Abstract
Background
Continued cigarette smoking after small cell lung cancer (SCLC) diagnosis has been shown to shorten patients’ survival, but little is known about the impact of smoking and cessation on quality of life (QOL) profile (e.g., overall QOL, pain, fatigue, cough, dyspnea, appetite change, and performance status) in SCLC survivors (who survived at least 6 months post initial diagnosis). In this study, we sought to evaluate the relationship between cigarette smoking and QOL profiles in SCLC patients.
Methods
A total of 223 survivors were classified into five groups: never smokers, former smokers (quit more than 1 year prior to diagnosis), recent quitters (quit within 1 year surrounding diagnosis), late quitters (quit after 1 year post diagnosis) and never quitters. One hundred and sixty-eight of these survivors were matched with 334 lung-cancer-free controls on age, gender, and smoking status for comparative analysis. QOL scales were scored from 0 (worse) to 100 (best). Conditional logistic regression, linear mixed-effect models, and Wilcoxon signed rank tests were used.
Results
SCLC survivors consistently showed a significant deficit in QOL profile; e.g., mean overall QOL in patients was 17.5 points worse than the controls (p < 0.0001). Among all smokers, former smokers reported the best QOL profile, while late or never quitters reported the worst. The recent quitters showed an improving trend in QOL profile and lower percent of reduced appetite (an average of 43%) compared to the late or never quitters (58%).
Conclusions
Our study confirmed the negative impact of smoking on SCLC survivors’ QOL and found that smoking cessation surrounding the time of diagnosis could improve overall QOL and symptoms. The findings of this study provide evidence for oncologists to recommend smoking cessation to their SCLC patients.
Keywords: Quality of life, Cigarette smoking, Small cell lung cancer, Symptom burden
1. Introduction
Health-related quality of life (QOL) has become a very important outcome for cancer therapy especially when the treatment intent is supportive or palliative or the survival benefits are modest. Therefore, although survival is generally the most important measurement for treatment efficacy, patients and physicians are giving greater consideration to multiple dimensions of QOL including symptom burden. Indeed, QOL at baseline may be as important as stage and performance status in predicting non-small cell lung cancer (NSCLC) survival,1 but the QOL of small cell lung cancer (SCLC) has not been well documented. SCLC represents approximately 13% of all lung cancer2 and is the most aggressive cell type among all subtypes. From the time of diagnosis, the median ranges of survival for limited stage and extensive stage SCLC are 15–20 months and 8–13 months, respectively.3,4 Despite that a modest improvement has been achieved in the past two decades in treating SCLC,5–7 treatment bears positive and negative effects on patients’ QOL. Specifically, side-effects from various therapies could severely deteriorate patients’ conditions.
Cigarette smoking is the most predominant risk factor in SCLC prognosis. A personal history of cigarette smoking has been associated with decreased overall survival among patients receiving treatment for both SCLC and non-small cell lung cancer (NSCLC).8–11 Our recent study of a series of 284 SCLC patients also showed that compared to continuing smokers (those who never quit smoking), patients who quit at or after diagnosis cut the risk of death by 45%,7 but little is known about smoking’s effects on patients’ QOL in SCLC survivors. The negative effects of cigarette smoking on QOL in general and specific disease populations have been well established, including NSCLC patients.12–19 In this study, we sought to determine the relationship between smoking and QOL profile, including overall QOL and selected symptoms, in a relatively large series of SCLC survivors. We hypothesised that cigarette smoking would have a negative effect on the QOL profile of SCLC survivors, and we specifically evaluated the differing effects of the timing of smoking cessation.
2. Methods and materials
2.1. Patients follow-up and clinical data
This study was undertaken within a prospectively followed lung cancer patient cohort at Mayo Clinic in Minnesota starting on January 1, 1997, and was approved by the Mayo Clinic Institutional Review Board. Informed consent was obtained from the subjects and/or proxies. Details of patient enrollment, diagnosis confirmation, follow-up procedures, data collection, and quality assurance have been previously reported.20,21 In brief, at enrolment, each patient’s medical record was reviewed and the diagnosis was confirmed. SCLC was classified into the two stage system: limited stage SCLC is defined as disease confined to the ipsilateral hemithorax and within a single radiotherapy port and extensive stage SCLC is defined as evident metastatic disease outside the ipsilateral hemithorax.22 To acquire complete follow-up information, all patients were actively followed by a series of questionnaires, including QOL and symptom questions beginning within 6 months post diagnosis and then annually thereafter. Of all patients in this study who answered their first questionnaire within 12 months post diagnosis, 9.4% answered between months 3–6 and 90.6% answered between months 7–12.
Information on current and past use of tobacco products was self-reported and collected each time during follow-up through a structured questionnaire. A never smoker was defined as one who had smoked less than 100 cigarettes in his or her lifetime. Current smokers were individuals who reported tobacco use within the same year of their diagnosis. In order to evaluate the impact of timing on the QOL profile in this study, we categorised survivors according to time of smoking cessation in relation to diagnosis time: early quitters (more than 1 year prior to the date of diagnosis), recent quitters (within 1 year surrounding diagnosis, either prior to or after the date of diagnosis), late quitters (after 1 year post diagnosis) and never quit.
2.2. Health-related quality of life (QOL) assessment
The QOL measure was a built-in component of the lung cancer follow-up questionnaire. The Lung Cancer Symptom Scale (LCSS) was used at the outset, starting in October 1999. In April 2005, the Linear Analogue Self-Assessment (LASA) was implemented and the redundant LCSS items were dropped to minimise patients’ burden yet expand QOL measures beyond symptoms. Both are previously validated QOL tools.23,24 For the purpose of this study, we focused on the five overlapping items between these two tools: pain, fatigue, cough, dyspnea and overall QOL. Two additional domains included were patient-reported Eastern Cooperative Oncology Group (ECOG) performance status (PS) and appetite change since diagnosis. Appetite was self-rated using a previously validated item.25 For each QOL item, patients were asked to provide their best answer during the past week, and we summarised all above-mentioned measures as ‘QOL profile’.
To understand the QOL profile of SCLC patients relative to the normal population, we compared each QOL item in SCLC patients who returned a questionnaire within 1 year post diagnosis to controls using a matched case–control design. Controls were from Mayo Clinicbased computed tomography (CT) scan trials of healthy heavy smokers over 50 years of age as described in previous publications,26 and pulmonary medicine patients who had a chest CT-scan and pulmonary function test; all individuals were free of any lung cancer at the time of the QOL data collection.
2.3. Statistical analyses
The primary goal of this study was to describe the health-related QOL profile in SCLC patients in relationship to smoking status and time of smoking cessation. Original scores of pain, fatigue, cough, dyspnea and global QOL were translated onto a 0–100 point scale for comparability and ease of interpretability,27 with higher scores representing better status. For example, higher overall QOL scores indicate better quality of life; higher pain scores correspond to less pain. PS was re-grouped into three levels: fully active versus light work restricted versus worse. Appetite change was summarised by two levels: increased or same versus reduced.
Patient characteristics were summarised by limited versus extensive stage SCLC, and the differences were compared using a Chi-square test (or Fisher’s exact test) for categorical variables and a two sample t-test (or Wilcoxon rank sum test) for continuous variables. Descriptive statistics are provided for each QOL item, PS, appetite change, and smoking status at each follow-up time point and these data are also presented graphically. A one-way analysis of variance (ANOVA) was used to test the difference among the five groups (year 1 through year 5) for continuous QOL items, and a Chi-square test was used for PS, appetite change, and smoking status. Changes from within 1 year versus over 2 years post diagnosis were calculated and tested using Wilcoxon signed rank tests. A change in the magnitude of one-half standard deviation (SD) of QOL, which was considered clinically meaningful,27 was examined overall and by smoking status to explore the impact of smoking status on patient QOL.
In the matched case–control analysis, each SCLC case was matched to 1 or 2 controls based on age (within 5 years, age at diagnosis for cases and at the time of QOL data collection for controls), gender, and smoking status (at diagnosis for cases and at time of QOL data collection for controls) using an optimal match algorithm.28 The same questionnaire excluding lung-cancer-specific items was used for controls. Conditional logistic regression was used to model categorical factors accounting for matching and linear mixed-effect models with matched set as random effect for continuous factors. All analyses were performed with SAS software, version 8.2 (SAS Institute, Cary, NC).
3. Results
3.1. SCLC patient cohort
A total of 223 patients with SCLC who were diagnosed between 1998 and 2008 and who have answered a follow-up questionnaire at least once were included in this study. At the time of diagnosis, 85 patients (38%) had extensive disease; the median age was 65 years (range, 37–89 years) and 54% were males; 3% were never smokers, 44% were former smokers, and 53% were current smokers. Forty-one percent of the patients quit smoking more than 1 year before diagnosis; an additional 32% quit before lung cancer treatment and 14% never quit smoking. The median follow-up time for living patients was 3 years with a range of 6 months to 10 years. Table 1 provides more information on patient characteristics and comparisons by disease stage.
Table 1.
Characteristics of 223 small cell lung cancer patients by stage.
| Limited stage (n = 138) | Extensive stage (n = 85) | Total (n = 223) | p-Value* | |
|---|---|---|---|---|
| Age at diagnosis | 0.5797a | |||
| N | 138 | 85 | 223 | |
| Mean (standard deviation (SD)) | 64.4 (9.8) | 65.2 (10.0) | 64.7 (9.8) | |
| Median | 65.0 | 66.0 | 65.0 | |
| Range | (38.0–87.0) | (37.0–89.0) | (37.0–89.0) | |
| Sex | 0.0223b | |||
| Female | 72 (52.2%) | 31 (36.5%) | 103 (46.2%) | |
| Male | 66 (47.8%) | 54 (63.5%) | 120 (53.8%) | |
| Race | 0.5388c | |||
| Non-Caucasian** | 8 (5.8%) | 3 (3.5%) | 11 (4.9%) | |
| Caucasian | 130 (94.2%) | 82 (96.5%) | 212 (95.1%) | |
| Smoking status at diagnosis | 0.8631c | |||
| Never smoker | 4 (2.9%) | 2 (2.4%) | 6 (2.7%) | |
| Former smoker | 59 (42.8%) | 40 (47.1%) | 99 (44.4%) | |
| Current smoker | 75 (54.3%) | 43 (50.6%) | 118 (52.9%) | |
| Pack-years of smokers | 0.1089c | |||
| Missing | 0 (–) | 1 (–) | 1 | |
| 0–40 | 34 (24.6%) | 27 (32.1%) | 61 (27.5%) | |
| 41–60 | 54 (39.1%) | 20 (23.8%) | 74 (33.3%) | |
| >60 | 46 (33.3%) | 35 (41.7%) | 81 (36.5%) | |
| Smoking cessation | 0.4507c | |||
| Early quitters (Quit ≥1 year before diagnosis) | 53 (38.4%) | 39 (45.9%) | 92 (41.3%) | |
| Recent quitters (Quit within 1-year of diagnosis) | 56 (40.6%) | 32 (37.7%) | 88 (39.5%) | |
| Late quitters (Quit ≥1 year after diagnosis) | 5 (3.6%) | 0 (0.0%) | 5 (2.2%) | |
| Never quitters | 20 (14.5%) | 12 (14.1%) | 32 (14.3%) |
p-Value for between stage comparison;
Wilcoxon rank sum;
Chi-square;
Fisher’s exact.
Non-Caucasian includes Hispanic and Alaskan/Native American.
3.2. Health-related QOL profile between SCLC patients and comparable controls free of lung cancer
In the analysis comparing baseline QOL to a control group free of lung cancer, 168 SCLC patients who returned a questionnaire within 1 year post diagnosis were successfully matched to 334 controls (166 cases each matched to two controls and two cases each matched to one control). Table 2 summarises characteristics and QOL comparison between the cases and matched controls. Pack-years of cigarette smoking history and smoking cessation were found to be significantly different between the two groups; therefore, these two factors were adjusted in the case–control comparison analysis. SCLC survivors consistently showed a statistically significant worse QOL compared to the matched controls (Table 2). For instance, the mean overall QOL was 63.4 in the SCLC survivors and 80.9 in the controls, a significant 17.5-point difference (p < 0.0001).
Table 2.
Characteristics of matched cases and controls.a
| Cases (n = 168) | Controls (n = 334) | p-Value | |
|---|---|---|---|
| Race | 0.6360e | ||
| Missing | 0 (–) | 1 (–) | |
| Non-Caucasian | 6 (3.6%) | 15 (4.5%) | |
| Caucasian | 162 (96.4%) | 318 (95.5%) | |
| Pack-years among smokers | 0.0004e | ||
| Missing | 0 (–) | 6 (–) | |
| 0–40 | 44 (26.2%) | 137 (41.8%) | |
| 41–60 | 59 (35.1%) | 97 (29.6%) | |
| >60 | 59 (35.1%) | 82 (25%) | |
| Smoking cessationb | <0.0001e | ||
| Early quitters (Quit ≥1 year before diagnosis/questionnaire) | 85 (50.6%) | 177 (53%) | |
| Recent quitters (Quit <1 year before diagnosis/questionnaire) | 64 (38.1%) | 20 (6%) | |
| Never quitters | 19 (11.3%) | 137 (41%) | |
| Overall quality of life (QOL)c, N | 163 | 334 | <0.0001f |
| Mean (SD) | 63.4 (23.2) | 80.9 (15.9) | <0.0001g |
| Range | (0.0–100.0) | (20.0–100.0) | |
| Pain, N | 163 | 333 | <0.0001f |
| Mean (SD) | 79.6 (23.0) | 69.2 (25.3) | <0.0001g |
| Range | (0.0–100.0) | (10.0–100.0) | |
| Fatigue, N | 168 | 334 | <0.0001f |
| Mean (SD) | 48.5 (23.0) | 63.8 (25.2) | <0.0001g |
| Range | (0.0–100.0) | (0.0–100.0) | |
| Coughing, N | 167 | 334 | 0.0059f |
| Mean (SD) | 68.6 (23.5) | 74.7 (22.9) | 0.0017g |
| Range | (5.0–100.0) | (0.0–100.0) | |
| Dyspnea, N | 168 | 333 | 0.0003f |
| Mean (SD) | 58.5 (26.9) | 67.8 (26.2) | 0.0022g |
| Range | (0.0–100.0) | (0.0–100.0) | |
| Eastern Cooperative Oncology Group (ECOG) performance statusc | <0.0001e | ||
| Missing | 2 (–) | 0 (–) | |
| Fully active | 21 (12.7%) | 185 (55.6%) | |
| Light work | 100 (60.2%) | 136 (40.8%) | |
| Unable to work and worse | 45 (27.1%) | 12 (3.6%) | |
| Appetite changec,d | <0.0001e | ||
| Missing | 5 (–) | 0 (–) | |
| Increased/same | 78 (47.9%) | 260 (77.8%) | |
| Reduced | 85 (52.1%) | 74 (22.2%) |
Matching variables: age, sex, and smoking status.
Time relative to diagnosis date for cases and date of questionnaire for controls.
Used the first questionnaire returned within the 1-year time frame for cases.
Appetite change since diagnosis for cases and appetite change since 1 year ago for controls.
Conditional logistic regression.
Mixed-effect model with matched set as a random effect.
Mixed-effect model with matched set as a random effect, includes pack-years and smoking cessation as covariates.
Table 3 shows the cross-sectional mean QOL profiles of SCLC survivors over a 5-year follow-up period; overall QOL and symptom scales of pain, fatigue, cough, and dyspnea were relatively stable; e.g., mean overall QOL was between 63.0 (year 1, SD 23.2) and 67.6 (year 5, SD 21.1). Pain seemed not to be a major problem in survivors, with mean scores ranging from 74.4 (SD 25.4) to 79.5 (SD 23.6). SCLC survivors scored consistently more severe on fatigue (mean scores between 48.5 and 52.7) and dyspnea (mean scores between 57.1 and 59.0). Coughing was moderate, with a lowest mean score of 66.4 at year 4. For the ECOG PS, most patients were fully active or capable of light work, with roughly one-third unable to work or having worse status (ranging between 25% at year 2 to 33.3% at year 3) except year 5 (10.7%). Higher percentages of reduced appetite were observed earlier in the follow-up period with 52% at year 1 and 32.1% at year 5.
Table 3.
Follow-up questionnaire variables (QOL, performance status (PS), appetite change, and smoking status).*
| 1 year (N = 177) | 2 years (N = 78) | 3 years (N = 54) | 4 years (N = 48) | 5+ years (N = 29) | p-Value** | |
|---|---|---|---|---|---|---|
| Overall QOL | 0.0382 | |||||
| N | 173 | 72 | 52 | 47 | 29 | |
| Mean (SD) | 63.0 (23.2) | 63.8 (25.4) | 64.8 (24.5) | 67.4 (26.8) | 67.6 (21.1) | |
| Range | (0.0–100.0) | (0.0–100.0) | (0.0–100.0) | (0.0–100.0) | (24.0–94.0) | |
| Pain | 0.1220 | |||||
| N | 172 | 71 | 51 | 46 | 29 | |
| Mean (SD) | 79.1 (23.6) | 76.0 (25.4) | 79.5 (23.6) | 77.9 (23.9) | 74.4 (25.4) | |
| Range | (0.0–100.0) | (6.0–100.0) | (3.0–100.0) | (3.0–100.0) | (14.0–100.0) | |
| Fatigue | 0.6735 | |||||
| N | 177 | 74 | 53 | 47 | 29 | |
| Mean (SD) | 48.5 (22.9) | 50.8 (27.1) | 52.7 (21.4) | 49.7 (21.3) | 49.1 (24.8) | |
| Range | (0.0–100.0) | (0.0–100.0) | (2.0–94.0) | (0.0–98.0) | (4.0–92.0) | |
| Coughing | 0.4874 | |||||
| N | 176 | 75 | 53 | 43 | 29 | |
| Mean (SD) | 69.4 (23.3) | 67.6 (24.2) | 72.1 (24.8) | 66.4 (24.1) | 71.2 (24.7) | |
| Range | (5.0–100.0) | (3.0–100.0) | (3.0–100.0) | (8.0–100.0) | (3.0–100.0) | |
| Dyspnea | 0.6662 | |||||
| N | 177 | 74 | 53 | 46 | 29 | |
| Mean (SD) | 59.0 (26.9) | 58.1 (27.7) | 57.6 (27.7) | 57.9 (28.7) | 57.1 (29.2) | |
| Range | (0.0–100.0) | (0.0–100.0) | (5.0–100.0) | (0.0–100.0) | (6.0–96.5) | |
| ECOG PS | 0.0154 | |||||
| Missing | 2 (–) | 2 (–) | 0 (–) | 0 (–) | 1 (–) | |
| Fully active | 22 (12.6%) | 17 (22.4%) | 7 (13%) | 8 (16.7%) | 11 (39.3%) | |
| Light work | 107 (61.1%) | 40 (52.6%) | 29 (53.7%) | 26 (54.2%) | 14 (50%) | |
| Unable to work and worse | 46 (26.3%) | 19 (25%) | 18 (33.3%) | 14 (29.2%) | 3 (10.7%) | |
| Appetite change since diagnosis | 0.0048 | |||||
| Missing | 4 (–) | 1 (–) | 3 (–) | 3 (–) | 1 (–) | |
| Increased/same | 83 (48%) | 46 (59.7%) | 31 (60.8%) | 28 (62.2%) | 19 (67.9%) | |
| Reduced | 90 (52%) | 31 (40.3%) | 20 (39.2%) | 17 (37.8%) | 9 (32.1%) | |
| Smoking status | 0.2508 | |||||
| Never smokers | 6 (3.4%) | 3 (3.8%) | 2 (3.7%) | 2 (4.2%) | 1 (3.4%) | |
| Former smokers | 140 (79.1%) | 60 (76.9%) | 41 (75.9%) | 35 (72.9%) | 24 (82.8%) | |
| Current smokers | 31 (17.5%) | 15 (19.2%) | 11 (20.4%) | 11 (22.9%) | 4 (13.8%) |
The table reports multiple responses per person, but all p-values were calculated after subsetting to each case’s first response.
The analysis of variance (ANOVA) method was used to test the difference among the five groups (1 year through 5 year) for continuous factors, and Chi-square test for categorical factors.
3.3. Impact of smoking status and smoking cessation on health-related QOL among SCLC survivors
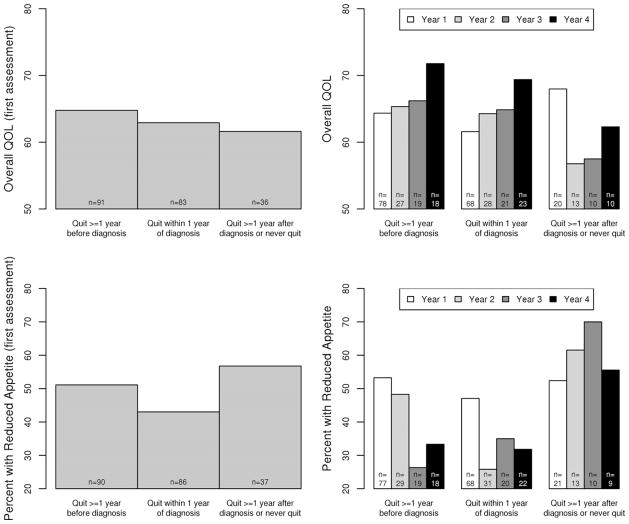
We analysed QOL profiles across the 5 years of follow-up for each symptom as well as overall QOL according to smoking status and the time of quitting smoking; data of year 4 and beyond were combined due to fewer survivors. Fig. 1 illustrates the key findings based on mean overall QOL (upper panel) and reduced appetite (lower panel) over follow-up years. Among the three subgroups of smokers, early quitters reported the best overall QOL, while recent quitters reported the best symptoms scores, e.g., the lowest percentage of reduced appetite. Late or never quitters reported the worst QOL, e.g., from year 2 to year 4 they had the lowest overall QOL (56–62) and the highest percentage of reduced appetite (55–70%). Overall, the recent quitters showed an improving trend of QOL profile and lower percentage of reduced appetite at first assessment (43%) compared to the late or never quitters (57%).
Fig. 1.
Time trend of overall quality of life (QOL) and reduced appetite among groups of patients by smoking cessation. Upper panel: mean overall QOL; lower panel: reduced appetite. (footnote: data of year 4 and beyond were combined.)
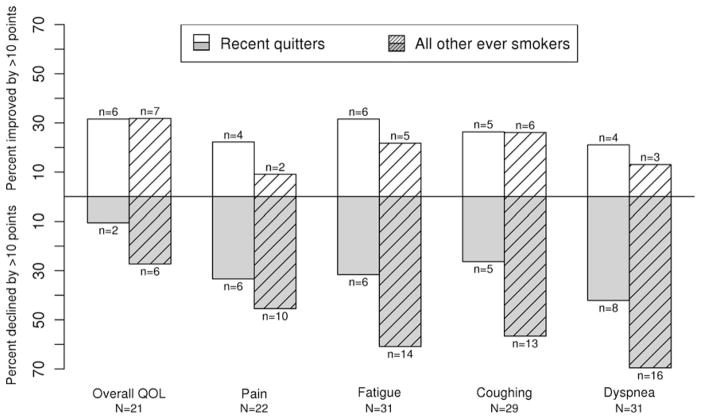
In order to assess the longitudinal changes of reported QOL over time in the same patients, we evaluated data from 45 patients who answered questionnaires within 1 year after diagnosis and beyond 2 years after diagnosis (Table 4). Among these 45 patients, 3 never smoked cigarettes (removed from further analysis due to small number), 19 were recent quitters (subgroup 1), and the remaining 23 were former smokers and late or never quitters (subgroup 2). Fig. 2 depicts the percentage of patients with clinically significant improvement or decline of their reported QOL profile over the two time intervals; specifically, for example, fatigue (middle panel) improved in 32% and declined in 32% in subgroup 1 although there was no statistically significant change in fatigue status between within-1-year and over-2-years; in subgroup 2, fatigue significantly declined in 61% and improved in only 22% of patients.
Table 4.
Difference in overall QOL and selected symptoms at within 1 year versus over 2 years post diagnosis of small cell lung cancer.
| QOL | Mean (SD) <1 year |
Mean (SD) ≥2 years |
Signed rank test p-value |
Percent (%) declined by ≥10 points | Percent (%) improved by ≥10 points |
|---|---|---|---|---|---|
| All patients (n = 45*) with <1 and ≥2 years QOL assessment | |||||
| Overall QOL | 65.8 (19.9) | 69.0 (22.5) | 0.14 | 18.18 | 31.82 |
| Pain | 81.7 (20.4) | 73.5 (22.5) | 0.02 | 37.21 | 13.95 |
| Fatigue | 54.5 (19.4) | 47.5 (23.7) | 0.06 | 44.44 | 24.44 |
| Coughing | 69.6 (20.1) | 66.2 (20.1) | 0.21 | 40.00 | 26.67 |
| Dyspnea | 68.9 (23.3) | 56.0 (26.0) | <0.01 | 55.56 | 17.78 |
| Sub-group 1** (n = 19): recent quitters | |||||
| Overall QOL | 61.9 (17.6) | 69.7 (20.8) | 0.07 | 10.53 | 31.58 |
| Pain | 76.3 (22.6) | 75.4 (20.9) | 0.49 | 33.33 | 22.22 |
| Fatigue | 50.2 (18.6) | 48.4 (26.8) | 0.84 | 31.58 | 31.58 |
| Coughing | 70.1 (18.6) | 70.3 (18.5) | 0.85 | 26.32 | 26.32 |
| Dyspnea | 68.0 (21.3) | 58.6 (29.8) | 0.17 | 42.11 | 21.05 |
| Sub-group 2*** (n = 23): early quitters, late or never quitters | |||||
| Overall QOL | 67.5 (22.0) | 66.2 (24.6) | 0.90 | 27.27 | 31.82 |
| Pain | 85.0 (18.9) | 69.2 (23.8) | 0.01 | 45.45 | 9.09 |
| Fatigue | 56.2 (19.4) | 44.7 (21.1) | 0.03 | 60.87 | 21.74 |
| Coughing | 68.4 (21.5) | 61.1 (20.7) | 0.07 | 56.52 | 26.09 |
| Dyspnea | 68.2 (25.5) | 51.5 (22.2) | 0.01 | 69.57 | 13.04 |
Three (3) of the 45 patients were never smokers and removed from the subgroup analysis regarding quitting smoking time.
Subgroup 1: recent quitters were patients who quit smoking within 1 year of diagnosis.
Subgroup 2: former smokers and late or never quitters were patients who were smokers, not in sub-group 1.
Fig. 2.
Difference in overall QOL and selected symptoms for patients with assessments within 1 year and over 2 years post diagnosis of small cell lung cancer (SCLC).
4. Discussion
Although it has been shown that SCLC patients who continue to smoke have poorer chances of survival than those who do not,7,9,11 the relationship between smoking cessation (timing and duration) and QOL in SCLC patients has not been adequately studied. In this matched analysis, we demonstrated that SCLC patients consistently showed a statistically significant worse QOL. Over the 5-year follow-up period, cross-sectional overall QOL and symptom scales of pain, fatigue, cough, and dyspnea were relatively stable. Smoking status had a significant impact on overall QOL and each individual symptom scale in SCLC patients, with the exception of year 1 after diagnosis. Never smokers reported in general the best QOL profile, while late or never quitters reported the worst. More importantly, recent quitters showed a trend of improvement in overall QOL and better scores on all symptoms in this study than never quitters.
The negative impact of smoking on QOL in both the general patient population and in specific subgroups has been reported in a number of studies.12–14,18 Other studies reporting similar findings were based on patients with head and neck cancer, asthma, HIV infection, or other conditions, where current smoking was negatively associated with multiple QOL scales including physical functioning, general health, vitality or energy, cognitive and social functioning, and role-emotional health.15–17,19
The first study of QOL of lung cancer survivors, composed of 142 NSCLC patients, found that smoking status was not predictive of QOL.29 However, this study included only prevalent long-term survivors and had a relatively small number of NSCLC patients; the authors suggested that further study is needed to address this question. In our previous study of the effect of cigarette smoking on QOL in a large series of lung cancer patients (92% with NSCLC),30 we found that continued cigarette smoking is related to a deficit in QOL measures. One caveat of this study was that SCLC was not separately studied due to small sample size, therefore the results were virtually driven by NSCLC patients.
In our current study, when comparing the baseline QOL of SCLC patients with the matched non-cancer controls, we unsurprisingly found that SCLC patients consistently showed a significantly worse overall QOL. This was the case with four of the five individual symptoms as well as PS and appetite. An unexpected finding was that the controls reported worse pain than the cases, which is believed to reflect one of the important characteristics of subjects in the control groups, i.e., they were heavy smokers participating in lung cancer screening trials or non-cancer patients with lung diseases.
Although the cross-sectional mean QOL and symptom scores were relatively stable during the course of the 5-year follow-up, significant differences were detected when the patient population was divided into five groups by smoking history. In line with the findings from the literature, we found that, except for year 1, never and former smokers with SCLC reported the best QOL in general, while late or never quitters reported the worst. To our best knowledge, our study is the first to confirm the negative impact of smoking on SCLC survivors’ QOL by the timing and duration of smoking cessation.
Several limitations should be recognised when interpreting the results in this study. First, only seven selected QOL and symptom scales were analysed: five overlapping items from LCSS and LASA plus patient-reported ECOG PS and appetite change. Further investigation is needed to explore other dimensions of QOL in SCLC patients. Second, we did not observe a meaningful difference in age and smoking cessation status at the time of diagnosis by stage of SCLC; however, this may reflect the cases enrolled in our institution, e.g., 62% being limited stage. Third, although QOL data were collected yearly after SCLC diagnosis, the number of patients who consistently responded to the survey each year is small, particularly among patients with extensive stage SCLC. Due to insufficient longitudinal data, the ability to reach a definitive conclusion about QOL trend during the course of the disease is limited. Finally, QOL data in the control group may not represent the general population; instead, they only represent ‘high-risk’ people who took CT-scans to rule out lung cancer or other lung conditions. Coupled with the pre-existing medical conditions of the controls, the strength of QOL comparisons between SCLC patients and ‘healthy’ controls was compromised.
In conclusion, our study confirmed the negative impact of smoking on SCLC survivors’ QOL and found that smoking cessation surrounding the time of diagnosis could improve overall QOL and symptoms. This information provides evidence for oncologists to recommend smoking cessation to their SCLC patients.
Acknowledgments
The authors thank Susan Ernst, M.A., for her technical assistance with the manuscript. This work was funded by the U.S. National Institutes of Health (Grant R01 CA 115857) and Mayo Clinic Foundation Funds.
Footnotes
Conflict of interest statement
All authors have no conflict of interest to disclose.
References
- 1.Hollen PJ, Gralla RJ, Cox C, Eberly SW, Kris MG. A dilemma in analysis: issues in the serial measurement of quality of life in patients with advanced lung cancer. Lung Cancer. 1997;18(2):119–36. doi: 10.1016/s0169-5002(97)00059-7. [DOI] [PubMed] [Google Scholar]
- 2.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 3.Seifter EJ, Ihde DC. Therapy of small cell lung cancer: a perspective on two decades of clinical research. Semin Oncol. 1988;15(3):278–99. [PubMed] [Google Scholar]
- 4.Osterlind K, Hansen HH, Hansen M, Dombernowsky P, Andersen PK. Long-term disease-free survival in small cell carcinoma of the lung: a study of clinical determinants. J Clin Oncol. 1986;4(9):1307–13. doi: 10.1200/JCO.1986.4.9.1307. [DOI] [PubMed] [Google Scholar]
- 5.Glisson BS, Hong WK. Survival after treatment of small-cell lung cancer: an endless uphill battle. J Natl Cancer Inst. 1997;89(23):1745–7. doi: 10.1093/jnci/89.23.1745. [DOI] [PubMed] [Google Scholar]
- 6.Janne PA, Freidlin B, Saxman S, et al. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer. 2002;95(7):1528–38. doi: 10.1002/cncr.10841. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Jiang R, Garces YI, et al. Prognostic factors for limited-stage small cell lung cancer: a study of 284 patients. Lung Cancer. 2010;67(2):221–6. doi: 10.1016/j.lungcan.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins SJ, Pereira JR. Clinical factors and prognosis in non-small cell lung cancer. Am J Clin Oncol. 1999;22(5):453–7. 1600. doi: 10.1097/00000421-199910000-00006. [DOI] [PubMed] [Google Scholar]; J Chen et al/European Journal of Cancer. 2012;48:1593–1601. doi: 10.1016/j.ejca.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Videtic GM, Stitt LW, Dar AR, et al. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. J Clin Oncol. 2003;21(8):1544–9. doi: 10.1200/JCO.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 10.Ebbert JO, Williams BA, Sun Z, et al. Duration of smoking abstinence as a predictor for non-small-cell lung cancer survival in women. Lung Cancer. 2005;47(2):165–72. doi: 10.1016/j.lungcan.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 11.Johnston-Early A, Cohen MH, Minna JD, et al. Smoking abstinence and small cell lung cancer survival. An association. JAMA. 1980;244(19):2175–9. [PubMed] [Google Scholar]
- 12.Tillmann M, Silcock J. A comparison of smokers’ and ex-smokers’ health-related quality of life. J Public Health Med. 1997;19(3):268–73. doi: 10.1093/oxfordjournals.pubmed.a024629. [DOI] [PubMed] [Google Scholar]
- 13.Wilson D, Parsons J, Wakefield M. The health-related quality-of-life of never smokers, ex-smokers, and light, moderate, and heavy smokers. Prev Med. 1999;29(3):139–44. doi: 10.1006/pmed.1999.0523. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell CJ, Hirdes JP. The prevalence of smoking and implications for quality of life among the community-based elderly. Am J Prev Med. 1993;9(6):338–45. [PubMed] [Google Scholar]
- 15.Sippel JM, Pedula KL, Vollmer WM, Buist AS, Osborne ML. Associations of smoking with hospital-based care and quality of life in patients with obstructive airway disease. Chest. 1999;115(3):691–6. doi: 10.1378/chest.115.3.691. [DOI] [PubMed] [Google Scholar]
- 16.Taira DA, Seto TB, Ho KK, et al. Impact of smoking on healthrelated quality of life after percutaneous coronary revascularization. Circulation. 2000;102(12):1369–74. doi: 10.1161/01.cir.102.12.1369. [DOI] [PubMed] [Google Scholar]
- 17.Turner J, Page-Shafer K, Chin DP, et al. Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. AIDS Patient Care STDS. 2001;15(12):615–24. doi: 10.1089/108729101753354617. [DOI] [PubMed] [Google Scholar]
- 18.Rachiotis G, Behrakis PK, Vasiliou M, Yfantopoulos J. Quality of life and smoking among industrial workers in Greece. Med Lav. 2006;97(1):44–50. [PubMed] [Google Scholar]
- 19.Duffy SA, Terrell JE, Valenstein M, et al. Effect of smoking, alcohol, and depression on the quality of life of head and neck cancer patients. Gen Hosp Psychiatry. 2002;24(3):140–7. doi: 10.1016/s0163-8343(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 20.Yang P, Allen MS, Aubry MC, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128(1):452–62. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 21.Jatoi A, Williams BA, Marks R, et al. Exploring vitamin and mineral supplementation and purported clinical effects in patients with small cell lung cancer: results from the Mayo Clinic lung cancer cohort. Nutr Cancer. 2005;51(1):7–12. doi: 10.1207/s15327914nc5101_2. [DOI] [PubMed] [Google Scholar]
- 22.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep. 1973;4(2):31–42. [PubMed] [Google Scholar]
- 23.Hollen PJ, Gralla RJ, Kris MG, Potanovich LM. Quality of life assessment in individuals with lung cancer: testing the Lung Cancer Symptom Scale (LCSS) Eur J Cancer. 1993;29A(Suppl 1):S51–8. doi: 10.1016/s0959-8049(05)80262-x. [DOI] [PubMed] [Google Scholar]
- 24.Grunberg SM, Groshen S, Steingass S, Zaretsky S, Meyerowitz B. Comparison of conditional quality of life terminology and visual analogue scale measurements. Qual Life Res. 1996;5(1):65–72. doi: 10.1007/BF00435970. [DOI] [PubMed] [Google Scholar]
- 25.Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst. 1990;82(13):1127–32. doi: 10.1093/jnci/82.13.1127. [DOI] [PubMed] [Google Scholar]
- 26.Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology. 2003;226(3):756–61. doi: 10.1148/radiol.2263020036. [DOI] [PubMed] [Google Scholar]
- 27.Sloan J, Symonds T, Vargas-Chanes D, Fridley B. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Inf J. 2003;37:23–31. [Google Scholar]
- 28.Ming K, Rosenbaum PR. A note on optimal matching with variable controls using the assignment algorithm. J Comput Graphical Stat. 2001;10(3):455–63. [Google Scholar]
- 29.Sarna L, Padilla G, Holmes C, et al. Quality of life of long-term survivors of non-small-cell lung cancer. J Clin Oncol. 2002;20(13):2920–9. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- 30.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126(6):1733–41. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]