Abstract
Tissue sealants have emerged in recent years as strong candidates for hemostasis. A variety of formulations are currently commercially available and though they satisfy many of the markets’ needs there are still key aspects of each that need improvement. Here we present a new class of blends, based on silk fibroin and chemically active polyethylene glycols (PEGs) with strong adhesive properties. These materials are cytocompatible, crosslink within seconds via chemical reaction between thiols and maleimides present on the constituent PEGs and have the potential to further stabilize through β-sheet formation by silk. Based on the silk concentration in the final formulation, the adhesive properties of these materials are comparable or better than the current leading PEG-based sealant. In addition, the silk-PEG based materials show decreased swelling and longer degradation times. Such properties would make them suitable for applications for which the current sealants are contraindicated.
Introduction
Despite significant progress in recent years, control of bleeding in the operating room still represents an issue. A series of hemostatic agents and tissue adhesives/sealants have been FDA approved and are currently used in the medical practice. While these products satisfy many of the practical requirements, there is still a need to improve the overall material properties and engineer a better product.
Hemostasis can be achieved by mechanical tamponade, by blood cloth formation or artificial wound closure 1. Currently available hemostatic materials are exploring the aforementioned mechanisms either alone or in combination. For instance, mechanical agents, available as sponges, foams or powders of gelatin, collagen, cellulose or other polysaccharide, achieve hemostasis through mechanical tamponade, by swelling at the site of bleeding and molding to the wound shape2. Earlier data suggested that platelet stimulation, aggregation, degranulation and release of clotting factors also occur when these materials are applied to the wound site3.
Blood clot formation can be initiated or achieved enzymatically by the use of thrombin, either alone or in combination with mechanical agents (i.e. bovine collagen sponges or porcine gelatin matrix) or fibrin sealants 2. Thrombin acts by activating platelets at the site of injury and by cleaving fibrinogen to fibrin. Fibrin, in turn, crosslinks into an insoluble network to which platelets adhere and form the homeostatic plug. When thrombin is used in conjunction with mechanical agents, they act synergistically to stop blood loss 4. Fibrin sealants formulated as mixtures of thrombin and fibrinogen recapitulate the last step of the coagulation cascade and supply exogenously the material needed for the formation of the blood cloth 5.
Finally, hemostasis can be attained by using reagents that self-crosslink while simultaneously covalently binding the adjacent tissue to physically close the wound site. In this category there are three types of adhesives currently available. The first class includes the cyanoacrylate-based adhesives that rapidly polymerize in situ in the presence of endogenous hydroxyl groups through an exotermic reaction 1,4–6. The second class comprises the bovine albumin and glutaraldehyde-based sealants 7. In this formulation, glutaraldehyde acts by linking amine groups of albumin to extracellular matrix proteins found at the wound site. Nevertheless, the presence of glutaraldehyde raises toxicity issues 7. Finally, the third class of crosslinking hemostatic reagents encompasses polyethylene glycol (PEG)-based sealants. In this class, several formulations are currently in use and their mechanism of action involves either photopolymerization or chemical crosslinking. Polyethylene glycol (PEG)-based products or biomaterials, in single-component formulations or in combination with other molecules, have been extensively used in the past decades for numerous biomedical applications 8–10. The exceptional biocompatibility and the chemical versatility make this polymer compatible with a large spectrum of biomedical applications 11.
Here we present the development and characterization of novel silk-PEG-based sealants with superior properties to CoSeal, the commercially available PEG-based leader. Silks have been widely employed for a variety of biomedical and biotechnological applications 12–16. The silks have an unusual amino acid sequence with the bulk of the protein organized into alanine and glycine-rich hydrophobic domains with large side chain amino acids clustered in chain-end hydrophilic blocks. Furthermore, structurally the hydrophobic blocks are organized into crystalline regions while hydrophilic blocks form amorphous regions 17. The uniqueness of silk fibroin is conferred by the ability of the crystalline regions to organize into crystalline β-sheets via intra- and inter- molecular hydrogen bonding and hydrophobic interactions. This structural change is exploited in many silk-based biomaterials since these formulations elicit excellent mechanical properties while still maintaining their biocompatibility 12–16.
Our hypothesis was that by combining silk, known for its mechanical strength and biocompatibility with PEGs that are able to rapidly crosslink, we would be able to obtain a sealant formulation with properties superior to currently available counterparts. Our rationale in choosing the system components was focused on: (a) - achieving a high extent of crosslinking by using multi-arm PEGs in the system; (b) attaining rapid crosslinking by carefully selecting the chemical functionalities involved in the crosslinking reaction; (c) improve the properties of the currently marketed PEG-based sealant (i.e. reduce the swelling, increase adhesiveness) by adding in an adhesive hydrophobic biopolymer (silk) 18,19. Based on the chosen components’ properties we anticipated that the silk-PEG based materials would be able to stabilize via a two-step process; the first would involve the rapid covalent crosslinking between the chemical functionalities (maleimide and thiol) present on the four-armed PEG molecules 20; second, the formed network could further be consolidated through the formation of β-sheets between silk fibroin chains.
Materials and Methods
Materials
The four arm-polyethylene glycol maleimide (MW = 10kDa) and four arm polyethylene glycol thiol (MW = 10kDa) were purchased from Nanocs Inc., New York, NY. Silk worm cocoons were obtained from School of Materials Engineering, Soochow University, Suzhou, China.
Silk fibroin extraction
Silk fibroin was obtained as previously described 16. Briefly, Bombyx mori cocoons were cleaned and cut into small pieces. In a subsequent degumming process sericin was removed by boiling the cocoons for 1 h in 0.2 M Na2CO3 solution. The silk fibroin was then dissolved in 9M LiBr at 60°C for 1 h to a concentration of 20% w/v then it was dialyzed against water (MWCO 3,500) for 72 h.
Gel formation and β-sheet content determination
Samples were prepared as described in Table 1, using 1X PBS, with pH 6–8 (this range was assessed based on the pH specificity of the crosslinking reaction). Gelation was assessed by a modified test tube inversion method 21. Specifically, if no fluidity or liquid accumulation to the bottom of the inverted vial/tube was observed after mixing the two components, it was concluded that a gel had formed. The mixing of solutions was achieved by brief vortexing at room temperature. To assess β-sheet formation, samples were incubated at 37°C covered with 1X PBS pH 7.4 or absolute ethanol treated for 15 min and analyzed by FTIR (Equinox 55 ATR-FTIR, Bruker, Billerica, MA).
Table 1.
Preparation of various silk-PEG material formulations
| Sample | Solution A | Solution B | Final composition (mix of 1:1 volume ratio of Solution A and Solution B) | |||
|---|---|---|---|---|---|---|
| 4-arm PEG-thiol (% w/v) | Silk fibroin (% w/v) | 4-arm PEG-maleimide (% w/v) | Silk fibroin (% w/v) | Total PEG (thiol + maleimide) (% w/v) | Silk fibroin (% w/v) | |
| CONTROL | 10 | - | 10 | - | 10 (5 + 5) | - |
| 5 % SILzK | 10 | 5 | 10 | 5 | 10 (5 + 5) | 5 |
| 10 % SILK | 10 | 10 | 10 | 10 | 10 (5 + 5) | 10 |
| 20 % SILK | 10 | 20 | 10 | 20 | 10 (5 + 5) | 20 |
CoSeal Preparation
CoSeal was prepared according to the manufacturer’s instructions. The kit contained two synthetic polyethylene glycols (PEGs) provided as powder in a syringe, and a liquid pouch containing two syringes - one with a dilute hydrogen chloride solution and one with a sodium phosphate/sodium carbonate solution. The two syringes containing the liquids were supplied pre-assembled into a housing designed to allow mixing of the powder syringe with the correct liquid (probably the buffer solution). The liquid was then transferred into the powder by forcefully depressing the plunger. The contents were mixed back and forth between the syringes at least 20 times, until the solid was completely dissolved. The syringe with the dissolved powder and the one containing the other liquid (probably the dilute hydrochloric acid solution) were then placed into a provided epoxy-like adaptor that allowed the simultaneous dispersion of the two solutions. Crosslinking and gelation of the dispersed liquids would occur within 5–10 seconds.
Swelling ratio determination
Gels (50 μl) were prepared in Transwell inserts with 8 μm pore membranes (Corning Inc., Corning, NY) to ensure maximum surface access to solvent, weighed then covered with 1X PBS, pH 7.4. Plates were then incubated at 37°C and 50 rpm and at intervals, the buffer was decanted, the gels blotted to remove excess solvent, and the gel containing inserts were weighed.
In vitro degradation
Gels (100 μL) were prepared similar to swelling determination. The volumes were increased to allow for detection of subtle changes in gel weight. Degradation was determined by incubating 0.1 mL precast gels in 1X PBS, pH 7.4 with or without 1 mg/mL (5 U/mg) Protease XIV (Sigma-Aldrich, St. Louis, MO) at 37°C and 50 rpm and daily weighing the gels for 10 d.
Adhesion tests
Dynamic Mechanical Analyses (DMA) in multiple extension mode (MEM) using an RSA III from TA Instruments (Delaware, U.S.A.) were performed to assess the adhesion of CoSeal and silk-PEG-based biomaterials to intestines or steel. For all measurements the gap between fixtures was set to 1 mm with a maximum allowed pull force of 0.05 N. The steel fixtures used were 8 mm in diameter. For all runs, the sample equilibration steps were followed by a strain-controlled dynamic time sweep test at low strain amplitude (1–5% strain at 1 Hz). Following, a transient tensile test at a constant transducer speed of 5 mm/min was collected until complete material-substrate seal failure. Three replicates were averaged to characterize each sample. For measurements of adhesions to intestinal mucosa, sausage casing was obtained from Whole Foods Market (Woburn, MA), cut into small rectangles that were then applied onto the steel plates. All tests were done at room temperature, regular humidity with tissue samples kept in sterile PBS until needed for the experiment. The tissue sections were handled and cut in wet state (in the presence of PBS), then applied onto the fixture and allowed to set for 30 sec - 1 minute prior to testing.
Cytocompatibility assay
Primary human cervical fibroblasts passage 8 (3 × 105 cells/well) were cultured under serum-free conditions on tissue culture plate, wells coated with PEG-only hydrogels and 10% silk-PEG hydrogels prepared in 1X PBS pH 6. Plates were then incubated for 48 h at 37°C/5% CO2. Cell viability was assessed by using a Live/Dead viability/cytotoxicity kit (Invitrogen, CA). Images were collected with a fluorescent microscope (Leica Microsystems, Wetzlar, Germany) at 100X magnification. Cells were a gift from Dr. Michael House from Tufts Medical Center.
Subcutaneous injections
Samples (200 μl each) were injected subcutaneously into the back of BALB/c mice (n = 4) and followed for local tissue reactions and degradation times for two weeks. Tissues from injection sites were processed for histological evaluation and stained with hematoxylin and eosin (H&E). Animal studies were conducted in accordance with Tufts IACUC approved protocols.
Statistical analysis
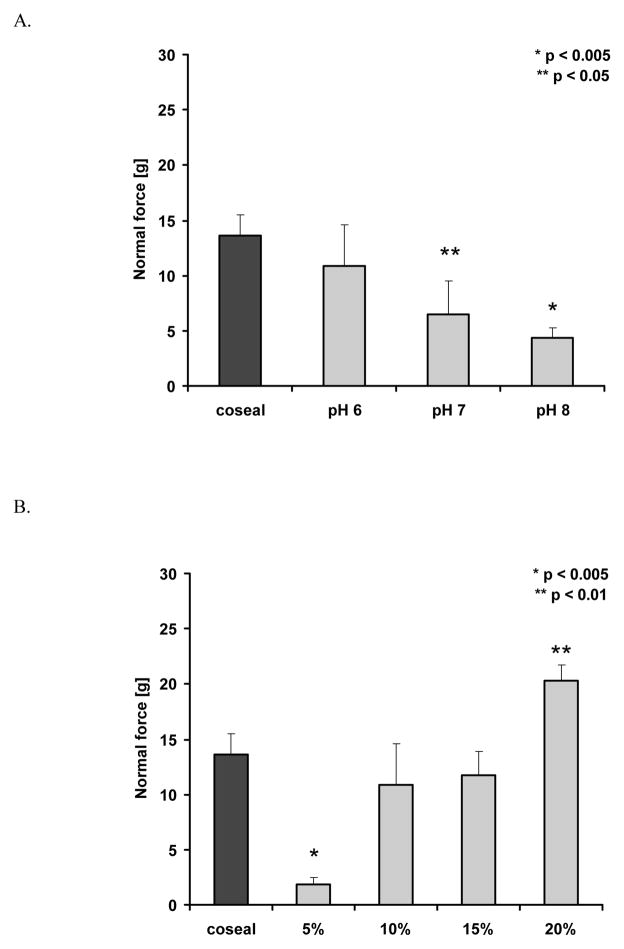
Values, represented as mean ± standard deviation (S.D.) were compared using Student’s t-test (2-tailed, type 3) with p < 0.05 or p < 0.01 considered statistically significant and p < 0.005 considered highly significant. For multiple data comparison (Figure 7A and 7B) the ANOVA Single Factor analysis was used.
Figure 7.
DMA measurements of adhesion. A. pH-dependence of adhesion for 10% silk-containing samples (n = 3) (ANOVA, P=0.009). B. Concentration dependence of adhesion at pH 6 (n = 3) (ANOVA, P= 0.00002). The statistics indicated in the figures were obtained with Student t-test.
Results
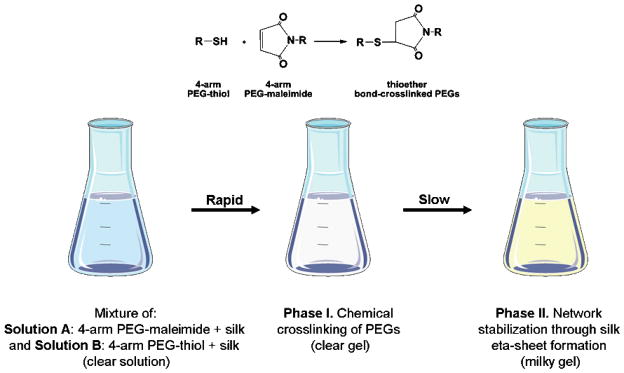
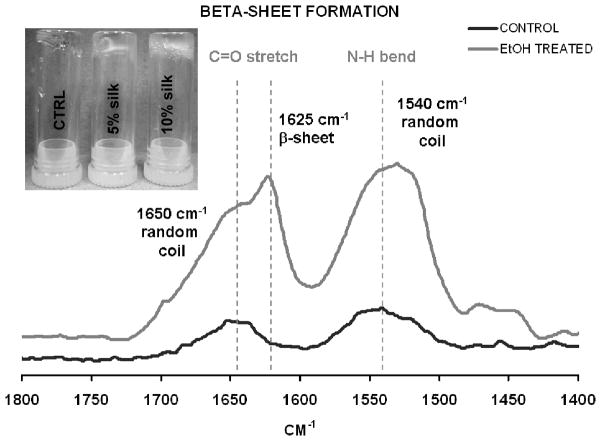
Various silk-PEG-based hydrogel formulations were obtained as described in Table 3. Based on the system design, a two-step gelation process was anticipated (Figure 1). The first phase would involve rapid gel formation via chemical reaction between the two PEGs. This step was confirmed by the formation of a gel within second after mixing 4-arm PEG-SH and 4-arm-PEG-maleimide containing samples, with or without silk 20 (Figure 2 -inset). Silk was expected to further be able to consolidate the crosslinked network through β-sheet formation. To confirm that in the blended formulations silk would still maintain its ability of to form beta-sheets, we designed a proof of concept experiment where we used ethanol as an “exogenous accelerator” for β-sheet formation. To this end, silk-containing (10%-silk-PEG) samples were treated with ethanol (known to induce β-sheet formation) or PBS (control) and monitored by FTIR, using the control sample as background. Alcohol treatment did induce β-sheet formation as evidenced by the appearance of the characteristic β-sheet peak (1625 cm−1) (Figure 2). This result confirmed the plausibility of our two-step stabilization hypothesis and that the blended silk maintained its ability to form β-sheets. Under physiological conditions (no ethanol treatment) no significant β-sheet was detectable in control and silk-containing samples after 24 h indicating that network stabilization through β-sheet formation would occur slowly in time (data not shown). To be noted, none of the further described experiments involved the use of ethanol.
Figure 1.
Graphic representation of the silk-PEG gel formation process.
Figure 2.
Proof of concept experiment illustrating the ability of silk to rapidly form β-sheets in the presence of EtOH. Confirmation of the gelation model, with a rapid chemical crosslinking step (inset) and β-sheet formation by silk (10% silk containing PEG samples were treated with EtOH to assess the β-sheet forming capacity of fibroin in the blended formulation).
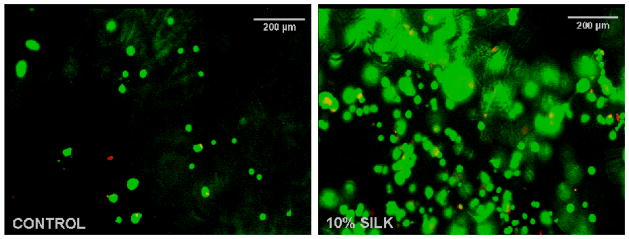
We next confirmed the cytocompatibility of the blends by casting gels and seeding them with cells without prior washing. These conditions would intimately mimic situations where the materials would be crosslinked in vivo without additional washing steps. Primary cervical fibroblasts cultured for 48 h on the control and silk containing samples were highly viable but displayed a rounded morphology on both materials most probably related to the lack of cell specific attachment sites. For the silk containing samples, this behavior has been previously documented by our group 16,22(Figure 3). In future studies, this property of the materials could be further exploited for surgical cytoadherence prevention and scar tissue formation prevention in in vivo settings.
Figure 3.
Cytocompatibility of PEG (left) and PEG-silk-based (right) biomaterials after 48 h cell culture (live cells – green, dead cells – red). The overall cell number on both control and silk containing materials were the same. However, the imaging fields were selected based on minimal background fluorescence, therefore the local cell numbers do not appear identical in the two images.
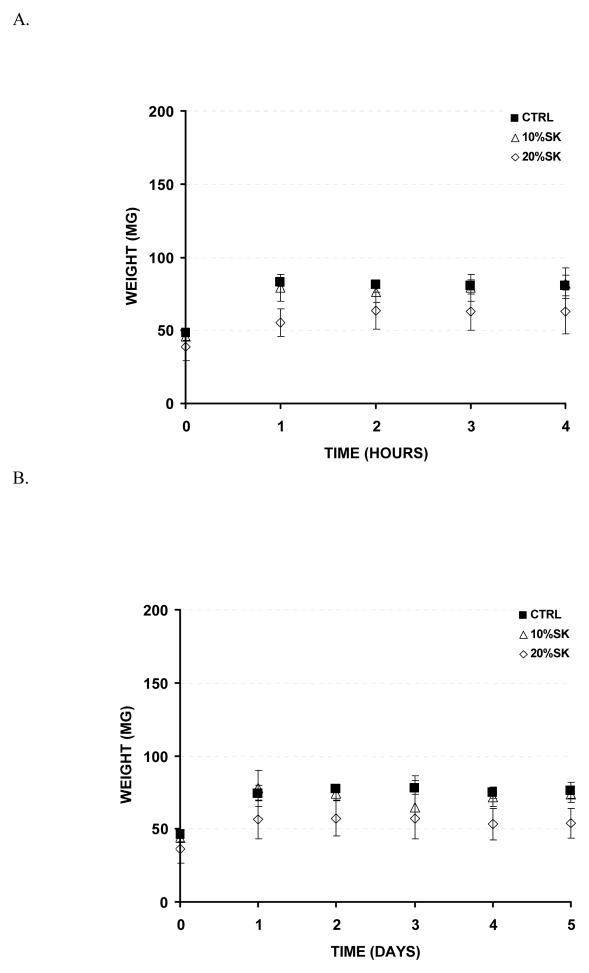
Next, to assess biomaterial swelling under physiological conditions, samples were prepared in Transwell inserts, covered with buffered saline solution and incubated at 37°C with gentle shaking. Within 2 h, the control (PEG-only) and 10% silk containing samples swell up to ~70 % of their original size, while the 20% silk containing samples only swelled up to ~60% (Figure 4). Sample swelling was monitored for up to day 5 of incubation without variations in the initially observed values.
Figure 4.
Swelling profile of silk-PEG-based materials. A. Swelling ratio throughout 4 h. B. Swelling profile throughout 5 d (n = 3).
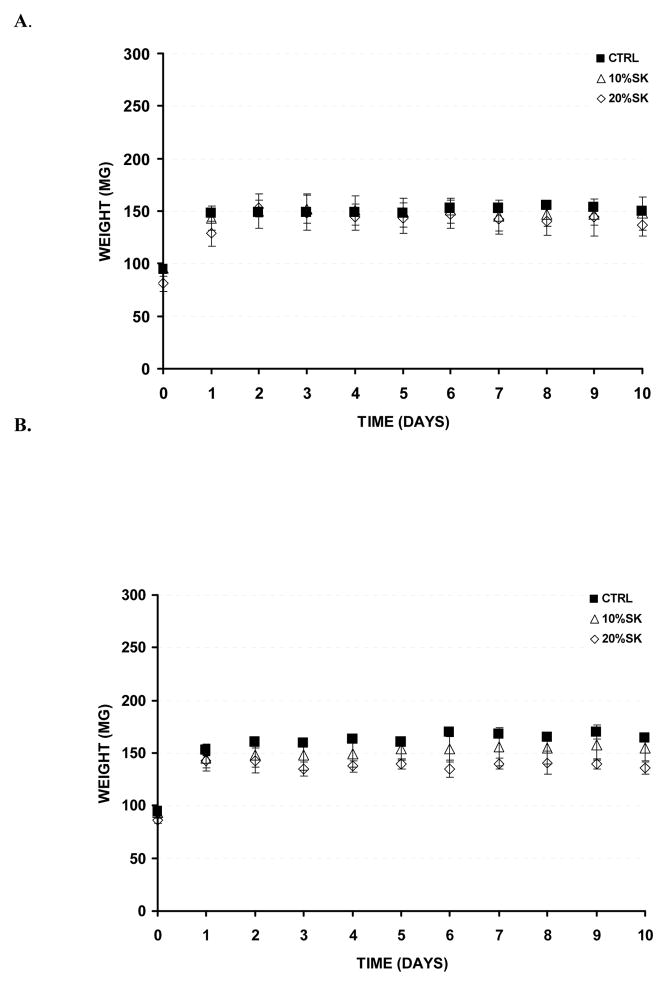
The stability of the materials was next assessed by conducting degradation experiments in the presence and absence of Protease XIV. Since mammalian systems do not express silk-specific degrading enzymes, Protease XIV has been used in most of published research as a hallmark silk-degrading enzyme 23. Dry weight degradation of the samples was avoided, since the wet state scenario is more pertinent to the events that would occur in vivo. Consistent with this, in both our control and protease containing degradation experiments, initial swelling of samples was noticed. The degradation percent was determined by normalizing the final recorded weight to the swollen sample weight (the maximum recorded weight). In the control experiment (PBS only), no significant hydrolytic degradation was noticed during the 14 d experimental process for any of the samples (control, 10% and 20% silk containing samples) (Figure 5A). Similarly, in the presence of Protease XIV, no significant enzymatic degradation was noticed during the 14 d experimental process for any of the samples (control, 10% and 20% silk containing samples) (Figure 5B). These results indicate that the silk-PEG hydrogels would fall apart very slowly in vivo and most likely would be present for months before degradation 23. In vivo, this property might translate to occurrence of complete wound healing prior to the disappearance of the tissue adhesive.
Figure 5.
In vitro degradation rates. A. Samples in 1X PBS, pH 7.4. B. Samples in Protease XIV (1 mg/mL, 5 U/mg) (n = 3). No significant degradation rates were noticed for any of the tested conditions.
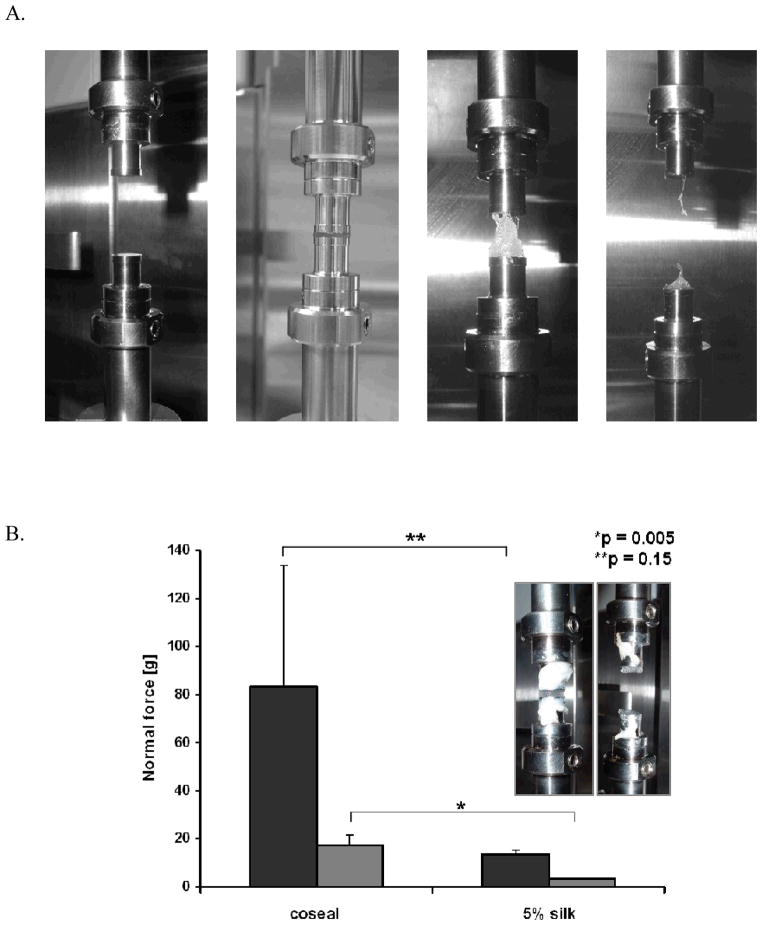
To test the adhesiveness of the silk-PEG materials, samples were compared to CoSeal in DMA measurements. The goal of these tests was to determine the pull force needed to break the contact between the sealant and the substrate as a measure of adhesiveness (Figure 6A). In our initial experimental design, bovine intestines (sausage casing) were selected as biological membrane model (substrate) for adhesion. However, the biological membranes were highly perishable and inconsistencies in surface properties or sample-to-sample surface property variations appeared to affect the reproducibility of our results and hindered the accurate data interpretation (Figure 6B). However, when CoSeal and 5% silk pH 7 were tested on steel, the data reproducibility was significantly increased. Moreover, even though the adhesion values were much lower on steel (approximately 6-fold for both CoSeal and silk-containing samples), the trends for the values on intestines and steel were comparable (Figure 6B). Based on this data we decided to complete the in vitro characterization of the materials adhesives properties on steel.
Figure 6.
A. Illustration of DMA measurement procedure (adhesion to steel) depicting the steel fixtures prior sample mounting (left) and the testing process. B. Comparison of CoSeal and 5% silk/PEG materials on intestines (dark grey) and steel (light grey), showing similar trends (n = 3). Inset – DMA setting for adhesion to intestines measurements. The indicated statistics were obtained with Student t-test.
Since the chemical reaction between the thiol and maleimide functionalities present on the PEGs is pH sensitive (basic pH values would favor the formation of disulfide bonds rather than thioether), we investigated the pH dependence of the adhesion for the silk containing samples and controls. The 10% silk containing sample was chosen as a generic representative for this assay. For silk-containing samples prepared in 1x PBS pH 6 the adhesion values to steel were comparable to that of CoSeal, while samples prepares in pH 7 or pH 8 buffers were surpassed by CoSeal (Figure 7A). Next the effect of silk concentration on adhesion was addressed. Comparable adhesion values were obtained for CoSeal, 10% and 15% silk containing samples. Significantly lower adhesion (p< 0.005) was observed for 5% silk samples, while the 20% silk containing samples outperformed CoSeal (p < 0.01) with approximately 50% increase in adhesiveness (Figure 7B). These results appear to indicate that silk containing samples, at 10% w/v or higher, could be competitive tissue sealants.
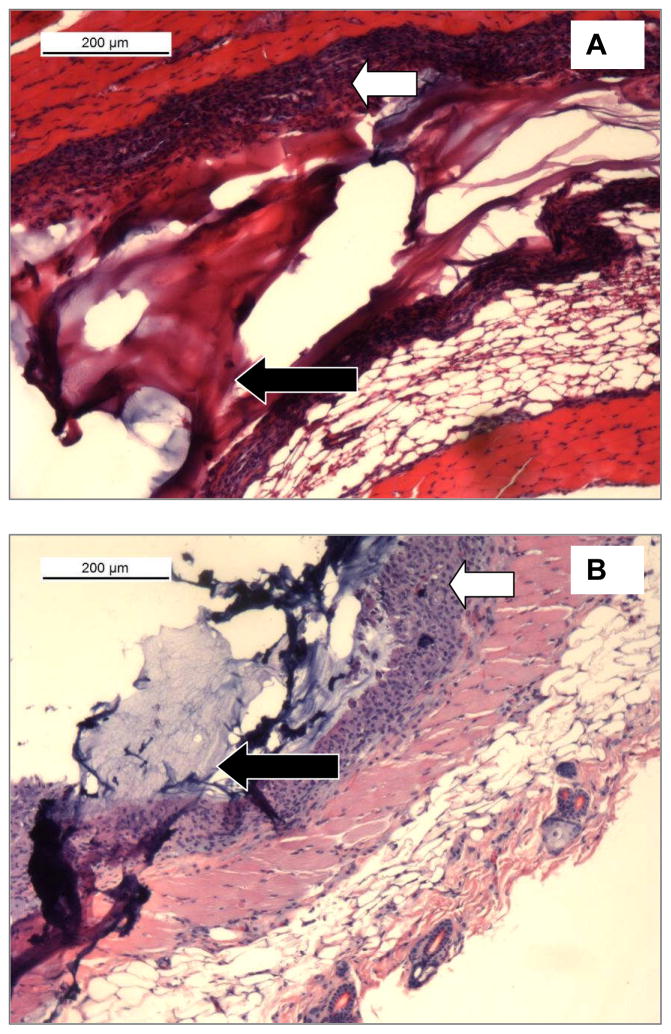
Next we addressed the in vivo compatibility of the silk-PEG blends. Materials (CoSeal and 5% silk/PEG) were then injected subcutaneously on the back of BALB/c mice and removed for histological analysis after two weeks. Both materials integrated well with the native tissue causing no significant inflammatory response and normal levels of fibrosis, as previously reported for CoSeal 10 (Figure 8).
Figure 8.
Analysis of tissue samples subcutaneously injected with A. - CoSeal or B. - 5% silk containing samples two weeks post-procedure. Black arrows indicate the injected material. White arrows indicate similar levels of fibrosis in the two samples, consistent with previously reported data on CoSeal 10.
Discussion
This study presents the design and in vitro characterization of PEG-silk based composites with adhesive properties. As a reference for our experiments, we chose CoSeal as it is the closest in formulation to our system and it emerged in 2003 as the leading crosslinking sealant. CoSeal is Food and Drug Administration (FDA)-approved and is comprised of a thiol-PEG and a succinimide-PEG component, a dilute hydrogen chloride solution and a sodium phosphate/sodium carbonate solution. This material is available as a two-component (liquid and powder), epoxy-like syringe that upon mixing crosslinks within 5–10 s to achieve hemostasis. In clinical trials, CoSeal was well tolerated by patients and its use lead to immediate sealing of oozing wounds in 50% of patients versus 26% that were cared for by standard treatments. For brisk bleeding, CoSeal was effective in 41% of cased compared to 3% immediate hemostasis seen with standard treatment. However, some of the major drawbacks of this product are its cumbersome reparation/reconstitution prior use and its swelling ratio. CoSeal reconstitution requires one of its components, supplied as a powder, to be mixed back and forth between two syringes at least 20 times until completely dissolved. Additionally, the swelling ratio of CoSeal, 400% of its original size/weight, limits its applicability to areas where nerve compression would not be an issue.
The results presented in our study indicate that on steel silk-PEG-based materials are comparable or better than CoSeal. Silk containing samples prepared in 1X PBS pH 6, at 10–15% w/v silk showed comparable adhesion values to the control, while the 20% w/v formulation showed approximately 50% increase in adhesiveness. Moreover, the silk-PEG based materials could overcome some of the major issues seen with CoSeal such as swelling and in vivo residence time. Our data also indicates that the swelling ratio of silk containing sealants is between 60–70% while CoSeal increases its size to up to 400% post-application. The difference in the swelling ratio between the 10% and 20% sample is related to the sample formulation and swelling assay set-up. Samples were dissolved in water but assayed for swelling in PBS to mimic physiological conditions. The 20% silk samples have less water compared to the 10 % silk sample – therefore upon ionic equilibration the end weight values for the 10% samples were higher than for the 20% ones.. This property of the silk-based sealants would increase their application spectra and allow them to be used in close spaces or in the vicinity of pressure sensitive structures such as nerves, where CoSeal use is contraindicated. The duration of the chemical crosslinking for our materials is comparable to CoSeal and in addition would be further consolidated in time via the secondary physical crosslinking reaction involving β-sheet formation in silk. The long in vivo residence time of the silk-PEG material is an additional attractive feature. CoSeal is degraded in approximately 1–2 weeks 10 while the silk-PEG-based materials would be present for a significantly longer time23, allowing for complete healing prior to full material resorption. For our experimental data, an array of silk concentrations ranging from 5% to 20% was tested. Decreased silk concentrations yield solutions with lower viscosities and require fewer raw materials; however the adhesive properties of the silk-containing materials were optimal at 10% w/v or higher. At these concentrations the solution viscosities are still suitable for injection. The pH values tested for material preparation were within the physiological range (6.0–8.0) and the optimal pH value for our system was 6.0 as it yielded materials with the highest adhesive properties. No significant β-sheet formation dependence on pH was detectable after material crosslinking (data not shown) suggesting that the pH value impacts primarily the chemical crosslinking step. This makes sense considering that a lower pH would preserve the free thiol functionalities from the 4-arm PEG-SH and prevent them from forming intra-or intermolecular disulfide bonds that would reduce the molecule’s capacity to react with the intended maleimide functionalities.
In the process of selecting a substrate for our adhesion tests (biological membrane versus steel), differences in key adhesion process players such as surface topology (smooth versus rugged), surface proteins, extent of hydration, etc. were considered. However, steel appeared more suitable for this preliminary study since variations in the properties of the biological membranes (due to the membrane physiology or surface fouling) would have caused interpretation errors and would have decreased the validity of our study. Overall, considering that the in vitro adhesion trends on steel correlated well with those observed on biological membranes (bovine intestines) and that the material characteristics were better than those of CoSeal, we would anticipate that silk-PEG sealants could outperform CoSeal in biological systems, but this assumption will need to be further confirmed in in vivo adhesion tests.
In contrast to CoSeal, the dual nature of silk-PEG-based materials and its two-step crosslinking mechanism would allow for a wide spectrum of applications and offer a series of advantages. These materials could be processed to be available as gels, mats, sponges, fibers and other material formats as previously reported for silk-based materials 24–27. Our cytocompatibility results further indicate that these systems might be suitable as anti-scar formation systems as they do not promote cell spreading. This feature, combined with their versatile processability could also expand the application spectrum of these materials (i.e. allow for production of anti-adhesive sheets or films, etc.). In subcutaneous injections in mice the tissue compatibility of silk-based sealants was comparable to CoSeal. Both materials were still present after two weeks. Previous studies reported that in subcutaneous implants of pre-made gels in rabbits on CoSeal completely degraded or resorbed after approximately two weeks 10. We speculate that the discrepancies noticed in degradation rates are due to the different experimental conditions (implants versus injections; pre-made gels versus in situ crosslinking; rabbits versus mice). Overall, the data herein appears to indicate that silk-PEG based composites could be strong competitors for bleeding control; nevertheless further in vivo studies will be conducted by our group to directly assess the hemostatic and sealant properties of these materials.
Conclusions
This work indicates that silk-PEG-based materials that rapidly crosslink via a chemical reaction between thiol and maleimide functionalities have biological, physical and mechanical properties that could allow them to be used as sealant and hemostats. Furthermore, the design of these systems allows for application-oriented material tailoring in terms of general formulations, adhesive properties and degradability. Additional in vivo hemostatic model experiments will be conducted to confirm the data presented here and to assess the orthotopic sealant capacities of the materials; however our data so far indicated that silk-PEG based materials would represent a novel, adaptable category of composites with competitive adhesion/tissue sealing properties.
Acknowledgments
We thank Dr. Michael House from Tufts Medical Center for providing the CoSeal for this study and Dr. Tuna Yucel for his initial assistance with DMA.
References
- 1.Wheat JC, Wolf JS., Jr Advances in bioadhesives, tissue sealants, and hemostatic agents. Urol Clin North Am. 2009;36(2):265–75. x. doi: 10.1016/j.ucl.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Spotnitz WD, Burks S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion. 2008;48(7):1502–16. doi: 10.1111/j.1537-2995.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins HP, Janda R, Clarke J. Clinical and experimental observations on the use of gelatin sponge or foam. Surgery. 1946;20(1):124–32. [PubMed] [Google Scholar]
- 4.Gill IS, Ramani AP, Spaliviero M, Xu M, Finelli A, Kaouk JH, Desai MM. Improved hemostasis during laparoscopic partial nephrectomy using gelatin matrix thrombin sealant. Urology. 2005;65(3):463–6. doi: 10.1016/j.urology.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Lowe J, Luber J, Levitsky S, Hantak E, Montgomery J, Schiestl N, Schofield N, Marra S. Evaluation of the topical hemostatic efficacy and safety of TISSEEL VH S/D fibrin sealant compared with currently licensed TISSEEL VH in patients undergoing cardiac surgery: a phase 3, randomized, double-blind clinical study. J Cardiovasc Surg (Torino) 2007;48(3):323–31. [PubMed] [Google Scholar]
- 6.Marcovich R, Williams AL, Rubin MA, Wolf JS., Jr Comparison of 2-octyl cyanoacrylate adhesive, fibrin glue, and suturing for wound closure in the porcine urinary tract. Urology. 2001;57(4):806–10. doi: 10.1016/s0090-4295(00)01075-x. [DOI] [PubMed] [Google Scholar]
- 7.Furst W, Banerjee A. Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann Thorac Surg. 2005;79(5):1522–8. doi: 10.1016/j.athoracsur.2004.11.054. discussion 1529. [DOI] [PubMed] [Google Scholar]
- 8.Preul MC, Campbell PK, Bichard WD, Spetzler RF. Application of a hydrogel sealant improves watertight closures of duraplasty onlay grafts in a canine craniotomy model. J Neurosurg. 2007;107(3):642–50. doi: 10.3171/JNS-07/09/0642. [DOI] [PubMed] [Google Scholar]
- 9.Torchiana DF. Polyethylene glycol based synthetic sealants: potential uses in cardiac surgery. J Card Surg. 2003;18(6):504–6. doi: 10.1046/j.0886-0440.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- 10.Wallace DG, Cruise GM, Rhee WM, Schroeder JA, Prior JJ, Ju J, Maroney M, Duronio J, Ngo MH, Estridge T, et al. A tissue sealant based on reactive multifunctional polyethylene glycol. J Biomed Mater Res. 2001;58(5):545–55. doi: 10.1002/jbm.1053. [DOI] [PubMed] [Google Scholar]
- 11.Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26(3):631–43. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bini E, Knight DP, Kaplan DL. Mapping domain structures in silks from insects and spiders related to protein assembly. J Mol Biol. 2004;335(1):27–40. doi: 10.1016/j.jmb.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann S, Foo CT, Rossetti F, Textor M, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Silk fibroin as an organic polymer for controlled drug delivery. J Control Release. 2006;111(1–2):219–27. doi: 10.1016/j.jconrel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence BD, Marchant JK, Pindrus MA, Omenetto FG, Kaplan DL. Silk film biomaterials for cornea tissue engineering. Biomaterials. 2009;30(7):1299–308. doi: 10.1016/j.biomaterials.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soffer L, Wang X, Zhang X, Kluge J, Dorfmann L, Kaplan DL, Leisk G. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J Biomater Sci Polym Ed. 2008;19(5):653–64. doi: 10.1163/156856208784089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54(1):139–48. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhou CZ, Confalonieri F, Jacquet M, Perasso R, Li ZG, Janin J. Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins. 2001;44(2):119–22. doi: 10.1002/prot.1078. [DOI] [PubMed] [Google Scholar]
- 18.Leisk GG, Lo TJ, Yucel T, Lu Q, Kaplan DL. Electrogelation for protein adhesives. Adv Mater. 22(6):711–5. doi: 10.1002/adma.200902643. [DOI] [PubMed] [Google Scholar]
- 19.Yucel T, Kojic N, Leisk GG, Lo TJ, Kaplan DL. Non-equilibrium silk fibroin adhesives. J Struct Biol. 170(2):406–12. doi: 10.1016/j.jsb.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderhooft JL, Mann BK, Prestwich GD. Synthesis and characterization of novel thiol-reactive poly(ethylene glycol) cross-linkers for extracellular-matrix-mimetic biomaterials. Biomacromolecules. 2007;8(9):2883–9. doi: 10.1021/bm0703564. [DOI] [PubMed] [Google Scholar]
- 21.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3(6):1304–11. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan DL. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J Biomed Mater Res A. 2003;67(2):559–70. doi: 10.1002/jbm.a.10120. [DOI] [PubMed] [Google Scholar]
- 23.Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. In vitro degradation of silk fibroin. Biomaterials. 2005;26(17):3385–93. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23(20):4131–41. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, Kaplan DL. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5(3):786–92. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27(16):3115–24. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5(3):718–26. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]