Abstract
Background and Purpose
Vascular endothelial growth factor (VEGF) expression is elevated in human brain arteriovenous malformations (bAVM). We have developed a bAVM model in the adult mouse by focal Alk1 gene deletion and human VEGF stimulation. We hypothesized that once the abnormal vasculature has been established; tonic VEGF stimulation is necessary to maintain the abnormal phenotype and VEGF antagonism by bevacizumab (Avastin) would reduce vessel density and attenuate the dysplastic vascular phenotype.
Methods
Angiogenesis and bAVM were induced by injection of adeno-associated viral vector expressing human VEGF (AAV-VEGF) alone into the brain of wild-type (WT) mice or with adenoviral vector expressing Cre recombinase (Ad-Cre) into Alk12f/2fmice. Six weeks later, bevacizumab or trastuzumab (Herceptin, bevacizumab control) were administered. Vessel density (VD), dysplasia index (DI), vascular cell proliferation and apoptosis, and human IgG were assessed (n=6/group).
Results
Compared to trastuzumab (15 mg/kg), administration of 5, 10 and 15 mg/kg of bevacizumab to AAV-VEGF treated WT mice reduced focal VD (p<0.05); administration of 5 mg/kg bevacizumab decreased proliferating vascular cells (p=0.04) and increased TUNEL-positive vascular cells (p=0.03). More importantly, bevacizumab (5 mg/kg) treatment reduced both VD (p=0.01) and DI (p=0.02) in our bAVM model. Human IgG was detected in the vessel wall and the parenchyma in the angiogenic foci of bevacizumab treated mice.
Conclusions
We provide proof-of-principle that, once abnormal AVM vessels have formed, VEGF antagonism may reduce the number of dysplastic vessels and should be further evaluated as a therapeutic strategy for the human disease.
Keywords: VEGF, arteriovenous malformation, mouse, brain, angiogenesis
Introduction
Brain arteriovenous malformations (bAVM) represent a relatively infrequent but important source of neurological morbidity in children and young adults,1, 2 primarily from new or recurrent intracranial hemorrhage (ICH). Current therapies consist of a combination of surgical resection, embolization and stereotactic radiotherapy, all with potentially high morbidities. Specific medical therapies are needed, especially for the large fraction of bAVM patients who are currently not offered treatment due to perceived high risks associated with available therapies. Studies of surgically-resected bAVM tissue suggest an active angiogenic and inflammatory lesion rather than a static congenital anomaly. We and others3–5 have shown that vascular endothelial growth factor (VEGF) level is increased in resected surgical specimens; VEGF signaling may present a target for medical intervention.
Patients with hereditary hemorrhagic telangiectasia (HHT, OMIM#187300) have a much higher prevalence of bAVM than the generation population, on the order of ≈1000-fold higher in HHT1 caused by endoglin (ENG) haploinsufficiency and ≈100-fold higher in HHT2 with ALK1 haploinsufficiency.2 Vascular lesions developed in various organs in Eng+/− 6 and Alk1+/− adult mice,7 but spontaneous lesions in the brain are quite modest, and only seen in older mice.8, 9 Our group demonstrated greatly increased brain microvascular dysplasia after virally-mediated VEGF stimulation in both Eng+/− and Alk1+/− mice.10
Somatic conditional deletion of Alk1 in adult mice induced arteriovenous fistulas and hemorrhage in the lung and gastrointestinal tract, but not in the skin or brain.11 Importantly, upon induction of skin wounding, Alk1-deleted mice developed arteriovenous fistula and AVM-like vessels around the wound. The combination of local angiogenic stimulation (Matrigel + VEGF/FGF) and Eng loss led to gross venous enlargement in the retina.12 These results suggest that angiogenic stimulation, in addition to genetic mutation, is required for the development of vascular malformations in adult mouse brain. Taken together, all these studies suggest that a paradigm for AVM pathogenesis may be an abnormal response to injury.13 In support of this notion, we recently developed a bAVM model in adult mice by focal Alk1 gene deletion combined with VEGF stimulation (virally-mediated human VEGF-A overexpression) which resembles various aspects of human bAVM including large irregular vasculature and arterio-venous shunting.14
Bevacizumab (Avastin) is a humanized monoclonal antibody that is directed against human VEGF-A.15 Bevacizumab binds to and neutralizes all VEGF-A isoforms and bioactive cleavage fragments.16 Bevacizumab sequesters VEGF, preventing the interaction of VEGF with its cell surface receptor.17 Bevacizumab has been used extensively to inhibit angiogenesis in cancer and other diseases with pathological angiogenesis.18–20
In this study we sought to test the hypothesis that, once the abnormal phenotype has been established in our bAVM mouse model, whether antagonism of VEGF by bevacizumab would attenuate the dysplastic vascular phenotype.
Materials and Methods
Viral Vector Transduction in the Mouse Brain
The mice were placed in a stereotactic frame (David Kopf Instruments, Tujunga, CA). A burr hole was drilled to the pericranium 2 mm lateral to the sagittal suture and 1 mm posterior to the coronal suture. A 10 μl syringe (Hamilton Company, NV) was inserted into the basal ganglia 3 mm under the cortex. Viral vectors were injected at a rate of 0.2 μl/min.21 Ad-Cre (adenoviral vector with CMV promoter driving Cre recombinase expression) was purchased from Vector Biolabs (Philadelphia, PA). AAV-VEGF and AAV-LacZ have been previously described.22, 23 WT mice received 2×109 genome copies (gcs) of AAV viral vectors in 2 μl of PBS. Alk12f/2f mice received 3 μl viral suspension containing 2×107 plaque forming unit (PFU) Ad-Cre and 2×109 gcs of AAV-VEGF.14
Antibody Treatment and Dosage
Six weeks after viral vector injections, WT and bAVM mice received intraperitoneal (IP) injections every other day for ten days of either bevacizumab (Avastin, Genentech/Roche Inc., South San Francisco, CA) (1, 5, 10, or 15 mg/kg), an anti-VEGF humanized monoclonal antibody or trastuzumab (Herceptin, Genentech/Roche Inc.) (5 or 15 mg/kg), an anti-HER2 humanized monoclonal antibody that will not affect endogenous mouse VEGF expression or the human VEGF expression mediated by AAV-VEGF.
Statistical Analysis
Analysis of vessel density was done using one-way ANOVA, followed by Bonferroni posthoc analysis to compare the means between groups. A dose inhibitory analysis was done with GraphPad Prism (GraphPad Software, Inc., San Diego, U.S.A.) to obtain a dose inhibitory curve and IC50 value. Sample sizes were n=6/group. Data are presented as mean ±SEM. A p value < 0.05 was considered statistically significant.
The Supplemental Methods section describes additional methods, please see http://stroke.ahajournals.org.
Results
Humanized Antibody Present in the Vessel Wall and Parenchyma at Angiogenic Foci of Bevacizumab Treated Mice
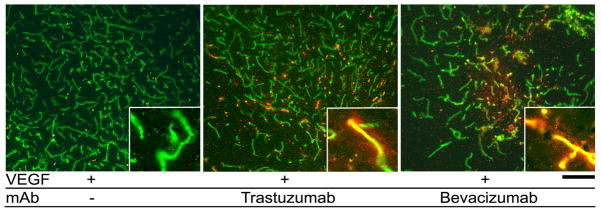
To test if IP injected bevacizumab and trastuzumab would reach the VEGF-stimulated angiogenic foci in the mouse brain, we stained the mouse brain with an antibody specific to human IgG. Positive staining was observed in the vessel wall and brain parenchyma in the VEGF-stimulated angiogenic region. No staining was detected in untreated mice (Figure 1). This indicates that the humanized antibodies can penetrate through the blood-brain barrier in a VEGF-induced angiogenic focus.
Figure 1.
Human antibody was detected in the brain parenchyma of mice treated with bevacizumab and trastuzumab. Vessels are labeled with lectin (green). Human IgG (red) was detected only in VEGF-stimulated regions of mice treated with bevacizumab and trastuzumab. Inserts show high magnification images of vessels. Scale bar: 100 μm.
Bevacizumab Inhibits Human VEGF-Induced Angiogenesis in Mouse Brain
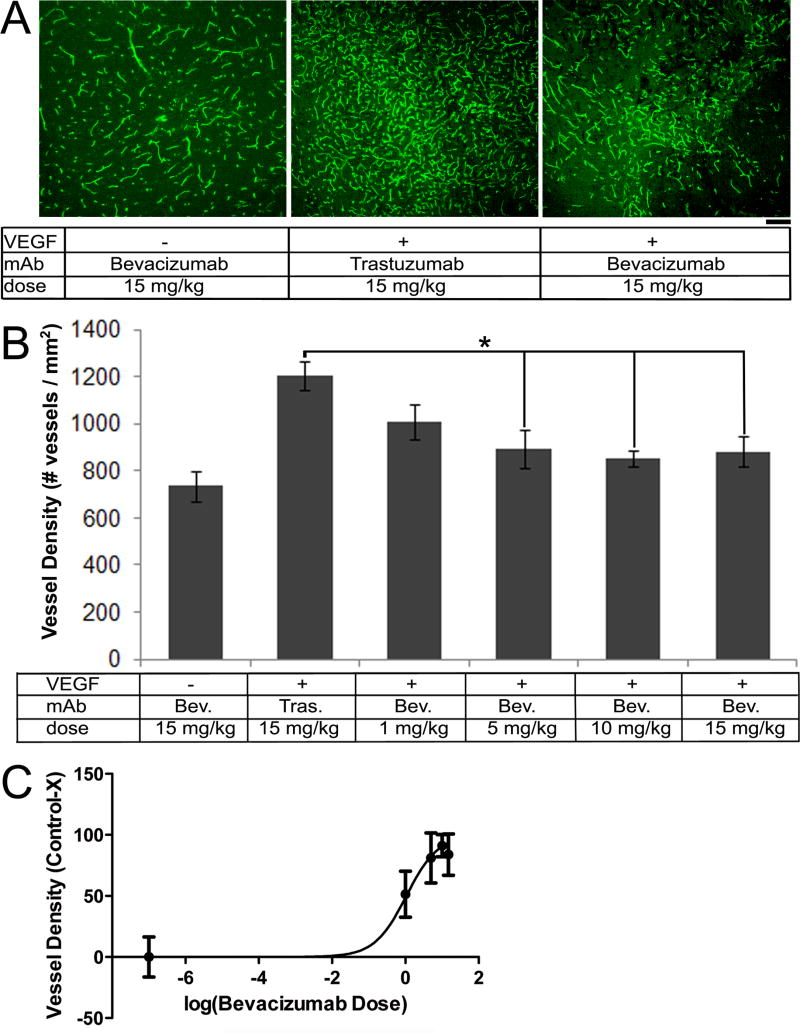
To determine the appropriate dose to use for our disease model, we treated WT mice with 1, 5, 10, and 15 mg/kg bevacizumab every other day for ten days six weeks after the injection of AAV-VEGF and assessed vessel density. AAV-VEGF stimulated angiogenesis as compared to AAV-LacZ injected controls (WT+VEGF: 1152±48 vessels/mm2 vs. WT+LacZ: 736±65 vessels/mm2, p=0.005). Administration of 5, 10 and 15 mg/kg of bevacizumab resulted in a reduction of vessel density in the AAV-VEGF stimulated angiogenic foci as compared to the trastuzumab treated group (15 mg/kg trastuzumab vs. 5 mg/kg bevacizumab: p=0.04, 10 mg/kg bevacizumab: p=0.01, 15 mg/kg bevacizumab: p=0.03). Administration of 1 mg/kg bevacizumab resulted in a trend towards reduction of vessel density as compared to trastuzumab treatment (p=0.07, Figure 2A and 2B). Trastuzumab (15 mg/kg) treated mice had a similar vessel density to untreated WT mice injected with AAV-VEGF alone (WT+VEGF: 1152±48 vessels/mm2 vs. WT+VEGF+Trastuzumab: 1205±63 vessels/mm2, p=0.59, Supplemental Figure S1); suggesting trastuzumab does not affect VEGF-induced brain angiogenesis. Analysis of the dose response effect predicted the inhibitory curve (IC50)of bevacizumab to inhibit brain angiogenesis is 1.06 mg/kg (95% CI: 0.36–3.10 mg/kg) (Figure 2C). Based on these data, 5 mg/kg bevacizumab was selected for subsequent experiments.
Figure 2.
Bevacizumab treatment reduced vessel density in VEGF-induced angiogenic foci. (A) Representative images of vessels labeled with lectin (green). Scale bar: 100 μm. (B) Bar graphs show quantification of vessel density. Administration of 5, 10, and 15 mg/kg bevacizumab significantly reduced vessel density as compared to trastuzumab treated control (*p<0.05). (C) Dose-inhibitory curve. The projected IC50 for bevacizumab anti-angiogenic effect is 1.06 mg/kg.
Bevacizumab Treatment Decreased Proliferating Vascular Cells and Increased TUNEL-Positive Vascular Cells in VEGF-Stimulated Angiogenic Foci
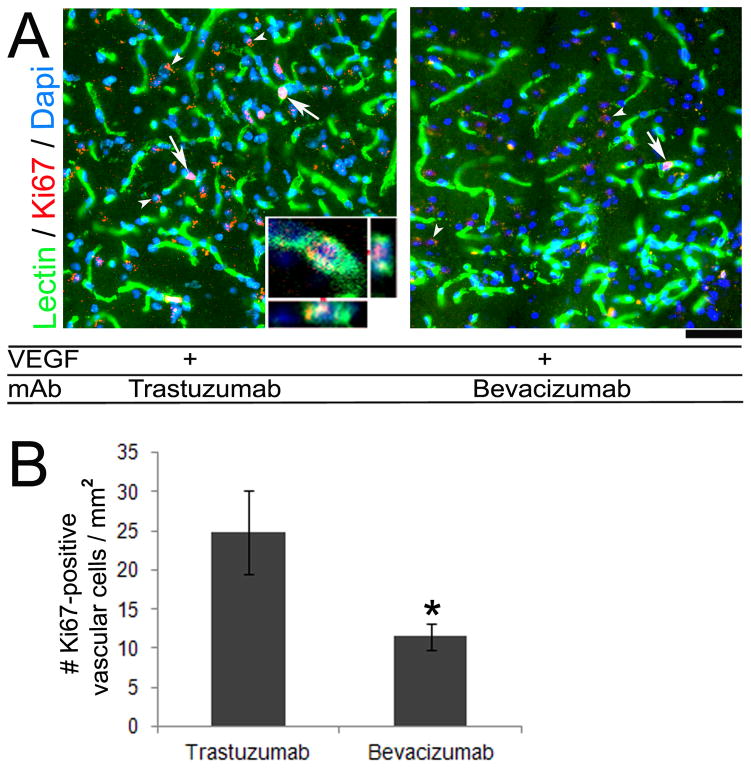
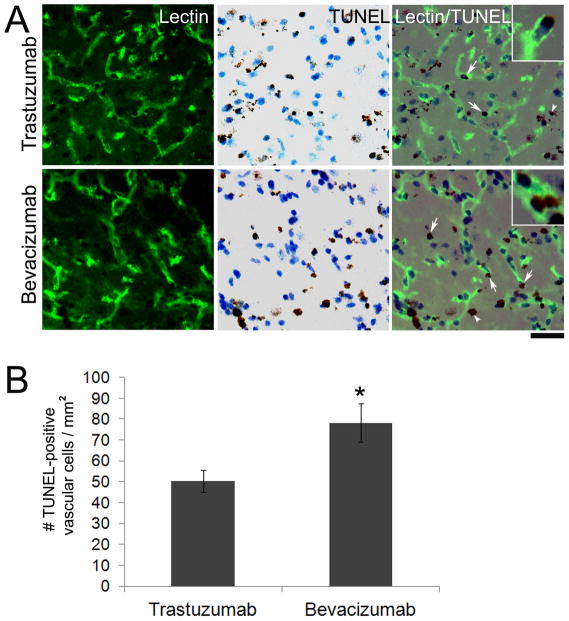
To determine the mechanism of the inhibition effect of bevacizumab on VEGF-induced brain angiogenesis, we assessed vascular cell proliferation and apoptosis within the VEGF-stimulated angiogenic foci. We found a 54% decrease of Ki67-positive cells co-localized with lectin-positive vascular cells in the angiogenic foci of bevacizumab (5 mg/kg) treated WT mice as compared to trastuzumab treated controls (bevacizumab: 11.5±1.7 cells/mm2 vs. trastuzumab: 24.8±5.4 cells/mm2, p=0.04) (Figure 3). WT mice with VEGF stimulation alone had 13.5±6.9 Ki67-positive vascular cells/mm2. In addition, bevacizumab treatment increased TUNEL-positive vascular cells in the angiogenic region by 36% as compared to trastuzumab controls (bevacizumab: 78.3±9.3 cells/mm2 vs. trastuzumab: 50.4±5.4 cells/mm2, p=0.03) (Figure 4). Thus, the reduced vessel density in bevacizumab treated mice is due to both a decrease in vascular cell growth and increase in vascular cells with damaged DNA.
Figure 3.
Bevacizumab treatment reduced vascular cell proliferation. (A) Representative images of sections co-stained with lectin for vessels (green) and Ki67 for proliferating nuclei (red). Proliferating cells on vessel walls (arrows) were reduced in bevacizumab treated group as compared to trastuzumab treated group. A few Ki67-positive cells were not associated with a vessel (arrowhead). Inset is a confocal image showing co-localization of a lectin-positive vascular cell and Ki67-positive nucleus. Scale bar: 20 μm. (B) Quantification demonstrated a significant reduction in proliferating vascular cells in the bevacizumab treated group as compared to trastuzumab (*p=0.04).
Figure 4.
Bevacizumab treatment increased TUNEL-positive cells on vessel wall. (A) Representative images show sections double stained with lectin (green, left), TUNEL (brown, middle), and merged (right). The bevacizumab treated group had more TUNEL-positive vascular cells (arrows) as compared to trastuzumab. A few TUNEL-positive cells were not associated with a vessel (arrowheads). Insets are high magnification images showing TUNEL-positive nuclei co-localized with lectin. Scale bar: 50 μm. (B) Bar graph shows quantification of TUNEL-positive vascular cells (*p=0.03).
Bevacizumab Treatment Reduced Vessel Density and Vascular Dysplasia in Mouse bAVM Model
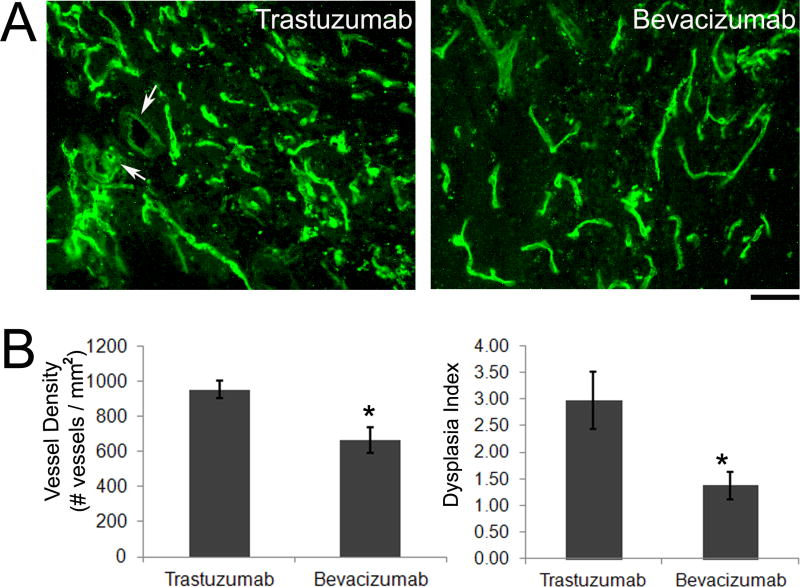
In our mouse bAVM model,14 we tested if bevacizumab treatment could reduce dysplastic vessels. We treated mice with bevacizumab (5 mg/kg) six weeks after the induction of the model (injection of Ad-Cre and AAV-VEGF into the brain) through IP injection every other day for 10 days. As compared to trastuzumab controls, bevacizumab treatment resulted in a 30% reduction of vessel density (trastuzumab: 952±51 vessels/mm2 vs. bevacizumab: 662±73 vessels/mm2, p=0.01) and a 54% reduction of dysplasia index (trastuzumab: 2.97±0.5 vs. bevacizumab: 1.37±0.3, p=0.02) (Figure 5), suggesting that reducing VEGF levels in the lesion leads to regression of dysplastic vessels.
Figure 5.
Bevacizumab treatment reduced vessel density and dysplasia in the adult mouse bAVM model. (A) Representative images show lectin-stained vessels. There are fewer irregular shaped large vessels (arrows) in the bevacizumab treated group as compared to trastuzumab. Scale bar: 50 μm. (B) Bar graph shows quantification of vessel density and dysplasia index. Bevacizumab treatment reduced vessel density (*p=0.01) and dysplasia index (*p=0.02) as compared to trastuzumab treatment.
Discussion
We demonstrate here that bevacizumab, a VEGF-specific antibody, is able to cross the blood-brain barrier in angiogenic foci following IP injection entering into the brain parenchyma. Bevacizumab treatment in WT mice attenuated VEGF-stimulated angiogenesis, reduced vascular cell proliferation, and increased TUNEL-positive vascular cells. Most importantly, bevacizumab treatment reduced vessel density and the number of dysplastic vessels in our adult mouse bAVM model. These observations provide proof-of-principle that, once the abnormal AVM vessels have been formed, VEGF antagonism may reduce the number of dysplastic vessels. The implication is that tonic VEGF stimulation is needed for maintenance of the abnormal phenotype in the mouse model. Whether the abnormal vessels in the lesion have regressed or have been remodeled into normal vessels after bevacizumab treatment needs to be analyzed in future studies. However, our data indicate that an anti-VEGF strategy could be a potential therapy for bAVM and should be further evaluated for the treatment of the human disease.
There are many questions that remain to be addressed in further studies. It is not currently known whether bevacizumab can cross the luminal surface in human bAVMs. We do not yet understand the extent to which our Alk1-deleted, VEGF-stimulated model of bAVM replicates the human disease in terms of natural history. Even if bevacizumab can penetrate the luminal surface in human bAVM and our bAVM model faithfully represents the human disease, it is still not clear whether the reversal of the phenotype will favorably alter the natural history of the human disease. Nonetheless, this is the first report of an approach to medical therapy in a disorder that is currently treatable only by surgical ablation, either by excision, irradiation or embolization.
In the normal vasculature, VEGF is required for the survival and maintenance of new vessels.24, 25 In this study, we used the AAV1 vector that transfects various cell types in the brain, including neurons, astrocytes, and endothelial cells.22 VEGF acts directly on endothelial cells26 and has been shown to be involved in endothelial cell proliferation and angiogenic remodeling.27 Without this tonic stimulation, the vessels regress. After VEGF stimulation, diminution of VEGF level abruptly induces vascular tree regression in chick chorioallantoic membrane by intussusceptive vascular pruning.28 It may be the case for the human disease, that tonic VEGF stimulation is needed for maintenance of the phenotype. This notion is consistent with the emerging view that bAVM is a dynamic, primarily post-natal disease process, rather than a static congenital anomaly.13, 29 BrdU pulse and chase technique will be used to study the dynamic of VEGF stimulation on endothelial cell proliferation and turnover in the bAVM model.
In our model of angiogenesis, we found more TUNEL-positive vascular cells concurrent with a decrease of proliferating vascular cells in bevacizumab treated mice, suggesting that blocking VEGF in an angiogenic foci increased DNA damage in vascular cells. It has been reported that in human bAVM, radiotherapy damaged endothelial cells shrink and detach from neighboring endothelial cells and basement membrane, permitting platelet infiltration with deposition of fibrin and hyaline.30–32 We did not analyze platelet infiltration and fibrin deposition in this study. The fact that we detected more TUNEL positive cells in the bevacizumab treated group than the control group suggests that tonic VEGF is important in maintaining a normal angiogenic process in the brain. Inhibition of VEGF in our bAVM model with bevacizumab led to a reduction in vascular density and irregular vessels, indicating that tonic VEGF stimulation is also necessary in maintaining the abnormal vascular phenotype in our bAVM model. Thus, reducing VEGF levels in human bAVM may reduce the lesional burden.
VEGF has various effects on the cerebrovasculature in addition to increased angiogenesis through endothelial cell proliferation and migration. One of these effects includes breakdown of the blood-brain barrier.33, 34 With VEGF stimulation in our bAVM model, the blood-brain barrier is likely to be compromised, allowing therapeutic antibodies to get into the brain. The presence of the human IgG in the parenchyma in VEGF induced angiogenic foci infers passage of IP-injected bevacizumab and trastuzumab across the blood-brain barrier which facilitates the antibody to interact with VEGF protein in the brain parenchyma.
In this study, we induced angiogenesis in the mouse brain with AAV-VEGF expressing human VEGF, thus replicating the VEGF elaboration in the human disease and allowing assessment of the effect of bevacizumab, an anti-human VEGF antibody in a mouse model. Although our bAVM model was induced by VEGF stimulation, we started bevacizumab treatment six weeks after Alk1 deletion and VEGF stimulation, when the lesion has already developed. We found reduction of VEGF in the lesion with bevacizumab treatment leads to regression of the established dysplastic vessels. There have been potential complications associated with the use of VEGF inhibition including bleeding, hypertension, and hemorrhage,35 with bevacizumab therapy having increased hemorrhage in cancer patients.36 However, VEGF-A inhibition by bevacizumab has been shown to have minimal neurotoxicity37 with a low risk of CNS hemorrhage.36 In fact, there has been recent interest in VEGF inhibition for clinical management of bAVM, if only for controlling perilesional edema.38 However, before bevacizumab can be developed as a therapeutic agent for bAVM, additional pre-clinical safety and efficacy studies will be necessary.
Some studies have shown reduced levels of VEGF-R1 and VEGF-R2 in human resected bAVM,39 and others describe a higher expression of VEGF in partially embolized AVMs, which might imply elevated VEGF in response to the embolic material rather than uniquely as a part of the disease pathogenesis.40 The expression of VEGF appears to be related to patient age, as younger patients have higher expression levels; those AVMs that recur after resection also appear to have higher VEGF.41 Taken together, there may be a range of VEGF expression in the lesional tissue that would result in varying degrees of responsiveness to bevacizumab therapy. Thus, in a possible future clinical study, bevacizumab dose might need to be adjusted to suit different groups of patients. We show that antagonism of the VEGF effect in the angiogenic foci or in a bAVM lesion reduced vessel number and dysplasia in the bAVM. Our results are consistent with the supposition that tonic VEGF stimulation is an important factor in maintaining the lesional phenotype in the nidus of the bAVM.
Conclusions
We present evidence that (1) in WT mice, IP injected bevacizumab can penetrate the blood-brain barrier in VEGF-induced angiogenic foci and inhibit angiogenesis; and (2) in our bAVM model, bevacizumab treatment can reduce vessel density and the number of dysplastic vessels. Our data suggest that bevacizumab, or a related strategy to abrogate VEGF signaling, merits further evaluation in pre-clinical models as a potential approach for the medical therapy of bAVM.
Supplementary Material
Acknowledgments
The authors thank Tony Pourmohamad, MA, for assistance with statistical analysis, Voltaire Gungab for assistance with manuscript preparation, and the other members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their support. We thank Genentech/Roche, South San Francisco, CA, for providing bevacizumab and trastuzumab; and Susan Desmond-Hellmann, MD, MPH, for facilitating transfer of reagents.
Sources of Funding
This study was supported in part by grants from the National Institutes of Health (T32 GM008440 to E.W., R01 NS027713 to W.L.Y., P01 NS044155 to W.L.Y. and H.S., and R21 NS070153 to H.S.), and from the American Heart Association (AHA 10GRNT3130004 to H.S.).
Footnotes
Disclosures
None
References
- 1.Mohr JP, Pile-Spellman J, Stein BM. Arteriovenous malformations and other vascular anomalies. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, editors. Stroke: Pathophysiology, Diagnosis, and Management. 3. Philadelphia: Churchill Livingstone; 1998. pp. 725–750. [Google Scholar]
- 2.Kim H, Marchuk DA, Pawlikowska L, Chen Y, Su H, Yang GY, et al. Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl. 2008;105:199–206. doi: 10.1007/978-3-211-09469-3_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto T, Lawton MT, Wen G, Yang GY, Chaly T, Jr, Stewart CL, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54:410–423. doi: 10.1227/01.neu.0000103421.35266.71. [DOI] [PubMed] [Google Scholar]
- 4.Rothbart D, Awad IA, Lee J, Kim J, Harbaugh R, Criscuolo GR. Expression of angiogenic factors and stuctural proteins in central nervous system vascular malformations. Neurosurgery. 1996;38:915–924. doi: 10.1097/00006123-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Jabbour MN, Elder JB, Samuelson CG, Khashabi S, Hofman FM, Giannotta SL, et al. Aberrant angiogenic characteristics of human brain arteriovenous malformation endothelial cells. Neurosurgery. 2009;64:139–146. doi: 10.1227/01.NEU.0000334417.56742.24. [DOI] [PubMed] [Google Scholar]
- 6.Torsney E, Charlton R, Diamond AG, Burn J, Soames JV, Arthur HM. Mouse model for hereditary hemorrhagic telangiectasia has a generalized vascular abnormality. Circulation. 2003;107:1653–1657. doi: 10.1161/01.CIR.0000058170.92267.00. [DOI] [PubMed] [Google Scholar]
- 7.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, et al. Activin receptor-like kinase 1 modulates transforming growth factor- beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satomi J, Mount RJ, Toporsian M, Paterson AD, Wallace MC, Harrison RV, et al. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke. 2003;34:783–789. doi: 10.1161/01.STR.0000056170.47815.37. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan S, Hanes MA, Dickens T, Porteous ME, Oh SP, Hale LP, et al. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet. 2003;12:473–482. doi: 10.1093/hmg/ddg050. [DOI] [PubMed] [Google Scholar]
- 10.Hao Q, Zhu Y, Su H, Shen F, Yang GY, Kim H, et al. VEGF induces more severe cerebrovascular dysplasia in Endoglin+/− than in Alk1+/− mice. Transl Stroke Res. 2010;1:197–201. doi: 10.1007/s12975-010-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119:3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, et al. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res. 2010;106:1425–1433. doi: 10.1161/CIRCRESAHA.109.211037. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl. 2011;111:83–92. doi: 10.1007/978-3-7091-0693-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69:954–962. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 16.Muller YA, Chen Y, Christinger HW, Li B, Cunningham BC, Lowman HB, et al. VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure. 1998;6:1153–1167. doi: 10.1016/s0969-2126(98)00116-6. [DOI] [PubMed] [Google Scholar]
- 17.Rosen LS. Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control. 2002;9:36–44. doi: 10.1177/107327480200902S05. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell A, Adams LA, MacQuillan G, Tibballs J, vanden Driesen R, Delriviere L. Bevacizumab reverses need for liver transplantation in hereditary hemorrhagic telangiectasia. Liver Transpl. 2008;14:210–213. doi: 10.1002/lt.21417. [DOI] [PubMed] [Google Scholar]
- 19.Flieger D, Hainke S, Fischbach W. Dramatic improvement in hereditary hemorrhagic telangiectasia after treatment with the vascular endothelial growth factor (VEGF) antagonist bevacizumab. Ann Hematol. 2006;85:631–632. doi: 10.1007/s00277-006-0147-8. [DOI] [PubMed] [Google Scholar]
- 20.Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007;18:502–508. doi: 10.1097/ICU.0b013e3282f0ca54. [DOI] [PubMed] [Google Scholar]
- 21.Hao Q, Su H, Marchuk DA, Rola R, Wang Y, Liu W, et al. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol. 2008;295:H2250–2256. doi: 10.1152/ajpheart.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006;11:3190–3198. doi: 10.2741/2042. [DOI] [PubMed] [Google Scholar]
- 23.Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 25.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69 (Suppl 3):11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 27.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 28.Hlushchuk R, Ehrbar M, Reichmuth P, Heinimann N, Styp-Rekowska B, Escher R, et al. Decrease in VEGF expression induces intussusceptive vascular pruning. Arterioscler Thromb Vasc Biol. 2011;31:2836–2844. doi: 10.1161/ATVBAHA.111.231811. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Pawlikowska L, Young WL. Genetics and vascular biology of brain vascular malformations. In: Mohr JP, Wolf PA, Grotta JC, Moskowitz MA, Mayberg M, von Kummer R, editors. Stroke: Pathophysiology, Diagnosis, and Management. 5. Chapter 12. Philadelphia: Churchill Livingstone Elsevier; 2011. pp. 169–186. [Google Scholar]
- 30.Schneider BF, Eberhard DA, Steiner LE. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997;87:352–357. doi: 10.3171/jns.1997.87.3.0352. [DOI] [PubMed] [Google Scholar]
- 31.Achrol AS, Guzman R, Varga M, Adler JR, Steinberg GK, Chang SD. Pathogenesis and radiobiology of brain arteriovenous malformations: implications for risk stratification in natural history and posttreatment course. Neurosurg Focus. 2009;26:E9. doi: 10.3171/2009.2.FOCUS0926. [DOI] [PubMed] [Google Scholar]
- 32.Chang SD, Shuster DL, Steinberg GK, Levy RP, Frankel K. Stereotactic radiosurgery of arteriovenous malformations: pathologic changes in resected tissue. Clin Neuropathol. 1997;16:111–116. [PubMed] [Google Scholar]
- 33.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen F, Walker EJ, Jiang L, Degos V, Li J, Sun B, et al. Coexpression of angiopoietin1 with VEGF increases the structural integrity of the blood-brain barrier and reduces atrophy volume. J Cereb Blood Flow Metab. 2011;31:2343–2351. doi: 10.1038/jcbfm.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roodhart JM, Langenberg MH, Witteveen E, Voest EE. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol. 2008;3:132–143. doi: 10.2174/157488408784293705. [DOI] [PubMed] [Google Scholar]
- 36.Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79:27–38. doi: 10.1159/000314980. [DOI] [PubMed] [Google Scholar]
- 37.Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 38.Williams BJ, Park DM, Sheehan JP. Bevacizumab used for the treatment of severe, refractory perilesional edema due to an arteriovenous malformation treated with stereotactic radiosurgery. J Neurosurg. 2012 doi: 10.3171/2012.1.JNS111627. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto T, Emala CW, Joshi S, Mesa-Tejada R, Quick CM, Feng L, et al. Abnormal pattern of Tie-2 and vascular endothelial growth factor receptor expression in human cerebral arteriovenous malformations. Neurosurgery. 2000;47:910–918. doi: 10.1097/00006123-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Sure U, Butz N, Siegel AM, Mennel HD, Bien S, Bertalanffy H. Treatment-induced neoangiogenesis in cerebral arteriovenous malformations. Clin Neurol Neurosurg. 2001;103:29–32. doi: 10.1016/s0303-8467(01)00112-3. [DOI] [PubMed] [Google Scholar]
- 41.Sonstein WJ, Kader A, Michelsen WJ, Llena JF, Hirano A, Casper D. Expression of vascular endothelial growth factor in pediatric and adult cerebral arteriovenous malformations: an immunocytochemical study. J Neurosurg. 1996;85:838–845. doi: 10.3171/jns.1996.85.5.0838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.