Abstract
We have reported that the acute postexercise increases in muscle protein synthesis rates, with differing nutritional support, are predictive of longer-term training-induced muscle hypertrophy. Here, we aimed to test whether the same was true with acute exercise-mediated changes in muscle protein synthesis. Eighteen men (21 ± 1 yr, 22.6 ± 2.1 kg/m2; means ± SE) had their legs randomly assigned to two of three training conditions that differed in contraction intensity [% of maximal strength (1 repetition maximum)] or contraction volume (1 or 3 sets of repetitions): 30%-3, 80%-1, and 80%-3. Subjects trained each leg with their assigned regime for a period of 10 wk, 3 times/wk. We made pre- and posttraining measures of strength, muscle volume by magnetic resonance (MR) scans, as well as pre- and posttraining biopsies of the vastus lateralis, and a single postexercise (1 h) biopsy following the first bout of exercise, to measure signaling proteins. Training-induced increases in MR-measured muscle volume were significant (P < 0.01), with no difference between groups: 30%-3 = 6.8 ± 1.8%, 80%-1 = 3.2 ± 0.8%, and 80%-3= 7.2 ± 1.9%, P = 0.18. Isotonic maximal strength gains were not different between 80%-1 and 80%-3, but were greater than 30%-3 (P = 0.04), whereas training-induced isometric strength gains were significant but not different between conditions (P = 0.92). Biopsies taken 1 h following the initial resistance exercise bout showed increased phosphorylation (P < 0.05) of p70S6K only in the 80%-1 and 80%-3 conditions. There was no correlation between phosphorylation of any signaling protein and hypertrophy. In accordance with our previous acute measurements of muscle protein synthetic rates a lower load lifted to failure resulted in similar hypertrophy as a heavy load lifted to failure.
Keywords: skeletal muscle, protein synthesis, motor unit, loading
heavier loading [usually expressed as percentage of a person's maximal strength or single repetition maximum (1RM)] is often recommended as the optimal way to maximize muscle hypertrophy with resistance training (1). However, there is very little empirical evidence to support this supposition and it is unclear as to the physiological mechanisms by which heavier training loads would provide a signal for greater muscle hypertrophy compared with, for example, a lighter load lifted to the point of fatigue; both conditions would result in a large amount of muscle fibers being recruited. As proof-of-principle, we recently tested this idea and demonstrated (9) that a single bout of resistance exercise performed at 30% of 1RM to the point of momentary muscle fatigue (failure) was equally as effective in stimulating myofibrillar protein synthesis rates (MPS) as loads lifted at 90% of 1RM (also lifted to fatigue). In addition, the 30%-1RM condition resulted in a more prolonged muscle protein synthetic response with a greater elevation of myofibrillar protein synthesis rates than the 90% of 1RM condition at 24 h after exercise (9). We have proposed that the acute exercise-induced increases in MPS that are further augmented with protein ingestion summate and lead to muscular hypertrophy (27). If such a thesis is valid then acute measures of protein synthesis would be, at least qualitatively if not quantitatively, predictive of long-term gains in muscle protein. There is support for this concept as we have shown that measures of acute postexercise MPS, with differing nutritional support (44), are qualitatively predictive of the training-induced phenotypic outcome (17) between young men consuming milk or soy. Therefore our previous results (9) suggest that resistance training performed at 30% of 1RM to fatigue should result in muscle hypertrophy after chronic resistance training that, based on its prolonged stimulation of myofibrillar protein synthesis, is at least equivalent or greater than the degree of hypertrophy resulting from training with heavy loads. Support for this thesis exists from previous training studies and from a number of studies using low loads with vascular occlusion showing equivalent hypertrophy as that with high loads (36–38).
Other than relative training load, another resistance training variable that is often considered important is the volume of work performed (1, 7, 25). Since fatigue limits the number of repetitions that can be completed in a set at a given load, varying the number of sets is a common way to adjust training volume. There is currently disagreement concerning the benefit of additional sets for increases in muscle hypertrophy (11, 29, 34). We have generated acute protein synthesis data from young men showing that 3 sets performed at 70% of 1RM to failure resulted in a greater and more prolonged myofibrillar protein synthetic rate compared with a single set condition (7). We speculate that if the model of summative changes leading to hypertrophy (27) is correct, then our data (7) would mean a greater hypertrophic response with 3 sets vs. 1 set of resistance exercise.
We also wished to test the thesis that early postexercise signaling responses, in particular that of p70S6-kinase (p70S6K), would be related to hypertrophy as has been shown in humans (39) and rodents (4). This is of interest since if the phosphorylation of one single protein is truly predictive of hypertrophy and strength gains then it is certainly worthy of great attention as to its exact mechanistic role in muscle hypertrophy. The overall purpose of the present study was to test whether the acute results we had observed previously (7, 9) would be reflected with longer-term resistance training adaptations. We employed acute measurements of protein signaling to evaluate the relevance of these variables in predicting phenotype.
METHODS
Subjects.
Eighteen healthy young men (21 ± 0.8 yr, 1.76 ± 0.04 m, 73.3 ± 1.4 kg; means ± SE) volunteered to participate in the study after being informed of the procedures and potential risks involved in the investigation. Subjects were recreationally active with no formal weightlifting experience or regular weightlifting activity over the last year. The protocol was approved by the Research Ethics Board of Hamilton Health Sciences and McMaster University and was written in accordance with standards set by the Declaration of Helsinki.
Experimental design.
Participants completed 10 wk of unilateral knee extension resistance training. Each leg was randomly assigned in counterbalanced fashion to one of three possible unilateral training conditions: one set of knee extension performed to voluntary failure at 80% of 1RM (80%-1); three sets of knee extension performed to the point of fatigue at 80% of 1RM (80%-3); or three sets performed to the point of fatigue with 30% of 1RM (30%-3). Each participant trained both legs and was therefore assigned to two of the three possible training conditions. Immediately after each training session subjects consumed a source of high-quality protein (PowerBar Protein Plus, 360 kcal, 3.5 g leucine, 30 g protein, 33 g carbohydrate, 11 g fat; Nestle Nutrition, Florham Park, NJ) in conjunction with ∼300 ml of water to standardize the postexercise meal and maximize training adaptations.
Before and after the training program, whole muscle volume was measured using magnetic resonance imaging (described below) and changes in muscle fiber area were determined by fiber planimetry with myosin ATPase histochemistry (described below). Knee extension performance was measured by 1RM, maximal voluntary isometric contraction (MVC), rate of isometric force development (RFD), and peak power (described below).
Prior to the first training session, participants fasted overnight and consumed a standard liquid meal (480 kcal, 20 g protein, 82 g carbohydrate, 8 g fat) 2 h prior to having resting bilateral muscle biopsies taken from the vastus lateralis. Biopsies were performed with a Bergström needle that was custom-modified for manual suction under local anesthesia (2% xylocaine). Tissue from this biopsy (∼80 mg) was used for myosin ATPase histochemistry and Western blotting analysis. Following these initial biopsies, subjects completed their prescribed training session for each leg and immediately consumed a PowerBar Protein Plus bar (Nestle Nutrition, Florham Park, NJ) and 300 ml of water. One hour following the completion of the exercise, bilateral biopsies were taken from the vastus lateralis, for the measurement of acute changes in anabolic signaling molecule phosphorylation status via Western blot. Following 10 wk of training a third set of muscle biopsies was taken from each vastus lateralis muscle and used for fiber area quantification.
Western blots.
A piece of wet muscle (≈20 mg) was homogenized by hand on ice using a Teflon pestle in a standard Western blotting homogenization buffer (10 μl/mg): 25 mM Tris (pH 7.2) buffer containing 1 mM Na3VO4, 50 mM NaF, 40 mM β-glycerolphosphate, 20 mM sodium pyrophosphate, 0.5% vol/vol Triton X-100, and Complete Protease Inhibitor Mini-Tabs (Roche, Indianapolis, IN) was used. The samples were centrifuged at 1,500 g at 4°C for 10 min. The resulting supernatant was removed and protein content was determined by the Bradford assay. Equal aliquots of protein were boiled in Laemmli sample buffer (250 mM Tris·HCl, pH 6.8; 2% SDS; 10% glycerol; 0.01% bromophenol blue; 5%β-mercaptoethanol) for 5 min. Samples (20 μg of protein) were loaded onto 7.5–10% SDS-polyacrylamide gels and run for 1.5 h at 150 V. Gels were then transferred to a PVDF membrane at 100 V for 1 h. Membranes were blocked with 5% skim milk powder (wt/vol) in Tris-buffered saline with 0.1% Tween (vol/vol) (TBST). Membranes were then incubated overnight in primary antibody at 4°C. The following phosphorylation sites were determined: p70S6K-1Thr389 (Santa Cruz Biotechnology, Santa Cruz, CA; no. 11759, 1:500 dilution in TBST), mTORSer2448 (Cell Signaling Technology, no. 2971; 1:1,000 dilution in TBST), Akt Ser473 (Cell Signaling Technology, no. 4056; 1:1,000 dilution in TBST). After washing in TBST, membranes were incubated in horseradish peroxidase-linked anti-rabbit IgG secondary antibody (GE Healthcare, Amersham Biosciences, Piscataway, NJ; no. NA934VS, 1:15,000 dilution in TBST), washed with TBST, and detected by chemiluminescence (SuperSignalWest Dura Extended Duration Substrate, Thermo Scientific, no. 34075). Images were developed using FluorChem SP Imaging system and quantified by spot densitometry using ImageJ software. All signaling protein phosphorylation responses were normalized to their respective total protein.
Magnetic resonance imaging.
Subjects rested in the supine position for 1 h prior to scanning to prevent the influence of fluid shifts on muscle volume. Also, no strenuous activity was allowed within 24 h of the scanning. MR imaging was performed in a 3-T HD scanner (Signa MRI System; GE Medical, Milwaukee, WI) at the Brain-Body Institute, Imaging Research Centre, St. Joseph's Healthcare (Hamilton, Ontario). Image acquisition was carried out using T1 fluid attenuation inversion recovery (FLAIR) in the axial plane with the following parameters: repetition time/echo time = 2,574 ms/6.7 ms; field of view = 25–30 cm; matrix size = range from 320/320 to 512/512 phase/frequency; inversion time = 958 ms; slice thickness = 5 mm. Thigh image acquisition utilized an eight-channel torso coil with two excitations. There was a 10 mm gap between slices. Quadriceps volume was calculated by multiplying the slice area by the distance between slices. Volume was measured from the first slice where the rectus femoris was visible to the first slice where the gluteus maximus was visible. ImageJ software (U. S. National Institutes of Health, Bethesda, MD) was used to determine the area of each slice. Pre- and postscans were performed at the same time of day and joint angle and leg compression was controlled.
Muscle function.
Subjects completed two testing sessions to assess knee extensor function before and after the 10-wk training period. The two sessions occurred in randomized order and were separated from each other and the first training session by 3 days. Each session started with two sets of submaximal dynamic knee extensions with a load designed not to be fatiguing. Session one consisted of three 5-s unilateral knee extension MVCs conducted on a Biodex dynamometer (Shirley, NY) with 1 min of rest between contractions. A knee angle of 90° was used. The highest recorded torque for each leg was taken as the MVC. The analog torque signal for the Biodex was sampled at 2,000 Hz with PowerLab 3 data acquisition system (ADInstruments, Bella Vista, Australia) and the RFD was calculated off-line by taking the first derivative with respect to time. The dynamometer was then set in isotonic mode and subjects were instructed to move the load as quickly as possible. Three trials at 20, 30, 40, and 50% of MVC were completed in a random order and the highest instantaneous power achieved in any of the trials was recorded as peak power. Subjects then completed a single set of knee extension to muscle fatigue for each leg. The load used for this test was 30% of 1RM for legs assigned to train with 80% of 1RM and 80% of 1RM for legs assigned to train with 30% of 1RM (to assess the endurance of each subject at their nontrained load). The second visit required subjects to complete a single set to fatigue using the percentage of 1RM which they would train with for the 10-wk study. Both the total number of repetitions completed and the total work (the product of repetitions and load) completed were recorded for each test.
Muscle fiber cross-sectional area (CSA).
Muscle fibers were oriented vertically by visual inspection and embedded in optimal cutting temperature (OCT) medium. The mounted muscle was frozen in isopentane cooled by liquid nitrogen and stored at −80°C until processing for cross-sectional area analysis. Cross sections (10 μm thick) were cut, mounted on glass slides, and stained using a myofibrillar ATPase histochemisty procedure that uses an acid preincubation pH of 4.6 to distinguish type I and type II fibers as described previously (24, 33). Pictures of the stained slides were taken using a light microscope and NIS Elements 3.0 Imaging Software (Nikon, NY). An average of 55 fibers of each type (type I and II) were outlined using ImageJ software (U. S. National Institutes of Health, Bethesda, MD) for each subject.
Statistics.
Our mixed design did not permit us to make within-subject comparisons; therefore, between-condition differences (muscle hypertrophy, anabolic signaling, and performance) were tested with a blocked two-factor (condition × time) analysis of variance with repeated measures on time, where applicable. A Tukey's post hoc test was used to test for significant interactions. For all analyses differences were considered significant at P < 0.05. All results are presented as means ± SE.
RESULTS
Muscle hypertrophy.
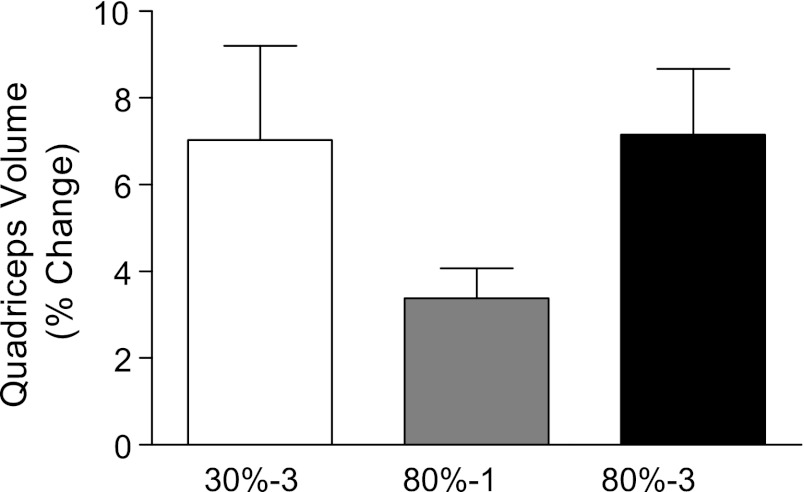
Prior to training, quadriceps muscle volume was 1,581 ± 242, 1,602 ± 215, and 1,529 ± 207 cm3 in the 30%-3, 80%-1, and 80%-3 groups, respectively (no differences between conditions at baseline). After 10 wk of training, the quadriceps muscle volume increased significantly in all groups (P < 0.001) to 1,676 ± 198, 1,651 ± 213, and 1,633 ± 198 cm3 in the 30%-3, 80%-1, and 80%-3 groups, respectively. Figure 1 depicts these data expressed as percentage change from baseline. Average type I and type II muscle fiber area increased with training (both P < 0.05), irrespective of training condition with no significant between-group differences (Table 1).
Fig. 1.
Percentage change in quadriceps muscle volume following 10 wk of resistance training. The 3 training condition groups differed in contraction intensity [% of maximal strength (1 repetition maximum)] or contraction volume (1 or 3 sets of repetitions): 30%-3, 80%-1, and 80%-3. There was a significant main effect for time (increase in quadriceps volume pre- to posttraining, P < 0.0001). N = 12 legs in each condition.
Table 1.
Changes in knee extension performance and vastus lateralis fiber area following 10 wk of resistance training
| MVC, N · m |
RFD, N · m/s |
Average Type I Fiber Area, μm2 |
Average Type II Fiber Area, μm2 |
Percentage Change in Fiber Size |
Knee Extension Peak Power, W |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Pre | Post* | Pre | Post* | Pre | Post* | Pre | Post* | Type I | Type II | Pre | Post* |
| 80%-1 set | 192 ± 37 | 248 ± 52 | 768 ± 150 | 1,259 ± 256 | 4,532 ± 739 | 5,163 ± 589 | 6,430 ± 869.5 | 7,604 ± 1,019 | 16 ± 7% | 20 ± 5% | 589 ± 88 | 808. ± 106 |
| 80%-3 sets | 188 ± 42 | 255 ± 47 | 817 ± 101 | 1,110 ± 234 | 4,268 ± 701 | 4,970 ± 882 | 5,873 ± 715.4 | 6,784 ± 1,019 | 17 ± 4% | 16 ± 5% | 626 ± 113.2 | 805 ± 124 |
| 30%-3 sets | 219 ± 37 | 278 ± 52 | 747 ± 186 | 1248 ± 335 | 4,315 ± 685 | 5,324 ± 1,167 | 6,052 ± 1,189.6 | 6,782 ± 1,100 | 30 ± 12% | 18 ± 8% | 607 ± 113.2 | 805 ± 124 |
Values are presented as means ± SE; N = 12 legs in each conditions for all comparisons. RFD, rate of force development; MVC, maximal voluntary contraction.
Significant main effect pre- vs. post (P < 0.05).
Western blots.
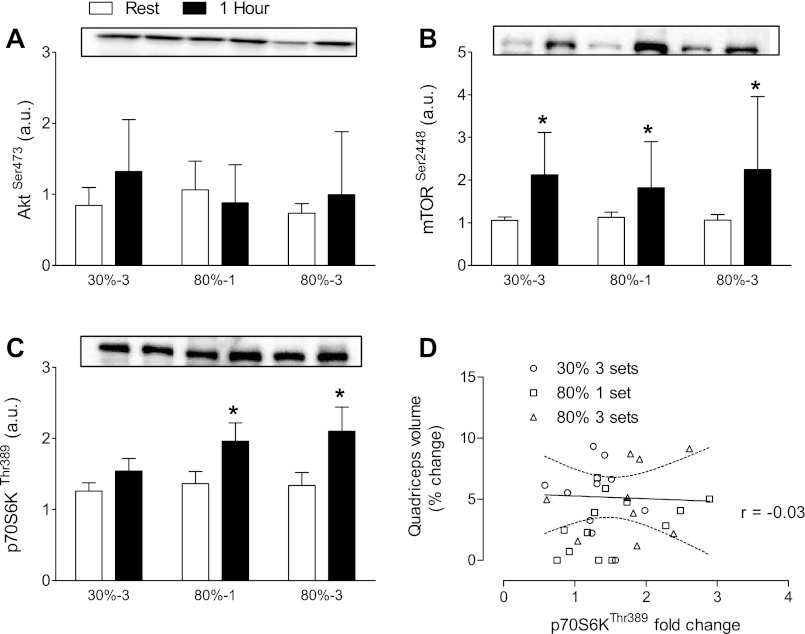
Phosphorylation of Akt at Ser473 was not elevated in any of the conditions. Phosphorylation of mTOR at Ser2448 was elevated above rest at 1 h postexercise in all conditions (P = 0.0002). p70S6K phosphorylation at Thr389 was elevated 1 h postexercise in the 80%-1 and 80%-3 groups, but not 30%-3. There was no correlation between the degree of p70S6K phosphorylation at Thr389 and changes in muscle volume within any training condition (all P > 0.3). Overall, there was no correlation between the degree of p70S6K phosphorylation at Thr389 and the magnitude of quadriceps hypertrophy (r = −0.03, P = 0.88; Fig. 2D). There was also no correlation between the degree of p70S6K phosphorylation at Thr389 and the magnitude of fiber hypertrophy for either fiber (data not shown).
Fig. 2.
Phosphorylated Akt, mammalian target of rapamycin (mTOR), and P70S6K at rest and 1 h following resistance exercise. A: Akt phosphorylated at Ser 473 expressed relative to total Akt. There are no significant differences between conditions. B: mTOR phosphorylated at Ser 2448 relative to total mTOR. *Main effect for greater phosphorylated mTOR 1 h following resistance exercise (P < 0.05). C: p70S6K phosphorylated at Thr 389 relative to total p70S6K. *Significant increases in phosphorylated p70S6K 1 h after 1 or 3 sets at 80% of one repetition maximum (1RM) but not 3 sets at 30% of 1RM (P < 0.05). D: correlation between p70S6K phosphorylation and muscle hypertrophy, r = −0.03 (P = 0.88). N = 12 legs in each condition.
Muscle function.
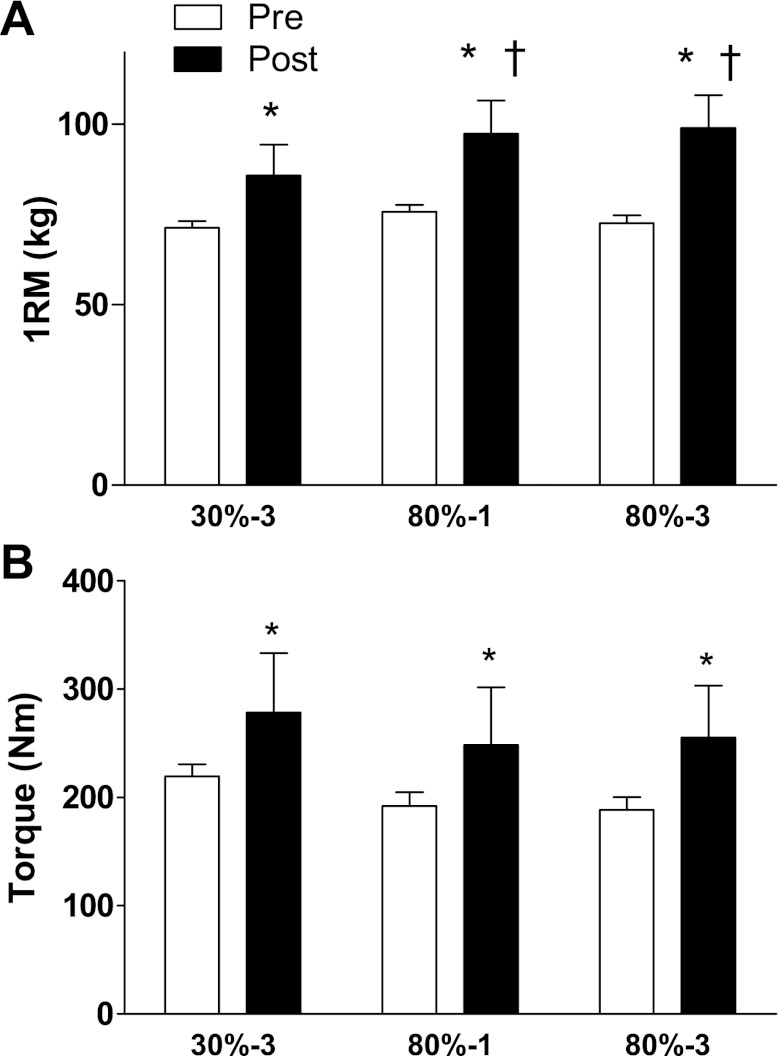
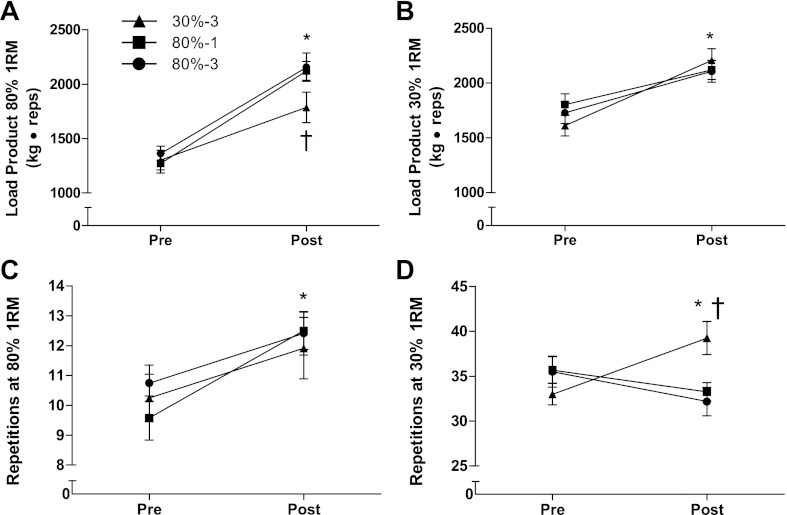
Initial unilateral knee extension 1RM was 71 ± 2 kg in the 30%-3 condition, 76 ± 2 kg in the 80%-1 condition, and 73 ± 2 kg in the 80%-1 condition. After the training period all conditions significantly increased in 1RM strength. The increase in 1RM strength was greater in the 80%-1 and 80%-3 conditions compared with the 30%-3 condition (P = 0.04; Fig. 3). MVC force, knee extension maximal power output, and RFD increased in all conditions with no between-condition differences (Table 1). The total work that could be completed with 30% of the subject's 1RM increased with no between-condition differences (Fig. 4). The total work that could be completed with 80% of the subject's 1RM increased in all groups. The magnitude of the increase was significantly less in the 30%-3 condition compared with the other conditions. The number of repetitions that could be performed with 80% of their current 1RM increased in all groups from 10 ± 1 (30%-3), 10 ± 1 (80%-1), and 11 ± 1 (80%-3) pretraining to 12 ± 1 (30%-3), 13 ± 1 (80%-1), and 12 ± 1 (80%-3) with no-between condition differences in the magnitude of the increase. The number of repetitions that could be performed with 30% of 1RM increased in the 30%-3 condition; the other conditions did not increase.
Fig. 3.
Knee extension strength. A: the maximal load that could be lifted prior to training and after 10 wk of training. *Significantly greater than prior to training (P < 0.05). †Significantly greater than the 3-sets at 30% of 1RM condition. B: single leg isometric knee extension torque before and after 10 wk of training. *Significantly greater than prior to training (P < 0.05). N = 12 legs in each condition.
Fig. 4.
Maximal work and repetitions with 30 and 80% of 1RM before and after 10 wk of resistance training. Load product (in arbitrary units) is the product of load in kg and repetitions completed. A: work completed at 80% of the subject's 1RM (determined within 1 wk of the test). *Significant main effect for an increase in work performed with 80% of 1RM pre- to posttraining (P < 0.05). †Significantly less work at 80% 1RM after training in the 3-sets at 30% of 1RM condition than the other two groups (P < 0.05). B: work completed with 30% of the subject's 1RM. *Significant main effect for an increase in work performed with 30% of 1RM pre- to posttraining (P < 0.05). C: maximal number of repetitions completed with 80% of 1RM (determined within 1 wk of the test). *Significant main effect for an increase in the number of repetitions performed with 80% of 1RM pre- to posttraining (P < 0.05). D: maximal number of repetitions completed with 30% of 1RM. *3-sets at 30% of 1RM condition completed more repetitions after training than before (P < 0.05). †3-sets at 30% of 1RM condition completed more repetitions after training than either of the other groups. N = 12 legs in each condition.
DISCUSSION
We have advocated a model of resistance exercise-induced human skeletal muscle hypertrophy that, when supported by adequate nutrition, arises over time due to summed incremental acute increases in muscle protein synthesis that occur after each training session (27, 28). Thus, as a proof-of-principle of this model, we tested here whether the acute changes we observed in two previous studies comparing different volumes of work (1 set vs. 3 sets of exercise) (7) and divergent intensities of work (high-load vs. low-load lifting) (9) would be borne out in a long-term study. We discovered that there was no difference in the magnitude of quadriceps muscle hypertrophy, as determined by both MRI and muscle fiber area, between legs that trained at 30% or 80% of 1RM after 10 wk of knee-extensor exercise. Interestingly, there was no statistical difference in the degree of quadriceps hypertrophy between the 80%-1 and 80%-3 conditions, despite a mean gain in quadriceps volume of ∼7% in the 80%-3 condition and only ∼3% in the 80%-1 condition (P = 0.18). However, the 80%-3 and 30%-3 showed more than double the average hypertrophy of the 80%-1 condition. These results, while not quantitatively congruent with our acute data (7, 9), are, we propose, broadly supportive of the framework we have proposed of how muscle hypertrophy arises (27, 28). Moreover, our results are actually congruent with a number of other lines of evidence showing that lifting lighter loads, so long as fatigue is induced, leads to roughly equivalent hypertrophy and strength gains (23, 36–38). There are of course a number of factors, beyond acute changes in muscle protein synthesis, which contribute to hypertrophy. In fact, when subjects are stratified as high and low responders, 20–25% subjects exhibit a very limited hypertrophic response where as the top 20–25% show robust muscle hypertrophy that is four to five times greater than that seen in low responders (17, 26). To date, factors such as changes in microRNA expression (12), satellite cell number (26), and intramuscular anabolic signaling protein activation (39) have been shown to be related to the variability in training response, but systemic hormonal factors do not play a role (41–43).
Perhaps the most interesting finding from our work is that hypertrophy in the 80%-3 and 30%-3 conditions was equivalent, which is in contrast to the range of lifting intensities usually prescribed to promote muscle hypertrophy (1, 10). However, the current recommendations (1, 10) ignore a large body of evidence showing that lower loads, when combined with vascular occlusion, promote equivalent hypertrophy and strength gains as that observed with conventional heavy training (36–38). These results indicate that at least in principle lower load are effective at inducing muscle hypertrophy. Moreover, most work that has investigated different relative resistance training loads has focused on muscle function measurements such as 1RM and relative endurance (5, 6, 13, 35). In one study by Campos and colleagues (10), 8 wk of training in a 20- to 28-repetition range did not elicit hypertrophy despite increases in 1RM strength and the number of repetitions that could be completed with 60% of 1RM (10). However, in a subsequent study in which the training methods employed previously by Campos et al. (10) were replicated, equivalent hypertrophy was found in high- and low-load training groups (23). It is often claimed (1, 10) that high relative training loads are necessary to induce hypertrophy as they are associated with full muscle fiber recruitment and activation of the type II fibers which are known to be more “responsive” to hypertrophy (40). This statement is, however, only accurate during a single effort (repetition) since Henneman's size principle of motor unit activation states that motor units are recruited in an orderly fashion from smallest to largest with increasing requirement for force generation (18, 19). Thus it is true that an isometric contraction performed at 30% 1RM will recruit less muscle than a contraction preformed at 80% of 1RM (2). In agreement, it has also been shown that nonfatiguing acute resistance exercise or chronic resistance training results in lower rates of MPS (21) and hypertrophy (20) compared with heavier resistance exercise loads, respectively. However, when a submaximal contraction is sustained, motor units that were initially recruited will fatigue (produce less force) or cease firing completely necessitating the recruitment of additional motor units (15) to sustain force generation. In this way, as the repetitions at lighter loads are repeated, the point of failure/fatigue ultimately necessitates near maximal motor unit recruitment to sustain muscle tension (16). Thus relatively lighter loads lifted to the point of failure would result in a similar amount of muscle fiber activation compared with heavier loads lifted to failure (18, 31); however, we acknowledge it is difficult to experimentally verify this motor unit recruitment strategy during voluntary dynamic contractions in humans. In the present study, the average area of both type I and II fibers increased equally with heavy and light relative loads, which is suggestive that both fiber types were recruited during training and to a roughly equivalent extent, at least insofar as the phenotypic hypertrophy response is an indication of this.
The regulation of muscle mass is the result of small changes in net muscle protein balance over time. MPS can be increased by resistance exercise alone; however, adequate and properly timed protein consumption is required for a positive net muscle protein balance and thus muscle hypertrophy (8). It is not completely clear how resistance exercise results in increases in MPS as this process is multifaceted. One potentially important step is the activation of p70S6K, which is a downstream protein target of mTORC1 that when activated upregulates initiation of mRNA translation and subsequently increases muscle protein synthesis rates (14). It has been reported that heavy resistance exercise results in an increase in p70S6K phosphorylation (7, 9, 42); our data, at least in the 80%-1 and 80%-3 conditions, broadly agree with these findings. We did not observe an increase in p70S6K phosphorylation in the 30%-3 condition at 1 h postexercise, but we have observed elevated phosphorylation of p70S6K at 4 h after exercise when subjects have completed the same protocol (9). Taken together these results suggest that heavy and light relative loads lifted until the point of failure may result in a different time course of anabolic signaling, with p70S6K phosphorylation occurring later after exercise with light compared with heavy relative loads. Previously, a significant correlation between early (1 h postexercise) phosphorylation of p70S6K and muscle hypertrophy was observed (39); our results, which include a larger sample size than the previous investigation, failed to support a similar relationship (Fig. 2D).
Although relative training load did not impact the magnitude of the hypertrophic response, it did have a clear impact on voluntary isotonic strength gains. Both the 80%-1 and 80%-3 conditions demonstrated a larger increase in 1RM strength compared with the 30%-3 group. These results suggest that practice with a heavy relative load is necessary to maximize gains in 1RM strength of the trained movement. These observations are in line with previous work that has shown that strength gains are specific to the movement that is trained (30) and strength gains are due to a combination of muscle hypertrophy and neural adaptations (32). It should be noted, however, that similar gains in MVC strength, maximal instantaneous power output, and rate of force development were seen across all three training conditions. These data show that hypertrophy is generally beneficial to all strength and power tests that engage the larger muscle. However, it appears that neural adaptations are largely specific to the movement and load used in training (32).
One potential limitation to the unilateral training model employed in this study is the cross education effect where resistance training of one muscle can lead to neurally mediated strength gains in the untrained contralateral muscle (22). The magnitude of the effect varies widely but averages ∼7% (22). However, to the author's knowledge there has been no attempt to quantify this effect when both limbs are training with different protocols. We found no correlation between isotonic strength gains in the left and right legs (r = 0.33, NS), suggesting that the cross education effect is minimal or nonexistent when both limbs are training with different protocols.
In summary, we report that similar resistance training-induced muscle hypertrophy can result from lifting loads to failure with higher (80% of 1RM) and lower (30% of 1RM) loads than are currently recommended for novice lifters (1). The results from our study also suggest that additional training volume in the form of more sets may result in greater muscle hypertrophy; however, due to the inherent variability in the individual response to resistance training, it appears that longer-term training studies may be required to manifest these differences more clearly. Importantly, these data support the concept that acute increases in rates of MPS are reasonable qualitative indexes of the amount of muscle protein gain with similar training as they appear to be with nutrition. However, these gains in muscle mass may be dependent in the adequate provision of amino acids (3). Finally, despite that lack of support for the idea of a hypertrophy-specific load and repetition range, these data confirm the specificity principle of training with regard to muscle strength and endurance.
GRANTS
This work was supported by a grant from the National Science and Engineering Research Council (NSERC) of Canada to S. M. Phillips. C. J. Mitchell and T. A. Churchward-Venne were supported by NSERC Postgraduate Scholarships while performing this work and D. D. West by a Canada Graduate Scholarship from the Canadian Institutes for Health Research (CIHR).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.J.M., T.A.C.-V., D.D.W., N.A.B., L.B., and S.M.P. conception and design of research; C.J.M., T.A.C.-V., D.D.W., N.A.B., L.B., S.K.B., and S.M.P. performed experiments; C.J.M., T.A.C.-V., D.D.W., N.A.B., L.B., and S.M.P. analyzed data; C.J.M., T.A.C.-V., D.D.W., N.A.B., L.B., S.K.B., and S.M.P. interpreted results of experiments; C.J.M. prepared figures; C.J.M., D.D.W., L.B., and S.M.P. drafted manuscript; C.J.M., T.A.C.-V., D.D.W., N.A.B., L.B., S.K.B., and S.M.P. edited and revised manuscript; C.J.M., T.A.C.-V., D.D.W., N.A.B., L.B., S.K.B., and S.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge Nestle Nutrition for the donation of the PowerBar Protein Plus bars for this study.
REFERENCES
- 1. ACSM American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41: 687–708, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Alkner BA, Tesch PA, Berg HE. Quadriceps EMG/force relationship in knee extension and leg press. Med Sci Sports Exerc 32: 459–463, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, Suetta C, Magnusson P, Aagaard P. The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism 54: 151–156, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Berger R. Optimum repetitions for the development of strength. Res Q 33: 1962 [Google Scholar]
- 6. Berger RA, Hardage B. Effect of maximum loads for each of ten repetitions on strength improvement. Res Q 38: 715–718, 1967 [PubMed] [Google Scholar]
- 7. Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol 588: 3119–3130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol 106: 1692–1701, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One 5: e12033, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 88: 50–60, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Carpinelli RN, Otto Strength training RM Single versus multiple sets. Sports Med 26: 73–84, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol 110: 309–317 [DOI] [PubMed] [Google Scholar]
- 13. Delorme TL. Restoration of muscle power by heavy-resistance exercises. J Bone Joint Surg Am 27: 645–667, 1945 [Google Scholar]
- 14. Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 106: 1374–1384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fallentin N, Jorgensen K, Simonsen EB. Motor unit recruitment during prolonged isometric contractions. Eur J Appl Physiol Occup Physiol 67: 335–341, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460: 549–572, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 86: 373–381, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957 [DOI] [PubMed] [Google Scholar]
- 19. Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 28: 560–580, 1965 [DOI] [PubMed] [Google Scholar]
- 20. Holm L, Reitelseder S, Pedersen TG, Doessing S, Petersen SG, Flyvbjerg A, Andersen JL, Aagaard P, Kjaer M. Changes in muscle size and MHC composition in response to resistance exercise with heavy and light loading intensity. J Appl Physiol 105: 1454–1461, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee M, Carroll TJ. Cross education: possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med 37: 1–14, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576: 923–933, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oates BR, Glover EI, West DW, Fry JL, Tarnopolsky MA, Phillips SM. Low-volume resistance exercise attenuates the decline in strength and muscle mass associated with immobilization. Muscle Nerve 42: 539–546, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Peterson MD, Rhea MR, Alvar BA. Applications of the dose-response for muscular strength development: a review of meta-analytic efficacy and reliability for designing training prescription. J Strength Cond Res 19: 950–958, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Phillips SM. Protein requirements and supplementation in strength sports. Nutrition 20: 689–695, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Phillips SM. Short-term training: when do repeated bouts of resistance exercise become training? Can J Appl Physiol 25: 185–193, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Ronnestad BR, Egeland W, Kvamme NH, Refsnes PE, Kadi F, Raastad T. Dissimilar effects of one- and three-set strength training on strength and muscle mass gains in upper and lower body in untrained subjects. J Strength Cond Res 21: 157–163, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Sale D, MacDougall D. Specificity in strength training: a review for the coach and athlete. Can J Appl Sport Sci 6: 87–92, 1981 [PubMed] [Google Scholar]
- 31. Sale DG. Influence of exercise and training on motor unit activation. Exerc Sport Sci Rev 15: 95–151, 1987 [PubMed] [Google Scholar]
- 32. Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc 20: S135–145, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Shepstone TN, Tang JE, Dallaire S, Schuenke MD, Staron RS, Phillips SM. Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J Appl Physiol 98: 1768–1776, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Starkey DB, Pollock ML, Ishida Y, Welsch MA, Brechue WF, Graves JE, Feigenbaum MS. Effect of resistance training volume on strength and muscle thickness. Med Sci Sports Exerc 28: 1311–1320, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Stone WC, Coulter SP. Strength/endurance effects from three resistance training protocols with women. J Strength Cond Res 8: 231–234, 1994 [Google Scholar]
- 36. Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 86: 308–314, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88: 2097–2106, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Tanimoto M, Ishii N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J Appl Physiol 100: 1150–1157, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102: 145–152, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Thorstensson A, Hulten B, von Dobeln W, Karlsson J. Effect of strength training on enzyme activities and fibre characteristics in human skeletal muscle. Acta Physiol Scand 96: 392–398, 1976 [DOI] [PubMed] [Google Scholar]
- 41. West DW, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, Baker SK, Phillips SM. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol 108: 60–67, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 587: 5239–5247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. West DW, Phillips SM. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol (November 22, 2011) doi:10.1007/s00421-011-2246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr 85: 1031–1040, 2007 [DOI] [PubMed] [Google Scholar]