Abstract
Cognitive control is required for correct performance on antisaccade tasks, including the ability to inhibit an externally driven ocular motor response (a saccade to a peripheral stimulus) in favor of an internally driven ocular motor goal (a saccade directed away from a peripheral stimulus). Healthy humans occasionally produce errors during antisaccade tasks, but the mechanisms associated with such failures of cognitive control are uncertain. Most research on cognitive control failures focuses on poststimulus processing, although a growing body of literature highlights a role of intrinsic brain activity in perceptual and cognitive performance. The current investigation used dense array electroencephalography and distributed source analyses to examine brain oscillations across a wide frequency bandwidth in the period before antisaccade cue onset. Results highlight four important aspects of ongoing and preparatory brain activations that differentiate error from correct antisaccade trials: (1) ongoing oscillatory beta (20–30 Hz) power in anterior cingulate before trial initiation (lower for error trials); (2) instantaneous phase of ongoing alpha/theta (7 Hz) in frontal and occipital cortices immediately before trial initiation (opposite between trial types); (3) gamma power (35–60 Hz) in posterior parietal cortex 100 ms before cue onset (greater for error trials); and (4) phase locking of alpha (5–12 Hz) in parietal and occipital cortices immediately before cue onset (lower for error trials). These findings extend recently reported effects of pre-trial alpha phase on perception to cognitive control processes and help identify the cortical generators of such phase effects.
Introduction
Response variability within individuals engaged in multi-trial cognitive control tasks might reveal critical information concerning regulation of both simple and complex behaviors. A well studied paradigm during which humans have performance variability is the antisaccade task, which requires withholding a prepotent response of glancing at a peripheral stimulus (cue) and generation of a glance to the same location in the opposite visual field (Hallett, 1978). An initial glance toward the cue constitutes an error of cognitive control. Even extensively practiced healthy individuals make errors on a nontrivial proportion of trials (Taylor and Hutton, 2009).
The current study sought to characterize brain states predisposing individuals to such errors of cognitive control. Investigations on nonhuman primates show that high levels of pre-cue intrinsic activity in retinotopic superior colliculus neurons precede antisaccade errors (Everling et al., 1998); this activity can be modulated by dorsolateral prefrontal cortex (Koval et al., 2011). Functional magnetic resonance imaging (MRI) studies of humans also suggest that pre-cue activation in frontal and cingulate cortices covaries with the proportion of correct antisaccades (Ford et al., 2005).
The precise characteristics of neural activity in a distributed cortical network can be studied using oscillatory measures of brain activity. For instance, the levels of high gamma (>60 Hz) and beta (10–20 Hz) activity in anterior cingulate and frontal cortices vary as a function of behavioral context (Rothe et al., 2011). Within the alpha band (8–12 Hz), parietal and frontal ocular motor structures are known to modulate visual cortices and perceptual performance (Capotosto et al., 2009). Finally, increases in broadband gamma (40–115 Hz) but decreases in alpha oscillations in parietal cortices support motor planning (Van Der Werf et al., 2008). These studies indicate function-related variance in frequency modulations of neural activity in brain regions supporting cognitive control. The current study specifically investigates such modulations within the context of an antisaccade task, specifically their value as pre-trial predictors of cognitive control failures.
Certain phases of alpha oscillations prior to stimulus onset affect visual perception (Mathewson et al., 2009) and ocular motor reaction time (Hamm et al., 2010; Drewes and VanRullen, 2011); this effect is moderated by attention (Busch and VanRullen, 2010). Given the association of antisaccade performance with the above listed processes, it is hypothesized that pre-trial alpha may predict antisaccade performance. Evaluating whether certain phases in ongoing alpha oscillations before response cue appearance predispose to errors of cognitive control may provide an important explanation for why antisaccade response variability persists in healthy individuals.
Oscillatory phase, inter-trial phase locking, and power in the time/frequency domain were measured using dense-array electroencephalography (EEG). An antisaccade “gap” paradigm, which involves a brief 200 ms disappearance of pre-trial fixation point before appearance of the directional response cue, was used to: (1) ensure a sufficient proportion of antisaccade errors across subjects (McDowell and Clementz, 1997); and (2) investigate both stochastic and preparatory neural events predicting cognitive control errors. Oscillations from theta through gamma (4–60 Hz) occurring during the pre-cue period were quantified. Furthermore, the cortical generators of phase and power effects were approximated using sLORETA-weighted accurate minimum norm or SWARM (Wagner et al., 2007) on complex operators derived from single trials (Palva et al., 2010) (where sLORETA is standardized low-resolution brain electromagnetic tomography).
Materials and Methods
Participants
Female participants (N = 17) were recruited through the undergraduate psychology research pool at the University of Georgia, Athens, GA (mean age = 19 years; SD = 1.0). Subjects were in good physical health, absent of known neurological hard signs, and provided informed consent. Exclusion criteria, which were assessed via individual interviews, included neurological disease, a history of severe head trauma or brain injury, and current or past drug or alcohol abuse. This project was approved by the University of Georgia Institutional Review Board.
Stimuli and procedure
Stimuli were presented on a 21 inch, flat-surface, high-resolution color monitor (60 Hz vertical refresh) situated 100 cm from the participant's nasion. The task and visual stimuli were the same for all trials (Fig. 1). To start the trial, a fixation cross within a diamond (2.5°) was presented at the center of the monitor and remained for 1600 ms. The fixation cross was extinguished and the screen remained blank for 200 ms (gap), at which point a 2.5° gray ring (cue) was presented for 1000 ms in one of two locations (8° from fixation, randomly determined) in the horizontal plane (one-half in each visual field). Participants were instructed to move their eyes to the mirror image location of the cue (same amplitude, opposite direction) as quickly and accurately as possible. Participants completed 144 saccade trials per session over four sessions.
Figure 1.
Displayed are the visual stimuli, their duration, and their temporal order during a typical trial (from left to right). Participants are required to maintain gaze in the center of the screen for the first two screens. In the third screenshot (cue period), a saccade 8° (deg) from the fixation point in the opposite direction of the cue is required. Arrows indicate initial gaze refixation during the cue period; a saccade to the donut is considered an error. COR, Correct; ERR, error.
Electrophysiological recording
EEG data were vertex referenced and recorded using a 256-sensor Geodesic Sensor Net and NetAmps 200 amplifiers (Electrical Geodesics Inc., EGI). Individual sensor impedances were kept below 50 kΩ (Ferree et al., 2001). In addition, an electrolyte bridge test was conducted between all pairs of sensors before recording (Tenke and Kayser, 2001), and if there was evidence of bridging, sensors were adjusted until bridging was no longer evident (this was rarely required). Data were sampled at 500 Hz with an analog filter bandpass of 0.1–200 Hz. Sensors located at the outer canthi of each eye and below and above both eyes recorded horizontal and vertical eye movements and eye blinks. After data collection, the three-dimensional locations of sensors were acquired using a photogrammetry rig (EGI).
Eye movement data
The position data from the horizontal eye sensors for individual trials were scored using routines written in MATLAB (The MathWorks) (Dyckman and McDowell; 2005). EOG signals were converted to degrees of visual angle, and velocity was calculated as the first derivative of location by time plots. Saccade onsets were defined as initial eye movement exceeding 20°/s for each trial. Trials with blinks and eye movements in the gap and late pre-gap period (from 1200 ms before cue appearance until saccade onset), trials with no saccades, and trials with saccade latencies earlier than 90 ms or later than 400 ms were eliminated. These latency cutoffs were determined based on an examination of both individual and group latency distributions and on expectations based on previously reported findings with this paradigm (Fischer and Weber, 1997). Trials were scored for correct or incorrect direction.
EEG data
EEG sensors located at the neck and cheeks were excluded, leaving 211 sensors for data analyses. Raw data were visually inspected offline for bad sensor recordings (BESA 5.1; MEGIS Software). Bad sensors (no more than 5% of sensors for any subject) were interpolated using a spherical spline interpolation method provided in BESA software. Cardiac artifacts, when present, were eliminated using the Independent Component Analysis module in EEGLAB 7.4 (Delorme and Makeig, 2004). The artifact-free data were then transformed to an average reference. Trials were segmented into 1200 ms epochs of 700 ms pre-cue (500 ms pre-gap) to 500 ms post-cue onset. Trials containing EEG activity exceeding ±150 μV in any sensor were eliminated from further processing. Overall, these and the above mentioned criteria resulted in the retention of 88% (range 81–95%) of the 574 total trials.
Time–frequency analyses
For each subject and each sensor, voltage data were converted to the time–frequency domain in 8 ms steps and 1 Hz steps using a modified Morlet wavelet transformation (Delorme and Makeig, 2004) from 700 ms pre-cue to 50 ms post-cue onset for each trial. The length of the wavelets increased linearly from 1 cycle at 4 Hz to 9 cycles at 60 Hz to optimize the tradeoff between temporal resolution at lower frequencies and stability at higher frequencies (Busch and VanRullen, 2010). At each time t and frequency f, the result of the wavelet transform for trial k is a complex number in which A represents the amplitude of the signal and ϕ its phase:
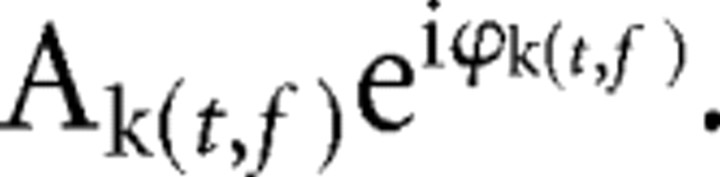 |
Power.
Oscillatory power (in decibels) was defined as 10 times the log10 of A squared in the equation above. Power was averaged across trials within response type (error versus correct), providing an index of the average magnitude of neural involvement at a given oscillatory frequency at a given time. Averaged power on error trials was subtracted from averaged power on correct trials to yield a single power difference distribution from −700 pre-cue to the time of cue onset for each subject and sensor.
Inter-trial phase coherence.
Inter-trial phase coherence (ITC) is a measure of the consistency of oscillatory phase across trials (Jammalamadaka and Sengupta, 2001) and indexes time locking of ongoing oscillatory brain processes to occurrence of an external event (e.g., a stimulus) without regard to the magnitude (power) of the oscillation (Moratti et al., 2007; Hamm et al., 2010). ITC was calculated, for each time–frequency point and trial type, by first dividing the complex results of the above described wavelet analysis by its absolute value. The resulting values were then summed across trials and divided by the corresponding number of trials in the trial type. The absolute value of this result is bound between 0 and 1 (1 indicating perfect phase alignment across trials at that frequency). Baseline (or random) ITC values vary depending on the number of trials involved in the calculation (Jammalamadaka and Sengupta, 2001; Hipp et al., 2011) and can be equated between conditions with unequal numbers of trials by subtracting baseline ITC from the ITC values calculated from the EEG for each trial type (Moratti et al., 2007):
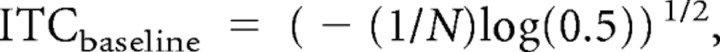 |
where N indicates the number of trials. Once adjusted, ITC values from error trials were subtracted from correct trials to yield a single ITC difference distribution, again from −700 ms pre-cue to the time of cue onset for each subject and sensor.
Phase differences.
Momentary phase of ongoing oscillations in the theta-alpha range (4–14 Hz) has been previously demonstrated to determine successful detection of visual targets (Busch et al., 2009; Mathewson et al., 2009; Busch and VanRullen, 2010). To examine whether the phase of ongoing oscillatory theta-alpha activity in the pre-cue period influenced subsequent performance on the antisaccade task, the time–frequency sensor space was searched (4–20 Hz) for points where phase “bifurcated” between the two trial types. Based on Busch et al. (2009), the phase bifurcation index (Φ) was calculated at each time–frequency sensor point using ITC as described above:
If error and correct trials have exactly equal mean phases and phase distributions, then phase bifurcation (Φ) will be 0. The upper bound of the phase bifurcation index is 1, which indicates perfectly opposite phase distributions between conditions. The hypothesis that error and correct trials will be distinguished by different pre-trial phases predicts a positive phase bifurcation value in the pre-trial period. Phase bifurcation was calculated from −700 ms pre-cue to the time of cue onset for each subject and sensor. The bandwidth was limited from 4–20 Hz for this analysis based on previous findings (Busch et al., 2009; Busch and VanRullen, 2010; Drewes and VanRullen, 2011).
Statistical analyses
First, to minimize familywise error rates, the power, ITC, and phase bifurcation value statistical comparisons were limited to 33 sensors based on a modified 10–20 system (Busch et al., 2009) involving an additional row of occipital sensors. Values were grouped into time–frequency bins of (100 ms)/(5 Hz) for power and ITC. Such a grouping is not appropriate for phase bifurcation, since the relationship of actual phase values in neighboring frequencies is complex.
Because error and correct trials differed in number for each participant, traditional dependent or within-subjects statistics may be inappropriate for hypothesis testing. As a result, power and ITC difference distributions and measures of phase bifurcation were ascribed probability estimates for each time–frequency point for each sensor and each subject using a set of bootstrap procedures (Busch et al., 2009; Busch and VanRullen, 2010). For each subject, the correct error status of all trials was shuffled 10,000 times. Correct minus error power, correct minus error ITC, and phase bifurcation distributions for every hypothesis test were then calculated and saved. These probability distributions were used to determine statistical significance (p values).
The p values for each time–frequency sensor point were then combined across subjects using Fisher's method (Fisher, 1925) to yield a χ2 for each time–frequency sensor point. To correct for the effect of multiple comparisons, the resulting distributions of p values were converted with the false discovery rate procedure (Hochberg and Benjamini, 1990) to adjusted p values, which minimized falsely rejected null hypotheses to 5%. Thus, the overall alpha level for the experiment was kept at 0.05. This procedure was completed for power, ITC, and phase bifurcation separately across time–frequency sensor distributions. Data from significant effect time/frequency regions (identified with white boundary lines) were plotted in Figures 2–4 using data from all 211 sensors.
Figure 2.
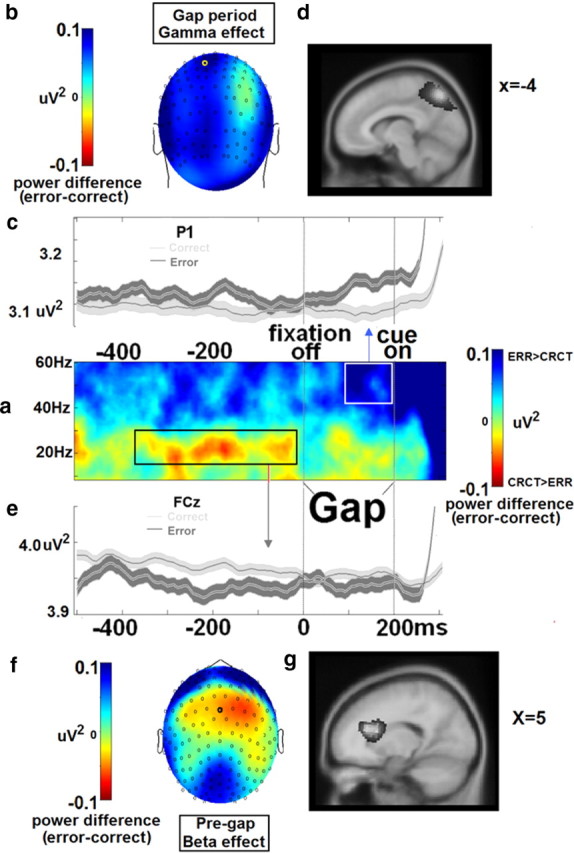
Power effects. a, Correct minus error trial difference in pre-cue time/frequency data. b–d, Shown above is the gamma effect (right box in a), which consisted of greater power 100–0 ms before cue onset on error trials and an estimated source location of posterior parietal cortex/precuneus, depicted in the topography (b), time course (c), and estimated cortical source location (d). e–g, Below is the beta effect (left box in a), which consisted of less power 400–0 ms before the gap onset on error trials and an estimated source location of anterior cingulate cortex depicted in the time course (e), topography (f), and the estimated cortical source location (g). Source images are in neurological convention (left is left); coordinates depicted in d and g are Talairach space.
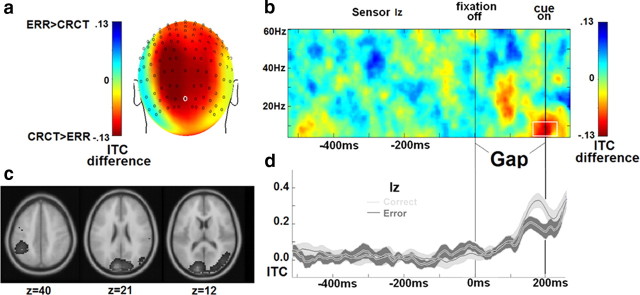
Figure 3.
ITC effects. Error trials are characterized by less ITC at cue onset, and this difference is present in sensor Iz and was localized to left inferior parietal lobule, cuneus, and right middle occipital gyrus. a–c, Differences between error (ERR) and correct (CRCT) trial ITC displayed as topography (a), a time–frequency distribution (b), and an estimated cortical source location (Talairach coordinates, neurological convention) (c). d, Time courses of ITC for each trial type are displayed. Results in a, c, and d are derived from data within the white square in b.
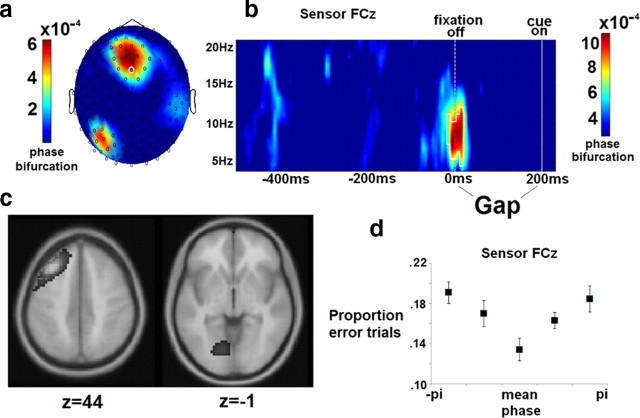
Figure 4.
Phase bifurcation effects. Alpha phase at the onset of the gap period (200 ms pre-cue) from 6 to 9 Hz originating in left prefrontal/premotor cortex and left visual cortex is different between error and correct trials. a–c, Phase bifurcation values displayed as topography (a), a time–frequency distribution (b), and an estimated cortical source location (Talairach coordinates, neurological convention) (c). d, A plot of proportion of error antisaccades as a function of instantaneous phase bin indicates that performance varies depending on a subject's pre-trial alpha phase. Results in a, c, and d are derived from data within the white square in b. Error bars in d reflect within-subjects standard errors (Cousineau, 2005).
Source analyses
At the time–frequency points showing significant power, ITC, or phase bifurcation effects in sensor space, we sought to describe the neural generators of these effects using realistic MRI-based forward models (calculated for all 211 sensors) and complex-valued sLORETA-weighted accurate minimum norm method or SWARM (Wagner et al., 2007) as implemented in CURRY (version 6.0; Compumedics Neuroscan).
An averaged magnetic resonance mage from the Montreal Neurological Institute (Collins et al., 1994) was used to construct a three-compartment boundary element method (BEM) model (Fuchs et al., 2001) before source localization. The MR images were segmented into skin surface, inside of the skull, and cortex. Standard homogeneous conductivities were assumed for the skin, skull, and brain (0.33, 0.0042, 0.33 S/m). For this BEM model, the average triangle edge lengths were 9 mm for the skin, 7 mm for the skull, and 5 mm for the brain compartment. Sensors were mapped to the head surface by first matching the fiducial locations (nasion, left and right preauricular points) from the EEG data collection session to the fiducial locations on the averaged segmented skin surface and then projecting the 211 sensors to the skin surface using a nonlinear least-square fitting procedure.
Because the effects of interest involve both phase and amplitude statistics, localization of sensor data into these BE -model volumes first required conversion of fully complex spectral values from sensor space to source space (Palva et al., 2010), rather than an estimation of the sources accounting for the largest sensor amplitudes as is afforded by beamforming approaches. Surface level complex values for each frequency band and time bin of interest were converted to source space using a SWARM inverse solution. SWARM is based on the standardized low-resolution brain electromagnetic tomography or sLORETA approach (Pascual-Marqui, 2002), which provides a highly accurate reconstruction of EEG sensor data and is more sensitive to deep sources than traditional minimum norm techniques (Soufflet and Boeijinga, 2005). sLORETA solutions, like beamformer solutions, yield pseudo statistics which reflect signal-to-noise ratios rather than estimated current strength; SWARM uses sLORETA to weight minimum norm acquired current values (Hämäläinen and Ilmoniemi, 1994), resulting in values scaled in amperes for each 5 mm3 voxel in brain space.
The SWARM procedure was performed separately for imaginary and real components of the surface level complex value. Imaginary valued SWARM solutions were multiplied by “i” and added to real-valued SWARM solutions to yield fully complex source solutions for each trial, subject, and time/frequency effect of interest. When appropriate, power, ITC, or phase bifurcation statistics were calculated as described above in brain space for each 5 mm3 voxel. These values were standardized within subjects across the brain and then averaged across subjects, resulting in final source solutions for each effect.
Results
Behavioral results
On average, participants made antisaccade errors on 12% of trials (SD = 5.0). Three subjects had fewer than 20 total error trials retained after artifact rejection, making them clear outliers from the rest of the group (range 35–100). These subjects were excluded, leaving 14 subjects for further comparisons. As reported previously (Hutton, 2008), reaction times were slower on correct (196 ms, SD = 19) compared to error trials (176 ms, SD = 26), F(1,13) = 9.19, p < 0.01.
Power
Analyses indicate two space–time–frequency clusters showing power differences between error and correct trials (Fig. 2a). First, present at FC3, FCZ, FC4, C3, CZ, and C4 sensors, participants displayed less beta range (19–29 Hz) power before antisaccade error trials in the 400 ms period immediately before gap onset (data from FCZ depicted in Fig. 2e–g). This effect was most prominent over the midline sensors in the last 100 ms before gap onset, χ2(28) = 52.9, p < 0.01, and was localized to medial frontal cortex with a peak in right anterior cingulate gyrus [Talairach coordinates: x = 5, y = 24, z = 18, Broadmann's area (BA) 24]. Second, distributed across multiple frontal and posterior sensors, participants displayed more gamma range (35–60 Hz) power before antisaccade error trials in the 100 ms period immediately before cue onset (data from P1 depicted in Fig. 2b–d). This effect was strongest in the left-central parietal and occipital regions, χ2(28) = 65.2, p < 0.001, and was localized to central lateral parietal cortices with a peak in the precuneus (Talairach coordinates: x = −4, y = −69, z = 46, BA 7).
ITC effects
Analyses indicate a single space–time–frequency cluster showing an ITC effect. Across multiple posterior sensors, participants displayed less theta-alpha band (4–14 Hz) ITC before antisaccade error trials in the 100 ms immediately before cue onset (Fig. 3). This effect was largest for Iz at cue onset, χ2(28) = 75.5, p < 0.001, with source analyses indicating three posterior maxima: left inferior parietal lobule (Talairach coordinates: x = −44, y = −47, z = 40, BA 40), central cuneus (Talairach coordinates: x = −9, y = −89, z = 21, BA 18), and right middle occipital gyrus (Talairach coordinates: x = 37, y = −74, z = 12, BA 19).
Phase effects
Analyses revealed a single space-time-frequency cluster showing significant positive phase bifurcation in the pre-cue period, indicating opposite phase distributions for error versus correct antisaccade trials (Fig. 4). This effect extended from −230 to −170 ms (peak at −210 ms) before cue onset (straddling the start of the gap period) and from 6 to 9 Hz (peaking at 7 Hz) with maximal effect over the frontal midline, χ2 (28) = 67.5, p < 0.001.
To further illustrate the difference in phase between error and correct antisaccade trials, single trial phase values from 7 Hz activity at 10 ms pre-gap onset were subtracted from each subject's mean phase across correct trials (Busch et al., 2009). Trials were then sorted into 1 of 5 separate phase bins, and each subject's proportion of correct trials was calculated within each bin (plotted in Fig. 4). Results from a repeated measures ANOVA demonstrated that proportion of error antisaccades depended significantly on the momentary oscillatory phase of low alpha oscillations at this pre-cue time point (F(4,13) = 5.13, p < 0.05).
Additionally, a circular-to-linear correlation coefficient (circ-r) between unadjusted phase and dummy-coded (1,0) trial type values was calculated (Zar, 1999) for each subject at 7 Hz and 10 ms pre-gap across all trials. Phase values across all trials were randomized 10,000 times and used to construct a random distribution of circ-r values. The p values, calculated as the percentage of random circ-r values greater than the actual circ-r value, were combined across subjects (Fisher, 1925) to yield a single significant χ2: χ2(28) = 52.38, p < 0.01. Overall, this series of analyses indicate that the probability with which a subject will complete an error antisaccade varies as a function of pre-trial alpha phase.
Source analyses localized this effect to two maxima: left middle frontal gyrus (Talairach coordinates: x = −35, y = 24, z = 44, BA 8/9) and lingual gyrus in medial left ventral occipital lobe (Talairach coordinates: x = −15, y = 70, z = −1, BA 18). The former is in the vicinity of dorsolateral prefrontal cortex, and the latter is in the vicinity of primary and secondary visual cortices.
Discussion
Results highlighted four aspects of oscillatory brain activity differentiating error trials from correct trials before the onset of a response cue. (1) Instantaneous phase at trial onset (200 ms before the response cue at the beginning of the gap) in alpha/theta oscillations within a prefrontal-occipital network was different between error and correct trials and covaried with the probability of antisaccade errors. Error trials were also characterized by (2) smaller magnitude of ongoing beta oscillations in anterior cingulate cortices in the 400 ms period before gap initiation, (3) increased gamma oscillations in posterior parietal cortex during the last 100 ms of the gap period (immediately before response cue onset), and (4) the absence of phase locking of alpha oscillations immediately before response cue onset. Theoretically, antisaccade errors can occur due to failure of saccadic inhibition (generation of a visually driven saccade) and/or failure to prepare and properly execute an internally calculated saccade (Carpenter and Williams, 1995; Hutton, 2008). While the complete information necessary to calculate the proper response was unknown to the participant before response cue onset, we demonstrated that the probability of the former type of failure was predicted by a participant's brain state before awareness of the correct saccade direction. This brain state, as well as the relevance of these findings for understanding cognitive control processes in general and visuospatial processing in particular, is discussed below.
Pre-trial alpha phase
Alpha oscillations, though initially thought to reflect idling in the brain, recently have been found to relate to executive (Klimesch, 1999) and visual attention processes (Ergenoglu et al., 2004; Hanslmayr et al., 2007). In visual cortices, alpha oscillations theoretically reflect cyclic fluctuation between low (inhibited) and high excitability states (Mathewson et al., 2009, 2011), operating as a mechanism of top-down visuospatial inhibition (Klimesch et al., 2007) in a spatially specific manner in the cerebral cortex (Capotosto et al., 2009). The current study advances this line of research by demonstrating effects of pre-trial phase in an ocular motor task. One study investigating pre-trial EEG during a simple lateralized visual detection task reported an effect of alpha phase on stimulus detection performance similar to the effect presented in the current study in that it was strongest (1) at the FCz sensor, (2) at 7.1–8.2 Hz (our peak effect was at 7 Hz), and (3) ∼150–200 ms before stimulus appearance (Busch et al. 2009). Subsequent studies have replicated this same pattern of frontally distributed EEG alpha phase effects and extended it to visual–spatial attention (Busch and VanRullen, 2010) and saccadic reaction time (Drewes and VanRullen, 2011). Yet, other investigations have found similar pre-trial alpha phase effects in occipital sensors (Mathewson et al., 2009, Dugué et al., 2011). Data presented here support the role of both frontal and occipital cortices in generation of the alpha phase effect, suggesting that alpha oscillatory phases relevant to visuospatial processing involve a distributed network of frontal and occipital-parietal regions, complementing findings of attention-related alpha power decreases (Capotosto et al., 2009).
Pre-trial beta power
Beta oscillations during the pre-trial period were lower in magnitude before error antisaccade trials; this effect was localized to the dorsal portion of anterior cingulate cortex (dACC). Pre-response activity in the dACC has been shown to facilitate the production of correct antisaccades (Phillips et al., 2011). More generally, dACC has been demonstrated to play a critical role in error monitoring and prevention (van Veen and Carter, 2006). Stability of beta rhythms reflects motor inhibition, whereas disruption reflects disinhibition (Zhang et al., 2008). The dACC's role in antisaccade production (McDowell et al., 2008), therefore, may involve ongoing or tonic beta band neural modulation, perhaps of motor brain structures, to monitor and reduce the probability of an error.
High levels of stable beta oscillations in the cerebral cortex have been theorized to support the maintenance of ongoing motor programs while attenuating the initiation of new movement (Engel and Fries, 2010). Indeed, motor response latencies are slowed when initiated during periods of enhanced beta activity (Gilbertson et al., 2005). Together with the fact that beta effects in the current study occurred while subjects maintained fixation, it can be hypothesized that anterior cingulate cortex supports correct completion of the antisaccade task through the tonic attenuation of motor excitability in favor of fixation maintenance.
Gap period gamma power
Because gamma oscillations generated in posterior parietal/precuneus regions increase in power before error compared to correct antisaccades (Fig. 2a), suppression of gap period parietal activity may be necessary for successful antisaccade performance. Parietal gamma oscillations in the 35–60 Hz range reflect local processing (Uhlhaas et al., 2011) and saccade preparation (Van Der Werf et al., 2008). Therefore it could be that an increase in gamma activity before an error reflects precocious motor activity, which increases the likelihood of the execution of the prepotent visually driven saccade (Everling et al., 1998). Manipulation of gap length in future saccade studies, however, will be necessary to determine whether gap period activities are related to trial onset or cue anticipation (Watanabe et al., 2010).
Interestingly, this gamma effect occurred on the same timescale (midway through the gap), and displayed similar scalp topography and an estimated cortical source generator as the express saccade effects reported by Hamm et al. (2010). This finding indicated, similar to what was postulated by Everling and Fischer (1998), that antisaccade errors were neurophysiologically similar to express saccades (rapidly generated saccades at nearly 60% of the response latency in the context of a “prosaccade” task). Interestingly, increased proportions of express saccades and antisaccade errors covary in clinical populations, including schizophrenia (Clementz, 1996; McDowell and Clementz, 1997; Reilly et al. 2008) and Parkinson's disease (Armstrong et al., 2002).Together, these data suggest that impaired modulation of anticipatory parietal gamma oscillations could constitute a clinically relevant biomarker.
Alpha phase locking
An increase in alpha band phase locking occurring at and immediately before the onset of the response cue was present before correct trials but absent or attenuated before error trials. This effect was localized to a constellation of posterior regions possibly comprising both sensory (visual cortex and middle occipital gyrus; see Fig. 3) and motor cortices (intraparietal sulcus, the putative location of parietal eye fields; McDowell et al., 2008). Spatially distributed reset of alpha phases may serve as a method to temporally coordinate cortical structures to optimize incoming signal processing in accordance with top-down goals (Klimesch et al., 2007). The phase locking identified in the current study may reflect temporally precise coordination of sensory and motor cortices (intraparietal sulcus, the putative location of parietal eye fields; McDowell et al., 2008) as an effort to preemptively reroute spatial information of the incoming sensory stimulus (the cue) to alternative spatial coordinates in the motor cortex. Timed alpha phase reset across occipital/parietal cortex may therefore constitute a cognitive control strategy for ensuring optimal cue processing and correct completion of the antisaccade task.
Alternatively, alpha oscillations have been associated with inhibitory processes. Under the thesis that error antisaccades are neurophysiologically similar to deliberate prosaccades (Hutton, 2008), Olk and Kingstone (2003) suggested that additional inhibitory processes present on correct antisaccade trials account for increased response latencies. Alpha coherence in the occipital parietal network could reflect a strategy for reducing the likelihood of an error saccade by reducing the input signal of the cue appearance (Clementz et al., 2010).
General discussion and conclusion
The current study highlights a number of important aspects of ongoing (alpha phase and beta power) and preparatory (gap period gamma power and alpha phase locking) brain activations that differentiate error from correct trials during a cognitive control task. A few points merit additional consideration. Activity during the 200 ms gap contains a mixture of evoked responses due to fixation offset and ongoing intrinsic activity. One analytical way to separate ongoing oscillations from those evoked by a stimulus is by baseline-adjusting stimulus period data using the prestimulus period. Such an adjustment assumes the absence of differences between conditions in the baseline period to avoid confounding evoked and ongoing differences. Because the current study found such differences, it was inappropriate to perform such an adjustment.
Nonetheless, ongoing oscillations by definition do not contain stimulus-locked phase resetting or evoked signals. Increases in ITC theoretically may contain both such activities (Martínez-Montes et al., 2008), but the late gap period increases in alpha range ITC seen for correct trials over error trials seem unlikely to reflect a simple difference in only ongoing activity. Increases in single trial power also may contain both such activities, even if they are not phase locked to a stimulus (Wang and Ding, 2011). As Watanabe et al. (2010) describe, manipulation of the gap interval in future studies will be necessary to model and characterize evoked fixation offset-related activity and differentiate it from motor preparatory activity. Indeed, ITC increases may reflect anticipatory brain activations rather than those that are stimulus evoked. Likewise, gamma-band single trial power increases may reflect anticipatory increases in ongoing brain oscillations and/or superimposition of temporally variable evoked responses due to gap onset (e.g., as measured by Wang and Ding, 2011).
Of greatest importance, the effects reported here reveal brain activations observed before the presentation of a response cue and, therefore, well before the participant actually made an error. These activations provide possible objectives for future studies investigating response variability and failures of cognitive control. Such findings may have particular relevance for populations characterized by cognitive control problems—such as those with schizophrenia or attention deficit hyperactivity disorder. These data also extend the reported effects of ongoing alpha phase on visual perception to the ocular motor system and identify their possible neural generators.
Footnotes
This work was supported by NIH Grants MH076998 and MH082514. We thank Noah Duncan and Emily Leonard Parks for their help with data collection.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Armstrong IT, Chan F, Riopelle RJ, Munoz DP. Control of saccades in Parkinson's disease. Brain Cogn. 2002;49:198–201. [PubMed] [Google Scholar]
- Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci U S A. 2010;107:16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377:59–62. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- Clementz BA. The ability to produce express saccades as a function of gap interval among schizophrenia patients. Exp Brain Res. 1996;111:121–130. doi: 10.1007/BF00229561. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Gao Y, McDowell JE, Moratti S, Keedy SK, Sweeney JA. Top-down control of visual sensory processing during an ocular motor response inhibition task. Psychophysiology. 2010;47:1011–1018. doi: 10.1111/j.1469-8986.2010.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subjects designs: a simpler solution to Loftus and Masson's method. Tutorials Quant Meth Psych. 2005;1:42–45. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Drewes J, VanRullen R. This is the rhythm of your eyes: the phase of ongoing electroencephalogram oscillations modulates saccadic reaction time. J Neurosci. 2011;31:4698–4708. doi: 10.1523/JNEUROSCI.4795-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué L, Marque P, VanRullen R. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J Neurosci. 2011;31:11889–11893. doi: 10.1523/JNEUROSCI.1161-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyckman KA, McDowell JE. Behavioral plasticity of antisaccade performance following daily practice. Exp Brain Res. 2005;162:63–69. doi: 10.1007/s00221-004-2105-9. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations–signalling the status quo? Current opinion in neurobiology. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Ergenoglu T, Demiralp T, Bayraktaroglu Z, Ergen M, Beydagi H, Uresin Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Brain Res Cogn Brain Res. 2004;20:376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Munoz DP. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J Neurophysiol. 1998;80:1584–1589. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clin Neurophysiol. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Effects of stimulus conditions on the performance of antisaccades in man. Exp Brain Res. 1997;116:191–200. doi: 10.1007/pl00005749. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical methods for research workers. Edinburgh: Oliver and Boyd; 1925. [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Wagner M, Kastner J. Boundary element method volume conductor models for EEG source reconstruction. Clin Neurophysiol. 2001;112:1400–1407. doi: 10.1016/s1388-2457(01)00589-2. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 2005;25:7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hamm JP, Dyckman KA, Ethridge LE, McDowell JE, Clementz BA. Preparatory activations across a distributed cortical network determine production of express saccades in humans. J Neurosci. 2010;30:7350–7357. doi: 10.1523/JNEUROSCI.0785-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bäuml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hipp JF, Engel AK, Siegel M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron. 2011;69:387–396. doi: 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Hutton SB. Cognitive control of saccadic eye movements. Brain Cogn. 2008;68:327–340. doi: 10.1016/j.bandc.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Jammalamadaka SR, Sengupta A. Topics in circular statistics. River Edge, NJ: World Scientific; 2001. [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Koval MJ, Lomber SG, Everling S. Prefrontal cortex deactivation in macaques alters activity in the superior colliculus and impairs voluntary control of saccades. J Neurosci. 2011;31:8659–8668. doi: 10.1523/JNEUROSCI.1258-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Montes E, Cuspineda-Bravo ER, El-Deredy W, Sánchez-Bornot JM, Lage-Castellanos A, Valdés-Sosa PA. Exploring event-related brain dynamics with tests on complex valued time-frequency representations. Stat Med. 2008;27:2922–2947. doi: 10.1002/sim.3132. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. J Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychol. 2011;2:99. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Exp Brain Res. 1997;115:333–344. doi: 10.1007/pl00005702. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratti S, Clementz BA, Gao Y, Ortiz T, Keil A. Neural mechanisms of evoked oscillations: stability and interaction with transient events. Hum Brain Mapp. 2007;28:1318–1333. doi: 10.1002/hbm.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olk B, Kingstone A. Why are antisaccades slower than prosaccades? A novel finding using a new paradigm. Neuroreport. 2003;14:151–155. doi: 10.1097/00001756-200301200-00028. [DOI] [PubMed] [Google Scholar]
- Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci U S A. 2010;107:7580–7585. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Phillips JM, Johnston K, Everling S. Effects of anterior cingulate microstimulation on pro- and antisaccades in nonhuman primates. J Cogn Neurosci. 2011;23:481–490. doi: 10.1162/jocn.2010.21482. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Reduced attentional engagement contributes to deficits in prefrontal inhibitory control in schizophrenia. Biol Psychiatry. 2008;63:776–783. doi: 10.1016/j.biopsych.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothé M, Quilodran R, Sallet J, Procyk E. Coordination of high gamma activity in anterior cingulate and lateral prefrontal cortical areas during adaptation. J Neurosci. 2011;31:11110–11117. doi: 10.1523/JNEUROSCI.1016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufflet L, Boeijinga PH. Linear inverse solutions: simulations from a realistic head model in MEG. Brain Topogr. 2005;18:87–99. doi: 10.1007/s10548-005-0278-6. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Hutton SB. The effects of task instructions on pro and antisaccade performance. Exp Brain Res. 2009;195:5–14. doi: 10.1007/s00221-009-1750-4. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clin Neurophysiol. 2001;112:545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W. A new look at gamma? High- (>60 Hz) gamma-band activity in cortical networks: function, mechanisms and impairment. Prog Biophys Mol Biol. 2011;105:14–28. doi: 10.1016/j.pbiomolbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Van Der Werf J, Jensen O, Fries P, Medendorp WP. Gamma-band activity in human posterior parietal cortex encodes the motor goal during delayed prosaccades and antisaccades. J Neurosci. 2008;28:8397–8405. doi: 10.1523/JNEUROSCI.0630-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clin EEG Neurosci. 2006;37:330–335. doi: 10.1177/155005940603700411. [DOI] [PubMed] [Google Scholar]
- Wagner M, Fuchs M, Kastner J. SWARM: sLORETA-weighted accurate minimum norm inverse solutions. International Congress Series. 2007;1300:185–188. [Google Scholar]
- Wang X, Ding M. Relation between P300 and event-related theta-band synchronization: a single-trial analysis. Clin Neurophysiol. 2011;122:916–924. doi: 10.1016/j.clinph.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hirai M, Marino RA, Cameron IG. Occipital-parietal network prepares reflexive saccades. J Neurosci. 2010;30:13917–13918. doi: 10.1523/JNEUROSCI.3884-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J. Biostatistical analysis. Ed 4. Upper Saddle River: Prentice Hall; 1999. [Google Scholar]
- Zhang Y, Chen Y, Bressler SL, Ding M. Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience. 2008;156:238–246. doi: 10.1016/j.neuroscience.2008.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]