Abstract
The molecular mechanisms that operate within the organ microenvironment to support metastatic progression remain unclear. Here we report that upregulation of the hyaluronan synthase HAS2 occurs in highly metastatic breast stem-like cancer cells (CSCs) defined by CD44+/CD24−/ESA+ phenotype, where it plays a critical role in the generation of a pro-metastatic microenvironment in breast cancer. HAS2 was critical for interaction of CSCs with tumor associated macrophages (TAMs), leading to enhanced secretion of PDGF-BB from TAMs which then activated stromal cells and enhanced CSC self-renewal. Loss of HAS2 in CSCs or treatment with 4-methylumbelliferone (4-MU), an inhibitor of hyaluronan synthases which blocks hyaluronan production, drastically reduced the incidence and growth of metastatic lesions in vitro or in vivo, respectively. Taken together, our findings demonstrate a critical role for HAS2 in the development of a pro-metastatic microenvironment and suggest that HAS2 inhibitors can act as anti-metastatic agents that disrupt a paracrine growth factor loop within this microenvironment.
Keywords: tumor associated macrophages, microenvironment, HAS2
Introduction
According to the cancer stem cell (CSC) model, the metastatic cells must have stem cell-like capability for their self-renewal and invasive abilities, in addition to the capacity to differentiate into a heterogeneous population of cancer cells. In the case of breast cancer, the CSCs which are significantly enriched for tumor-initiating capacity were isolated from breast cancer patients using specific surface markers such as CD24−/low/CD44+ and EpCAM+ (ESA) (1, 2) and, more recently, by means of expression of aldehyde dehydrogenase 1 (ALDH1) activity (3). These cells have indeed shown to be highly tumorigenic and also reported to have invasive and metastatic properties (4–7), and therefore, the existence of these CSCs at an early stage of cancer may well explain the clinical observation of early stage metastasis.
Metastatic tumor cells generally establish colonization in a relatively organ-specific manner, i.e. breast cancer preferentially metastasizes to the bone and lung; however, the mechanism of this organ specificity is still not well understood (8, 9). Metastatic tumor cells are believed to be “guided” by a chemo-attractant to distant organs, and they become responsive to a specific growth factor by adapting themselves in the different microenvironments. This “seed and soil” theory has been extensively interrogated, and many such factors were identified (10). If the metastatic CSCs indeed exist, it is of paramount interest to know whether the “seed and soil” theory is also applicable to these cells, and if so, what the tissue-specific factors are and what signaling promotes metastatic behavior of these cells. It is well recognized that CSCs, like embryonic stem cell, require niche which provides appropriate environment for self-renewal of these cells (11). However, the type of cells constituting the niche and factors involved in this microenvironment is as yet poorly understood. It is not known whether cancer stem cells merely “adapt” to the existing niche for normal stem cell or they more aggressively “generate” such environment through reciprocal interaction with stromal cells (12). A variety of stromal cells in the surrounding environment are recruited to the primary and metastatic lesions, and these include fibroblast, endothelial cells, mast cell and mescenchymal stem cells as well as macrophages and neutrophils (13). Macrophages, also called “tumor-associated macrophages (TAMs)” which are classified into M2 subtypes have been shown to promote tumor angiogenesis, invasion, intravasation and metastasis in addition to their immunosuppressive role (14). They are likely to be key components of the niche for the CSCs; however, the exact role of this cell type in metastatic sites is not yet well defined. In order to address these critical questions we isolated CSCs that are defined by CD44+/CD24−/ESA+ phenotype_from various breast cell lines and examined genome expression profile. We found that the hyaluronan synthase 2 (HAS2) gene was specifically up-regulated in the CSCs from highly metastasizing variants and that this gene was capable of enhancing invasion, and tumor growth in the bone environment. We also demonstrated that HAS2 stimulates interaction of CSCs and TAMs, which significantly promoted proliferation of the CSCs by stimulating stromal cells in bone. Importantly, we also showed that 4-methylumbelliferone (4-MU) which can block hyaluronan synthesis by inhibition of hyaluronan synthases (HASs) drastically reduced the incidence and growth of metastatic lesions in our metastatic animal model using CSCs, suggesting the potential utility of 4-MU as an anti-metastatic drug by blocking the tumor microenvironment.
Materials and Methods
Cells and Cell Culture
Human breast carcinoma cell lines, MDA-MB-231 and MCF7, immortalized epithelial cell line, MCF10A, bone marrow fibroblast cell lines, HS5 and HS27A, osteoblast cell line, hFOB1.19, and monocyte cell line, THP1, were purchased from ATCC. MCF10DCIS.com cells were purchased from Asterand. All these cell lines from the commercial sources were obtained in 2008–2009 and they were subjected to master cell bank generation. 231BoM-1833, 231BrM-2a, CN34, CN34-BoM2d, CN34-BrM2c and MCF7-BoM2d cell lines were kindly provided by Dr. Joan Massagué in 2009 and they are the sole source of these cell lines. 231BoM-1833, 231BrM-2a and MCF7-BoM2d were authenticated by conducting Affymetrix expression array analysis. Authenticity of CN34, CN34-BoM2d and CN34-BrM2c was confirmed by qRT-PCR analysis for the expression signature of 20 genes. The immortalized mouse bone microvascular endothelial cell (mBMEC) was a gift from Dr. Isaiah Fidler in 2009, and the authenticity of the cell line was confirmed by FACS (VCAM-1, E-selecting and PCR (H-2Kb-tsA58). After the authentication of these cell lines, they were subjected to master cell bank generation, and all cell culture experiments were conducted with cells at less than 8 passages, and they were routinely tested for the absence of Mycoplasma.
CSCs isolation by MACS
CSCs were isolated by the magnetic-activated cell sorting (MACS) system (Miltenyi Biotec) using antibodies to CD24 (Stem cell technologies), CD44 (Biolegend) and ESA (GeneTex). Detailed sorting conditions by MACS system are also described in Supplemental Data.
Gene-expression microarray profiling
RNA was extracted from isolated CSCs using the RNeasy mini kit (Qiagen) followed by DNase treatment and re-purified using the RNA cleanup kit (Qiagen). Labeling and hybridization of the samples to Human gene 1.0ST chip (Affymetrix) by the CFG Microarray Core Facility (New York). Normalization of the chip was performed using RMA algorithm in Expression consol software (Affymetrix). These expression data were submitted to the NCBI Gene Expression Omnibus (GEO) under accession number GSE25976. For cancer cohort analysis, we chose 10 breast cancer microarray cohorts data that contain the information of patient survival statuses (Table S1).
Cytokine and growth factor antibody array analysis
Cytokines and growth factor antibody analysis was performed using AAH-CYT-5 and AAH-GF-1 (RayBiotech) according to the manufacturer's protocol.
Cell adhesion assay and transmigration assay
CSCs were seeded on the monolayer of mBMEC, and they were allowed to adhere for 30 min. Plates were then washed and the firefly luciferase activity in CSCs was measured. CSCs were also labeled with Cell tracker green (Invitrogen) and cells were seeded on the mBMEC monolayer. Cells were then washed followed by counting the number of adhered cells. For transmigration assay, CSCs were labeled with Cell tracker green and cells were seeded into the trans-well insert with the monolayer of mBMEC. After 48 hours, labeled cells that had migrated through the membranes were counted.
In vivo tumor cell survival assay
CSCs were labeled with Cell tracker green and 106 cells were intravenously injected through the tail vein into nude mice. After 48 hrs, rhodamine-lectin was injected through i.v. The mice were then sacrificed and the lungs were removed. The tissue was snap-frozen and cryosectioned. The fluorescent image was visualized by microscopy.
Immunocyto- and Immunohisto-chemistry
For immunocytochemistry, CSCs were added on the TAM monolayer and they were incubated for 48 hrs. The cells were fixed and stained with antibodies for CD68 and PDGF-BB followed by counter staining with DAPI. Immunohistochemical analysis was performed for paraffin-embedded specimens of breast cancer using anti-HAS2 antibody.
Animal Experiments
For orthotopic tumor growth, CSCs were mixed with Matrigel and they were injected into mammary glands of nude mice. For experimental metastasis assay, CSCs were injected into the left cardiac ventricles of the mice. Tumor growth was then monitored using Xenogen bioimager. To examine the effect of bone microenvironment on tumor growth, CSCs with or without TAM were co-injected directly into the tibial bones and the mice were monitored for tumor growth.
Results
HAS2 is over-expressed in CSCs from metastatic breast cancer cell
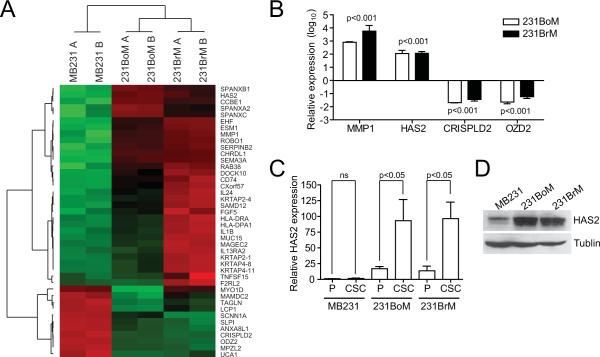
To understand the role of CSCs in the process of tumor metastasis, we first isolated CSC populations from well established model cell lines of breast cancer using CD24, CD44 and EpCAM antibodies (Figure S1A). Overall yield of CSCs ranged from 2% to 10% (Figure S1B) and the CSCs population after sorting was confirmed by FACS (Figure S1C). We evaluated their “tumor initiating” and “metastasis initiating” abilities in nude mice and found that the CSCs indeed showed significantly stronger abilities of tumorigenesis and metastasis than the corresponding non-CSCs and unsorted populations (Table 1, Table S3 and S4). We then performed global expression profile analysis for the CSCs. A comparison of transcriptional profiles uncovered 42 genes whose mRNA were up- or down-regulated at least 10-fold in CSCs of highly metastatic cell lines (231BoM and 231BrM) compared to the CSCs of MB231 (Figure 1A). We also carried out genes set enrichment analysis. Interestingly, we found that embryonic stem cells-associated gene sets (15) were significantly enriched in CSCs of the highly metastatic cell lines, while the polycomb-target gene sets (15) were significantly repressed (Figure S1D), suggesting the strong correlation of these signatures with the metastatic abilities of CSCs. To examine the clinical relevance of the 42 genes, we first chose 13 genes that were highly up- or down-expressed in both CSCs of metastatic cells. We then examined the relationship between the expression of these genes and overall- and metastasis-free survival of breast cancer patients using existing data base (Table S1). As shown in Table 2 and S5, up-regulation of two genes (MMP1 and HAS2) and down-regulation of two genes (CRISPLD2 and ODZ2) were positively and negatively correlated with poor overall- and metastasis-free survival, respectively. We also examined the expression of these genes by qRT-PCR and confirmed that the expressions of the genes were indeed significantly altered in CSCs from 231BoM and 231BrM compared to that of MB231 (Figure 1B). Notably, recent evidence suggests that the HAS2 gene expression is significantly correlated with tumorigenicity and tumor progression in several cancers, and therefore, HAS2 is of considerable interest for further study. When we examined the expression of HAS2 in both CSCs and parental cells by qRT-PCR, HAS2 gene expression was shown to be specifically overexpressed in isolated CSCs from metastatic variant cell lines and these results were further confirmed by Western blot (Figures 1C, 1D and S1E). We also found that only HAS2 among all tested genes for hyaluronan processing enzymes was specifically up-regulated in CSCs from metastatic variants (Figure S1D).
Table 1.
Limiting dilution analysis for tumor incidence of CSCs in nude mice.
| Strain | Population | Number of tumors/number of injections |
CSC frequency (95% confidence interval) | ||||
|---|---|---|---|---|---|---|---|
| Cells per injection | |||||||
| 10,000 | 1,000 | 100 | 10 | ||||
| MB231 | Unsorted | 2/4 | 1/4 | 0/2 | 1/10,720 | (1/3,203-1/35,879) | |
| Stem cells | 6/6 | 5/6 | 2/6 | 1/448**/$$ | (1/183-1/1,097) | ||
| Non-stem cells | 1/2 | 0/2 | 0/2 | 1/16,705 | (1/2,356-1/118,284) | ||
|
| |||||||
| 231BoM | Unsorted | 6/6 | 6/7 | 3/5 | 0/3 | 1/334 | (1/140-1/844) |
| Stem cells | 5/5 | 11/11 | 9/11 | 6/11 | 1/37**/$$ | (1/19-1/72) | |
| Non-stem cells | 2/4 | 0/4 | 0/4 | 1/1,671 | (1/419-1/6,668) | ||
|
| |||||||
| 231BrM | Unsorted | 4/4 | 1/4 | 0/4 | 1/277 | (1/86-1/895) | |
| Stem cells | 4/4 | 5/6 | 2/6 | 1/45*/$$ | (1/19-1/110) | ||
| Non-stem cells | 5/6 | 1/6 | 0/6 | 1/569 | (1/236-1/1374) | ||
: P<0.05 (Unsorted vs stem cells)
: P<0.0001 (Unsorted vs stem cells)
: P<0.0001 (Non-stem cells vs stem cells)
Figure 1. HAS2 gene is upregulated in CSCs from metastatic breast cancer cells.
(A) A heatmap was generated for the genes that were significantly up- or down-regulated at least 10 times in CSCs among 231BoM, 231BrM and MB231. (B) The expression of MMP1, HAS2, CRISPLD2 and OZD2 was examined by qRT-PCR for CSCs prepared from these three cell lines. The expression level of CSCs of MB231 was set as 0 in log10-scale (n=3). (C) HAS2 expressions in both parental cells (P) and CSCs (CSC) from each cell line were measured by qRT-PCR (n=3). (D) Western blotting for HAS2 protein in CSCs was performed.
Table 2.
Survival analysis of genes which are up- or down-regulated in metastatic CSCs using multiple breast cancer cohorts.
| Gene | FC in BoM SC | FC in BrM SC | Overall survival | MET-free survival | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Good Prog. | Poor Prog. | Good Prog. | Poor Prog. | |||
| MMP1 | 47.46 | 103.90 | 0/8 | 5/8 | 0/8 | 5/8 |
| SERPINB2 | 33.11 | 58.15 | 1/8 | 1/8 | 1/8 | 1/8 |
| EHF | 23.31 | 69.62 | 0/7 | 0/7 | 2/7 | 0/7 |
| CHRDL1 | 15.31 | 17.46 | 1/7 | 1/7 | 0/7 | 1/7 |
| SPANXB1 | 18.46 | 10.37 | 1/6 | 2/6 | 1/6 | 3/6 |
| HAS2 | 17.77 | 12.01 | 0/8 | 2/8 | 0/8 | 3/8 |
| CCBE1 | 16.96 | 10.33 | 0/2 | 0/2 | 0/2 | 0/2 |
|
| ||||||
| ANXA8L1 | −11.42 | −16.94 | 0/2 | 0/2 | 0/2 | 0/2 |
| CRISPLD2 | −14.90 | −17.84 | 1/6 | 0/6 | 1/6 | 0/6 |
| UCA1 | −19.57 | −14.81 | 0/2 | 0/2 | 0/2 | 0/2 |
| ODZ2 | −21.35 | −26.39 | 2/3 | 0/3 | 1/3 | 0/3 |
| LCP1 | −49.54 | −14.16 | 0/8 | 1/8 | 1/8 | 0/8 |
| MPZL2 | −38.25 | −50.60 | 0/7 | 1/7 | 0/7 | 1/7 |
4-MU blocks HAS2-mediated metastasis of CSCs in vivo
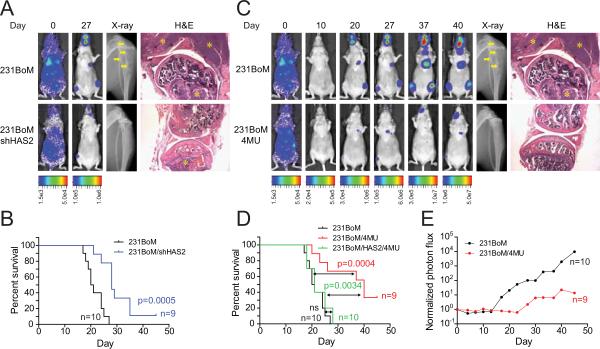
To further examine the role of HAS2 in tumor metastasis in vivo, we prepared CSCs from 231BoM and implanted them into nude mice by intra-cardiac injection. At day 27, metastatic tumors were clearly visible in tibial bones and jaws, and they generated large osteolytic bone lesions. On the other hand, knockdown of HAS2 significantly suppressed the metastatic spread of tumor cells (Figure 2A and Figure S2A). As shown in Figure 2B, mice inoculated with CSCs of 231BoM carrying shRNA to HAS2 (231BoM-shHAS2) had significantly improved the metastasis-free survival rate. Next, we investigated the effect of hyaluronan synthases inhibitor, 4-methylumbelliferone (4-MU), on the metastatic ability of CSCs by intracardially injecting CSCs of 231BoM to the mice followed by daily administration of 4-MU. We found that 4-MU significantly suppressed the incidence of metastasis of CSCs to the bones and also significantly improved metastasis-free survival (Figures 2C, 2D and 2E). The 4-MU treatment did not affect the body weight of these mice and did not show noticeable toxic effects. It is known that 4-MU can also inhibit UDP-glucuronyltransferases (UGT) and thereby affect synthesis of a number of glycosaminoglycans such as heparan sulfate (HS) and chondroitin sulfate (CS) as well as hyaluronan (HA). To examine a possible off-target effect of 4-MU, we first constructed the 231BoM cell line which ectopically expressed HAS2, and CSCs prepared from this cell line were injected into mice followed by treatment with 4-MU. We found that 4-MU significantly delayed the onset of bone metastasis of 231BoM cells; however, this effect of 4-MU was significantly suppressed by the over-expression of HAS2 (Figure 2D). These results strongly suggest that the effect of 4-MU on metastasis is mainly through inhibition of HA synthesis, at least with the dose used for this experiment. We have also estimated the concentration of 4-MU in the circulation as approximately 0.3 mM in these animals, based on the data from a previous study (16). Accordingly, we treated 231BoM cells with 4-MU at 0.5 mM and measured the concentration of HA, HS and CS by ELISA. We found that the 4-MU treatment significantly reduced HA but not HS or CS (Figures D2B, S2C and S2D). Moreover, overexpression of HAS2 gene in this cell significantly enhanced HA production, while the 4-MU treatment with this concentration did not affect HA (Figures S2B). Furthermore, we evaluated effects of shRNA to xylosyltransferase I (XYLT1) on glycosaminoglycan synthesis and on bone metastasis. XYLT1 is capable of transferring UDP-xylose to serine residues of an acceptor protein, during the initial step of glycosaminoglycan biosynthesis. We found that knockdown of XYLT1 significantly suppressed the production of HS and CS as expected, while the same shRNA did not have any effect on HA production (Figures S2B, S2C and S2D). We then intracardially injected CSCs prepared from 231BoM cell carrying shXYLT1 into nude mice. Interestingly, we found that the knockdown of XYLT1 did significantly suppress bone metastasis, but the extent of the suppression was far less than the treatment with 4-MU (Figure S2E). Together, our results suggest that the suppressive effect of 4-MU on bone metastasis is mainly due to the inhibition of HA synthesis with the concentration used in our experiments. 4-MU does affect metastasis which is induced by other glycosaminoglycan synthesis; however, this effect is considered to be minor at this concentration of 4-MU.
Figure 2. HAS2 enhances metastasis in vivo.
(A) 5 × 104 cells of CSC were intracardially injected into nude mice. Images are bioluminescent, radiographic, and H&E analysis of bone lesions from representative mice in each group. The osteolytic lesions in the X-ray image are indicated by arrows. Tumors in H&E stained photos are indicated by asterisk. (B) Kaplan-Meier analysis for metastasis-free survival of these animals was performed (n=9–10 per group). (C) CSCs of 231BoM were intracardially injected into the nude mice. The animals were then fed with or without 4-MU (400 mg/kg/day, every day for 45 days). (D) Kaplan-Meier analysis for metastasis-free survival of these animals was performed (n=9–10 per group). Black; CSCs of 231BoM (n=10), red; CSCs of 231BoM with treatment of 4-MU (n=9), green; CSCs of 231BoM expressing HAS2 with treatment of 4-MU (n=10). (E) The metastatic growth of tumors in tibia with or without the treatment of 4-MU was monitored by Xenogen bioimager. (n=9–10 per group).
HAS2 promotes metastatic functions by enhancing adhesion of CSCs to endothelial cells
To understand the exact roles of HAS2 in metastatic CSCs, we first examined the expression of cell surface HA of CSCs and found significantly larger pericellular HA matrix in CSCs of 231BoM compared with that of MB231 (Figure. 3A). Knockdown of HAS2 or treatment with 4-MU significantly reduced the HA matrix. (Figure. 3A). Next, the CSCs were isolated from MB231 and 231BoM, and their abilities to adhere to bone microvessel endothelial cells and transmigrate through endothelial cell monolayer were assayed. As shown in Figure 3B, CSCs from 231BoM showed significantly greater ability to adhere to the endothelial cells compared to that from MB231 in two different assays (Figure 3B). In addition, CSCs from 231BoM showed significantly greater ability to transmigrate through endothelial cell compared to that from MB231 (Figure 3C). When the same assay was done using HAS2 inhibitors, shHAS2 and 4-MU, the adhesive ability (Figure 3B) and transmigration (Figure 3C) of the CSCs was significantly abrogated. These results suggest that HAS2 plays an important role in adhesion of CSCs to endothelial cells and extravasations. We therefore examined the behavior of CSCs in blood vessels in animals by labeling the CSCs with Cell tracker dye followed by injecting them through the tail-vein. We found that more CSCs of 231BoM were retained in the microcapillary of the lung than that of MB231 after 48 hours and that knockdown of HAS2 or treatment with 4-MU significantly decreased the retention of CSCs in the microcapillaries (Figures 3D and S3). These results suggest that CSCs with higher amount of hyaluronan have better survival advantage in microcapillaries, possibly due to the ability of the HAS2 expressing cell to adhere to endothelial cells and evade anoikis.
Figure 3. HAS2 enhances adhesion and invasion abilities of CSCs.
(A) Particle exclusion assay for CSCs was performed, and areas of the halos were quantified (n=5). (B) CSCs were labeled with the Cell tracker green and subjected to cell adhesion assay using bone-derived endothelial cells. The amount of attached CSCs was assayed by measuring the luciferase activity (left panel). The actual number of attached CSCs was visually measured by counting the cells under a fluorescent microscope (right panel) (n=3). (C) The ability of trans-endothelial migration of the CSCs that were treated with sh-HAS or 4MU was measured (n=9). (D) CSCs were labeled with Cell tracker dye and injected into nude mice via tail vein. After 48 hours, mice were injected with rhodamine conjugated lectin via tail vein. Ten minutes later, mice were sacrificed and the lungs were then removed, sectioned and visualized under microscope. The number of CSCs in the lungs was counted by fluorescent microscopy (right panel). Additional photos were shown in Figure S3 (n=6). *** indicates p<0.0001, ** indicates p<0.001, * indicates p<0.05.
HAS2 promotes CSCs growth by enhancing interaction with TAM
Tumor associated macrophages (TAMs) are often abundantly found around tumor cells and they are considered to play a critical role in tumor growth (14). TAMs also express the major surface receptor of hyaluronan, CD44, and are capable of interacting with the tumor cells. Therefore, we sought a possibility that the interaction of TAM and CSCs through hyaluronan and CD44 provides a niche for proliferation of CSCs. To test this possibility, we first generated TAM by incubating human monocytes with IL-4, IL-13 and the CM of 231BoM (Figure 4A). We then co-injected TAMs and CSCs of 231BoM directly into the tibial bones of mice. As shown in Figure 4B, the co-injection of TAMs with CSCs significantly augmented the growth of tumor in the tibiae, which strongly supports the idea that TAMs plays a critical role in CSCs growth of breast cancer. We also found that the treatment with 4-MU or knockdown of HAS2 significantly attenuated the growth of CSCs in the bones (Figure 4B). It is plausible that TAMs are able to release specific secretory factors to support the growth of CSCs in the bones upon interaction with CSCs through hyaluronan. To test this hypothesis, we first collected CM from the culture of TAMs and CSCs of 231BoM alone, and from the co-culture of both cells. These CM were then applied to the antibody array membranes. As shown in Figure 4C, we found that PDGF-BB was specifically released in the CM when TAMs and CSCs were co-cultured. We then co-cultured TAMs and CSCs followed by sorting these cells by FACS and assaying the PDGF-BB expression by qRT-PCR. We found that PDGF-BB was indeed significantly up-regulated in TAMs only when it was co-cultured with CSCs of 231BoM cells, but not when TAMs was co-cultured with CSCs from 231BoM-shHAS2 (Figure 4D). We also performed immunofluorescence analysis for the co-culture and found that PDGF-BB was expressed in TAMs specifically when they adhered with CSCs (Figure 4E and S4A). These results indicate that PDGF-BB is specifically expressed and released from TAMs when it directly interacts with CSCs and that blocking the HAS2 expression in CSCs significantly abrogates the induction of PDGF-BB in TAMs. We then tested a possibility that PDGF-BB released from TAMs directly stimulates the proliferation of CSCs by treating CSCs with PDGF-BB; however, the PDGF-BB treatment did not show any significant effect on the growth of CSCs (Figure S4B). Thus, the effect of PDGF-BB on CSC, if any, is likely to be indirect. We then analyzed the expression profile of PDGFR-beta to which PDGF-BB has the highest affinity, in various types of cells using GEO database, and found that PDGFR-beta is most highly expressed in the stromal cells (Figure S4C). Therefore, we hypothesized that PDGF-BB from TAMs indirectly affect CSCs through activation of stromal cells. To test this hypothesis, we treated the bone marrow derived fibroblasts (HS5 and HS27A), osteoblast (hFOB1.19) and bone marrow derived human mesenchymal stem cell (BM-hMSC) with PDGF-BB, and collected their CM. We then added the CM to CSCs of 231BoM followed by measuring cell proliferation and found that CM from PDGF-BB-treated stromal cells significantly enhanced the proliferation of CSCs (Figure 4), suggesting that PDGF-BB-activated the stromal cells to secrete a factor which in turn stimulates the CSCs proliferation. To further confirm this notion, we co-cultured TAMs and CSCs followed by collecting CM. The stromal cells were then treated with the CM and the secondary CM was collected. CSCs were then cultured with the secondary CM followed by assaying for proliferation. We found that this secondary CM significantly enhanced the proliferation of CSCs (Figures S4D–G), suggesting that the interaction of CSCs and TAM secretes PDGF-BB which then activates stromal cells to secrete growth stimulating factor(s) for CSCs. To identify such factor(s), we collected CM from stromal cells after treating them with PDGF-BB followed by cytokine antibody array analysis. We found that FGF7, FGF9, eotaxins and HGF were specifically secreted in the CM of three different cells when they were treated with PDGF-BB (Figure 4G). Among these factors, FGF7 and FGF9 are known to play important roles in embryonic development as well as in expansion and maintenance of CSCs population in breast cancers. Therefore, we examined the effects of these factors on CSCs and found that FGF9 significantly enhanced the proliferation (Figure 4H, S5A and S5C), amount of the population (Figure 4I, S5B and S5D) and sphere formation (Figure 4J) of CSCs, while treatment with FGF receptor inhibitor, PD173074, significantly suppressed these properties of CSCs (Figure 4H, 4I, 4J and Figure S5A–D). To further examine the effect of PDGF-BB, we introduced shPDGFB into TAM. Similarly, we introduced shRNA to both FGF7 and FGF9 into BM-hMSC cells. These cells and CSCs were then co-injected into mouse tibiae. We found that the knockdown of PDGFB in TAM indeed significantly abrogated the tumor growth promoting effect of TAM in the bone (Figure 4K). Furthermore, knockdown of FGF7 and FGF9 in BM-hMSC also significantly suppressed growth promoting effect of BM-hMSC (Figure 4L). To examine whether HAS2 expression in CSCs also directly affects the ability of stromal cells to promote CSC proliferation, we collected CM of co-cultured CSCs and stromal cells. CSCs were then treated with the CM and they were measured for proliferation. We found that the CM of co-cultured CSCs from 231BoM-shHAS2 and stromal cells did not affect the proliferation of CSCs treated with the CM from CSCs and stromal cells (Figure S4H). Therefore, the effect of HAS2 appears to be limited in stimulating TAMs to produce PDGF-BB. These results further support our notion that the direct interaction of TAMs and CSCs through hyaluronan stimulates the secretion of PDGF-BB which in turn activates stromal cells to secrete FGF7 and FGF9 that stimulate proliferation and self-renewal of CSCs.
Figure 4. TAMs promote the growth of CSCs through activation of bone stromal cells.
(A) Primary human monocytes were treated with IL-4, IL-13 and CM of 231BoM for 7 days and analyzed by FACS. The morphology of the cells was also shown. (B) CSCs of 231BoM or 231BoM-shHAS2 were co-injected with (right tibia) or without (left tibia) TAMs in the same animals. They were treated with or without 4-MU for 30 days. The normalized bioluminescent values were represented. P-value was calculated by Wilcoxon rank sum test (n=12–17). (C) CM from co-culture of TAMs and CSCs were subjected to Growth factor antibody array analysis. The position of PDGF-BB is indicated by a red box. (D) TAMs were co-cultured with CSCs from 231BoM or 231BoM-shHAS2 for 2 days. TAMs were then sorted by FACS, and the expression of PDGFB gene was quantified by qRT-PCR (n=3). (E) Immunofluorescent image of co-culture of TAMs with CSCs of 231BoM. (F) CSCs of 231BoM were treated with CM from various stromal cells that were pretreated with or without PDGF-BB (100 ng/ml) followed by measuring the growth of CSCs by the MTS assay (n=6). (G) CM from HS5, hFOB1.19 and BM-hMSC treated with 100 ng/ml of PDGF-BB were individually subjected to Cytokine antibody array analysis. (H) Growth rate of CSCs of 231BoM treated with FGF9 or FGF7 and a combination with FGFR inhibitor (PD173074) was measured by the MTS assay (n=3). (I) CSC population of 231BoM cells by the same treatment as described in (H) was measured by FACS (n=3). (J) Sphere formation in suspension culture of MCF7-BoM2d cells was measured as the average number of spheres per 500 cells and the results were plotted (n=6). (K) CSCs of 231BoM were co-injected with TAMs/sh-scramble (left tibia) or TAMs/shPDGFB (right tibia) in the same animals (n=5). (L) CSCs of 231BoM were co-injected with BM-hMSC/sh-scramble (left tibia) or BM-hMSC/shFGF7&shFGF9 (right tibia) in the same animals (n=5). The normalized bioluminescent values were represented.
HAS2 expression correlates with metastasis status of breast cancer patients
To further validate the clinical significance of HAS2 in breast cancer progression, we performed IHC analysis for specimens from breast cancer patients at various stages (Figure 5A). Our results indicate that the HAS2 expression was significantly up-regulated in breast cancers (Figure 5B upper) and positively correlated with the incidence of metastasis (Figure 5B lower). Importantly, HAS2 expression showed significant correlation with overall survival of the patients (Figure 5 C). To further examine the clinical significance of HAS2 and in combination with fifty genes that were identified by our array analysis for CSCs (Table S6), we evaluated the prognostic value of these genes as a combined signature in breast cancer patients. As shown in Figure 5D, we found that this gene set signature including HAS2 was significantly associated with overall survival and distant metastasis-free survival of breast cancer patients.
Figure 5. Prognosis value of HAS2 protein in patients.
(A) Immunohistochemical analysis for HAS2 expression was performed for clinical specimens from breast cancer patients at various stages. DCIS: ductal carcinoma in situ. The specificity of this antibody was shown in Figure S6. (B) The expression of HAS2 was examined in 81 patients (upper panel). The relationship of patient overall survival and the expression of HAS2 was examined in 47 patients (lower panel). (C) The relationship of HAS2 expression and patient overall survival was also examined by Kaplan-Meier analysis (n=31). P-value was calculated by log-rank test. (D) Kaplan-Meier analyses for overall (upper panel) and relapse-free (lower panel) survival of Desmedt breast cancer cohort was performed using the gene set signature that were indicated in Table S7. (E) Proposed model of paracrine loop for breast cancer progression in bones.
Discussion
In this report, we have demonstrated that CSCs isolated from metastatic breast tumor cells indeed show significantly higher tumorigenic as well as metastatic abilities compared to that from low-metastatic cells. CD44+/CD24−/ESA+ cells have both tumor initiating ability in vivo and self-renewal capability in vitro, and these are the most critical characteristics to define cancer stem cells. Theoretically, metastatic tumor cells should have these characteristics because metastasized tumor cells must re-initiate their growth at the distant organs. However, these markers per se are not necessarily correlated with the aggressiveness of the cancer cells, and it is likely that these markers and “stemness” are pre-requisites for metastatic tumor cells but not sufficient conditions for metastasis. In fact, we have shown in this report that HAS2 is a necessary factor to endow CSC with metastatic phenotype. Our results also indicate that the HAS2 gene is significantly up-regulated in the metastatic CSCs and that hyaluronan plays a critical role in generating a favorable microenvironment by promoting the interaction of TAM and CSCs followed by secretion of PDGF-BB by TAMs. This growth factor then activates other stromal cells that in turn augment the growth of CSCs by secreting FGF7 and FGF9 in the bone. Therefore, HAS2 plays a pivotal role in orchestrating the cascade event to structure the niche of CSCs in the bone (Figure 5E). Importantly, our results clearly indicate that blocking HAS2 expression in CSCs by a small molecule serves as an effective strategy for the treatment of metastatic disease.
Aberrant expression of hyaluronan in tumor cells and adjacent stroma has been known to be linked to tumor progression and poor survival of cancer patients (17, 18). In animal experiments, ectopic expression of HAS2 was indeed shown to promote invasiveness and metastatic ability of various cancer cell lines (19–22). HAS2 is responsible for the synthesis of large molecular weight hyaluronan and is involved in a variety of cellular functions including proliferation, differentiation and inflammation (23). One of the major receptors of hyaluronan is CD44 which is abundantly expressed on monocytes and macrophage lineage, and therefore, CSCs with a high level of HAS2 are likely to interact with other CD44-positive cells in the microenvironment which may endow CSCs with growth advantage. We have indeed shown that the metastatic CSCs which express high-level of HAS2 directly interact with TAMs to promote secretion of PDGF-BB. This result is consistent with the previous finding that monocytes are often recruited preferentially to stromal regions in hyaluronan-rich tumors (24) and that hyaluronan treatment of the monocytes can induce M2 conversion into TAMs (25). Therefore, the direct interaction of CSCs and TAMs is the critical step for remodeling the tumor microenvironment, and how TAMs promote CSCs growth is an intriguing question. Although previous reports indicate that TAMs secrete various cytokines and growth factors that augment the tumor growth (14), we found that neither CM of TAMs nor PDGF-BB alone showed little effect on the growth of CSCs in vitro. However, TAMs significantly enhanced the growth of CSCs in the animal, suggesting that the effect of TAMs on CSCs is indirect and other environmental factors or cells are involved. We indeed found that PDGF-BB secreted from TAMs was able to activate mesenchymal stem cells, osteoblasts, as well as bone stromal cells that in turn promoted the growth and self renewal of CSCs by secreting other growth factors and cytokines such as FGF7 and FGF9. It should be noted that PDGF-BB was also shown to stimulate both osteoblasts and osteoclasts and hence promote bone metastasis of tumor cells (26–28). It is also known that breast cancer patients who have high expression of PDGF in the tumor are associated with poor prognosis (29). Collectively, our results indicate that the initial contact of CSCs and TAMs and the following secretion of PDGF-BB triggers a series of cascade events for remodeling the microenvironment which involves mesenchymal stem cells, osteoblasts and bone stromal cells through intrinsic autocrine factors in a positive feedback loop. Furthermore, we have shown that PDGF-BB indeed activates these stromal cells to secrete FGF7 and FGF9 that are capable of enhancing self-renewal of CSCs in the bone microenvironment. Note that fibroblast growth factors are commonly used to expand the embryonic stem cells (30), suggesting that these factors have important roles in self-renewal of stem-like cells. More recently, FGF9 was found to promote the growth of CSC population in primary breast cancers (31). We have also shown that tumor growth is significantly enhanced by co-inoculation of stromal cells into the tibial bones, while the knockdown of these growth factors in the stromal cells significantly suppressed tumor growth. Therefore, HAS2 and its product, hyaluronan, are considered as master regulators for generating metastatic niche for CSCs. In this context, it is of significant interest to understand how the expression of HAS2 in CSCs is controlled by the environmental factors of niche. HAS2 has previously been shown to be up-regulated by TGF-beta and osteopontin that play roles in bone regeneration (32, 33). We have also shown here that BMP7 can significantly enhance the expression of HAS2 through activation of SMAD1 pathway as shown in Figure S7. Notably, BMP7 is one of the critical factors for bone regeneration and it is highly up-regulated in metastatic breast cancer (34–36), although the function of BMP7 is context dependent and it can serve as both promoter and suppressor depending on the stage and type of tumor (37). These results imply that bone regenerative conditions may favor creating propitious microenvironment for the growth of metastatic CSCs in the bone, although this idea needs to be further tested.
As we have shown that HAS2 and PDGF play major roles in generating the auto-looped microenvironment for CSCs, intervention of the HAS2-PDGF axis offers a window of therapeutic opportunity for metastatic disease. We have indeed shown that 4-MU can significantly suppress the incidence of metastasis and growth of CSCs in the bone due to specific inhibition of HA synthesis in our animal model. 4-MU is a natural compound and abundantly exists in many edible plants such as broccoli and celery (38) and the anti-cancer activity of this compound has been observed in prostate cancers and melanomas (39, 16). In fact, 4-MU is already approved by FDA and is currently under phase II clinical trial for the treatment of Hepatitis B and C infections. Therefore, 4-MU and a combination with other inhibitors for the auto-loop network of the CSCs microenvironment are considered to be promising therapeutic and preventive measures for metastatic breast cancer.
Supplementary Material
Acknowledgments
Financial support This work was supported by NIH (R01CA124650, R01CA129000 to KW), Department of Defense (BC085424, BC085590 to KW, BC096982 to AK) and Susan G. Komen (KG080477 to HO).
Footnotes
Conflict of interest The authors have declared that no conflict of interest exists.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–88. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–26. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 3.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Lippitt JM, Guzman-Ramirez N, et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010;70:5163–73. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 7.Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–15. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 10.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–7. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 11.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 12.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An emryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshihara S, Kon A, Kudo D, Nakazawa H, Kakizaki I, Sasaki M, et al. A hyaluronan synthase suppressor, 4-methylumbelliferone, inhibits liver metastasis of melanoma cells. FEBS Lett. 2005;579:2722–6. doi: 10.1016/j.febslet.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 17.Auvinen P, Tammi R, Parkkinen J, Tammi M, Agren U, Johansson R, et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol. 2000;156:529–36. doi: 10.1016/S0002-9440(10)64757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, et al. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol Chem. 2001;276:11922–32. doi: 10.1074/jbc.M008432200. [DOI] [PubMed] [Google Scholar]
- 19.Simpson MA, Reiland J, Burger SR, Furcht LT, Spicer AP, Oegema TR, Jr, et al. Hyaluronan synthase elevation in metastatic prostate carcinoma cells correlates with hyaluronan surface retention, a prerequisite for rapid adhesion to bone marrow endothelial cells. J Biol Chem. 2001;276:17949–57. doi: 10.1074/jbc.M010064200. [DOI] [PubMed] [Google Scholar]
- 20.Udabage L, Brownlee GR, Waltham M, Blick T, Walker EC, Heldin P, et al. Antisense-mediated suppression of hyaluronan synthase 2 inhibits the tumorigenesis and progression of breast cancer. Cancer Res. 2005;65:6139–50. doi: 10.1158/0008-5472.CAN-04-1622. [DOI] [PubMed] [Google Scholar]
- 21.Cook AC, Chambers AF, Turley EA, Tuck AB. Osteopontin induction of hyaluronan synthase 2 expression promotes breast cancer malignancy. J. Biol Chem. 2006;281:24381–9. doi: 10.1074/jbc.M602694200. [DOI] [PubMed] [Google Scholar]
- 22.Bharadwaj AG, Kovar JL, Loughman E, Elowsky C, Oakley GG, Simpson MA. Spontaneous metastasis of prostate cancer is promoted by excess hyaluronan synthesis and processing. Am J Pathol. 2009;174:1027–36. doi: 10.2353/ajpath.2009.080501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M, et al. Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res. 2010;70:7073–83. doi: 10.1158/0008-5472.CAN-09-4687. [DOI] [PubMed] [Google Scholar]
- 25.Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110:587–95. doi: 10.1182/blood-2007-01-068031. [DOI] [PubMed] [Google Scholar]
- 26.Franchimont N, Canalis E. Platelet-derived growth factor stimulates the synthesis of interleukin-6 in cells of the osteoblast lineage. Endocrinology. 1995;136:5469–75. doi: 10.1210/endo.136.12.7588297. [DOI] [PubMed] [Google Scholar]
- 27.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21:3763–70. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 29.Seymour L, Bezwoda WR. Positive immunostaining for platelet derived growth factor (PDGF) is an adverse prognostic factor in patients with advanced breast cancer. Breast Cancer Res Treat. 1994;32:229–33. doi: 10.1007/BF00665774. [DOI] [PubMed] [Google Scholar]
- 30.Gotoh N. Control of stemness by fibroblast growth factor signaling in stem cells and cancer stem cells. Curr Stem Cell Res Ther. 2009;4:9–15. doi: 10.2174/157488809787169048. [DOI] [PubMed] [Google Scholar]
- 31.Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, et al. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci USA. 2010;107:21737–42. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andhare RA, Takahashi N, Knudson W, Knudson CB. Hyaluronan promotes the chondrocyte response to BMP-7. Osteoarthritis Cartilage. 2009;17:906–16. doi: 10.1016/j.joca.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook AC, Chambers AF, Turley EA, Tuck AB. Osteopontin induction of hyaluronan synthase 2 expression promotes breast cancer malignancy. J Biol Chem. 2006;281:24381–9. doi: 10.1074/jbc.M602694200. [DOI] [PubMed] [Google Scholar]
- 34.Alarmo EL, Korhonen T, Kuukasjarvi T, Huhtala H, Holli K, Kallioniemi A. Bone morphogenetic protein 7 expression associates with bone metastasis in breast carcinomas. Ann Oncol. 2008;19:308–14. doi: 10.1093/annonc/mdm453. [DOI] [PubMed] [Google Scholar]
- 35.Alarmo EL, Parssinen J, Ketolainen JM, Savinainen K, Karhu R, Kallioniemi A. BMP7 influences proliferation, migration, and invasion of breast cancer cells. Cancer Lett. 2009;275:35–43. doi: 10.1016/j.canlet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 37.Masuda H, Fukabori Y, Nakano K, Shimizu N, Yamanaka H. Expression of bone morphogenetic protein-7 (BMP-7) in human prostate. Prostate. 2004;59:101–6. doi: 10.1002/pros.20030. [DOI] [PubMed] [Google Scholar]
- 38.Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today. 2008;13:161–71. doi: 10.1016/j.drudis.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Lokeshwar VB, Lopez LE, Munoz D, Chi A, Shirodkar SP, Lokeshwar SD, et al. Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res. 2010;70:2613–23. doi: 10.1158/0008-5472.CAN-09-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.