behavioural response was driven by either of these pigments, the degree of photophobia should reflect this corrected irradiance, irrespective of wavelength. We therefore determined whether the behavioural data measured across all three wavelengths could be fitted with a single curve following such corrections. We found that this was not the case when corrections were applied based upon the predicted sensitivity of either rod opsin or exo-rod opsin (F-test, P>0.05). This analysis therefore indicates that the spectral sensitivity of the phototactic response is not consistent with the predicted in vivo spectral response properties of either pigment.
Many freshwater fish have photopigments that utilise 11-cis 3-dehydroretinal either in addition to or instead of 11-cis retinal as the chromophore (Toyama et al., 2008). The λmax of a pigment using this so-called A2 chromophore (porphyropsin) is typically shifted to longer wavelengths than when it incorporates the corresponding A1 pigment (rhodopsin) (Parry et al., 2003). However, the native chromophore content for P. andruzzii is unknown. Thus, we assessed whether either rod or exo-rod opsins could explain the behavioural data if they used an A2 chromophore. Rather than empirically determining the absorbance properties of rod and exo-rod pigments reconstituted with A2, we used the method of Parry and Bowmaker (Parry and Bowmaker, 2000) to adjust the measured A1 absorption curves (Fig. 4B). The effect was to shift the predicted λmax of each pigment to longer wavelengths (λmax=528 and 524 nm for rod opsin and exo-rod opsin, respectively). These putative A2 absorbance spectra were corrected for skin/skull absorbance, which further shifted the λmax to 533 and 542 nm (Fig. 4B). We then determined whether correcting irradiances at each of the three behavioural assay wavelengths according to the predicted in vivo spectral sensitivity of these pigments would enable those data to be fit by a single curve. The irradiance–response relationships at the three wavelengths could be fit with a single curve (F-test, P>0.05) following corrections appropriate to either A2 pigment (although the F-statistic was lowest for the exo-rod pigment). We therefore conclude that the A2 versions of either or both of these pigments could underlie the observed behavioural spectral sensitivity.
DISCUSSION
Despite exhibiting complete anophthalmia (Berti et al., 2001), P. andruzzii shows a clear photophobic behaviour. Negative phototaxis is one of the factors proposed as promoting the colonization of hypogean habitats by surface-dwelling teleost fish (Langecker, 1992). Indeed, photophobic behaviour has been reported in several cavefish, such as the Mexican cave tetras, Astyanax mexicanus (Langecker, 1989), the Arabian barb, Garra barreimiae (Timmermann and Plath, 2009), and the African cyprinids Caecobarbus geertsii (Thinès, 1953; Thinès, 1955) and Barbopsis devecchii (Ercolini and Berti, 1978). We have shown that the threshold of the photophobic response in P. andruzzii changes as a function of wavelength, being highest at the longest wavelength tested (644 nm). Indeed, the threshold for a significant response was three orders of magnitude higher for this long wavelength (1017–1016 quanta m−2 s−1) compared with short wavelength stimuli (1014–1013 quanta m−2 s−1). Previous studies in other troglobiont fish did not describe a tendency for a maximum sensitivity to distinct wavelengths (Langecker, 1992). However, many investigations did not equalize the irradiances of the different monochromatic light sources used, making it difficult to compare spectral sensitivity among different species.
The threshold of encephalic photoreception has been intensively investigated in eyed fish. For instance, the cyprinids Phoxinus phoxinus and Couesius plumbeus react behaviourally to light stimuli of 1013–1012 quanta m−2 s−1 within a spectral range of 530–742 nm (de la Motte, 1964; Kavaliers, 1980). Thus, the thresholds that we have reported in P. andruzzii are consistent with a hypothesis where encephalic photoreception represents the basis for negative phototaxis.
It has been shown that epigean morphs of A. mexicanus possess an intact rod opsin gene (Yokoyama et al., 1995), and microspectrophotometric study of F2 individuals derived from a hypogean × epigean background suggests that intact rod and exo-rod opsin genes are present in the hypogean genome of this species (Parry et al., 2003). In an attempt to elucidate the mechanism that underlies the observed encephalic photoreception in P. andruzzii, we have successfully cloned, functionally expressed and reconstituted two opsins (rod and exo-rod) from this purely hypogean teleost. Our ability to express, reconstitute and photo-bleach the resultant pigments, coupled with the high levels of sequence conservation and an apparent absence of significant substitutions at key transduction interaction motifs, suggests that both opsins are likely to be functional. The A1 λmax values that we have measured for both the rod and exo-rod photopigments are typical of what might be expected for a freshwater fish utilizing the 11-cis retinal chromophore (Tarttelin et al., 2011). However, we predict that the incorporation of the A2 chromophore 11-cis 3-dehydroretinal as a porphyropsin will red-shift the λmax for both pigments (Parry et al., 2003).
The rod and exo-rod opsin cDNA sequences were both derived from brain tissue. Although we have not determined the specific sites of expression of these two opsins in the brain of P. andruzzii, the presence of both is perhaps not unexpected given that immunolabelling with CERN-886 antibody has shown ‘rod opsin’ expression in both the pineal gland and habenula of the European minnow, P. phoxinus (Alvarez-Viejo et al., 2004). Expression of rod and exo-rod opsin in the pineal gland is variable amongst teleost species. The pineal glands of D. rerio (Mano et al., 1999), Salmo salar (Philp et al., 2000a) and Plecoglossus altivelis (Minamoto and Shimizu, 2003) express only exo-rod opsin, whereas that of Conger myriaster (Zhang et al., 2002) expresses rod opsin. Although the pineal gland of P. altivelis only expresses exo-rod opsin, RT-PCR has shown rod opsin to be expressed in the telencephalon and diencephalon (Masuda et al., 2003). Though the light-detecting function of the P. andruzzii pineal gland remains unclear, preliminary neuroanatomical observations (C.B. and E.F., unpublished) have revealed that this pineal gland is reduced in size compared with that of other teleosts.
Our data cannot exclude the possibility that additional encephalic photopigments such as VA-opsin (Philp et al., 2000b) or one of the various forms of melanopsin (Drivenes et al., 2003; Davies et al., 2011) govern photobehavioural responses in P. andruzzii. However, a comparison of our molecular and behavioural data indicates that rod and exo-rod photopigments with A2 as a chromophore could be responsible for negative phototaxis. Interestingly, P. andruzzii is sympatric with the isopod Acanthastenasellus forficuloides (Chelazzi and Messana, 1985) and the amphipod Afridiella pectinicauda (Ruffo, 1982). Phreatichthys andruzzii is a likely predator of both species and thus it is tempting to speculate that these crustaceans might serve as a natural dietary source of vitamin A2 (Suzuki et al., 1984).
ACKNOWLEDGEMENTS
We are grateful to Ron H. Douglas (City University London, UK) for the spectral transmission assay of the skull and skin. We also thank Andrea Margutti for technical assistance.
FOOTNOTES
FUNDING
This work was supported by funding from the University of Ferrara [to C.B.], the Wellcome Trust [grant number WT078808 to R.J.L. and J.B.], the Karlsruhe Institute of Technology [to N.S.F.], and the VIGONI program of the Deutscher Akademischer Austausch Dienst and the Ateneo Italo Tedesco – Ministero Istruzione Universitá Ricerca [to C.B. and N.S.F.]. J.B. is grateful for support from the National Institute for Health Research Manchester Biomedical Research Centre and the Manchester Academic Health Science Centre. Deposited in PMC for release after 6 months.
REFERENCES
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403-410 [DOI] [PubMed] [Google Scholar]
- Alvarez-Viejo M., Cernuda-Cernuda R., Alvarez-López C., García-Fernández J. M. (2004). Identification of extraretinal photoreceptors in the teleost Phoxinus phoxinus. Histol. Histopathol. 19, 487-494 [DOI] [PubMed] [Google Scholar]
- Berti R., Durand J. P., Becchi S., Brizzi R., Keller N., Ruffat G. (2001). Eye degeneration in the blind cave-dwelling fish Phreatichthys andruzzii. Can. J. Zool. 79, 1278-1285 [Google Scholar]
- Bertolucci C., Foà A. (2004). Extraocular photoreception and circadian entrainment in nonmammalian vertebrates. Chronobiol. Int. 21, 501-519 [DOI] [PubMed] [Google Scholar]
- Cavallari N., Frigato E., Vallone D., Fröhlich N., Lopez-Olmeda J. F., Foà A., Berti R., Sánchez-Vázquez F. J., Bertolucci C., Foulkes N. S. (2011). A blind circadian clock in cavefish reveals that opsins mediate peripheral clock photoreception. PLoS Biol. 9, e1001142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L., Messana G. (1985). Acanthastenasellus forficuloides n. gen. n. sp., a stenasellid isopod (Asellota) from Somalian phreatic layer. Monit. Zool. Ital. 20, 43-54 [Google Scholar]
- Davies W. I., Zheng L., Hughes S., Tamai T. K., Turton M., Halford S., Foster R. G., Whitmore D., Hankins M. W. (2011). Functional diversity of melanopsins and their global expression in the teleost retina. Cell. Mol. Life Sci. 68, 4115-4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Motte I. (1964). Untersuchungen zur vergleichenden Physiologie der Lichtempfindlichkeit geblendeter Fische. Z. Vgl. Physiol. 49, 58-90 [Google Scholar]
- Drivenes Ø., Søviknes A. M., Ebbesson L. O. E., Fjose A., Seo H.-C., Helvik J. V. (2003). Isolation and characterization of two teleost melanopsin genes and their differential expression within the inner retina and brain. J. Comp. Neurol. 456, 84-93 [DOI] [PubMed] [Google Scholar]
- Ercolini A., Berti R. (1975). Light sensitivity experiments and morphology studies of the blind phreatic fish Phreatichthys andruzzii Vinciguerra from Somalia. Monit. Zool. Ital. 6, 29-43 [Google Scholar]
- Ercolini A., Berti R. (1978). Morphology and response to light of Barbopsis devecchii Di Caporiacco (Cyprinidae) microphthalmic phreatic fish from Somalia. Monit. Zool. Ital. 10, 299-314 [Google Scholar]
- Foster R. G., Grace M. S., Provencio I., Degrip W. J., Garcia-Fernandez J. M. (1994). Identification of vertebrate deep brain photoreceptors. Neurosci. Biobehav. Rev. 18, 541-546 [DOI] [PubMed] [Google Scholar]
- Govardovskii V. I., Fyhrquist N., Reuter T., Kuzmin D. G., Donner K. (2000). In search of the visual pigment template. Vis. Neurosci. 17, 509-528 [DOI] [PubMed] [Google Scholar]
- Hunt D. M., Dulai K. S., Partridge J. C., Cottrill P., Bowmaker J. K. (2001). The molecular basis for spectral tuning of rod visual pigments in deep-sea fish. J. Exp. Biol. 204, 3333-3344 [DOI] [PubMed] [Google Scholar]
- Kato S., Loeb N. G. (2003). Twilight irradiance reflected by the earth estimated from Clouds and the Earth's Radiant Energy System (CERES) measurements. J. Clim. 16, 2646-2650 [Google Scholar]
- Kavaliers M. (1980). Retinal and extraretinal entrainment action spectra for the activity rhythms of the lake chub, Couesius plumbeus. Behav. Neural Biol. 30, 56-67 [DOI] [PubMed] [Google Scholar]
- Langecker T. G. (1989). Studies on the light reaction of epigean and cave populations of Astyanax fasciatus (Characidae, Pisces). Mem. Biospeol. 16, 169-176 [Google Scholar]
- Langecker T. G. (1992) Light sensitivity of cave vertebrates – behavioral and morphological aspects. In The Natural History of Biospeleology (ed. Camachio A. I.), pp. 295-326 Madrid, Spain: Museo Nacional de Ciencias Naturales; [Google Scholar]
- Lythgoe J. N. (1988) Light and vision in the aquatic environment. In Sensory Biology of Aquatic Animals (ed. Atema J., Fay R. R., Popper A. N., Tavolga W. N.), pp. 57-82 New York: Springer-Verlag; [Google Scholar]
- Mano H., Kojima D., Fukada Y. (1999). Exo-rhodopsin: a novel rhodopsin expressed in the zebrafish pineal gland. Brain Res. Mol. Brain Res. 73, 110-118 [DOI] [PubMed] [Google Scholar]
- Masuda T., Iigo M., Mizusawa K., Aida K. (2003). Retina-type rhodopsin gene expressed in the brain of a teleost, ayu (Plecoglossus altivelis). Zoolog. Sci. 20, 989-997 [DOI] [PubMed] [Google Scholar]
- Minamoto T., Shimizu I. (2003). Molecular cloning and characterization of rhodopsin in a teleost (Plecoglossus altivelis, Osmeridae). Comp. Biochem. Physiol. 134B, 559-570 [DOI] [PubMed] [Google Scholar]
- Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., et al. (2000). Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739-745 [DOI] [PubMed] [Google Scholar]
- Parry J. W., Bowmaker J. K. (2000). Visual pigment reconstitution in intact goldfish retina using synthetic retinaldehyde isomers. Vision Res. 40, 2241-2247 [DOI] [PubMed] [Google Scholar]
- Parry J. W., Peirson S. N., Wilkens H., Bowmaker J. K. (2003). Multiple photopigments from the Mexican blind cavefish, Astyanax fasciatus: a microspectrophotometric study. Vision Res. 43, 31-41 [DOI] [PubMed] [Google Scholar]
- Pasqualetti M., Bertolucci C., Ori M., Innocenti A., Magnone M. C., De Grip W. J., Nardi I., Foà A. (2003). Identification of circadian brain photoreceptors mediating photic entrainment of behavioural rhythms in lizards. Eur. J. Neurosci. 18, 364-372 [DOI] [PubMed] [Google Scholar]
- Peirson S. N., Halford S., Foster R. G. (2009). The evolution of irradiance detection: melanopsin and the non-visual opsins. Philos. Trans. R. Soc. B 364, 2849-2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp A. R., Bellingham J., Garcia-Fernandez J., Foster R. G. (2000a). A novel rod-like opsin isolated from the extra-retinal photoreceptors of teleost fish. FEBS Lett. 468, 181-188 [DOI] [PubMed] [Google Scholar]
- Philp A. R., Garcia-Fernandez J. M., Soni B. G., Lucas R. J., Bellingham J., Foster R. G. (2000b). Vertebrate ancient (VA) opsin and extraretinal photoreception in the Atlantic salmon (Salmo salar). J. Exp. Biol. 203, 1925-1936 [DOI] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365-386 [DOI] [PubMed] [Google Scholar]
- Ruffo S. (1982). Studi sui Crostacei Anfipodi. 92. Nuovi Anfipodi di acque sotterranee della Somalia. Monit. Zool. Ital. 3, 97-113 [Google Scholar]
- Scharrer E. (1928). Die Lichtempfindlichkeit Blinder Elritzen. (Untersuchungen Über das Zwischenhirn der Fische I.) Z. Vgl. Physiol. 7, 1-38 [Google Scholar]
- Shand J., Foster R. G. (1999) The extraretinal photoreceptors of non-mammalian vertebrates. In Adaptive Mechanisms in the Ecology of Vision (ed. Archer S., Djamgoz M., Loew E.), pp. 197-222 Dordrecht: Kluwer Academic Publishers; [Google Scholar]
- Suzuki T., Makino-Tasaka M., Eguchi E. (1984). 3-Dehydroretinal (vitamin A2 aldehyde) in crayfish eye. Vision Res. 24, 783-787 [DOI] [PubMed] [Google Scholar]
- Tarttelin E. E., Fransen M. P., Edwards P. C., Hankins M. W., Schertler G. F., Vogel R., Lucas R. J., Bellingham J. (2011). Adaptation of pineal expressed teleost exo-rod opsin to non-image forming photoreception through enhanced Meta II decay. Cell. Mol. Life Sci. 68, 3713-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinès G. (1953) Recherches expérimentales sur la photosensibilité du poisson aveugle Caecobarbus geertsii Blgr. Ann. Soc. R. Zool. Belg. 84, 231-265 [Google Scholar]
- Thinès G. (1955). Étude comparative de la photosensibilité des poissons aveugles Caecobarbus geertsii Blgr et Anoptichthys jordani Hubbs et Innes. Ann. Soc. R. Zool. Belg. 85, 35-58 [Google Scholar]
- Timmermann M., Plath M. (2009). Phototactic response and light sensitivity in an epigean and a hypogean population of a barb (Garra barreimiae, Cyprinidae). Aquat. Ecol. 43, 539-547 [Google Scholar]
- Toyama M., Hironaka M., Yamahama Y., Horiguchi H., Tsukada O., Uto N., Ueno Y., Tokunaga F., Seno K., Hariyama T. (2008). Presence of rhodopsin and porphyropsin in the eyes of 164 fishes, representing marine, diadromous, coastal and freshwater species – a qualitative and comparative study. Photochem. Photobiol. 84, 996-1002 [DOI] [PubMed] [Google Scholar]
- Underwood H., Groos G. (1982). Vertebrate circadian rhythms: retinal and extraretinal photoreception. Experientia 38, 1013-1021 [DOI] [PubMed] [Google Scholar]
- von Frisch K. (1911). Beiträge zur Physiologie der Pigmentzellen in der Fischhaut. Pflugers Arch. 138, 319-387 [Google Scholar]
- Yokoyama R., Knox B. E., Yokoyama S. (1995). Rhodopsin from the fish, Astyanax: role of tyrosine 261 in the red shift. Invest. Ophthalmol. Vis. Sci. 36, 939-945 [PubMed] [Google Scholar]
- Zhang H., Futami K., Yamada Y., Horie N., Okamura A., Utoh T., Mikawa N., Tanaka S., Okamoto N., Oka H. P. (2002). Isolation of freshwater and deep-sea type opsin genes from the common Japanese conger. J. Fish Biol. 61, 313-324 [Google Scholar]