Abstract
BACKGROUND
Patients with tuberculosis (TB) often suffer from profound malnutrition.
OBJECTIVE
To examine the patterns and predictors of change in nutritional and hemoglobin status during and after TB treatment.
METHODS
A total of 471 HIV-positive and 416 HIV-negative adults with pulmonary TB were prospectively followed in Dar es Salaam, Tanzania. All patients received 8 months TB treatment following enrollment.
RESULTS
About 40% of HIV-positive and 47% of HIV-negative TB patients had BMI <18.5 kg/m2 at baseline. About 94% of HIV-positive and 84% of HIV-negative participants were anemic at baseline. Both HIV-positive and HIV-negative patients experienced increases in BMI and hemoglobin concentrations over the course of TB treatment. Among HIV-positive patients, older age, low CD4 cell counts, and high viral load were independently associated with a smaller increase in BMI from baseline to 8 months. Female sex, older age, low CD4 cell counts, previous TB infection, and less money spent on food were independently associated with a smaller improvement in hemoglobin among HIV-positive patients during treatment.
CONCLUSION
HIV- positive TB patients, especially those with low CD4 cell counts, showed poor nutritional recovery during TB treatment. Adequate nutritional support should be considered during TB treatment.
Keywords: tuberculosis, HIV, malnutrition, anemia
INTRODUCTION
Tuberculosis (TB) continues to be a major public health problem across the world. It is estimated that 9.4 million people develop active TB and 1.3 million people die from TB every year (1). TB is the leading cause of death among HIV-positive individuals in sub-Saharan Africa (2). Patients with TB often suffer from profound malnutrition, and co-infection with HIV may exacerbate the extent of malnutrition (3–6). Malnourished status, often measured as body mass index (BMI) < 18.5 kg/m2, has been associated with the severity of disease among TB patients (7, 8). Low BMI status is an independent predictor of mortality among TB patients, especially HIV/TB-co-infected patients (9–11). Moreover, patients with poor weight gains during TB treatment are at an increased risk of treatment failure and relapse (12, 13). Anemia is also a highly common nutritional complication among TB patients and a strong risk factor for mortality (11). A recent study from India reported that malnutrition and anemia were extremely prevalent among HIV-positive TB patients even after the completion of TB treatment (5). In spite of the known detrimental effects of malnutrition, few studies have longitudinally examined nutritional status during TB treatment in the context of HIV infection (14, 15).
The aim of our study was to investigate baseline predictors of change in BMI and hemoglobin concentrations during and after TB treatment in a cohort of HIV-positive and HIV-negative patients with pulmonary TB in Tanzania. The current observational study was conducted among patients participating in a micronutrient supplementation trial. In the trial, micronutrient supplementation significantly reduced the risk of early TB recurrences; however, supplementation showed no overall effects on BMI and hemoglobin status (16).
METHODS
Study population and procedures
The study population consisted of 887 patients with pulmonary TB who enrolled in a clinical trial that examined the effect of micronutrient supplements on TB treatment outcomes, mortality, morbidity and nutritional status (16). Participants were recruited from five outpatient TB clinics in Dar es Salaam, Tanzania between April 2000 and April 2005. Eligible participants were men and non-pregnant women aged 18 to 65 years who had at least two positive sputum smears for acid-fast bacilli, had not received TB treatment during the previous year, and intended to stay in Dar es Salaam for 2 years. We excluded any patients with a Karnofsky performance score < 40% or hemoglobin concentration of < 7 g/dL from the trial. Within strata of HIV status, participants were randomly assigned to receive a daily dose of micronutrients (vitamins A, B-complex, C, and E, and selenium) or placebo. Immediately following enrollment, all patients received 8 months of directly observed, short course (DOTS) anti-TB treatment in accordance with the Tanzania National TB and Leprosy Programme at the time of the study. Patients received a daily combination of rifampicin, isoniazid, pyrazinamide, and ethambutol at the health facility under direct observation of a health worker for the first 2 months. In the subsequent 6 months, patients self-administered isoniazid and ethambutol at home. Antiretroviral therapy was not available at the time of the study.
At the baseline visit, trained research nurses obtained information about demographic and socioeconomic characteristics. Study physicians performed a complete physical examination and, in HIV-positive patients, assessed the stage of HIV disease based on the World Health Organization (WHO) staging system (17). Trained nurses obtained anthropometric measurements at the baseline visit and during monthly visits. Height was measured to the nearest 0.1 cm (Seca Bodymeter 206 stadiometers) and weight was measured to the nearest 100 g (Seca 700 balance beam scales). BMI (kg/m2) was calculated as body weight divided by height squared. Blood specimens for hemoglobin measurements were obtained at baseline and follow-up visits at approximately 2, 8, 14, and 20 months.
All participants provided written informed consent. The study protocol was approved by the Institutional Review Boards at the Muhimbili University of Health and Allied Sciences, the Tanzanian National AIDS Control Program, and the Harvard School of Public Health.
Laboratory methods
HIV-1 infection was determined by two sequential ELISAs (Wellcozyme, Murex Biotech Ltd, Dartford, UK and Enzygnost anti-HIV1+2 Behring Marburg, Germany), and discrepant results were resolved by a Western blot test (Genetic Systems, Redmond, WA, USA). We measured CD4 T-lymphocyte cell counts using either the FACScount or FACSCAN systems (Becton-Dickinson, San Jose, CA, USA) and plasma viral load using the Amplicor HIV-1 monitor test version 1.5 assay (Roche Diagnostics Corp., Indianapolis, IN, USA).
Statistical analysis
We used linear regression models to examine the associations between baseline predictors and the average changes in BMI and hemoglobin concentrations from baseline to 8 months and from baseline to 14 months (18). A robust empirical variance structure was employed to overcome deviations from normality. Baseline predictors of interest were sociodemographic characteristics including sex, age, marital status, education, number of household assets (from a list that included sofa, television, radio, refrigerator, and fan), money spent on food per person per day, and frequency of purchasing meat including red meat, fish, or poultry. Predictors of interest also included clinical and laboratory measures, namely Karnofsky performance score, previous diagnosis with TB, the number of Mycobacterium tuberculosis bacilli per sputum smear field, and CD4 cell counts. Viral load and HIV clinical disease stage were also examined among HIV-positive patients. Multivariate regression models were built to estimate the adjusted difference in change in nutritional status. We analyzed separately by HIV status, because of differences in immunological profiles and possibly differential associations between baseline predictors and change in nutritional status. Predictors with p < 0.20 in univariate analyses were considered as candidates for the multivariate model, and predictors with p < 0.05 along with age, sex, treatment assignment, and other relevant factors were retained in the final multivariate model. We also examined change in BMI or hemoglobin status by baseline nutritional status in the univariate analysis (18). Malnutrition was defined as BMI < 18.5 kg/m2. Anemia was defined as hemoglobin < 13.0 g/dL for men and < 12.0 g/dL for women based on the WHO criteria (19). Severe anemia was defined as < 8.5 g/dL. We visually examined changes in BMI and hemoglobin levels by HIV status by fitting a restricted cubic splines model which provides flexible smooth curves (20). All statistical analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC, USA).
RESULTS
Of 887 patients with pulmonary TB, 471 were HIV-positive and 416 were HIV-negative (Table 1). The majority of participants were men (58% of HIV-positive and 76% of HIV-negative patients). The average age was 32 years (SD = 9) at baseline. About 40% of HIV-positive and 47% of HIV-negative TB patients were malnourished (BMI < 18.5 kg/m2) at the time of diagnosis. Almost all patients were anemic (94% of HIV-positive and 84% of HIV-negative patients). Most patients reported experiencing weight loss (81%), chronic cough (88%), and fever (63%). Among HIV-positive patients, 37% had CD4 counts < 200 cells/mm3 and 9% were in WHO HIV clinical disease stage 4. The number of patients with measurements at 8 and 14 months was 642 (72%) and 492 (55%) for anthropometry, and 537 (61%) and 427 (48%) for hemoglobin measurements.
Table 1.
Characteristics at the time of diagnosis with TB
| Baseline characteristics | HIV-positive patients
|
HIV-negative patients
|
||
|---|---|---|---|---|
| Men (n = 273) | Women (n = 198) | Men (n = 317) | Women (n = 99) | |
| Age (years) | 36.3 ± 8.51 | 31.0 ± 7.4 | 30.0 ± 8.7 | 29.9 ± 10.1 |
| Completed more than 5 years of primary education [n (%)] | 228 (83.5%) | 150 (75.8%) | 258 (81.4%) | 68 (68.6%) |
| Money spent on food (TSh)2, [median (IQR)] | 500 (333, 900) | 388 (250, 600) | 500 (286, 800) | 333 (200, 500) |
| Height (cm) | 166.9 ± 7.1 | 155.4 ± 6.5 | 166.9 ± 6.5 | 155.5 ± 6.5 |
| Weight (kg) | 53.5 ± 7.4 | 48.0 ± 9.4 | 52.2 ± 6.5 | 46.7 ± 8.1 |
| Body mass index (kg/m2) | 19.2 ± 2.3 | 19.8 ± 3.5 | 18.7 ± 2.1 | 19.3 ± 3.4 |
| Wasting3 [n (%)] | 109 (40.4%) | 76 (38.8%) | 151 (47.6%) | 46 (46.5%) |
| Mid-upper arm circumference (cm) | 23.4 ± 2.3 | 23.4 ± 3.1 | 23.1 ± 2.4 | 23.2 ± 3.5 |
| Hemoglobin (g/dL) | 10.3 ± 1.9 | 9.2 ± 1.5 | 11.3 ± 1.7 | 10.3 ± 1.3 |
| Anemia4 [n (%)] | 256 (93.8%) | 189 (95.5%) | 263 (83.0%) | 87 (87.9%) |
| Karnofsky score <70% [n (%)] | 36 (13.2%) | 32 (16.2%) | 20 (6.3%) | 11 (11.2%) |
| CD4 cell counts (/mm3) | 339 ± 240 | 299 ± 245 | 687 ± 247 | 764 ± 282 |
| Viral load (copies/mm3), [median (IQR)] | 61,800 (11,500, 207,000) | 53,700 (13,500, 179,000) | - | - |
Footnotes for Table 1.
Mean ± SD for such value, otherwise noted.
Household income spent on food per person per day in Tanzanian shillings (TSh).
Wasting was defined as BMI < 18.5 kg/m2.
Anemia was defined as hemoglobin <13.0 g/dL for men and <12.0 g/dL for women.
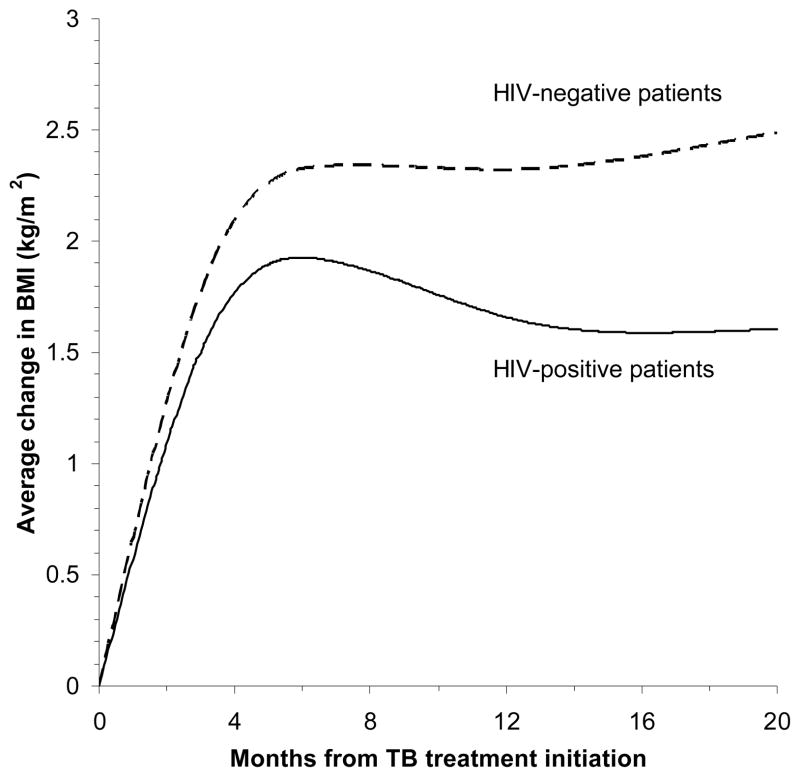
Among all participants, mean BMI gradually increased until around 6 months during TB treatment, and these gains persisted beyond treatment (Figure 1). On average, BMI increased by 2.0 kg/m2 among HIV-positive patients from baseline to 8 months, and by 2.7 kg/m2 among HIV-negative TB patients. At the end of treatment, 19% of HIV-positive patients and 12% of HIV-negative patients still had BMI < 18.5 kg/m2. During treatment, HIV-negative patients gained an average of 7.4 kg and HIV-positive patients gained an average of 5.3 kg. Among HIV-positive patients, older age, low CD4 cell counts, and high viral load were significantly associated with a smaller increase in BMI from baseline to 8 months in the multivariate model (Table 2). In univariate analysis, HIV-positive patients with BMI <18.5 kg/m2 at baseline tended to show a greater increase in BMI from over the 8 month treatment period, compared those who had BMI ≥18.5 kg/m2 (crude difference = 0.4 kg/m2, 95% CI = −0.1, 0.8; p = 0.06). Socioeconomic status and indicators of TB severity were not associated with the change in BMI during and after TB treatment among HIV-positive patients. Among HIV-negative patients, low Karnofsky performance score (< 70%) was significantly associated with greater increase in BMI from baseline to 8 months. However, all other variables were not associated with change in BMI (Table 3).
Figure 1.
Change in BMI by HIV status from TB treatment initiation. The curves were adjusted for age, sex, and CD4 cell counts. The average increase in BMI from baseline to 8 months was lower among HIV-positive patients than among HIV-negative patients (adjusted difference = −0.5 kg/m2; 95% CI = −0.8, −0.2 kg/m2; p = 0.004).
Table 2.
Baseline predictors of change in BMI (kg/m2) among HIV-positive TB patients from initiation to end of TB treatment (8 months) and post-treatment (14 months after baseline).
| Baseline predictors | N (%) | BMI at baseline1 | Change in BMI from 0 to 8 months (n = 328)
|
Change in BMI from 0 to 14 months (n = 241)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change from 0 to 8 months | Adjusted difference (95% CI)2 | p | p, test for trend3 | Change from 0 to 14 months | Adjusted difference (95% CI)2 | p | p, test for trend3 | |||
| Sex | ||||||||||
| Male | 273 (58.0%) | 19.2 ± 0.1 | 1.9 ± 0.1 | Reference | 1.8 ± 0.2 | Reference | ||||
| Female | 198 (42.0%) | 19.8 ± 0.3 | 2.1 ± 0.2 | 0.1 (−0.3, 0.5) | 0.67 | 2.2 ± 0.2 | 0.3 (−0.2, 0.7) | 0.30 | ||
| Age | 0.02 | 0.04 | ||||||||
| < 30 years | 158 (33.6%) | 19.2 ± 0.2 | 2.3 ± 0.2 | Reference | 2.3 ± 0.2 | Reference | ||||
| 30 – 39 years | 200 (42.5%) | 19.4 ± 0.2 | 1.9 ± 0.2 | −0.5 (−0.9, 0.0) | 0.06 | 2.0 ± 0.2 | −0.2 (−0.8, 0.4) | 0.52 | ||
| ≥ 40 years | 113 (24.0%) | 19.8 ± 0.3 | 1.8 ± 0.2 | −0.7 (−1.3, −0.2) | 0.01 | 1.5 ± 0.2 | −0.7 (−1.4, −0.1) | 0.02 | ||
| Marital status | ||||||||||
| Married | 230 (48.8%) | 19.7 ± 0.2 | 1.9 ± 0.2 | Reference | 1.7 ± 0.2 | Reference | ||||
| Cohabiting | 37 (7.9%) | 19.4 ± 0.4 | 1.6 ± 0.3 | −0.5 (−1.2, 0.3) | 0.22 | 2.1 ± 0.3 | 0.2 (−0.6, 0.9) | 0.62 | ||
| Single | 144 (30.6%) | 19.2 ± 0.2 | 2.0 ± 0.2 | −0.2 (−0.7, 0.3) | 0.52 | 2.1 ± 0.3 | 0.1 (−0.6, 0.7) | 0.83 | ||
| Divorced/widowed | 60 (12.7%) | 19.1 ± 0.4 | 2.9 ± 0.3 | 1.1 (0.4, 1.7) | 0.001 | 2.6 ± 0.3 | 0.8 (0.2, 1.5) | 0.01 | ||
| CD4 count | 0.009 | 0.09 | ||||||||
| < 100 cells/mm3 | 69 (18.6%) | 19.7 ± 0.4 | 1.5 ± 0.4 | −0.9 (−1.7, 0.0) | 0.06 | 1.8 ± 0.3 | −0.5 (−1.3, 0.2) | 0.18 | ||
| 100 – 200 cells/mm3 | 70 (18.8%) | 19.4 ± 0.4 | 1.6 ± 0.3 | −0.7 (−1.2, −0.1) | 0.02 | 1.7 ± 0.3 | −0.4 (−1.1, 0.3) | 0.22 | ||
| 200 – 350 cells/mm3 | 89 (23.9%) | 19.0 ± 0.3 | 2.0 ± 0.3 | −0.3 (−0.9, 0.2) | 0.26 | 2.0 ± 0.3 | −0.3 (−1.0, 0.4) | 0.43 | ||
| ≥ 350 cells/mm3 | 144 (38.7%) | 19.6 ± 0.2 | 2.4 ± 0.2 | Reference | 2.2 ± 0.2 | Reference | ||||
| Viral load | 0.004 | 0.08 | ||||||||
| ≥ 100,000 copies/mm3 | 146 (38.4%) | 19.2 ± 0.2 | 1.5 ± 0.2 | −1.1 (−1.6, −0.5) | <0.001 | 1.4 ± 0.2 | −0.9 (−1.6, −0.2) | 0.01 | ||
| 10,000 – 100,000 copies/mm3 | 149 (39.2%) | 19.5 ± 0.2 | 2.1 ± 0.2 | −0.4 (−1.0, 0.2) | 0.15 | 2.1 ± 0.2 | −0.2 (−0.9, 0.5) | 0.57 | ||
| < 10,000 copies/mm3 | 85 (22.4%) | 19.7 ± 0.3 | 2.7 ± 0.2 | Reference | 2.4 ± 0.3 | Reference | ||||
| HIV disease stage | ||||||||||
| Stage 3 | 313 (91.0%) | 19.6 ± 0.2 | 1.9 ± 0.1 | Reference | 1.9 ± 0.1 | Reference | ||||
| Stage 4 | 31 (9.0%) | 18.6 ± 0.5 | 1.6 ± 0.5 | −0.2 (−1.1, 0.7) | 0.60 | 2.0 ± 0.4 | 0.3 (−0.4, 1.3) | 0.43 | ||
Footnotes for Table 2.
Mean ± SE for all such value.
Adjusted differences in change in BMI were estimated from the multivariate linear regression model with robust empirical variance that includes sex, age, marital status, CD4 cell counts, HIV disease stage, and treatment assignment. Estimates for viral load were obtained from separate multivariate model. Education level, number of household assets, money spent on food per day, frequency of meat purchase, density of acid-fast bacilli in the smear, Karnofsky performance score, and previous diagnosis with TB were not associated with change in BMI, and thus, they were not included in the multivariate model.
P-value for test for trend was obtained from analyzing median values within quartiles.
Table 3.
Baseline predictors of change in BMI (kg/m2) among HIV-negative TB patients from initiation to end of TB treatment (8 months) and post-treatment (14 months after baseline).
| Baseline predictors | N (%) | BMI at baseline1 | Change in BMI from 0 to 8 months (n = 314)
|
Change in BMI from 0 to 14 months (n = 252)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change from 0 to 8 months | Adjusted difference (95% CI)2 | p | p, test for trend3 | Change from 0 to 14 months | Adjusted difference (95% CI)2 | p | p, test for trend3 | |||
| Sex | ||||||||||
| Male | 317 (76.2%) | 18.7 ± 0.1 | 2.7 ± 0.1 | Reference | 2.5 ± 0.1 | Reference | ||||
| Female | 99 (23.8%) | 19.4 ± 0.3 | 3.0 ± 0.2 | 0.3 (−0.2, 0.8) | 0.22 | 3.3 ± 0.3 | 0.8 (0.1, 1.5) | 0.02 | ||
| Age | 0.79 | 0.03 | ||||||||
| < 30 years | 241 (57.9%) | 18.7 ± 0.2 | 2.8 ± 0.1 | Reference | 2.6 ± 0.2 | Reference | ||||
| 30 – 39 years | 112 (26.9%) | 19.2 ± 0.2 | 2.7 ± 0.2 | −0.2 (−0.7, 0.6) | 0.38 | 2.4 ± 0.3 | −0.5 (−1.2, 0.2) | 0.17 | ||
| ≥ 40 years | 63(15.1%) | 19.2 ± 0.4 | 2.8 ± 0.3 | −0.1 (−0.7, 0.6) | 0.86 | 3.4 ± 0.3 | 0.6 (−0.2, 1.4) | 0.12 | ||
| Marital status | ||||||||||
| Married | 179 (43.0%) | 19.1 ± 0.2 | 2.9 ± 0.2 | Reference | 3.1 ± 0.2 | Reference | ||||
| Cohabiting | 29 (7.0%) | 19.5 ± 0.5 | 3.0 ± 0.3 | 0.0 (−0.7, 0.7) | 0.96 | 2.4 ± 0.7 | −0.6 (−2.4, 1.1) | 0.48 | ||
| Single | 175 (42.1%) | 18.5 ± 0.2 | 2.6 ± 0.2 | −0.4 (−0.9, 0.1) | 0.13 | 2.3 ± 0.2 | −0.8 (−1.4, −0.2) | 0.01 | ||
| Divorced/widowed | 33 (7.9%) | 19.2 ± 0.5 | 2.2 ± 0.4 | −0.8 (−1.6, 0.1) | 0.07 | 2.4 ± 0.7 | −0.6 (−2.4, 1.1) | 0.12 | ||
| CD4 count | 0.22 | 0.08 | ||||||||
| < 750 cells/mm3 | 236 (64.0%) | 19.0 ± 0.2 | 2.8 ± 0.1 | 0.3 (−0.4, 0.8) | 0.19 | 3.0 ± 0.2 | 0.6 (0.0, 1.2) | 0.06 | ||
| ≥ 750 cells/mm3 | 133 (36.0%) | 18.8 ± 0.2 | 2.6 ± 0.2 | Reference | 2.3 ± 0.3 | Reference | ||||
| Karnofsky performance score | 0.02 | 0.04 | ||||||||
| ≥ 70% | 384 (92.5%) | 19.0 ± 0.1 | 2.7 ± 0.1 | Reference | 2.6 ± 0.1 | Reference | ||||
| < 70% | 31 (7.5%) | 17.1 ± 0.3 | 3.3 ± 0.3 | 0.5 (0.0, 1.1) | 0.05 | 3.6 ± 0.5 | 1.1 (0.1, 2.0) | 0.03 | ||
Footnotes for Table 3.
Mean ± SE for all such value.
Adjusted differences in change in BMI were estimated from the multivariate linear regression model that includes sex, age, marital status, CD4 cell counts, Karnofsky performance score, and treatment assignment. Other variables were not associated with change in BMI in univariate analysis (p> 0.20).
P-value for test for trend was obtained from median values within quartiles. Karofsky score was entered as a continuous variable.
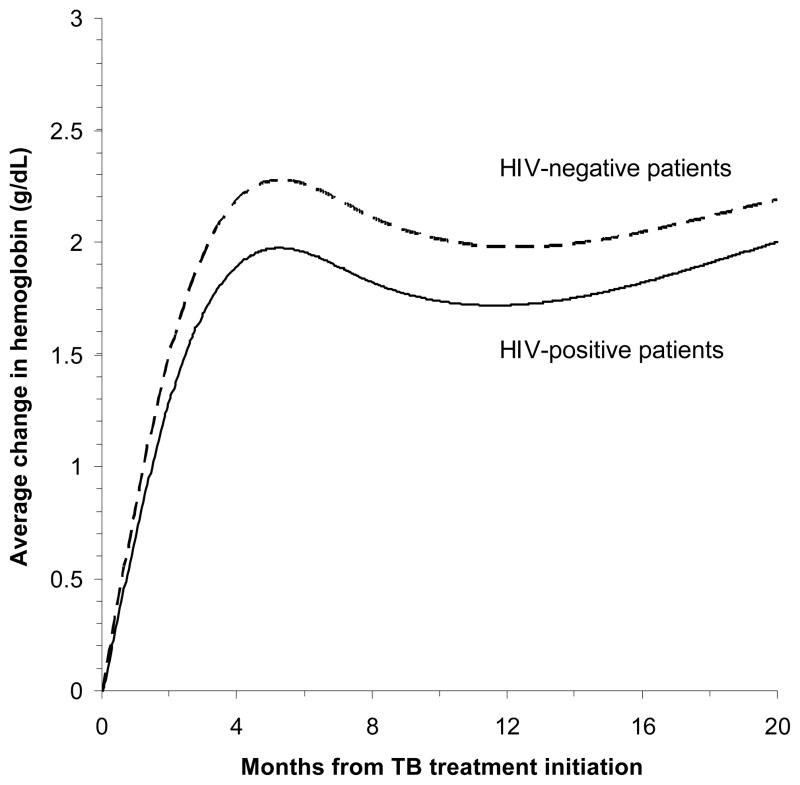
Both HIV-positive and HIV-negative patients showed a rapid rise in hemoglobin levels from baseline to 8 months (increase by 1.7 g/dL vs. 2.3 g/dL, respectively; Figure 2). However, 71% of HIV-positive patients and 31% of HIV-negative patients still remained anemic at the end of 8 month treatment. Among HIV-positive patients, female sex, older age, low CD4 cell counts, advanced HIV clinical stage, previous TB infection, and less money spent on food per person were significantly associated with a smaller increase in hemoglobin from baseline to 8 months in the multivariate model (Table 4). HIV-positive patients with severe anemia (< 8.5 g/dL) at baseline showed a larger increase in hemoglobin than HIV-positive patients who had hemoglobin ≥ 8.5 g/dL (crude difference = 2.0 g/dL, 95% CI = 1.4, 2.5; p < 0.001). Among HIV-negative patients, female sex was the only variable associated with smaller increases in hemoglobin concentrations during and after TB treatment (Table 5).
Figure 2.
Change in hemoglobin concentrations by HIV status from TB treatment initiation. The curves were adjusted for age, sex, and CD4 cell counts. HIV infection was not associated with a change in hemoglobin concentration from baseline to 8 months (adjusted difference = −0.1 g/dL; 95% CI = −0.5, 0.2 g/dL; p = 0.44).
Table 4.
Baseline predictors of change in hemoglobin levels (g/dL) among HIV-positive TB patients from initiation to end of TB treatment (8 months) and post-treatment (14 months after baseline).
| Baseline predictors | N (%) | HB at baseline1 | Change in hemoglobin from 0 to 8 months (n = 272)
|
Change in hemoglobin from 0 to 14 months (n = 214)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change from 0 to 8 months | Adjusted difference (95% CI)2 | p | p, test for trend3 | Change from 0 to 14 months | Adjusted difference (95% CI)2 | p | p, test for trend3 | |||
| Sex | ||||||||||
| Male | 273 (58.0%) | 10.3 ± 0.1 | 2.0 ± 0.2 | Reference | 2.1 ± 0.2 | Reference | ||||
| Female | 198 (42.0%) | 9.2 ± 0.1 | 1.3 ± 0.2 | −1.0 (−1.5, −0.5) | <0.001 | 1.5 ± 0.2 | −1.0 (−1.5, −0.5) | <0.001 | ||
| Age | 0.005 | <0.001 | ||||||||
| < 30 years | 158 (33.6%) | 9.6 ± 0.1 | 1.9 ± 0.2 | Reference | 2.3 ± 0.3 | Reference | ||||
| 30 – 39 years | 200 (42.5%) | 9.8 ± 0.1 | 1.8 ± 0.2 | −0.3 (−0.8, 0.2) | 0.23 | 1.9 ± 0.2 | −0.6 (−1.2, 0.0) | 0.07 | ||
| ≥ 40 years | 113 (24.0%) | 10.3 ± 0.2 | 1.4 ± 0.3 | −0.8 (−1.5, −0.1) | 0.03 | 1.1 ± 0.3 | −1.4 (−2.1, −0.7) | <0.001 | ||
| Marital status | ||||||||||
| Married | 230 (48.8%) | 10.0 ± 0.1 | 1.6 ± 0.2 | Reference | 1.7 ± 0.2 | Reference | ||||
| Cohabiting | 37 (7.9%) | 10.0 ± 0.3 | 1.6 ± 0.4 | 0.0 (−0.9, 0.8) | 0.94 | 1.8 ± 0.5 | 0.2 (−0.7, 1.0) | 0.72 | ||
| Single | 144 (30.6%) | 9.5 ± 0.2 | 2.1 ± 0.2 | 0.4 (−0.2, 1.0) | 0.23 | 2.2 ± 0.3 | 0.3 (−0.4, 1.0) | 0.35 | ||
| Divorced/widowed | 60 (12.7%) | 9.9 ± 0.3 | 1.6 ± 0.4 | 0.3 (−0.5, 1.2) | 0.42 | 1.7 ± 0.4 | 0.6 (−0.3, 1.5) | 0.21 | ||
| CD4 count | 0.16 | 0.50 | ||||||||
| < 100 cells/mm3 | 69 (18.6%) | 9.2 ± 0.2 | 1.7 ± 0.4 | 0.1 (−0.7, 1.0) | 0.78 | 1.6 ± 0.4 | −0.1 (−0.9, 0.6) | 0.70 | ||
| 100 – 200 cells/mm3 | 70 (18.8%) | 9.9 ± 0.2 | 0.8 ± 0.4 | −1.1 (−1.9, −0.4) | 0.004 | 1.3 ± 0.3 | −0.4 (−1.1, 0.4) | 0.32 | ||
| 200 – 350 cells/mm3 | 89 (23.9%) | 9.8 ± 0.2 | 1.6 ± 0.3 | −0.3 (−0.9, 0.4) | 0.38 | 1.5 ± 0.3 | −0.4 (−1.2, 0.4) | 0.32 | ||
| ≥ 350 cells/mm3 | 144 (38.7%) | 10.2 ± 0.2 | 2.1 ± 0.2 | Reference | 2.0 ± 0.2 | Reference | ||||
| HIV clinical stage | ||||||||||
| Stage 3 | 313 (91.0%) | 9.9 ± 0.1 | 1.8 ± 0.2 | Reference | 1.9 ± 0.2 | Reference | ||||
| Stage 4 | 31 (9.0%) | 9.5 ± 0.3 | 0.9 ± 0.6 | −0.9 (−2.0, 0.1) | 0.07 | 1.6 ± 0.4 | −0.3 (−1.2, 0.6) | 0.50 | ||
| Previous TB infection | ||||||||||
| Yes | 233 (49.6%) | 10.0 ± 0.1 | 1.5 ± 0.2 | −0.5 (−1.0, 0.0) | 0.03 | 1.8 ± 0.2 | −0.1 (−0.6, 0.5) | 0.89 | ||
| No | 237 (50.4%) | 9.6 ± 0.1 | 2.0 ± 0.2 | Reference | 1.9 ± 0.2 | Reference | ||||
| Money spent on food | 0.05 | 0.58 | ||||||||
| < 250 TSh | 97 (22.9%) | 9.6 ± 0.2 | 1.4 ± 0.3 | −0.6 (−1.3, 0.1) | 0.07 | 1.5 ± 0.2 | −0.5 (−1.1, 0.2) | 0.15 | ||
| 250 – 500 TSh | 113 (26.7%) | 9.9 ± 0.2 | 1.6 ± 0.2 | −0.2 (−0.7, 0.4) | 0.52 | 2.0 ± 0.3 | 0.0 (−0.4, 0.8) | 0.54 | ||
| ≥ 500 TSh | 214 (50.5%) | 10.0 ± 0.1 | 1.9 ± 0.2 | Reference | 1.8 ± 0.2 | Reference | ||||
Footnotes for Table 4.
Mean ± SE for all such value.
Adjusted differences in change in hemoglobin were estimated from the multivariate linear regression model that includes sex, age, marital status, CD4 cell counts, HIV clinical stage, previous TB infection, money spent on food, and treatment assignment. Other variables were not associated with change in hemoglobin.
P-value for test for trend was obtained from median values within quartiles.
Table 5.
Baseline predictors of change in hemoglobin levels (g/dL) among HIV-negative TB patients from initiation to end of TB treatment (8 months) and post-treatment (14 months after baseline).
| Baseline predictors | N (%) | HB at baseline1 | Change in hemoglobin from 0 to 8 months (n = 267)
|
Change in hemoglobin from 0 to 14 months (n = 213)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change from 0 to 8 months | Adjusted difference (95% CI)2 | p | p, test for trend3 | Change from 0 to 14 months | Adjusted difference (95% CI)2 | p | p, test for trend3 | |||
| Sex | ||||||||||
| Male | 317 (76.2%) | 11.3 ± 0.1 | 2.5 ± 0.1 | Reference | 2.3 ± 0.1 | Reference | ||||
| Female | 99 (23.8%) | 10.3 ± 0.1 | 1.6 ± 0.2 | −1.0 (−1.4, −0.5) | <0.001 | 1.4 ± 0.2 | −0.8 (−1.3, −0.3) | 0.003 | ||
| Age | 0.96 | 0.85 | ||||||||
| < 30 years | 241 (57.9%) | 11.1 ± 0.1 | 2.2 ± 0.1 | Reference | 2.0 ± 0.2 | Reference | ||||
| 30 – 39 years | 112 (26.9%) | 11.2 ± 0.2 | 2.2 ± 0.3 | −0.2 (−0.9, 0.4) | 0.46 | 2.0 ± 0.2 | −0.1 (−0.8, 0.6) | 0.84 | ||
| ≥ 40 years | 63(15.1%) | 10.8 ± 0.2 | 2.4 ± 0.2 | −0.1 (−0.7, 0.5) | 0.76 | 2.2 ± 0.3 | 0.3 (−0.5, 1.1) | 0.43 | ||
| Marital status | ||||||||||
| Married | 179 (43.0%) | 11.2 ± 0.1 | 2.3 ± 0.2 | Reference | 2.1 ± 0.2 | Reference | ||||
| Cohabiting | 29 (7.0%) | 10.6 ± 0.3 | 2.1 ± 0.4 | −0.2 (−0.9, 0.6) | 0.70 | 1.9 ± 0.6 | 0.0 (−1.1, 1.0) | 0.97 | ||
| Single | 175 (42.1%) | 11.2 ± 0.1 | 2.2 ± 0.2 | −0.2 (−0.7, 0.4) | 0.61 | 2.1 ± 0.2 | 0.0 (−0.6, 0.7) | 0.96 | ||
| Divorced/widowed | 33 (7.9%) | 10.4 ± 0.2 | 2.7 ± 0.4 | 0.3 (−0.3, 1.0) | 0.33 | 1.5 ± 0.6 | −0.7 (−1.8, 0.5) | 0.24 | ||
| CD4 count | 0.65 | 0.15 | ||||||||
| < 750 cells/mm3 | 236 (64.0%) | 11.1 ± 0.1 | 2.3 ± 0.1 | 0.1 (−0.4, 0.5) | 0.75 | 2.3 ± 0.2 | 0.5 (0.0, 1.0) | 0.04 | ||
| ≥ 750 cells/mm3 | 133 (36.0%) | 11.2 ± 0.1 | 2.2 ± 0.2 | Reference | 1.7 ± 0.2 | Reference | ||||
| Previous TB infection | ||||||||||
| Yes | 302 (73.1%) | 11.2 ± 0.1 | 2.1 ± 0.1 | −0.4 (−0.8, 0.1) | 0.10 | 2.0 ± 0.1 | −0.3 (−0.8, 0.3) | 0.35 | ||
| No | 111 (26.9%) | 10.9 ± 0.2 | 2.5 ± 0.2 | Reference | 2.3 ± 0.3 | Reference | ||||
| Money spent on food per person per day | 0.48 | 0.70 | ||||||||
| < 250 TSh | 92 (25.5%) | 10.8 ± 0.2 | 2.2 ± 0.2 | −0.1 (−0.6, 0.4) | 0.60 | 2.2 ± 0.2 | 0.0 (−0.6, 0.6) | 0.94 | ||
| 250 – 500 TSh | 94 (26.0%) | 11.1 ± 0.2 | 2.0 ± 0.2 | −0.2 (−0.7, 0.4) | 0.24 | 1.6 ± 0.2 | −0.4 (−1.0, 0.2) | 0.15 | ||
| ≥ 500 TSh | 175 (48.5%) | 11.3 ± 0.1 | 2.5 ± 0.2 | Reference | 2.3 ± 0.2 | Reference | ||||
Footnotes for Table 5.
Mean ± SE for all such value.
Adjusted differences in change in hemoglobin were estimated from the multivariate linear regression model that includes sex, age, marital status, CD4 cell counts, previous TB infection, money spent on food, and treatment assignment. Other variables were not associated with change in hemoglobin.
P-value for test for trend was obtained from median values within quartiles. Karofsky score was entered as a continuous variable.
DISCUSSION
In a cohort of patients with pulmonary TB in Tanzania, about half of patients were malnourished and almost all patients were anemic at the time of diagnosis. Both HIV-positive and HIV-negative patients experienced increases in BMI and hemoglobin concentrations during TB treatment. However, low CD4 cell counts among HIV-positive patients were associated with smaller increases in BMI and hemoglobin levels during TB treatment.
Malnutrition is common among TB patients, with prevalence estimates ranging from 21% in Peru (BMI < 18.5 kg/m2) to 61% in Malawi and 62% in Uganda (BMI < 19.0 in Malawi and Uganda) (7, 13, 21). Most TB patients experience substantial weight gain during TB treatment (22, 23). Reported average weight gain during TB treatment include 4.9 kg in Indonesia, 5.7 kg in Guinea-Bissau, and 6.9 kg in Tanzania (22–24). It is noteworthy that patients in our study continued to have low BMI status even after the completion of treatment. Full recovery in nutritional status may take more time beyond the completion of treatment. Weight gain during treatment may be mostly due to accumulated fat mass, and patients may fail to restore body protein by the end of treatment (25). Understanding the body composition of TB patients is important and it needs to be examined in future research (26, 27).
In our study, HIV-positive patients with CD4 counts <200 cells/mm3 showed suboptimal improvements in BMI and hemoglobin levels during TB treatment. Anorexia may lead to poor nutritional status by reducing energy intake. Poor nutritional recovery may be also due to delayed clearance of mycobacterial infection, other co-existing opportunistic infections, and excess production of TNF-α, which may alter metabolism and impair weight gain. Proinflammatory cytokines play an important role in the intracellular killing of mycobacteria; however, excessive production leads to tissue damage and weight loss (28). Overproduction of TNF-α may activate NFκB, which in turn enhances HIV replication and may accelerate HIV disease progression (29). Highly active antiretroviral therapy (HAART) is extremely important among HIV-positive patients during TB treatment. At the time of the study, HAART was not available to most HIV-positive participants. WHO now recommends that all HIV-positive individuals with active TB, irrespective of CD4 cell count, should receive antiretroviral treatment as soon as possible after starting TB treatment (30).
Anemia is a highly common hematologic complication among TB patients and is a strong risk factor for mortality (11). Almost all patients were anemic at the time of diagnosis. Previous studies in sub-Saharan Africa also found a high prevalence of anemia; 88% of HIV-positive and 77% of HIV-negative TB patients were anemic in Malawi (21, 31). We found that the majority of HIV-positive patients remained anemic even after treatment. HIV-associated anemia has been found to be associated with poor quality of life, HIV disease progression, and mortality (32). Anemia in TB may largely be due to chronic inflammation. Studies showed that a decline in the serum concentrations of C-reactive protein, the acute phase reactants, coincided with the increase in hemoglobin concentration during treatment (22, 33). Excessive production of pro-inflammatory cytokines, such as IL-6, TNF-α and IFN-γ, contributes to anemia through reduced production of erythropoietin, suppressed response of bone marrow to erythropoietin, and altered iron metabolism, which may in turn impair erythropoiesis (34). Poor quality diet could also account for anemia, and in fact, we observed that patients who spent less money on food had poorer recovery of hemoglobin levels. Women showed a smaller average increase in hemoglobin which may be due to menstrual blood loss and poor quality diet.
Patients with low socioeconomic status may have insufficient dietary intakes and poor quality diets, and may be vulnerable to food insecurity; however, measures of socioeconomic status and frequency of meat purchase were not associated with change in nutritional status in our study. A recent review concluded that there is insufficient evidence to suggest whether incorporating nutritional supplements during TB treatment may improve clinical outcomes (35). However, micronutrient supplements reduced the risk of early recurrence in our study population and the role of nutritional support merits further research (16).
Our study has limitations worth noting. One limitation is that HIV-positive patients were not receiving antiretroviral therapy concurrently with TB treatment. Our findings need to be confirmed under concomitant treatment with antiretroviral therapy. Our study did not examine body composition and further research is needed to fully understand the extent of nutritional recovery. Our analysis did not include patients who died before 8 or 14 months or patients without measurements. Because these patients may have poor nutritional status, excluding them from the analysis may have attenuated the associations between some of the baseline predictors and change in BMI or hemoglobin. Nevertheless, relatively small proportion of patients (7.6% of HIV-positive and 0.7% of HIV-negative) died during the first 8 months of TB treatment in the study. Our study may have limited generalizability because the study excluded patients with hemoglobin <7.0 g/dL and Karnofsky score <40%, and included only sputum-smear positive pulmonary TB patients. Another limitation may be that participants received previous 8 months TB regimen. The National TB Programme in Tanzania recently changed the TB treatment regimen from an 8 months regimen to a 6 months regimen which includes 6 months of rifampicin.
In conclusion, both HIV-positive and HIV-negative patients experienced increases in BMI and hemoglobin concentrations during TB treatment. Because the majority of patients have already lost substantial weight by the time they present to a clinic, early and intensified identification of TB cases and treatment is essential. As malnutrition continues to place a tremendous burden in resource limited countries, research is needed to examine the benefits of incorporating nutritional supplements or food packages. Nutritional support may be essential in combination with initiation of antiretroviral therapy for HIV-positive TB patients.
Acknowledgments
We would like to thank the patients who participated in this study. We are grateful to the study coordinator, Dorothy Mallya, study physicians, nurses, laboratory and administrative staff at Muhimbili University of Health and Allied Sciences, and the TB clinics in Dar es Salaam for their valuable contribution to the study. We also thank Dr. Anne Goldfield for important intellectual contributions (Harvard Medical School) and Ellen Hertzmark (Harvard School of Public Health) for statistical assistance.
KK conducted the data analyses, interpreted the data, and wrote the initial draft of the manuscript. EV, FMM, ES, WU, RJB and WWF contributed to the study design, implemented the study, and interpreted the data. DS provided statistical guidance in the data analyses and interpreted the data. All authors participated in the manuscript preparation and have read and approved the final manuscript.
This study was supported by the National Institute of Allergy and Infectious Diseases (UO1 AI 45441-01).
Footnotes
None of the authors had any conflicts of interest.
References
- 1.WHO. WHO Report. 2009. Global tuberculosis control - epidemiology, strategy, financing. [Google Scholar]
- 2.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. Aids. 2001;15(2):143–52. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 3.van Lettow M, Fawzi WW, Semba RD. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev. 2003;61(3):81–90. doi: 10.1301/nr.2003.marr.81-90. [DOI] [PubMed] [Google Scholar]
- 4.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–98. [PubMed] [Google Scholar]
- 5.Swaminathan S, Padmapriyadarsini C, Sukumar B, Iliayas S, Kumar SR, Triveni C, et al. Nutritional status of persons with HIV infection, persons with HIV infection and tuberculosis, and HIV-negative individuals from southern India. Clin Infect Dis. 2008;46(6):946–9. doi: 10.1086/528860. [DOI] [PubMed] [Google Scholar]
- 6.Semba RD, Darnton-Hill I, de Pee S. Addressing tuberculosis in the context of malnutrition and HIV coinfection. Food Nutr Bull. 2010;31(4):S345–64. [PubMed] [Google Scholar]
- 7.van Lettow M, Kumwenda JJ, Harries AD, Whalen CC, Taha TE, Kumwenda N, et al. Malnutrition and the severity of lung disease in adults with pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2004;8(2):211–7. [PubMed] [Google Scholar]
- 8.Villamor E, Saathoff E, Mugusi F, Bosch RJ, Urassa W, Fawzi WW. Wasting and body composition of adults with pulmonary tuberculosis in relation to HIV-1 coinfection, socioeconomic status, and severity of tuberculosis. Eur J Clin Nutr. 2006;60(2):163–71. doi: 10.1038/sj.ejcn.1602281. [DOI] [PubMed] [Google Scholar]
- 9.Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96(3):291–4. doi: 10.1016/s0035-9203(02)90103-3. [DOI] [PubMed] [Google Scholar]
- 10.Gustafson P, Gomes VF, Vieira CS, Samb B, Naucler A, Aaby P, et al. Clinical predictors for death in HIV-positive and HIV-negative tuberculosis patients in Guinea-Bissau. Infection. 2007;35(2):69–80. doi: 10.1007/s15010-007-6090-3. [DOI] [PubMed] [Google Scholar]
- 11.Ciglenecki I, Glynn JR, Mwinga A, Ngwira B, Zumla A, Fine PE, et al. Population differences in death rates in HIV-positive patients with tuberculosis. Int J Tuberc Lung Dis. 2007;11(10):1121–8. [PubMed] [Google Scholar]
- 12.Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med. 2006;174(3):344–8. doi: 10.1164/rccm.200511-1834OC. [DOI] [PubMed] [Google Scholar]
- 13.Krapp F, Veliz JC, Cornejo E, Gotuzzo E, Seas C. Bodyweight gain to predict treatment outcome in patients with pulmonary tuberculosis in Peru. Int J Tuberc Lung Dis. 2008;12(10):1153–9. [PubMed] [Google Scholar]
- 14.Kennedy N, Ramsay A, Uiso L, Gutmann J, Ngowi FI, Gillespie SH. Nutritional status and weight gain in patients with pulmonary tuberculosis in Tanzania. Trans R Soc Trop Med Hyg. 1996;90(2):162–6. doi: 10.1016/s0035-9203(96)90123-6. [DOI] [PubMed] [Google Scholar]
- 15.Zachariah R, Spielmann M, Harries A, Salaniponi F. Malnutrition in tuberculosis patients on admission and weight-gain in relation to HIV Status in Thyolo District. Malawi Medical Journal. 2001;13(4):12–13. [Google Scholar]
- 16.Villamor E, Mugusi F, Urassa W, Bosch RJ, Saathoff E, Matsumoto K, et al. A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. J Infect Dis. 2008;197(11):1499–505. doi: 10.1086/587846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Proposed ‘World Health Organization staging system for HIV infection and disease’: preliminary testing by an international collaborative cross-sectional study. The WHO International Collaborating Group for the Study of the WHO Staging System. AIDS. 1993;7(5):711–8. [PubMed] [Google Scholar]
- 18.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 19.WHO. A guide for programme managers. 2001. Iron Deficiency Anaemia - Assessment, Prevention, and Control. [Google Scholar]
- 20.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 21.Shah S, Whalen C, Kotler DP, Mayanja H, Namale A, Melikian G, et al. Severity of human immunodeficiency virus infection is associated with decreased phase angle, fat mass and body cell mass in adults with pulmonary tuberculosis infection in Uganda. J Nutr. 2001;131(11):2843–7. doi: 10.1093/jn/131.11.2843. [DOI] [PubMed] [Google Scholar]
- 22.Karyadi E, West CE, Schultink W, Nelwan RH, Gross R, Amin Z, et al. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr. 2002;75(4):720–7. doi: 10.1093/ajcn/75.4.720. [DOI] [PubMed] [Google Scholar]
- 23.Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multi-vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br J Nutr. 2006;95(4):762–70. doi: 10.1079/bjn20051684. [DOI] [PubMed] [Google Scholar]
- 24.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–50. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 25.Schwenk A, Hodgson L, Wright A, Ward LC, Rayner CF, Grubnic S, et al. Nutrient partitioning during treatment of tuberculosis: gain in body fat mass but not in protein mass. Am J Clin Nutr. 2004;79(6):1006–12. doi: 10.1093/ajcn/79.6.1006. [DOI] [PubMed] [Google Scholar]
- 26.Mupere E, Zalwango S, Chiunda A, Okwera A, Mugerwa R, Whalen C. Body composition among HIV-seropositive and HIV-seronegative adult patients with pulmonary tuberculosis in Uganda. Ann Epidemiol. 2010;20(3):210–6. doi: 10.1016/j.annepidem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Praygod G, Range N, Faurholt-Jepsen D, Jeremiah K, Faurholt-Jepsen M, Aabye MG, et al. Weight, body composition and handgrip strength among pulmonary tuberculosis patients: a matched cross-sectional study in Mwanza, Tanzania. Trans R Soc Trop Med Hyg. 2011;105(3):140–7. doi: 10.1016/j.trstmh.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Ellner JJ. Review: the immune response in human tuberculosis--implications for tuberculosis control. J Infect Dis. 1997;176(5):1351–9. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 29.Lawn SD, Shattock RJ, Acheampong JW, Lal RB, Folks TM, Griffin GE, et al. Sustained plasma TNF-alpha and HIV-1 load despite resolution of other parameters of immune activation during treatment of tuberculosis in Africans. Aids. 1999;13(16):2231–7. doi: 10.1097/00002030-199911120-00005. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Antiretoviral therapy for HIV infection in adullts and asolescents: recommendations for a public health approach. 2010
- 31.van Lettow M, West CE, van der Meer JW, Wieringa FT, Semba RD. Low plasma selenium concentrations, high plasma human immunodeficiency virus load and high interleukin-6 concentrations are risk factors associated with anemia in adults presenting with pulmonary tuberculosis in Zomba district, Malawi. Eur J Clin Nutr. 2005;59(4):526–32. doi: 10.1038/sj.ejcn.1602116. [DOI] [PubMed] [Google Scholar]
- 32.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):27S–43S. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Lawn SD, Obeng J, Acheampong JW, Griffin GE. Resolution of the acute-phase response in West African patients receiving treatment for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4(4):340–4. [PubMed] [Google Scholar]
- 34.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 35.Abba K, Sudarsanam TD, Grobler L, Volmink J. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev. 2008;(4):CD006086. doi: 10.1002/14651858.CD006086.pub2. [DOI] [PubMed] [Google Scholar]